Abstract
Transcription factors are proposed as suitable targets for the control of traits such as yield or food quality in plants. This study reports the results of a functional genomics research effort that identified ATHB17, a transcription factor from the homeodomain-leucine zipper class II family, as a novel target for the enhancement of photosynthetic capacity. It was shown that ATHB17 is expressed natively in the root quiescent centre (QC) from Arabidopsis embryos and seedlings. Analysis of the functional composition of genes differentially expressed in the QC from a knockout mutant (athb17-1) compared with its wild-type sibling revealed the over-representation of genes involved in auxin stimulus, embryo development, axis polarity specification, and plastid-related processes. While no other phenotypes were observed in athb17-1 plants, overexpression of ATHB17 produced a number of phenotypes in Arabidopsis including enhanced chlorophyll content. Image analysis of isolated mesophyll cells of 35S::ATHB17 lines revealed an increase in the number of chloroplasts per unit cell size, which is probably due to an increase in the number of proplastids per meristematic cell. Leaf physiological measurements provided evidence of improved photosynthetic capacity in 35S::ATHB17 lines on a per unit leaf area basis. Estimates of the capacity for ribulose-1,5-bisphosphate-saturated and -limited photosynthesis were significantly higher in 35S::ATHB17 lines.
Key words: Chloroplast number, chloroplast size, homeodomain-leucine zipper, leaf development, photosynthetic capacity, transcription factor.
Introduction
Homeodomain-leucine zipper (HD-Zip) proteins fall into four classes (I–IV), and all contain a DNA-binding homeodomain coupled to a leucine zipper motif, which is thought to facilitate homo- or heterodimerization (Ariel et al., 2007; Ciarbelli et al., 2008; Elhiti and Stasolla, 2009; Harris et al., 2011). HD-Zip class II proteins are implicated in mediating the response of plants to a range of environmental conditions as well as in regulating developmental processes (Ariel et al., 2007; Ciarbelli et al., 2008; Elhiti and Stasolla, 2009; Harris et al., 2011; Ruberti et al., 2012). Five HD-Zip class II genes are known to respond to changes in light quality (Ciarbelli et al., 2008). For the remaining class II HD-Zip genes, little is known about their regulation other than that they are not responsive to changes in light quality (Ciarbelli et al., 2008). The light responsiveness of members of the γ subclass (ATHB2, ATHAT1, and ATHAT2) has been investigated the most thoroughly. For example, when grown in white light, ATHB2 expression is induced by shading (Steindler et al., 1997). ATHB2 and ATHAT1 may play roles in the shade-avoidance response, as their expression is induced by treatment with far-red-rich light, implicating a possible role for phytochrome in the regulation of these two genes (Ciarbelli et al., 2008).
Light quality and intensity can also affect plastid number. For example, in plants grown under high-intensity white light, chloroplast number increases with a relative decrease in plastid size (Butterfass, 1979). Chloroplast division is a complex process that involves multiple distinct steps, which are controlled by different nuclear genes (reviewed by Pyke, 1999; Osteryoung and McAndrew, 2001; Aldridge et al., 2005; Pogson and Albrecht, 2011). Higher-plant chloroplasts develop in young mesophyll cells from small plastid initials called proplastids. Proplastids are present in meristematic cells and divide in association with cell division. This division ensures that a constant proplastid number (about 14; Pyke, 1999) is maintained in all meristematic cells. As post-meristematic cells differentiate and expand, proplastids increase in size, develop into chloroplasts, and undergo approximately three rounds of division to produce the final complement of chloroplasts in a fully expanded mesophyll cell (Pyke, 1999; Aldridge et al., 2005).
Here, we present results obtained with ATHB17 (AT2G01430), which encodes a member of the α subclass of the class II HD-Zip protein family of transcription factors (Riechmann et al., 2000; Ariel et al., 2007; Ciarbelli et al., 2008; Elhiti and Stasolla, 2009; Harris et al., 2011). This study describes the expression pattern of ATHB17 and characterizes the phenotypes of ATHB17-overexpressing plants and athb17 knockout plants. The results describe the application of ATHB17 to regulate leaf morphology and chlorophyll content, the number and size of chloroplasts, and the capacity for photosynthesis in Arabidopsis leaves.
Materials and methods
Plant material and growth conditions
ATHB17-overexpressing lines were created in the Arabidopsis thaliana Heynh. ecotype Col-0. The ATHB17 (AT2G01430) clone was PCR amplified from a diversified cDNA library created using mRNA from different Arabidopsis tissues. This clone contained an isoleucine substituted for methionine at position 227 and also contained 64bp of the native 5′-untranslated region (5′-UTR) immediately upstream of the start ATG and 116bp of the native 3′-UTR immediately downstream of the stop codon. Arabidopsis plants were transformed by the floral dip method (Bechtold et al., 2003) using Agrobacterium carrying a transformation construct containing a kanamycin resistance gene driven by the nopaline synthase (NOS) promoter and the ATHB17 clone downstream from the cauliflower mosaic virus 35S promoter. A control line generated by transforming Col-0 with an empty transformation construct was used to determine the effect of ATHB17 overexpression.
Between 20 and 40 independent primary transformants were isolated on selection medium and transplanted into soil. For bulk seed production, plants were grown under 24h light, in growth rooms (80–110 μmol m–2 s–1, 22 °C). Transformants were PCR genotyped to confirm that they harboured the correct transgene using forward (5′-GCAAGTGGATTGATGTGATATC-3′) and reverse (5′-TGAATTCTTCATCGTCTCCGTCTTC-3′) primers. The majority of primary transformants showed alterations in leaf shape and dark green coloration, and in some instances reductions in plant size and alterations in inflorescence morphology were apparent. Expression of ATHB17 mRNA transcripts was verified by reverse transcription-PCR (RT-PCR) on RNA extracted from leaves of 40-d-old plants (see below). Lines that showed overexpression of ATHB17 by RT-PCR versus the control line were selected and used in subsequent experiments.
For morphological analysis and photosynthetic measurements, seeds were surface sterilized and stratified at 4 °C for 3 d. Seeds were sown on 80% Murashige and Skoog (MS) medium plus vitamins, 1% sucrose and kanamycin (35mg l–1) and placed in a growth chamber (ATC 26, Controlled Environments; photosynthetic photon flux (PPF) of approximately 120–150 μmol m–2 s–1; 10 h:14h light:dark; 22 °C:19 °C day:night). After 6–10 d of growth on selection medium, seedlings were transplanted into autoclaved Promix soil and grown in an ATC 26growth chamber as above. After 1 week of growth on soil, plants were given weekly fertilizer treatments with 0.4 or 0.8g l–1 of Peter’s fertilizer. Plants were about 30–42 d old when used for experiments.
To examine the endogenous expression of ATHB17 transcripts by in situ RNA–RNA hybridization, seeds were prepared as above and germinated on solid 80% MS medium (80% MS plus vitamins, 0.3% sucrose, and 1% BactoAgar) with a 16 h:8h light:dark photoperiod for 6 d. Seedlings were then transferred to vertical plates and grown for an additional 6 d. Seedlings that were 12 d old were used for in situ analysis of shoot apices and roots. Soil-grown plants were used to analyse the later stages of Arabidopsis growth. Seeds were germinated directly in soil after 2 d of stratification in the dark at 4 °C. Plants were grown in a 16 h:8h light:dark photoperiod at 22 °C in a growth chamber. Inflorescence, floral meristems, and developing seeds were collected from 4–5-week-old plants.
For laser-capture microdissection, 11-d-old seedlings (grown as above for in situ analysis) from athb17-1 and its wild-type sibling were used for collection of specific root tissues.
RNA isolation and quantitative RT-PCR (qRT-PCR)
Expression of the ATHB17 mRNA transcripts in athb17 mutant and overexpressing plants was examined by qRT-PCR. RNA was isolated from frozen root tips or 14-day-old seedlings using a Qiagen RNeasy Plant Mini kit and reverse transcribed (Superscript II RT; Invitrogen) using a mixture of oligo(dT) and random hexamer primers (60 and 40% respectively). cDNA templates were then analysed by real-time PCR (7900 HT Fast Real-Time PCR; Applied Biosystems) using a SYBR Green PCR Mix (Applied Biosystems).
