Abstract
Lesion mimic mutants (LMMs) are a class of mutants in which hypersensitive cell death and defence responses are constitutively activated in the absence of pathogen attack. Various signalling molecules, such as salicylic acid (SA), reactive oxygen species (ROS), nitric oxide (NO), Ca2+, ethylene, and jasmonate, are involved in the regulation of multiple pathways controlling hypersensitive response (HR) activation, and LMMs are considered useful tools to understand the role played by the key elements of the HR cell death signalling cascade. Here the characterization of an Arabidopsis LMM lacking the function of the FZL gene is reported. This gene encodes a membrane-remodelling GTPase playing an essential role in the determination of thylakoid and chloroplast morphology. The mutant displayed alteration in chloroplast number, size, and shape, and the typical characteristics of an LMM, namely development of chlorotic lesions on rosette leaves and constitutive expression of genetic and biochemical markers associated with defence responses. The chloroplasts are a major source of ROS, and the characterization of this mutant suggests that their accumulation, triggered by damage to the chloroplast membranes, is a signal sufficient to start the HR signalling cascade, thus confirming the central role of the chloroplast in HR activation.
Key words: Arabidopsis thaliana, chloroplast, double mutants, expression analysis, lesion mimic mutants (LMMs), reactive oxygen species (ROS)
Introduction
Programmed cell death (PCD) is a metabolically active and genetically controlled process leading to cell death (Jones, 2001). In plants, this process occurs not only during normal development and senescence, but also during interactions with the environment in biotic and abiotic stress responses (van Doorn et al., 2011). One of the most studied forms of PCD is the cell death associated with the defence pathway known as the hypersensitive response (HR) (Lam et al., 2001)
When plants are attacked by pathogens, a first basal response, the so-called PAMP-triggered immunity (PTI) (Jones and Dangl, 2006), is activated after the recognition of conserved microbial/pathogen-associated molecular patterns (MAMPs/PAMPs). Pathogens can, however, overcome PTI by secreting effector proteins in the plant cell. In response, cells have developed specific receptors that recognize the effectors and activate a second layer of immunity, the effector-triggered immunity (ETI) (Jones and Dangl, 2006). ETI, a more rapid and robust defence response than PTI, is specifically set up when a pathogen avr (avirulence) gene is recognized by the complementary R (resistance) gene of the plant in a gene-for-gene interaction (Ellis et al., 2000), and the death of cells challenged by the pathogen (HR cell death) is often used as a barrier to limit pathogen growth in plant tissues (Tsuda and Katagiri, 2010). This response is also characterized by the activation of a complex defence system including the following: rapid ROS (reactive oxygen species) accumulation, production of defence molecules, cell wall strengthening, and activation of the expression of defence genes, with the aim of stopping not only pathogen diffusion but also further attacks by different pathogens (Muthamilarasan and Prasad, 2013). In particular, the so-called pathogen-related proteins (PRs) are a wide and heterogeneous group of proteins induced in cells under attack by pathogens, that have been demonstrated to act specifically in the control of pathogen infection (Van Loon et al., 2006).
The structure of the R protein appears to determine which are the positive regulators required for HR signalling: the TIR-NBS-LRR (Toll interleukin1 receptor–nucleotide-binding–leucine-rich repeat) class of R genes require EDS1 (Enhanced Disease Susceptibility1) and PAD4 (Phytoalexin Deficient4) genes, while the R genes encoding CC-NB-LRR (coiled-coil–nucleotide binding–leucine-rich repeat) proteins require the NDR1 (Non-Race Specific Disease Resistance1) gene (Aarts et al., 1998; Lorrain et al., 2003).
The aberrant regulation of HR characterizes the lesion mimic mutants (LMMs), a group of mutants showing discrete leaf lesions and activation of defence responses in the absence of pathogen attack. This type of mutant thus appears to be a powerful tool to identify genes involved in the regulation of the cell death programme, to dissect the signalling pathways activated in this process, and to discover the cross-talk between them (Walbot et al., 1983; Greenberg and Ausubel, 1993; Dietrich et al., 1994; Greenberg et al., 1994; Lorrain et al., 2003).
The LMMs can be grouped into two classes: the initiation mutants, characterized by the presence of discrete lesions that identify functions related to the initiation phase of lesion formation; and the propagation mutants, characterized by the uncontrolled spread of the lesions and identifying functions related to the containment of HR cell death signalling (Lorrain et al., 2003).
The constitutive expression of biochemical and molecular markers associated with HR is one of the hallmarks of the LMMs: they are diagnostic of the activation of defence responses and give precise indications of which, among the different defence pathways, are specifically activated in the LMM analysed (Lorrain et al., 2003). In fact, several different signalling molecules are known to be involved in the HR pathway: salicylic acid (SA) (Gaffney, 1993; Devadas et al., 2002), ROS (Jabs et al., 1996; Levine et al., 1996; Zurbriggen et al., 2010), nitric oxide (NO) (Delledonne et al., 1998), ethylene, and jasmonate (JA) (Dong, 1998; Greenberg et al., 2000), and the activation of each of these pathways can be revealed by the analysis of specific markers.
The central role played by SA in plant–pathogen interactions has been demonstrated by the use of transgenic plants expressing the bacterial NahG gene (encoding salicylate hydroxylase) that were unable not only to accumulate SA but also to activate defence responses after pathogen attack (Gaffney et al., 1993). This evidence is supported by the higher level of SA generally found in LMMs in comparison with the wild type, and the suppression of lesion formation when different LMMs are crossed with NahG transgenic plants (Lorrain et al., 2003).
The characterization of the sid2 (salicylic acid induction deficient2) mutant and its allelic eds16 (enhanced disease susceptibility16), defective in SA biosynthesis and with enhanced susceptibility to pathogens, supported a central role for the enzyme isochorismate synthase, encoded by the SID2 gene, in pathogen-stimulated SA biosynthesis (Wildermuth et al., 2001). The EDS5 (SID1) gene is also involved in pathogen-mediated SA biosynthesis (Nawrath et al., 2002), and recently it has been suggested to be responsible for SA transport (Yamasaki et al., 2013).
The isolation of the npr1 (non expresser of PR genes1) mutant, unable to activate PR gene expression, allowed the identification of the essential role played by NPR1 in SA signalling, downstream of the R gene-mediated defence responses, but an NPR1-independent SA signalling pathway has also been reported (Shah, 2003; Dong, 2004).
Associated with hypersensitive cell death, pathogen attacks often trigger, in uninfected tissue, a sort of broad spectrum immunity to subsequent infections, called SAR (systemic acquired resistance) (Fu and Dong, 2013). The accumulation of the signalling molecule SA (Gaffney et al., 1993) and the expression of a group of disease-related genes, in particular PR genes (Van Loon et al., 2006), GST (glutathione S-transferase), PRXc (peroxidase C), and Pal1 (phenylalanine ammonia lyase1) (Ward et al., 1991; Greenberg et al., 1994; Maleck et al., 2000), are known to be linked to the establishment of SAR.
The earlier signals generally reported to be connected with HR execution are the rapid rise of cytoplasmic Ca2+ and the production of ROS, generated not only at the cytoplasmic level, by the action of NADPH oxidases, but also in mitochondria and chloroplasts. In particular chloroplasts are thought to be the initial source of ROS immediately after pathogen recognition, thence this signalling is spread to the apoplast and then to the adjacent cells, leading to the selected death of the cells challenged by the pathogen (Zurbriggen et al., 2010).
In chloroplasts during HR, the generation of ROS can be the consequence of EEE (excess excitation energy); that is, photon intensity is in excess of that required for CO2 fixation, or can be the product of chlorophyll catabolism. This is supported by data reporting that mutations in genes involved in EEE dissipation (LSD1) or in chlorophyll catabolism (ACD1 and ACD2) resulted in light-dependent lesion mimic phenotypes caused by photo-oxidative damage with formation of ROS (Mach et al., 2001; Pružinská et al., 2003; Mateo et al., 2004).
In this work, an Arabidopsis mutant with the typical appearance of the LMMs (i.e. characterized by the presence, early during development, of chlorotic lesions on rosette leaves, and the constitutive activation of defence responses) is described. Both lesion formation and defence response activation are SA dependent, requiring the functions of EDS16, PAD4, and NPR1 genes, but are ethylene–JA independent.
Sequence analysis showed that the mutation was in the At1g03160 gene encoding an FZO-like protein (FZL), playing a unique role in the determination of thylakoid and chloroplast morphology (Gao et al., 2006), and histological analysis confirmed the presence of chloroplasts with altered morphology in fzl-Ler (mutation fzl in Landsberg erecta ecotype) mutants.
Data are presented showing that in the fzl-Ler mutant the loss of chloroplast integrity is linked to the activation of defence responses, and it is suggested that a chloroplast-generated signal plays a central role in the signalling cascade leading to defence activation and HR cell death.
Materials and methods
Plant material
The fzl-Ler mutant was initially isolated during the generation of the transposant lines of the Exotic collection (http://Arabidopsis.info/CollectionInfo?id=31; last accessed 18 July 2013), ecotype Landsberg erecta (Ler). Because, as shown in the Results, the Ds element did not co-segregate with the fzl-Ler mutation, the characterization of this mutant was performed on the fzl-Ler line obtained after the segregation of the Ds element.
The two T-DNA insertion lines of the Salk collection: Salk_033745 and Salk_009051 (provided by the NASC, Nottingham Arabidopsis Stock Centre, http://nasc.nott.ac.uk/) (Alonso et al., 2003) correspond to the fzl mutant in the Columbia ecotype previously characterized (Gao et al., 2006). In all the experiments, both the T-DNA insertional lines were used, always obtaining comparable results, so for this reason herein the fzl-Col mutant is referred to without further specifications.
The mutants eds5, eds16, ein2, etr1, jar1, npr1, pad4, and vad1 were provided by the NASC.
Plant growth conditions
Arabidopsis thaliana plants were grown in soil (Vegetal Radic, Tercomposti, Calvisano Brescia, Italy) in a greenhouse or in vitro in a growth chamber.
The seeds for in vitro growth were surface sterilized in 95% ethanol, soaked for 6min in 40% bleach, 0.1% Tween-20, and washed twice in sterile distilled water. The seeds were then sown in Murashige–Skoog medium (MS; SIGMA M-5524), supplemented with 0.7% Bacto agar (Difco) and 1% sucrose. The growth conditions in the greenhouse were 16h light (100µmol m–2 s–1 light intensity), 22 °C temperature, 60% humidity, while in the phytochamber (for in vitro growth) they were 16h light (100µmol m–2 s–1 light intensity), 22 °C temperature, and 40% humidity.
