Abstract
Background
The aim of this study was to investigate the effects of smokeless tobacco (Maras powder, Nicotiana rustica Linn) on carotid intima media thickness.
Material/Methods
The study included 32 Maras powder users presenting to our Family Medicine outpatient clinic and 30 non-users of Maras powder as a control group. Carotid intima media thickness was measured by duplex ultrasonography.
Results
All the participants were male. The mean duration of Maras powder use was 27.3±11.6 years. Carotid intima media thickness was 0.73±0.20 mm in the Maras powder users and 0.49±0.14 mm in the controls. Blood pressure measured before Maras powder use in Maras powder users was similar to that measured in the control group (p>0.05). Systolic blood pressures were 136.6±12.4 mmHg and 109.7±9.7 mmHg after 30–60 minutes using Maras powder in the Maras powder users and in the controls, respectively. Diastolic blood pressures were 87.2±6.1 mmHg and 62.8±8.1 mmHg after 30–60 minutes using Maras powder in the Maras powder users and the controls, respectively. Carotid intima media thickness was significantly correlated with systolic blood pressure (r=0.613, p<0.001) and diastolic blood pressure (r=0.612, p<0.001).
Conclusions
Carotid intima media thickness was higher in Maras powder users than in nonusers of the powder. Increased carotid intima media thickness can be associated with an immediate increase in systolic and diastolic blood pressures. Therefore, attempts to increase public awareness about smoking should also be directed towards prevention of Maras powder use.
Keywords: Maras powder, carotid intima media thickness, subclinical atherosclerosis, Nicotiana rustica Linn
Background
Tobacco use is still an important cause of morbidity and mortality over the world [1]. Smoking not only affects many organ systems, but also causes hypoxia in vascular structures and thus formation of atheromas in the intima and deterioration of existing atheromas. In addition, it increases low-density lipoprotein (LDL) cholesterol and VLDL cholesterol, while it decreases high-density lipoprotein (HDL) cholesterol. For these reasons, smoking is known to initiate and accelerate atherosclerotic process through many mechanisms [2,3].
Maras powder, or Nicotiana rustica Linn, is obtained by crushing leaves of a wild tobacco plant into powder and mixing it with ashes of oak, walnut, and vine stems in the ratio of one-half or one-third [4]. Nicotine concentrations in tobacco used to produce Maras powder are 8 to 10 times higher than those in tobacco used to produce cigarettes. Tobacco used to make Maras powder is smokeless and consumed by chewing and sucking. It is widely consumed in the eastern Mediterranean region of Turkey. In fact, the rate of Maras powder use has been reported to be 4% and 16.8% in 2 studies carried out in our country [5,6]. The way Maras powder is consumed is known to increase oxidative stress and thus quicken the atherosclerotic process [7,8].
Clinically evident atherosclerosis is preceded by preclinical changes in the arterial wall, specifically, intima-media complex thickening and plaque formation [9]. After the first report of intima-media thickness measurement by Pignoli et al, carotid intima-media thickness (CIMT) has begun to be used as a reliable and reproducible method in the diagnosis of atherosclerosis [10,11].
We aimed to determine the effects of Maras powder use on CIMT.
Material and Methods
Study population
Thirty-two Maras powder users presenting to the family medicine outpatient clinic to seek help giving up smoking between January 2013 and March 2013 were selected. The control group comprised 30 age-matched healthy volunteers. Medical history was taken and detailed physical examinations were performed in all subjects. The inclusion criterion was using Mara powder for at least 3 years. A package of Maras powder was considered as sufficient to provide 20 uses of the powder.
Data about demographic characteristics, biochemical parameters, lipid values, and electrocardiograms of the study sample were obtained. Exclusion criteria were: patients with hypertension (systolic >140 mmHg/diastolic 90 mmHg), diabetes mellitus, chronic obstructive lung disease, coronary artery disease, chronic renal failure, obesity, and osteoporosis (diseases likely to affect CIMT), history of taking medications for the above-mentioned diseases, and history of smoking and alcohol consumption. There were similar blood lipid levels and BMI between in the study and control groups.
Written informed consent was obtained from each subject and the institutional ethics committee approved the study protocol. Data about socio-demographic features, Maras powder use, and history of diseases were collected with a questionnaire.
