Abstract
Delirium and poor sleep quality are common and often co-exist in hospitalized patients. A link between these disorders has been hypothesized but whether this link is a cause and effect relationship or simply an association resulting from shared mechanisms is yet to be determined. Potential shared mechanisms include: abnormalities of neurotransmitters, tissue ischemia, inflammation, and sedative exposure. Sedatives, while decreasing sleep latency, often cause a decrease in slow wave sleep and stage REM sleep and therefore may not provide the same restorative properties as natural sleep. Mechanical ventilation, an important cause of sleep disruption in ICU patients, may lead to sleep disruption not only from the discomfort of the endotracheal tube but also as a result of ineffective respiratory efforts and by inducing central apnea events if not properly adjusted for the patient’s physiologic needs. When possible, efforts should be made to optimize the patient-ventilator interaction to minimize sleep disruptions.
Keywords: Sleep, delirium, sedation, mechanical ventilation
INTRODUCTION
Sleep is an essential biologic function that is important for both physiologic rest and emotional wellbeing. Hospitalized patients often have poor quality sleep experiencing difficulty with sleep initiation, sleep fragmentation, early morning awakenings, and decreased total sleep time. The patient’s underlying illness, pain, environmental noise and artificial light, patient care activities, and medication effects all are potential contributors to their sleep disruption. Even the sedative and analgesic medications administered in an attempt to promote sleep and comfort can lead to abnormal sleep patterns that may not provide the same restorative function as natural sleep.
Normal sleep architecture consists of two major sleep stage categories - non-rapid eye movement sleep (NREM) and rapid eye movement sleep (REM). NREM is further subdivided into three stages of progressively deeper sleep which are N1, N2, and N3 [also known as slow wave sleep (SWS)]. During NREM sleep, brain activity is decreased and there is a corresponding decrease in metabolic rate. In contrast, during REM sleep the brain is active and there is skeletal muscle atonia. Though the functions of the different sleep stages remain largely unknown, both NREM and REM sleep are thought to be involved in memory consolidation.1
Delirium is common in hospitalized patients and occurs in 11% of elderly emergency department patients,2 in 37% of post operative patients,3 and in as many as 80% of critically ill patients receiving mechanical ventilation.4 Older patients are more susceptible with up to 50% of geriatric patients developing delirium at some point in their hospital course.5;6 The pathogenesis of delirium is poorly understood. Hypotheses regarding contributing etiologies have included neurotransmitter imbalances, proinflammatory cytokines, tissue hypoxia, and sleep deprivation.7–9
Delirium is characterized by inattention, fluctuating mental status, disorganized thinking and an altered level of consciousness – findings which are also characteristics of sleep deprivation. Sleep disturbances are common in delirious patients. And, while sleep deprivation is regarded to be a potentially modifiable risk factor for the development of delirium, it is also likely that delirium itself contributes to sleep disturbances.
This article reviews (1) the potential relationship between sleep deprivation and delirium, (2) the modification of sleep with sedative administration, and (3) the effects of mechanical ventilation on sleep quality.
SLEEP – DELIRIUM INTERACTIONS
Hospital-related sleep disturbances
Poor sleep quality is a problem that affects many hospitalized adult patients and that is yet underreported and not properly considered by the majority of health professionals. Baseline acute disease, change of sleep habits, use of sedatives and other medications, environmental noise and artificial light can be listed among the main causes of this problem. An association has been shown between sleep disturbances and the number of chronic diseases, the presence of pain, use of tricyclic antidepressants, and the length of hospital stay.10
Almost 50% of patients admitted in general hospital medical wards experience insomnia, excessive daytime sleepiness, or both.11 Few, if any of these sleep problems are ever reported in the medical records. The use of hypnotic medications both prior to hospital admission and also during the hospital stay is common; therefore, physicians should be aware of not only the direct adverse effects of these medications but also the potential effects of sedative-hypnotic withdrawal in hospitalized patients.
Intensive care units (ICU) are the hospital setting where the problem of sleep disturbances likely reaches its highest level and represents the source of most relevant data available in the literature. During their ICU stay, patients often experience prolonged sleep onset, fragmented sleep, poor sleep efficiency and decreased amounts of REM sleep.12 The overall sleep time during a 24-hour period can be within normal limits (7–9 hours), but almost 50% of this time is spent in shorts bouts during the day rather than a consolidated nocturnal sleep, so that patients have difficulty achieving the REM and N3 (SWS) sleep stages.13 ICU patients complain of long periods of wakefulness; the sleep architecture in these patients is remarkable for increased stage N1 (light) sleep, decreased amounts of N2, N3, and notably a marked reduction REM stage sleep. In contrast to healthy individuals who experience an average of 20% to 25% REM sleep, ICU patients may experience little or no stage REM sleep during their ICU stay.14;15
Sleep deprivation can cause both physiological and behavioral consequences. The most relevant physiologic consequences are an impaired immune response,16 an alteration in metabolic and endocrine systems,17 an increase in pain sensitivity,18 and changes in cardiac modulations from sympathetic and parasympathetic systems.19 The behavioral changes include impaired attention, mood and psychomotor performance, increased daytime sleepiness, sensation of fatigue, and irritability.20
Delirium and possible links to sleep disturbances
Delirium is a form of brain dysfunction whose main features are acute onset of a fluctuating awareness, inattention, disorganized thinking, and an altered level of consciousness. These same features can occur to varying degrees as a result of sleep deprivation. Delirium can be either hypoactive, hyperactive, or mixed in character. The hypoactive form occurs frequently and though known to be associated with poor outcomes, it too often goes unrecognized by the physicians and nurses caring for these patients.
Both sleep disturbances and delirium are very common in hospitalized patients, especially critically ill patients in the ICU environment. A possible link between these two disorders has been hypothesized in terms of a common pathophysiologic pathway, shared mechanisms, and a potential cause-effect relationship.21
Studies conducted mainly in cardiac surgical patients indicate that sleep deprivation can either cause delirium,22 be a result of it,23 or may simply lower the clinical threshold for delirium. Decreased SWS and decreased stage REM sleep have been hypothesized as contributing factors for the development of delirium. While a recent study of ICU patients demonstrated an association between delirium and severe REM sleep reduction (<6% of total sleep time), a cause and effect relationship was not established.15 Although the association between sleep disturbance and delirium has been studied for many years, at present it is difficult to define the true relationship between them. Perhaps, though, it is possible to view their relationship as part of a shared pathophysiologic pathway.
Although the mechanism of delirium is not completely understood, a neurotransmitter imbalance is one of the leading hypotheses, with dopamine and acetylcholine felt to be the most important neurotransmitters involved. An imbalance of these same neurotransmitters also occurs in association with sleep deprivation.24 During delirium the levels of acetylcholine are generally thought to be low and those of dopamine high, though a few reports tend to hypothesize the opposite imbalance (high acetylcholine and low dopamine).25 The importance of dopamine on the development of delirium, in particular, seems to be supported by the therapeutic effect of haloperidol, a powerful dopamine blocker.
