Abstract
To accelerate domestication of Miscanthus, an important energy crop, 244 replicated genotypes, including two different species and their hybrids, were analysed for morphological traits and biomass yield over three growing seasons following an establishment phase of 2 years in the largest Miscanthus diversity trial described to date. Stem and leaf traits were selected that contributed both directly and indirectly to total harvested biomass yield, and there was variation in all traits measured. Morphological diversity within the population was correlated with dry matter yield (DMY) both as individual traits and in combination, in order to determine the respective contributions of the traits to biomass accumulation and to identify breeding targets for yield improvement. Predictive morphometric analysis was possible at year 3 within Miscanthus sinensis genotypes but not between M. sinensis, Miscanthus sacchariflorus, and interspecific hybrids. Yield is a complex trait, and no single simple trait explained more than 33% of DMY, which varied from 1 to 5297g among genotypes within this trial. Associating simple traits increased the power of the morphological data to predict yield to 60%. Trait variety, in combination, enabled multiple ideotypes, thereby increasing the potential diversity of the crop for multiple growth locations and end uses. Both triploids and interspecific hybrids produced the highest mature yields, indicating that there is significant heterosis to be exploited within Miscanthus that might be overlooked in early selection screens within years 1–3. The potential for optimizing biomass yield by selecting on the basis of morphology is discussed.
Key words: bioenergy, biomass yield, domestication, Miscanthus, morphology, trait diversity.
Introduction
In response to the challenges of maintaining energy security, mitigating climate change, and reducing the impact of peak oil in the face of increased demand, it is vital that sustainable bio-based energy and bulk chemicals are developed to substitute for petroleum-based products. Meeting this challenge will require the rapid improvement of entirely novel crops optimized for harvestable biomass. The targeted and accelerated domestication of dedicated energy crops over a small number of years represents an unprecedented challenge to plant breeding and requires the application of interdisciplinary approaches.
Although there are many potential sources of plant biomass, there is a specific need for dedicated biomass crops that perform well on suboptimal land, so as to minimize conflict with food production, and with low demand for energy-intensive inputs such as fertilizers. Harvested plant biomass primarily comprises fixed carbon, usually in the form of complex or simple polysaccharides and the energy-rich polymer lignin, ideally with little associated protein (Gomez et al., 2008). The benefits of increasing harvestable yield in biomass crops include improved land use efficiency, economic viability, and the capture of more atmospheric carbon. However, increasing yield in biomass crops will largely target different traits to those modified during the domestication of many food crops, as domestication for food uses has focused largely on enhancing grain production, especially in cereals, at the expense of overall biomass accumulation (Sang, 2011). The use of high-energy food crops to produce bioenergy is inefficient when intensive annual agronomic practices are accounted for and reduces the availability of high-quality land for food production (Valentine et al., 2012). To optimize biomass production, dedicated crops with efficient energy capture are required in which the photosynthate is optimized and partitioned predominantly to the harvestable vegetative structures, i.e. primarily to the stems.
In recent years, C4 grasses, in particular members of the perennial genus Miscanthus, have been identified as energy crops with global potential and are therefore excellent targets for improvement (reviewed recently by Brosse et al., 2012, and van der Weijde et al., 2013). Miscanthus originates from diverse climates ranging from tropical Africa and South-East Asia up to Siberia, and is a highly productive temperate biomass crop due to its rapid biomass accumulation in temperate climates with low input requirements. Currently a single clone, Miscanthus × giganteus (M. × giganteus) is grown commercially. In order to diversify the crop and breed novel high-yielding varieties optimized for different environments and end uses, it is imperative to evaluate and utilize the wide genetic diversity present within the genus. Sixteen species of Miscanthus are currently described on GrassBase—The Online World Grass Flora (Clayton et al., 2006 onwards). Among those with potential for development as biomass crops are Miscanthus sinensis and Miscanthus sacchariflorus, which each have very wide distributions including both tropical and temperate regions within Asia, and Miscanthus floridulus, which is limited to low altitudes and tropical areas (Clifton-Brown et al., 2008b ). Miscanthus lutarioriparius, a very tall type, is localized to one region of China and has previously been considered a subspecies of M. sacchariflorus. It is likely that breeding programmes will incorporate these diverse species to different degrees, both for suitability to the growing environment and in terms of biomass properties for end-use applications.
If Miscanthus is to make a significant contribution to providing sufficient biomass to fuel a low-carbon bioeconomy, it will need to be domesticated within the next two decades. Domestication is, in effect, the reduction in genetic variation within a population to increase the frequency of desired traits over undesired or neutral traits.
Unlike the domestication of the cereals, which was based purely on phenotypic (ideotype) selection, the domestication of Miscanthus will be genetically based, with genotypes conferring the desired traits in a range of environments being selected for recurrent selection (Johnson et al., 1992). The majority of Miscanthus germplasm is either directly collected from the wild or is no more than one or two generations removed. In contrast to recent crop breeding, energy crops must be domesticated to retain and improve their innate resource use efficiency so that they are high yielding over successive growing seasons without the requirement for environmentally and economically costly fossil-fuel-based inputs. Since the widespread application of nitrogen fertilizers, crop breeding has focused almost entirely on selecting for yield increase with fertilizer rather than optimizing the plant’s inherent nitrogen-use efficiency. There is consequently an inherent dependence on fertilizer usage for the majority of the world’s food production, which is expensive both economically and environmentally. In domesticating a novel crop for biomass production, not only do we need to focus on different morphological traits, but we must also be mindful to ensure that our primary selections for biomass yield are not compromising other aspects of sustainable crop production in the future.
In terms of domestication then, Miscanthus breeders are seeking to reduce the frequency of alleles conferring undesirable traits in the breeding populations. Increasing the frequency of beneficial alleles and reducing the frequency of detrimental alleles is achieved through repeated cycles of recombination and selection. Typically, a breeding cycle takes up to 7 years (Casler, 2012); however, at least a few hundred generations may have been required for domestication in the past (Burger et al., 2008). Increasing the selection pressure increases the rate of genetic gain (Moose & Mumm, 2008) but may also increase the concurrent loss of desirable variation in other traits.
Biomass accumulation is a complex process comprising multiple structural component traits. A key factor in breeding is to understand the correlations between traits and the extent to which they can be uncoupled. It is imperative that the interactions between traits, both positive and negative correlations, are well understood because selection for one trait can have profound effects on another (Farrar et al., 2012).
A thorough understanding of the key traits that contribute to harvestable biomass yield in these grasses is required in order to drive genetic gain for accelerated domestication. High yield in terms of harvestable biomass per plant is a composite of multiple simple traits, which not only act in combination but also interact in complex ways, thereby enabling the selection of high-yielding plants via different trait-optimization strategies (i.e. through multiple ideotypes). The aims of this study were: (i) to determine the traits contributing to high biomass yields; (ii) to define high-yielding ideotypes for Miscanthus; and (iii) to identify genotypes for introduction into recurrent selection cycles for increased biomass production.
The ideotype should comprise the smallest possible number of simple traits that can be screened for maximum yield improvement. It was therefore essential to identify and dissect a range of composite morphological yield traits, and determine to what extent they can be optimized independently, or whether different traits are linked by physiological or genetic constraints. In order to determine the key traits comprising high yield in Miscanthus that may be used to predict high-yielding individuals, the phenotypic relationships were considered.
In 2004, a replicated trial of 244 genotypes, including M. sinensis and M. sacchariflorus species and a number of intra- and interspecific hybrids (including M.×giganteus), was planted in Aberystwyth to assess the diversity within genotypes previously brought to Europe, mainly by taxonomists and the horticultural industry. The genotypes were therefore pre-screened for survival in Europe and consequently limited to temperate types. The majority of the genotypes were diploid, but some tetraploid and triploid types were represented. Although the plants within this trial do not represent the full potential of Miscanthus for bioenergy applications, they provide an excellent resource in which to study diversity and to link plant morphology traits to yield in a temperate climate. The data described here represent mature plants during the third, fourth and fifth years of growth, as years 1 and 2 were considered to be of limited predictive value for long-term yield projections (Lewandowski et al., 2000).