Measurements
Blood pressure was measured with sphygmomanometer on both right and left arms while the patients were sitting after 10 minutes of resting. Measurements were made 3 times and the median of 3 measurements was obtained. In the Maras Powder users group, we measured blood pressure values before using Maras powder and 30–60 minutes after using Maras powder. Body weight was measured to the nearest 0.5 kg in light clothing without shoes. Height was measured to the nearest 0.5 cm. Body mass index (BMI) was calculated as kilograms divided by square meters (kg/m2).
Biochemical analyses and complete blood count
After 12 hours of fasting, 8 ml blood was drawn from the antecubital vein with minimal tourniquet method from each patient. Total cholesterol, triglycerides, LDL cholesterol, HDL cholesterol, and blood sugar concentrations were measured.
Evaluation of CIMT
Duplex ultrasound scanning was performed by a single trained radiologist using a high-end ultrasound machine (Aplio XG machine, Toshiba Medical Systems, Co, Ltd., Otawara, Japan), with 7 MHz or 10 MHz linear-array transducers. Subjects were examined in the supine position with their neck extended and their head turned 45° to the left or right. Ultrasound scans of the right and left last distal centimeter of the common carotid arteries (CCAs), the bifurcation, and of the first proximal centimeter of the internal carotid arteries in 3 different projections (anterior, lateral, and posterior) were performed. All subjects had a CIMT measurement at the far wall of both distal segments of the CCAs, and the measurements were made at the time of scanning on frozen images of longitudinal scans by using an automated border-detection program of the instrument. Two carotid segments for each projection were examined and the maximal CIMT value of each segment was measured. The CIMT values for the 3 different projections and for the right and left carotid arteries were averaged to obtain the mean maximum CIMT.
Statistical analyses
Statistical analyses were performed using SPSS software version 15. All continuous variables are expressed as mean ± standard deviation; categorical variables are defined as percentages. Mean values of continuous variables were compared between groups using the t test or Mann-Whitney U test, according to whether they were normally distributed or not, as tested by the Kolmogorov-Smirnov test. Pearson’s correlation coefficients were used to assess the strength of the relationship between continuous variables. Before and after using Maras powder, mean blood pressure values in the Maras powder users group were analyzed by paired t test. A p-value of less than 0.05 was considered to show a statistically significant result.
Results
The mean age of the patients included in the study was 46.3+11.6 years (min=22; max=64). The mean age of Maras powder users was 45.4±11.3 years (min=27; max=67). All patients included in the study were male. BMI was 27.1±3.0 in the control group and 26.5±6.3 in the Maras powder users, without a significant difference (p=0.657). The mean duration of Maras powder use was 27.3±11.6 years (min=3, max=48). The mean amount of Maras powder consumed a day was 1 package.
There was a significant difference in CIMT between the Maras powder users and the controls (p<0.001). It was higher in the Maras powder users (Table 1).
Table 1.
Blood pressure, lipid profile, fasting glucose concentrations and carotid intima media thickness in patients included in the study.
| Parameters | Groups | p-value | |
|---|---|---|---|
| Controls (mean ±SD) | Maras powder users (mean ±SD) | ||
| BMI (kg/m2) | 27.1±3.0 | 26.5±6.3 | 0.657 |
| Systolic blood pressure (mmHg) | 109.7±9.7 | 136.6±12.4* | <0.001 |
| Diastolic blood pressure (mmHg) | 62.8±8.1 | 87.7±6.1* | <0.001 |
| Total cholesterol (mg/dl) | 184.4±36.9 | 166.8±45.1 | 0.112 |
| LDL-cholesterol (mg/dl) | 111.3±30.1 | 108.7±44.1 | 0.794 |
| HDL-cholesterol (mg/dl) | 44.6±11.4 | 41.2±8.4 | 0.212 |
| Triglycerides (mg/dl) | 141.8±100.2 | 154.0±106.1 | 0.654 |
| Fasting blood glucose level (mg/dl) | 92.6±12.9 | 103.2±36.7 | 0.143 |
| CIMT (mm) | 0.49±0.14 | 0.73±0.20 | <0.001 |
Blood pressure levels of 30–60 minutes after using Maras powder.