In addition to acetylcholine and dopamine, there is evidence that other neurotransmitters such as tryptophan can play a significant role in delirium. Tryptophan, a serotonin precursor, was reduced in a population of cardiac surgery patients with delirium.26 Importantly, it appears that an abnormal tryptophan metabolism can modulate the type of delirium, i.e. hyperactive or hypoactive.27 Tryptophan, moreover, is tightly connected to melatonin a hormone involved in the regulation of circadian rhythm that has also been linked to delirium. Tryptophan is a direct precursor of melatonin; melatonin is secreted by the pineal gland and metabolized by the liver to 6-hydroxymelatonin and then conjugated with sulphuric acid to 6-sulfatoxymelatonin (excreted in the urine and sometimes used to in clinical trials to assess melatonin secretion).
There is increasing interest in melatonin and its role in postoperative and critical illness. Critically ill patients undergoing mechanical ventilation exhibit a derangement of circadian rhythm and decreased melatonin production.28;29 Moreover, the involvement of melatonin in surgical stress, sepsis and delirium is becoming more and more evident. In particular, post-operative patients in whom melatonin plasma levels were measured for two days after surgery, showed a correspondence between abnormal melatonin levels and delirium. Patients without postoperative delirium had normal plasma levels, while those who developed delirium had plasma melatonin levels lower than preoperative values if their operative course was uncomplicated or increased melatonin levels if they had further complications like pneumonia or sepsis.30 A possible derangement, not only in melatonin secretion, but also in melatonin metabolism can also be suspected from the observation of Balan, who showed that patients with hypoactive or hyperactive delirium had, respectively, increased or decreased urinary 6-sulfatoxymelatonin levels.31 The role of melatonin in the regulation of the sleep-wake cycle, resetting of circadian rhythm disturbances and its extensive antioxidant activity have potential applications in critical care patients.
Other possible mechanisms believed to contribute to the occurrence of delirium are acute inflammation and ischemia. The anatomical pathway thought to be involved in delirium includes brain areas like the thalamus, prefrontal, posterior parietal and fusiform cortex and the basal ganglia.25 Hence, in theory, ischemia in one of these areas can lead to delirium. Systemic inflammation has also been implicated in the pathogenesis of delirium. It has been observed that delirious patients after hip replacement surgery had higher serum levels of C-reactive protein.32 Other inflammatory cytokines, such as IL-6 and IL-8 are associated with and can possibly induce delirium, either directly or through a neurotransmitter imbalance.33–36
Another link between sleep disturbances and delirium comes from the observation that delirium, in a population of elderly patients undergoing knee replacement surgery, occurred more frequently among those with obstructive sleep apnea.37 The mechanism whereby sleep apnea increases the risk of delirium is not clear but we do know that oxygen desaturations occur during apneic phases and that hypoxia is one of the possible causes of delirium. Moreover, the cyclic oxygen desaturations and restorations during apneic episodes can cause an increase of inflammatory cytokines; and, sleep apnea, per se, can be viewed as an inflammatory process, with increased levels of interleukins and tumor necrosis factor.38 As stated above, the occurrence of delirium may be facilitated by ischemia and inflammation. Moreover, a connection has been found between sleep disordered breathing and dementia-like cognitive impairment, and patients affected by dementia are more prone to develop delirium,39 thus representing the rationale for a further link between sleep disruption and delirium.
Further evidence of a connection between sleep disorders and delirium comes from the current preventive strategies for delirium. The Hospital Elder Life Program (HELP) tested by the Yale Delirium Prevention trial40 assessed six risk factors (dehydration, sleep deprivation, immobility, visual impairment, cognitive impairment and hearing impairment) at the time of hospital admission. When one of these risk factors was determined to be present, it was treated with a targeted intervention by a dedicated team. The nonpharmacologic sleep protocol included sips of warm milk, back and shoulder rubs and relaxing music when going to sleep. This protocol not only reduced the need of sedative and hypnotic drugs, but also reduced the incidence of delirium.41 As a further reinforcement of this evidence, the guidelines for prevention of delirium issued by the National Institute for Health and Clinical Excellence (NICE) recommend efforts to improve sleep quality (i.e. avoiding unnecessary nursing or staff procedures during night-time and reducing environmental noise as much as possible) as a mainstay measure to reduce the incidence of delirium in hospitalized patients.42
The existence of a link between delirium and sleep disruption is very likely though it is not yet evident whether the former can be the cause of the latter and vice-versa; or, whether more likely they simply share a common pathophysiologic pathway. This last mechanism appears to be the most likely explanation, involving the above mention complex pathways between ischemia/inflammation, hypoxia, neurotransmitter imbalance, and abnormalities of tryptophan/melatonin metabolism (see Figure 1). The potential role of sedative medications will be discussed in the following section.
Figure 1.
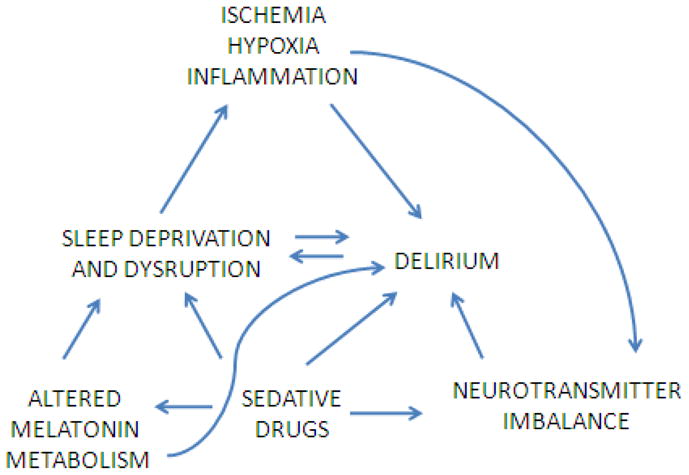
Possible common pathophysiological pathways between delirium and sleep disruption
SEDATION, SLEEP AND DELIRIUM
Critically ill patients, are almost universally administered sedative-hypnotics and often at high doses. These medications are given to aid with the comfort and anxiety of patients as well as to facilitate procedures and mechanical ventilation. Additionally, these medications are often administered in an attempt to improve the sleep of ICU patients. The relationship between sedation and sleep is however complicated. While sedatives produce a state that can physically resemble sleep, there are in fact significant differences between pharmacologically-induced sleep and natural sleep that may be important clinically.
For years sleep has been used as a metaphor for sedation; and indeed, sleep and sedation do have several similarities. Both lead to decreased responsiveness to external stimuli, decreased muscle tone, and respiratory depression. There are, however, many important differences. While the functions of sleep are not fully known, it is an essential biologic function necessary for life. Sleep occurs spontaneously and is reversible by external stimuli whereas sedation is not. Moreover, natural sleep is characterized by a circadian rhythm and a cyclical progression through the sleep stages with classic EEG patterns for each sleep stage. Sedation effects are medication-specific and dose dependent rather than cyclical and will frequently lead to atypical EEG patterns not observed in natural sleep.
Most sedatives and analgesics are known to cause a decrease in SWS and REM sleep, the stages that are considered the most restorative. Whether sedatives provide the same restorative properties as natural sleep is largely unknown and is often debated as few studies have addressed this question. Studies conducted on rodents indicate that sedation with propofol for 12 hours is not associated with an increase in sleep need compared to baseline and also that sedation with propofol appears to allow for recovery from sleep deprivation.43;44 In human study, day-surgery patients undergoing sedation with propofol for one hour were found to have a longer nocturnal sleep latency but a shorter latency to stage N2 sleep and no difference in total sleep time (TST) or sleep efficiency.45 However, this lack of difference in TST following sedation may not have reached significance due to the relatively short sedation period.
