Abstract
Purpose
The incidence of pediatric adrenocortical tumors (ACTs) is remarkably high in southern Brazil, where more than 90% of patients carry the germline TP53 mutation R337H. We assessed the impact of early detection of this mutation and of surveillance of carriers.
Patients and Methods
Free newborn screening was offered at all hospitals in the state of Paraná. Parents of positive newborns were tested, and relatives in the carrier line were offered screening. Positive newborns and their relatives age < 15 years were offered surveillance (periodic clinical, laboratory, and ultrasound evaluations). ACTs detected by imaging were surgically resected.
Results
Of 180,000 newborns offered screening, 171,649 were screened, and 461 (0.27%) were carriers. As of April 2012, ACTs had been diagnosed in 11 of these carriers but in only two neonatally screened noncarriers (P < .001); six patient cases were identified among 228 carrier relatives age < 15 years (total, 19 ACTs). Surveillance participants included 347 (49.6%) of 699 carriers. Tumors were smaller in surveillance participants (P < .001) and more advanced in nonparticipants (four with stage III disease; two deaths). Neonatally screened carriers also had neuroblastoma (n = 1), glioblastoma multiforme (n = 1), choroid plexus carcinoma (n = 2), and Burkitt lymphoma (n = 1). Cancer histories and pedigrees were obtained for 353 families that included 1,704 identified carriers. ACTs were the most frequent cancer among carrier children (n = 48).
Conclusion
These findings establish the prevalence of the TP53 R337H mutation in Paraná state and the penetrance of ACTs among carriers. Importantly, screening and surveillance of heterozygous carriers are effective in detecting ACTs when readily curable.
INTRODUCTION
Childhood adrenocortical tumors (ACTs) are rare1 but remarkably frequent in southeastern and southern Brazil,2–4 where > 90% of patients carry a point mutation in exon 10 of TP53, causing the substitution of histidine for arginine (R337H).3 Other low-penetrance TP53 mutations associated with pediatric ACTs suggest that their aggregate frequency worldwide is higher than previously thought.5–9 In the R337H cluster area, ACTs3,10 and choroid plexus carcinoma (CPC)11,12 are the most frequent cancers in carriers age < 15 years, whereas breast, brain, and stomach cancers are most common at older ages.5
In this age group and this region of Brazil, more deaths occur as a result of ACTs than phenylketonuria, cystic fibrosis, or sickle cell disease, which are typically included in neonatal screening. ACTs are easily curable by resection when small, but advanced-stage ACTs carry a poor prognosis.4 Because this mutation is accurately and inexpensively detectable, we investigated the feasibility, cost, and effectiveness of neonatal screening and surveillance of young carriers for ACTs in the state of Paraná, Brazil.
PATIENTS AND METHODS
Study Population
Paraná has approximately 10.5 million residents (35% age < 15 years; approximately 80% urban), whose ancestry is mainly European (77% white; 18% mixed race [African, Asian, Native American]). Mean monthly household income is US$660.13
Screening
Newborn screening (NBS) for the TP53 R337H mutation was offered free at all Paraná hospitals. Of 180,000 newborns offered screening, 171,649 were screened with written consent. All mothers were precounseled about the high incidence and clinical manifestations of ACTs, their association with the mutation, and known health implications for carriers. Mothers were given contact information for remaining questions. Parents of positive newborns received information about ACTs, genetic counseling, and consultation with a study physician. With consent, positive newborns underwent confirmatory testing, and both parents were tested to determine the carrier line. All available relatives from the carrier side were then offered testing. Parents of positive relatives age < 15 years were invited to consent to surveillance; older relatives were counseled and referred to a medical provider. The cancer history of each family was taken by a trained physician or genetic specialist.
Detection of TP53 R337H Mutation
The Data Supplement describes the assay protocol in detail. Briefly, blood samples were blotted directly onto filter paper and processed in 96-well microplates. A 447-bp DNA segment encompassing TP53 exon 10 was amplified by polymerase chain reaction. The amplicon was incubated with HhaI endonuclease. Electrophoresis yielded a single 447-bp fragment (indicating the R337H allele) or two fragments of 154 and 293 bp (wild-type allele). For confirmation, exon 10 was sequenced in all R337H-positive neonatal specimens.
ACT Surveillance
Figure 1 provides a schema of screening and surveillance. ACT surveillance included family education and periodic physical examination, including abdominal ultrasonography and serum dehydroepiandrosterone sulfate, total testosterone, and cortisol assays. Frequency of surveillance was determined by age (Fig 1). Abdominal magnetic resonance or computed tomography imaging was recommended when any hormone level remained elevated after 15 to 30 days. Laparotomy was performed only if imaging clearly demonstrated an adrenal mass; otherwise, children were referred to pediatric endocrinologists. Screening, surveillance, and treatment (for uninsured children) were free of charge.
Fig 1.
Flowchart of screening and surveillance protocols; TP53 R337H screening (after precounseling) and adrenocortical tumor (ACT) surveillance; 15 years of follow-up planned (2006 to 2021). Surveillance procedures were scheduled at staggered times to allow broader coverage. Although our surveillance included testosterone and cortisol, only dehydroepiandrosterone sulfate (DHEAS) provided predictive value.