Primers used for the RT-PCR were: ATHB17 transformation construct (5′-TTCGTGTAAATACTAAGAGACTCTGTTCCG-3′ and 5′-TGCCATAATACTCAAACTCAGTAGGA-3′), ATHB17 native expression (5′-AGGTGGTTTGGTTCATTAACGGAAG-3′ and 5′-GCAGCGAGGACACATAGTAAGGC-3′), ATHB2 (5′-ACATG AGCCCACCCACTACTTTGAC-3′ and 5′-CAGGAGCCCACGC ATTGACC-3′), ATHAT2 (5′-AGCACAATACTCTCAATCCCA-3′ and 5′-CTGCTTTAACTTTGTCCTTGCTC3′), ATHAT1 (5′-GAA CACAACACTCTCAATCCC-3′ and 5′-TTCAAGTATTCGCAAT CTACCTCC-3′) and a mid-level constitutive control gene AT2G32170 (5′-GCAGGAATAACAGAAGGTTTCTCTATGTGT-3′ and 5′-AT GAGCAGTGTCGATGAAAAAGCA-3′; Czechowski et al., 2005). Expression levels were calculated based on ΔC t, the difference in cycle threshold (C t) between the gene of interest and the control, with signals normalized to AT2G32170, a mid-level constitutive control gene.
Protoplast-based transcriptional repression assay
The cDNA sequence encoding the ATHB17 protein was cloned downstream of the cauliflower mosaic virus 35S promoter and a translational enhancer from the 5′-leader of tobacco mosaic virus (Skuzeski et al., 1990). All promoter sequences were PCR amplified and cloned upstream of the GUS reporter gene. The Agrobacterium NOS transcriptional terminator was used in all reporter and effector plasmids (Skuzeski et al., 1990). The sequence length of each promoter was as follows: ATHAT1 (1821bp), ATHB2 (1500bp), and AGL8 (1833bp). The nucleotide position of each promoter was the number of bases upstream of the translation start site of the respective genes. The 35S::CAT reporter gene construct encoding chloramphenicol acetyltransferase (CAT) has been described previously (Tiwari et al., 2006).
Protoplasts were prepared from leaves of 3–4-week-old Arabidopsis plants using cellulase (Research Products International) and macerozyme (SERVA Electrophoresis; Tiwari et al., 2006). For the assay, 200 μl of protoplast cells was co-transfected with 10 μg of the ATHAT1::GUS, ATHB2::GUS, or AGL8::GUS reporter gene construct and either 10 μg of the 35S::CAT control construct or a 35S::ATHB17 construct. Plasmid DNA (EndoFree Plasmid Maxi; Qiagen) was introduced into protoplasts by incubating with 40% polyethylene glycol for 30min. After polyethylene glycol removal, protoplasts were kept in darkness at room temperature for 18–20h. Cells were lysed and incubated with 1mM 4-methylumbelliferyl-β-d-glucuronide (Gold Biotechnology) at 37 °C for 1h. β-Glucuronidase (GUS) activity was quantified by taking fluorescence measurements using a Synergy HT Microplate Reader (BioTek). GUS measurements for each construct were averaged (n=3).
In situ RNA–RNA hybridization
DNA templates were generated by PCR using gene-specific primers (ATHB17-forward, 5′-CTTCGATCCTAGCTCTTAAGAACC-3′, and ATHB17-reverse, 5′- GTAACCGATTCATGTCTAGCC-3′) with incorporation of a T7 promoter sequence (5′-CTAATACGACTCA CTATAGGG-3′) on one primer (Baklanov et al., 1996; Iacobuzio-Donahue et al., 2002). To generate template for the sense and antisense probes, the T7 promoter sequence was incorporated at the 5′ end of ATHB17-forward and the 5′ end of ATHB17-reverse, respectively.
Digoxigenin (DIG)-labelled RNA probes were generated by in vitro transcription using T7 RNA polymerase (Life Technologies) except that a 1:2 ratio of UTP:DIG-11-UTP (Roche Applied Science) was used during the reaction. Fixation of tissues, preparation of sections, hybridization, and washes were carried out as described by Jeffrey Long (http://www.its.caltech.edu/~plantlab/protocols/insitu.pdf, last accessed 26 July 2013) with the following modifications: (i) RNAase treatment was omitted; (ii) the blocking reagent was 1% blocking reagent in 1× maleic acid buffer (Roche Applied Science); (iii) the wash buffer contained 1× maleic acid buffer with 0.03–0.05% Tween 20 (v/v) (Roche Applied Science); and (iv) sections were mounted using Immu-Mount (Thermo Scientific).
Preparation of paraffin sections and laser-capture microdissection
Primary and lateral root tips from 11-d-old seedlings were used for the collection of specific root tissues. Preparation of paraffin-embedded tissues (Kerk et al., 2003; Jiao et al., 2009) was performed with minor modifications. Specifically, root tips were fixed in ice-cold fixative (70% ethanol, 15% acetic acid, 0.6% trehalose) for approximately 3h, and then treated twice with 75% alcohol for 30min each and 90% alcohol for 45min. Stepwise infiltration of paraffin was done with a TP1020 automatic tissue processor (Leica Microsystems): 100% ethanol for 1h, 0.2% Eosin Y (in 100% ethanol) for 30min, 100% ethanol for 2h, ethanol/Histo-Clear series (3:1, 1:1, 1:3, 0:1, 0:1, 1–2h each), and paraffin (for 4 and 8h with vacuum). Paraffin sections (8 μm) were prepared as described previously (Jiao et al., 2009).
Paraffin sections were deparaffinized in Histo-Clear and air dried before slides were viewed using the fluorescence mode and the rhodamine reflector of a PALM MicroBeam System (Carl Zeiss) to facilitate the identification of different cell types. The root cap, quiescent centre (QC), epidermis/endodermis/cortex, and stele tissues were collected into adhesive caps (Carl Zeiss). Three biological replicates, each pooled from 40–60 roots, were collected for each sample
Microarray analysis
Total RNA was prepared and amplified and cRNA was generated from microdissected tissues using commercial kits (PicroPure RNA, RiboAmp HS RNA, and Turbo Labeling Biotin kits, respectively; Life Technologies). Transcript profiling experiments were performed using a custom full-genome Arabidopsis Affymetrix GeneChip (mbiATh1a520184) microarray and analysed according to Holtan et al. (2011). Calculations for the over-representation analysis were performed according to Holtan et al. (2011).
The Affymetrix data files (.CEL), pre-processed profiles, and supplementary files described herein have been deposited at the National Center for Biotechnology Information (http://www.ncbi.nlm.nih.gov/geo, last accessed 26 July 2013) under GEO accession no. GSE48127.
Preparation and analysis of mesophyll cell suspensions for chloroplast counting
Chloroplasts were counted using Nomarski differential interference contrast optics (Leica DMR-HC; Leica Microsystems) in individual fixed mesophyll cells obtained by the maceration of prepared leaf tissue (Pyke and Leech, 1991).
Estimating biochemical limitations to light-saturated photosynthesis
The protocol of Long and Bernacchi (2003) guided the generation of plots of light-saturated photosynthesis (A sat) and the rate of linear electron transport through photosystem II (J PSII) to substomatal [CO2] (C i) for the youngest, fully expanded leaves of plants for a control line and two 35S::ATHB17 lines using an infra-red gas analyser with a modulated chlorophyll a fluorimeter (LI-6400 XT; Licor). Initial measurements were made at an atmospheric [CO2] (C a) of 400 μmol of CO2 mol–1, after 40min exposure to a PPF of 700 μmol m–2 s–1. After the initial measurement, further measurements were made after three stepwise decreases in C a to 150, 100, and 50 μmol of CO2 mol–1. After a second measurement at 400 μmol of CO2 mol–1, three final measurements were made after stepwise increases in C a to 600, 700, and 800 μmol of CO2 mol–1. Measurements for all three lines were made at the same leaf–air water vapour pressure deficit of about 1 kPa and relative humidity of about 50%.