Materials and methods
Genetic resources
A total of 244 Miscanthus genotypes were assembled from smaller collections within Europe, including 102 genotypes from known collection points in China, Japa,n and South Korea, latitude range 32.3–43.6° N. Four clonal replicates were planted in a randomized trial as described previously (Clifton-Brown et al., 2008a ; Jensen et al., 2011). The germplasm collection comprised 187 M. sinensis genotypes, 35 M. sacchariflorus genotypes, and 22 interspecific hybrid (henceforth referred to as hybrid) genotypes, including diploid, triploid, and tetraploid genotypes (Table 1).
Table 1.
Frequency of species and ploidy in the trial genotypesThe replicated trial consisted of a total of 244 genotypes of different ploidy levels, comprising M. sinensis, M. sacchariflorus, and their hybrids.
| Diploid | Triploid | Tetraploid | Total | |
|---|---|---|---|---|
| M. sinensis | 184 | 3 | 0 | 187 |
| M. sacchariflorus | 18 | 1 | 16 | 35 |
| Hybrid | 10 | 8 | 4 | 22 |
| Total | 212 | 12 | 20 | 244 |
Trial conditions
The trial was established on a sloping field (52° 26’ N 04° 01’ W) near Aberystwyth on the west coast of Wales, exposed to winds from the south and west. The soil is classified as a dystric cambisol and a dystric gleysol depending on spatial variation in drainage (FAO, 1988) with a stone fraction (particles >2mm) of approximately 50% of the soil mass in the 0–40cm layer.
Climate data (rainfall, temperature, and radiation) were obtained from the Gogerddan weather station (52° 25’ N 04° 01’ W). Average monthly rainfall for the years 2007, 2008, and 2009 was 109, 113, and 98%, respectively, of the long-term monthly average for Gogerddan (86.5cm). Monthly average maximum and minimum temperatures for 2007, 2008, and 2009 were similar to the long-term mean. The average annual temperature of 2007–2009 was 10.5 °C compared with the long-term average of 9.7 °C. Soil temperatures between 2007 and 2009 at a depth of 5cm did not fall below –1 °C. Solar radiation in 2007 and 2009 was higher than the long-term average of 9.4 MJ m–2 d–1 (104 and 105%, respectively), while 2008 was the same as the long-term average (Jensen et al., 2011; Robson et al., 2012).
Phenotyping the structural components of Miscanthus
Following an establishment phase of two growing seasons, extensive phenotyping of mature plants was undertaken in year 3 (Y3) (2007), Y4 (2008), and Y5 (2009), which are considered to be the first ‘mature’ years under UK conditions (Clifton-Brown et al., 2008a ). A theoretical model describing the impact of trait variation on yield in Miscanthus guided trait selection. Stem traits were considered to be direct components of biomass, and other traits including leaf development (leaf length and width) and plant stature were hypothesized to affect light capture and water relations. Other key yield components—flowering time, emergence, and senescence—were also measured and have been described previously (Jensen et al., 2011; Robson et al., 2012, 2013). Canopy height measurements were taken at fortnightly intervals throughout the growing season as the height at which the majority of light was intercepted by the canopy. At the end of the growing season (October), the following biomass component traits were measured: basal diameter (the diameter of the clump at ground level), transect count (the number of stems counted along a transect inserted through the middle of the clump at approximately half canopy height), stem diameter (the average of three stem diameters taken at mid-internode at approximately half canopy height), and tallest stem (the height to the highest part of the stem, excluding the flower, if present). Additional morphometric measurements were taken at least once during this time period: leaf length (length of the leaf blade from petiole to tip, excluding the leaf sheath), leaf width (width of the leaf blade at approximately half leaf length), and plant stature (stem angle and leaf angle).
Dry matter yield (DMY)
The biomass yield of each mature plant was analysed in the February following the growing season. Harvest was delayed to spring to reflect current management practice. The spring harvest improves biomass quality in terms of moisture and nutrient content, despite a concurrent loss of biomass yield relative to the peak yield (Clifton-Brown et al., 2007). Plants were harvested at a height of approximately 5cm from the soil surface and the whole above-ground biomass was passed through a silage chopper. The chopped plant material was collected in a plastic sack and weighed to determined total fresh weight (FWtotal). A subsample from the bulk sample of approximately 250g was removed, placed in a paper bag, and weighed to determine the subsample wet weight (FWsubsample). The subsample was then dried to a constant weight at 60 °C and the percentage dry weight calculated (DWsubsample). The percentage dry weight was used to calculate the total dry weight of the bulk sample: DWtotal=FWtotal×(DWsubsample/FWsubsample).
Statistical analysis
The morphological traits were measured and considered as explanatory variables in several linear regression models for the total DMY. There were two intrinsic traits, species and ploidy, which were invariant for each plant. In addition, the year and replicate block were considered as two further traits, which partly captured any otherwise unmeasured environmental effects, specifically developmental and climatic differences between years, and spatial differences between replicates. The different linear models were used to compare the ability of individual explanatory variables and subsets of explanatory variables to predict DMY. To improve model fit and predictive ability, transformations of variables were considered. For all regression models, adjusted r 2 values are reported to indicate predictive ability. Furthermore, to aid comparison of nested models (i.e. variables included in one model are a subset of another), the Akaike information criterion (AIC) is reported as a measure of model fit in selected cases.
All tabulations, plots and, statistical analyses were performed in the GNU R statistical software (R Development Core Team, 2012). Linear regressions were performed using the lm command.
Results
Trait diversity within Miscanthus species
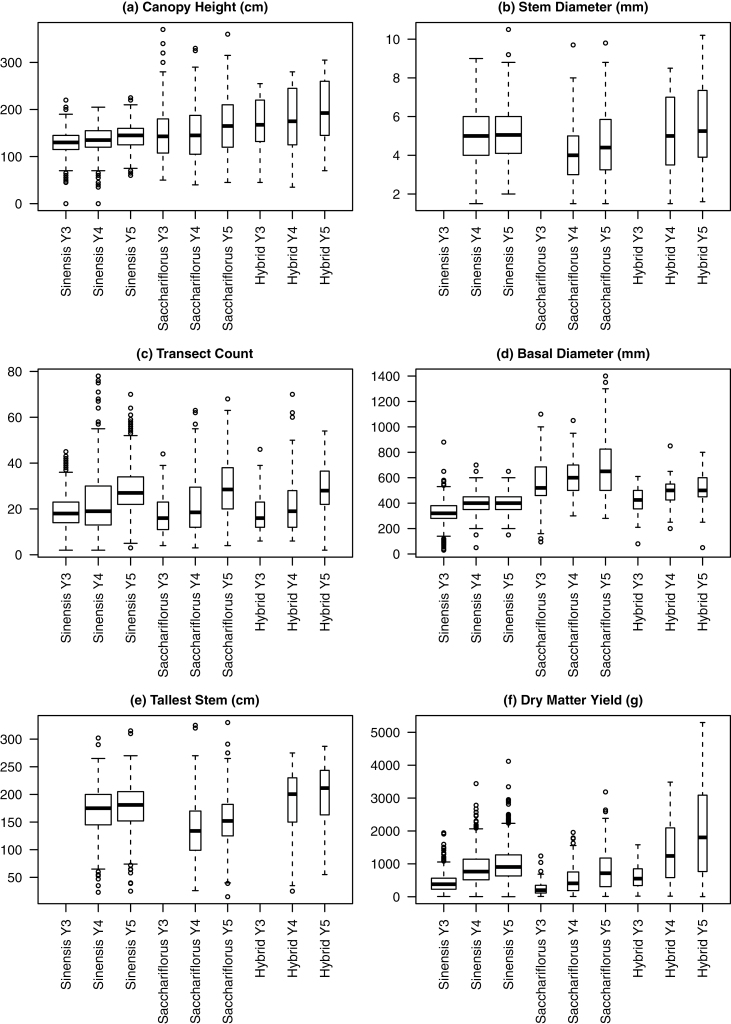
Overall morphological diversity within the trial was extremely high with tallest stem measurements ranging from 15 to 330cm, stem diameter from 1.5 to 10.5mm, leaf length from 12 to 100cm, leaf width from 0.2 to 3.6cm, and transect counts of 2 to 78. M. sinensis and M. sacchariflorus and their interspecific hybrids exhibited different morphologies. M. sinensis plants typically were shorter (canopy height <2 m) with a clumped base, while M. sacchariflorus plants could be taller with a spreading base. The hybrids were the tallest group on average, and had an intermediate base. Despite representing the largest group (n=187), the M. sinensis group show the smallest trait diversity in terms of canopy height and basal diameter (Fig. 1a, d).
