Abstract
Nonalcoholic steatohepatitis (NASH) is typically associated with pro-apoptotic caspase activation. A potential role for pro-inflammatory caspases remains incompletely understood. Our aims were to examine a potential role of caspase 1 in the development of liver damage and fibrosis in NASH. C57BL/6 wild-type (WT), developed marked steatohepatitis, HSC activation, fibrosis, and increased hepatic caspase 1 and IL-1β expression when placed on the methioninecholine deficient (MCD) diet. Marked caspase 1 activation was detected in the liver of MCD-fed mice. Hepatocyte and non-parenchymal fractionation of the livers further demonstrated that caspase 1 activation after MCD feeding was mainly localized to non-parenchymal cells. Caspase 1-knockout (Casp1−/−) mice on the MCD diet showed marked reduction in mRNA expression of genes involved in inflammation and fibrogenesis (TNFα was 7.6-fold greater in WT vs. Casp1−/−MCD-fed mice; F4/80 was 1.5-fold greater in WT vs. Casp1−/− MCD-fed mice; α-SMA was 3.2-fold greater in WT vs. Casp1−/− MCD-fed mice; Collagen 1-alpha was 7.6–fold greater in WT vs. Casp1−/− MCD-fed mice; TGFβ was 2.4-fold greater in WT vs. Casp1−/− MCD-fed mice; CRP2 was 3.2-fold greater in WT vs. Casp1−/− MCD-fed mice). Furthermore, Sirius red staining for hepatic collagen deposition was significantly reduced in Casp1−/− mice MCD-fed mice compared to WT MCD-fed animals. However, serum aminotransferase (ALT) levels, caspase 3 activity and TUNEL positive cells were similar in Casp1−/− and WT mice on the MCD diet. Selective Kupffer cell depletion by clodronate injection markedly suppressed MCD-induced caspase 1 activation and protected mice from fibrogenesis and fibrosis associated with this diet. Conclusion: this study uncovers a novel role for caspase 1 in inflammation and fibrosis during NASH development.
Keywords: Nonalcoholic fatty liver disease (NAFLD), nonalcoholic steatohepatitis (NASH), inflammasome, caspases, inflammation, fibrosis
Nonalcoholic Fatty Liver Disease (NAFLD) is currently the most common form of chronic liver disease affecting both adults and children, and is strongly associated with obesity and insulin resistance (1, 2). One in three adults and one in ten children or adolescents in the United States have hepatic steatosis, a stage within the spectrum of NAFLD, that is characterized by triglyceride accumulation in liver cells and follows a benign non-progressive clinical course (3, 4). Nonalcoholic steatohepatitis (NASH) is defined as lipid accumulation with evidence of cellular damage, inflammation and different degrees of scarring or fibrosis (5). NASH is a serious condition as approximately 25% of these patients progress to cirrhosis and its feared complications of portal hypertension, liver failure and hepatocellular carcinoma (6-8). The pathogenesis of NAFLD/NASH in particular the mechanisms responsible for liver injury and disease progression remains incompletely understood but are of significant biomedical importance as identification of these processes may help to identify novel diagnostic and therapeutic targets for this highly prevalent and potentially serious disease.
Since the original description that caspase activation and hepatocyte apoptosis are characteristic pathologic features in the liver of NASH patients (9), a growth of data have demonstrated a key role for caspase-dependent cell death in NASH pathogenesis (10-13). Caspases are a family of cysteine proteases with unique substrate specificities that play a central role in the apoptotic machinery (14, 15). They are synthesized as inert zymogens and upon receipt of apoptotic stimuli, cells activate initiator caspases such as caspase 1, 2, 8, 9, and 10 that, in turn, proteolytically cleave and activate effector caspases including caspase 3, 6, and 7. Caspases have been further categorized as either proinflammatory or proapoptotic, depending upon their participation in these cellular programs. The proinflammatory caspases include caspase 1, 11 and 12 in mouse and caspase 1, 4, and 5 in human (16). Targeting caspase activity and apoptosis has gained significant attention for developing of novel therapeutic diagnostic strategies for NASH patients. Recent data suggest that pan-caspase inhibition protects again diet-induced steatohepatitis in different dietary murine models (17-19). These pan-caspase inhibitors, not only inhibit caspase-mediated cellular apoptosis, but also block the caspase 1-dependent processing and activation of various proteins with functions in inflammation and tissue repair during tissue damage. However, the contribution of caspase 1-dependent processes to liver injury and fibrosis remains unclear. In the present study we examined the occurrence and significance of caspase 1 activation in NASH.
Experimental Procedures
Animal Studies
These experimental protocols were approved by the Institutional Animal Care and Use Committee at the Cleveland Clinic. Male C57BL/6 mice, 20 to 25 g of body weight, were purchased from Jackson Laboratory. C57BL/6 Caspase 1 (Casp1−/−) knockout mice (generously provided by Dr. Richard Flavell from Yale University, New Haven, CT) were described previously (20, 21). Mice were placed on a methionine and choline deficient (MCD) diet (TD 90262, Teklad Mills), which has been extensively shown to result in steatosis associated with significant inflammation and progressive fibrosis pathologically similar to human severe steatohepatitis (22, 23). In this model steatosis results from both decreased mitochondrial oxidation of fatty acids and decreased export of fatty acids in the form of very low density lipoprotein (24). Identical groups of animals (n = 5 - 7 in each group) received a standard diet consisting of 5% fat (TD 2918, Teklad Mills, Madison, WI) to act as controls (CTL). Total body weight was measured at 0, 1, 3 and 6 weeks. Animals in each group were sacrificed after 6 weeks on respective diets.
In selective studies, C57BL/6 male mice of 20-25 g in weight were placed on the MCD diet for the intravenous injection of liposomes encapsulating phosphate (PBS) or clodronate (CLOD) (n=3-7 in each group). After 5 weeks of MCD diet, animals were injected twice 5 days apart with 0.1 ml per 10 g of body weight of a 1 mg/ml suspension of liposomes as previously described(25). Animals in each group were sacrificed after 6 weeks on respective treatments.
Cell Lines and Culture
Primary mouse hepatocytes and total non-parenchymal cells were isolated from C57BL/6 mice on a 6-week MCD diet. The mice were anesthetized and the livers were perfused with warm oxygenated Hanks (-) with 1mM EGTA and 10mM HEPES followed by Williams E media containing 10mg collagenase per mouse. Hepatocytes were collected after centrifugation and resulting cell suspensions were used to collect total non-parenchymal cells with a Percoll gradient centrifugation. Caspase 1 and IL-1β expression was determined by western blot analysis as detailed below.