The curve-fitting software of Sharkey et al. (2007) and kinetic constants described therein were used to make in vivo estimates of: (i) the maximum rate of ribulose-1,5-bisphosphate (RuBP)-saturated photosynthesis (V c,max), from the three measurements of A sat and C i made at the lowest C a levels; and (ii) the light-saturated capacity for RuBP regeneration, calculated and expressed in terms of electron flow required to support the modelled rate of A sat (J max), from the three highest Ca levels. Estimates of J PSII were estimated as the product of the operating efficiency of PSII and the fraction of incident PAR absorbed by PSII (Genty et al. 1989). The fraction of the 700 μmol m–2 s–1 of PAR incident upon the leaf in the leaf chamber that was transmitted through leaves was measured by pressing a quantum sensor (LI 190; Licor) to the centre of the abaxial side of the leaf. No measure of reflectance from the adaxial leaf surface was made; consequently absorption was calculated as 1 – transmission for the determination of J PSII. We assumed that 50% of incident PAR was absorbed by PSII for the purposes of calculating J PSII.
Chlorophyll assays
Chlorophyll content was measured using a SPAD 502 (Konica Minolta).
Data analysis
Mesophyll cell plan areas, chloroplast number per cell, and individual chloroplast plan areas were measured directly from the microscope image using ImageJ (Abramoff et al., 2004). Data from leaves were averaged to give a mean and standard error (SE). Significance was tested with a Student’s t-test and a standard linear regression model using R (version 2.2.0; R Development Core Team, 2007).
The hypothesis that 35S::ATHB17 plants had increased V c,max and J max compared with the empty-vector control plants was tested with a one-tailed Student’s t-test using Excel (Microsoft). Data analysis was performed on six replicate samples unless otherwise stated and an effect was described as statistically significant when P < 0.05.
Results and discussion
ATHB17 is expressed in the QC of the primary and lateral roots
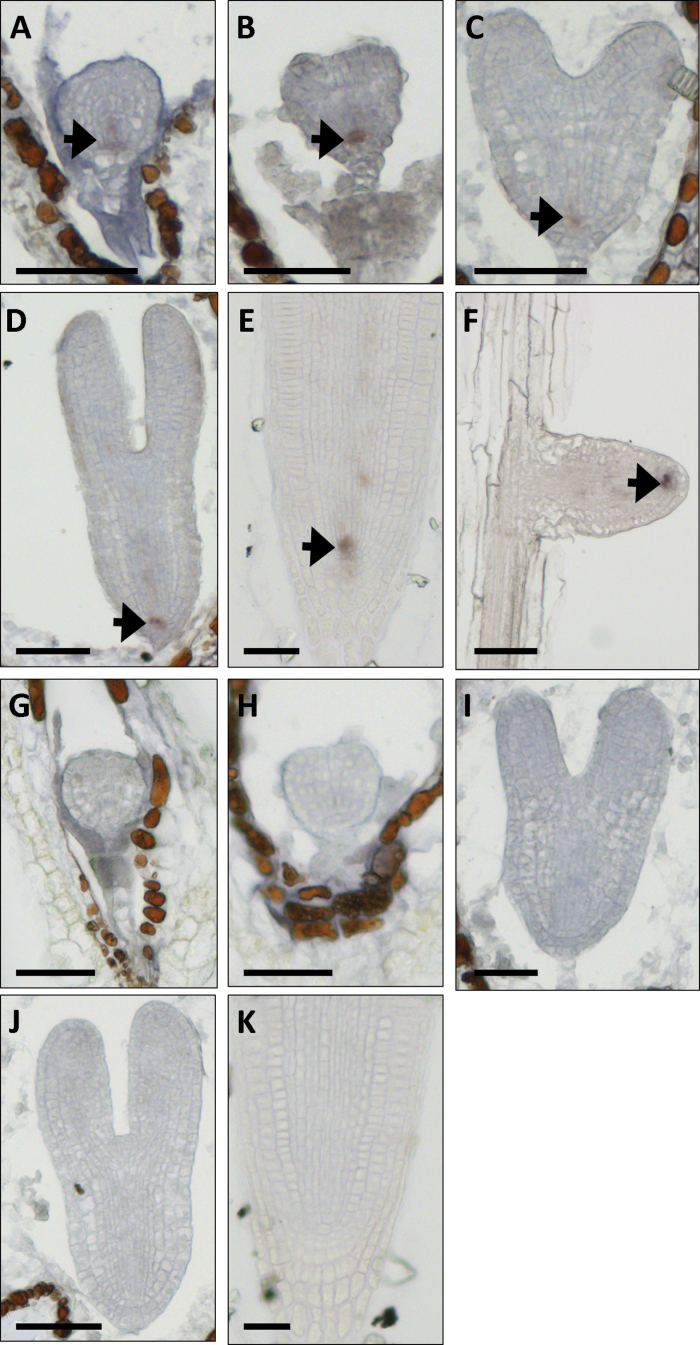
We analysed the spatial expression pattern of ATHB17 by in situ RNA–RNA hybridization (Fig. 1A–F) using DIG-labelled antisense and sense probes targeted to a unique region of the ATHB17 coding sequence (nt 173–353). Of the tissues examined (roots, shoot apical meristems, inflorescence meristems, floral meristems, and developing embryos), hybridization was only detected in specific cells in embryonic, primary, and lateral roots. In a survey across a developmental series, ATHB17 signal was first detected in the base of globular stage embryos, and was then restricted at the transition stage to the lens-shaped cell, which gives rise to the QC. In serial sections from older embryos and the primary root tip of seedlings (Fig. 1A–E) as well as the tips of lateral roots (Fig. 1F), ATHB17 expression continued to be localized specifically to the small number of cells that comprised the QC. No signal was detected using the control sense probe (Fig. 1G–K).
Fig. 1.
In situ hybridization using DIG-labelled antisense and sense probe targeting nt 173–353 of the ATHB17 coding sequence. (A–F) Antisense probe, (G–K) sense probe. (A–D, G–J) Embryos at the globular stage (A, G), transition stage (B, H), heart stage (C, I), and linear cotyledon stage (D, J). (E, K) Primary root tip. (F) Lateral root tip. In each panel, the region of greatest hybridization signal is highlighted with a black arrow. Bars, 50 μm.
The root is comprised of several tissues that undergo predictable divisions. Initials for all root tissues surround a group of four to seven mitotically less active cells known as the QC. The QC is important for proper root development and regulates processes and events in neighbouring cells for proper meristem establishment and maintenance (Doerner, 1998; Jiang and Feldman, 2005). The root QC-specific expression of ATHB17 implied that ATHB17 plays a role in the regulation of root growth and development.
Comparison of root QC transcript profiles between athb17-1 and its wild-type sibling
We screened T-DNA insertion lines (Alonso et al., 2003) and identified three different ATHB17 alleles (Supplementary Fig. S1 at JXB online). RT-PCR analysis indicated a significant reduction of the normal ATHB17 transcript in the line designated athb17-1, containing a T-DNA insertion of 162bp into the third intron of ATHB17 (Supplementary Fig. S2A at JXB online). Western blot analysis confirmed the absence of ATHB17 in the root tips of athb17-1, but not in athb17-2 and athb17-3 (Supplementary Fig. S2B). The athb17-1 line was used for further analysis.
We examined how ATHB17 regulates gene expression by transcript profiling of athb17-1 and its wild-type sibling. To increase our sensitivity to effects caused by the loss of ATHB17, we analysed tissue samples (collected by laser-capture microdissection) that were highly enriched with QC cells. To validate that we were able to enrich for QC cells by laser microdissection, a small panel of genes was selected and their transcript levels analysed by qRT-PCR across four distinct tissue types dissected from the root tip: QC, root cap, stele, and epidermis. The expression of known QC-localized genes [PLETHORA 1 (PLT1), WIP4, and ATMYB34; Aida et al., 2004; Nawy et al., 2005] was enriched in QC samples (Supplementary Fig. S3A at JXB online). On the other hand, genes known to be expressed in stele (AT5G25490; Nawy et al., 2005) or root cap tissue (ATMDK-20; del Campillo et al., 2004; Lilley et al., 2011) displayed stele- or root cap-enriched expression, respectively (Supplementary Fig. S3B, C).