Surveillance was conducted at the study site in Curitiba or by local pediatricians provided with surveillance guidelines and real-time Web-based consultation ( www.curadotca.org.br). Most tumor resections were performed in Curitiba.
Table 1 lists characteristics of surveillance participants and nonparticipants for analysis. We employed stringent criteria for the participant group to rigorously assess the effectiveness of surveillance in detecting small, curable tumors. Participants whose scheduled examinations were delayed > 3 months or delayed > 2 months twice within 1 year were grouped with nonparticipants for analysis.
Table 1.
Definition of Surveillance Cohorts As Analyzed
| Definition |
|---|
| Surveillance participants (n = 346 R337H heterozygotes) |
| 1. Children in full compliance with surveillance program (n = 248), including those identified and examined by trained public clinics |
| 2. Children who missed some appointments but underwent ≥ two medical examinations and hormone tests per year (n = 42) |
| 3. Children who were excluded for noncompliance but who, after counseling, returned to full compliance (n = 56); none showed evidence of tumor development at time of readmission to surveillance |
| Surveillance nonparticipants (n = 351 R337H heterozygotes) |
| 1. Newborns whose parents could not be located (n = 31); their mothers received general information about mutation and ACTs during consent for NBS |
| 2. Children whose parents declined to participate in surveillance (n = 130); these parents consented to confirmatory testing and received additional information about implications of mutation and clinical features of ACTs |
| 3. Children whose parents wanted to participate in surveillance but were unable to comply with schedule (n = 81); these children had too few medical visits and hormone tests to meet inclusion criteria |
| 4. Children enrolled onto surveillance program who subsequently became noncompliant (n = 109); their parents were informed of implications of mutation and clinical features of ACTs |
Abbreviations: ACT, adrenocortical tumor; NBS, newborn screening.
Research Participant Protection
This study was approved by the Ethics Committees for Research With Human Subjects of the Hospital de Clínicas (Federal University of Paraná) and Hospital Pequeno Príncipe (Curitiba). Participation of all maternity centers was approved by the Brazilian Federal Ethics Committee (Brasília). The St Jude Children's Research Hospital institutional review board approved participation of its faculty members.
Newly delivered mothers received pretest counseling; they were informed that they would receive clinical and laboratory information and genetic counseling if their child tested positive, and members of the affected parental line would be offered free R337H testing. Parents of positive children were informed about the early clinical manifestations of ACTs, and family cancer histories were taken. Relatives of the carrier parent were given an informed consent document, print and digital video descriptions of the surveillance program and clinical manifestations of ACTs, and a Web site ( www.curadotca.org.br) for additional information. Separate written informed consent for surveillance was obtained from a parent or legal guardian.
Statistical Analysis
We assumed that the R337H mutation (resulting from a founder effect14) had reached steady-state in Paraná. The mean population of Paraná during 2005 to 2009 and the frequency of mutation-positive newborns were used to estimate the population frequency of the mutation. We calculated the annual incidence of ACTs in children age 0 to 4 years from the mean birth rates (2005 to 2009) in the 22 Paraná health districts and the observed annual incidence of ACTs.
The cumulative age-specific penetrance of ACTs in children identified as carriers at birth was analyzed from surveillance cohort data by the method of Kalbfleisch and Prentice.15 ACT-free time was calculated from birth to ACT diagnosis or most recent follow-up. We analyzed penetrance in consecutive 2-year age categories by computing the linearly interpolated cumulative incidence curve. SE and 95% CIs were calculated for each age segment by bootstrap sampling with 10,000 repetitions.
The Wilcoxon rank sum test was used to compare age at diagnosis, tumor volume, and tumor weight in surveillance participants versus nonparticipants, as defined in Table 1, excluding homozygous R337H carriers and noncarrier screened newborns who developed ACTs. Fisher's exact test was used to compare the distribution of sex, disease stage, and initial treatment in surveillance participants and nonparticipants. A P value < .05 was considered statistically significant.
RESULTS
Incidence of R337H Mutation and Pediatric ACTs
Between December 2005 and March 2010, 171,649 mothers (approximately 22% of 761,650 expected births) consented to NBS. The mean number of children age > 1 year during 2005 to 2009 (ie, number of births per year) was 152,330. Approximately 21% to 26% of viable newborns were screened.