Histopathology, Immunostaining and Serum Assays
Blood samples and liver tissue were collected under deep anesthesia after a 5-h fast as previously described in detail (26). Liver tissue was fixed in 10% Formalin and embedded in Tissue Path (Fisher Scientific, Pittsburgh, PA). Hematoxylin and eosin as well as Oil Red O-stained liver specimens were evaluated by light microscopy. Liver triglyceride determinations were performed using a specific kit following manufacturer’s instructions (Pointe Scientific). Serum alanine aminotransferase determinations were performed using a commercial kit (Sigma Diagnostics). Individual features including degree of steatosis, inflammation, and ballooning were assessed in the MCD and CTL-fed animals by an experienced pathologist (BGP) in a blinded fashion. Steatosis, inflammation, and ballooning were scored based on NAFLD activity score (NAS)(27).
Assessment of hepatic caspase-1 activation and cellular localization
Caspase 1 activity was determined using 200 μg whole liver protein with Caspase 1 Fluorometric Assay Kit (cat. ab39412) purchased from Abcam. Immunohistochemistry for caspase 1 was performed using paraffin embedded liver tissue using standard DAB technique. The following primary antibodies were used: rabbit anti-Caspase 1 (cat. 06-503 dilution 1:80) purchased from Millipore.
Apoptosis Assessment
Tissue sections (4 μm) were prepared, and terminal deoxynucleotidyl transferase dUTP nick-end labeling (TUNEL) assay was performed following manufacturer’s instructions (in situ cell death detection kit; Roche Molecular Biochemicals, Mannheim, Germany). Caspase activation was quantified by immunostaining for active caspase 3 using a cleaved caspase-3 antibody (Cell Signaling). Hepatocyte apoptosis in liver sections was quantitated by counting the number of TUNEL-positive or active caspase 3 –positive cells in 5 random microscopic fields (40x), as previously described (12).
Determination of Liver Fibrosis
Liver fibrosis was quantified with Sirius Red. Direct Red 80 and Fast Green FCF (color index 42053) were provided by Sigma-Aldrich. Liver sections were incubated in the dark for 2 h at room temperature with an aqueous solution of saturated picric acid containing 0.1% Fast Green FCF and 0.1% Direct Red (26). Stained slides were washed slowly under running distilled water for 6 min, dehydrated (3 min for each step), mounted, and examined by light microscopy. Red-stained collagen fibers were quantitated by digital image analysis, excluding blood vessels.
Immunoblot and Immunoprecipitation Analysis
500 μg of total liver protein was immunoprecipitated with 5 μg rabbit anti-caspase-1 antibody (cat. 06-503) purchased from Millipore. Immunoblot analysis was performed using 30-40 μg whole liver lysate, 20 μg hepatocyte or non-parenchymal fraction, or immunoprecipitated lysate. Whole liver lysate and hepatocyte/non-parenchymal samples were resolved by 12-15% SDS-PAGE and immunoprecipitated liver lysate samples were resolved by a 4-20% gradient gel (cat. EC60285BOX) purchased from Invitrogen, transferred to nitrocellulose membrane, and blotted with appropriate primary antibodies. The membrane was incubated with peroxidase-conjugated secondary antibody (dilution 1:10,000 (BIOSOURCE International, Camarillo, CA), and the bound antibody was visualized using a chemiluminescent substrate (ECL, Amersham Biosciences) and Kodak X-OMAT film (Eastman Kodak, Rochester, NY). The following primary antibodies were used: rabbit anti-Caspase 1 (cat. 06-503 dilution 1:80) purchased from Millipore, rabbit anti-IL1β (cat. Ab9722, dilution 1:5000) purchased from Abcam; rabbit anti-apoptosis-associated speck-like protein containing a caspse recruitment domain (ASC) (cat. ab64808 dilution 1:1000) purchased from Abcam; rabbit anti-alpha smooth muscle actin (cat. ab5694 dilution 1:500) purchased from Abcam.
Real-time PCR
Total RNA was isolated from liver tissue using RNeasy Tissue Mini kit (Qiagen, Valencia, CA). The reverse transcript (the cDNA) was synthesized from 1 μg of total RNA using the iScript cDNA Synthesis kit (Bio-Rad). Real-time PCR quantification was performed. Briefly, 25 μl of reaction mix contained: cDNA, Syber Green buffer, Gold Taq polymerase, dNTPs, and primers at final concentrations of 200 μm. The sequences of the primers used for quantitative PCR were as follows: αSMA 5′-GTC CCA GAC ATC AGG GAG TAA and 5′-TCG GAT ACT TCA GCG TCA GGA; COL1A1 5′-CAA GAA CAG CAA CGA GTA CCG and GTC ACT GGT CAA CTC CAG CAC; TGF-β 5′-CTCCCGTGGCTTCTAGTGC and 5′-GCCTTAGTTTGGACAGGATCTG; TNFα 5′-CCC TCA CAC TCA GAT CAT CTT CT and 5′-GCT ACG ACG TGG GCT ACA G; F4/80 5′-CCCCAGTGTCCTTACAGAGTG and 5′-GTGCCCAGAGTGGATGTCT; CD11c 5′-CTG GAT AGC CTT TCT TCT GCT G and 5′-GCA CAC TGT GTC CGA ACT CA; ASC 5′ CTT GTC AGG GGA TGA ACT CAA AA and 5′ GCC ATA CGA CTC CAG ATA GTA GC; Casp1 5′ ACA AGG CAC GGG ACC TAT G and 5′ TCC CAG TCA GTC CTG GAA ATG; IL-1 5′ GCA ACT GTT CCT GAA CTC AAC T and 5′ ATC TTT TGG GGT CCG TCA ACT; IL-18 5′ GAC TCT TGC GTC AAC TTC AAG G and 5′ CAG GCT GTC TTT TGT CAA CGA; CRP2 5′ GCTACGGAAAG AAGTATGGACC and 5′ CTCAGTCAAGTTGTAGACTCC.18S ribosomal RNA 5′-and ACG GAA GGG CAC CAC CAG GA 5′-CAC CAC CAC CCA CGG AAT CG was used as an endogenous control. RT-PCR was performed in the Mx3000P cycler (Stratagene): 95 °C for 10 min, 40 cycles of 15 s at 95 °C, 30 s at 60 °C, 30 s at 72 °C followed by 1 min at 95 °C, 30 s at 55 °C and 30 s at 95 °C. The fold change over control samples was calculated using CT, ΔCT, and ΔΔCT values using MxPro software (Stratagene).