Fig. 1.
Summary of measured traits as boxplots for all plants by species (M. sinensis, M. sacchariflorus, and hybrid) and year (Y3, Y4, and Y5).
Effect of maturity on different species
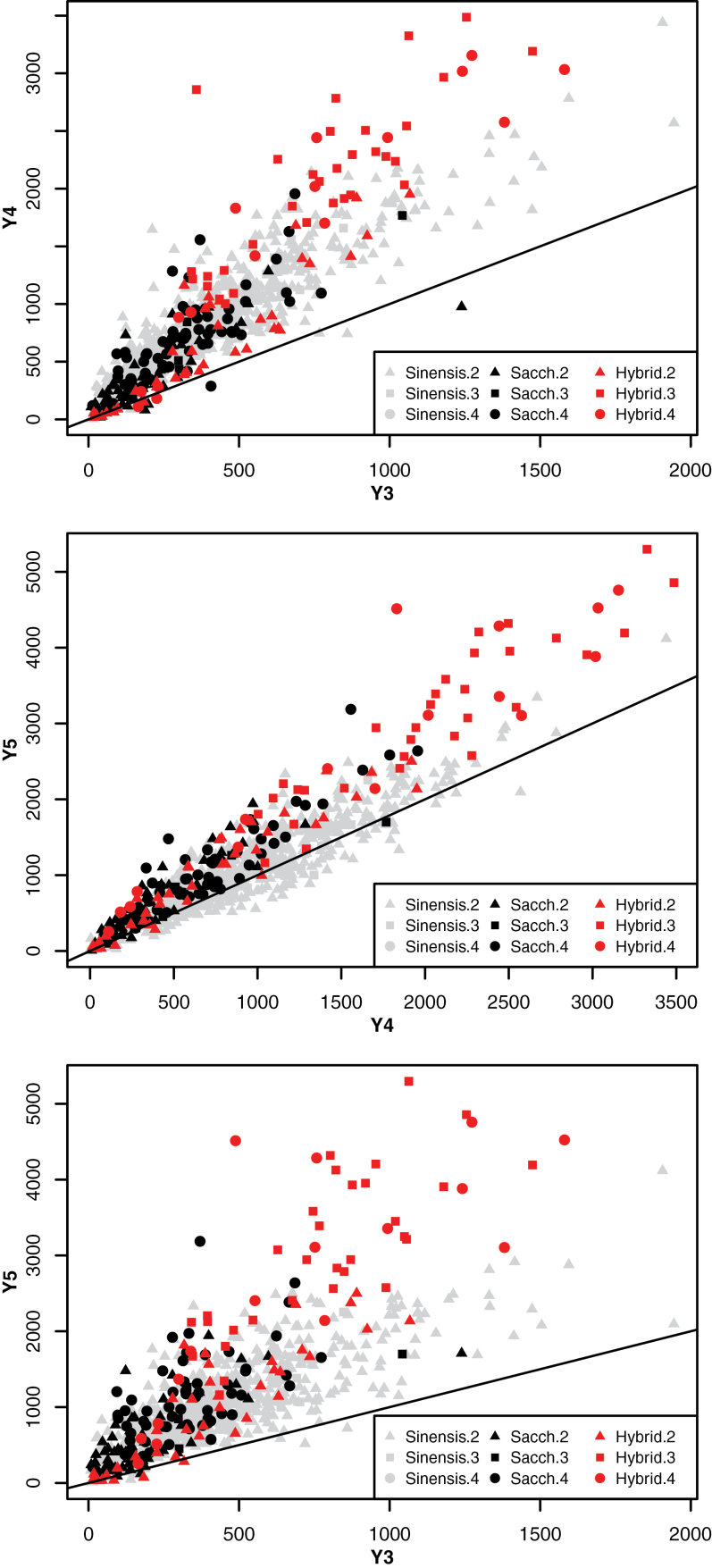
The first recorded harvest occurred following the third growing season (Y3); however, the DMY continued to increase over the 3 years of this study (Y3–5, Fig. 1f). DMY increased between Y3 and Y5 for all but 15 out of 976 plants, indicating that all genotypes used in this experiment had not achieved maximum yield potential at Y3. Plotting the DMY for each plant in Y3 against Y4, Y4 against Y5, and Y3 against Y5 demonstrated that M. sinensis plants had a lower DMY gain between Y4 and Y5 compared with M. sacchariflorus and hybrid genotypes (Fig. 2). This indicated that M. sinensis reached maximum yield faster, and that the yield of both M. sacchariflorus and hybrids continued to increase annually until at least Y5 under UK conditions.
Fig. 2.
Year-on-year trends for DMY for all plants by species: M. sinensis (grey), M. sacchariflorus (black), and hybrid (blue); and by ploidy: diploid (triangle), triploid (square), and tetraploid (circle). The line of equality, x=y, is represented by a black line.
Effect of intrinsic and environmental traits on DMY
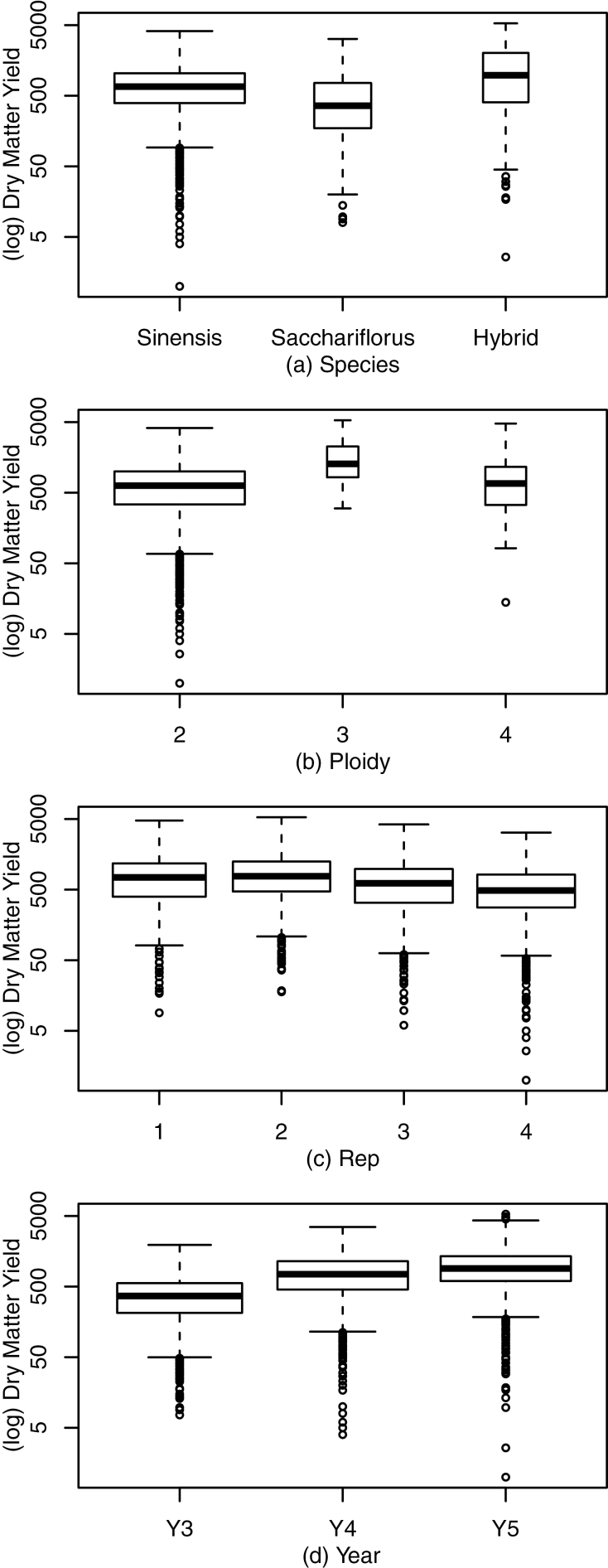
DMY varied from 1 to 5297g in the trial over the 3 years. DMY means for M. sinensis, M. sacchariflorus, and hybrids were calculated using all 244 genotypes in the trial. There were more M. sinensis genotypes in the trial, reflecting the relative availability of the different species in Europe at the time the experiment was established (Table 1; Clifton-Brown et al., 2008a ). Furthermore, there was a non-normal distribution of traits, including yield, within the trial. The effects of the intrinsic and environmental traits on the DMY were considered using the natural logarithm of the DMY due to the skew in distribution of DMY. The distribution of log(DMY) is shown as box and whisker plots in Fig. 3, subgrouped by species, ploidy, replicate block, and year; the width of each box corresponds to the size of each subgroup (see Table 1).
