Abstract
The role of many genes and interactions among genes involved in flowering time have been studied extensively in Arabidopsis, and the purpose of this study was to investigate how effectively results obtained with the model species Arabidopsis can be applied to the Brassicacea with often larger and more complex genomes. Brassica rapa represents a very close relative, with its triplicated genome, with subgenomes having evolved by genome fractionation. The question of whether this genome fractionation is a random process, or whether specific genes are preferentially retained, such as flowering time (Ft) genes that play a role in the extreme morphological variation within the B. rapa species (displayed by the diverse morphotypes), is addressed. Data are presented showing that indeed Ft genes are preferentially retained, so the next intriguing question is whether these different orthologues of Arabidopsis Ft genes play similar roles compared with Arabidopsis, and what is the role of these different orthologues in B. rapa. Using a genetical–genomics approach, co-location of flowering quantitative trait loci (QTLs) and expression QTLs (eQTLs) resulted in identification of candidate genes for flowering QTLs and visualization of co-expression networks of Ft genes and flowering time. A major flowering QTL on A02 at the BrFLC2 locus co-localized with cis eQTLs for BrFLC2, BrSSR1, and BrTCP11, and trans eQTLs for the photoperiod gene BrCO and two paralogues of the floral integrator genes BrSOC1 and BrFT. It is concluded that the BrFLC2 Ft gene is a major regulator of flowering time in the studied doubled haploid population.
Key words: Brassica rapa, candidate gene mapping, expression quantitative trait loci (eQTL), FLOWERING LOCUS C (FLC)., flowering time, gene expression networks, genome triplication.
Introduction
In flowering plants, the change from vegetative to reproductive development is a major transition that is sensitive to various seasonal climatic signals (Andres and Coupland, 2012). Controlling the timing of this transition is especially important in crop plants as it determines the geographical range where the crop can be cultivated and ensures high agricultural productivity. Genetic factors that control flowering time are well studied in the model plant Arabidopsis. Control of flowering in Arabidopsis is the result of an interaction of environmental and physiological factors in different pathways: the vernalization pathway, photoperiod/circadian clock pathway, autonomous pathway, ageing pathway, ambient temperature pathway, and gibberellin pathway (Roux et al., 2006; Alonso-Blanco et al., 2009; Fornara et al., 2010). In total, >300 genes have been implicated in the control of flowering in Arabidopsis, and the key regulatory components involved in different pathways and their interactions are well summarized (Fornara et al., 2010).
Polyploidy [whole-genome duplication (WGD)] has played an important role in evolution and genetic diversity of angiosperm genomes (De Bodt et al., 2005; Flagel and Wendel, 2009). WGD events are generally followed by changes in gene expression and widespread gene loss (Sankoff et al., 2010). The Brassica genus is closely related to the model species Arabidopsis thaliana, both members of the Brassicaceae family. Six Brassica species are cultivated worldwide: three diploids, B. rapa (AA, 2n=20), B. nigra (BB, 2n=16), and B. oleracea (CC, 2n=18); and three amphidiploids, B. juncea (AABB, 2n=36), B. napus (AACC, 2n=38), and B. carinata (BBCC, 2n=34). Comparative genetic and physical mapping and genome sequencing studies have confirmed the syntenic relationship between Arabidopsis and the triplicated genome of B. rapa, with subgenomes having evolved by genome fractionation (Park et al., 2005; Wang et al., 2011; Cheng et al., 2012; Tang et al., 2012).
The paleohexaploid crop B. rapa displays extreme morphological diversity, and includes leafy vegetables, turnips, and oil types that all differ based on which organs are consumed (Zhao et al., 2005; Bonnema et al., 2011). Flowering time is an important developmental trait, and wide variation exist both between and within B. rapa morphotypes. Previous studies on flowering time regulation in Brassica have identified some major flowering candidate genes such as FLOWERING LOCUS C (FLC), CONSTANS (CO), and FLOWERING LOCUS T (FT). In Arabidopsis, the FLC MADS-box gene is a target of the autonomous flowering time and vernalization pathways that acts in a dosage-dependent manner to repress flowering (Michaels and Amasino, 1999). No null allele or copy number variation of FLC has been identified in Arabidopsis ecotypes (Michaels et al., 2003). Four FLC paralogues (FLC1, FLC2, FLC3, and FLC5) each in B. rapa (Schranz et al., 2002) and in B. oleracea (Okazaki et al., 2007) have been cloned. Co-localization of FLC paralogues with quantitative trait loci (QTLs) for flowering time in B. rapa (Lou et al., 2007; Yuan et al., 2009) and study of BrFLC expression in Chinese cabbage (Kim et al., 2007) and in B. napus (Tadege et al., 2001; Zou et al., 2012) indicates that the Brassica FLC genes act similarly to Arabidopsis FLC. Co-localization of a major QTL for both flowering time and vernalization response with the BrFLC2 locus and correlation between BrFLC2 transcript levels, vernalization treatments, and flowering time also suggested that the role of BrFLC2 in B. rapa is comparable with that of FLC in Arabidopsis (Zhao et al., 2010). However, in another study using a collection of B. rapa accessions and a Chinese cabbage double haploid (DH) population, a splicing site mutation in BrFLC1 was significantly associated with flowering time (Yuan et al., 2009). This illustrates that in different genetic backgrounds, different FLC paralogues have major effects on flowering time. In addition, six FT paralogues in B. napus have been mapped; three of them were associated with two major QTL clusters for flowering time (Wang et al., 2009).