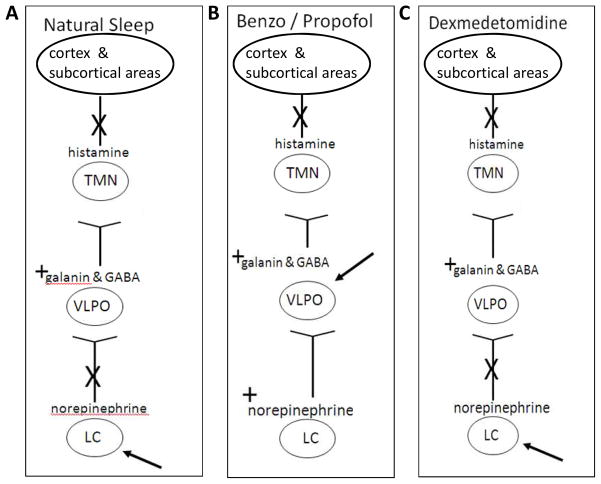
There is evidence that sedatives exert their effect at least in part by acting on the neuronal pathways governing natural sleep and therefore it may be helpful to review some of the basics of these neuronal sleep pathways. The sleep/wake state is regulated by a complex interaction of neurotransmitters produced by clusters of neurons located in the reticular formation of the brain stem, midbrain, thalamus, and hypothalamus with connections to the brain cortex. The neurotransmitters norepinephrine, serotonin, acetylcholine, and histamine all play an important role in maintenance of wakefulness. Important nuclei of the sleep pathway include (1) the locus coeruleus (LC) located in the pons and the principle site of norepinephrine production in the brain, (2) the ventrolateral preoptic nucleus (VLPO) of the hypothalamus which produces the sleep active neurotransmitters, γ–aminobutyric acid (GABA) and galanin, and (3) the tuberomammillary nucleus (TMN), the site of histamine production.
The natural sleep/wake state is controlled in a flip-flop manner where each state inhibits the activity of the other (i.e. the wake active neurons inhibit the activity of neurons producing sleep neurotransmitters and vice-versa).46 At sleep onset, there is an inhibition of norepinephrine release from the LC which leads to an increase in GABA and galanin release from the ventrolateral preoptic nucleus (VLPO) (Figure 2A). GABA then inhibits the release of histamine, a wake promoting neurotransmitter, from the TMN. Serotonin producing neurons located in the medial and dorsal raphe also contribute to the maintenance of a quiet awake state. And also, acetylcholine is important in maintaining wakefulness and attention. Other neurotransmitters including dopamine, orexin, and adenosine also play a significant role in the regulation of the sleep/wake state.
Figure 2. The neuronal pathways of natural sleep, GABA agonists, and dexmedetomidine.
A, In natural sleep, norepinephrine release from the LC is inhibited and this leads to a release of the sleep promoting neurotransmitters, GABA and galanin from the VLPO which then inhibits the release of histamine from the TMN leading to a decrease in consciousness. B, The GABA agonist sedatives interact with the sleep pathway downstream at the level of the VLPO and therefore norepinephrine release from the LC is not inhibited. C, Dexmedetomidine interacts at the level of the LC, and like in natural sleep, the release of norepinephrine from the LC is inhibited.
Definition of abbreviations: LC = locus coeruleus, GABA = γ-aminobutyric acid, VLPO = ventrolateral preoptic nucleus, TMN = tuberomammillary nucleus
Sedatives cause their effects by intersecting the neuronal sleep pathway at various locations. The most commonly used sedatives, benzodiazepines and propofol, exert their effect by activating GABAA receptors in the VLPO,47 thereby suppressing histamine release from the TMN (Figure 2B). But because these sedatives act downstream from the LC, norepinephrine release from the LC is not inhibited. This is hypothesized to lead to an excessive of this neurotransmitter which may contribute to the delirium seen with these medications. Alternatively, dexmedetomidine binds to the α-2 receptors in the LC resulting in a decrease in noradrenergic activity/release (Figure 2C). This different site of action has been hypothesized to be one reason that sedation with dexmedetomidine is associated with a decreased incidence of delirium when compared to benzodiazepines.
Effects of sedating medications
The GABA agonists, benzodiazepines and propofol, are the most commonly used and the currently recommended first line agents for sedation in critically ill patients.48;49 Benzodiazepine administration results in a decrease in sleep latency but adversely affects sleep architecture by significantly decreasing SWS and also reducing stage REM sleep. Animal studies have indicated that benzodiazepines may also affect sleep by inhibiting melatonin synthesis by the pineal gland.50 Both lorazepam and midazolam, the most commonly used benzodiazepines in the ICU, have been shown to be strong independent risk factors for the development of delirium.51;52 Whether the increased incidence of delirium occurring with benzodiazepine use is secondary to primary effects on sleep (decreased SWS and REM sleep), to changes in neurotransmitter imbalance (ongoing release of norepinephrine from the LC), or to other mechanisms remains to be determined.
Propofol is believed to bind to the GABAA receptor at a site distinct from the benzodiazepines.53 Propofol, like the benzodiazepines, is a potent suppresser of SWS. At high doses propofol will induce EEG burst suppression. Burst suppression has been previously shown to be both an independent risk factor for mortality in patients and is also associated with an increase likelihood of post-coma delirium.54;55
Dexmedetomidine, in contrast to the GABA agonists, binds to the α-2 receptors in the LC and causes a decrease in noradrenergic release from this nucleus similar to natural sleep. The decrease in noradrenergic release disinhibits the VLPO neurons that inhibit the arousal pathways thus inducing sedation.56 Sedation with dexmedetomidine more closely resembles natural sleep than sedation with the previously discussed GABA agonists, benzodiazepines and propofol. Patients sedated with dexmedetomidine are responsive to stimuli in a manner similar to natural sleep.57 In contrast to GABA agonists, sedation with demedetomidine leads to an increase in delta frequency EEG activity resembling naturally occurring SWS. Sedation with dexmedetomidine has been demonstrated in two separate studies to result in less brain dysfunction/delirium than the more commonly used GABA agonists, lorazepam and midazolam.58;59
Opioid analgesics are commonly used in conjunction with sedatives in ICU patients to decrease pain and to aid with sedation. They bind the μ receptors of the ponto-thalamic arousal pathway, the neuronal pathway important in REM generation, as opposed to the hypothalamic pathway which is the site of action of GABA and α-2 agonists.60 Opioids suppress REM sleep and SWS in a dose dependent manner. A single lose dose of opioid administered to healthy adult volunteers was associated with a 30% – 50% reduction in SWS.61 Despite these effects on sleep architecture in volunteers and evidence that opioids can be a risk factor for delirum, 62–64 opioids may, in fact, improve sleep and decrease the risk of delirium in patients who are actively experiencing pain. In a study of burn patients requiring mechanical ventilation, opioid exposure was shown to be associated with a decreased risk for delirium.52 Opioid based sedation protocols are increasingly being utilized.
Antipsychotics are recommended for use in critically ill patients in an attempt to decrease and treat delirium48 but are also administered for their sedative effects. Haloperidol, the most commonly used typical antipsychotic in this setting, has been shown to increase sleep efficiency, TST, and have little or no effect on SWS.65 The atypical antipsychotics, olanzapine and resperidone, are also frequently used in ICU patients. They have been shown to increase TST, sleep efficiency, and SWS in healthy volunteers.65 However, these agents should be used with caution given their significant side effect profile.
