Abstract
Background
Bone marrow stem cell treatment has been proven a promising therapeutic strategy and showed significant results given the strong immune modulating properties. We have investigated the safety and efficacy of autologous bone marrow stem cell transplantation through liver puncture in two patients with recently diagnosed type 1 diabetes mellitus.
Material/Methods
The procedure was approved by the Institutional Ethics Committee. In 2011, in three young patients, type 1 diabetes mellitus diagnosis was confirmed, with the presence of positive antibodies and ketoacidosis. Two patients was treated with autologous bone marrow stem cell stimulated with filgrastim and transplantation, through liver puncture, as immune modulators. One patients was treated with conventional treatment and participate in this experiment as a control group. The families of the patients signed the informed consent. No specific statistical analysis was performed.
The patients had less than 8 years old, diagnosis of type 1 diabetes for less than 60 days, body mass index less than 22 kg/m2, normal complete blood count, coagulation and renal function, no lesions in target organs, glycosylated hemoglobin (HbA1c) level less than 13.70%, c-peptide level less than 0.67 ng/ml, positive results of Islets Cells Antibody (ICA), Glutamic Acid Decarboxylase (GAD) and insulin antibody.
Results
In two patients treated, the follow up at 12 months showed negative value in ICA, GAD and anti insulin antibody levels, with an increased levels of c peptide and decreased levels of blood glucose and HbA1c.
Conclusions
Treatment with autologous bone marrow stem cells is easy and effective as it reversed the production and effect of anti pancreatic islet antibody and significantly resulted in an increased c-peptide concentration.
Keywords: type 1 diabetes, bone marrow transplantation, bone marrow stem cells
Background
Type 1 diabetes mellitus (T1D) is an autoimmune disease that affects children and young adults. Specific auto-antibodies attack the insulin-secreting pancreatic cells and result in the destruction of pancreatic islets. This causes a decrease in insulin secretion and requirements for exogenous insulin to regulate glucose metabolism. The attack of antibodies is the characteristic feature in the first stage of T1D and the summation of antibodies involved has important prognostic implications as it directly relates to the disease severity [1,2]. The level of antibodies is reported as an independent risk variable in T1D without complications or fulminant T1D [3]. Several treatments have been tried to stop the autoimmune attack in the early stages of T1D but none of them showed promising results. Encouragingly, a large number of studies have demonstrated the immunomodulating and anti-inflammatory properties of stem cells [4]. Furthermore, bone marrow stem cells (BMSCs) display immune modulating properties through CD4+ and CD8+ T cells via surface molecular program death ligand-1 (PD-L1) and secrete nitric oxide (NO), as well as the proliferation suppression of mitogen phytohemagglutinin (PHA)-stimulated lymphocytes [4,5].
It has been reported that the proliferation of T cell clones stimulated by the antigen-presenting cells (APCs) and different doses of glutamic acid decarboxylase (GAD) peptide were markedly and specifically decreased in the presence of BMSCs as compared to the control group, indicating that BMSCs have a potential to suppress the GAD-specific T cell proliferation [6]. The role of regulatory T cells (Tregs) is also well known in T1D. Tregs represent the specialized T cell population that plays a crucial role in maintaining homeostasis and self-tolerance through their inhibitory impact on auto-reactive effector T cells [6]. Although defects in effector T cells or APCs could play a role, increasing evidence both in human trials and animal models demonstrates that the abnormalities of Tregs, either in cell quantity or function, are also associated with the initiation and progression of T1D [6,7]. Mechanistic studies showed that the control of diabetes was correlated with systemic immune alterations, including restoration of T lymphocytes helper (Th) 1 and 2, cytokine balance in blood, and local regulation in pancreatic islets through a unique distributional pattern of transforming growth factor-β1 (‘a TGF-β1 ring’) that may protect islet β cells against the infiltrated lymphocytes. Haller et al observed that autologous cord blood transfusion was safe and slowed the loss of endogenous insulin production in children with T1D, due to the highly functional populations of Tregs in cord blood [8]. The ability of stem cells to protect and recover the integrity of blood vessel formation [9,10] and cell membrane laminas in the pancreatic islets also plays important roles in the treatment of T1D [11–13].