Jansen and Nap (2001) proposed the term ‘Genetical–Genomics’, in which mRNA transcript abundances can be treated as quantitative traits to map expression QTLs (eQTLs). In this way, eQTLs can be classified as cis-acting (if the gene and QTL co-localize) and trans-acting (no co-localization). Recently this approach has been widely applied in different organisms (Keurentjes et al., 2007; Zhang et al., 2011) to gain insight into the genetics of traits of interest. In a genetical–genomics study in Arabidopsis, regulatory networks of genes involved in the transition to flowering have been constructed by using eQTLs identified in a genome-wide gene expression study of a recombinant inbred line (RIL) population (Keurentjes et al., 2007).
To understand the genetic architecture of flowering time by a genetical–genomics approach in B. rapa, a large number of genetic markers were developed based on Arabidopsis flowering time genes (Ft genes) that were identified in BLAST searches against the Chinese cabbage Chiifu-401 genome sequence (Wang et al., 2011), and could address whether Ft genes are preferentially retained in the B. rapa genome. A subset of these genes were mapped in a B. rapa DH population (DH68) developed from a cross between a Yellow sarson (YS-143) and a Pak choi (PC-175), which is a cross reciprocal to an earlier studied population DH38 (Lou et al., 2007; Zhao et al., 2010). These markers connect the physical and genetic B. rapa maps, which is a prerequisite for genetical–genomic studies. To understand how flowering time is regulated when many Ft genes are retained in multiple copies, and to reveal the roles of these genes in the specific population and growth conditions studied here, flowering QTLs were identified in the DH68 population and leaves of young DH plants were profiled for whole-genome transcript variation using a distant pair design with the Cogenics 60-mer oligo Microarray (Trick et al., 2009). A genome-wide eQTL analysis was performed and gene networks related to flowering time were constructed to explore the intra- or inter-relationship of genes involved in flowering time pathways. Finally, correlations between transcript abundance and flowering time variation, combined with genetic positional information of flowering QTLs and eQTLs allowed the Ft genes that regulate flowering time to be defined and to gain insight into the complex flowering time network in the paleohexaploid crop B. rapa.
Materials and methods
Plant materials and growth conditions
A B. rapa DH population DH68 of 163 DH lines was established from three F1 plants of a cross between Yellow sarson YS-143 (accession no. FIL500) as the female parent and Pak choi PC-175 (cultivar: Nai Bai Cai; accession no. VO2B0226) as the male parent. A set of 92 DH lines was evaluated for flowering time from September to December 2008. Seedlings were germinated on wet filter paper at 25 °C in the dark for 2 d, and then transplanted to nursery trays to be cultured in a greenhouse for 2 weeks. Plants with 4–5 true leaves were transplanted to soil (pots of Ø 17cm). Plants were grown in a heated greenhouse (18–21 °C) at Wageningen, The Netherlands. When the days were shorter than 16h, artificial light was supplied until a photoperiod (200 μmol m–2 s–1) of 16h. DH lines were planted in three blocks arranged in a randomized design, and flowering time was scored for one plant per block.
Flowering time was defined as number of days from transplanting to appearance of the first open flower.
Development of genetic markers for genes involved in flowering time regulation
A total of 365 Arabidopsis genes were selected from the literature (Roux et al., 2006; Brachi et al., 2010; Fornara et al., 2010) and databases (http://www.arabidopsis.org/) that are described as being implicated in flowering time control in Arabidopsis. Using the Arabidopsis gene sequence as query, homologous genes in B. rapa were identified in BLASTn searches against the annotated Chinese cabbage Chiifu genome sequence (http://brassicadb.org/brad/index.php) (Wang et al., 2011) (Supplementary Table S1 available at JXB online). All B. rapa annotations matching the same Arabidopsis genes generated by BLASTP best hit were considered as B. rapa paralogues (cut-off E-value e–5). Gene structures were predicted by sequence comparison with the Arabidopsis CDS by DNASTAR Lasergene 9.0 (Lasergene, USA) (Supplementary Table S1).
