Summary
While the functional interplay between mutant and wild type Ras in driving tumor initiation and growth has been described, a clear picture of the precise ramifications and mechanisms of this association remains elusive, with sometimes conflicting conclusions. A report in this issue of Cancer Discovery tackles this question and their findings may have important implications for therapeutic strategies to block mutant Ras for cancer treatment.
INTRODUCTION
Wild type (WT) Ras proteins (H-Ras, N-Ras and K-Ras) function as regulated binary switches that cycle between an inactive GDP-bound and an active GTP-bound state (1). In normal resting cells, Ras is predominantly GDP-bound and inactive. In response to growth factor stimulation and activation of Ras-selective guanine nucleotide exchange factors (RasGEFs; e.g., Sos1), Ras rapidly cycles to the GTP-bound state. Activated Ras-GTP then binds and activates downstream effectors that include the Raf serine/threonine kinases and the class I phosphoinositol 3-kinases, leading to the phosphorylation and activation of the ERK and AKT serine/threonine kinases, respectively. The activation state is transient, with Ras-selective GTPase activating proteins (RasGAPs; e.g., neurofibromin) stimulating hydrolysis of the bound GTP, rapidly returning Ras to the inactive GDP-bound state, terminating effector signaling. Current evidence suggests that Ras-GDP is simply an inactive protein. However, whether Ras-GDP preferentially interacts with other proteins and has functions distinct from those of Ras-GTP remains an unresolved issue.
Mutationally activated forms of Ras are found in ~30% of human cancers (1). These cancers harbor single amino acid substitutions, most commonly at G12, G13 or Q61, that impair the intrinsic and GAP-stimulated GTP hydrolysis activities, rendering mutant Ras proteins persistently GTP-bound in the absence of extracellular stimuli. Mutant Ras proteins are then persistently bound to their effectors, causing persistent effector signaling. However, persistent Ras signaling may be detrimental to the cancer cell and mechanisms to dampen ERK activation include upregulated expression of ERK phosphatases (e.g., Dusp6) or ERK phosphorylation of upstream components, also attenuating ERK activation.
Early ectopic expression studies in NIH 3T3 mouse fibroblasts conveyed the perception that the presence of mutationally activated Ras rendered WT Ras dispensable for cancer cell growth. This was best demonstrated by studies utilizing the dominant negative Ras S17N mutant, which forms a nonproductive complex with RasGEFs, thus preventing WT Ras activation and causing growth inhibition. The actions of Ras S17N could be overcome by concurrent expression of mutationally activated Ras (2). Similarly, introduction of mutations in Ras that impaired RasGEF interactions did not impair mutant Ras transforming activity (3). These studies suggested that mutant Ras is fully active and function and independent of RasGEF activity, and consequently, independent of WT Ras activity.
FINDINGS
In this issue of Cancer Discovery, McCormick and colleagues addressed the issue of whether the remaining two WT Ras isoforms contribute to tumor cell growth in the context of mutationally activated Ras (4). While this is not a new concept (5), they take a different approach to evaluate this issue. Three tumor cell lines were studied, each with either mutant H-Ras, K-Ras or N-Ras. There is conflicting evidence for the role of the WT counterpart of the mutant Ras isoform, for example, evidence that the WT RAS allele can act as a tumor suppressor gene and antagonize the oncogenic properties of its mutationally activated counterpart (6), and that human tumor cell lines are often homozygous for mutant RAS (COSMIC). Therefore, to avoid that potential complication, cell lines were chosen that are homozygous for the mutant Ras isoform: KRAS G12C mutant MIA PaCa-2 pancreatic, HRAS G12V mutant T24 bladder and NRAS Q61H mutant RD rhabdomyosarcoma cell lines.