The ability of stem cells to suppress or re-educate activated T lymphocytes have been described recently. One clinical trial confirmed that co-culturing patients’ T lymphocytes with cord blood stem cells (CB-SCs) altered the patients’ immune response, leading to clinically relevant improvements in the autoimmune process [14]. Specifically, this trial also provided evidence that CB-SCs educated effector T cells and/or Tregs, resulting in lasting changes in the expression of co-stimulating molecules, increasing the population of Tregs, and restoring the Th1/Th2/Th3 cytokine balance, each of which is expected to improve the control of autoimmunity in T1D [14].
In this study, we report the treatment and follow-up results of 2 patients with recently diagnosed T1D, who underwent autologous BMSC implantation within 3 months after the defined diagnosis. The evolution of these 2 patients was compared with 1 patient with similar clinical characteristics who did not receive stem cell treatment.
Material and Methods
Protocol
Three patients (Patient 1, Patient 2, and Patient 3) with recently diagnosed T1D were enrolled in this study. The diagnosis was confirmed with the presence of positive antibodies and ketoacidosis as a complication of T1D. The patients were hospitalized for initial treatment with insulin and discharged with the adequate blood glucose control and insulin dosage scheme after family education. Patients 1 and 2 were referred to our hospital to assess the possibility of stem cell therapy when no further complications were presented. Autologous BMSCs were prepared and transplanted through liver puncture as immune modulators after the 2 families agreed with the treatment protocol and signed the informed consent. The family of Patient 3 consented to participate in this study as a control subject and the patient received the same standard treatment as the other 2 patients mentioned above but without autologous BMSC transplantation. The protocol was approved by the Institutional Ethics Committee.
BMSC preparation and transplantation
Bone marrow stimulation was performed using Filgrastim (10 ug/kg/day, subcutaneous, a granulocyte colony-stimulating factor (G-CSF) on the first 4 days and bone marrow was extracted on the 5th day. Cellular fractions were immediately separated by centrifugation (at 6000 rpm for 10 min.) and no in vitro cell culture procedure was made. Under general anesthesia, stem cells were implanted into the 6th segment of the liver through an ultra-fine needle guided by ultrasound parenchymal puncture. The volume injected was 10 ml of autologous plasma and BMSCs. The qualified autologous BMSCs were collected and identified as mononuclear cells (MNCs) >180×106/kg, CD34+ cells >0.22%. The patients were monitored for 1 day after the procedure and could return home if no complications were presented.
Follow-up
The subjects were phoned every 48 hours during the first 3 months after the cell implantation. Clinical evaluations were performed at baseline (pre-treatment), 6 months (6 m) and 12 months (12 m) following the treatment, including adverse events, daily insulin dose, CBC, renal function test and measurements of C-peptide (normal value 0.90–7.10 ng/ml, method: chemiluminescence), glycosylated hemoglobin (HbA1C) (normal value 4.20–6.00%, method: liquid chromatography), ICA anti-islet antibody (normal value negative, Juvenile Diabetes Foundation Units (JDF), material: blood, method: immunofluorescence). GAD antibody (normal value: equal to or less than 1.0 U/ml, method: radio immune analysis), anti-insulin antibody (normal value: equal to or less than 0.4 U/ml, method: radio immune analysis), and abdominal ultrasound.