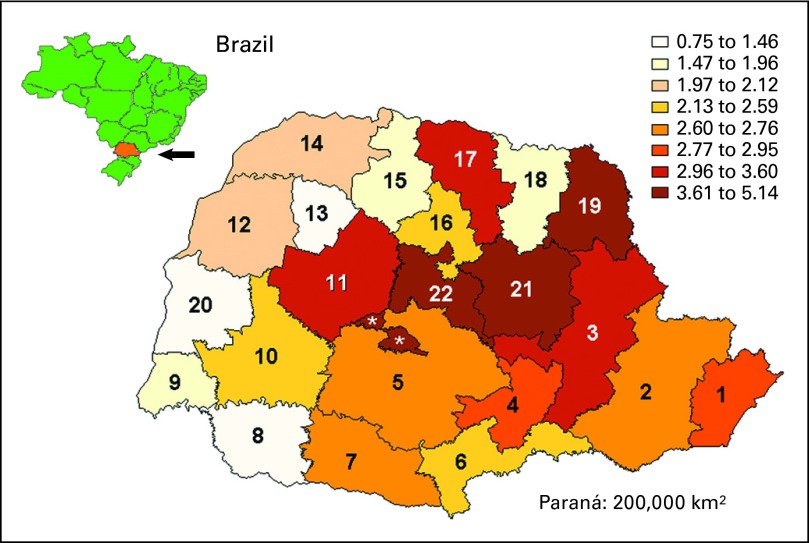
Of the 171,649 newborns tested, 461 (0.27%) carried the R337H mutation. Therefore, 0.27% (95% CI, 0.24% to 0.29%) is the estimated prevalence of the mutation in newborns in Paraná. Prevalence according to health district differed widely (P = .020), ranging from 0.075% (95% CI, 0.001% to 0.27%) to 0.51% (95% CI, 0.30% to 0.81%; Fig 2).
Fig 2.
Prevalence (per 1,000) of TP53 R337H germline mutation in 22 health districts of southern Brazilian state of Paraná (population 10.5 million), estimated on basis of newborn screening. Estimated range of prevalence in each district is color coded. (*) Analyzed with health district 22.
During the study period, 11 children heterozygous for the R337H mutation at birth developed ACTs; therefore, the estimated penetrance of ACTs (follow-up, 3.0 to 6.7 years) was 2.39%. The estimated cumulative penetrance (mean ± SE) was 0.7% ± 0.49% at 2 years and 2.21% ± 0.9% at 5 years of age. Because approximately 80% of pediatric R337H-associated ACTs develop during the first 5 years, only a few more patient cases are expected.
Two ACTs were diagnosed among the 171,188 children negative for the mutation at birth (wild-type germline TP53 confirmed by sequencing; Table 2; Fig 3). Frequency of ACT differed significantly between newborns negative (two of 171,188) and positive (11 of 461) for the mutation (P < .001). Overall sensitivity of screening was 84.6% (11 of 13); specificity was 99.7% (171,186 of 171,636); positive predictive value was 2.4% (11 of 461); negative predictive value was 100% (171,186 of 171,188; Fig 3). Assuming that the 171,649 tested newborns represented a random sample of children age ≤ 5 years in Paraná, the 13 patient cases of ACT among children screened at birth indicated a mean annual incidence of 27.6 per million children age ≤ 5 years (95% CI: 20.8 to 34.4 × 10−6, large sample approximation method; 21.1 to 35.3 × 10−6, exact method).
Table 2.
Clinical and Biologic Features of 19 Patients With Childhood ACTs During Study Period
| Patient No. | Age at Diagnosis (months) | Sex | Clinical Presentation* | Tumor |
Disease Stage† | Initial Treatment |
Outcome | Follow-Up (months)§ | |||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Weight (g) | Volume (cm3) | Histology | Resection | Chemotherapy*‡ | |||||||
| Surveillance participants | |||||||||||
| 1NBS | 28 | F | P1, C+ | 30 | 35 | Carcinoma | I | Yes | No | NED | 47 |
| 2NBS | 23 | F | P2, C+ | 35 | 52 | Carcinoma | I | Yes | No | NED | 48 |
| 3NBS | 3 | F | P2, Cushing | 45 | 50 | Carcinoma | I | Yes | No | NED | 46 |
| 4NBS | 15 | F | P2, C+ | 20 | 27 | Carcinoma | I | Yes | No | NED | 35 |
| 5RS | 28 | F | P2, C+, comedones | 22 | 37 | Carcinoma | I | Yes | No | NED | 36 |
| 6NBS | 23 | F | None | 17 | 18 | Carcinoma | I | Yes | No | NED | 35 |
| 7NBS | 22 | M | None | 1 | 0.6 | Adenoma | I | Yes | No | NED | 31 |
| Surveillance nonparticipants | |||||||||||
| 8RS | 6 | F | P2, C++, Cushing | 40 | 46 | Carcinoma | I | Yes | No | NED | 70 |
| 9NBS | 11 | F | P3, C++, acne | 160 | 160 | Carcinoma | II | Yes | No | NED | 64 |
| 10NBS | 10 | M | None | 580 | 828 | Carcinoma adherent to liver and right kidney | II | Yes | Yes | NED | 64 |
| 11NBS | 6 | F | P3, C+, comedones | 65 | 64 | Carcinoma | I | Yes | No | Relapse 5 and 16 months after diagnosis | 56 |
| 12NBS | 14 | F | P4, C+, acne | 90 | 137 | Carcinoma | III (spillage) | Yes | Yes | NED | 59 |
| 13NBS | 13 | F | P2, C+, acne | 60 | NA | Adenoma | I | Yes | No | NED | 34 |
| 14RS | 43 | M | P3, MPSE | NA | 205 | Carcinoma (spillage) | III | Yes | Yes | NED | 29 |
| 15RS | 41 | F | P3, C++ | 780 | NA | Carcinoma | III (extensive regional involvement) | Yes | Yes | Relapse 10 months after diagnosis; death resulting from disease 17 months after diagnosis | |
| Homozygous R337H | |||||||||||
| 16RS | 112 | M | None | 31 | 38.7 | Carcinoma | I | Yes | No | Local relapse 11 months after diagnosis | 24 |
| 17RS | 42 | F | P4, C++ | NA | 150 | Carcinoma | III (spillage) | Yes | Yes | Death resulting from toxicity 12 months after diagnosis | |
| Wild-type TP53 | |||||||||||
| 18NBS | 6 | F | NA | 100 | 266 | Carcinoma | II | Yes | No | NED | 41 |
| 19CNBS | 10 | F | NA | 70 | 100 | Carcinoma | I | Yes | No | NED | 53 |
Abbreviations: ACT, adrenocortical tumor; DHEAS, dehydroepiandrosterone sulfate; MPSE, modest penile-scrotal enlargement; NA, not available; NBS, newborn screening; NED, no evidence of disease; RS, relative screening.