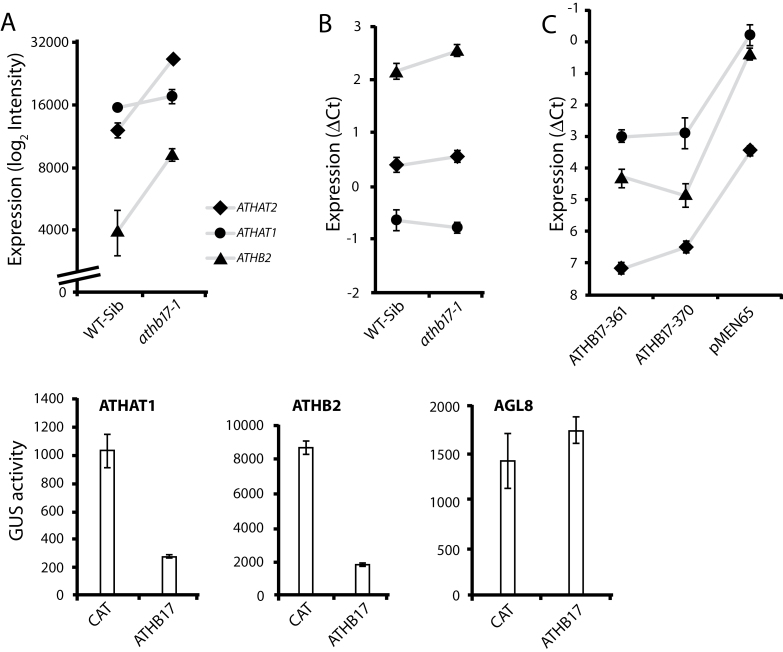
Of the three class II HD-Zip genes expressed in the root QC (Nawy et al., 2005), ATHB2 and ATHAT2 showed increased expression in the QC of athb17-1 roots relative to the wild-type control, while ATHAT1 did not (Fig. 2A). We confirmed the effect on ATHB2 and lack of effect on ATHAT1 by performing qRT-PCR using RNA isolated from whole root tips of athb17-1 and its wild-type sibling (Fig. 2B). The more subtle effect on ATHAT2 observed in the array experiments could not be confirmed in whole root tips. The array and qRT-PCR results suggested that ATHB17 functions to repress the expression of ATHB2 and ATHAT2 in the QC, or that ATHB17 may act to repress an activator of these genes. Indeed, in seedlings of 35S::ATHB17 lines in which ATHB17 was constitutively expressed at high levels, ATHB2, ATHAT1, and ATHAT2 were all strongly repressed (Fig. 2C). We tested whether ATHB17 could regulate the expression of ATHB2 and ATHAT1 using a transient expression assay. We co-transfected constructs encoding ATHB17 or CAT and pATHB2::GUS or pATHAT1:GUS reporter genes (Fig. 2D) into Arabidopsis mesophyll protoplasts. The pAGL8::GUS reporter gene was used as a non-specific reporter that was not expected to be regulated by ATHB17 protein. The transcription of the reporter gene driven by either class II HD-Zip promoter was strongly repressed in the presence of ATHB17. Thus, class II HD-Zip gene expression within the root QC was regulated by other class II HD-Zip proteins and may function as a negative regulatory loop in which homeostasis is mutually controlled (Ariel et al., 2007; Sorin et al., 2009; Harris et al., 2011). The specific expression of ATHB17 in the root QC suggested that ATHB17 may be involved in some aspects of root development. However, the roles that ATHB2, ATHAT1, ATHAT2, and ATHB17 play in affecting root growth are unknown at this time.
Fig. 2.
Expression levels of ATHAT2, ATHAT1, and ATHB2 in various Arabidopsis tissues. Microarray analysis indicated that the transcript levels of selected class II HB-Zip genes were altered in the QC-enriched samples of athb17-1 compared with its wild-type sibling. (A) ATHAT2 and ATHB2 expression, but not ATHAT1 expression, was induced in the athb17-1 QC. (B) qRT-PCR was used to verify the altered expression pattern observed in panel A. (C) ATHAT1, ATHAT2, and ATHB2 expression is significantly repressed in seedlings of two 35S::ATHB17 lines (line 361 and line 370). (D) ATHB17 also repressed the transcription of ATHAT1 and ATHB2 in Arabidopsis mesophyll protoplasts. Full-length ATHB17 or CAT was co-transfected with the pATHAT1::GUS or pATHB2::GUS reporter gene into Arabidopsis mesophyll protoplasts. The pAGL8::GUS reporter gene was used as a non-specific reporter as it was not expected to be regulated by ATHB17. The data are means ±standard deviation (SD) of three replicates. PCR comparisons were made after normalization to a constitutive control gene by the ΔCt method.
Several related biological processes are subtly perturbed in athb17-1
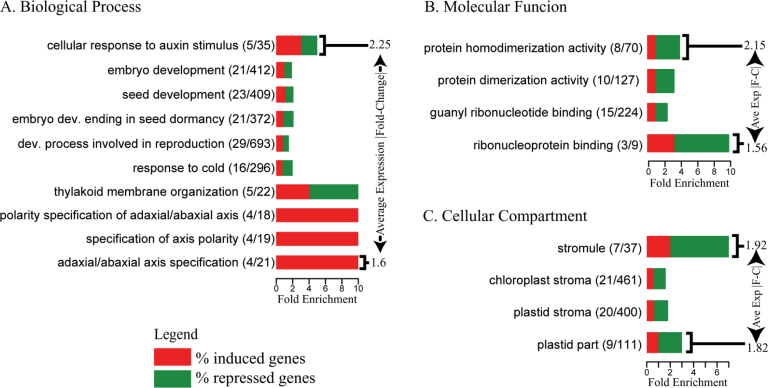
Gene set over-representation analysis using gene ontology (GO) terms was used to determine which biological processes and molecular functions showed the greatest differences between athb17-1 and its wild-type sibling (Fig. 3). Genes differentially expressed between athb17-1 and its wild-type sibling with annotation to biological processes that included responses to auxin stimulus, embryo development, and axis polarity were over-represented. Functional analyses indicate that some HD-Zip class II genes (ATHB2, ATHB4, and ATHAT2) are linked to auxin (Steindler et al., 1999; Sawa et al., 2002), consistent with our observations on the expression of class II HD-Zip genes in athb17-1 QC cells.
Fig. 3.
Functional category analysis of genes differentially expressed in the root QC of athb17-1 compared with the wild-type sibling line. Over-representation of terms from different aspects of GO, including (A) Biological Process, (B) Molecular Function, and (C) Cellular Compartment are shown separately. Based on analysis of all GO terms annotated to Arabidopsis genes (Berardini et al., 2004), the categories listed above were over-represented (P <0.01) among significantly mis-expressed genes. For each over-represented GO category, we have shown the number of genes with significantly altered expression and the total number of genes annotated to the term. The horizontal length of the bar indicates the ratio of the number of genes annotated to the term that have significant expression changes relative to the number of genes from that category that would have been expected to show such changes by chance, while the vertical length of the bar represents the magnitude of the average absolute fold change of these genes. The percentage of induced and repressed genes annotated to each GO term is indicated by red and green, respectively. See Supplementary Table S1 at JXB online for a list of genes over-represented in each of the GO categories.
The presence of genes in GO categories related to embryo or seed development and axis polarity (Fig. 3) was interesting considering that, while class II HD-Zips are implicated in the integration of shade-avoidance responses and hormone-mediated growth (Sawa et al., 2002; Sorin et al., 2009), class III HD-Zip proteins and KANADI are characterized as developmental regulators during embryogenesis, the establishment of the apical meristem, lateral root formation, and the determination of leaf polarity (Hawker and Bowman, 2004; Prigge et al., 2005; Izhaki and Bowman, 2007). The expression of PHAVOLUTA (AT1G30490), KANADI 2 (AT1G32240), and REVOLUTA (AT5G60690) was induced in the QC of athb17-1 roots (Supplementary Table S2 at JXB online). A common link between class II/III HD-Zip proteins and KANADI-related proteins is auxin. Auxin concentration and flux mediated by PIN-FORMED1 (PIN1) contribute to embryo patterning during embryogenesis and the establishment of the apical–basal axis and bilateral symmetry (Izhaki and Bowman, 2007).