Statistical Analysis
All data were expressed as the mean ± S.E.M.. unless otherwise indicated. Differences between 3 or more groups were compared by an ANOVA analysis followed by a post hoc Newman Keuls test parametric test or the Kruskall-Wallis nonparametric test Differences between two groups of normalized data were compared by a two-sided students t test. Differences were considered to be statistically significant at p < 0.05. All statistical analysis was performed using GraphPad Prism 4.0c.
Results
Hepatic Caspase-1 activation is a prominent pathological feature in experimental NASH
To investigate the role of caspase-1 activation in the pathogenesis of NASH, we initially placed C57BL/6 mice on the methionine and choline deficient (MCD) diet, which has been extensively shown to be associated with progressive fibrosing steatohepatitis pathologically similar to human severe steatohepatitis (22, 23). After 6 weeks on the respective diets we observed significant hepatic fat accumulation induced by MCD feeding (Fig. 1A), in conjunction with an increased in histological parameters of liver injury including hepatic inflammation, and hepatocyte ballooning (Fig. 1A and Table 1). Expression of caspase 1 was significantly increased in mice on the MCD feeding compared to controls (Fig. 1B), This was accompanied by increased mRNA levels of ASC, a key component of the inflammasome, the protein platform that activates caspase 1, as well as IL-1 but not of IL-18 (Fig. 1B). These changes in the livers of mice fed the MCD diet were associated with a marked increased in inflammasome activation (Fig. 1C and D), as well as caspase 1 protein expression and activity levels (Fig. 1E - G), Consistent with these data, liver active mature IL-1β protein expression was also significantly increased in MCD-fed animals compared to CTL mice (Fig. 1H and I). Immunohistochemical analysis of liver sections from the two groups of mice showed that caspase 1 was primarily localized to non-parenchymal sinusoidal cells, and to a lesser extent to hepatocytes (Fig. 1J). This was further confirmed by fractionation of the liver tissue from MCD fed animals into hepatocytes and total non-parenchymal cells further demonstrating that caspase 1 and IL1β protein expression was markedly enhanced in the non-parenchymal fraction compared to hepatocytes (Fig. 1K).
Fig. 1. Hepatic caspase 1 activation during diet-induced steatohepatitis.
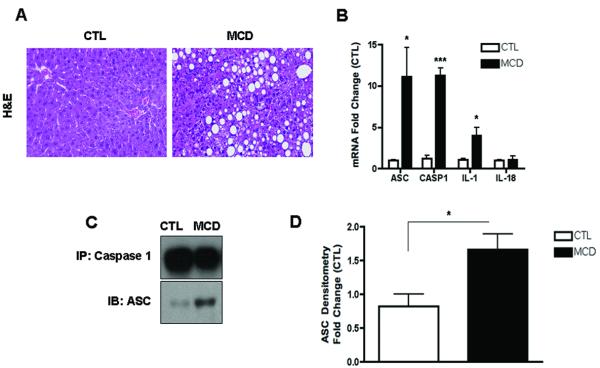
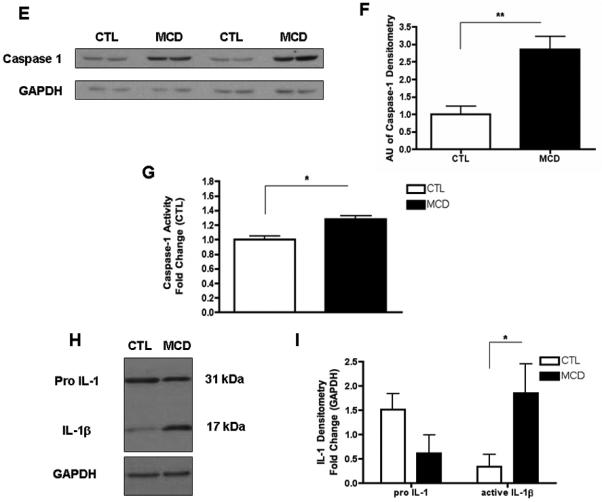
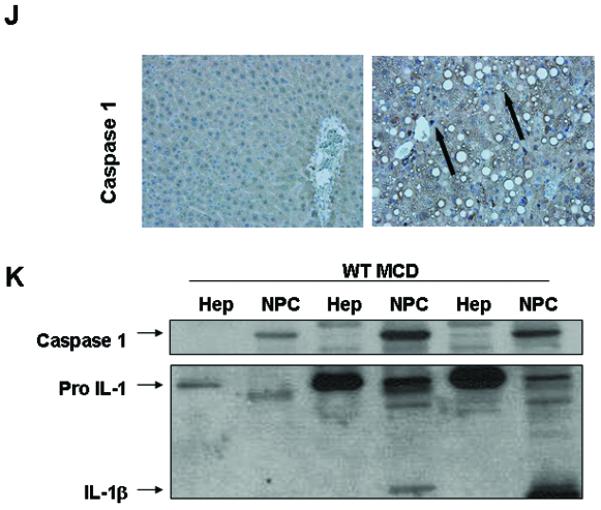
(A) C57BL/6 mice were placed on a methionine and choline deficient (MCD) diet or a control (CTL) diet for 6 weeks (n = 5-7 in each group). Representative microphotographs of Hematoxylin & Eosin (H&E) staining from mice on the two groups (40x). (B) RT PCR analysis of inflammasome components apoptosis speck-like protein containing a CARD (ASC), NACHT, LRR and PYD domains-containing protein 3 (NALP3), caspase 1 (CASP1), interleukin-1 (IL-1) and interleukin-18 (IL-18) in liver WT mice (n=4 in each group). (C) Western blot of ASC on caspase 1 immunoprecipitated whole liver lysates in WT MCD-fed mice compared to WT CTL-fed mice and (D) densitometric analysis of ASC (n=4). (E) Western blot of caspase 1 on whole liver lysates in WT MCD-fed mice compared to WT CTL-fed mice and (F) corresponding densitometric analysis to GAPDH (n=4). (G) Caspase-1 activity assay was performed on whole liver lysates of WT MCD-fed mice and compared to CTL-fed mice (n=9 in each group). (H) Western blots of IL-1 displays pro IL-1 processing into active IL-1β in whole liver lysates of MCD-fed mice compared to CTL-fed mice and corresponding (I) densitometric analysis to GAPDH. (J) Representative microphotograph of caspase 1 immunohistochemistry in paraffin-embedded liver sections of MCD-fed mice compared to CTL-fed animals (Magnification 40x). (K) Western blot of caspase 1 and IL-1 was performed on cell lysates from isolated primary hepatocytes and total non-parenchymal cells from MCD-fed animals. Results are expressed as mean ± S.E.M.. * P < 0.05; ** P < 0.01; compared to controls.