Results
Participant characteristics
Patient 1 was a female Caucasian, 6 years old, who presented with symptoms of irritability, feeding intolerance, polydipsia, and polyuria for 3 days. Physical examination showed normal growth and development and no obvious abnormal signs. There was no previous medical history and no other disease. Laboratory data: normal results of CBC, renal function (normal value: urea 8–38 mg/dl; creatinine 0.30–1.00 mg/dl, method: colorimetric), thyroid function, and thyroid hormone antibodies (T3 normal value 0.94–2.41 ng/ml, T4: 5.6–14.9 ug/ml, TSH: NV 0.70–6.40 uUI/ml, method: chemiluminescence, anti-peroxidase: NV 0–34 UI/ml, anti-thyroglobulin: NV 0–115 UI/ml); glycemia 162 mg/dl (normal value: 70–100 mg/dl, method: enzymatic), ketonemia (+), glycosuria >500 mg/dl (normal value: 0–15 mg/dl, method: test strips), HbA1c 10%, C-peptide 0.62 ng/ml, islet antibody 20 U/JDF, GAD antibody 6.6 U/ml, insulin antibody 0.4 U/ml (Table 1).
Table 1.
Laboratory date before and after treatment (6 and 12 months).
| Variables (normal value) | Patient 1 | Patient 2 | Patient 3 | ||||||
|---|---|---|---|---|---|---|---|---|---|
| Before | 6 m | 12 m | Before | 6 m | 12 m | Before | 6 m | 12 m | |
| Blood glucose (mg/dl) (70–100 mg/dl) | 162 | 130 | 135 | 506 | 120 | 110 | 300 | 130 | 120 |
| Glycated hemoglobin (%) (4.2–6.0%) | 10 | 8.9 | 8 | 13.7 | 7 | 7 | 12 | 9 | 10 |
| C Peptide (ng/ml) (0.90–7.10 ng/ml) | 0.62 | 1.17 | 1.17 | 0.44 | 0.73 | 0.44 | 0.42 | 0.33 | 0.2 |
| Daily insulin dose (U/day) (negative) | 5 | 5 | 10 | 7 | 6 | 6 | 5 | 10 | 15 |
| Anti-Islets antibody (U/JDF) (negative) | 20 | 0 | 0 | 40 | 0 | 5 | 33 | 57 | 80 |
| Anti GAD antibody (U/ml) (equal or less than 1.0 U/ml) | 6.6 | 7.8 | 5.5 | 2.7 | 3.5 | 3.2 | 10.6 | 12 | 35 |
| Anti-insulin antibody(U/ml) (equal or less than 0.4 U/ml) | 0.4 | 41 | 41 | 0.4 | 0.6 | 0.4 | 0.8 | 4 | 60 |
Patient 2 was a female Caucasian, 8 years old. She presented with symptoms of polydipsia, polyuria, and comatose state for 1 week. On physical examination she showed normal growth and development and no signs or symptoms of any other disease. There was no previous medical history of any other disease.
Laboratory Data: glycemia: 506 mg/dl, ketonemia positive, glycosuria >900 mg/dl, glycosylated hemoglobin A1c 13.70%, C-peptide: 0.44 ng/ml, islet antibody 40 U/JDF, GAD antibody: 2.7 U/ml, insulin antibody (Table 1): 0.4 U/ml, normal blood count, normal renal function, normal thyroid function, and normal thyroid hormone antibodies.
Patient 3 was a female Caucasian, 7 years old. She presented symptoms of polydipsia, polyuria, and irritability for 2 weeks. On physical examination she showed normal growth and development and no signs or symptoms of other diseases. There was no previous medical history of any other disease.
Laboratory data: hyperglycemia: 300 mg/dl, ketonemia: positive, glycosuria >800 mg/dl, glycosylated hemoglobin 12%, C-peptide of 0.42 ng/ml, islet antibody: 33 U/JDF, GAD antibody: 4.4 U/ml, insulin antibody: 0.8 U/ml (Table 1). She had normal blood count, normal renal function, normal thyroid function, and normal thyroid hormone antibodies.
Safety outcomes
Bone pain and nausea related to bone marrow stimulation before cells implantation and bruising at the site of bone marrow puncture were observed during the treatment course, which were tolerable and quickly subsided without treatment. There were no reports of any death, lymphoproliferative disease, tumor, or infection post-transplantation. The results of CBC, renal function and abdominal ultrasound were normal in all subjects during the follow-ups.