P1 to P5 indicates Tanner stage; C+, clitoral enlargement 5 to 10 mm; C++, clitoral enlargement 10 to 15 mm. All surveillance participants (Nos. 1 to 7) presented with increased DHEAS levels (41, 114, 175, 271, 115, 30, and 40 μg/dL, respectively) at ACT diagnosis, but only one presented with increased cortisol level (No. 3). Testosterone was elevated in participants Nos. 2, 3, 4, and 5 (30, 364, 48, and 78 ng/dL, respectively).
Staging system adapted.4
Cisplatin, etoposide, doxorubicin, and mitotane.
From time of diagnosis to most recent contact.
Fig 3.
Mutation screening and adrenocortical tumor (ACT) detection in surveillance versus nonsurveillance groups as defined for outcome analysis. Newborns and relatives positive for TP53 R337H mutation and children diagnosed with ACT during study period are shown according to analysis; those not eligible for analysis as surveillance participants listed as not in ACT surveillance group. (*) Two relatives age < 15 years who were homozygous for R337H mutation had ACTs but were excluded from analysis (one would have been assigned to surveillance group and one to nonsurveillance group). (†) Two newborns who did not carry R337H mutation later developed ACTs.
Girls showed a much higher frequency of ACTs. Fifteen of the 19 screened children who developed ACTs were girls; four were boys (P = .012). Among surveillance participants, the R337H mutation was equally distributed among boys (n = 170) and girls (n = 177), but the estimated cumulative incidence (mean ± SE) of ACTs at age 5 years was 3.65% ± 1.5% in girls versus 0.65% ± 0.65% in boys (P = .06, Wilcoxon rank sum test).
Comparison of Surveillance Cohorts
Table 1 defines the surveillance cohorts as analyzed. Reasons for not consenting to surveillance or for incomplete adherence included low education and socioeconomic level, long travel distance to the study center, inability to leave work for the child's appointments, and perceived low risk of ACTs. As shown in Figure 3, participants comprised 346 carriers (290 identified at birth; 56 identified at age < 15 years). As of April 2012, seven of these children (six identified as newborns) had developed ACTs. Carriers in the nonparticipant group comprised 171 children identified at birth and 180 identified at age < 15 years. Eight of these 352 children (five identified at birth) had ACTs. The two neonatally screened noncarrier patients with ACTs and the two relatives homozygous for R337H were excluded from group comparisons.
Median age at diagnosis of ACTs (17 carcinomas; two adenomas) was 15 months (range, 3 to 112 months; Table 2) and did not differ significantly between surveillance participants and nonparticipants (Table 3). However, median tumor weight (P = .003) and volume (P = .007) at diagnosis were dramatically lower among surveillance participants (P < .001). Moreover, mild virilization was typically observed in the surveillance cohort (five of eight), whereas advanced virilization was predominant in nonparticipants. None of the surveillance participants experienced relapse, and all remained disease free 31 to 48 months after diagnosis. Conversely, two of the eight nonparticipants experienced relapse, and one died as a result of progressive disease. Four of eight nonparticipant children required intensive adjuvant chemotherapy for extensive local disease.
Table 3.
Selected Clinical Features of Children With ACTs According to Participation in Surveillance
| Feature | Surveillance Group (n = 7) | Nonsurveillance Group (n = 8) | P |
|---|---|---|---|
| Age at diagnosis, years | .999* | ||
| Median | 1.91 | 1.27 | |
| Range | 0.18 to 2.92 | 0.81 to 3.43 | |
| Sex | |||
| Male | 1 | 2 | .999† |
| Female | 6 | 6 | |
| Tumor weight, g | .003* | ||
| Median | 22.0 | 90.0 | |
| Range | 0.51 to 45.0 | 40.0 to 780.0 | |
| Tumor volume, cm3 | .007* | ||
| Median | 35.0 | 148.5 | |
| Range | 0.6 to 52.0 | 46.0 to 828.0 | |
| Disease stage, No. of patients | .077† | ||
| I | 7 | 4 | |
| II | 0 | 2 | |
| III | 0 | 2 | |
| Initial treatment, No. of patients | .077† | ||
| Surgery alone | 7 | 4 | |
| Surgery plus chemotherapy | 0 | 4 |
Abbreviation: ACT, adrenocortical tumor.
Wilcoxon rank sum test.