For each gene/paralogue, three sets of primers were designed based on the genomic sequence of candidate genes to amplify 84–619bp fragments uniquely (Supplementary Table S2 at JXB online). The primers are named following the gene nomenclature system of Brassica ordered from left to right (genus–species–gene–name–genome–locus) (Østergaard and King, 2008). Primer pairs that gave a clear single band on agarose gel that was polymorphic between the parents of DH68 using the LightScanner scoring were profiled over the DH68 population. LightScanner is an extremely sensitive, accurate, and reliable technique for genotyping studies (http://www.biofiredx.com/MutationDiscovery/index.html) (Idaho Technology Inc., USA).
Genetic map position of gene-targeted makers
Genomic DNA was extracted from fresh leaves of the 163 DH68 lines according to the procedure described by Beek et al. (1992). PCR was performed in 10 μl volumes: 2 μl of 5× PCR buffer, 0.4 μl of 2mM of dNTP, 0.5 μl of 2.5mM of both forward and reverse primers, 1 μl of 1×LC-green Plus, 0.1 μl of 1U of Phire enzyme, 1 μl of 50ng of genomic DNA, and 4.5 μl of double-distilled water. PCRs were performed on a 7300 Thermo cycler (Bio-RAD, USA). The temperature cycling protocol consists of an initial denaturation step at 98 °C for 30 s, followed by 40 cycles of denaturation at 98 °C for 10 s, annealing at 60 °C for 10 s, and an extension at 72 °C for 30 s. This PCR was followed by another denaturation step at 94 °C for 30 s, followed by cooling down to 25 °C for 30 s to facilitate heteroduplex formation. Following PCR, each product was transferred to a 96-well LightScanner® System (Idaho Technology Inc., USA) for high resolution melting (HRM) analysis and covered with a mineral oil overlay. Samples were heated from 70 °C up to 96 °C with a ramp rate of 0.10 °C s–1. LightScanner software (Version 2.0) was used for data analysis following the protocols described in Montgomery et al. (2007). A saturating DNA binding dye is introduced during DNA amplification, which enables differentiation of PCR products based on their dissociation behaviour as they are subjected to increasing temperatures. Melting profiles were calibrated by internal oligonucleotide controls, and then normalized, temperature shifted, grouped. and displayed as fluorescence versus temperature plots (Montgomery et al., 2007).
A total of 125 flowering time gene-targeted (FTGT) markers together with AFLP (amplified fragment length polymorphism), SSR (simple sequence repeat), and InDel markers were used to construct the genetic map using the program Joinmap 4.0 (Kyazma, Wageningen, The Netherlands) (http://www.kyazma.nl). The 10 linkage groups of B. rapa were named A01–A10 corresponding to the reference map (http://brassicadb.org/brad/geneticMarker.php?chr=ALL). To investigate the quality of the genetic map, the marker order of the genetic and physical Chiifu-401 map was compared by placing the genetically mapped candidate Ft genes on the physical map using annotated gene position number by blasting the Brassica database (http://brassicadb.org/brad/searchAll.php) (Supplementary Table S1 at JXB online).
Flowering time QTL analysis
Flowering time QTLs (flowering QTLs) were identified by composite interval mapping using the software MAPQTL 5.0 (Van Ooijen, 2004). A permutation test was applied to each data set (1000 repetitions) to decide the LOD (logarithm of odds) thresholds (P=0.05). In this study, a LOD value of 3.0 was used as a significant threshold. Flowering QTLs were graphically displayed using Map chart 2.2 (Voorrips, 2002).
RNA isolation and microarray design
The plants of the DH lines were grown under the following conditions: 16h light and temperature between 18 °C and 21 °C. After a week, germinated seedlings were transplanted and randomly distributed over three different blocks. Five weeks after transplanting, the third and fourth leaves of each replicate were collected in the morning (10:00–12:00h) and placed in liquid nitrogen to be further ground and stored at –70 °C. At that moment, none of the plants was switched to flowering/bolting, so all were still in the vegetative stage when sampling the leaves. Each replicate was ground individually and the mix with equally weighted amounts of the three replicates was used for RNA extraction and transcriptome profiling.
Total RNA was extracted by using the TRIZOL reagent (Invitrogen), from ~300mg of frozen leaf material of 92 DH68 lines. The first strand of cDNA was synthesized from 1 μg of total RNA using the I Amplification Grade kit (Invitrogen) according to the manufacturer’s instructions.