Efficacy outcomes
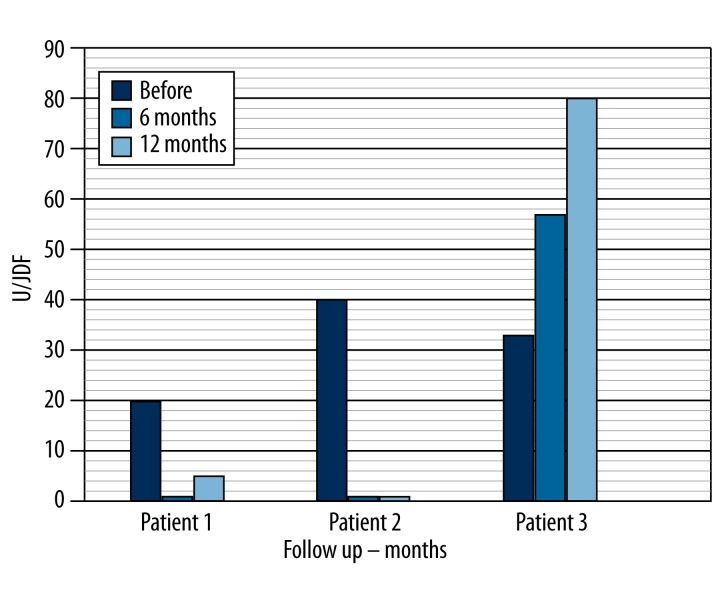
Islet cells, GAD and insulin antibodies: The subjects showed positive results for ICA (20, 40 and 33 U/JDF) before cell implantation. At 6-month follow-up, Patients 1 and 2 showed negative results for ICA antibodies. At 12 months post-treatment, Patient 1 showed negative values and Patient 2 shows slightly increased values (5U/JDF) (Table 1, Figure 1). Patient 3 showed an increase in the measurements of ICA at 6-month (57 U/JDF) and 12-month (80 U/JDF) follow-up. All 3 patients showed positive results for GAD (6.6 U/JDF – 2.7 U/JDF and 4.4 U/JDF) before treatment. At 6 months, Patients 1 and 2 showed an increase in GAD antibodies (7.8 U/JDF and 3.5 U/JDF). At 12 months, we observed a decrease in the levels of GAD antibodies (5.5 and 3.2 U/JDF) in these patients. Patient 3 showed an increase in GAD antibodies at 6-month (12 U/JDF) and 12-month (35 U/JDF) follow-up. Patients 1 and 2 showed negative results for anti-insulin (0.4 U/JDF – 0.4 U/JDF) before treatment. At 6 and 12 months, Patient 1 showed an increase in anti-insulin antibodies (41 U/JDF). In Patient 2 we observed an increase at 6 months (0.6 U/JDF) and a decrease in levels of anti-insulin antibodies at 12 months (0.4 U/JDF). Patient 3 showed an increase in anti-insulin antibodies at 6-month (4 U/JDF) and 12-month (60 U/JDF) follow-up (Table 1).
Figure 1.
Follow up antibody pancreatic islets after immunologic treatment.