A number of genes with GO terms specifying plastid-related processes and constituent parts including thylakoid membrane organization, stroma, stromule (Kohler and Hanson, 2000), and the term ‘plastid part’ were significantly over-represented, with more genes repressed than induced in the athb17-1 mutant.
A whole-plant phenotype comparison among wild-type, 35S::ATHB17, and athb17-1 Arabidopsis plants
The athb17-1 plants displayed no clear differences from wild-type controls when grown on plates to examine potential root phenotypes or when grown in soil to examine overall morphology and chlorophyll content (Supplementary Fig. S4 at JXB online), which suggested that ATHB17 is functionally redundant with other class II HD-Zip proteins in the root QC. The primary and branch root lengths as well as branch root number were similar in both athb17-1 and its wild-type sibling (Supplementary Fig. S4A–C). No detectable rosette phenotype was observed (Supplementary Fig. S4D, E). On the other hand, athb17-1 may produce a conditional phenotype or our experimental growth conditions may not have been sensitive enough to detect a discernible phenotype.
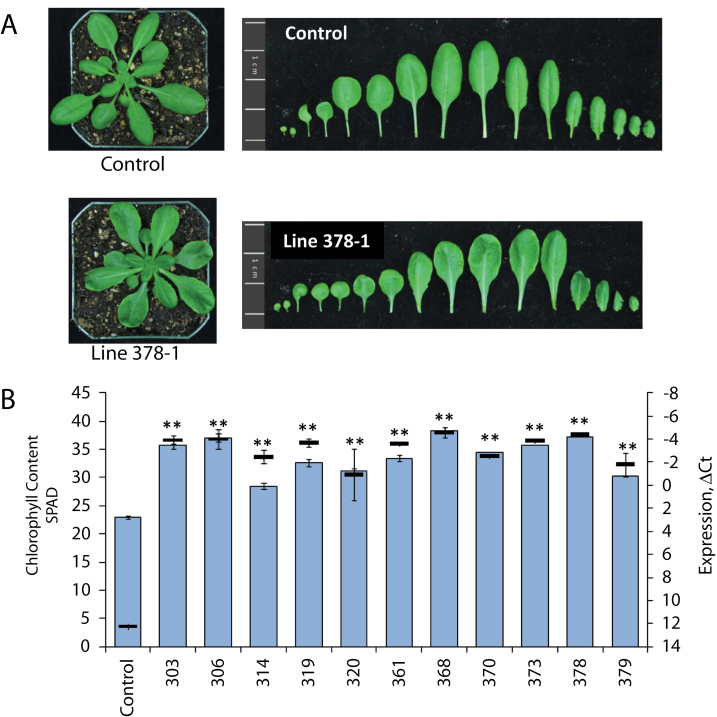
In contrast, overexpression of ATHB17 produced marked phenotypic effects; in particular, the leaves of the transgenic lines were consistently visibly darker green (Fig. 4A). A series of 35S::ATHB17 lines was examined for this phenotype; chlorophyll content as measured by soil plant analysis development (SPAD) was higher in all lines (Fig. 4B) and was positively correlated with ATHB17 mRNA levels. Chlorophyll levels in leaves as measured by SPAD from athb17-1 plants were comparable to those seen in empty-vector control leaves (Supplementary Fig. S4F). Leaf blade development in 35S::ATHB17 lines was also de-repressed along the petiole, creating a loss of distinct proximal and distal zones and resulting in a slight leaf blade on petiole phenotype that resulted in a rounder appearance (Fig. 4A; Ha et al., 2003; Hepworth et al., 2005). We have suggested that, within the root QC, ATHB17 with ATHB2, ATHAT1, and ATHAT2 form a negative regulatory loop. It has been widely demonstrated that the expression of class II HD-Zip genes is mutually regulated, in that when one class II HD-ZIP gene is overexpressed, the expression of other class II HD-Zip genes is repressed (Ohgishi et al., 2001; Sawa et al., 2002; Sorin et al., 2009; Harris et al., 2011). The phenotypes observed with constitutive overexpression of ATHB17 could result from repression of other class II HD-Zip genes or other native targets during embryo development and growth during the seedling and rosette stages. The slight blade on petiole phenotype (Ha et al., 2003; Hepworth et al., 2005) suggested that subtle alterations in leaf development are caused by ATHB17 overexpression. Leaf pattern formation is dependent on the establishment of the abaxial/adaxial axis because loss of either abaxial or adaxial identity perturbs leaf blade outgrowth and symmetry. Consequently, some leaf phenotypes in 35S::ATHB17 plants may result from perturbations in abaxial/adaxial polarity mediated by effects on auxin transport/signalling or the disruption of a complex regulatory network consisting of various class II and class III HD-Zips plus KANADI-like proteins (Harris et al., 2011; Brandt et al., 2012).
Fig. 4.
(A) Plants (30 d old) grown under short-day conditions illustrating that the transgenic plants (T3) had leaves that were darker green, and rounder with a slight leaf blade on petiole phenotype.(B) A high correlation was found between chlorophyll content determined by SPAD (blue) and ATHB17 transgene expression (black, shown relative to the constitutive control gene AT2G32170) was demonstrated across 11 T2 35S::ATHB17 lines. Results are shown as the mean±SE (n=20). **P <0.05.
Chloroplast and cell characteristics of 35S::ATHB17 plants
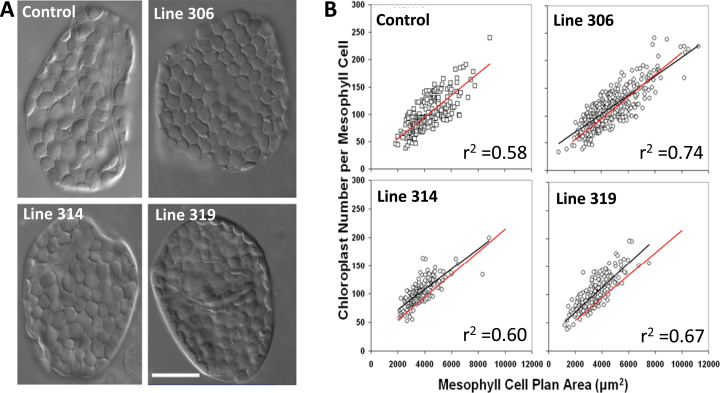
Representative cellular phenotypes of typical mature leaf mesophyll cells of an empty-vector control line and three 35S::ATHB17 lines (306, 314, and 319; T2 generation) are illustrated in Fig. 5A. Control and 35S::ATHB17-overexpressing lines showed a close relationship between plastid number and mesophyll cell size (Fig. 5B). 35S::ATHB17 plants accumulated more plastids per unit cell area compared with the control line (Table 1). The distribution of chloroplast plan area in fully expanded mesophyll cells was also investigated and is shown in Table 1. 35S::ATHB17 lines exhibited a slight reduction in plastid plan area compared with the control line. The proportion of mesophyll surface area covered by chloroplasts was consistent between 35S::ATHB17-overexpressing lines and the control line (data not shown). This may result from compensation between chloroplast number and chloroplast size; i.e. 35S::ATHB17 mesophyll cells have more chloroplasts per cell than a wild-type control line, but the chloroplasts are somewhat smaller.
Fig. 5.
Isolated leaf mesophyll cells (A) and chloroplast number per mesophyll cell versus cell size (B) from the youngest fully expanded leaves of 40-d-old control or 35S::ATHB17 lines. (A) Mesophyll cells were viewed with Nomarski differential interference contrast optics. Bar, 25 μm. (B) Each data point represents the measurement from one cell. The solid red line in each figure represents the regression line for the empty-vector control line, while the solid black line is the regression of the transgenic in each panel. Values for r 2 are indicated on the graphs.
Table 1.