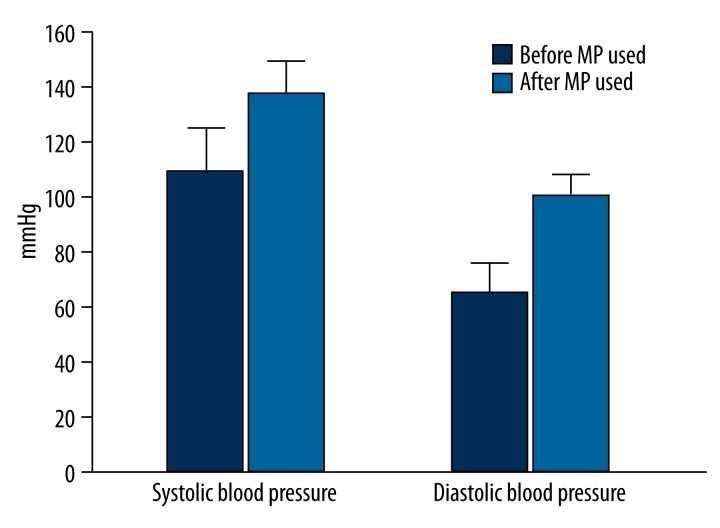
Before using Maras powder, the mean systolic blood pressure was 110.3±9.4 and the mean diastolic blood pressure was 62.8±8 in the Maras powder users. A comparison of blood pressures measured before and after using Maras powder in the Maras powder users is shown in Figure 1.
Figure 1.
Comparison of blood pressure levels before and after using Maras powder in Maras powder users.
There were no significant differences in terms of BMI, LDL cholesterol, HDL cholesterol, triglycerides, total cholesterol, and fasting blood sugar levels between the controls and Maras powder users (p>0.05). However, systolic and diastolic blood pressures measured after using Maras powder in the Maras powder users significantly differed from those measured in the control group (p<0.001). Maras powder use was found to increase systolic and diastolic blood pressures after using Maras powder (Table 1).
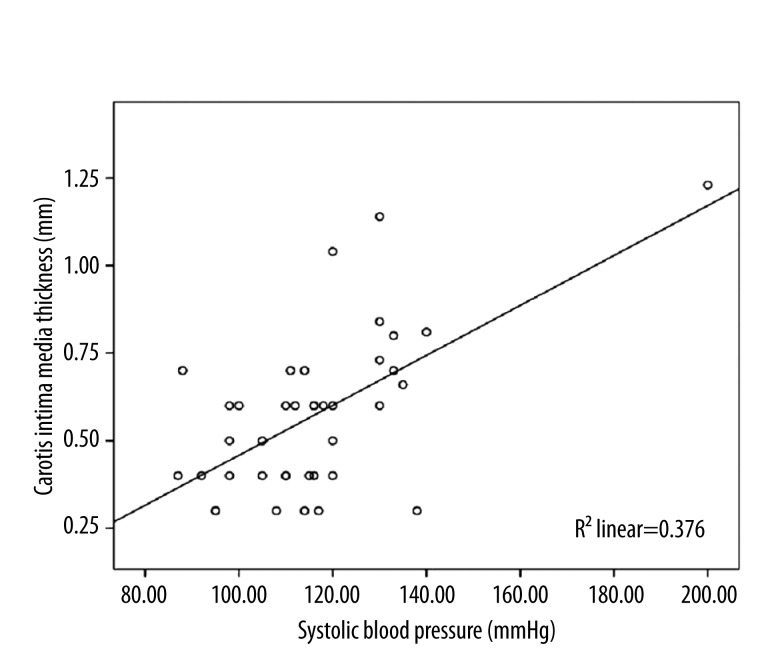
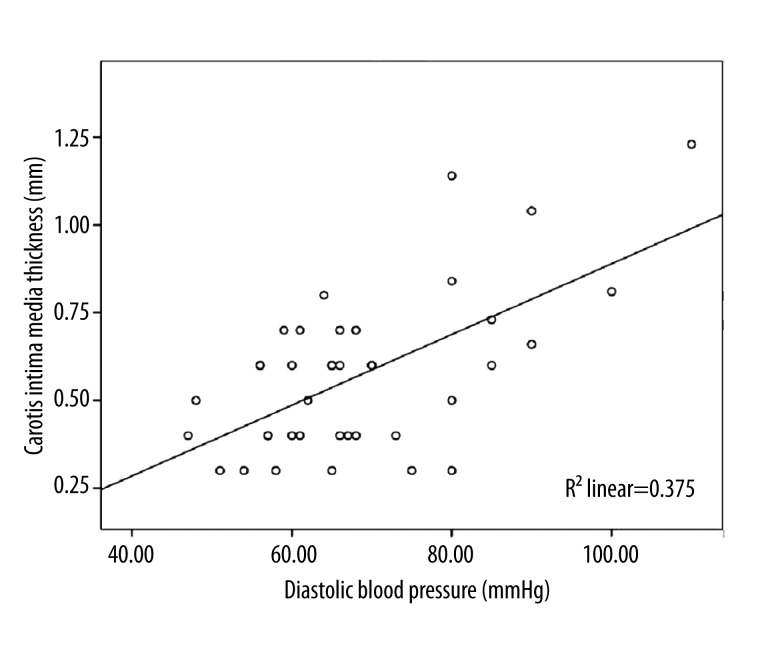
Results of correlation analysis of variables likely to be associated with CIMT are presented in Table 2. CIMT was significantly correlated with systolic blood pressure (r=0.613, p<0.001) and diastolic blood pressure (r=0.612, p<0.001), (Figures 2 and 3). There was not a significant correlation between CIMT and duration of Maras powder use and amount of Maras powder consumed (r=0.212; p=0.253) (Table 2).
Table 2.
Results of Pearson correlation analysis on all patients included in the study.
| Parameters | CIMT | |
|---|---|---|
| r | p-value | |
| BMI | 0.35 | 0.006 |
| Systolic Blood Pressure | 0.613 | <0.001 |
| Diastolic Blood Pressure | 0.612 | <0.001 |
| Total cholesterol | 0.177 | 0.188 |
| LDL-cholesterol | 0.319 | 0.015 |
| HDL-cholesterol | −0.240 | 0.072 |
| Triglycerides | 0.260 | 0.049 |
| Fasting blood glucose level | 0.384 | 0.003 |
Figure 2.
Significant positive correlation between carotid intima media thickness and systolic blood pressure measured after using Maras powder (r=0.613, p<0.001).
Figure 3.
Significant positive correlation between carotid intima media thickness and diastolic blood pressure measured after using Maras powder (r=0.612, p<0.001).
Discussion