C-peptide
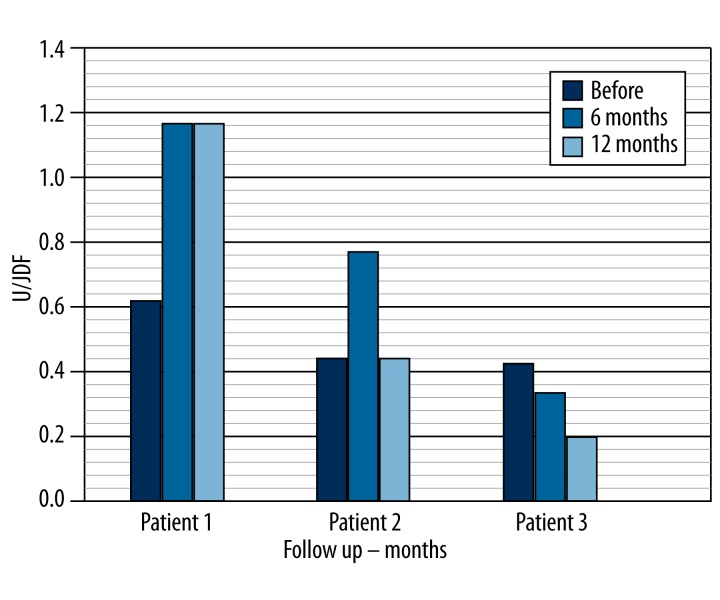
All subjects had C-peptide values less than 0.9 ng/ml before cell implantation (0.62 ng/ml, 0.44 ng/ml, and 0.42 ng/ml). At 6 and 12 months post-treatment, Patient 1 showed an elevation of C-peptide (1.17 ng/ml and 1.17 ng/ml) and Patient 2 had an elevation of C-peptide at 6 months, which disappeared at 12 months (0.7 ng/ml and 0.44 ng/ml). Patient 3 showed a C-peptide value less than 0.9 ng/ml at 6 and 12 months post-treatment (0.33 ng/ml and 0.2 ng/ml) (Table 1, Figure 2).
Figure 2.
Follow up c-peptide after immunologic treatment.
Daily insulin dose
The daily insulin dose utilized by all enrolled subjects was less than 8 UI/day before cell implantation. At 6 months (5 and 6 UI/day) and 12 months (10 and 6 UI/day) post-treatment, Patients 1 and 2 showed a very low daily insulin dose. Patient 3 showed an increase in daily insulin requirements at 6-month (10 UI/day) and 12-month (15 UI/day) follow-up (Table 1).
Glycated hemoglobin A1C (HbA1c)
The enrolled patients were between 10% and 13.7% before cell implantation. At 6 and 12 months treatment all patients showed lower levels as compared to the previous measurements, but these levels were still not normal (Table 1).
Discussion
Type 1 diabetes mellitus is a metabolic disorder characterized by elevated blood glucose levels as a result of decreased insulin secretion. Its pathophysiology involves the destruction of β-pancreatic cells, with an absolute insulin deficiency and permanent dependence on exogenous insulin. The cause in 90% of cases is considered autoimmune, as determined by the presence of anti-islet antibodies (ICA), glutamic acid decarboxylase antibodies (anti-GAD antibodies), and anti-insulin antibodies, and about 10% of cases are idiopathic. These antibodies are elevated during years of evolution of the disease and spontaneously follow a downward curve [15–17]. There is no medical treatment available at present to alter this evolution of the disease.
The prevalence of diabetes mellitus for all age groups worldwide was estimated to be 2.8% in year 2000 and is projected to be 4.4% by 2030 [15–17]. It is quite clear that the statistics show a growing disease without curative treatments, which develops into serious complications and justifies research and development of alternative therapeutic strategies.
The clinical picture refers to the classical triad of polyphagia, polyuria, and polydipsia, accompanied by an emaciated nutritional status and hyperglycemia.
Ketoacidosis, a major complication in many patients, is evidenced by dehydration, acidic respiration, nausea, and vomiting [18].
Recently it was demonstrated that different types of stem cells, including mesenchymal stem cells, have properties allowing them to regulate the function of immune cells. The therapeutic use of mesenchymal stem cells in autoimmune diseases is being studied in different pre-clinical and clinical experiments in adults and children [19–27]. In addition, other studies showed that mesenchymal cells also secrete peptides that act as modulating factors for local micro-immunity [28–30]. The molecules secreted by mesenchymal cells are predominantly anti-inflammatory in nature. Mesenchymal cells play a central role in immunologic homeostasis and several studies have shown that mesenchymal cells (MSCs) can secrete specific peptides, such as hepatocyte growth factor, that can contribute to the creation of a local immunosuppressive environment. Similarly, transforming growth factor-1 is also involved in T cell suppression by working with hepatocyte growth factor in promoting the allo-escaping phenotype [31]. Di Nicola et al showed that neutralizing antibodies to hepatocyte growth factor and transforming growth factor-1 restored the proliferative response in mixed lymphocyte reactions [31]. Other suggested factors include interferon (INF), tumor necrosis factor, and interleukin (IL)-2 [32,33]. Interleukin-10 also seems to be constitutively expressed by MSCs and has a well documented role in T cell regulation and in the promotion of the suppressor phenotype by antagonizing the action of IL-12 during induction of the inflammatory immune responses.