Fig. 3.
Comparison of log(DMY) across experimental traits of species (a), ploidy (b), and intrinsic traits of replicate block (c) and year (d). Widths of boxes indicate the number of plants within each group (see Table 1).
Hybrid plants exhibited a higher median yield than parental species (Fig. 3a) and triploid plants exhibited a higher median yield than either diploids or tetraploids (Fig. 3b). There was a small replicate effect, with replicate 4 yielding less on average than replicates 1, 2, and 3 (Fig. 3c). These observations were consistent with the geography of the trial site, as replicate 4 was at the top of a hill and experienced more wind and more rapid water drainage than the lower replicates. Perhaps most striking was the year-on-year increase in DMY (Fig. 3d).
Developing an ideotype
Linear regression of biomass traits—phenotypic correlations
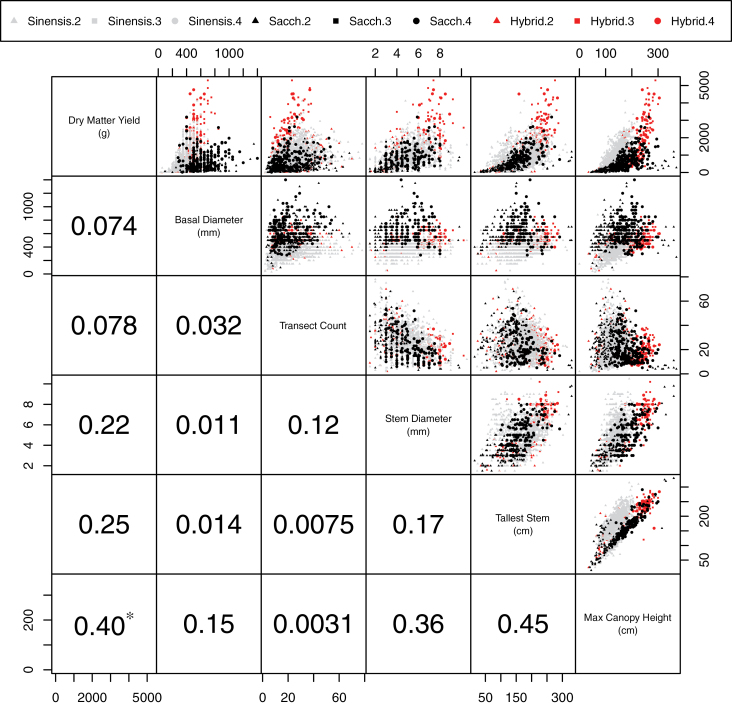
Pairwise plots between the untransformed measured traits (excluding leaf width and leaf length) and Pearson’s correlation r 2 coefficient between traits are shown in Fig. 4; no single trait predicted yield with any accuracy. The different species displayed different plant forms; M. sinensis had small basal diameters and high stem numbers (transect count), while M. sacchariflorus types had larger basal diameters and were otherwise somewhat diverse for morphological characters. The hybrid group was highly morphologically diverse, despite being small in number and containing the majority of high-yielding plants. There was an overall relationship between plant height (tallest stem/canopy height) and stem diameter, indicating that the former may be physically restricted by the latter. Conversely, there was a predominantly negative relationship between number of stems (transect count) and stem diameter. Basal diameter was not correlated with stem diameter, which would be expected if stem number were consistent among genotypes but is unsurprising given the high variation observed in the number of stems (transect count=2–78). Plant height (tallest stem/canopy height) and transect count appeared to be independent of one another, especially in the M. sacchariflorus group, although there were no plants within the trial with high numbers of tall stems.
Fig. 4.
Pairwise trait plots for all replicates and associated r 2 values between traits for Y4 and Y5 by species: M. sinensis (grey), M. sacchariflorus (black), and hybrid (red); and by ploidy: diploid (triangle), triploid (square), and tetraploid (circle). The asterisk indicates the highest trait association with yield.
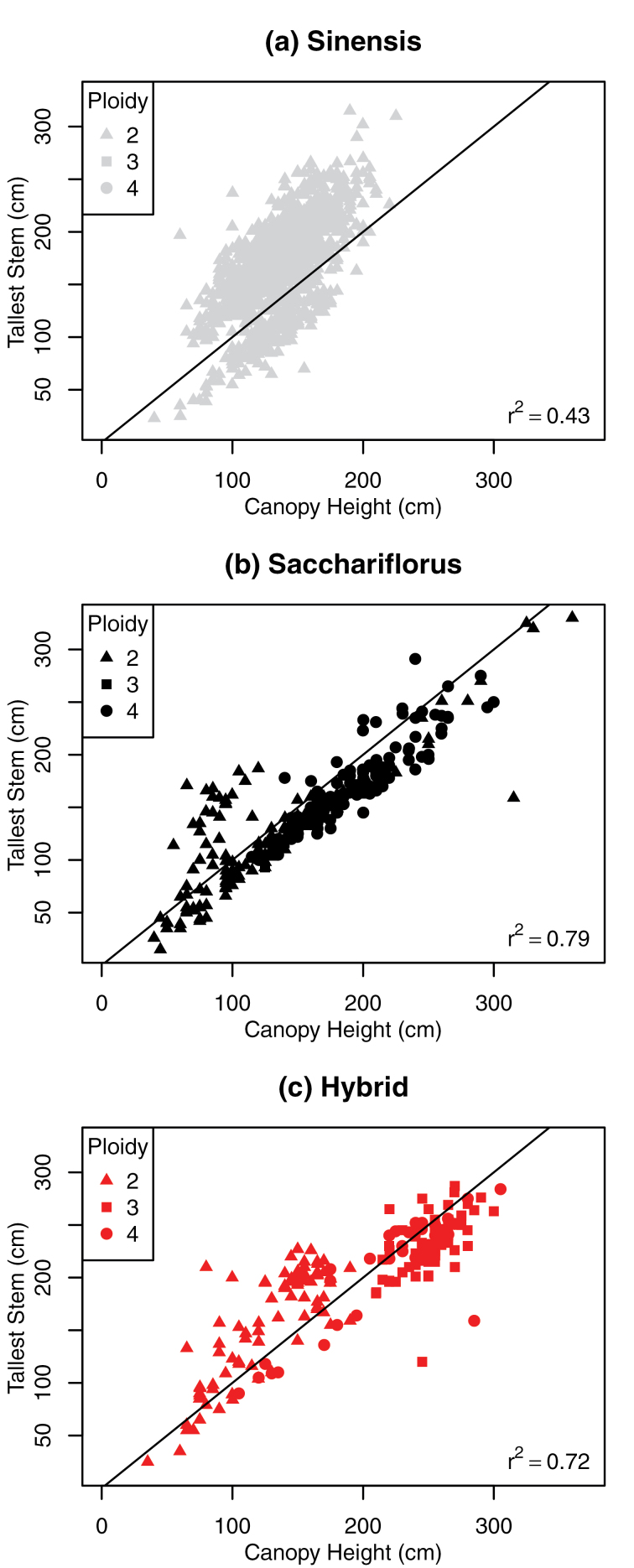
Canopy height had the highest correlation with yield, but this is a complex trait comprising stem and leaf height/length and angle traits. The simple trait with the highest correlation to yield was tallest stem, which was clearly a component of canopy height but was substantially less correlated with yield. Further analysis of the relationship between canopy height and tallest stem revealed that there were broadly two linear relationships between the two traits, which were represented differently within the three species groups (Fig. 5); this may represent the phenology of the plant as the majority of M. sinensis flowered in this trial while the majority of the other species did not.