It has been reported that Maras powder use increases oxidative stress and thus quickens atherosclerosis in addition to its negative effects on many tissues and organ systems [7,8,12–14].
In the present study, we found that Maras powder use significantly increased CIMT. In fact, it was 0.73 mm in the Maras powder users and 0.49 mm in the controls. To our knowledge, there have not been any studies investigating the effects of Maras powder use on CIMT. In a study examining the effects of tobacco use, especially effects of smoking on CIMT, smoking was shown to affect lipid profiles and increase CIMT [15]. Several studies also revealed that tobacco use caused hypertension and increased oxidative stress and, as a result, atherosclerosis developed [16–18]. In the present study, increased CIMT might be related to an acute increase in blood pressure due to use of Maras powder.
In this study, Pearson correlation analysis showed a significant correlation between CIMT and systolic blood pressure and diastolic blood pressure. Other variables likely to be associated with CIMT such as BMI, LDL-cholesterol, and blood sugar levels were significantly but weakly correlated with CIMT. It has been reported in several studies that blood pressure and lipid profile disorders increase CIMT [16–19].
In a study on effects of Maras powder use on biochemical and hematological parameters, Maras powder users had significantly higher concentrations of total cholesterol, LDL cholesterol, and triglycerides than controls [13]. However, we did not find a significant difference in total cholesterol, HDL cholesterol, LDL cholesterol, and triglycerides between the Maras powder users and the controls. This can be explained by the fact that gender and age groups in this study differed from those in other studies.
In this study, Maras powder use was found to increase systolic blood pressure and diastolic blood pressure, which is congruent with the literature. It has also been shown that increased CIMT was associated with acute hypertension [15–18]. It has been thought that Maras powder use increased CIMT by means of its effects on systolic and diastolic blood pressures. As reported in the literature, this can be explained by the fact that nicotine stimulates sympathic nerve endings and increases adrenaline release, which in turn increases blood pressure [19,20].