We believe that bone marrow stem cell transplantation directly into the liver parenchyma provides conditions similar to the culture media, where the implanted cells stay in contact for more than 4 days, the same as a culture medium, and may stimulate the cellular differentiation and modulation of the immune system.
The implanted bone marrow cells could stimulate the secretion of hepatocyte growth factor and other chemokines, which could modulate the action of antigen-presenting cells and lymphocytes and may reverse the production of antibodies, as described in the preclinical experiments.
We observed a reduction in anti-islet (ICA) and GAD antibodies, which remained during the follow-up at 12 months, and noted that the negative results for antibodies is associated with increased C peptide, decreased requirement for daily insulin dose, and decreased concentrations of glycosylated hemoglobin (HbA1c).
At 12 months, in Patient 1 we observed a small increase in anti-insulin antibody, which we consider insignificant since it only reaches 20% of the level observed before cell treatment. The patient who was treated with insulin only (Control case/Patient 3) showed positive and continuously increasing antibodies and decreased C-peptide measurements, which continued to increase in daily insulin dose. However, the daily insulin requirement was lower in patients treated with stem cells (Patients 1 and 2). Clearly, the bone marrow stem cells implantation in 2 consecutive patients had an effect on the production of pancreatic antibodies, pancreatic function, and metabolic control after 12-month follow-up.
The proposed treatment is easy to perform and has no ethical issues. No complications were observed during the implantation procedure or 12 months of follow-up. We believe that the cell implantation clearly influenced the suppression of anti-islet antibody and reversed its effects. The exact mechanism of action of stem cells is unclear but we could not determine any other means of change in antibody levels.
This therapeutic method appears to be an effective alternative for the treatment of recently diagnosed type 1 diabetes. Despite the small number of patients treated, the results, which are unprecedented in the history of medicine, are very impressive. This information should be verified in clinical studies attempting verify our results, which could mean a cure for type 1 diabetes mellitus.
Conclusions
Treatment with autologous bone marrow stem cells is easy and reversed the production and effect of anti-pancreatic islet antibody and resulted in an increased C-peptide concentration. Treatment with autologous bone marrow stem cells, performed soon after diagnosis of the disease, suppress the immune attack and pancreatic damage. We believe the effects, which started at 4 months after the treatment and continued up to 12 months, involve a change in the disease prognosis and could mean a delay in disease evolution.