Fig. 5.
Pairwise trait plots between tallest stem and canopy height for all replicates for Y4 and Y5 by species: M. sinensis (grey), M. sacchariflorus (black), and hybrid (red); and by ploidy: diploid (triangle), triploid (square) and tetraploid (circle).
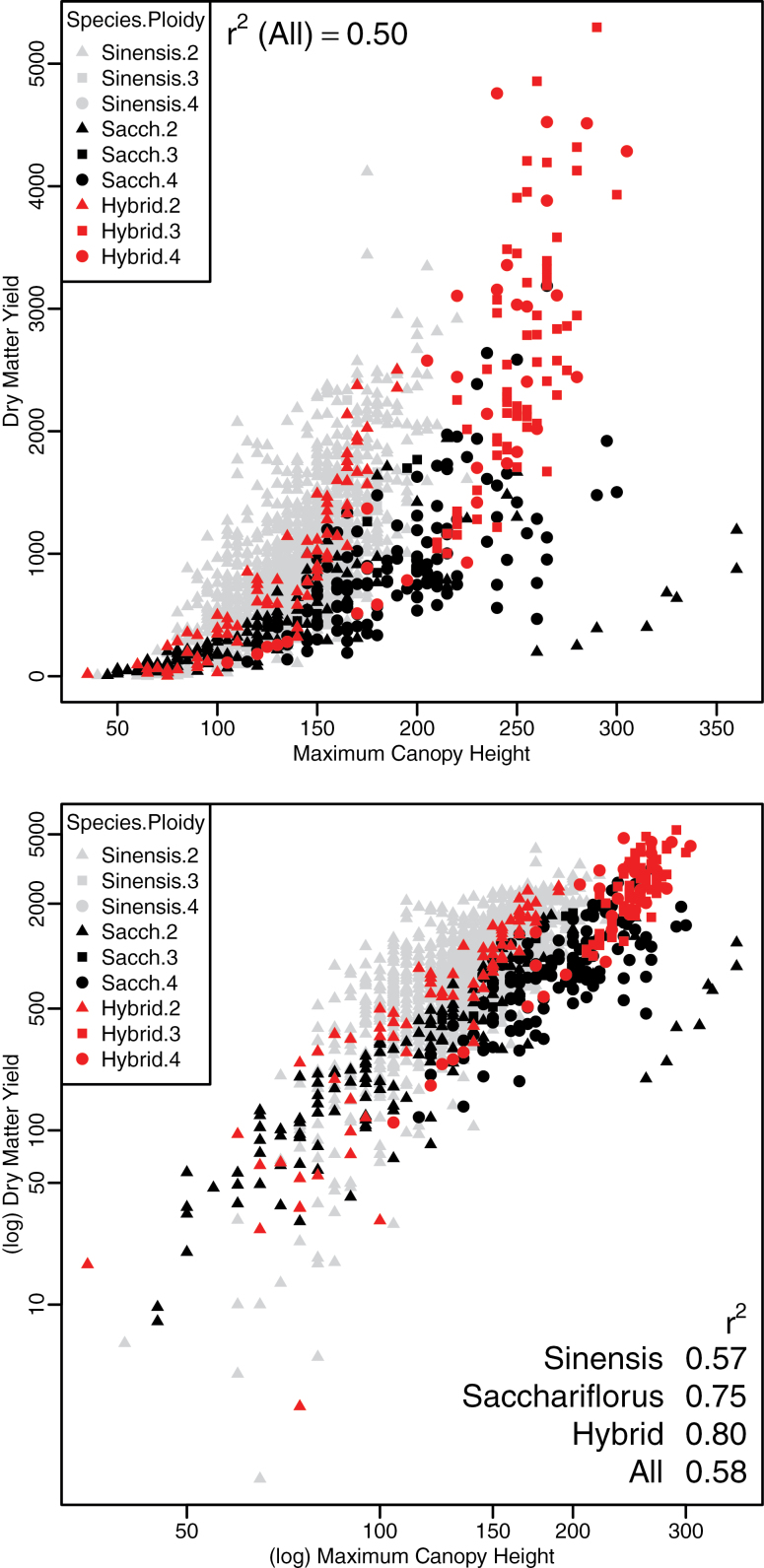
Explanatory plots of the data demonstrated that the association between each trait and DMY was non-linear in many cases, including the relationship of DMY to maximum canopy height, especially for high-yielding plants (Fig. 6, upper plot). Using log transformation (Fig. 6, lower plot), the association was reasonably linear, although there was departure for smaller yields, which were of less value in terms of optimizing the model for high-yielding Miscanthus. Log transformations were used for all traits for subsequent linear modelling. Furthermore, the exploratory analysis (Figs 1–3) indicated that the majority of plants were not yet mature in Y3 for the majority of plants, so Y4 and Y5 data only were included in subsequent analysis.
Fig. 6.
Pairwise trait plots between dry DMY and canopy height for all replicates for Y4 and Y5 for untransformed (upper plot) and log transformed (lower plot) data by species: M. sinensis (grey), M. sacchariflorus (black), and hybrid (red); and by ploidy: diploid (triangle), triploid (square), and tetraploid (circle).
The morphological diversity observed within the trial indicated that multiple simple traits may be optimized independently of one another to increase yield. The four simple traits measured in Y4 and Y5 were correlated with yield both individually and in combination to determine the combination of measurements that best predicted yield (Table 2). Although a combination of all four gave the highest prediction (r 2=0.601), removing basal diameter did not have a great impact (r 2=0.589). However, no other combination had the predictive power of canopy height alone: r 2 log(maximum canopy height) vs log(DMY)=0.55.
Table 2.
Comparison of simple linear models for log(DMY)Comparison of models for log(DMY) (Y3 and Y4) using one-time measurement traits, i.e. excluding maximum canopy height as it is a function of multiple measurements. The adjusted r 2 values indicate the proportion of variability in DMY explained by the included traits (the first four rows correspond to first column entries in Table 3). The AIC compares models, accounting for how well they fit the observed data and how many traits are included; lower values indicate better-fitting models.
| Models for log(DMY) using: | |||||
|---|---|---|---|---|---|
| log(basal diameter) | log(transect count) | log(tallest stem) | log(stem diameter) | Adjusted r 2 | AIC |
| –890.7 | |||||
| ✓ | 0.078 | –1042.0 | |||
| ✓ | 0.068 | –1021.9 | |||
| ✓ | 0.339 | –1670.2 | |||
| ✓ | 0.227 | –1376.2 | |||
| ✓ | ✓ | 0.448 | –2007.9 | ||
| ✓ | ✓ | 0.442 | –1987.0 | ||
| ✓ | ✓ | 0.422 | –1923.3 | ||
| ✓ | ✓ | 0.276 | –1497.4 | ||
| ✓ | ✓ | 0.443 | –1990.7 | ||
| ✓ | ✓ | 0.413 | –1891.6 | ||
| ✓ | ✓ | ✓ | 0.448 | –2007.9 | |
| ✓ | ✓ | ✓ | 0.465 | –2067.4 | |
| ✓ | ✓ | ✓ | 0.442 | –1987.0 | |
| ✓ | ✓ | ✓ | 0.589 | –2563.6 | |
| ✓ | ✓ | ✓ | ✓ | 0.601 | –2620.6 |
Statistical yield modelling incorporating additional morphometric measurements
Independent linear models regressing log(DMY) against each explanatory variable in turn were performed. Of these, only log(tallest stem) and log(stem diameter) explained more than 10% of the observed variation in log(DMY), (adjusted r 2 >0.1; Table 3). The individual trait that explained the most variation was the logarithm of the maximum canopy height (adjusted r 2=0.55), but this is a complex trait comprising aspects of stem and leaf morphology. Tallest stem, as would be predicted due to the strong pairwise correlation with maximum canopy height, was the most predictive single simple trait (adjusted r 2=0.339; Table 3).