In the high temperature experiment, the temperature was 28 °C for the treatment, and 22 °C for the control; in the low light growth experiment, the light intensity was 50 µmol m–2 s–1 for the treatment and 100 µmol m–2 s–1 for the control.
Genetic analysis
For double mutants analysis, fzl-Ler plants, used as pollen donor, were crossed with the mutants eds5, eds16, ein2, etr1, jar1, npr1, pad4 and vad1. The genotype of double mutants was determined by cleaved amplified polymorphic sequence (CAPS and dCAPS) analysis as described previously (Resnick et al., 2006; Stein et al., 2006).
The fzl-Ler mutation was selected by CAPS analysis: using the primers EcoRVFor 5′-GAGCAACAACGTTGCCAAACAC-3′ and EcoRVRev 5′-ACTGCGATGGTAGAATTTTGAATTACTGA-3′, and the enzyme EcoRV, the wild-type DNA yielded a single band of 102bp, and the fzl-Ler allele yielded two bands of 71bp and 31bp.
Histochemistry
Callose and autofluorescence detection were performed as reported by Dietrich and colleagues (1994). Evan’s blue staining was performed as reported by Iriti and colleagues (2003). 3,3′-Diaminobenzidine (DAB) staining was performed as reported by Murgia and colleagues (2004).
Cell death quantification
Cell death was quantified by electrolyte leakage measurement as previously reported (Roberts et al., 2013).
H2O2 quantification
H2O2 was quantified as previously reported (Shi et al., 2013).
Salicylic acid and salicylic acid glucoside measurement
Free and total SA were extracted and measured from 2g of dried tissue (3-week-old rosette), as previously described (Toiu et al., 2011).
Chloroplast analysis
Chloroplasts of individual fixed mesophyll cells (Pyke and Leech, 1991) were observed using a Zeiss Axiophot D1 microscope equipped with differential interference contrast (DIC) optics.
RNA isolation and expression analyses
The tissues were collected from wild-type and mutant plants, grown in vitro or in soil as specified in the different experiments.
The expression analyses were performed by the RT–PCR technique as previously reported (Landoni et al., 2010), or, in the case of double mutants, by real-time RT–PCR as previously reported (Lazzeri et al., 2012). The sequences of the oligonucleotides used are reported in Supplementary Table S1 available at JXB online for RT–PCR and in Supplementary Table S2 for real-time RT–PCR.
Positional cloning of the fzl-Ler mutant
The mutant which was isolated was crossed to the Col ecotype, the F1 progeny (phenotype wild type). were allowed to self-fertilize, and in the F2 population the LMM phenotype, as expected, segregated 3:1 (1625:533, P > 0.70). DNA was collected from single F2 plants showing the LMM phenotype, from the F1 and from the parental lines, and positional cloning as reported by Lukowitz and colleagues (2000) was started. By the analysis of the collected recombinants with the Mapmaker program (Lander et al., 1987), it was found that the LMM mutation mapped within an interval of 5.4 cM flanked by the two SSLP (simple sequence length polymorphism) markers NF21B7 and ACC2 (TAIR SSLP collection: www.Arabidopsis.org).
DNA library preparation and sequencing
DNA libraries with an insert size of 250bp were prepared starting from 10 µg of genomic DNA using a Paired-End DNA Sample Prep Kit (Illumina Inc., San Diego, CA, USA). Library quality control was performed with a High Sensitivity DNA Kit (Agilent, Wokingham, UK). Libraries were sequenced with an Illumina GAIIx sequencer (Illumina Inc., San Diego, CA, USA), and 75–100bp paired-end sequences were generated.
Data analysis
The alignment against the reference genome (TAIR9 version of the A. thaliana genome) was performed with GenomeMapper v. 0.4, allowing for up to four mismatches and one gap in the reads. Data were processed using the SHORE v.0.4 pipeline (Ossowski et al., 2008). Trimmed reads shorter than 50bp were discarded. The variants were called with ShoreMap v. 1.1 in the genomic interval determined by positional cloning (Schneeberger et al., 2009). Known Ler variants were excluded from the analysis.
Complementation of the fzl-Ler mutant
The 5900bp PCR-amplified fragment containing the FZL coding region and a sequence of ~1000bp 5′ of the ATG was amplified with the primers 3160-1F/1R using the Phusion™ DNA Polymerase (Fynnzymes) and cloned in the pCR-XL-TOPO vector (Invitrogen). The SacI/XbaI fragment was then cloned in the binary vector PZP221 that was used to transform fzl-Ler plants via Agrobacterium tumefaciens (strain GV3101) as previously reported (Clough and Bent, 1998). Transformed plants were selected on MS medium containing 100 µg ml–1 gentamycin sulphate.
Results
Isolation and phenotypic characterization of a new lesion mimic mutant
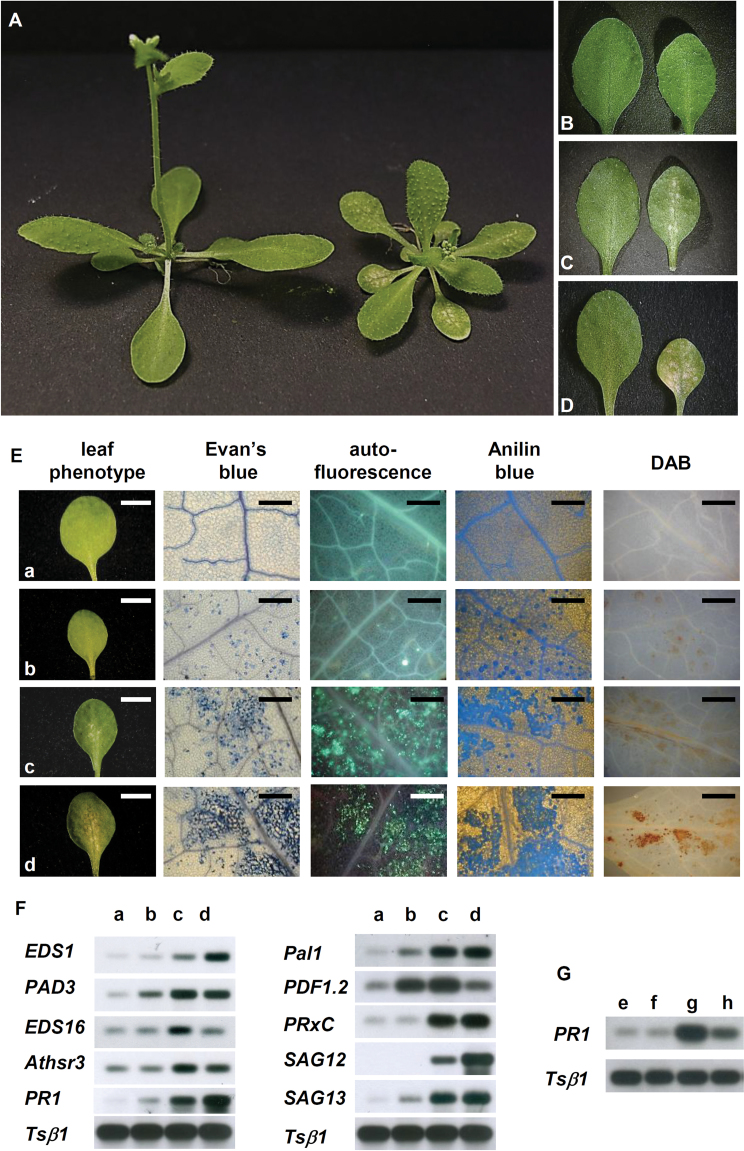
During the generation of a gene-trapping collection of A. thaliana based on the Ac/Ds transposon system of maize (Sundaresan et al., 1995), a mutant showing chlorotic lesions on rosette leaves and reduced plant size was isolated (Fig. 1A). The lesions began to appear as small chlorotic spots close to the central vein of the rosette leaves, starting from 2–3 weeks after germination, then within a week to 10 d they enlarged to cover the entire leaf blade (Fig. 1B–D). The mutant seedlings also showed delayed development, with a marked reduction of the rosette size (Fig. 1A).
Fig. 1.
Phenotype of the mutant and analyses of the biochemical and molecular markers associated with defence responses. (A) Three-week-old wild-type (left) and mutant (right) plants. (B–D) Three-week-old wild-type (left) and mutant (right) leaves: while all the wild-type leaves are green, in the mutant the third pair of leaves are green (b, right), the second show small chlorotic spots (c, right), and the first show large chlorotic spots (d, right). (E) Histochemical analysis of 3-week-old wild-type and mutant leaves: in the first column is shown the phenotype of leaves analysed (a, wild-type green leaf; b, mutant green leaf; c, mutant leaf with small chlorotic spots; d, mutant leaf with large chlorotic spots), in the second column staining with Evan’s blue, in the third natural autofluorescence, in the fourth aniline blue staining, and in the fifth DAB staining. Bars indicate 5mm in the first column, 500 µm in the second, third, and fourth columns, and 1mm in the fifth column. (F) RT–PCR analysis of genes associated with plant defence responses in 3-week-old leaves of a wild-type and mutant plant (a, wild-type green leaf; b, mutant green leaf; c, mutant leaf with small chlorotic spots; d, mutant leaf with large chlorotic spots). (G) Analysis of SAR activation in the fzl-Ler mutant by comparison of the expression levels of the PR1 gene in wild-type rosette leaves (e), wild-type cauline leaves (f), mutant rosette leaves (g), and mutant cauline leaves (h).
Taken together, the phenotypic alterations displayed by this mutant were reminiscent of the class of LMMs, characterized by the misregulation of the HR (Lorrain et al., 2003).
To test whether the mutant that was isolated could really be considered a member of this group of mutants, the presence of some biochemical markers that are known to be constitutively expressed in the LMMs was sought. In particular, the presence of callose, revealed by aniline blue staining, secondary metabolites, revealed by their natural fluorescence, H2O2 accumulation, revealed by DAB staining, and dead cells, revealed by Evan’s blue staining, was looked for.
These analyses were performed on 3-week-old rosette leaves of mutant plants that showed different degrees of lesion development (green leaves, leaves with small chlorotic spots, and leaves with large chlorotic spots) and on 3-week-old wild-type leaves (green) (Fig. 1E, first column).