There remains much to be learned regarding the interplay between sleep, sedative-hypnotics, and delirium. It has, however, become increasingly clear that the sedatives historically administered to aid with sleep in ICU patients actually lead to harm by increasing time on mechanical ventilation, increasing incidence of delirium, and importantly increasing risk of death.66–68 Therefore efforts should be made to minimize sedative exposure when possible.
SLEEP AND MECHANICAL VENTILATION
Mechanical ventilation has been proposed as one of the risk factors for development of delirium and the sub-syndromal delirium forms.4;69;70 Sleep fragmentation due poor patient-ventilator interaction may contribute to the occurrence of brain dysfunction seen in mechanically ventilated patients. The sleep state frequently represents a specific challenge for physicians involved in the care of patients with acute or chronic respiratory failure. Indeed, for each patient, physicians should be able to assess these issues: (1) co-existence of sleep disordered breathing [i.e. sleep apnea, REM-related alveolar hypoventilation], (2) presence of non-respiratory sleep disorders [insomnia or periodic limb movements], (3) the specific setting of the ventilator during sleep, and (4) sleep disturbances induced by mechanical ventilation. The approach to these problems is different according to the patient’s clinical status (acute or acute-on-chronic respiratory failure vs. chronic respiratory failure) and the mode of mechanical ventilation (invasive vs. non-invasive).
Recent surveys found a high prevalence of sleep disordered breathing, mainly obstructive sleep apnea (OSA), in patients admitted in hospital for severe illness requiring ICU treatment. Kaw et al reported that obstructive sleep apnea is associated with an increasing risk of ICU admission in the post-operative period.71 And, Diaz-Abad et al found a high prevalence of unrecognized sleep-disordered breathing in patients who are candidates for decannulation after weaning from prolonged mechanical ventilation.72 Similarly, insomnia and periodic leg movements as well as other sleep disorders are very common in elderly patients and in those with chronic disease like COPD, making it difficult to discriminate the effect of mechanical ventilation on sleep quality.73
While mechanical ventilation certainly affects sleep quality, sleep quality has also been shown to affect the success of ventilator therapies. Roche Campo et al reported that late noninvasive ventilation failure in elderly patients with acute hypercapnic respiratory failure was associated with early sleep disturbances. They found that patients failing noninvasive ventilation had poorer sleep quality with greater circadian sleep-cycle disruption and less nocturnal REM sleep compared with patients successfully treated with non-invasive ventilation.74
Ventilatory parameters are mainly determined empirically, based on diurnal arterial blood gas variations or the patient’s tolerance when awake and conscious. However during sleep, patients may have a profound modification in the recruitment of the respiratory muscles leading to worsening of alveolar hypoventilation.75 This is particularly the case in patients with severe respiratory muscle weakness. Sustained SpO2 desaturations (>10 min) can be seen in these patients as the result of this residual hypoventilation. On the other hand, excessive inspiratory support may induce the development of periodic breathing or central apneas, especially with pressure support ventilation (PSV).76 Indeed, setting the inspiratory support too high compared to the patient’s demand during sleep may result in passive hyperventilation. In this way, the level of PCO2 may diminish during sleep, triggering central apnea, which in turn induces arousals and awakenings.
The presence of ineffective respiratory efforts, mouth leaks in patients receiving non-invasive ventilation (NIV), and auto-triggering also reduce the effectiveness of mechanical ventilation. A phenomenon of missing or ineffective efforts has been shown to occur both in ventilator-dependent COPD patients and in others with difficult weaning from ventilator. This was attributed to several factors including: a reduction in respiratory drive, reduction in respiratory muscle strength, increased inspiratory load due to augmented upper airway resistance and mouth leaks. These factors all contribute to the inability to trigger the ventilator adequately.77
Fanfulla et al found that the patients with recurrent ineffective breathing during sleep were those with the highest level of inspiratory assistance. High levels of inspiratory assistance, that produce larger tidal volumes, may induce dynamic hyperinflation which has also been implicated in the genesis of ineffective efforts.78 Furthermore, in PSV and assisted volume-cycled ventilation, the end of the ventilator’s inflation cycle is not always synchronized with the end of the patient’s inspiratory effort. As a result, inflation may continue into the phase of neural expiration. When the ventilator cycle extends into neural expiration, the time available for expiratory flow, before the next inspiratory effort, is reduced. If passive functional residual capacity is not reached during the shortened expiratory phase, dynamic hyperinflation can occur or worsen. Dynamic hyperinflation increases the work of breathing and makes it difficult for the patient to trigger the ventilator making the occurrence of ineffective breathing more likely.
Patients with the coexistence of acute respiratory failure and obstructive sleep apnea require specific assessment when NIV is identified as the treatment of choice. Two different goals should be reached: the first is to stabilize upper airway and the second is to provide adequate inspiratory support to maintain optimal alveolar ventilation. Intermittent obstruction of the upper airway is frequent in patients during NIV.79 Moreover, upper airways obstruction may also occur in patients without coexistence of OSA and is generally related to episodes of intermittent obstruction at the glottic level reflecting cyclic glottic closure induced by hyperventilation.80
The exact role of mechanical ventilation in sleep fragmentation in ICU patients remains poorly understood. Mechanical ventilation has been shown to disrupt sleep in patients with acute or chronic respiratory failure though several different mechanisms: level of inspiratory support, development of central apneas and/or Cheyne-Stokes breathing, patient-ventilator asynchronies (particularly with ineffective inspiratory efforts), and the presence of an endotracheal tube. Parthasarathy et al. found that critically ill patients experience greater fragmentation of sleep during PSV than during assist-control ventilation (ACV) because of the development of central apneas.76 Bosma et al., found that patient-ventilator discordance causes sleep disruption and that proportional assist ventilation seems more efficacious than PSV in matching the patient’s ventilatory requirements with ventilator assistance, therefore resulting in fewer patient-ventilator asynchronies and a better quality of sleep.81 In contrast, Cabello et al, in a study designed to compare the influence of three common ventilation modes, observed that the ventilatory mode did not influence sleep pattern.82 The three mode of ventilation were ACV, clinically adjusted PSV, and automatically adjusted PSV, which offers a continuous adaptation of the PS level. A possible explanation of these results, clearly different with those previously published, is that the Authors take care to avoid excess of inspiratory support as demonstrated by similar minute ventilation and tidal volume observed with the three different ventilators.
In a study designed to compare two noninvasive pressure support ventilation settings, one based on clinical parameters and the other based on physiological requirements as estimated from an assessment of the patient’s respiratory effort and mechanics, Fanfulla et al showed that the physiological based setting was associated with improvements in sleep quantity and quality.83 They found a statistically significant association between the reduction of ineffective respiratory efforts and an improvement in sleep quality. However, when evaluating the effects of mechanical ventilation on sleep in the clinical arena, other sources of variability should be taken into account: the physicians’ approach may vary greatly; clinical severity of the patients (increased respiratory drive during acute respiratory failure and during acute inflammatory states); aetiology of acute respiratory failure; different mechanical respiratory properties; methods used for monitoring patient-ventilator interaction.