Fisher's exact test.
Family Cancer History
Cancer histories and complete pedigrees were obtained for 353 families. When one parent was unavailable and the other was a noncarrier, the carrier line was identified by screening relatives. In the carrier lines, 3,523 relatives were tested; 1,704 (48%) carried the mutation, and 2,238 declined testing or were unavailable. Three children were homozygous for the R337H allele. Two of them (Table 2; patients 16 and 17) had ACTs, and one child developed CPC.
A variety of cancers were reported in 605 individuals (including probands) in the carrier lines. However, cancer distribution was inconsistent: 30.8% (109 of 353) had no family history of cancer, 27.4% (97 of 353) had ≥ three patient cases and met the criteria for Li-Fraumeni–like syndrome,16 and 41.6% (143 of 353) fit no clinical criteria for a cancer syndrome.
The most frequently reported childhood cancers in the carrier lines were ACTs (n = 48) and brain tumors (n = 6). In the 48 children with ACTs, genomic DNA from available blood (n = 31) or tumor samples (n = 8) consistently contained the R337H mutation. Five other cancers were identified among carriers screened at birth: neuroblastoma (n = 1), glioblastoma multiforme (n = 1), CPC (n = 2), and Burkitt lymphoma (n = 1). The most frequently reported adult cancers were breast (n = 93), stomach (n = 81), and brain (n = 32) tumors. ACTs were reported in three adults.
Cost Effectiveness
Our results suggest that 90% to 95% of children with ACTs would be cured without morbidity as a result of screening (newborns and relatives) followed by surveillance (Data Supplement). ACTs associated with homozygous R337H, other rare TP53 mutations, or wild-type TP53 may have a different natural history but would account for few patient cases. Without screening and surveillance, only 50% of children with ACTs survive, and many require intensive, toxic chemotherapy. The overall cost of fully implemented screening and surveillance is approximately US$1,000,000, and six lives would be saved (40% of 15 patient cases), at a cost of US$166,000 per life saved.
DISCUSSION
Our surveillance program was highly effective in detecting ACTs of low weight and volume. Remarkably, maximum tumor weight was only 45 g among surveillance participants, compared with 780 g among nonparticipants. Age at diagnosis was not related to tumor weight or volume.
An important strength of our surveillance approach was the selection of children on the basis of a genetic abnormality predisposing to ACTs, allowing sensitive monitoring throughout the primary age range of the tumor. The interval between surveillance procedures reflected our observation that mild virilizing signs of short duration (1 to 4 months) are usually associated with small (stage I) ACTs. All seven tumors in R337H-heterozygous surveillance participants were small (stage I) when detected, and as in our previous study,17 all children survived 36 to 60 months postdiagnosis. In contrast, the eight ACTs in nonparticipants were associated with advanced virilization, significantly greater tumor weight (P = .003) and volume (P = .007) at diagnosis, and more adverse events. Relapse and survival rates could not be compared in participants versus nonparticipants because of the small number of patients. The similar frequency of ACTs in surveillance participants and nonparticipants indicates the absence of selection bias. Two R337H-homozygous children experienced local relapse within 1 year of diagnosis (Table 2; patient 16). This limited observation suggests that the ACT phenotype may be more aggressive in R337H-homozygous children.
The impact of screening alone, accompanied by information about ACTs and R337H, could not be directly assessed because of the small size of this category. Historically, however, advanced-stage disease has seemed to be more frequent among unscreened and uninformed families.4 For example, four of five carriers with ACTs reported in 2011 had advanced ACTs at diagnosis; all of these families were rural and unaware of ACTs or the TP53 R337H mutation.18 These findings suggest that identification of newborn carriers and education of parents about the signs and symptoms of ACTs are useful.
Identification of the TP53 R337H mutation at birth, followed by close biochemical, radiologic, and clinical monitoring, seems to be cost effective for identifying and optimally treating early-stage ACTs, with no loss of quality of life. All seven surveillance participants who had ACTs had no clinical signs or only mild virilization at diagnosis. The estimated cost per life saved was approximately US$166,000, less than the cost of managing children with leukemia who need hematopoietic stem-cell transplantation. Moreover, because children are expected to live approximately 60 to 70 years, the cost per year of life saved per child was approximately US$2,371.
Only approximately 50% of the parents of mutation-positive children consented to ACT surveillance. Incomplete participation substantially reduces the benefits of screening. The underlying reasons for nonparticipation are unclear but are likely to involve socioeconomic and cultural factors. Future efforts may increase surveillance participation. For example, with consent, community health agents and physicians could be alerted to and monitor childhood carriers. Periodic updates about carriers' health could be sought from parents and providers, also facilitating preventive care for adult carriers. In our program, all carriers age > 25 years who accepted genetic counseling were encouraged to undergo periodic cancer examinations.
The marked predominance of girls among our patients with ACTs is unexplained. Embryonic adrenocortical methylation patterns may be sex dependent and may cooperate with the R337H mutation to increase predisposition to tumorigenesis. DNA methylation is physiologically altered in XX embryonic stem-cell lines, explaining the instability of their karyotype.19
To our knowledge, no other report to date has identified such a large number of carriers (n = 1,704) of a single TP53 point mutation in a small portion of the general population. We monitored R337H carriers between ages 2 months and 15 years on the basis of epidemiologic data.4,18,20 Biochemical and imaging surveillance of carriers is known to improve survival in Li-Fraumeni families,21 and all surveillance participants survived in our study.