In mutant plants, the biochemical markers analysed are present not only in and around the chlorotic lesions but also in completely green leaves (Fig. 1E, second row). In this tissue, however, although no chlorotic lesions were detected by macroscopic observation, the Evan’s blue staining revealed the presence of dead cells, singly or in small groups. The blue spots are localized close to the central vein, in the positions where the chlorotic lesions will subsequently appear during development of the mutant phenotype (Fig. 1E, second column). In wild-type leaves, neither dead cells nor other HR-specific biochemical markers were detected (Fig. 1E, first row).
The expression of a group of defence-related genes known to be generally constitutively expressed in LMMs was then analysed in mutant and in wild-type plants. For all the genes analysed, no or low expression was detected in wild-type leaves, while in mutant leaves the amount of these transcripts was higher than in the wild type, generally further increasing with the development of the lesions (Fig. 1F).
It was then checked whether, as observed for other LMMs (Dietrich et al., 1994; Devadas et al., 2002), PR genes, considered to be the executors of SAR (Fu and Dong, 2013), are also expressed in tissues that never develop lesions. The expression of PR1 (Ward et al., 1991) was analysed in 3- to 4-week-old rosette leaves, that appeared completely green in wild-type plants and with chlorotic spots in mutants, and in cauline leaves, that appeared completely green in both wild-type and mutant plants.
While in wild-type rosette and cauline leaves, no PR1 expression was detected, in mutant leaves PR1 transcript is present not only in rosette leaves, but also in cauline leaves (Fig. 1G), even if the level of expression, quantified by real-time RT–PCR, is slightly lower than in green rosette leaves (data not shown). Further experiments will be needed to test the real activation of SAR in fzl-Ler mutants.
These data indicated that the defence response pathway was constitutively activated in mutant plants, and, together with the histochemical data and the phenotypic traits previously described, suggested that the mutant which was isolated could be considered a propagative LMM.
Genetic analysis and identification of the fzl-Ler mutation
The mutant, isolated as a homozygous recessive mutant, was crossed with its parental ecotype Ler, and the F1 progeny, which showed a wild-type phenotype, were allowed to self-pollinate. The segregation of the mutant phenotype was analyzed in the F2 generation and a 3:1 ratio was observed (169:59, P > 0.9), as expected for a recessive mutation affecting a single locus.
The Ds association with the mutant phenotype was investigated by PCR amplification of the GUS (β-glucuronidase) reporter gene included in the Ds element (Sundaresan et al., 1995): an F2 population of 312 plants was analysed, and it was found that the mutation was not tagged by the Ds transposon, so positional cloning of this gene was set up. The mutant, in the original background (Ler), was crossed to a Columbia wild-type plant and it was found that the mutant phenotype was still perfectly recognizable in the mixed background of the resulting F2. Therefore, 536 mutant plants were isolated from this segregant population and were analysed with SSLP molecular markers (Lukowitz et al., 2000).
The mutated locus was mapped in a 5.4 cM region flanked by the SSLP markers NF21B7 and ACC2 on chromosome 1. Further, SSLP/CAPS analysis failed to narrow down this genetic interval; therefore, a deep sequencing approach was used to isolate the mutation.
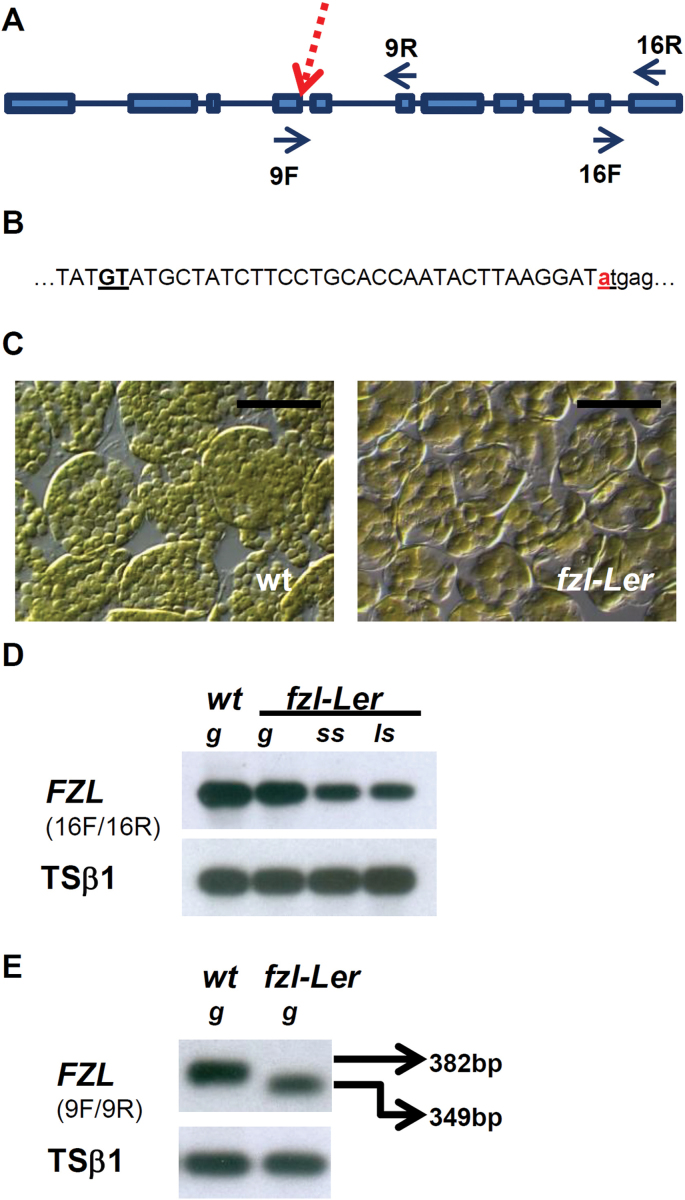
Massive parallel DNA sequencing was performed of mutant and Ler plants generating 33 792 085 and 33 337 253 paired-end reads of 75–100bp, respectively. The alignment of the genomic sequence obtained from mutant and Ler plant revealed a mutation (G→A) in the 5′ splice site of the fourth intron of the FZL gene (Ossowski et al., 2008), encoding a plant-specific member of the dynamin superfamily of membrane-remodelling GTPases, playing a unique role in the determination of chloroplast and thylakoid morphology (Gao et al., 2006) (Fig. 2A, B).
Fig. 2.
Molecular characterization of the fzl-Ler mutation. (A) Schematic representation of the FZL gene. Boxes indicate exons, lines indicate introns, the dotted arrow indicates the site of fzl-Ler mutation (first base of fourth intron), and arrows indicate the positions of the primers used for the RT–PCR analysis represented in D (primers 16F/16R) and E (primers 9F/9R). (B) Partial genomic sequence of the fourth exon (upper case letters) and fourth intron (lower case letters): the mutated base (a instead of the wild-type g) is indicated in red, and the activated cryptic site is underlined. (C) Chloroplast morphology of mesophyll cells of 3-week-old wild-type and fzl-Ler plant leaves. Bars=50 µm. (D) RT–PCR analysis of FZL gene expression in wild-type and fzl-Ler plants, using the primers FZL-16F/16R. (E) Analysis by RT–PCR of splicing of the fourth intron. Using the primers 9F/9R, a PCR product from the wild-type allele of 382bp is amplified and then sequenced, while from the fzl-Ler allele a PCR product of 349bp is amplified and then sequenced. g, green leaves; ss, leaves with small chlorotic spots; ls, leaves with large chlorotic spots.
Because of the role played by FZL, the chloroplast structure was analysed in the isolated mutant and an alteration in the chloroplast morphology was found, very similar to that reported for the previously characterized T-DNA insertional mutants in the FZL gene (lines Salk_033745 and Salk_009051) (Gao et al., 2006) (Fig. 2C). The alteration of chloroplast morphology was already present very early during leaf development (12-day-old leaves) and the same level of alteration was present in 3-week-old leaves with or without chlorotic spots, suggesting the involvement of a developmental signal for HR cell death activation in fzl mutants.
Surprisingly the fzl mutants described by Gao and colleagues did not display a lesion mimic phenotype, but were characterized by delayed flowering and pale green leaves. Supposing that, together with the different environmental conditions, the different ecotypes in which these mutations were isolated (Columbia for the T-DNA insertional mutants, Landsberg erecta for the LMM) could account for the different phenotypes displayed, the LMM that was isolated is referred to as fzl-Ler and the T-DNA insertional mutant is referred to as fzl-Col.
Expression analysis of the FZL gene
The expression profile of the FZL gene was analysed in 3-week-old mutant and wild-type leaves. The RT–PCR analysis showed a decrease of FZL expression in the fzl-Ler mutant (Fig. 2D), in agreement with the previously reported effect of mutations in the 5′ intron splicing site (Brown et al., 1996).
The fzl-Ler cDNA was then analysed by sequencing the region amplified with the primers FZL9F/9R, to check if the mutation interferes with the correct splicing of the fourth intron. It was found that this mutation caused the activation of the next upstream cryptic splicing site in the fourth exon, thus causing the deletion of 33 nucleotides in the mature RNA (Fig. 2E) and the loss of 11 amino acids in the FZL protein. The deleted amino acids belong to the predicted domain with GTPase activity, where the deletion of the conserved Lys362 has been shown to modify the localization of FZL, with the consequent loss of function of the FZL protein (Gao et al., 2006); therefore, the molecular alteration found in the fzl-Ler mutant is compatible with the severe phenotype observed.
Genetic complementation of the fzl-Ler mutation.