The key finding is that both mutant and WT Ras proteins contribute to tumor cell anchorage-dependent proliferation (Figure 1). siRNA silencing of the mutant Ras isoform alone caused a near-complete suppression of proliferation. By comparison, the combined suppression of the remaining two WT Ras isoforms caused a less drastic but significant reduction in tumor cell proliferation. These results suggested that WT and mutant Ras may have distinct functions required for tumor cell growth. Since both WT Ras isoforms were suppressed, the study did not address whether each WT isoform served redundant or distinct functions. In a similar analysis, Counter and colleagues found that stable shRNA suppression of either HRAS or NRAS alone reduced the tumorigenic growth of MIA PaCa-2 cells in SCID mice (5), suggesting distinct roles for each WT Ras isoform.
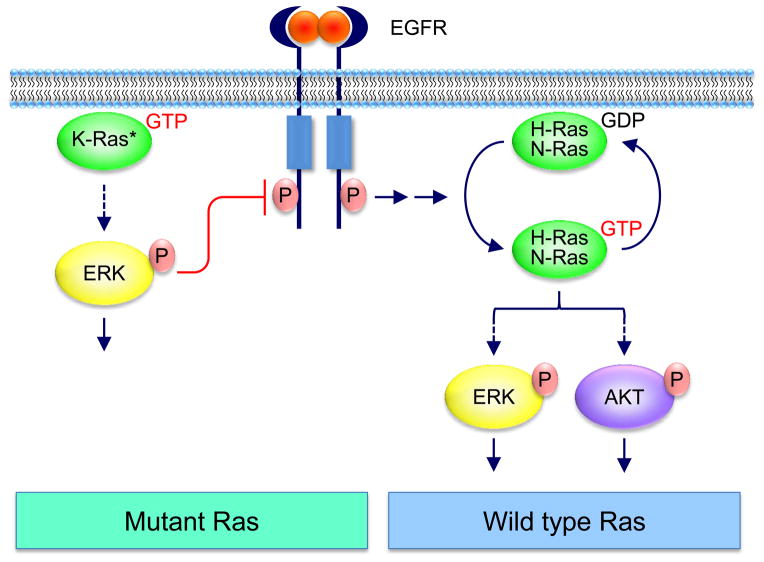
Figure 1.
Functional interplay between WT and mutant Ras. Shown in this figure are the signaling interactions between mutationally activated K-Ras and WT H-Ras and N-Ras. Similar interactions are also seen human tumor cells harboring mutated H-Ras or N-Ras and the remaining two WT Ras isoforms. siRNA silencing of either mutant (*) KRAS or concurrently WT HRAS and NRAS reduces tumor cell proliferation.
To determine whether the distinct roles of WT and mutant Ras can be explained by differences in effector signaling, the levels of activated ERK (pERK) and AKT (pAKT) were evaluated. Depletion of the mutant Ras isoform caused a partial reduction in basal pERK levels in all three tumor lines, but surprisingly, an elevation in pAKT was seen in T24 and RD cells. In contrast, depletion of the WT Ras isoforms increased pERK in MIA PaCa-2 and RD cells, suggesting that WT Ras may antagonize mutant Ras and ERK signaling. Depletion of WT Ras did not cause the same pAKT increase seen with silencing of mutant Ras. While these analyses indicate that WT and mutant Ras do not regulate effector signaling by the same mechanisms, they also suggest that the requirement for WT Ras for proliferation cannot be attributed simply to their regulation of ERK and/or AKT activation.
A second key finding was that depletion of mutant Ras increased signaling (pERK and pAKT) through the EGF receptor (EGFR). Thus, mutant Ras antagonizes EGFR signaling, possibly through ERK-mediated phosphorylation of EGFR at an inhibitory T669 residue. The sensitivity of other receptor tyrosine kinases may also be antagonized by mutant Ras. This result suggests that cancer cells may undergo reprogramming when mutant Ras function is blocked, leading to increased EGFR signaling and activation of WT Ras, and thus bypassing the block in mutant Ras signaling. This additionally suggested that inhibition of EGFR may block this reprogramming mechanism. Consistent with this possibility the treatment of cells depleted of mutant H-Ras with the erlotinib EGFR inhibitor caused a further reduction in T24 cell proliferation that was associated with enhanced apoptosis. EGFR dependency of T24 cells correlated with AKT activation. This synergy between mutant Ras suppression and EGFR inhibition, however, was not seen with the mutant K-Ras or N-Ras tumor lines, possibly because the increased activities of other RTKs may be associated with loss of mutant Ras function. Alternatively, the different isoforms of mutant Ras or cancers of different tissue origin may not behave identically when mutant Ras activity is blocked.