In the present study, we did not find a significant difference in blood sugar concentrations, considered one of the conditions increasing CIMT, between the Maras powder users and the controls. In a similar study, Maras powder use did not affect blood sugar concentrations [7], which is consistent with the present study.
In the present study, there was not a significant correlation between duration and amount of Maras powder used and CIMT. However, the small sample size of this study might have had an influence on this result. In addition, studies on effects of smoking on CIMT have revealed that passive smoking contributed to the positive correlation between duration of smoking and CIMT [21,22]. However, the fact that there is no passive Maras powder (as in passive smoking) exposure might have played a role in our results.
Limitations of the study
The major limitation of this study is its cross-sectional design. Another important limitation is that the sample size of the study was small since the patients both smoking and using Maras powder, which constituted a large number of the patients, were not included in the study. Another important limitation of the study was that we did not investigate markers for oxidative stress likely to elucidate the association between Maras powder use and CIMT. Investigation of these markers may explain the mechanism whereby Maras powder increases CIMT.
Conclusions
We showed that Maras powder use increased CIMT, which is an indicator of subclinical atherosclerosis. Increased CIMT might be associated with an acute increase in blood pressure due to use of Maras powder. However, further studies with larger sample sizes are needed to clarify the association between Maras powder use and pathogenesis of atherosclerosis. In addition, development of public health policies is of great importance in terms of prevention of Maras powder use. In addition, long-term Maras powder users should be screened and early diagnosis and treatment of possible conditions should be performed. This issue should also be investigated through studies with larger samples.
Acknowledgements
This manuscript was presented as an oral presentation at the 12th National Congress of Turkish Family Medicine, Kusadası, Turkey, on 15–19 May 2013.
Footnotes
Competing interests
The authors declare they have no competing interests.
Source of support: Departmental sources
Funding
We did not receive any funding for this study from grants or foundations.
References
- 1.Bartecchi CE, MacKenzie TD, Robert W. The human costs of tobacco use. Schrie. N Engl J Med. 1994;330:907–12. doi: 10.1056/NEJM199403313301307. [DOI] [PubMed] [Google Scholar]
- 2.Shennan NM, Seed M, Wynn V. Variation in serum lipid and lipoprotein levels associated with changes in smoking behaviour in non obese Caucasian males. Atherosclerosis. 1985;58:17–25. doi: 10.1016/0021-9150(85)90052-8. [DOI] [PubMed] [Google Scholar]
- 3.Stein EA, Myers GL. Lipids, lipoproteins and apolipoproteins. In: Burtis CA, Ashwood ER, editors. Tietz textbook of clinical chemistry. 2nd edition. Philadelphia: WB Saunders; 1994. pp. 1002–87. [Google Scholar]
- 4.Aral M, Ekerbicer H, Celik M, et al. Comparison of effects of smoking and smokeless tobacco “Maras powder” use on humoral immune system parameters. Mediators Inflamm. 2006;3:85019. doi: 10.1155/MI/2006/85019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Kafas A. Master’s Thesis. Institute of Science, Department of Agricultural Economics, Kahramanmaras Sutcu Imam University; Turkey: 2011. [last accessed: 13.09.2013]. Analysis of factors affecting cigarete smoking and Maras powder use among adults in the urban area of Kahramanmaras. https://tez.yok.gov.tr/UlusalTezMerkezi/SearchTez. [Google Scholar]