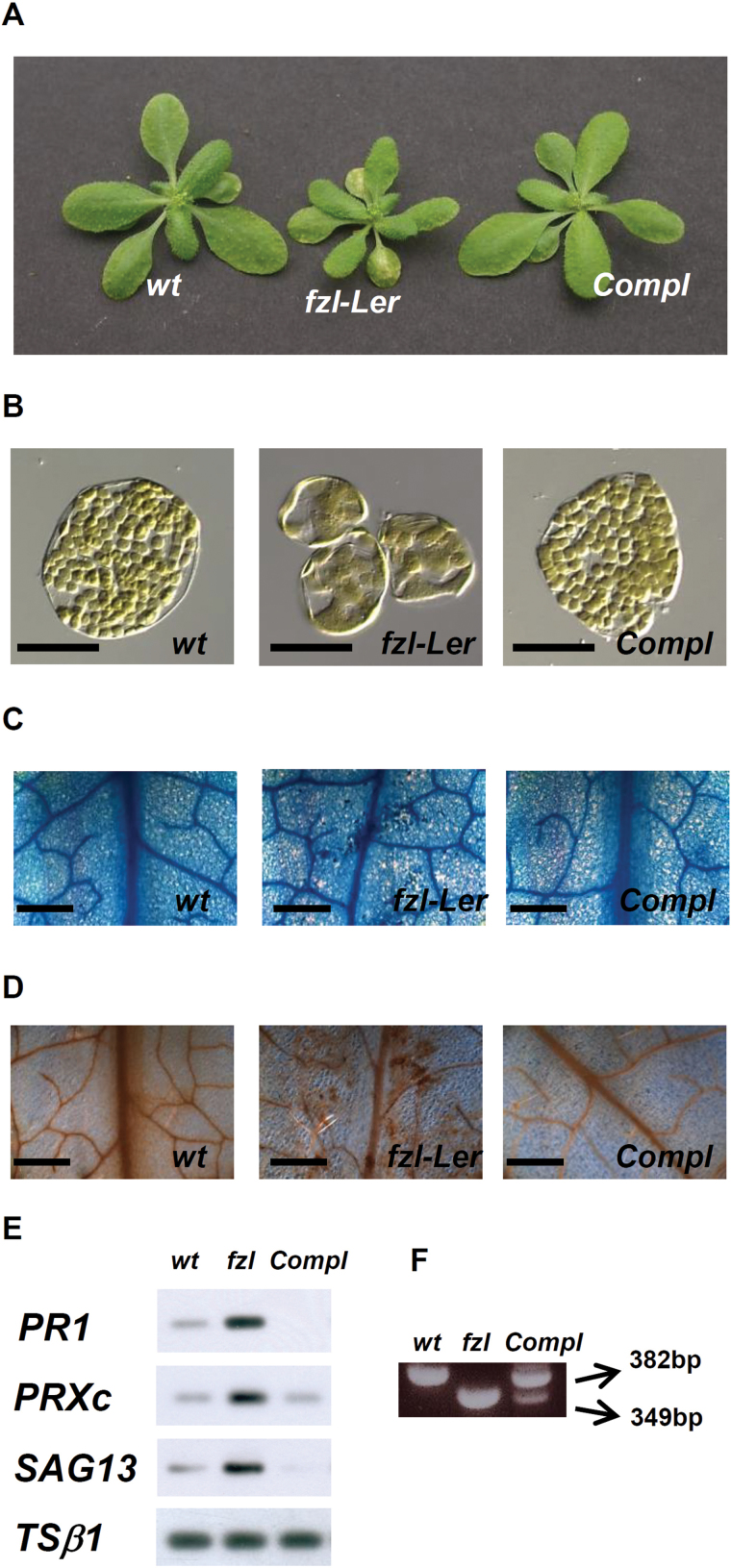
To confirm that the mutation in the FZL gene was responsible for the mutant phenotype observed, mutant plants were transformed with the complete FZL genomic sequence. The phenotype of transformed plants was indistinguishable from the wild type at the macroscopic (no chlorotic lesions were present) (Fig. 3A), microscopic (no alterations in chloroplast morphology) (Fig. 3B), histochemical (no cell death and ROS accumulation) (Fig. 3C, D), and molecular level (no induction of disease response genes) (Fig. 3E), indicating that the mutant phenotype was associated with the mutation of the FZL gene.
Fig. 3.
Genetic complementation of the fzl-Ler phenotype. Phenotype (A), chloroplast morphology (B), Evan’s blue (C), and DAB (D) staining of 3-week-old plants of the wild type, fzl-Ler, and fzl-Ler complemented with the wild-type sequence of the FZL gene (Compl). Bars in B=50 µm; in C and D=500 µm. (E) Expression analysis of a group of defence-associated genes in 3-week-old plants of the wild type, fzl-Ler, and fzl-Ler complemented with the wild-type sequence of the FZL gene (Compl). (F) PCR analysis of the FZL transcript in wild-type, fzl-Ler, and complemented plants (Compl). The primers used (3160-9F/10R) allowed the amplification of two distinct PCR products of 382bp and 349bp corresponding to the wild-type and the fzl-Ler allele, respectively.
Using PCR analysis, it was also checked whether the fourth intron was spliced correctly, and in the complemented plants, as expected, two different mature RNAs were found, the wild-type form, generated by correct splicing, and corresponding to a PCR product of 382bp, and the mutated form produced by the activation of the cryptic site of splicing and corresponding to a PCR product of 349bp (Fig. 3F).
Effect of the environment on the fzl phenotype
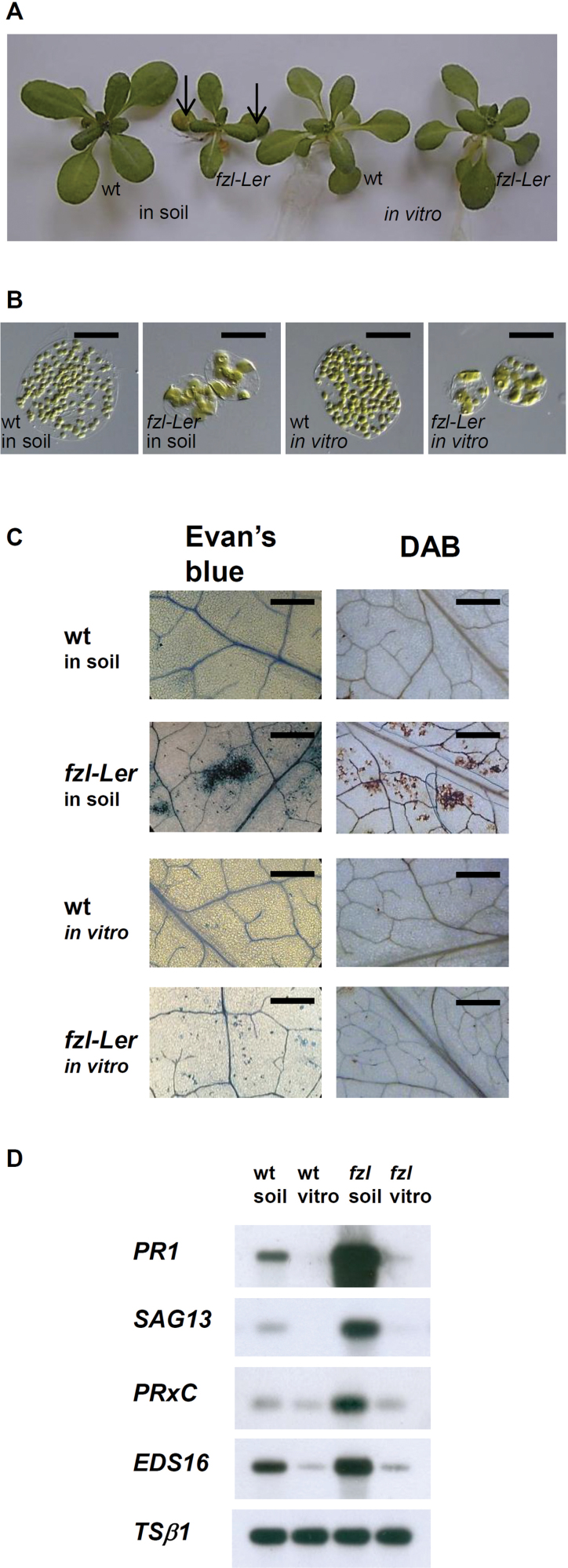
One of the characteristics shared by the LMMs described in the literature is the effect of environmental conditions in modulating the appearance of the lesions. The effect of growth under in vitro conditions (Brodersen et al., 2002; Lorrain et al., 2004) on the fzl-Ler phenotype was analysed, and it was found that fzl-Ler seedlings appeared indistinguishable from the wild type (Fig. 4A). Nevertheless microscopic analysis revealed that the chloroplasts of mutant plants grown in vitro showed the same morphological alterations observed in fzl-Ler plants grown in soil (Fig. 4B). The expression of some of the previously analysed biochemical and molecular markers associated with HR was also checked, and while in fzl-Ler mutants grown in vitro no DAB staining was detected, the Evan’s blue staining revealed the presence of some cell death (Fig. 4C). No significant difference was detected between wild-type and mutant leaves grown in vitro regarding the expression of the genes PR1, PRXc, SAG13, and EDS16 (Fig. 4D).
Fig. 4.
Effect of environmental conditions on the fzl-Ler phenotype. Phenotype (A), chloroplast morphology of mesophyll cells (B), Evans’s blue and DAB staining (C), and expression analysis (D) of 3-week-old wild-type and fzl-Ler plants grown in soil or in vitro. In A, arrows indicate the lesions present on fzl-Ler rosette leaves. Bars in B=50 µm, in C=500 µm.
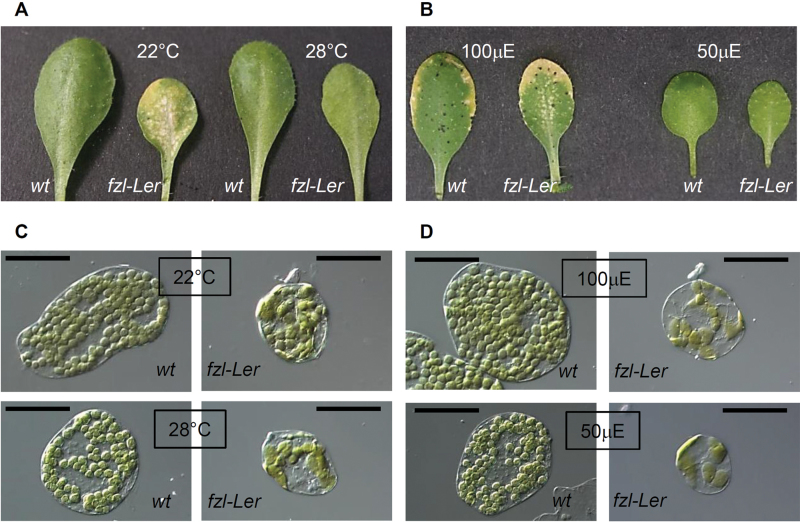
Supposing that temperature, light, humidity and nutrient availability are the conditions that, in addition to the sterility, constitute the difference between in vitro and soil growth, these different conditions were tested one by one. The conditions that, at least partially, were able to suppress the mutant phenotype were growth at high temperature and at low light intensity (Fig. 5). In fact, when the plants were grown in soil at 28 °C or at a light intensity of 50 µE, the mutant leaves displayed a pale green colour instead of the typical chlorotic spots (Fig. 5A, B) even though histological analysis revealed that the chloroplasts still showed the morphological alterations observed in mutant plants grown under standard conditions (Fig. 5C, D).