Footnotes
Source of support: Departmental sources
References
- 1.Gianani R, Campbell-Thompson M, Sarkar SA, et al. Dimorphic histopathology of long-standing child- hood-onset diabetes. Diabetologia. 2010;53:690–98. doi: 10.1007/s00125-009-1642-y. [DOI] [PubMed] [Google Scholar]
- 2.Madsbad S, Krarup T, Regeur L, et al. Insulin secretory reserve in insulin dependent patients at time of diagnosis and the first 180 days of insulin treatment. Acta Endocrinologica. 1980;95:359–63. doi: 10.1530/acta.0.0950359. [DOI] [PubMed] [Google Scholar]
- 3.Sue M, Yoshihara A, Otani T, et al. Characteristics of fulminant type 1 diabetes mellitus. Med Sci Monit. 2008;14(10):CS97–101. [PubMed] [Google Scholar]
- 4.Zhao Y, Huang Z, Qi M, et al. Immune regulation of T lymphocyte by a newly characterized human umbilical cord blood stem cell. Immunol Lett. 2007;108:78–87. doi: 10.1016/j.imlet.2006.10.007. [DOI] [PubMed] [Google Scholar]
- 5.Uccelli A, Moretta L, Pistoia V. Mesenchymal stem cells in health and disease. Nat Rev Immunol. 2008;8:726–36. doi: 10.1038/nri2395. [DOI] [PubMed] [Google Scholar]
- 6.Bluestone JA, Tang Q, Sedwick CE. T regulatory cells in autoimmune diabetes: past challenges, future prospects. J Clin Immunol. 2008;28:677–84. doi: 10.1007/s10875-008-9242-z. [DOI] [PubMed] [Google Scholar]
- 7.Alonso N, Soldevila B, Sanmarti A, et al. Regulatory T cells in diabetes and gastritis. Autoimmun Rev. 2009;8:659–62. doi: 10.1016/j.autrev.2009.02.014. [DOI] [PubMed] [Google Scholar]
- 8.Haller MJ, Viener HL, Wasserfall C, et al. Autologous umbilical cord blood infusion for type 1 diabetes. Exp Hematol. 2008;36:710–15. doi: 10.1016/j.exphem.2008.01.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Brissova M, Shostak A, Shiota M, et al. Pancreatic islet production of vascular endothelial growth factor – a is essential for islet vascularization, revascularization, and function. Diabetes. 2006;55:2974–85. doi: 10.2337/db06-0690. [DOI] [PubMed] [Google Scholar]
- 10.Brissova M, Powers AC. Revascularization of transplanted islets: can it be improved? Diabetes. 2008;57:2269–71. doi: 10.2337/db08-0814. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Virtanen I, Banerjee M, Palgi J, et al. Blood vessels of human islets of Langerhans are surrounded by a double basement membrane. Diabetologia. 2008;51:1181–91. doi: 10.1007/s00125-008-0997-9. [DOI] [PubMed] [Google Scholar]
- 12.Kragl M, Lammert E. Basement membrane in pancreatic islet function. Adv Exp Med Biol. 2010;654:217–34. doi: 10.1007/978-90-481-3271-3_10. [DOI] [PubMed] [Google Scholar]
- 13.Irving-Rodgers HF, Ziolkowski AF, Parish CR, et al. Molecular composition of the peri-islet basement membrane in NOD mice: a barrier against destructive insulitis. Diabetologia. 2008;51:1680–88. doi: 10.1007/s00125-008-1085-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Zhao Y, Jiang Z, Zhao T, et al. Reversal of type 1 diabetes via islet beta cell regeneration following immune modulation by cord blood-derived multipotent stem cells. BMC Med. 2012;10:3. doi: 10.1186/1741-7015-10-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Wild S, Roglic G, Green A, et al. Global Prevalence of Diabetes. Estimates for the year 2000 and projections for 2030. Diabetes Care. 2004;27:1047–53. doi: 10.2337/diacare.27.5.1047. [DOI] [PubMed] [Google Scholar]
- 16.Onkamo P, Väänänen S, Karvonen M, Tuomilehto J. Worldwide increase in incidence of Type I diabetes - the analysis of the data on published incidence trends. Diabetologia. 1999;42:1395–403. doi: 10.1007/s001250051309. [DOI] [PubMed] [Google Scholar]