Studies of the newest modes of ventilation, such as adaptive support ventilation and proportional assist ventilation with load adjustable gain factors and neutrally adjusted ventilatory assist, have demonstrated an improvement of patient ventilator interaction when compared to the more traditional ventilator modes. However, data on sleep quality during these modes of ventilation are still limited and further investigation is needed.81;83–86
PRACTICE POINTS.
Sleep complaints are common in hospitalized patients.
There is an association between poor sleep quality and delirium but a cause and effect relationship has not been established.
Sedating medications cause alterations in sleep architecture with most causing a decrease SWS and stage REM sleep.
Patient – ventilator interactions can differ depending on whether the patient is awake or asleep and should be monitored during sleep in patients requiring long-term mechanical ventilation.
Upper airway obstructive events during sleep occur frequently in patients treated with non-invasive mechanical ventilation, especially those with signs or symptoms of obstructive sleep apnea or with obesity-hypoventilation syndrome.
RESEARCH AGENDA.
Are sleep alterations, which are common in hospitalized patients, a cause delirium?
Does sedation provide the same restorative benefits as natural sleep?
How and when should sleep quality be assessed in patients admitted to ICU?
Is polysomnography or cardio-respiratory monitoring the gold standard for the assessment of patient-ventilator interaction?
SUMMARY.
Poor sleep quality is common in hospitalized patients and leads to emotional distress, fatigue, and can potentially affect the patient’s recovery from illness. Sleep disturbances and delirium frequently coexist and while a cause and effect relationship has not been established, it is likely that both can cause and/or worsen the other. Commonly used sedatives cause abnormalities of sleep architecture (decreases in SWS and stage REM sleep) and are also known risk factors for delirium representing a potential for a shared mechanism. Therefore, whenever possible exposure to these medications should be minimized. As a prominent cause of sleep disruptions in ICU patients, mechanical ventilation settings should be adjusted to optimize patient-ventilator synchrony baring in mind that the optimal settings during sleep may not be the same as when the patient is awake.
The extent that sleep disruption contribute to hospital and ICU delirium continues to be debated. Regardless, the comfort of our patients is reason enough to make efforts to promote better sleep in this environment.
Acknowledgments
Funding/Support: Dr. Watson received support from the National Institutes of Health (MO1 RR-00095) and the Vanderbilt CTSA grant UL1 RR024975-01 from NCRR/NIH.
Footnotes
CONFLICT OF INTEREST
Dr. Watson has received an unrestricted research grant for an investigator initiated study from Aspect Medical Systems, Inc. and honorarium from Hospira Inc.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Stickgold R, Walker MP. Sleep-dependent memory consolidation and reconsolidation. Sleep Med. 2007;8:331–343. doi: 10.1016/j.sleep.2007.03.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Han JH, Morandi A, Zhou C, Callison C, Ely EW, Storrow AB, Dittus RS, Habermann R, Schnelle J. Delirium in the nursing home patients seen in the emergency department. J Am Geriatr Soc. 2009;57:889–894. doi: 10.1111/j.1532-5415.2009.02219.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Dyer CB, Ashton CM, Teasdale TA. Postoperative delirium: a review of 80 primary data collection studies. Arch Intern Med. 1995;155:461–465. doi: 10.1001/archinte.155.5.461. [DOI] [PubMed] [Google Scholar]
- 4.Ely EW, Shintani A, Truman B, Speroff T, Gordon SM, Harrell FE, Jr, Inouye SK, Bernard GR, Dittus RS. Delirium as a predictor of mortality in mechanically ventilated patients in the intensive care unit. JAMA. 2004;291:1753–1762. doi: 10.1001/jama.291.14.1753. [DOI] [PubMed] [Google Scholar]
- 5.Inouye SK, Rushing JT, Foreman MD, Palmer RM, Pompei P. Does delirium contribute to poor hospital outcomes? a three-site epidemiologic study. J Gen Intern Med. 1998;13:234–242. doi: 10.1046/j.1525-1497.1998.00073.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Francis J, Kapoor WN. Delirium in hospitalized elderly. J Gen Intern Med. 1990;5:65–79. doi: 10.1007/BF02602312. [DOI] [PubMed] [Google Scholar]
- 7.Inouye SK. Delirium in older persons. N Engl J Med. 2006;354:1157–1165. doi: 10.1056/NEJMra052321. [DOI] [PubMed] [Google Scholar]
- 8.Inouye SK, Bogardus ST, Williams CS, Leo-Summers L, Agostini JV. The role of adherence on the effectiveness of nonpharmacologic interventions: evidence from the delirium prevention trial. Arch Intern Med. 2003;163:958–964. doi: 10.1001/archinte.163.8.958. [DOI] [PubMed] [Google Scholar]
- 9.Weinhouse GL, Schwab RJ, Watson PL, Patil N, Vaccaro B, Pandharipande P, Ely EW. Bench-to-bedside review: Delirium in ICU patients - importance of sleep deprivation. Crit Care. 2009;13:234. doi: 10.1186/cc8131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Frighetto L, Marra C, Bandali S, Wilbur K, Naumann T, Jewesson P. An assessment of quality of sleep and the use of drugs with sedating properties in hospitalized adult patients. Health Qual Life Outcomes. 2004;2:17. doi: 10.1186/1477-7525-2-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Meissner HH, Riemer A, Santiago SM, Stein M, Goldman MD, Williams AJ. Failure of physician documentation of sleep complaints in hospitalized patients. West J Med. 1998;169:146–149. [PMC free article] [PubMed] [Google Scholar]
- 12.Weinhouse GL, Schwab RJ. Sleep in the critically ill patient. Sleep. 2006;29:707–716. doi: 10.1093/sleep/29.5.707. [DOI] [PubMed] [Google Scholar]
- 13.Cooper AB, Thornley KS, Young GB, Slutsky AS, Stewart TE, Hanly PJ. Sleep in critically ill patients requiring mechanical ventilation. Chest. 2000;117:809–818. doi: 10.1378/chest.117.3.809. [DOI] [PubMed] [Google Scholar]
- 14.Freedman NS, Gazendam J, Levan L, Pack AI, Schwab RJ. Abnormal sleep/wake cycles and the effect of environmental noise on sleep disruption in the intensive care unit. Am J Respir Crit Care Med. 2001;163:451–457. doi: 10.1164/ajrccm.163.2.9912128. [DOI] [PubMed] [Google Scholar]