Fig. 5.
Effect of high temperature (28 °C) and low light intensity (50 µE) on the fzl-Ler phenotype. Phenotype of 4-week-old leaves of the wild type and fzl-Ler grown at 22 °C or 28 °C (A) and at a light intensity of 100 µE or 50 µE (B). Chloroplast morphology of mesophyll cells of 3-week-old wild-type or fzl-Ler plants grown at 22 °C or 28 °C (C) and at a light intensity of 100 µE or 50 µE (D). Bars=50 µm.
Effect of the genetic background on the fzl phenotype
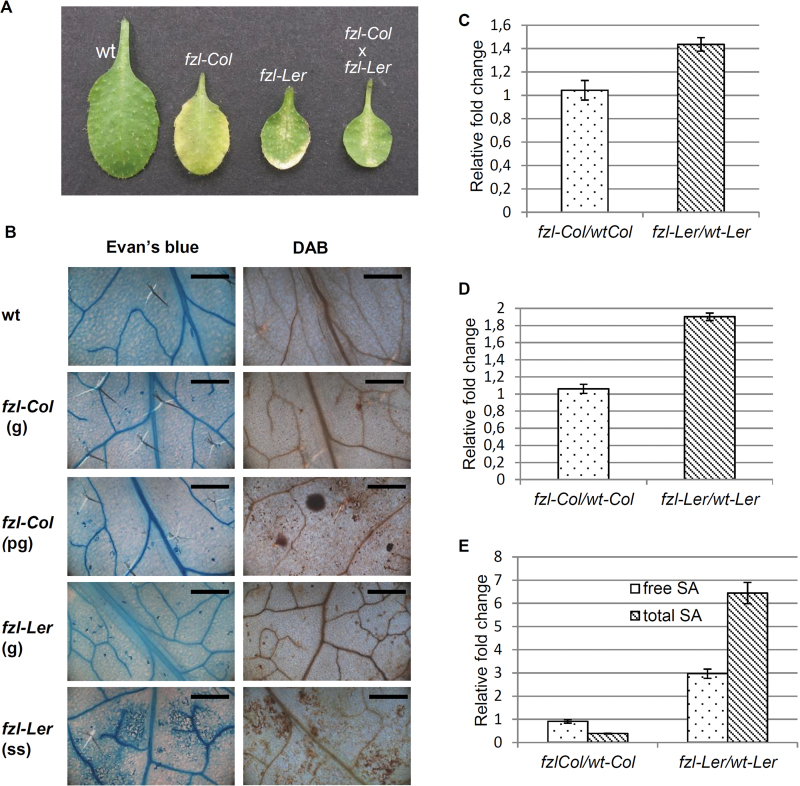
To understand the different effects of the fzl mutation on the Columbia/Landsberg ecotypes, the phenotype (Fig. 6A), the accumulation of the biochemical markers associated with HR (Fig. 6B–D), and the levels of free and total SA in fzl-Col mutants versus fzl-Ler mutants (Fig 6E) were compared.
Fig. 6.
Comparison of the fzl mutant phenotype in the two ecotypes Columbia (fzl-Col) and Landsberg (fzl-Ler). (A) Phenotype of 3-week-old leaves, from left to right: wild type, fzl-Col, fzl-Ler, and F1 progeny obtained by crossing fzl-Col×fzl-Ler. (B) Evan’s blue and DAB staining on 3-week-old leaves of the wild type, fzl-Col, and fzl-Ler. g, green leaves; pg, pale green leaves; ss, leaves with small spots. Bars=500 µm. (C) Cell death quantification by electrolyte leakage measurement. Data reported refer to the time point 30min, but a similar trend was observed at the successive time points analysed (data not shown). Values are expressed as fold change relative to the wild type and are the mean of three replicates. Bars represent the standard error. (D) H2O2 content quantified as fold change relative to the wild type. Values are the mean of three replicates. Bars represent the standard error. (E) free and total SA measurement. Values are the mean of three replicates. Bars represent the standard error.
The histochemical results showed that while in wild type leaf, H2O2 and dead cells are never present, in fzl-Col mutants these markers can be detected, and their level increased with the severity of the phenotype, even if the higher levels displayed in fzl-Ler mutants are never reached in fzl-Col mutants (Fig. 6B).
The quantification assays of H2O2 and cell death in fzl mutants confirmed the high level of both in the fzl-Ler mutant (80% and 40% higher, respectively, than in the relative wild type) while only a very small or no increase in these parameters was observed for the fzl-Col mutant in comparison with the control. A similar result was obtained with free and total SA quantification: while the fzl-Ler mutant accumulated a higher level (3- and 6-fold, respectively) of these molecules in comparison with the wild type, no difference (free SA) or a decrease (total SA) were detected when comparing the fzl-Col mutant with the wild type.
The fzl-Ler mutant was also crossed with fzl-Col mutants, and it was found that in the F1, the mutant phenotype was characterized by the presence of chlorotic spots very similar to those shown by the fzl-Ler mutant (Fig. 6A).
Analysis of double mutants
To check which among the signalling pathways known to be involved in HR regulation and execution are required for the determination of the fzl-Ler mutant phenotype, the fzl-Ler mutant was crossed with mutants altered in these pathways.
The fzl-Ler mutant was in the Ler background while all the other signalling mutants used in this analysis were in the Col background, but it had already been verified that the fzl-Ler mutation was still perfectly recognizable in the mixed background Ler/Col when the segregant population for the positional cloning of the fzl-Ler mutation was generated. Double mutants, single mutants, and the wild type were then compared by the analysis of the phenotype (Fig. 7) and the expression level of some defence-related genes previously used to characterize the single mutant fzl-Ler (Fig. 8).
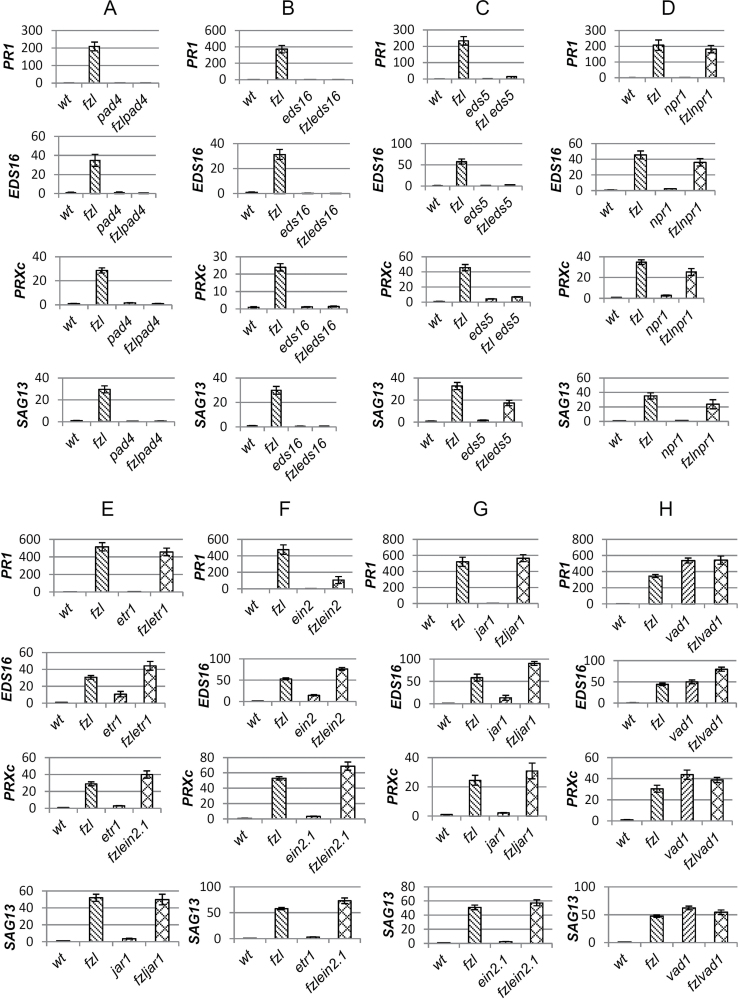
Fig. 7.
Phenotypic analysis of 3- to 4-week-old double mutants obtained by crossing fzl-Ler with mutants in the signalling pathways activated in HR. The Ler ecotype is used as the wild type.
Fig. 8.
Expression analysis by real-time RT-PCR of a group of defence-associated genes in 3- to 4-week-old wild-type, fzl-Ler, single mutant, and double mutant plants whose phenotype is represented in Fig. 7. The Ler ecotype is used as the wild type. On the y-axis is represented the relative expression level of the genes analysed using Tsβ1 as the endogenous control. Bars represent the standard errors of measurements performed in triplicate.
The double mutant fzl-Ler/pad4 showed no lesion formation; its size was comparable with that of the single pad4 mutant (Fig. 7A), and no induction of the defence-associated genes analysed was detected (Fig. 8A), suggesting a role for PAD4 (Wiermer et al., 2005) in both the defence response and cell death activation.
To determine the role of SA in lesion formation and defence response activation, fzl-Ler plants were crossed with the two mutants eds16 and eds5 (Wildermuth et al., 2001; Nawrath et al., 2002). In fzl-Ler/eds16 plants, the fzl-Ler phenotype was completely suppressed (Fig. 7B) and the expression of defence-related genes was not induced (Fig. 8B), while in fzl-Ler/eds5 plants the lesion formation was only delayed, the lesion size reduced (Fig. 7C), and the defence-related genes were induced to a level similar to that observed in the single fzl-Ler mutant (Fig. 8C).
Under standard growth conditions, the fzl-Ler/npr1 mutant showed a wild-type phenotype (Fig. 7D) (even though sometimes in suboptimal environmental conditions, such as high temperature and low water availability, some lesions appeared), and expression analysis showed enhanced expression of defence-related genes (Fig. 8D).
To check the involvement of the ethylene–JA pathways in fzl-Ler lesion formation, the double mutants fzl-Ler/etr1, fzl-Ler/ein2, and fzl-Ler/jar1 were generated. In all these double mutants, the timing of the appearance and the development of the lesions was similar to that observed in the single mutant fzl-Ler (Fig. 7E–G), and the expression level of defence-associated genes was the same as that shown by the fzl-Ler single mutant (Fig. 8E–G).
The fzl-Ler mutant was also crossed with the propagative LMM vad1 (vascular associated death1) (Lorrain et al., 2004; Bouchez et al., 2007) to analyse the effect of the interaction of two different LMMs. In fzl-Ler/vad1 plants, the mutant phenotype was more severe than that in the two single mutants, the lesions appeared earlier and their propagation was more rapid (Fig. 7H), while the expression level of the defence-associated genes was similar in the two single and in the double mutant (Fig. 8H).