- 17.Achenbach P, Bonifacio E, Koczwara K, Ziegler A-G. Natural History of Type 1 Diabetes. Diabetes. 2005;54(Suppl 2):S25–S31. doi: 10.2337/diabetes.54.suppl_2.s25. [DOI] [PubMed] [Google Scholar]
- 18.Palmer JP. C-peptide in the natural history of type 1 diabetes. Diabetes Metab Res Rev. 2009;25(4):325–28. doi: 10.1002/dmrr.943. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Abdi R, Fiorina P, Adra CN, et al. Immunomodulation by mesenchymal stem cells: a potential therapeutic strategy for type 1 diabetes. Diabetes. 2008;57:1759–67. doi: 10.2337/db08-0180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Aguayo-Mazzucato C, Bonner-Weir S. Stem cell therapy for type 1 diabetes mellitus. Nat Rev Endocrinol. 2010;6:139–48. doi: 10.1038/nrendo.2009.274. [DOI] [PubMed] [Google Scholar]
- 21.Zhao Y, Glesne D, Huberman E. A human peripheral blood monocyte-derived subset acts as pluripotent stem cells. Proc Nat Acad Sci USA. 2003;100:2426–31. doi: 10.1073/pnas.0536882100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Zhao Y, Lin B, Dingeldein M, et al. New type of human blood stem cell: a double-edged sword for the treatment of type 1 diabetes. Transl Res. 2010;155:211–16. doi: 10.1016/j.trsl.2010.01.003. [DOI] [PubMed] [Google Scholar]
- 23.Zhou Q, Brown J, Kanarek A, et al. In vivo reprogramming of adult pancreatic exocrine cells to beta-cells. Nature. 2008;455:627–32. doi: 10.1038/nature07314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Petrovsky N. Immunomodulation with microbial vaccines to prevent type 1 diabetes mellitus. Nat Rev Endocrinol. 2010;6:131–38. doi: 10.1038/nrendo.2009.273. [DOI] [PubMed] [Google Scholar]
- 25.Pittenger MF, MacKay AM, Beck SC, et al. Multilineage potential of adult human mesenchymal stem cells. Science. 1999;284:143–47. doi: 10.1126/science.284.5411.143. [DOI] [PubMed] [Google Scholar]
- 26.Nauta AJ, Fibbe WE. Immunomodulatory properties of mesenchymal stromal cells. Blood. 2007;110(10):3499–506. doi: 10.1182/blood-2007-02-069716. [DOI] [PubMed] [Google Scholar]
- 27.Habib H, Halawa T, Atta H. Therapeutic applications of mesenchymal stroma cells in pediatric diseases: Current aspects and future perspectives. Med Sci Monit. 2011;17(11):RA233–39. doi: 10.12659/MSM.882036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Rasmusson I. Immune modulation by mesenchymal stem cells. Exp Cell Res. 2006;312:2169–79. doi: 10.1016/j.yexcr.2006.03.019. [DOI] [PubMed] [Google Scholar]
- 29.Le Blanc K, Tammik C, Rosendahl K, et al. HLA expression and immunologic properties of differentiated and undifferentiated mesenchymal stem cells. Exp Hematol. 2003;31:890–96. doi: 10.1016/s0301-472x(03)00110-3. [DOI] [PubMed] [Google Scholar]
- 30.Leblanc K, Ringden O. Immunomodulation by mesenchymal stem cells and clinical experience. J Intern Med. 2007;262:509–25. doi: 10.1111/j.1365-2796.2007.01844.x. [DOI] [PubMed] [Google Scholar]
- 31.Di Nicola M, Carlo-Stella C, Magni M, et al. Human bone marrow stromal cells suppress T lymphocyte proliferation induced by cellular or non-specific mitogenic stimuli. Blood. 2002;99:3838–43. doi: 10.1182/blood.v99.10.3838. [DOI] [PubMed] [Google Scholar]
- 32.Tse WT, Pendleton JD, Beyer WM, et al. Suppression of allogeneic T-cell proliferation by human marrow stromal cells: implications in transplantation. Transplantation. 2003;75:389–97. doi: 10.1097/01.TP.0000045055.63901.A9. [DOI] [PubMed] [Google Scholar]
- 33.LeBlanc K, Tammik L, Sundberg B, et al. Mesenchymal stem cells inhibit and stimulate mixed lymphocyte cultures and mitogenic responses independently of the major histocompatibility complex. Scand J Immunol. 2003;57:11–20. doi: 10.1046/j.1365-3083.2003.01176.x. [DOI] [PubMed] [Google Scholar]