Table 1.
| WT | Casp1 -/- | |||
|---|---|---|---|---|
|
|
||||
| CTL | MCD | CTL | MCD | |
| Steatosis | 0.0 ± 0.0 | 2.8 ± 0.2 *** | 0.3 ± 0.3 | 3.0 ± 0.0 *** |
| Inflammation | 0.0 ± 0.0 | 1.3 ± 0.2 ** | 0.3 ± 0.0 | 0.3 ± 0.3 # |
| Ballooning | 0.0 ± 0.0 | 0.7 ± 0.2 * | 0.0 ± 0.0 | 1.0 ± 0.0 * |
| NAS | 0.0 ± 0.0 | 4.8 ± 0.5 *** | 0.3 ± 0.3 | 4.3 ± 0.3 *** |
NAS (NAFLD Activity Score)
Mean ± SEM
* = Comparison of CTL and MCD
# = Comparison of WT MCD and Casp1 -/- MCD
* p < 0.05 ** p < 0.01 *** p < 0.001
# p < 0.05 between WT MCD and Casp1 -/- MCD
Caspase-1 suppression is associated with dissociation between hepatic triglyceride accumulation and inflammatory activity
Having established the presence of inflammasome activation and increased caspase-1 activity in the liver of MCD fed mice, we next sought to investigate whether caspase-1 participates in NASH development using caspase-1 knock out mice (Casp1−/−). We first investigated whether Casp1−/− mice are resistant to hepatic steatosis and inflammation. C57BL/6 wild-type, Casp1 knock out mice were placed on either a MCD diet, or control (CTL) diet (n = 5 - 7 in each group) for 6 wks. WT mice and Casp1−/− mice had similar weight changes on the MCD-diet (Fig. 2A). However, microscopic examination of H&E and oil red-O staining showed that Casp1−/− mice on the MCD-diet developed more significant macrovesicular hepatic steatosis compared to the WT mice on this diet (Fig. 2B, C). Consistently with these results, hepatic triglyceride levels were significantly more elevated in Casp1−/− mice compared to WT mice on the MCD diet (Fig. 2D). Histological examination of liver samples of other individual features associated with NASH demonstrated a decrease in inflammatory foci resulting in lower inflammatory activity scores in the Casp1−/− mice on the MCD diet compared to WT mice on this diet (Table 1). However, the total NAFLD activity score (NAS) was similar in the two groups of mice as mainly as a result of higher degree of steatosis as well as higher levels of hepatocyte ballooning present in MCD-fed Casp1−/− mice (Table 1). Serum ALT levels were similarly elevated in Casp1−/− mice and WT mice on the MCD diet compared to animals fed the control diet (Fig. 2E). We next examined the inflammatory state of the liver at the molecular and cellular level, and found a marked reduction in mRNA levels of TNF-α, F4/80 and CD11c in the MCD-fed Casp1−/− mice compared to the WT animals on the MCD diet (Fig. 2F, G, H).
Fig. 2. Caspase 1 suppression is associated with dissociation between hepatic triglyceride accumulation and inflammatory activity in diet-induced steatohepatitis.
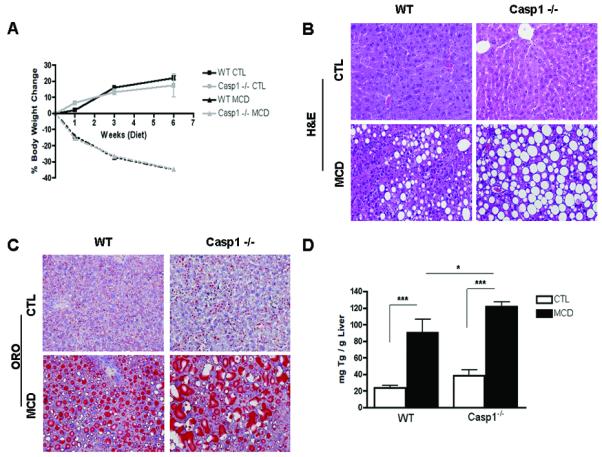
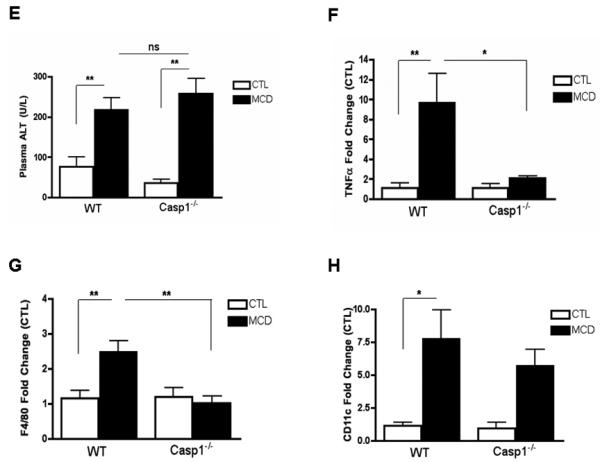
(A) Body weight was measured at weeks 0, 1, 3, and 6 of WT and Casp1−/− mice on a 6-week CTL or MCD diet (n = 5-7 in each group). (B, C) Representative images of H&E and Oil red O staining (ORO) staining of liver tissue displays the severity of cellular injury, further investigated by: (D) hepatic triglyceride (TG) (n=4-5) and (E) serum alanine aminotransferase (ALT) levels (n=5-6). (F) RT PCR analysis of pro-inflammatory cytokine tumor necrosis factor (TNFα) (n = 3-4), (G) macrophage marker F4/80 (n=6-8) and CD11c (n=4-5) in liver of Casp1−/− mice compared to WT mice. Results are expressed as mean ± S.E.M. * P < 0.05; ** P < 0.01; ***P < 0.001 compared to controls.