Discussion
The characterization of an Arabidopsis mutant which displayed chlorotic lesions on rosette leaves and reduced size caused by a single recessive mutation in the FZL gene, encoding a GTPase involved in the determination of thylakoid and chloroplast morphology (Gao et al., 2006), is reported. This mutant was isolated in the Ler ecotype and, because of the different phenotype shown by the previously described fzl insertional mutants, isolated in the Col background (Gao et al., 2006), the mutant was named fzl-Ler.
Histochemical analysis showed the presence, in and around the lesions, of the biochemical markers typically associated with the activation of the defence programme, and expression analysis revealed the constitutive activation of genes known to be markers of HR. These traits are generally associated with the LMMs previously described in the literature (reviewed by Lorrain et al., 2003), so it is suggested that fzl-Ler is a member of this class of mutants.
The analysis of double mutants showed that the loss of EDS16, EDS5, and PAD4 functions resulted in the reduction/absence of both the lesions and the defence programme activation, suggesting a central role for SA in the HR cell death process.
The absence of lesions and the high level of expression of the HR marker genes in the double mutant fzl-Ler/npr1 suggested that in the fzl-Ler mutant cell death activation is NPR1 dependent, while an NPR1-independent pathway is involved in defence gene activation. A dual role for NPR1 has also been reported previously for the mutant vad1 (Lorrain et al., 2004); in this case, NPR1 function was required for defence activation but not for cell death.
The fzl-Ler mutant was crossed not only with mutants altered in defence signalling pathways but also with the propagative LMM vad1 (Lorrain et al., 2004; Bouchez et al., 2007), with the aim of checking the existence of cross-talk between the signalling pathways activated in these two mutants that look very similar in some aspects. Both are propagative LMMs, and both require SA and ROS in the signalling cascade leading to the mutant phenotype. However, for other traits, they look the opposite of one another: vad1 lesions are associated with the vascular system, whereas the fzl-Ler lesions are close to the veins that however are never affected by cell death; and vad1 phenotypes require the activation of the ethylene pathway, while fzl-Ler phenotypes are completely independent from this signalling.
The two mutations fzl-Ler and vad1 resulted in the additive phenotype of the double mutant fzl-Ler/vad1, thus suggesting that the two single mutants are altered in different signalling pathways, but are acting additively in the double mutant to activate defence responses and HR cell death.
It has been previously hypothesized that some LMMs may derive from a metabolic imbalance, as in the case of organelle malfunctioning (Mur et al., 2008). In the case of the fzl-Ler mutant, it was shown that a mutation causing the alteration of chloroplast morphology is linked to an LMM phenotype. More specifically, in the fzl-Ler mutant, a branch of the HR signalling pathway is constitutively activated: this is SA/ROS dependent, requiring the genes EDS5, EDS16, PAD4, and NPR1, cross-talking with the senescence signalling, and is independent from the ethylene–JA pathway.
Since the first reports on LMMs (Walbot et al., 1983), but also recently (Yamaguchi et al., 2012; Wituszynska et al., 2013), it has been highlighted that not only environmental conditions (temperature, light, humidity, etc.) but also the genetic background is an important factor influencing the LMM phenotype. It is therefore not surprising that the fzl mutation in the Columbia ecotype was reported to cause pale leaves and delayed flowering (Gao et al., 2006) while it was found that in the Ler background the loss of function of the FZL gene determined a typical LMM phenotype.
The observation that the phenotype displayed by the fzl-Ler mutant partially recovered by high temperature or low light treatments was very similar to the fzl-Col phenotype, suggested that the fzl-Col mutant could be seen as the ‘mild’ version of the typical LMM phenotype shown by the fzl-Ler. This difference can be due to both the different type of mutation present in the FZL gene in the two backgrounds and to the natural variation existing among these ecotypes that has been previously reported to account for differences in the lipid composition of thylakoid membranes (Yin et al., 2012), ROS-scavenging activities (Nagata et al., 2003), R genes (Tahir et al., 2013), and susceptibility to, and symptoms after, bacterial (Buell and Sommerville, 1997; Godiard et al., 2003), fungal (Denby et al., 2004; Chen et al., 2006; Birker et al., 2009), and viral infections (Kaneko et al., 2004; Sicard et al., 2008).
For a more complete dissection of the effect of the genetic background on the LMM phenotype it will also be interesting to analyse the effect of temperature and light intensity on fzl-Col mutants.
DAB staining showed H2O2 accumulation in fzl-Ler green leaves, at the sites where subsequently the lesions will appear, thus suggesting a central role for ROS signalling in cell death initiation in fzl-Ler mutants. In wild type non-stressed cells, ROS are produced as normal by-products of aerobic metabolism; to prevent ROS accumulation and the consequent oxidative cell damage, the equilibrium between ROS production and scavenging is strictly regulated (Apel and Hirt, 2004).
Moreover it is known that during senescence the tightly regulated chloroplast dismantling process has the function of avoiding the release of the potentially phototoxic chlorophyll, thus suggesting that in plants, cell death can be regulated through the control of chloroplast integrity (Gray et al., 2002).
Stress conditions, both biotic and abiotic, cause increased ROS production (Møller and Sweetlove, 2010) and after pathogen attack their role in the defence response is due not only to their effect as anti-microbial compounds but also to their role as signalling molecules leading to HR (Jabs et al., 1996).
A mechanism for chloroplast generation of ROS during HR is presented in the model proposed by Zurbriggen and collaborators, in which chloroplasts are the initial source of ROS after pathogen attack through the shutdown of electron utilization in the chloroplast stroma, determining the over-reduction of the photosynthetic electron transport chain and EEE in the thylakoids: the signalling then is spread, by the activation of NADPH oxidases, to the apoplast and to the adjacent cells, leading to HR cell death (Zurbriggen et al., 2009, 2010).
Alterations in both biosynthesis and breakdown of chlorophyll pathways have been reported to generate the accumulation of phototoxic intermediates, resulting in light-dependent lesion mimic phenotypes (Kruse et al., 1995; Hu et al., 1998; Ishikawa et al., 2001; Mach et al., 2001; Pružinská et al., 2003). Interestingly, while mutations in the biosynthetic pathway generally result in the initiative lesion mimic phenotype (Hu et al., 1998; Ishikawa et al., 2001), mutations in the catabolic pathway are associated with propagative phenotypes (Mach et al., 2001; Pružinská et al., 2003).
The Arabidopsis propagative LMM acd1 and its maize orthologue lls1 are deficient in PAO (pheophorbide a oxygenase), the key enzyme of chlorophyll catabolism: these mutants accumulate the phototoxic chlorophyll catabolite pheide a that in a light-dependent manner allows the production of ROS, the presumed diffusible signal responsible for the spread of the lesions (Pružinská, et al., 2003). The first morphological alteration reported in lls1 mutants is the loss of structural integrity of chloroplast and thylakoid membranes in mesophyll cells (Gray et al., 2002), which, by causing the leakage of phototoxic chlorophyll intermediates, determine the propagative cell death (Pružinská et al., 2003).
However, alterations in chlorophyll metabolism cannot account for all the events of the release of phototoxic compounds from the chloroplast. Chlorophyll degradation occurs during all the phases of the life cycle of the plant for the normal turnover of chlorophyll, not only during senescence; furthermore, both biotic and abiotic stress can damage plant cells, resulting in chlorophyll release from the thylakoid membranes (Takamiya et al., 2000). Moreover, it is known that damaged chloroplasts not only activate a retrograde signalling to down-regulate the nuclear genes encoding the photosynthetic apparatus, but also, through the accumulation of 1O2 –, are able to trigger cell death signalling pathways (Galvez-Valdivieso and Mullineaux, 2010).
Two recent studies (Kim et al., 2012; Nomura et al., 2012) suggested a role for the chloroplast in the signalling cascade leading to PCD, while Noshi and colleagues demonstrated that chloroplastic H2O2 enhances the levels of SA and the response to SA (Noshi et al., 2012).
Recently it has also been reported that another mutation in a gene encoding a chloroplast membrane protein (AtLrgB) resulted in an LMM phenotype (Yamaguchi et al., 2012; Yang et al., 2012). The mutant phenotypes are completely rescued when the plants are grown under continuous light; thus, the authors suggested that the AtLrgB gene, in contrast to fzl-Ler, is not essential for chloroplast development (Yamaguchi et al., 2012).
The hypothesis from the present findings is that the loss of integrity of the chloroplast membrane system observed in the fzl-Ler mutant, determining the interruption of the electron transport chain and the release of chlorophyll phototoxic intermediates/catabolites, might be responsible for the release of a ROS-based signalling, which, overlapping with the signalling generated by a pathogen attack, turned on the HR signalling cascade, resulting in the activation of defence programmes and cell death. This is in agreement with the observations that the fzl-Ler phenotype is partially light dependent, as reported for other propagative LMMs in which the HR signalling is triggered by the photoactivation of chlorophyll catabolites (Mach et al., 2001; Pružinská et al., 2003), and may be partially reversed by high temperatures, known to down-regulate ROS production by the activation of scavenging enzymes during HR (Kiraly et al., 2008).
Further work will be focused on the identification of the chloroplast-derived signal generated in fzl-Ler mutants and on its specific role in HR cascade activation.
The fzl-Ler mutation also appears to be a useful tool with potential to unravel the mechanisms underlying two agronomic traits of fundamental importance in breeding programmes aimed at enhancing plant productivity, namely pathogen resistance and the control of the timing of leaf senescence.
Supplementary data
Supplementary data are available at JXB online.
Table S1. Oligonucleotides used for RT–PCR analysis.
Table S2. Oligonucleotides used for real-tme RT–PCR analysis.