Childhood screening based on familial cancer history or features of Li-Fraumeni or Li-Fraumeni–like syndrome is inappropriate in southern Brazil,22 because > 30% of our carrier families (three- to four-generation pedigrees) had no history of cancer. Furthermore, the cancer profile of the carrier lines differed markedly from that of Li-Fraumeni families,5,23–25 particularly among children.25 We observed no childhood soft tissue or bone sarcoma; 84% of all tumors were ACTs, and 11.5% were brain tumors, including CPC. Interestingly, 63% of reported patient cases of CPC in Paraná state12,26 and 65% in São Paulo11 were positive for the R337H mutation, suggesting that this mutation increases predisposition to CPC. However, the cancer histories of our 353 families showed ACTs (48 patient cases) to be much more prevalent than CPC (two patient cases). The cost effectiveness of surveillance for CPC in R337H carriers remains to be determined. It is possible that R337H carriers are predisposed to other Li-Fraumeni–component tumors, including sarcomas, at older ages. Taken together, our findings support the proposed role of age-, sex-, and tissue-specific factors in the cancer predisposition profiles of different TP53 mutations.24 Longer follow-up of more carrier newborns is needed to confirm the incidence of ACTs and of other tumors and lifetime risk of cancer.
Our findings apply to a single state and unique TP53 mutation associated with a rare childhood tumor. However, this mutation has a similar estimated frequency in a Brazilian population of almost 70 million,2,10,11,27 thus providing translational research opportunities to improve prevention and treatment of p53-associated disorders.28 Importantly, a subset of R337H carriers seems to have a lifetime predisposition to common malignancies. Our findings have broad implications for many areas of biomedical and public health research, including NBS, cancer epidemiology, and carcinogenesis.
To our knowledge, our study is the first to use neonatal genomic DNA testing to select children for surveillance for a specific malignancy. Other pediatric cancer screening programs have been less successful. For example, catecholamine screening and surveillance for neuroblastoma (highly curable if detected early29,30) yielded many false positives and identified patients who regressed spontaneously. Furthermore, only children age < 1 year underwent surveillance, whereas older children had more-aggressive neuroblastomas. Our results provide a framework for studies of the general use of screening in regions with high R337H prevalence12 or with other frequent tumor-predisposing mutations. If full surveillance is not feasible for R337H carriers, parental counseling and enhanced health care contact could be rapidly implemented. The R337H assay used in our study is suitable for mass screening and is feasible within the Brazilian public health system. We propose collaborations among governmental and nongovernmental agencies, academic centers, and community health care providers31 to reduce mortality and morbidity in identified carriers.
Supplementary Material
Acknowledgment
We thank all public clinics that contributed to surveillance; Ety Cristina C. Forte, José Alvaro Carneiro, and Drs Maria Helena P. Del Valle, Carlos A. Longui, Tatiane S. Silva, Iglenir João Cavalli, Flora M. Watanabe, Euripedes Ferreira, Ana Paula K. Pedrobom, Edna K. Carboni, Paulo Carboni Júnior (in memoriam), Donizetti D. Giamberardino Filho, Robson C. Coelho, Carlos Moreira Jr, Emilia M. Pinto, Carlos Rodriguez-Galindo, and Leslie Robison; the many physicians, nurses, and social assistants from the state of Paraná for their contributions to this study; Sharon Naron for editing the manuscript; and Cheng Cheng for statistical analysis support.
Glossary Terms
- Amplicon:
The DNA product of a polymerase chain reaction, usually an amplified segment of a gene or DNA.
- Carrier line:
Refers to the paternal or maternal side of a family that harbors an inherited mutation.
- Cluster:
The unexpectedly high incidence of a particular disease or phenotype (usually caused by a founder mutation) within a defined geographic area.
- Penetrance:
The likelihood that a given gene mutation will produce disease. This likelihood is calculated by examining the proportion of people with the particular genetic mutation who show symptoms of disease.
Footnotes
Supported by grants from the Brazilian State of Paraná Secretary of Science, Technology and Higher Education (2005 and 2008), Secretary of Health (2009), and Araucária Foundation (2009); Brazilian National Council for Research and Development (2007 and 2009); Coordenadoria de Aperfeiçomento de Pessoal de Ensino Superior (No. 806/Nanobiotec 2009); Raul Carneiro Hospital Association for Childhood Protection; Paraná Association for Childhood Protection Against Cancer; American Lebanese Syrian Associated Charities; French National Center for Scientific Research through the International Associated Laboratories; Institut National du Cancer; and European Network for the Study of Adrenal Tumors (No. 259735), National Institutes of Health, National Cancer Institute (No. CA021765).
Terms in blue are defined in the glossary, found at the end of this article and online at www.jco.org.
The views expressed here are those of the authors and should not be interpreted to reflect the views or policies of the funding agencies. The funding institutions had no role in study design, data collection or analysis, decision to publish, or preparation of the manuscript.