Table 3.
Complex linear models for log(DMY)Linear models for log(DMY) (Y4 and Y5) showing the effect of each trait separately (unadjusted column) and then adjusting for the inclusion of all other traits.
| Trait | Separate trait effects (unadjusted) | Linear model including all traits | |||||
|---|---|---|---|---|---|---|---|
| Coefficient | SE | P value | Adjusted r 2 | Coefficient | SE | P value | |
| Intercept/base line | –6.753 | 0.366 | <0.001 | ||||
| Sacchariflorus | –0.919 | 0.075 | <0.001 | 0.0782 | –0.685 | 0.058 | <0.001 |
| Sinensis | –0.420 | 0.062 | <0.001 | 0.0782 | 0.005 | 0.05 | 0.926 |
| Ploidy 3 | 0.943 | 0.080 | <0.001 | 0.0699 | 0.269 | 0.059 | <0.001 |
| Ploidy 4 | 0.205 | 0.063 | 0.001 | 0.0699 | 0.336 | 0.056 | <0.001 |
| Leaf angle 0.5 | 0.203 | 0.064 | 0.002 | 0.0087 | –0.031 | 0.053 | 0.563 |
| Leaf angle 1 | 0.294 | 0.069 | <0.001 | 0.0087 | –0.095 | 0.058 | 0.101 |
| Stem angle 2 | 0.311 | 0.038 | <0.001 | 0.0364 | 0.066 | 0.028 | 0.018 |
| Stem angle 3 | 0.320 | 0.066 | <0.001 | 0.0364 | 0.068 | 0.043 | 0.112 |
| Stem angle 4 | –0.092 | 0.161 | 0.567 | 0.0364 | –0.238 | 0.097 | 0.014 |
| log(basal diameter) | 0.754 | 0.060 | <0.001 | 0.0776 | 0.627 | 0.049 | <0.001 |
| log(transect count) | 0.411 | 0.035 | <0.001 | 0.0677 | 0.604 | 0.024 | <0.001 |
| log(tallest stem) | 1.450 | 0.048 | <0.001 | 0.3390 | 0.958 | 0.041 | <0.001 |
| log(stem diameter) | 1.220 | 0.052 | <0.001 | 0.2270 | 0.731 | 0.048 | <0.001 |
| log(leaf width) | 0.513 | 0.044 | <0.001 | 0.0656 | –0.016 | 0.038 | 0.680 |
| log(leaf length) | 0.610 | 0.054 | <0.001 | 0.0628 | 0.448 | 0.040 | <0.001 |
| Adjusted r 2=0.672 | |||||||
| AIC=–2976.5 | |||||||
A linear model incorporating all traits is presented in the second column of Table 3, which results in a very high adjusted r 2 of 0.672. Thus, without any considerations apart from log transformations, 67% of the variation in yield was explained. Thus, a complex model including intrinsic traits (genotype and ploidy) as well as non-intrinsic traits (basal diameter, transect count, stem diameter, tallest stem, leaf length and width, and leaf and stem angle) gave a prediction of <10% more than a simple model that consisted of three simple traits: transect count, tallest stem, and stem diameter.
Discussion
Few studies have been conducted to date including diverse Miscanthus genotypes, as most research has focused on the commercial clone M. × giganteus. These studies have been reviewed recently (Anderson et al., 2011; van der Weijde et al., 2013) and demonstrate both the potential for growing Miscanthus over a very wide geographical area, across Asia, Europe and the USA, and also the major effect of climate on yield. Most trials including M. × giganteus have been conducted in Europe where harvested yields have been reported as 10–30 t DW ha–1; the highest yields (up to 44 t DW ha –1 at the end of the growing season post-senescence) were reported from a trial in Illinois (Heaton et al., 2008). Comparisons between Miscanthus and other species such as bamboo (Hong et al., 2011), giant reed (Arundo donax; Angelini et al., 2009) and other C4 grasses (reviewed by van der Weijde et al., 2013) consistently show Miscanthus to be among the most productive plant species for biomass production. In practice. a mixture of crops will be developed for use in different locations and for different end uses, and Miscanthus is likely to be a key constituent of the mix.
The main factor limiting the deployment of Miscanthus to date is the lack of domesticated material of sufficiently predictable high yield, despite a wealth of natural diversity available. Morphological evaluation is a relatively rapid method for screening diverse material to be entered into restricted breeding cycles for rapid domestication and crop improvement.
Defining the ideotype
Simple correlations
For a given plant, there were mechanistic and biological relationships between several of the measured traits, most obviously between maximum canopy height and tallest stem. Maximum canopy height was the single measured trait that best described yield (r 2=0.4, increasing to r 2=0.55 for log values), and this correlation was stronger for M. sacchariflorus and the hybrid populations independently (Fig. 6, lower panel). Canopy height is a complex trait comprising stem height, leaf length, and stem and leaf angle, so, while being relatively easy to measure in the field, it is likely to require further dissection to identify individual genes regulating the components of this trait for targeted selection. Tallest stem has the highest correlation of the simple traits with yield (r 2=0.25; Fig. 4), which might be expected given that stem is a component of canopy height. While tallest stem was highly correlated with canopy height (r 2=0.45; Fig. 4), further analysis revealed that there were in fact at least two different relationships between the traits, which may represent the phenology of the plant (Fig. 5). The most likely explanation is that there is a linear relationship between canopy height and tallest stem for vegetative growth, which is perturbed by the extension of the panicle above the canopy; for example, Zub et al. (2011) reported an average of 30cm between canopy and panicle height. The majority of M. sacchariflorus types do no flower under UK conditions and demonstrate a simple 1:1 relationship between tallest stem and canopy height (Fig. 5b), while the second, less-well-defined relationship was observed for the majority of M. sinensis types that did flower in this trial (Fig. 5a). These data indicate that the mass of flowering stem above the vegetative canopy does not constitute a significant amount of biomass yield and that a new measurement ‘tallest stem to uppermost true leaf’ may be a valid simple trait to measure in future.
The two most informative traits following canopy height/tallest stem were stem diameter and transect count. While it was not surprising that a greater stem diameter was indicative of higher biomass yield, it was also confounded by the mechanics of stem growth, as a correlation existed between stem height and stem diameter (r 2=0.17). Interestingly, the correlation was higher for canopy height and stem diameter (r 2=0.36; Fig. 4), i.e. the additional flower stalk did not require a substantial stem diameter to support it, again indicating that the flowering stem did not contribute much in the way of biomass.
Complex correlations
Taking the simple traits both individually and in combination, it was possible to construct a simple model to predict optimization strategies for increasing yield. With the potential exception of transect count and stem diameter, the majority of traits did not appear to be negatively correlated, indicating that each could be optimized without unintended yield loss. Transect count, tallest stem, and stem diameter measurements predicted approximately 60% of yield, which increased to <70% with the addition of a further seven traits. Thus, these three measurements can account for the majority of the heritable yield, with environmental factors, or unmeasured traits, accounting for approximately one-third of the variation. The predictive value of the three traits may increase slightly if the tallest stem measurement were substituted with a new measurement, ‘tallest stem to uppermost true leaf’, to avoid the confounding effect of the flowering stem. Any pair of these traits, with the exception of basal diameter and stem diameter, gave an adjusted r 2 of 0.41–0.45.