References
- Aarts N, Metz M, Holub E, Staskawicz BJ, Daniels MJ, Parker JE. 1998. Different requirements for EDS1 and NDR1 by disease resistance genes define at least two R gene-mediated signalling pathways in Arabidopsis . Proceedings of the National Academy of Sciences, USA 95, 10306–10311 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alonso JM, Stepanova AN, Leisse TJ, Kim CJ, Chen H, Shinn P, Stevenson DK, Zimmerman J, Barajas P, Cheuk R, Gadrinab C, Heller C, Jeske A, Koesema E, Meyers CC, Parker H, Prednis L, Ansari Y, Choy N, Deen H, Geralt M, Hazari N, Hom E, Karnes M, Mulholland C, Ndubaku R, Schmidt I, Guzman P, Aguilar-Henonin L, Schmid M, Weigel D, Carter DE, Marchand T, Risseeuw E, Brogden D, Zeko A, Crosby WL, Berry CC, Ecker JR. 2003. Genome-wide insertional mutagenesis of Arabidopsis thaliana . Science 301, 653–657 [DOI] [PubMed] [Google Scholar]
- Apel K, Hirt H. 2004. Reactive oxygen species: metabolism, oxidative stress, and signal transduction. Annual Review of Plant Biology 55, 373–399 [DOI] [PubMed] [Google Scholar]
- Birker D, Heidrich K, Takahara H, Narusaka M, Deslandes L, Narusaka Y, Reymond M, Parker JE, O’Connell R. 2009. A locus conferring resistance to Colletotrichum higginsianum is shared by four geographically distinct Arabidopsis accessions. The Plant Journal 60, 602–613 [DOI] [PubMed] [Google Scholar]
- Bouchez O, Huard C, Lorrain S, Roby D, Balagué C. 2007. Ethylene is one of the key elements for cell death and defence response control in the Arabidopsis lesion mimic mutant vad1 . Plant Physiology 145, 465–477 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brodersen P, Petersen M, Pike HM, Olszak B, Skov S, Ødum N, Jørgensen LB, Brown RE, Mundy J. 2002. Knockout of Arabidopsis ACCELERATED-CELL-DEATH11 encoding a sphingosine transfer protein causes activation of programmed cell death and defense. Genes and Development 16, 490–502 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown JWS. 1996. Arabidopsis intron mutations and pre-mRNA splicing. The Plant Journal 10, 771–780 [DOI] [PubMed] [Google Scholar]
- Buell CR, Somerville SC. 1997. Use of Arabidopsis recombinant inbred lines reveals a monogenic and a novel digenic resistance mechanism to Xanthomonas campestris pv campestris. The Plant Journal 12, 21–29 [DOI] [PubMed] [Google Scholar]
- Chen X, Steed A, Harden C, Nicholson P. 2006. Characterization of Arabidopsis thaliana–Fusarium graminearum interactions and identification of variation in resistance among ecotypes. Molecular Plant Pathology 7, 391–403 [DOI] [PubMed] [Google Scholar]
- Clough SJ, Bent AF. 1998. Floral dip: a simplified method for Agrobacterium-mediated transformation of Arabidopsis thaliana . The Plant Journal 16, 735–743 [DOI] [PubMed] [Google Scholar]
- Delledonne M, Xia YJ, Dixon RA, Lamb C. 1998. Nitric oxide function as a signal in plant disease resistance. Nature 394, 585–588 [DOI] [PubMed] [Google Scholar]
- Denby KJ, Kumar P, Kliebenstein DJ. 2004. Identification of Botrytis cinerea susceptibility loci in Arabidopsis thaliana. The Plant Journal 38, 473–486 [DOI] [PubMed] [Google Scholar]
- Devadas S K, Enyedi A, Raina R. 2002. The Arabidopsis hrl1 mutation reveals novel overlapping roles for salicylic acid, jasmonic acid and ethylene signalling in cell death and defence against pathogens. The Plant Journal 30, 467–480 [DOI] [PubMed] [Google Scholar]
- Dietrich RA, Delaney TP, Uknes SJ, Ward ER, Ryals JA, Dangl JL. 1994. Arabidopsis mutants simulating disease resistance response. Cell 20, 565–577 [DOI] [PubMed] [Google Scholar]
- Dong X. 1998. SA, JA, ethylene, and disease resistance in plants. Current Opinion in Plant Biology 1, 316–323 [DOI] [PubMed] [Google Scholar]
- Dong X. 2004. NPR1, all things considered. Current Opinion in Plant Biology 7, 547–552 [DOI] [PubMed] [Google Scholar]
- Ellis J, Dodds P, Pryor T. 2000. Structure, function and evolution of plant disease resistance genes. Current Opinion in Plant Biology 3, 278–284 [DOI] [PubMed] [Google Scholar]
- Fu ZQ, Dong X. 2013. Systemic acquired resistance: turning local infection into global defense. Annual Review of Plant Biology 64, 839–863 [DOI] [PubMed] [Google Scholar]
- Gaffney T, Friedrich L, Vernooij B, Negrotto D, Nye G, Uknes S, Ward E, Kessmann H, Ryals J. 1993. Requirement of salicylic acid for the induction of systemic acquired resistance. Science 261, 754–756 [DOI] [PubMed] [Google Scholar]
- Galvez-Valdivieso G, Mullineaux PM. 2010. The role of reactive oxygen species in signalling from chloroplasts and the nucleus. Physiologia Plantarum 138, 430–439 [DOI] [PubMed] [Google Scholar]
- Gao H, Sage TL, Osteryoung KW. 2006. FZL, an FZO-like protein in plants, is a determinant of thylakoid and chloroplast morphology. Proceedings of the National Academy of Sciences, USA 103, 6759–6764 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Godiard L, Sauviac L, Torii KU, Grenon O, Mangin B, Grimsley NH, Marco Y. 2003. ERECTA, an LRR receptor-like kinase protein controlling development pleiotropically affects resistance to bacterial wilt. The Plant Journal 36, 353–365 [DOI] [PubMed] [Google Scholar]
- Gray J, Janick-Buckner D, Buckner B, Close PS, Johal GS. 2002. Light-dependent death of maize lls1 cells is mediated by mature chloroplasts. Plant Physiology 130, 1894–1907 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Greenberg JT, Ausubel FM. 1993. Arabidopsis mutants compromised for the control of cellular damage during pathogenesis and aging. The Plant Journal 4, 327–341 [DOI] [PubMed] [Google Scholar]
- Greenberg JT, Guo A, Klessing DF, Ausubel FM. 1994. Programmed cell death in plants: a pathogen-triggered response activated coordinately with multiple defense functions. Cell 77, 551–563 [DOI] [PubMed] [Google Scholar]
- Greenberg JT, Silverman FP, Liang H. 2000. Uncoupling salicylic acid-dependent cell death and defense-related responses from disease resistance in the Arabidopsis mutant acd5. Genetics 156, 341–350 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hu G, Yalpani N, Briggs SP, Jonhal S. 1998. A porphyrin pathway impairment is responsible for the phenotype of a dominant disease lesion mimic mutant in maize. The Plant Cell 10, 1095–1105 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iriti M, Rabotti G, De Ascensao A, Faoro F. 2003. Benzothiadiazole-induced resistance modulates ozone tolerance. Journal of Agricultural and Food Chemistry 51, 4308–4314 [DOI] [PubMed] [Google Scholar]
- Ishikawa A, Okamoto H, Iwasaki Y, Ashai T. 2001. A deficiency in coproporphyrinogen III oxidase causes lesion formation in Arabidopsis. The Plant Journal 27, 89–99 [DOI] [PubMed] [Google Scholar]
- Jabs T, Dietrich RA, Dangl JL. 1996. Initiation of runaway cell death in an Arabidopsis mutant by extracellular superoxide. Science 273, 1853–1856 [DOI] [PubMed] [Google Scholar]
- Jones AM. 2001. Programmed cell death in development and defense. Plant Physiology 125, 94–97 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones DG, Dangl JL. 2006. The plant immune system. Nature 444, 323–329 [DOI] [PubMed] [Google Scholar]
- Kaneko YH, Inukai T, Suehiro N, Natsuaki T, Masuta C. 2004. Fine genetic mapping of the TuNI locus causing systemic veinal necrosis by turnip mosaic virus infection in Arabidopsis thaliana . Theoretical and Applied Genetics 10, 33–40 [DOI] [PubMed] [Google Scholar]
- Kim C, Meskauskiene R, Zhang S, Lee KP, Lakshmanan Ashok M, Blajecka K, Herrfurth C, Feussner I, Apel K. 2012. Chloroplasts of Arabidopsis are the source and a primary target of a plant-specific programmed cell death signaling pathway. The Plant Cell 24, 3026–39 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kiraly l, Hafez YM, Fodor J, Kiraly Z. 2008. Suppression of tobacco mosaic virus-induced hypersensitive-type necrotization in tobacco at high temperatures is associated with downregulation of NADPH oxidase and superoxide and stimulation of dehydroascorbate reductase. Journal of General Virology 89, 799–808 [DOI] [PubMed] [Google Scholar]
- Kruse E, Mock H-P, Grimm B. 1995. Reduction of coproporphyrinogen oxidase level by antisense RNA synthesis leads to deregulated gene expression of plastid proteins and affects the oxidative defense system. EMBO Journal 15, 3712–3720 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lander ES, Green P, Abrahamson J, Barlow A, Day MJ, Lincoln SE, Newberg L. 1987. Mapmaker: an interactive computer package for constructing primary genetic linkage maps of experimental and natural populations. Genetics 121, 174–181 [DOI] [PubMed] [Google Scholar]
- Landoni M, De Francesco A, Galbiati M, Tonelli C. 2010. A loss-of-function mutation in Calmodulin2 gene affects pollen germination in Arabidopsis thaliana . Plant Molecular Biology 74, 235–247 [DOI] [PubMed] [Google Scholar]
- Lam E, Kato N, Lawton M. 2001. Programmed cell death, mitochondria and the plant hypersensitive response. Nature 411, 548–853 [DOI] [PubMed] [Google Scholar]
- Lazzeri V, Calvenzani V, Petroni K, Tonelli C, Castagna A, Ranieri A. 2012. Carotenoid profiling and biosynthetic gene expression in flesh and peel of wild-type and hp-1 tomato fruit under UV-B depletion. Journal of Agricultural and Food Chemistry 60, 4960–4969 [DOI] [PubMed] [Google Scholar]
- Levine A, Pennell RI, Alvarez ME, Palmer R, Lamb C. 1996. Calcium-mediated apoptosis in a plant hypersensitive disease resistance response. Current Biology 6, 427–437 [DOI] [PubMed] [Google Scholar]
- Lorrain S, Vailleau F, Balagué C, Roby D. 2003. Lesion mimic mutants: keys for deciphering cell death and defence pathways in plants? Trends in Plant Science 8, 263–271 [DOI] [PubMed] [Google Scholar]