HSC activation and collagen deposition induced by the MCD diet are decreased by caspase 1 suppression independent of caspase 3 activation and apoptosis
The findings of a key role of caspase 1 in hepatocyte injury and inflammatory signaling, two events that have been linked to HSC activation, led us to further examine the role of caspase 1 in fibrogenesis and fibrosis induced by the MCD diet. While, as expected after six weeks on the MCD diet wild type animals showed a marked increase in the mRNA expression of genes involved in HSC activation and fibrogenesis, such as TGFβ, αSMA, COL1A1, and CRP2 (Fig. 3A -D). These changes were significantly reduced in the Casp1−/− MCD-fed mice. In addition, αSMA protein expression was increased in WT MCD-fed mice, while it was significantly reduced in Casp1−/− MCD-fed mice (Fig. 3E, F). More importantly, an almost four-fold increase in collagen deposition as demonstrated by Sirius red staining of liver tissue coupled to quantitation by digitized image analysis was present in WT animals on the MCD diet compared to the wild type animals on the control diet (Fig. 3G, H), while these increases in collagen deposition were significantly less prominent in the Casp1−/− mice although still higher than in mice on the CTL diet (Fig. 3G, H). We next quantified the amount of hepatocellular cell death present in the various groups of mice. Caspase 3 activation and TUNEL-positive hepatocytes were increased to a similar extend in both WT animals and Casp1−/− mice on the MCD diet compared to animals on the CTL diet (Fig. 4A – D).
Fig. 3. HSC activation and collagen deposition during diet-induced steatohepatitis are markedly decreased in Casp1−/−.
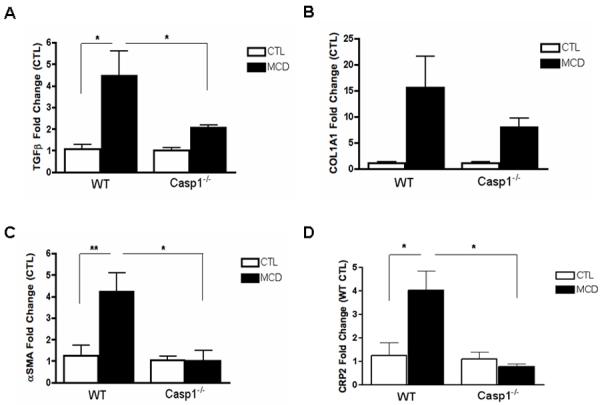
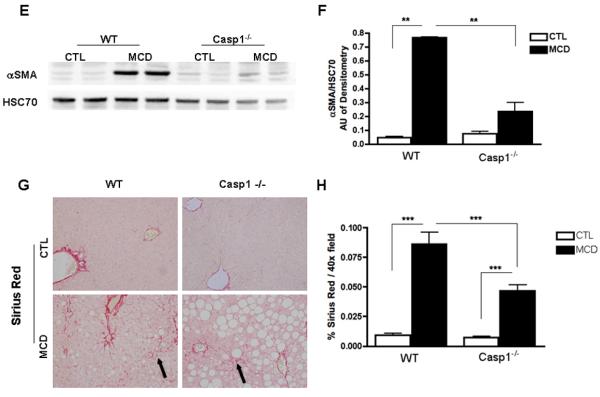
(A-D) RT-PCR analysis of HSC activation markers transforming growth factor beta (TGFβ), collagen type I alpha 1 (COL1A1), alpha smooth muscle actin (αSMA), cysteine- and glycine-rich protein 2 (CRP2), mRNA expression in liver of Casp1−/− mice compared to WT mice (n = 5-7 in each group). (E) Western blot of αSMA on whole liver lysates in WT and Casp1−/− MCD-fed mice compared to CTL-fed mice and (F) corresponding densitometric analysis to HSC70 (n=3). (G) Collagen fibers were stained with Sirius red and (H) quantified using the surface area stained per 40x field area (n = 5-7 in each group) excluding blood vessels. Results are expressed as mean ± S.E.M. * P < 0.05; ** P < 0.01; ***P < 0.001 compared to controls.
Fig. 4. Protection from MCD-diet induced fibrosis in Casp1−/− is independent of caspase 3 activation and hepatocellular apoptosis.
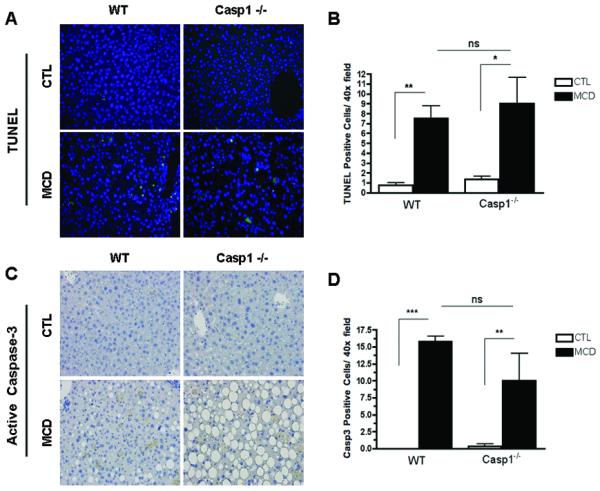
(A, B) Representative microphotographs of TUNEL staining, and active caspase 3 immunohistochemistry (40x). (C, D) Hepatocyte apoptosis in liver sections was quantified by counting the number of TUNEL positive cells, and cleaved caspase 3-positive cells in 5 random microscopic fields (40x). Results are expressed as mean ± S.E.M. * P < 0.05; ** P < 0.01 compared to controls.
Taken together, these observations suggest that during NASH development, caspase 1 activation in hepatocytes plays an important role in hepatocellular injury, inflammatory signaling, HSC activation, and hepatic fibrosis, independent of caspase 3 activation and hepatocellular apoptosis.
Selective Kupffer cell depletion in MCD-induced steatohepatitis reduces caspase 1 activation and protects against fibrogenesis and fibrosis
The findings of inflammasome activation and marked increased in caspase 1 and IL-1β mainly from non-parenchymal cells of the liver on MCD-fed animals in conjuction with the fact that the inflammasome is primarily present in monocytes and macrophages led us to the hypothesis that Kupffer cells, the liver resident macrophages, are the main source for these changes. To test this hypothesis we next examine the effects of selective depletion of Kupffer cells on caspase 1 expression and MCD induced liver damage. C57BL/6 WT mice on a 6-week MCD diet were injected twice during the last week of the diet with clodronate (CLOD) or PBS vehicle (PBS) liposomes intravenously. Hepatic steatosis was unchanged between the CLOD and PBS treated MCD-fed mice (Fig. 5A). Hepatic F4/80 immunostaining demonstrated the effectiveness of CLOD treatment in the depletion of Kupffer cells in the liver (Fig 5A). This was further confirmed by measurement of mRNA levels by RT-PCR of TNFα, F4/80 and CD68 which were markedly decreased in the CLOD-treated group compared to PBS-treated MCD-fed mice (Fig 5B). More importantly, caspase 1 protein expression was also significantly reduced in CLOD-treated MCD-fed mice as detected by both immunohistochemistry of liver tissue and immunblot analysis of whole liver lysates (Fig 5C - E). These changes were associated with a decrease in the expression of genes involved in fibrogenesis (Fig. 5F) as well as a dramatically reduced in collagen deposition in CLOD-treated MCD-fed mice (Fig 5G). These data strongly suggest that Kupffer cells are the main cellular source of active caspase 1 in MCD-diet induced steatohepatitis.