A confounding aspect of morphological selection for yield is the effect of environmental variation on these traits. In a trial of 93 wild-collected Miscanthus populations comprising M. sinensis, M. saccharflorus, and M. lutarioriparius, Yan et al. (2012) demonstrated site×population interactions for most M. sinensis and M. saccharflorus traits. This emphasizes the importance of comparing data from trials at different locations. Comparisons of trait and yield data from different trials may not be simple due to different phenotyping methodology and yield measurements; for example, other studies have taken biomass measurements from a number of stems either at peak biomass or at the end of the growing season. In this study, we analysed whole plant biomass at the commercial time of harvest following winter. The diverse morphologies of the material within this trial are, however, well suited to provide robust relationships between constituent traits and yield.
The basic ideotype for high-yielding plants can be considered to comprise tall plants (above 1.5 m) with thick stems (at least 5mm) and, theoretically, high stem numbers. No plants in the trial used in this study exhibited both very tall and very numerous stems, but it should be possible to select for such plants, or attempt to generate them by crossing tall and highly tillering individuals and analysing the progeny. However, it may be that there is an optimal range for stem number, above which light interception to the canopy cannot be increased. Adding additional traits such as leaf length and width, and stem and leaf angle increased the yield prediction to 67% (Table 3). Given the diversity within the traits, it should be possible to generate high-yielding plants with different combinations of stem height, number, and diameter, for example M. sinensis plants tend to be shorter than M. sacchariflorus and hybrids, and so are likely to require higher tiller numbers to achieve high yields. Thus, there is considerable scope for diversity within the crop and hence targeted breeding for alternative end uses such as power generation or liquid fuel production.
Potential for early morphometric prediction
As for other perennial plants, the possibility of a juvenile phase in Miscanthus severely hinders early phenotypic selection of mature traits. Years 1 and 2 were not measured in this experiment, as they have previously been considered ‘immature’ for Miscanthus in the UK, and were considered to constitute an establishment phase during which time there was little economic value to harvesting the crop. However, in this trial, the yield in Y3 was significantly lower than for Y4 and Y5, reflecting the fact that the plants had not all reached their full yield potential by Y3 in Aberystwyth. The data shown in Fig. 1 indicate that there are strong differences between M. sacchariflorus, M. sinensis, and hybrid groups, and that, while M. sinensis types may be approaching maturity at Y3, for both M. sacchariflorus and interspecific hybrids this may not occur until at least Y4, i.e. M. sinensis types may reach their yield potential at least a year earlier than M. sacchariflorus and the hybrids. Furthermore, while it may be possible to select high-yielding genotypes within species at Y3, it is not possible to predict the highest yielding genotypes within a mixed population.
The maturation phase in Miscanthus appears to consist firstly of individual ramets reaching a certain phenotype, in terms of height and diameter, while yield increases in subsequent years are primarily due to the production of increased numbers of ramets, as captured by the transect count measurement (Fig. 1). In a smaller study of 20 clones, Zub et al. (2011) identified plant height and shoot diameter as the morphological traits best correlated with yield in Y2 and Y3. This has important implications in terms of selecting genes for targeted improvement in a molecular breeding approach, as it may be these ‘mature’ traits that have the greatest potential in terms of long-term yield increase once the obvious increases in canopy height have been made.
Genetic gain
In domesticating a new crop, one of the greatest challenges is to reduce the genetic complexity and select only those alleles conferring desirable traits. In order to make rapid gains, stringent criteria must be applied to eradicate excess allelic diversity from the breeding pool: the more stringent the selection, the more rapid the rate of improvement (Moose & Mumm, 2008). The primary traits defining biomass yields are those intrinsic to yield, i.e. stem height, diameter, and number, and these three traits alone predicted 59% of yield. However, the addition of traits such as leaf length and width, and stem and leaf angle increased the yield prediction to 67%. It is likely that, once the variation within the intrinsic traits is sufficiently reduced (to taller, thicker, more numerous stems), these other traits will play a greater role in yield optimization, for example through optimizing light capture by the canopy. In practice, the reduction in complexity (primarily via morphological selection) has led to genetic bottlenecking in the majority of modern crops, which is problematic for continued improvement. Historically, selection was based predominantly on traits associated with yield gains and agronomic practice, thereby inadvertently reducing natural variation for other important traits such as stress resistance (Doebley et al., 2006). While yield increase is the primary aim, it is important to ensure that diversity for other desirable traits such as nitrogen-use efficiency, abiotic stress tolerance, composition, and longevity is retained for subsequent selection, so ideally one would select very few plants with multiple good traits.
Phenotypic and genetic correlations in plants are poorly understood (Waitt & Levin, 1998) and are complicated by the low heritability of key traits determining yield. Perennial plants demonstrate high levels of developmental plasticity, enabling them to survive throughout the year and over multiple growing seasons. Although this trial was conducted in a single location, significant differences were observed across the replicates, indicating that environmental variation was not eliminated in this experiment.
Implications for domestication/breeding of Miscanthus as a novel crop for biomass production
The domestication of Miscanthus will differ from historic crop domestications in a number of ways. Not only are different traits being selected for, but following collection and characterization of wild material, promising genotypes from extremely diverse origins can be crossed whereas only closely located neighbours would have come into contact in early domestication events. Additionally, genetic gains may be made rapidly by isolation of diverse individuals targeted for recurrent selection without genetic drag from unselected genotypes as would have happened in the past.
Direct selection of desirable alleles is confounded by both genetic linkage, which requires high levels of recombination to uncouple genetically linked traits, and the complex nature of yield traits. Not only are the majority of yield-associated traits highly polygenic, but they are also phenotypically responsive to environmental conditions (Fig. 3c). In order to accelerate the domestication of robust high-yielding Miscanthus for growth over a wide range of geographies, trials are essential to determine which genotypes are consistently high yielding, and which are adapted to certain locations and climates. A suite of traits is desirable in an energy crop, including high establishment rate, cold tolerance, water-use efficiency, nitrogen-use efficiency, and optimized phenology. A direct way to select genotypes with a range of desirable traits is to gather diverse germplasm and screen for consistent high yield in diverse environments under the intended growing conditions, i.e. in plots on marginal land without addition of nitrogen fertilizer. As this is resource intensive, a morphological pre-screen is of great value in selecting high-yielding genotypes for multi-location testing. The selection of relatively few robust morphological traits that predict high yield is therefore of value for accelerating the selection of parents and progeny within the crossing cycle.
In addition to the variation observed between the parental species and their hybrids, there were also differences between plants of different ploidy levels, with triploid and tetraploid hybrids producing the highest yields in this trial. This raises the possibility that additional yield gains may be accumulated through exploitation of heterosis in Miscanthus. One option is to optimize yield within isolated groups within species and then intercross between groups to exploit heterosis, as is employed in maize breeding. This approach has been highly successful in switchgrass, with observations of 30–35% F1 heterosis superiority with respect to the best parent, even without selection for specific recombining ability (Casler, 2012).
The final critical difference between historic and modern domestication/breeding is the application of molecular markers. There is much discussion about how next-generation technologies can assist breeding (Moose & Mumm, 2008; Flavell, 2010, Casler, 2012). Methods range from simple selection of desirable candidate gene alleles to genome-wide selection of high-density molecular markers associated with desirable traits. In either case, existing models are based on breeding crops in which genetic diversity is much reduced in relation to their wild progenitors, and good phenotype–genotype associations are required. In focusing on simple traits in this study, we intend to enable targeted identification of genes regulating these traits through association studies and comparative genomics, in particular through exploitation of the synteny between Miscanthus and Sorghum genomes (Rooney et al., 2007; Ma et al., 2012; Olson et al., 2012). In the long term, molecular selection of one kind or another will play a vital role in Miscanthus breeding; however, the short-term gains to be made via phenotypic selection should not be overlooked. The application of morphometric selection of Miscanthus for high yield simultaneously identifies genotypes for introduction into accelerated selection cycles and paves the way for phenotype–genotype studies, which will further accelerate the domestication and optimization of this promising crop and its relatives.