- Lorrain S, Lin B, Auriac MC, Kroj T, Saindrenan P, Nicole M, Balagué C, Roby D. 2004. VASCULAR ASSOCIATED DEATH1, a novel Gram domain-containing protein, is a regulator of cell death and defense responses in vascular tissues. The Plant Cell 16, 2217–2232 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lukowitz W, Gilmor CS, Schible WE. 2000. Positional cloning in Arabidopsis. Why it feels good to have a genome initiative working for you. Plant Physiology 123, 795–805 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mach JM, Castillo AR, Hoogstraten R, Greenberg JT. 2001. The Arabidopsis accelerated cell death gene ACD2 encodes red chlorophyll catabolite reductase and suppresses the spread of disease symptoms. Proceedings of the National Academy of Sciences, USA 98, 771–776 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maleck K, Levine A, Eulgem T, Morgan A, Schmid J, Lawton KA, Dangl JL, Dietrich RA. 2000. The transcriptome of Arabidopsis thaliana during systemic acquired resistance. Nature Genetics 26, 403–410 [DOI] [PubMed] [Google Scholar]
- Mateo A, Muhlenbock P, Rusterucci C, Chang CC, Miszalski Z, Karpinska B, Parker JE, Mullineaux PM, Karpinski S. 2004. LESION SIMULATING DISEASE 1 is required for acclimation to conditions that promote excess excitation energy. Plant Physiology 136, 2818–2830 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Møller IM, Sweetlove LJ. 2010. ROS signalling—specificity is required. Trends in Plant Science 15, 370–374 [DOI] [PubMed] [Google Scholar]
- Mur LA, Kenton P, Lloyd AJ, Ougham H, Prats E. 2008. The hypersensitive response; the centenary is upon us but how much do we know? Journal of Experimental Botany 59, 501–520 [DOI] [PubMed] [Google Scholar]
- Murgia I, de Pinto MC, Delledonne M, Soave C, De Gara L. 2004. Comparative effects of various nitric oxide donors on ferritin regulation, programmed cell death, and cell redox state in plant cells. Journal of Plant Physiology 161, 777–783 [DOI] [PubMed] [Google Scholar]
- Muthamilarasan M, Prasad M. 2013. Plant innate immunity: an updated insight into defence mechanism. Journal of Biosciences 38, 433–449 [DOI] [PubMed] [Google Scholar]
- Nagata T, Todoriki S, Masumizu T, Suda I, Furuta S, Du Z, Kikuchi S. 2003. Levels of active oxygen species are controlled by ascorbic acid and anthocyanin in Arabidopsis. Journal of Agricultural and Food Chemistry 51, 2992–2999 [DOI] [PubMed] [Google Scholar]
- Nawrath C, Heck S, Parinthawong N, Métraux J-P. 2002. EDS5, an essential component of salicylic acid-dependent signalling for disease resistance in Arabidopsis, is a member of the MATE transporter family. The Plant Cell 14, 275–286 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nomura H, Komori T, Uemura S, et al. 2012. Chloroplast-mediated activation of plant immune signalling in Arabidopsis. Nature Communications 3, 926. [DOI] [PubMed] [Google Scholar]
- Noshi M, Maruta T, Shigeoka S. 2012. Relationship beween chloroplastic H2O2 and the salicylic acid response. Plant Signaling and Behavior 7, 944–946 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ossowski S, Schneeberger K, Clark RM, Lanz C, Warthmann N, Weigel D. 2008. Sequencing of natural strains of Arabidopsis thaliana with short reads. Genome Research 18, 2024–2033 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pružinská A, Tanner G, Anders I, Roca M, Hörtensteiner S. 2003. Chlorophyll breakdown: pheophorbide a oxygenase is a Rieske-type iron–sulfur protein, encoded by the accelerated cell death 1 gene. Proceedings of the National Academy of Sciences, USA 100, 15259–15264 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pyke KA, Leech RM. 1991. Rapid image analysis screening procedure for identifying chloroplast number mutants in mesophyll cells of Arabidopsis thaliana (L.) Heiynh. Plant Physiology 96, 1193–1195 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Resnick JS, Wen CK, Shockey JA, Chang C. 2006. REVERSION-TO-ETHYLENE SENSITIVITY1, a conserved gene that regulates ethylene receptor function in Arabidopsis. Proceedings of the National Academy of Sciences, USA 103, 7917–7922 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roberts M, Tang S, Stallmann A, Dangl JL, Bonardi V. 2013. Genetic requirements for signaling from an autoactive plant NB-LRR intracellular innate immune receptor. PLoS Genetics 9, e1003465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schneeberger K, Ossowski S, Lanz C, Juul T, Petersen AH, Nielsen K L, Jørgensen J-E, Weigel D, Andersen SU. 2009. SHOREmap: simultaneous mapping and mutation identification by deep sequencing. Nature Methods 6, 550–551 [DOI] [PubMed] [Google Scholar]
- Shah J. 2003. The salicylic acid loop in plant defense. Current Opinion in Plant Biology 6, 365–371 [DOI] [PubMed] [Google Scholar]
- Shi H, Ye T, Chen F, Cheng Z, Wang Y, Yang P, Zhang Y, Chan Z. 2013. Manipulation of arginase expression modulated abiotic stress tolerance in Arabidopsis: effect on arginine metabolism and ROS accumulation. Journal of Experimental Botany 64, 1367–1379 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sicard O, Loudet O, Keurentjes JJ, Candresse T, Le Gall O, Revers F, Decroocq V. 2008. Identification of quantitative trait loci controlling symptom development during viral infection in Arabidopsis thaliana. Molecular Plant-Microbe Interactions 21, 198–207 [DOI] [PubMed] [Google Scholar]
- Stein M, Dittgen J, Sanchez-Rodriguez C, Hou BH, Molina A, Schulze-Lefert P, Lipka V, Somerville S. 2006. Arabidopsis PEN3/PDR8, an ATP binding cassette transporter, contributes to nonhost resistance to inappropriate pathogens that enter by direct penetration. The Plant Cell 18, 731–746 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sundaresan V, Springer P, Volpe T, Haward S, Jones JD, Dean C, Ma H, Martienssen R. 1995. Patterns of gene action in plant development revealed by enhancer trap and gene trap transposable elements. Genes and Development 9, 1797–1810 [DOI] [PubMed] [Google Scholar]
- Takamiya KI, Tsuchija T, Otha H. 2000. Degradation pathway(s) of chlorophyll: what has gene cloning revealed? Trends in Plant Science 5, 426–431 [DOI] [PubMed] [Google Scholar]
- Tahir J, Watanabe M, Jing HC, Hunter DA, Tohge T, Nun es-Nesi A, Brotman Y, Fernie AR, Hoefgen R, Dijkwel PP. 2013. Activation of R-mediated innate immunity and disease susceptibility is affected by mutations in a cytosolic O-acetylserine (thiol) lyase in Arabidopsis. The Plant Journal 73, 118–130 [DOI] [PubMed] [Google Scholar]
- Toiu A, Vlase L, Oniga I, Benedec D, Tãmaş M. 2011. HPLC analysis of salicylic derivatives from natural product. Farmacia 59, 106–112 [Google Scholar]
- Tsuda K, Katagiri F. 2010. Comparing signalling mechanisms engaged in pattern-triggered and effector-triggered immunity. Current Opinion in Plant Biology 13, 459–465 [DOI] [PubMed] [Google Scholar]
- van Doorn WG, Beers EP, Dangl JL, et al. 2011. Morphological classification of plant cell death. Cell Death and Differentiation 18, 1241–1246 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Loon LC, Rep M, Pieterse CMJ. 2006. Significance of inducible defense-related proteins in infected plants. Annual Review of Phytopathology 44, 135–162 [DOI] [PubMed] [Google Scholar]
- Walbot V, Hoisington DA, Neuffer MG. 1983. Disease lesion mimic mutations. In: Kosuge T, Meridith C, eds. Genetic engineering of plants. New York: Plenum Publishing Company, 431–442 [Google Scholar]
- Ward E, Uknes SJ, Williams SC, Dincher SS, Wiederhold DL, Alexander DC, Ahl-Goi P, Métraux J-P, Ryals JA. 1991. Coordinate gene activity in response to agents that induce systemic acquired resistance. The Plant Cell 3, 1085–1094 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wiermer M, Feys BJ, Parker JE. 2005. Plant immunity: the EDS1 regulatory node. Current Opinion in Plant Biology 8, 383–389 [DOI] [PubMed] [Google Scholar]
- Wildermuth MC, Dewdney J, Wu G, Ausubel FM. 2001. Isochorismate synthase is required to synthesize salicylic acid for plant defence. Nature 414, 562–565 [DOI] [PubMed] [Google Scholar]
- Wituszynska W, Slesak I, Vanderauwera S, et al. 2013. Lesion simulating disease1, enhanced disease susceptibility1, and phytoalexin deficient4 conditionally regulate cellular signaling homeostasis, photosynthesis, water use efficiency, and seed yield in Arabidopsis. Plant Physiology 161, 1795–805 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamaguchi M, Takechi K, Myouga F, Imura S, Sato H, Takio S, Shinozaki K, Takano H. 2012. Loss of the plastid envelope protein AtLrgB causes spontaneous chlorotic cell death in Arabidopsis thaliana . Plant and Cell Physiology 53, 125–134 [DOI] [PubMed] [Google Scholar]
- Yamasaki K, Motomura Y, Yagi Y, Nomura H, Kikuchi S, Nakai M, Shiina T. 2013. Chloroplast envelope localization of EDS5, an essential factor for salicylic acid biosynthesis in Arabidopsis thaliana. Plant Signalling and Behavior 8, e23603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang Y, Jin H, Chen Y, Lin W, Wang C, Chen Z, Han N, Bian H, Zhu M, Wang J. 2012. A chloroplast envelope membrane protein containing a putative LrgB domain related to the control of bacterial death and lysis is required for chloroplast development in Arabidopsis thaliana. New Phytologist 193, 81–95 [DOI] [PubMed] [Google Scholar]
- Yin L, Fristedt R, Herdean A, Solymosi K, Bertrand M, Andersson MX, Mamedov F, Vener AV, Schoefs B, Spetea C. 2012. Photosystem II function and dynamics in three widely used Arabidopsis thaliana accessions. PLoS One 7, e46206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zurbriggen MD, Carrillo N, Hajirezarei M-R. 2010. ROS signalling in the hypersensitive response. Plant Signaling and Behavior 5, 393–396 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zurbriggen MD, Carrillo N, Tognetti VB, Melzer M, Peisker M, Hause B, Hajirezarei M-R. 2009. Chloroplast-generated reactive oxygen species play a major role in localized cell death during the non-host interaction between tobacco and Xanthomonas campestris pv. vesicatoria . The Plant Journal 60, 962–973 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.