Fig. 5. Kupffer cell depletion abrogates caspase 1-mediated diet-induced steatohepatitis.
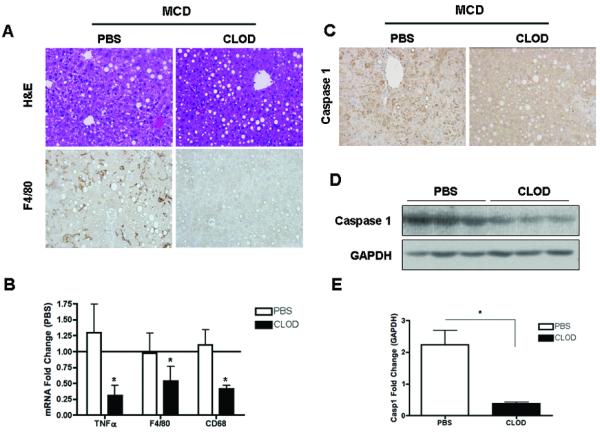
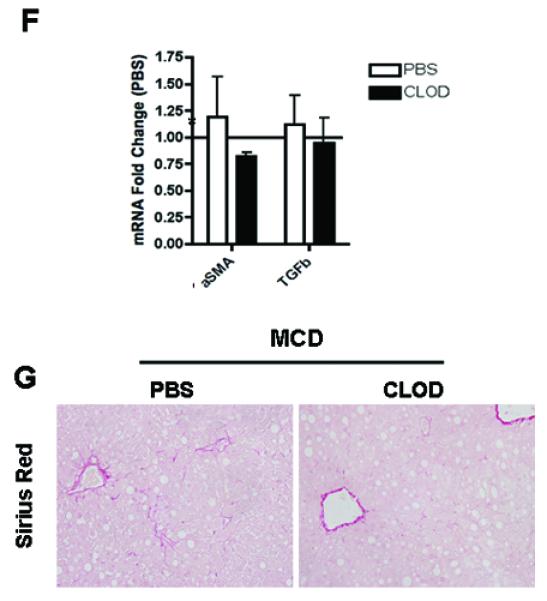
(A) Representative photomicrographs of H&E, and F4/80 immunostaining in the liver of PBS (n=7) or CLOD –treated (n=3) MCD-fed mice. (B) RT-PCR analysis of tumor necrosis factor (TNFα), and macrophage markers F4/80 and CD68 expression in the liver of PBS (n=7) or CLOD –treated (n=3) MCD-fed mice. (C) Representative photomicrographs of caspase 1 immunostaining, on liver tissue from the two groups of mice, and (D, E) Western blot analysis of caspase 1 performed on whole liver tissue lysates from PBS (n=7) or CLOD –treated (n=3) MCD-fed mice with densitometric analysis. (F) RT-PCR analysis of fibrogenesis genes αSMA and TGFβ expression in the liver of PBS (n=5) or CLOD –treated (n=3) MCD-fed mice (G) Collagen fibers were stained with Sirius red. Results are expressed as mean ± S.E.M. * P < 0.05 compared to controls.
Discussion
The principal findings of this study relate to the role of caspase 1 during NASH development. The results demonstrate that NASH induced by MCD feeding is associated with caspase 1 activation in the liver, while caspase 1 suppression is associated with dissociation between hepatic triglyceride accumulation and inflammatory activity, and protects against HSC activation and fibrosis development. These effects were independent of caspase-3 activation and hepatocyte cell death. Furthermore, we identified Kupffer cells as a key cellular source of active caspase 1 in during MCD diet-induced steatohepatitis.
Since the original description that caspase-3 and -7 activation and TUNEL positive cells are a characteristic pathologic features in the liver of NASH patients (9), a growth of data mainly from experimental studies have suggested that caspase activation, mainly caspase-3 is a key process involved in NASH pathogenesis (10). As a result, the targeting of caspase activity has gained significant attention for developing of both novel therapeutic as well as diagnostic strategies for NASH patients. A recent pre-clinical study tested a pan-caspase inhibitor VX-166 in an animal model of NASH(19). Obese leptin receptor deficient db/db mice were fed methionine and choline-deficient (MCD) diet to induce NASH and liver fibrosis. Mice gavaged daily with VX-166 showed a marked reduction in hepatic caspases activity, decreased levels of mature IL-1β and IL-18 in the liver, and liver fibrosis.
Caspases belong to a family of highly conserved cysteine-dependent aspartate-specific acid proteases that use a cysteine residue as the catalytic nucleophile and share a stringent specificity for cleaving their substrates after aspartic acid residues in target proteins (15). They are synthesized as inert zymogens and upon receipt of apoptotic stimuli, cells activate initiator caspases such as, caspase 1, 2, 8, 9, and 10 that, in turn, proteolytically cleave and activate effector caspases including caspase 3, 6, and 7 (16). Caspases have been further categorized as either proinflammatory or proapoptotic, depending upon their participation in these cellular programs. The proinflammatory caspases include caspase 1, 11 and 12 in mouse and caspase 1, 4, and 5 in human. The relative contribution of pro-apoptotic versus pro-inflammatory caspases to liver pathology during NASH development as well as in the protective effects of pan-caspase inhibitors remain incompletely understood. Our results confirmed the findings from Witek and colleagues (19) demonstrating that MCD feeding results in marked caspase-1 activation. Our current data further extend these observations by demonstrating that activated cleaved caspase 1 localized predominalty on non-parenchymal sinusoidal cells in the liver, and to a lesser extent to hepatocytes. Suppression of caspase-1 activation resulted in decreased tissue inflammation despite an increase in triglyceride deposition and hepatic steatosis. Moreover, while as expected after six weeks on the MCD diet wild type animals showed a marked increase in the expression of various genes involved in HSC activation and fibrogenesis, these changes were significantly reduced in the Casp1−/− mice. More importantly, wild-type animals on the MCD diet but not Casp1−/− mice showed a significant increase in collagen deposition. These changes were independent of hepatocyte caspase-3 activation, hepatocellular injury as evidence by ballooning degeneration of hepatocytes and elevation of serum transaminases, and cell death which occurred to a similar extent in both WT animals and Casp1−/− mice on the MCD diet. The precise mechanisms resulting in the catalytic processing of pro-caspase-1 into its enzymatically active form during NASH development will require further investigation but multiple processes that are known to induce the assembly of the inflammasome, the caspase-1-activating complex, such as increase in reactive oxygen species production, and lysosomal permabilization and release of cathepsins into the cytosol are known to be present in both experimental model of NASH as well as humans with this condition (13, 28).