Acknowledgements
The authors would like to thank the harvesting team and farm staff at Aberystwyth University for their valuable contributions to the work. This work was supported by the Biotechnology and Biological Sciences Research Council (BBSRC) (Institute career path fellowship BB/E024319/2 to K.F., grant number BB/E014933/1, BBSRC Institute Strategic Programme Grant ‘Energy Grasses & Biorefining’ (BB/J0042/1)) and Department for Environment Food and Rural Affairs (DEFRA) (NF0426). S.R.W. and K.K. were supported by Mathematics in the Plant Sciences Study Group Series (supported by the Centre for Plant Integrative Biology and GARNet)
Glossary
Abbreviations:
- AIC
Akaike information criterion
- DMY
dry matter yield
- DW
dry weight
- FW
fresh weight
- Y
year.
References
- Anderson E, Arundale R, Maughan M, Oladeinde A, Wycislo A, Voigt T. 2011. Growth and agronomy of Miscanthus × giganteus for biomass production. Biofuels 2, 167–183 [Google Scholar]
- Angelini LG, Ceccarinia L, Nassi o Di Nassoa N, Bonarib E. 2009. Comparison of Arundo donax L. and Miscanthus×giganteus in a long-term field experiment in Central Italy: analysis of productive characteristics and energy balance. Biomass and Bioenergy 33, 635–643 [Google Scholar]
- Brosse N, Dufour A, Meng X, Sun Q, Ragauskas A. 2012. Miscanthus: a fast-growing crop for biofuels and chemicals production. Biofuels, Bioproducts and Biorefining 6, 580–598 [Google Scholar]
- Burger JC, Chapman MA, Burke JM. 2008. Molecular insights into the evolution of crop plants. American Journal of Botany 95, 113–122 [DOI] [PubMed] [Google Scholar]
- Casler MD. 2012. Switchgrass breeding, genetics, and genomics. In: Monti A, ed. Switchgrass, green energy and technology. London: Springer-Verlag, 29–53 [Google Scholar]
- Clayton WD, Vorontsova MS, Harman KT, Williamson H. (2006. onwards). GrassBase—The Online World Grass Flora. http://www.kew.org/data/grasses-db.html Last accessed 9 September, 2013.
- Clifton-Brown JC, Breuer J, Jones MB. 2007. Carbon mitigation by the energy crop Miscanthus . Global Change Biology 13, 2296–2307 [Google Scholar]
- Clifton-Brown JC, Chiang Y-C, Hogkinson TR. 2008b. Miscanthus: genetic resources and breeding potential to enhance bioenergy production. In: Vermerris W, ed. Genetic Improvement of Bioenergy Crops. New York: Springer Science+Business Media, 295–308 [Google Scholar]
- Clifton-Brown JC, Robson PRH, Allison GG, et al. 2008a. Miscanthus: breeding our way to a better future. In: Booth E, Green M, Karp A, Shield I, Stock D, Turley D, eds. Aspects of Applied Biology 90, Biomass and Energy Crops III. Wellesbourne, UK: Association of Applied Biologists, 199–206 [Google Scholar]
- Doebley JF, Gaut BS, Smith BD. 2006. The molecular genetics of crop domestication. Cell 127, 1309–1321 [DOI] [PubMed] [Google Scholar]
- FAO 1988. FAO/Unesco Soil Map of the World, revised legend, with corrections and updates. World Soil Resources Report 60, FAO, Rome. Reprinted with updates as Technical Paper 20. Wageningen, Netherlands: ISRIC. [Google Scholar]
- Farrar K, Bryant B, Turner L, Gallagher JA, Thomas A, Farrell M, Humphreys MO, Donnison IS. 2012. Breeding for bio-ethanol production in Lolium perenne L.: association of allelic variation with high water-soluble carbohydrate content. Bioenergy Research 5, 149–157 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Flavell R. 2010. From genomics to crop breeding. Nature Biotechnology 28, 144–145 [DOI] [PubMed] [Google Scholar]
- Gomez LD, Steele-King CG, McQueen-Mason SJ. 2008. Sustainable liquid biofuels from biomass: the writing’s on the walls. New Phytologist 178, 473–485 [DOI] [PubMed] [Google Scholar]
- Heaton EA, Dohleman FG, Long SP. 2008. Meeting US biofuel goals with less land: the potential of Miscanthus . Global Change Biology 14, 2000–2014 [Google Scholar]
- Hong C, Fang J, Jin A, Cai J, Guo H, Ren J, Shao Q, Zheng B. 2011. Comparative growth, biomass production and fuel properties among different perennial plants, bamboo and Miscanthus . Botanical Review 77, 197–207 [Google Scholar]
- Jensen E, Farrar K, Thomas-Jones S, Hastings A, Donnison IS, Clifton-Brown J. 2011. Characterization of flowering time diversity in Miscanthus species. Global Change Biology Bioenergy 3, 387–400 [Google Scholar]
- Johnson JJ, Alldredge J R, llllrich SE, Dangi OP. 1992. Replacement of replications with additional locations for grain sorghum cultivar evaluation. Crop Science 32, 43–46 [Google Scholar]
- Lewandowski I, Clifton-Brown JC, Scurlock JMO, Huisman W. 2000. Miscanthus: European experience with a novel energy crop. Biomass and Bioenergy 19, 209–227 [Google Scholar]
- Ma XF, Jensen E, Alexandrov N, et al. 2012. High resolution genetic mapping by genome sequencing reveals genome duplication and tetraploid genetic structure of the diploid Miscanthus sinensis . PLoS ONE 7, e33821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moose SP, Mumm RH. 2008. Molecular plant breeding as the foundation for 21st century crop improvement. Plant Physiology 147, 969–977 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Olson SN, Ritter K, Rooney W, Kemanian A, McCarl BA, Zhang Y, Hall S, Packer D, Mullet J. 2012. High biomass yield energy sorghum: developing a genetic model for C4 grass bioenergy crops. Biofuels, Bioproducts and Biorefining 6, 640–655 [Google Scholar]
- R Core Team 2012. R: a language and environment for statistical computing. Vienna, Austria: R Foundation for Statistical Computing; http://www.R-project.org/ [Google Scholar]
- Robson PR, Clifton-Brown JC, Donnison IS, Mos M. 2012. Phenotypic variation in senescence in Miscanthus: towards optimising biomass quality and quantity. Bioenergy Research 5, 95–105 [Google Scholar]
- Robson RH, Farrar K, Gay AP, Jensen EF, Clifton-Brown JC, Donnison IS. 2013. Variation in canopy duration in the energy crop Miscanthus: reveals complex associations with yield. Journal of Experimental Botany 64, 2373–2383 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rooney WL, Blumenthal J, Bean B, Mullet JE. 2007. Designing sorghum as a dedicated bioenergy feedstock. Biofuels, Bioproducts and Biorefining 1, 147–157 [Google Scholar]
- Sang T. 2011. Toward the domestication of lignocellulosic energy crops: learning from food crop domestication. Journal of Integrated Plant Biology 53, 96–104 [DOI] [PubMed] [Google Scholar]
- Valentine J, Clifton-Brown J, Hastings A, Robson P, Allison GG, Smith P. 2012. Food vs. fuel: the use of land for lignocellulosic ‘next generation’ energy crops that minimize competition with primary food production. Global Change Biology Bioenergy 4, 1–19 [Google Scholar]
- van der Weijde T, Alvim Kamei CL, Torres AF, Vermerris W, Dolstra O, Visser RGF, Trindade LM. 2013. The potential of C4 grasses for cellulosic biofuel production. Frontiers in Plant Science 4, 107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Waitt DE, Levin DA. 1998. Genetic and phenotypic correlations in plants: a botanical test of Cheverud’s conjecture. Heredity 80, 310–319 [Google Scholar]
- Yan J, Chen W, Luo F, et al. 2012. Variability and adaptability of Miscanthus species evaluated for energy crop domestication. Global Change Biology Bioenergy 4, 49–60 [Google Scholar]
- Zub HW, Arnoult S, Brancourt-Hulmel M. 2011. Key traits for biomass production identified in different Miscanthus species at two harvest dates. Biomass and Bioenergy 35, 637–651 [Google Scholar]