- 6.Sucaklı MH, Kahraman H, Çelik M, Keten HS. An Evaluation of Knowledge, Attitudes and Behavior regarding Smoking and Smokeless Tobacco (Maras Powder) Use among High School Children. 12th National Congress of Turkish Family Medicine; Kusadası, Turkey. 15–19 May 2013; [last accessed: 25.08.2013]. p. 12. http://www.aile2013.org/Presentation_2013.pdf. [Google Scholar]
- 7.Guven A, Tolun F. Effects of smokeless tobacco “Maras Powder” use on nitric oxide and cardiovascular risk parameters. Int J Med Sci. 2012;9(9):786–92. doi: 10.7150/ijms.4563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kilinc M, Okur E, Kurutas EB, et al. The effects of Maras powder (smokeless tobacco) on oxidative stress in users. Cell Biochem Funct. 2004;22:233–36. doi: 10.1002/cbf.1093. [DOI] [PubMed] [Google Scholar]
- 9.Gepner AD, Keevil JG, Wyman RA, et al. Use of carotid intima-media thickness and vascular age to modify cardiovascular risk prediction. J Am Soc Echocardiogr. 2006;19:1170–74. doi: 10.1016/j.echo.2006.04.009. [DOI] [PubMed] [Google Scholar]
- 10.Pignoli P, Tremoli E, Poli A, et al. Intimal plus medial thickness of the arterial wall: a direct measurement with ultrasound imaging. Circulation. 1986;74:1399–406. doi: 10.1161/01.cir.74.6.1399. [DOI] [PubMed] [Google Scholar]
- 11.Steg PG, Bhatt DL, Wilson PW, et al. REACH Registry Investigators. One-year cardiovascular event rates in outpatients with atherothrombosis. JAMA. 2007;297:1197–206. doi: 10.1001/jama.297.11.1197. [DOI] [PubMed] [Google Scholar]
- 12.Kılınç M, Okur E, Yıldırım I, et al. The investigation of the effect of Maras Powder (Smokeless Tobacco) on hematological parameters. Turk J Haematol. 2004;21:131–36. [PubMed] [Google Scholar]
- 13.Güven A, Köksal N, Büyükbeşe MA, et al. Effects of using a different kind of smokeless tobacco on cardiac parameters: “Maras powder”. Anadolu Kardiyol Derg. 2003;3:230–35. [PubMed] [Google Scholar]
- 14.Büyükbese MA, Koksal N, Guven A, Cetinkaya A. Effects of smokeless tobacco “Maras Powder” use on respiratory functions. Tohoku J Exp Med. 2004;204:173–78. doi: 10.1620/tjem.204.173. [DOI] [PubMed] [Google Scholar]
- 15.Woo KS, Chook P, Raitakari OT, et al. Westernization of Chinese adults and increased subclinical atherosclerosis. Arterioscler Thromb Vasc Biol. 1999;19:2487–93. doi: 10.1161/01.atv.19.10.2487. [DOI] [PubMed] [Google Scholar]
- 16.Oren A, Vos LE, Uiterwaal CS, et al. Cardiovascular risk factors and increased carotid intima-media thickness in healthy young adults: the Atherosclerosis Risk in Young Adults (ARYA) Study. Arch Intern Med. 2003;163:1787–92. doi: 10.1001/archinte.163.15.1787. [DOI] [PubMed] [Google Scholar]
- 17.De Waart FG, Smilde TJ, Wollersheim H, et al. Smoking characteristics, antioxidant vitamins, and carotid artery wall thickness among life-long smokers. J Clin Epidemiol. 2000;53:707–14. doi: 10.1016/s0895-4356(99)00198-5. [DOI] [PubMed] [Google Scholar]
- 18.Liang LR, Wong ND, Shi P, et al. Cross-sectional and longitudinal association of cigarette smoking with carotid atherosclerosis in Chinese adults. Prev Med. 2009;49:62–67. doi: 10.1016/j.ypmed.2009.05.006. [DOI] [PubMed] [Google Scholar]
- 19.Mercado C, Jaimes EA. Cigarette smoking as a risk factor for atherosclerosis and renal disease: novel pathogenic insights. Curr Hypertens Rep. 2007;9:66–72. doi: 10.1007/s11906-007-0012-8. [DOI] [PubMed] [Google Scholar]
- 20.Edvinsson ML, Andersson SE, Xu CB, Edvinsson L. Cigarette smoking leads to reduced relaxant responses of the cutaneous microcirculation. Vasc Health Risk Manag. 2008;4:699–704. doi: 10.2147/vhrm.s2285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Mahmoud MZ. Effects of cigarettes smoking on common carotid arteries intima media thickness in current smokers. Ozean J Appl Sci. 2012;5:259–69. [Google Scholar]
- 22.Howard G, Burke G, Szklo M, et al. Active and passive smoking are associated with increased carotid wall thickness. The atherosclerosis risk in communities study. Arch Intern Med. 1994;154:1277–82. [PubMed] [Google Scholar]