Our key finding of a mixed pattern of cellular expression of caspase-1 in this model of steatohepatitis, in conjuction with the recent report that fatty acids may induce activation of caspase 1 in isolated hepatocytes(29) led us to further investigate the potential cellular source of this protease. Interestingly, we observed that selective depletion of Kupffer cells by clodronate treatment markedly reduced the protein expression of caspase 1 in the liver of MCD-fed animals as well as significantly reduced the amount of collagen deposition pro-inflammatory cytokine TNFα in a similar manner to the Casp1−/− mice on the MCD diet. These results strongly suggest that Kupffer cells are a key cellular source of active caspase 1 in MCD-induced steatohepatitis., which plays an important role in the pathogenesis of this model through inflammation and hepatic stellate cell activation and fibrogenesis. Future studies such as those using cell-type specific caspase 1 knock out mice will be required to further dissect the role of caspase 1 activation in other cell types in the liver in vivo and their potential distinct role in the pathophysiologic changes observed during NASH development.
In summary, the current studies uncover the role of hepatic caspase 1 activation in experimental NASH. The results support a model in which during the development of NASH, caspase 1 activation in Kupffer cells results in induction of pro-inflammatory signaling and hepatic stellate cell activation which are then responsible for collagen deposition and fibrosis (Fig. 6). These data provide new insights into the pathogenesis of liver damage in NASH, and identify potential novel molecular targets for therapeutic intervention in this highly common and potentially serious disease.
Fig. 6. Proposed model for role of inflammasome and caspase 1 activation in tissue damage and fibrosis in steatohepatitis.
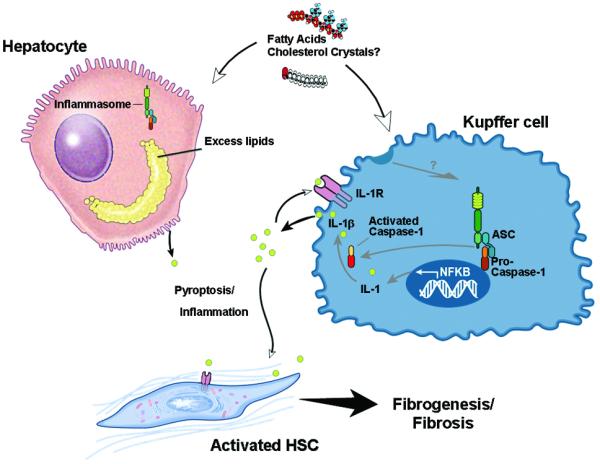
In the process of NASH development, different lipotoxic substances such as cholesterol crystals, or free fatty acids may induce the processing and activation of caspase 1 predominantly in Kupffer cells in the liver, and to a lesser extent in hepatocytes. Hepatic caspase 1 activation induce cleavage of IL-1 into its mature form IL-1beta resulting in increased inflammation and tissue damage including the activation of hepatic stellate cells. This results in fibrogenesis and fibrosis development. Therapy target at inhibiting the inflammasome, or caspase 1 activation may be a novel therapeutic target for treatment of NASH.
Acknowledgements
This work was supported by NIH grants (DK076852) and (DK082451) to AEF.
Abreviations
- MCD
Methionine-choline deficient diet
- NAFLD
Nonalcoholic fatty liver disease
- NASH
nonalcoholic steatohepatitis
- ALT
alanine aminotransferase
- TUNEL
terminal deoxynucleotidyl transferase dUTP nick-end labeling
References
- 1.Wieckowska A, Feldstein AE. Nonalcoholic fatty liver disease in the pediatric population: a review. Curr Opin Pediatr. 2005;17:636–641. doi: 10.1097/01.mop.0000172816.79637.c5. [DOI] [PubMed] [Google Scholar]
- 2.Angulo P. Nonalcoholic fatty liver disease. N Engl J Med. 2002;346:1221–1231. doi: 10.1056/NEJMra011775. [DOI] [PubMed] [Google Scholar]
- 3.Browning JD, Szczepaniak LS, Dobbins R, Nuremberg P, Horton JD, Cohen JC, Grundy SM, et al. Prevalence of hepatic steatosis in an urban population in the United States: impact of ethnicity. Hepatology. 2004;40:1387–1395. doi: 10.1002/hep.20466. [DOI] [PubMed] [Google Scholar]
- 4.Schwimmer JB, Deutsch R, Kahen T, Lavine JE, Stanley C, Behling C. Prevalence of fatty liver in children and adolescents. Pediatrics. 2006;118:1388–1393. doi: 10.1542/peds.2006-1212. [DOI] [PubMed] [Google Scholar]
- 5.Brunt EM, Neuschwander-Tetri BA, Oliver D, Wehmeier KR, Bacon BR. Nonalcoholic steatohepatitis: histologic features and clinical correlations with 30 blinded biopsy specimens. Hum Pathol. 2004;35:1070–1082. doi: 10.1016/j.humpath.2004.04.017. [DOI] [PubMed] [Google Scholar]
- 6.Adams LA, Lymp JF, St Sauver J, Sanderson SO, Lindor KD, Feldstein A, Angulo P. The natural history of nonalcoholic fatty liver disease: a population-based cohort study. Gastroenterology. 2005;129:113–121. doi: 10.1053/j.gastro.2005.04.014. [DOI] [PubMed] [Google Scholar]
- 7.Matteoni CA, Younossi ZM, Gramlich T, Boparai N, Liu YC, McCullough AJ. Nonalcoholic fatty liver disease: a spectrum of clinical and pathological severity. Gastroenterology. 1999;116:1413–1419. doi: 10.1016/s0016-5085(99)70506-8. [DOI] [PubMed] [Google Scholar]
- 8.Ekstedt M, Franzen LE, Mathiesen UL, Thorelius L, Holmqvist M, Bodemar G, Kechagias S. Long-term follow-up of patients with NAFLD and elevated liver enzymes. Hepatology. 2006;44:865–873. doi: 10.1002/hep.21327. [DOI] [PubMed] [Google Scholar]
- 9.Feldstein AE, Canbay A, Angulo P, Taniai M, Burgart LJ, Lindor KD, Gores GJ. Hepatocyte apoptosis and fas expression are prominent features of human nonalcoholic steatohepatitis. Gastroenterology. 2003;125:437–443. doi: 10.1016/s0016-5085(03)00907-7. [DOI] [PubMed] [Google Scholar]