The mechanism by which RTK signaling causes WT Ras activation was not addressed. Whether the mechanisms described in two previous studies, where either endothelial nitric oxide synthase or Sos1 were identified as mechanisms that promoted WT Ras activation in cells harboring mutant Ras (5, 7), are relevant for this current report is not clear.
INTERPRETATION
The main implication from this study is that an effective therapeutic strategy to block mutant Ras-driven cancer growth may require concurrent inhibition of mutant as well as WT Ras function, and that inhibitors of RTKs may facilitate that strategy. Not surprisingly, with now strong evidence for reprogramming signaling mechanisms that overcome protein kinase inhibitors, cancer cells are likely to adapt to the loss of mutant Ras. The recent studies characterizing small molecule inhibitors of RasGEF interaction with Ras raised the issue of whether this was a worthwhile strategy to target mutant Ras-containing tumors (8, 9). The findings in this report argue that such inhibitors may be effective, but through their inhibition of WT rather than mutant Ras function. An issue that remains unclear is whether the effectors that support the requirements for mutant and WT Ras in cancer cell proliferation are distinct.
References
- 1.Cox AD, Der CJ. Ras history: The saga continues. Small GTPases. 2010;1:2–27. doi: 10.4161/sgtp.1.1.12178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Feig LA, Cooper GM. Inhibition of NIH 3T3 cell proliferation by a mutant ras protein with preferential affinity for GDP. Mol Cell Biol. 1988;8:3235–43. doi: 10.1128/mcb.8.8.3235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Quilliam LA, Kato K, Rabun KM, Hisaka MM, Huff SY, Campbell-Burk S, et al. Identification of residues critical for Ras(17N) growth-inhibitory phenotype and for Ras interaction with guanine nucleotide exchange factors. Mol Cell Biol. 1994;14:1113–21. doi: 10.1128/mcb.14.2.1113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Young A, Lou D, McCormick F. Oncogenic and wild-type Ras play divergent roles in the regulation of mitogen-activated protein kinase signaling. Cancer Discov. 2012;3:112–23. doi: 10.1158/2159-8290.CD-12-0231. [DOI] [PubMed] [Google Scholar]
- 5.Lim KH, Ancrile BB, Kashatus DF, Counter CM. Tumour maintenance is mediated by eNOS. Nature. 2008;452:646–49. doi: 10.1038/nature06778. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Zhang Z, Wang Y, Vikis HG, Johnson L, Liu G, Li J, et al. Wildtype Kras2 can inhibit lung carcinogenesis in mice. Nat Genet. 2001;29:25–33. doi: 10.1038/ng721. [DOI] [PubMed] [Google Scholar]
- 7.Jeng HH, Taylor LJ, Bar-Sagi D. Sos-mediated cross-activation of wild-type Ras by oncogenic Ras is essential for tumorigenesis. Nat Commun. 2012;3:1168. doi: 10.1038/ncomms2173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Maurer T, Garrenton LS, Oh A, Pitts K, Anderson DJ, Skelton NJ, et al. Small-molecule ligands bind to a distinct pocket in Ras and inhibit SOS-mediated nucleotide exchange activity. Proc Natl Acad Sci U S A. 2012;109(14):5299–304. doi: 10.1073/pnas.1116510109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Sun Q, Burke JP, Phan J, Burns MC, Olejniczak ET, Waterson AG, et al. Discovery of small molecules that bind to K-Ras and inhibit Sos-mediated Activation. Angew Chem Int Ed Engl. 2012;51(25):6140–3. doi: 10.1002/anie.201201358. [DOI] [PMC free article] [PubMed] [Google Scholar]