Authors' disclosures of potential conflicts of interest and author contributions are found at the end of this article.
AUTHORS' DISCLOSURES OF POTENTIAL CONFLICTS OF INTEREST
The author(s) indicated no potential conflicts of interest.
AUTHOR CONTRIBUTIONS
Conception and design: Raul C. Ribeiro, Bonald C. Figueiredo
Collection and assembly of data: Gislaine Custódio, Guilherme A. Parise, Nilton Kiesel Filho, Heloisa Komechen, Cesar C. Sabbaga, Roberto Rosati, Leila Grisa, Ivy Z.S. Parise, Mara A.D. Pianovski, Carmem M.C.M. Fiori, Jorge A. Ledesma, José Renato S. Barbosa, Francisco R.O. Figueiredo, Elis R. Sade, Humberto Ibañez, Sohaila B.I. Arram, Sérvio T. Stinghen, Luciano R. Mengarelli, Mirna M.O. Figueiredo, Danilo C. Carvalho, Sylvio G.A. Avilla, Thiago D. Woiski, Lisiane C. Poncio, Geneci F.R. Lima, Bonald C. Figueiredo
Data analysis and interpretation: Gislaine Custódio, Guilherme A. Parise, Nilton Kiesel Filho, Jorge A. Ledesma, Sylvio G.A. Avilla, Roberto Pontarolo, Enzo Lalli, Yinmei Zhou, Gerard P. Zambetti, Raul C. Ribeiro, Bonald C. Figueiredo
Manuscript writing: All authors
Final approval of manuscript: All authors
REFERENCES
- 1.Ries LAG, Smith MA, Gurney JG, et al. Cancer Incidence and Survival Among Children and Adolescents: United States SEER Program 1975-1995. http://seer.cancer.gov/publications/childhood/
- 2.Pianovski MA, Maluf EM, de Carvalho DS, et al. Mortality rate of adrenocortical tumors in children under 15 years of age in Curitiba, Brazil. Pediatr Blood Cancer. 2006;47:56–60. doi: 10.1002/pbc.20624. [DOI] [PubMed] [Google Scholar]
- 3.Ribeiro RC, Sandrini F, Figueiredo B, et al. An inherited p53 mutation that contributes in a tissue-specific manner to pediatric adrenal cortical carcinoma. Proc Natl Acad Sci U S A. 2001;98:9330–9335. doi: 10.1073/pnas.161479898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Michalkiewicz E, Sandrini R, Figueiredo B, et al. Clinical and outcome characteristics of children with adrenocortical tumors: A report from the International Pediatric Adrenocortical Tumor Registry. J Clin Oncol. 2004;22:838–845. doi: 10.1200/JCO.2004.08.085. [DOI] [PubMed] [Google Scholar]
- 5.Figueiredo BC, Sandrini R, Zambetti GP, et al. Penetrance of adrenocortical tumours associated with the germline TP53 R337H mutation. J Med Genet. 2006;43:91–96. doi: 10.1136/jmg.2004.030551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Varley JM, McGown G, Thorncroft M, et al. Are there low-penetrance TP53 Alleles? Evidence from childhood adrenocortical tumors. Am J Hum Genet. 1999;65:995–1006. doi: 10.1086/302575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.West AN, Ribeiro RC, Jenkins J, et al. Identification of a novel germ line variant hotspot mutant p53–R175L in pediatric adrenal cortical carcinoma. Cancer Res. 2006;66:5056–5062. doi: 10.1158/0008-5472.CAN-05-4580. [DOI] [PubMed] [Google Scholar]
- 8.Gonzalez KD, Noltner KA, Buzin CH, et al. Beyond Li Fraumeni syndrome: Clinical characteristics of families with p53 germline mutations. J Clin Oncol. 2009;27:1250–1256. doi: 10.1200/JCO.2008.16.6959. [DOI] [PubMed] [Google Scholar]
- 9.Pinto EM, Ribeiro RC, Figueiredo BC, et al. TP53-associated pediatric malignancies. Genes Cancer. 2011;2:485–490. doi: 10.1177/1947601911409745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Latronico AC, Pinto EM, Domenice S, et al. An inherited mutation outside the highly conserved DNA-binding domain of the p53 tumor suppressor protein in children and adults with sporadic adrenocortical tumors. J Clin Endocrinol Metab. 2001;86:4970–4973. doi: 10.1210/jcem.86.10.7957. [DOI] [PubMed] [Google Scholar]
- 11.Seidinger AL, Mastellaro MJ, Paschoal Fortes F, et al. Association of the highly prevalent TP53 R337H mutation with pediatric choroid plexus carcinoma and osteosarcoma in southeast Brazil. Cancer. 2011;117:2228–2235. doi: 10.1002/cncr.25826. [DOI] [PubMed] [Google Scholar]
- 12.Custodio G, Taques GR, Figueiredo BC, et al. Increased incidence of choroid plexus carcinoma due to the germline TP53 R337H mutation in southern Brazil. PLoS One. 2011;6:e18015. doi: 10.1371/journal.pone.0018015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Instituto Brasileiro de Geografia e Estatística. http://www.ibge.gov.br/home.