- 15.Trompeo AC, Vidi Y, Locane MD, Braghiroli A, Mascia L, Bosma K, Ranieri VM. Sleep disturbances in the critically ill patients: role of delirium and sedative agents. Minerva Anestesiol. 2011;77:604–612. [PubMed] [Google Scholar]
- 16.Benca RM, Quintans J. Sleep and Host Defenses: A Review. Sleep. 1997;20:1027–1037. [PubMed] [Google Scholar]
- 17.Spiegel K, Leproult R, Van CE. Impact of sleep debt on metabolic and endocrine function. Lancet. 1999;354:1435–1439. doi: 10.1016/S0140-6736(99)01376-8. [DOI] [PubMed] [Google Scholar]
- 18.Lautenbacher S, Kundermann B, Krieg JC. Sleep deprivation and pain perception. Sleep Med Rev. 2006;10:357–369. doi: 10.1016/j.smrv.2005.08.001. [DOI] [PubMed] [Google Scholar]
- 19.Zhong X, Hilton HJ, Gates GJ, Jelic S, Stern Y, Bartels MN, Demeersman RE, Basner RC. Increased sympathetic and decreased parasympathetic cardiovascular modulation in normal humans with acute sleep deprivation. J Appl Physiol. 2005;98:2024–2032. doi: 10.1152/japplphysiol.00620.2004. [DOI] [PubMed] [Google Scholar]
- 20.Bonnet MH, Arand DL. Clinical effects of sleep fragmentation versus sleep deprivation. Sleep Med Rev. 2003;7:297–310. doi: 10.1053/smrv.2001.0245. [DOI] [PubMed] [Google Scholar]
- 21.Figueroa-Ramos MI, Arroyo-Novoa CM, Lee KA, Padilla G, Puntillo KA. Sleep and delirium in ICU patients: a review of mechanisms and manifestations. Intensive Care Med. 2009;35:781–795. doi: 10.1007/s00134-009-1397-4. [DOI] [PubMed] [Google Scholar]
- 22.Sveinsson IS. Postoperative psychosis after heart surgery. J Thorac Cardiovasc Surg. 1975;70:717–726. [PubMed] [Google Scholar]
- 23.Harrell RG, Othmer E. Postcardiotomy confusion and sleep loss. J Clin Psychiatry. 1987;48:445–446. [PubMed] [Google Scholar]
- 24.Gabor JY, Cooper AB, Hanly PJ. Sleep disruption in the intensive care unit. Curr Opin Crit Care. 2001;7:21–27. doi: 10.1097/00075198-200102000-00004. [DOI] [PubMed] [Google Scholar]
- 25.Trzepacz PT. Is there a final common neural pathway in delirium? Focus on acetylcholine and dopamine. Seminars in Clinical Neuropsychiatry. 2000;5:132–148. doi: 10.153/SCNP00500132. [DOI] [PubMed] [Google Scholar]
- 26.Van Der Mast RC, van den Broek WW, Fekkes D, Pepplinkhuizen L, Habbema JD. Is delirium after cardiac surgery related to plasma amino acids and physical condition? J Neuropsychiatry Clin Neurosci. 2000;12:57–63. doi: 10.1176/jnp.12.1.57. [DOI] [PubMed] [Google Scholar]
- 27.Lewis MC, Barnett SR. Postoperative delirium: the tryptophan dyregulation model. Med Hypotheses. 2004;63:402–406. doi: 10.1016/j.mehy.2004.01.033. [DOI] [PubMed] [Google Scholar]
- 28.Mundigler G, Delle-Karth G, Koreny M, Zehetgruber M, Steindl-Munda P, Marktl W, Ferti L, Siostrzonek P. Impaired circadian rhythm of melatonin secretion in sedated critically ill patients with severe sepsis. Crit Care Med. 2002;30:536–540. doi: 10.1097/00003246-200203000-00007. [DOI] [PubMed] [Google Scholar]
- 29.Gehlbach BK, Chapotot F, Leproult R, Whitmore H, Poston J, Pohlman M, Miller A, Pohlman AS, Nedeltcheva A, Jacobsen JH, et al. Temporal Disorganization of circadian rhythmicity and sleep-wake regulation in mechanically ventilated patients receiving continuous intravenous sedation. Sleep. 2012 doi: 10.5665/sleep.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Shigeta H, Yasui A, Nimura Y, Machida N, Kageyama M, Miura M, Menjo M, Ikeda K. Postoperative delirium and melatonin levels in elderly patients. Am J Surg. 2001;182:449–454. doi: 10.1016/s0002-9610(01)00761-9. [DOI] [PubMed] [Google Scholar]
- 31.Balan S, Leibovitz A, Zila SO, Ruth M, Chana W, Yassica B, Rahel B, Richard G, Neumann E, Blagman B, et al. The relation between the clinical subtypes of delirium and the urinary level of 6-SMT. J Neuropsychiatry Clin Neurosci. 2003;15:363–366. doi: 10.1176/jnp.15.3.363. [DOI] [PubMed] [Google Scholar]
- 32.Beloosesky Y, Grinblat J, Pirotsky A, Weiss A, Hendel D. Different C-reactive protein kinetics in post-operative hip-fractured geriatric patients with and without complications. Gerontology. 2004;50:216–222. doi: 10.1159/000078350. [DOI] [PubMed] [Google Scholar]
- 33.Flacker JM, Wei JY. Endogenous anticholinergic substances may exist during acute illness in elderly medical patients. J Gerontol A Biol Sci Med Sci. 2001;56:M353–M355. doi: 10.1093/gerona/56.6.m353. [DOI] [PubMed] [Google Scholar]
- 34.van Munster BC, Bisschop PH, Zwinderman AH, Korevaar JC, Endert E, Wiersinga WJ, Oosten HE, Goslings JC, Rooij SE. Cortisol, interleukins and S100B in delirium in the elderly. Brain Cogn. 2010;74:18–23. doi: 10.1016/j.bandc.2010.05.010. [DOI] [PubMed] [Google Scholar]
- 35.Plaschke K, Fichtenkamm P, Schramm C, Hauth S, Martin E, Verch M, Karck M, Kopitz J. Early postoperative delirium after open-heart cardiac surgery is associated with decreased bispectral EEG and increased cortisol and interleukin-6. Intensive Care Med. 2010 doi: 10.1007/s00134-010-2004-4. [DOI] [PubMed] [Google Scholar]
- 36.van Gool WA, van de BD, Eikelenboom P. Systemic infection and delirium: when cytokines and acetylcholine collide. Lancet. 2010;375:773–775. doi: 10.1016/S0140-6736(09)61158-2. [DOI] [PubMed] [Google Scholar]
- 37.Bateman BT, Eikermann M. Obstructive sleep apnea predicts adverse perioperative outcome: evidence for an association between obstructive sleep apnea and delirium. Anesthesiology. 2012;116:753–755. doi: 10.1097/ALN.0b013e31824b96e1. [DOI] [PubMed] [Google Scholar]
- 38.Dyugovskaya L, Lavie P, Lavie L. Increased adhesion molecules expression and production of reactive oxygen species in leukocytes of sleep apnea patients. Am J Respir Crit Care Med. 2002;165:934–939. doi: 10.1164/ajrccm.165.7.2104126. [DOI] [PubMed] [Google Scholar]
- 39.Yaffe K, Laffan AM, Harrison SL, Redline S, Spira AP, Ensrud KE, Ancoli-Israel S, Stone KL. Sleep-disordered breathing, hypoxia, and risk of mild cognitive impairment and dementia in older women. JAMA. 2011;306:613–619. doi: 10.1001/jama.2011.1115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.McDowell JA, Mion LC, Lydon TJ, Inouye SK. A nonpharmacologic sleep protocol for hospitalized older patients. J Am Geriatr Soc. 1998;46:700–705. doi: 10.1111/j.1532-5415.1998.tb03803.x. [DOI] [PubMed] [Google Scholar]