Mean chloroplast plan area, mean mesophyll plan area, and plastid number for populations of mesophyll cells from the youngest fully expanded leaves of 40-d-old control and 35S::ATHB17 Arabidopsis plantsChloroplast plan areas were measured by image analysis of fixed isolated cells from three 35S::ATHB17-overexpressing lines (T2 generation) and an empty-vector control line (pMEN65) in two independent experiments. Plastid number per cell (±SE) was determined from a regression line of chloroplast number per cell on mesophyll cell plan area using a value of 1000 μm2 for the mesophyll cell plan area (Fig. 5). The y-intercept of the regression line was used to estimate the number of proplastids (±SE) in very small, meristematic cells. For Experiments 1 and 2, mean chloroplast plan area is the mean ±SE of 600 chloroplasts from 30 or 60 different mesophyll cells, respectively. **P <0.05.
| Genotype | Mean chloroplast plan area (μm2) | Plastids | |
|---|---|---|---|
| No. chloroplasts per 1000 μm2 mesophyll cell plan area | Estimated number proplastids per meristematic cell | ||
| Experiment 1 | |||
| pMEN65 | 39.8±0.4 | 35±2 | 14±2 |
| 35S::ATHB17 line 306 | 35.9±0.3** | 51±1** | 33±1** |
| 35S::ATHB17 line 314 | 35.0±0.3** | 55±1** | 37±1** |
| 35S::ATHB17 line 319 | 38.3±0.4** | 48±1** | 25±1** |
| Experiment 2 | |||
| pMEN65 | 36.7±0.4 | 44±1 | 22±2 |
| 35S::ATHB17 line 306 | 32.7±0.3** | 70±1** | 48±1** |
| 35S::ATHB17 line 314 | 32.7±0.3** | 67±1** | 44±1** |
| 35S::ATHB17 line 319 | 30.5±0.4** | 57±1** | 34±1** |
Based on published data (Pyke and Leech, 1992; Pyke, 1997), the smallest mesophyll cells (500–800 μm2, representative of post-meristematic cells) of fully expanded mature leaves have approximately 10–20 chloroplasts. However, based on our regression analysis (Fig. 5B), the number of plastids in the smallest cells of 35S::ATHB17 plants was greater than that found in the youngest cells of an empty-vector control line. Therefore, the number of proplastids per meristematic cell is potentially greater in 35S::ATHB17 plants than in controls (Table 1). These data suggest that ATHB17 overexpression regulates proplastid division rather than chloroplast division.
Light-saturated photosynthetic capacity is increased in 35S::ATHB17 Arabidopsis plants
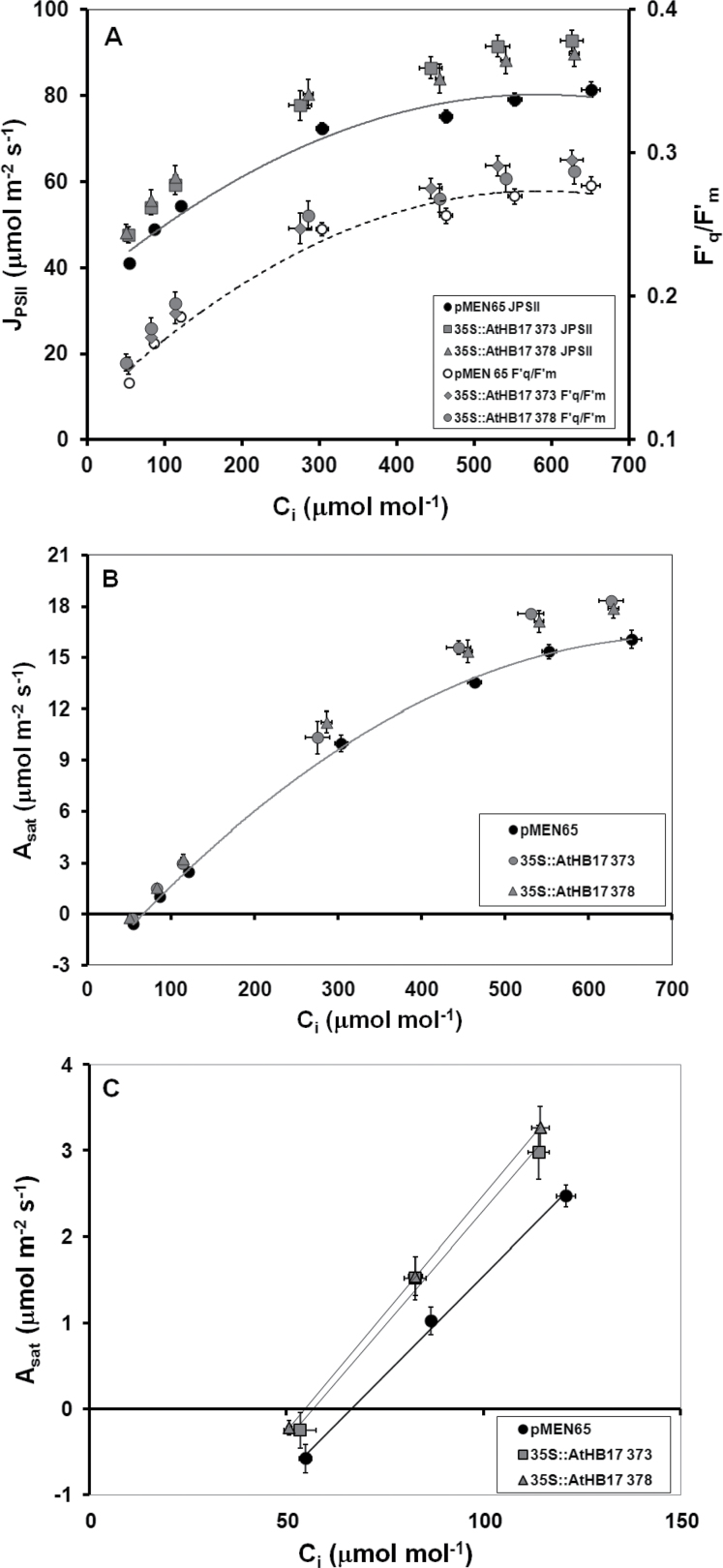
Two well-characterized 35S::ATHB17 lines (373-3 and 378-1; T3 generation) were studied to investigate whether increases in leaf chlorophyll content would impact photosynthesis in Arabidopsis. For both 35S::ATHB17 lines, leaf SPAD was significantly increased, by 36% relative to the control line (Table 2). Increased leaf chlorophyll was consistent with a significant 6% decrease in transmission of PAR through the leaves in the 35S::ATHB17 lines (Table 2). The decrease in transmission of PAR through the 35S::ATHB17 leaves was probably due to increased PAR absorption, an effect that could be reconciled with a significant increase in J PSII of up to 14% in the two 35S::ATHB17 lines, relative to the control line, over a range of C i (Fig. 6A). This increase in J PSII was largely the consequence of increased light absorption, with increases in the operating efficiency of PSII (F′q/F′m) in the 35S::ATHB17 lines typically being smaller over the same range of C i (Fig. 6A).
Table 2.
Key components of photosynthetic capacity are increased in 35S::ATHB17 linesSample means ±SE are shown for leaf SPAD, percentage transmission of PAR, and estimates of V c,max and J max. Data were collected on at least five replicate plants for the empty-vector control and two 35S::ATHB17 lines. The means of each data set collected for the two 35S::ATHB17 lines were significantly different from the control line (P <0.05; two-tailed Student’s t-test).
| Genotype | SPAD | Transmission (%) | V c,max | J max |
|---|---|---|---|---|
| pMEN65 | 31.2±0.5 | 12.7±0.5 | 28.8±1.1 | 80.4±2.3 |
| 35S::ATHB17 line 373-3 | 43.9±2.0 | 5.4±0.5 | 33.1±1.6 | 90.2±2.3 |
| 35S::ATHB17 line 378-1 | 42.4±1.4 | 5.8±0.4 | 33.6±2.1 | 87.5±2.7 |
Fig. 6.