- 10.Cazanave SC, Gores GJ. Mechanisms and clinical implications of hepatocyte lipoapoptosis. Clin Lipidol. 2010;5:71–85. doi: 10.2217/clp.09.85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Feldstein A, Gores GJ. Steatohepatitis and apoptosis: therapeutic implications. Am J Gastroenterol. 2004;99:1718–1719. doi: 10.1111/j.1572-0241.2004.40573.x. [DOI] [PubMed] [Google Scholar]
- 12.Feldstein AE, Canbay A, Guicciardi ME, Higuchi H, Bronk SF, Gores GJ. Diet associated hepatic steatosis sensitizes to Fas mediated liver injury in mice. J Hepatol. 2003;39:978–983. doi: 10.1016/s0168-8278(03)00460-4. [DOI] [PubMed] [Google Scholar]
- 13.Feldstein AE, Werneburg NW, Canbay A, Guicciardi ME, Bronk SF, Rydzewski R, Burgart LJ, et al. Free fatty acids promote hepatic lipotoxicity by stimulating TNF-alpha expression via a lysosomal pathway. Hepatology. 2004;40:185–194. doi: 10.1002/hep.20283. [DOI] [PubMed] [Google Scholar]
- 14.Chowdhury I, Tharakan B, Bhat GK. Caspases - an update. Comp Biochem Physiol B Biochem Mol Biol. 2008;151:10–27. doi: 10.1016/j.cbpb.2008.05.010. [DOI] [PubMed] [Google Scholar]
- 15.Li J, Yuan J. Caspases in apoptosis and beyond. Oncogene. 2008;27:6194–6206. doi: 10.1038/onc.2008.297. [DOI] [PubMed] [Google Scholar]
- 16.Weber IT, Fang B, Agniswamy J. Caspases: structure-guided design of drugs to control cell death. Mini Rev Med Chem. 2008;8:1154–1162. doi: 10.2174/138955708785909899. [DOI] [PubMed] [Google Scholar]
- 17.Anstee QM, Concas D, Kudo H, Levene A, Pollard J, Charlton P, Thomas HC, et al. Impact of pan-caspase inhibition in animal models of established steatosis and non-alcoholic steatohepatitis. J Hepatol. 2010 doi: 10.1016/j.jhep.2010.03.016. in press. [DOI] [PubMed] [Google Scholar]
- 18.Barreyro FJ, Holod S, Finocchietto PV, Avagnina A, Camino AM, Biondo CM, Aquino JB, et al. PF-03491390 Pan-caspase inhibitor decreases liver injury and fibrosis in a murine model of non-alcoholic steatohepatitis (NASH) j Hepatol. 2010;52:S303. doi: 10.1111/liv.12570. [DOI] [PubMed] [Google Scholar]
- 19.Witek RP, Stone WC, Karaca FG, Syn WK, Pereira TA, Agboola KM, Omenetti A, et al. Pan-caspase inhibitor VX-166 reduces fibrosis in an animal model of nonalcoholic steatohepatitis. Hepatology. 2009;50:1421–1430. doi: 10.1002/hep.23167. [DOI] [PubMed] [Google Scholar]
- 20.Kuida K, Lippke JA, Ku G, Harding MW, Livingston DJ, Su MS, Flavell RA. Altered cytokine export and apoptosis in mice deficient in interleukin-1 beta converting enzyme. Science. 1995;267:2000. doi: 10.1126/science.7535475. [DOI] [PubMed] [Google Scholar]
- 21.Li P, Allen H, Banerjee S, Franklin S, Herzog L, Johnston C, McDowell J, et al. Mice deficient in IL-1 beta-converting enzyme are defective in production of mature IL-1 beta and resistant to endotoxic shock. Cell. 1995;80:401. doi: 10.1016/0092-8674(95)90490-5. [DOI] [PubMed] [Google Scholar]
- 22.Dela Pena A, Leclercq I, Field J, George J, Jones B, Farrell G. NF-kappaB activation, rather than TNF, mediates hepatic inflammation in a murine dietary model of steatohepatitis. Gastroenterology. 2005;129:1663–1674. doi: 10.1053/j.gastro.2005.09.004. [DOI] [PubMed] [Google Scholar]
- 23.Den Boer M, Voshol PJ, Kuipers F, Havekes LM, Romijn JA. Hepatic steatosis: a mediator of the metabolic syndrome. Lessons from animal models. Arteriosclerosis, thrombosis, and vascular biology. 2004;24:644. doi: 10.1161/01.ATV.0000116217.57583.6e. [DOI] [PubMed] [Google Scholar]
- 24.London RM, George J. Pathogenesis of NASH: animal models. Clinics in liver disease. 2007;11:55–74. doi: 10.1016/j.cld.2007.02.010. [DOI] [PubMed] [Google Scholar]
- 25.Van Rooijen N, Sanders A. Kupffer cell depletion by liposome-delivered drugs: comparative activity of intracellular clodronate, propamidine, and ethylenediaminetetraacetic acid. Hepatology. 1996;23:1239–1243. doi: 10.1053/jhep.1996.v23.pm0008621159. [DOI] [PubMed] [Google Scholar]
- 26.Canbay A, Guicciardi ME, Higuchi H, Feldstein A, Bronk SF, Rydzewski R, Taniai M, et al. Cathepsin B inactivation attenuates hepatic injury and fibrosis during cholestasis. Journal of Clinical Investigation. 2003;112:152–159. doi: 10.1172/JCI17740. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kleiner DE, Brunt EM, Van Natta M, Behling C, Contos MJ, Cummings OW, Ferrell LD, et al. Design and validation of a histological scoring system for nonalcoholic fatty liver disease. Hepatology. 2005;41:1313–1321. doi: 10.1002/hep.20701. [DOI] [PubMed] [Google Scholar]
- 28.Li Z, Berk M, McIntyre TM, Gores GJ, Feldstein AE. The lysosomalmitochondrial axis in free fatty acid-induced hepatic lipotoxicity. Hepatology. 2008;47:1495–1503. doi: 10.1002/hep.22183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Csak T, Ganz M, Pespisa J, Kodys K, Dolganiuc A, Szabo G. Fatty acid and endotoxin activate inflammasomes in mouse hepatocytes that release danger signals to stimulate immune cells. Hepatology. 54:133–144. doi: 10.1002/hep.24341. [DOI] [PMC free article] [PubMed] [Google Scholar]