- 14.Pinto EM, Billerbeck AE, Villares MC, et al. Founder effect for the highly prevalent R337H mutation of tumor suppressor p53 in Brazilian patients with adrenocortical tumors. Arq Bras Endocrinol Metabol. 2004;48:647–650. doi: 10.1590/s0004-27302004000500009. [DOI] [PubMed] [Google Scholar]
- 15.Kalbfleisch JD, Prentice RL. Hoboken, NJ: Wiley; 2002. The Statistical Analysis of Failure Time Data. [Google Scholar]
- 16.Birch J, Heighway J, Teare MD, et al. Linkage studies in a Li-Fraumeni family with increased expression of p53 protein but no germline mutation in p53. Br J Cancer. 1994;70:1176–1181. doi: 10.1038/bjc.1994.468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Michalkiewicz EL, Sandrini R, Bugg MF, et al. Clinical characteristics of small functioning adrenocortical tumors in children. Med Pediatr Oncol. 1997;28:175–178. doi: 10.1002/(sici)1096-911x(199703)28:3<175::aid-mpo3>3.0.co;2-g. [DOI] [PubMed] [Google Scholar]
- 18.Custódio G, Komechen H, Figueiredo FR, et al. Molecular epidemiology of adrenocortical tumors in southern Brazil. Mol Cell Endocrinol. 2012;351:44–51. doi: 10.1016/j.mce.2011.10.019. [DOI] [PubMed] [Google Scholar]
- 19.Zvetkova I, Apedaile A, Ramsahoye B, et al. Global hypomethylation of the genome in XX embryonic stem cells. Nat Genet. 2005;37:1274–1279. doi: 10.1038/ng1663. [DOI] [PubMed] [Google Scholar]
- 20.Wasserman JD, Zambetti GP, Malkin D. Towards an understanding of the role of p53 in adrenocortical carcinogenesis. Mol Cell Endocrinol. 2012;351:101–110. doi: 10.1016/j.mce.2011.09.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Villani A, Tabori U, Schiffman J, et al. Biochemical and imaging surveillance in germline TP53 mutation carriers with Li-Fraumeni syndrome: A prospective observational study. Lancet Oncol. 2011;12:559–567. doi: 10.1016/S1470-2045(11)70119-X. [DOI] [PubMed] [Google Scholar]
- 22.Achatz MI, Hainaut P, Ashton-Prolla P. Highly prevalent TP53 mutation predisposing to many cancers in the Brazilian population: A case for newborn screening? Lancet Oncol. 2009;10:920–925. doi: 10.1016/S1470-2045(09)70089-0. [DOI] [PubMed] [Google Scholar]
- 23.Birch JM, Alston RD, McNally RJ, et al. Relative frequency and morphology of cancers in carriers of germline TP53 mutations. Oncogene. 2001;20:4621–4628. doi: 10.1038/sj.onc.1204621. [DOI] [PubMed] [Google Scholar]
- 24.Kleihues P, Schäuble B, zur Hausen A, et al. Tumors associated with p53 germline mutations: A synopsis of 91 families. Am J Pathol. 1997;150:1–13. [PMC free article] [PubMed] [Google Scholar]
- 25.Ruijs MW, Verhoef S, Rookus MA, et al. TP53 germline mutation testing in 180 families suspected of Li-Fraumeni syndrome: Mutation detection rate and relative frequency of cancers in different familial phenotypes. J Med Genet. 2010;47:421–428. doi: 10.1136/jmg.2009.073429. [DOI] [PubMed] [Google Scholar]
- 26.Torres LF, de Noronha L, Scheffel DL, et al. Choroid plexus tumours: Epidemiologic comparative study of 24 cases [in Portuguese] Arq Neuropsiquiatr. 2004;62:127–130. doi: 10.1590/s0004-282x2004000100022. [DOI] [PubMed] [Google Scholar]
- 27.Palmero EI, Schüler-Faccini L, Caleffi M, et al. Detection of R337H, a germline TP53 mutation predisposing to multiple cancers, in asymptomatic women participating in a breast cancer screening program in Southern Brazil. Cancer Lett. 2008;261:21–25. doi: 10.1016/j.canlet.2007.10.044. [DOI] [PubMed] [Google Scholar]
- 28.Vousden KH, Lane DP. p53 in health and disease. Nat Rev Mol Cell Biol. 2007;8:275–283. doi: 10.1038/nrm2147. [DOI] [PubMed] [Google Scholar]
- 29.Schilling FH, Spix C, Berthold F, et al. Neuroblastoma screening at one year of age. N Engl J Med. 2002;346:1047–1053. doi: 10.1056/NEJMoa012277. [DOI] [PubMed] [Google Scholar]
- 30.Soderstrom L, Woods WG, Bernstein M, et al. Health and economic benefits of well-designed evaluations: Some lessons from evaluating neuroblastoma screening. J Natl Cancer Inst. 2005;97:1118–1124. doi: 10.1093/jnci/dji203. [DOI] [PubMed] [Google Scholar]
- 31.Grosse SD, Schechter MS, Kulkarni R, et al. Models of comprehensive multidisciplinary care for individuals in the United States with genetic disorders. Pediatrics. 2009;123:407–412. doi: 10.1542/peds.2007-2875. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.