- 41.Inouye SK, Bogardus ST, Jr, Charpentier PA, Leo-Summers L, Acampora D, Holford TR, Cooney LM., Jr A multicomponent intervention to prevent delirium in hospitalized older patients. N Engl J Med. 1999;340:669–676. doi: 10.1056/NEJM199903043400901. [DOI] [PubMed] [Google Scholar]
- 42.O’Mahony R, Murthy L, Akunne A, Young J. Synopsis of the National Institute for Health and Clinical Excellence Guideline for Prevention of Delirium. Ann Intern Med. 2011;154:746–751. doi: 10.7326/0003-4819-154-11-201106070-00006. [DOI] [PubMed] [Google Scholar]
- 43.Tung A, Lynch JP, Mendelson WB. Prolonged sedation with propofol in the rat does not result in sleep deprivation. Anesth Analg. 2001;92:1232–1236. doi: 10.1097/00000539-200105000-00028. [DOI] [PubMed] [Google Scholar]
- 44.Tung A, Takase L, Fornal C, Jacobs B. Effects of sleep deprivation and recovery sleep upon cell proliferation in adult rat dentate gyrus. Neuroscience. 2005;134:721–723. doi: 10.1016/j.neuroscience.2005.06.008. [DOI] [PubMed] [Google Scholar]
- 45.Ozone M, Itoh H, Yamadera W, Ohbuchi K, Hayashida K, Sasaki M, Ushijima S, Toriumi K, Takinami M, Tanifuji Y. Changes in subjective sleepiness, subjective fatigue and nocturnal sleep after anaesthesia with propofol. Psychiatry Clin Neurosci. 2000;54:317–318. doi: 10.1046/j.1440-1819.2000.00694.x. [DOI] [PubMed] [Google Scholar]
- 46.Saper CB, Scammell TE, Lu J. Hypothalamic regulation of sleep and circadian rhythms. Nature. 2005;437:1257–1263. doi: 10.1038/nature04284. [DOI] [PubMed] [Google Scholar]
- 47.Tobler I, Kopp C, Deboer T, Rudolph U. Diazepam-induced changes in sleep: role of the alpha 1 GABA(A) receptor subtype. Proc Natl Acad Sci USA. 2001;98:6464–6469. doi: 10.1073/pnas.111055398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Jacobi J, Fraser GL, Coursin DB, Riker RR, Fontaine D, Wittbrodt ET, Chalfin DB, Masica MF, Bjerke HS, Coplin WM, et al. Clinical practice guidelines for the sustained use of sedatives and analgesics in the critically ill adult. Crit Care Med. 2002;30:119–141. doi: 10.1097/00003246-200201000-00020. [DOI] [PubMed] [Google Scholar]
- 49.Patel RP, Gambrell M, Speroff T, Scott TA, Pun BT, Okahashi J, Strength C, Pandharipande P, Girard TD, Burgess H, et al. Delirium and sedation in the intensive care unit: survey of behaviors and attitudes of 1384 healthcare professionals. Crit Care Med. 2009;37:825–832. doi: 10.1097/CCM.0b013e31819b8608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Djeridane Y, Touitou Y. Chronic diazepam administration differentially affects melatonin synthesis in rat pineal and Harderian glands. Psychopharmacology (Berl) 2001;154:403–407. doi: 10.1007/s002130000631. [DOI] [PubMed] [Google Scholar]
- 51.Pandharipande P, Shintani A, Peterson J, Pun BT, Wilkinson GR, Dittus RS, Bernard GR, Ely EW. Lorazepam is an independent risk factor for transitioning to delirium in intensive care unit patients. Anesthesiology. 2006;104:21–26. doi: 10.1097/00000542-200601000-00005. [DOI] [PubMed] [Google Scholar]
- 52.Pandharipande P, Cotton BA, Shintani A, Thompson J, Pun BT, Morris JA, Jr, Dittus R, Ely EW. Prevalence and risk factors for development of delirium in surgical and trauma intensive care unit patients. J Trauma. 2008;65:34–41. doi: 10.1097/TA.0b013e31814b2c4d. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Freitas ER, Soares BG, Cardoso JR, Atallah AN. Incentive spirometry for preventing pulmonary complications after coronary artery bypass graft. Cochrane Database Syst Rev. 2007:CD004466. doi: 10.1002/14651858.CD004466.pub2. [DOI] [PubMed] [Google Scholar]
- 54.Watson PL, Shintani AK, Tyson R, Pandharipande PP, Pun BT, Ely EW. Presence of electroencephalogram burst suppression in sedated, critically ill patients is associated with increased mortality. Crit Care Med. 2008;36:3171–3177. doi: 10.1097/CCM.0b013e318186b9ce. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Andresen J, King MS, Davidson MA, Girard TD, Pandharipande PP, Ely EW, Watson PL. Deeper sedation during coma as measured by bispectral index monitoring is associated with delirium upon emergence from coma. Intensive Care Med. 2010 Ref Type: Abstract. [Google Scholar]
- 56.Nelson LE, Lu J, Guo T, Saper CB, Franks NP, Maze M. The α2-adrenoceptor agonist dexmedetomidine converges on an endogenous sleep-promoting pathway to exert its sedative effects. Anesthesiology. 2003;98:428–436. doi: 10.1097/00000542-200302000-00024. [DOI] [PubMed] [Google Scholar]
- 57.Hall JE, Uhrich TD, Barney JA, Arain SR, Ebert TJ. Sedative, amnestic, and analgesic properties of small-dose dexmedetomidine infusions. Anesth Analg. 2000;90:699–705. doi: 10.1097/00000539-200003000-00035. [DOI] [PubMed] [Google Scholar]
- 58.Pandharipande PP, Pun BT, Herr DL, Maze M, Girard TD, Miller RR, Shintani AK, Thompson JL, Jackson JC, Deppen SA, et al. Effect of sedation with dexmedetomidine vs lorazepam on acute brain dysfunction in mechanically ventilated patients: the MENDS randomized controlled trial. JAMA. 2007;298:2644–2653. doi: 10.1001/jama.298.22.2644. [DOI] [PubMed] [Google Scholar]
- 59.Riker RR, Shehabi Y, Bokesch PM, Ceraso D, Wisemandle W, Koura F, Whitten P, Margolis BD, Byrne DW, Ely EW, et al. Dexmedetomidine vs midazolam for sedation of critically ill patients: a randomized trial. JAMA. 2009;301:489–499. doi: 10.1001/jama.2009.56. [DOI] [PubMed] [Google Scholar]
- 60.Keifer JC, Baghdoyan HA, Lydic R. Sleep disruption and increased apneas after pontine microinjection of morphine. Anesthesiology. 1992;77:973–982. doi: 10.1097/00000542-199211000-00021. [DOI] [PubMed] [Google Scholar]
- 61.Dimsdale JE, Norman D, DeJardin D, Wallace MS. The effect of opioids on sleep architecture. J Clin Sleep Med. 2007;3:33–36. [PubMed] [Google Scholar]
- 62.Marcantonio ER, Juarez G, Goldman L, Mangione CM, Ludwig LE, Lind L, Katz N, Cook EF, Orav EJ, Lee TH. The relationship of postoperative delirium with psychoactive medications. JAMA. 1994;272:1518–1522. [PubMed] [Google Scholar]
- 63.Dubois MJ, Bergeron N, Dumont M, Dial S, Skrobik Y. Delirium in an intensive care unit: a study of risk factors. Intensive Care Med. 2001;27:1297–1304. doi: 10.1007/s001340101017. [DOI] [PubMed] [Google Scholar]