Plot of J PSII and F′v/F′m (A) and A sat (B, C) against C i for select 38-d-old 35S::ATHB17 lines and an empty-vector control line (pMEN65). The data shown in (C) give an expanded view of the CO2-limited part of the larger A sat/C i response curve shown in (B). The photosynthetic phenotypes reported have proved to be highly penetrant across multiple 35S::ATHB17 lines, and the effects described were repeatable in multiple independent experiments. Results are shown as the mean±SE (n=6) from a representative experiment.
The plots of J PSII and A sat (Fig. 6B) against increasing C i provided evidence of increased photosynthetic capacity in both 35S::ATHB17 lines. A progressive stimulation of A sat relative to the control line was seen as C i increased in both 35S::ATHB17 lines (Fig. 6B). AT the three highest Ci values, both A sat and J PSII were significantly higher in the 35S::ATHB17 lines; however, while A sat was still rising in all lines, J PSII was reaching, or had reached, a plateau. Increasing A sat over a range of C i where J PSII is at a plateau is indicative of A sat being limited by the capacity to regenerate RuBP in the Calvin cycle (Long and Bernacchi, 2003). For both 35S::ATHB17 lines, J max estimated from these three data points was significantly higher than for the control line (Table 2). At the three lowest Ci values, A sat and J PSII increased linearly with increasing C i, observations indicative of A sat being limited by the activity of ribulose-1,5-bisphosphate carboxylase oxygenase (Rubisco; V c,max). For both 35S::ATHB17 lines, V c,max estimated from these three points was also significantly higher than in the control line (Fig. 6 and Table 2). Stomatal conductance, measured at ambient C a, was reduced by 17% in the 35S::ATHB17 373 line and by 9% in the 35S::ATHB17 378 line; however, neither effect was statistically significant (P >0.2; data not shown).
Much research effort is currently focused on increasing the C3 photosynthetic capacity. Increasing the maximum activation state of Rubisco, the capacity to regenerate RuBP in the Calvin cycle, and conductance of CO2 to the sites of carboxylation are all key components of the overall strategy (Long et al., 2006; Zhu et al., 2010; Raines, 2011). This study provides evidence that modulation of ATHB17 activity can increase the biochemical capacity for photosynthesis in Arabidopsis, and could improve leaf photosynthesis at current and predicted future atmospheric CO2 concentrations.
Conclusion
In the QC, ATHB17 appears to be part of a complex negative regulatory loop with other class II HD-Zip genes. Based on microarray data, this regulatory loop controls processes such as auxin transport, embryo development, and axis polarity. Constitutive overexpression of ATHB17, on the other hand, modulates leaf development, producing changes in leaf morphology, plastid number and size, and the capacity for photosynthesis. Such results underscore the utility of functional genomics research to identify candidates for the modification of complex traits in plants.
Supplementary data
Supplementary data are available at JXB online.
Supplementary Fig. S1. Loss-of-function mutants for ATHB17.
Supplementary Fig. S2. Expression levels of ATHB17 in the corresponding T-DNA insertion lines represented in Supplementary Fig. S1.
Supplementary Fig. S3. Quantitative RT-PCR analysis to analyse the differential expression of various genes using RNA isolated from the root tip, quiescent centre (QC), and stele.
Supplementary Fig. S4. When grown under our experimental conditions, athb17-1 does not produce a visible phenotype.
Supplementary Table S1. Genes found to be significantly regulated, where the Benjamini–Hochberg-corrected P value is less than 0.05 and the fold change is greater than 1.3, are shown.
Supplementary Table S2. Genes found to be significantly regulated, which underlie the over-representation presented in Fig. 3, are shown.
Acknowledgements
We thank the Arabidopsis Biological Resource Center (The Ohio State University) for providing the Salk T-DNA lines used this study.
Glossary
Abbreviations:
- Ct
cycle threshold
- Asat
rate of light-saturated photosynthesis
- Ca,
ambient [CO2]
- CAT
chloramphenicol acetyltransferase
- Ci
substomatal [CO2]
- DIG
digoxigenin
- GO
gene ontology
- GUS
β-glucuronidase
- HD-Zip
homeodomain-leucine zipper
- JPSII
rate of linear electron transport through photosystem II
- MS
Murashige and Skoog
- NOS
nopaline synthase
- PAR
photosynthetically active radiation
- PPF
photosynthetic photon flux
- QC
quiescent centre
- qRT-PCR
quantitative reverse transcription-PCR
- RuBP
ribulose-1,5-bisphosphate
- Rubisco
ribulose-1,5-bisphosphate carboxylase oxygenase
- SD
standard deviation
- SE
standard error
- SPAD
soil plant analysis development
- UTR
untranslated region
- Vc,max
the maximum rate of RuBP-saturated photosynthesis.
References
- Abramoff MD, Magelhaes PJ, Ram SJ. 2004. Image processing with ImageJ. Biophotonics International 11, 36–42 [Google Scholar]
- Aida M, Beis D, Heidstra R, Willemsen V, Blilou I, Galinha C, Nussaume L, Noh YS, Amasino R, Scheres B. 2004. The PLETHORA genes mediate patterning of the Arabidopsis root stem cell niche. Cell 119, 109–120 [DOI] [PubMed] [Google Scholar]
- Aldridge C, Maple J, Moller SG. 2005. The molecular biology of plastid division in higher plants. Journal of Experimental Botany 56, 1061–1077 [DOI] [PubMed] [Google Scholar]
- Alonso JM, Stepanova AN, Leisse TJ, et al. 2003. Genome-wide insertional mutagenesis of Arabidopsis thaliana . Science 301, 653–657 [DOI] [PubMed] [Google Scholar]
- Ariel FD, Manavella PA, Dezar CA, Chan RL. 2007. The true story of the HD-Zip family. Trends in Plant Science 12, 419–426 [DOI] [PubMed] [Google Scholar]
- Baklanov MM, Golikova LN, Malygin EG. 1996. Effect on DNA transcription of nucleotide sequences upstream to T7 promoter. Nucleic Acids Research 24, 3659–3660 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bechtold N, Jolivet S, Voisin R, Pelletier G. 2003. The endosperm and the embryo of Arabidopsis thaliana are independently transformed through infiltration by Agrobacterium tumefaciens . Transgenic Research 12, 509–517 [DOI] [PubMed] [Google Scholar]
- Berardini TZ, Mundodi S, Reiser L, et al. 2004. Functional annotation of the Arabidopsis genome using controlled vocabularies. Plant Physiology 135, 745–755 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brandt R, Salla-Martret M, Bou-Torrent J, et al. 2012. Genome-wide binding-site analysis of REVOLUTA reveals a link between leaf patterning and light-mediated growth responses. The Plant Journal 72, 31–42 [DOI] [PubMed] [Google Scholar]
- Butterfass T. 1979. Patterns of chloroplast reproduction. Cell Biology Monographs, Vol. 6 Vienna and New York: Springer Verlag [Google Scholar]
- Ciarbelli AR, Ciolfi A, Salvucci S, Ruzza V, Possenti M, Carabelli M, Fruscalzo A, Sessa G, Morelli G, Ruberti I. 2008. The Arabidopsis homeodomain-leucine zipper II gene family: diversity and redundancy. Plant Molecular Biology 68, 465–478 [DOI] [PubMed] [Google Scholar]
- Czechowski T, Stitt M, Altmann T, Udvardi MK, Scheible WR. 2005. Genome-wide identification and testing of superior reference genes for transcript normalization in Arabidopsis . Plant Physiology 139, 5–17 [DOI] [PMC free article] [PubMed] [Google Scholar]
- del Campillo E, Abdel-Aziz A, Crawford D, Patterson SE. 2004. Root cap specific expression of an endo-β-1,4-d-glucanase (cellulase): a new marker to study root development in Arabidopsis . Plant Molecular Biology 56, 309–323 [DOI] [PubMed] [Google Scholar]
- Doerner P. 1998. Root development: quiescent center not so mute after all. Current Biology 8, R42–R44 [DOI] [PubMed] [Google Scholar]