- 64.Pisani MA, Murphy TE, Araujo KL, Van Ness PH. Factors associated with persistent delirium after intensive care unit admission in an older medical patient population. J Crit Care. 2010 doi: 10.1016/j.jcrc.2010.02.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Gimenez S, Clos S, Romero S, Grasa E, Morte A, Barbanoj MJ. Effects of olanzapine, risperidone and haloperidol on sleep after a single oral morning dose in healthy volunteers. Psychopharmacology (Berl) 2007;190:507–516. doi: 10.1007/s00213-006-0633-7. [DOI] [PubMed] [Google Scholar]
- 66.Kollef MH, Levy NT, Ahrens TS, Schaiff R, Prentice D, Sherman G. The use of continuous i.v. sedation is associated with prolongation of mechanical ventilation. Chest. 1998;114:541–548. doi: 10.1378/chest.114.2.541. [DOI] [PubMed] [Google Scholar]
- 67.Kress JP, Pohlman AS, O’Connor MF, Hall JB. Daily interruption of sedative infusions in critically ill patients undergoing mechanical ventilation. N Engl J Med. 2000;342:1471–1477. doi: 10.1056/NEJM200005183422002. [DOI] [PubMed] [Google Scholar]
- 68.Girard TD, Kress JP, Fuchs BD, Thomason JW, Schweickert WD, Pun BT, Taichman DB, Dunn JG, Pohlman AS, Kinniry PA, et al. Efficacy and safety of a paired sedation and ventilator weaning protocol for mechanically ventilated patients in intensive care (Awakening and Breathing Controlled trial): a randomised controlled trial. Lancet. 2008;371:126–134. doi: 10.1016/S0140-6736(08)60105-1. [DOI] [PubMed] [Google Scholar]
- 69.Lat I, McMillian W, Taylor S, Janzen JM, Papadopoulos S, Korth L, Ehtisham A, Nold J, Agarwal S, Azocar R, et al. The impact of delirium on clinical outcomes in mechanically ventilated surgical and trauma patients. Crit Care Med. 2009;37:1898–1905. doi: 10.1097/CCM.0b013e31819ffe38. [DOI] [PubMed] [Google Scholar]
- 70.Harlan R, Oberman A, Grimm R, Rosati RA. Chronic congestive heart failure in coronary artery disease: Clinical criteria. Ann Intern Med. 1977;86:133–138. doi: 10.7326/0003-4819-86-2-133. [DOI] [PubMed] [Google Scholar]
- 71.Kaw R, Michota F, Jaffer A, Ghamande S, Auckley D, Golish J. Unrecognized sleep apnea in the surgical patient: implications for the perioperative setting. Chest. 2006;129:198–205. doi: 10.1378/chest.129.1.198. [DOI] [PubMed] [Google Scholar]
- 72.Diaz-Abad M, Verceles AC, Brown JE, Scharf SM. Sleep-disordered breathing may be under-recognized in patients who wean from prolonged mechanical ventilation. Respir Care. 2012;57:229–237. doi: 10.4187/respcare.01260. [DOI] [PubMed] [Google Scholar]
- 73.Valipour A, Lavie P, Lothaller H, Mikulic I, Burghuber OC. Sleep profile and symptoms of sleep disorders in patients with stable mild to moderate chronic obstructive pulmonary disease. Sleep Med. 2011;12:367–372. doi: 10.1016/j.sleep.2010.08.017. [DOI] [PubMed] [Google Scholar]
- 74.Roche CF, Drouot X, Thille AW, Galia F, Cabello B, d’Ortho MP, Brochard L. Poor sleep quality is associated with late noninvasive ventilation failure in patients with acute hypercapnic respiratory failure. Crit Care Med. 2010;38:477–485. doi: 10.1097/CCM.0b013e3181bc8243. [DOI] [PubMed] [Google Scholar]
- 75.Becker HF, Piper AJ, Flynn WE, McNamara SG, Grunstein RR, Peter JH, Sullivan CE. Breathing during sleep in patients with nocturnal desaturation. Am J Respir Crit Care Med. 1999;159:112–118. doi: 10.1164/ajrccm.159.1.9803037. [DOI] [PubMed] [Google Scholar]
- 76.Parthasarathy S, Tobin MJ. Effect of ventilator mode on sleep quality in critically ill patients. Am J Respir Crit Care Med. 2002;166:1423–1429. doi: 10.1164/rccm.200209-999OC. [DOI] [PubMed] [Google Scholar]
- 77.Georgopoulos D, Prinianakis G, Kondili E. Bedside waveforms interpretation as a tool to identify patient-ventilator asynchronies. Intensive Care Med. 2006;32:34–47. doi: 10.1007/s00134-005-2828-5. [DOI] [PubMed] [Google Scholar]
- 78.Fanfulla F, Taurino AE, Lupo ND, Trentin R, D’Ambrosio C, Nava S. Effect of sleep on patient/ventilator asynchrony in patients undergoing chronic non-invasive mechanical ventilation. Respir Med. 2007;101:1702–1707. doi: 10.1016/j.rmed.2007.02.026. [DOI] [PubMed] [Google Scholar]
- 79.Rabec C, Rodenstein D, Leger P, Rouault S, Perrin C, Gonzalez-Bermejo J. Ventilator modes and settings during non-invasive ventilation: effects on respiratory events and implications for their identification. Thorax. 2011;66:170–178. doi: 10.1136/thx.2010.142661. [DOI] [PubMed] [Google Scholar]
- 80.Delguste P, Aubert-Tulkens G, Rodenstein DO. Upper airway obstruction during nasal intermittent positive-pressure hyperventilation in sleep. Lancet. 1991;338:1295–1297. doi: 10.1016/0140-6736(91)92593-q. [DOI] [PubMed] [Google Scholar]
- 81.Bosma K, Ferreyra G, Ambrogio C, Pasero D, Mirabella L, Braghiroli A, Appendini L, Mascia L, Ranieri VM. Patient-ventilator interaction and sleep in mechanically ventilated patients: pressure support versus proportional assist ventilation. Crit Care Med. 2007;35:1048–1054. doi: 10.1097/01.CCM.0000260055.64235.7C. [DOI] [PubMed] [Google Scholar]
- 82.Cabello B, Thille AW, Drouot X, Galia F, Mancebo J, d’Ortho MP, Brochard L. Sleep quality in mechanically ventilated patients: comparison of three ventilatory modes. Crit Care Med. 2008;36:1749–1755. doi: 10.1097/CCM.0b013e3181743f41. [DOI] [PubMed] [Google Scholar]
- 83.Fanfulla F, Delmastro M, Berardinelli A, Lupo ND, Nava S. Effects of different ventilator settings on sleep and inspiratory effort in patients with neuromuscular disease. Am J Respir Crit Care Med. 2005;172:619–624. doi: 10.1164/rccm.200406-694OC. [DOI] [PubMed] [Google Scholar]
- 84.Ambrogio C, Koebnick J, Quan SF, Ranieri M, Parthasarathy S. Assessment of sleep in ventilator-supported critically III patients. Sleep. 2008;31:1559–1568. doi: 10.1093/sleep/31.11.1559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Delisle S, Ouellet P, Bellemare P, Tetrault JP, Arsenault P. Sleep quality in mechanically ventilated patients: comparison between NAVA and PSV modes. Ann Intensive Care. 2011;1:42. doi: 10.1186/2110-5820-1-42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Carlucci A, Fanfulla F, Mancini M, Nava S. Volume assured pressure support ventilation - Induced arousals. Sleep Med. 2012 doi: 10.1016/j.sleep.2012.02.006. [DOI] [PubMed] [Google Scholar]