- Elhiti M, Stasolla C. 2009. Structure and function of homodomain-leucine zipper (HD-Zip) proteins. Plant Signaling and Behavior 4, 86–88 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Genty B, Briantais JM, Baker NR. 1989. The relationship between the quantum yield of photosynthetic electron transport and quenching of chlorophyll fluorescence. Biochimica et Biophysica Acta 990, 87–92 [Google Scholar]
- Ha CM, Kim GT, Kim BC, Jun JH, Soh MS, Ueno Y, Machida Y, Tsukaya H, Nam HG. 2003. The BLADE-ON-PETIOLE 1 gene controls leaf pattern formation through the modulation of meristematic activity in Arabidopsis . Development 130, 161–172 [DOI] [PubMed] [Google Scholar]
- Harris JC, Hrmova M, Lopato S, Langridge P. 2011. Modulation of plant growth by HD-Zip class I and II transcription factors in response to environmental stimuli. New Phytologist 190, 823–837 [DOI] [PubMed] [Google Scholar]
- Hawker NP, Bowman JL. 2004. Roles for class III HD-Zip and KANADI genes in Arabidopsis root development. Plant Physiology 135, 2261–2270 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hepworth SR, Zhang Y, McKim S, Li X, Haughn GW. 2005. BLADE-ON-PETIOLE-dependent signaling controls leaf and floral patterning in Arabidopsis . Plant Cell 17, 1434–1448 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holtan HE, Bandong S, Marion CM, et al. 2011. BBX32, An Arabidopsis B-box protein, functions in light signaling by suppressing HY5-regulated gene expression and interacting with STH2. Plant Physiology 156, 2109–2123 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iacobuzio-Donahue CA, Ryu B, Hruban RH, Kern SE. 2002. Exploring the host desmoplastic response to pancreatic carcinoma: gene expression of stromal and neoplastic cells at the site of primary invasion. American Journal of Pathology 160, 91–99 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Izhaki A, Bowman JL. 2007. KANADI and class III HD-Zip gene families regulate embryo patterning and modulate auxin flow during embryogenesis in Arabidopsis . Plant Cell 19, 495–508 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jiang K, Feldman LJ. 2005. Regulation of root apical meristem development. Annual Review of Cell and Developmental Biology 21, 485–509 [DOI] [PubMed] [Google Scholar]
- Jiao Y, Tausta SL, Gandotra N, et al. 2009. A transcriptome atlas of rice cell types uncovers cellular, functional and developmental hierarchies. Nature Genetics 41, 258–263 [DOI] [PubMed] [Google Scholar]
- Kerk NM, Ceserani T, Tausta SL, Sussex IM, Nelson TM. 2003. Laser capture microdissection of cells from plant tissues. Plant Physiology 132, 27–35 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kohler RH, Hanson MR. 2000. Plastid tubules of higher plants are tissue-specific and developmentally regulated. Journal of Cell Science 113, 81–89 [DOI] [PubMed] [Google Scholar]
- Lilley CJ, Wang D, Atkinson HJ, Urwin PE. 2011. Effective delivery of a nematode-repellent peptide using a root-cap-specific promoter. Plant Biotechnology Journal 9, 151–161 [DOI] [PubMed] [Google Scholar]
- Long SP, Bernacchi CJ. 2003. Gas exchange measurements, what can they tell us about the underlying limitations to photosynthesis? Procedures and sources of error. Journal of Experimental Botany 54, 2393–2401 [DOI] [PubMed] [Google Scholar]
- Long SP, Zhu XG, Naidu SL, Ort DR. 2006. Can improvement in photosynthesis increase crop yields? Plant, Cell & Environment 29, 315–330 [DOI] [PubMed] [Google Scholar]
- Nawy T, Lee JY, Colinas J, Wang JY, Thongrod SC, Malamy JE, Birnbaum K, Benfey PN. 2005. Transcriptional profile of the Arabidopsis root quiescent center. Plant Cell 17, 1908–1925 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ohgishi M, Oka A, Morelli G, Ruberti I, Aoyama T. 2001. Negative autoregulation of the Arabidopsis homeobox gene ATHB-2 . The Plant Journal 25, 389–398 [DOI] [PubMed] [Google Scholar]
- Osteryoung KW, McAndrew RS. 2001. The plastid division machine. Annual Review of Plant Physiology and Plant Molecular Biology 52, 315–333 [DOI] [PubMed] [Google Scholar]
- Pogson BJ, Albrecht V. 2011. Genetic dissection of chloroplast biogenesis and development: an overview. Plant Physiology 155, 1545–1551 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prigge MJ, Otsuga D, Alonso JM, Ecker JR, Drews GN, Clark SE. 2005. class III homeodomain-leucine zipper gene family members have overlapping, antagonistic, and distinct roles in Arabidopsis development. Plant Cell 17, 61–76 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pyke KA. 1997. The genetic control of plastid division in higher plants. American Journal of Botany 84, 1017–1027 [PubMed] [Google Scholar]
- Pyke KA. 1999. Plastid division and development. Plant Cell 11, 549–556 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pyke KA, Leech RM. 1991. Rapid image analysis screening procedure for identifying chloroplast number mutants in mesophyll cells of Arabidopsis thaliana (L.) Heynh. Plant Physiology 96, 1193–1195 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pyke KA, Leech RM. 1992. Chloroplast division and expansion is radically altered by nuclear mutations in Arabidopsis thaliana . Plant Physiology 99, 1005–1008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- R Development Core Team 2007. R: a language and environment for statistical computing. http://www.R-project.org, last accessed 26 July 2013.
- Raines CA. 2011. Increasing photosynthetic carbon assimilation in C3 plants to improve crop yield: current and future strategies. Plant Physiology 155, 36–42 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Riechmann JL, Heard J, Martin G, et al. 2000. Arabidopsis transcription factors: genome-wide comparative analysis among eukaryotes. Science 290, 2105–2110 [DOI] [PubMed] [Google Scholar]
- Ruberti I, Sessa G, Ciolfi A, Possenti M, Carabelli M, Morelli G. 2012. Plant adaptation to dynamically changing environment: the shade avoidance response. Biotechnology Advances 30, 1047–1058 [DOI] [PubMed] [Google Scholar]
- Sawa S, Ohgishi M, Goda H, Higuchi K, Shimada Y, Yoshida S, Koshiba T. 2002. The HAT2 gene, a member of the HD-Zip gene family, isolated as an auxin inducible gene by DNA microarray screening, affects auxin response in Arabidopsis . The Plant Journal 32, 1011–1022 [DOI] [PubMed] [Google Scholar]
- Sharkey TD, Bernacchi CJ, Farquhar GD, Singsaas EL. 2007. Fitting photosynthetic carbon dioxide response curves for C3 leaves. Plant, Cell & Environment 30, 1035–1040 [DOI] [PubMed] [Google Scholar]
- Skuzeski JM, Nichols LM, Gesteland RF. 1990. Analysis of leaky viral translation termination codons in vivo by transient expression of improved β-glucuronidase vectors. Plant Molecular Biology 15, 65–79 [DOI] [PubMed] [Google Scholar]
- Sorin C, Salla-Martret M, Bou-Torrent J, Roig-Villanova I, Martinez-Garcia JF. 2009. ATHB4, a regulator of shade avoidance, modulates hormone response in Arabidopsis seedlings. The Plant Journal 59, 266–277 [DOI] [PubMed] [Google Scholar]
- Steindler C, Carabelli M, Borello U, Morelli G, Ruberti I. 1997. Phytochrome A, phytochrome B and other phytochrome(s) regulate ATHB-2 gene expression in etiolated and green Arabidopsis plants. Plant, Cell & Environment 20, 759–763 [Google Scholar]
- Steindler C, Matteucci A, Sessa G, Weimar T, Ohgishi M, Aoyama T, Morelli G, Ruberti I. 1999. Shade avoidance responses are mediated by the ATHB-2 HD-zip protein, a negative regulator of gene expression. Development 126, 4235–4245 [DOI] [PubMed] [Google Scholar]
- Tiwari S, Wang S, Hagen G, Guilfoyle TJ. 2006. Transfection assays with protoplasts containing integrated reporter genes. Methods in Molecular Biology 323, 237–244 [DOI] [PubMed] [Google Scholar]
- Zhu XG, Long SP, Ort DR. 2010. Improving photosynthetic efficiency for greater yield. Annual Review of Plant Biology 61, 235–261 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.