Agilent Cogenics 105K Brassica oligo-arrays (Agilent Technologies; Trick et al., 2009), which contain 96 557 features, assembled using expressed sequence tag (EST) sequences mainly from B. napus, B. rapa, and B. oleracea resources, were used for two-colour microarray experiments. Distant pair design was applied for the two-colour microarray hybridization studies of DH lines as described by Fu and Jansen (2006) and implemented in the R package designGG (http://www.rug.nl/research/bioinformatics/). All microarray experiments were performed according to the manufacturer’s manual (Agilent Technologies).
eQTL analyses
eQTL analysis was performed using the basic single marker regression procedure present in R/QTL. Expression was measured using two-colour array technology and, for the mapping, the ratios between two genotypes, Yi = á+ âGi+Error (Yi=probe intensity, Gi=genetic effect), were used. In this model, the genetic effect was annotated for the expression ratios as described in Fu and Jansen (2006); â is the effect of the different allele (1 for A>B 0 for A=B and –1 for A<B). This model was evaluated at each marker to get an estimate of the allelic effect on the expression probes. This resulted in a P-value, which was transformed into –log10 (P-value) (equivalent to LOD score). The eQTLs with LOD >3.0 were considered significant eQTLs. Several genetically linked markers with LOD >3.0 probably represent a single locus explaining variation in expression of the respective probe, but using this analysis these are described as several eQTLs. All 96 557 expression probes were annotated against the genome sequences of B. rapa (Wang et al., 2011), by blasting their 60-mer oligonucleotide sequence against both genomic (scaffolds) and coding (gene models) sequences of the B. rapa Chiifu genome with the allowance of only one mismatch (http://Brassicadb.org/Brad/search All.php). eQTLs (local eQTLs) were defined as cis when the eQTL mapped on the same chromosome as the physical location of the oligonucleotide, and as trans eQTLs (distant eQTLs) when the eQTL mapped on a different chromosome from the physical location of the probe.
Quantitative real-time PCR (RT-qPCR)
Expression of 16 genes was analysed using real-time PCR. A number of genes were selected because they were important Ft genes that were not represented on the Cogenics Microarray. These genes were: FT_A02, FT_A07(1), FT_A07(2), SOC1_A03, SOC1_A04, SOC1_A05, CO_A10, and CCA1_A05, and the two FLC paralogues FLC2_A02 and FLC3_A03. Real-time PCR was performed with paralogue-specific primers for all four BrFLC genes, and this was compared with microarray expression values for FLC1_A10 and FLC5_A03. A few other single-copy genes with eQTLs detected using the microarray profiles were selected to compare results obtained from the microarray and real-time PCR (ARR3_A09, FRL2_A09, and CAM1_A07). In addition, two single candidate Ft genes BrCCA1 and BrLD, which had no eQTLs on the microarray, were also examined by RT-qPCR. Transcripts of all 16 genes were profiled with two technical replications using the RNA samples of the 92 DH68 lines that were previously used for microarray analysis. RT-qPCR was carried out in 96-well optical reaction plates using the iQ™ SYBR® Green Supermix (Bio-Rad, www.Bio-rad.com). All the cycle threshold (C t) values from one gene were determined at the same threshold fluorescence value of 0.2 using the ΔΔC t method (Livak and Schmittgen, 2001). The reference gene glyceraldehyde-3-phosphate dehydrogenase (GAPDH) and gene-specific primers for the RT-qPCR are listed in Supplementary Table S3 at JXB online.
Expression correlation and genetic network analyses
Pearson’s correlation among Ft genes with eQTLs was computed based on microarray signal intensities (log2 scale), RT-qPCR (log2 values), and flowering time phenotypes (days) of each DH line to identify co-expression patterns among gene probes and flowering time. Using the heat map tool, including hierarchical clustering among the genes and flowering time phenotype, the correlation between gene expression and phenotype was visualized.
Similarly, a gene network based on co-regulation patterns among genes was constructed to identify regulatory gene hubs of flowering time pathways. Pearson’s correlation was calculated by using eQTL LOD profiles of genes represented in the microarray, RT-qPCR values, and flowering time phenotype; correlations >0.3 between genes were shown in the network. Visualization of the co-regulation network was carried out by using the open source visualization software Cytoscape 2.8.2 (www.cytoscape.org) to analyse the genetic interaction networks (Shannon et al., 2003). The calculations of Pearson’s correlation and heat map was done in open statistical software R 2.13.1 (Ihaka and Gentleman, 1996).
Results
Mapping of flowering time genes
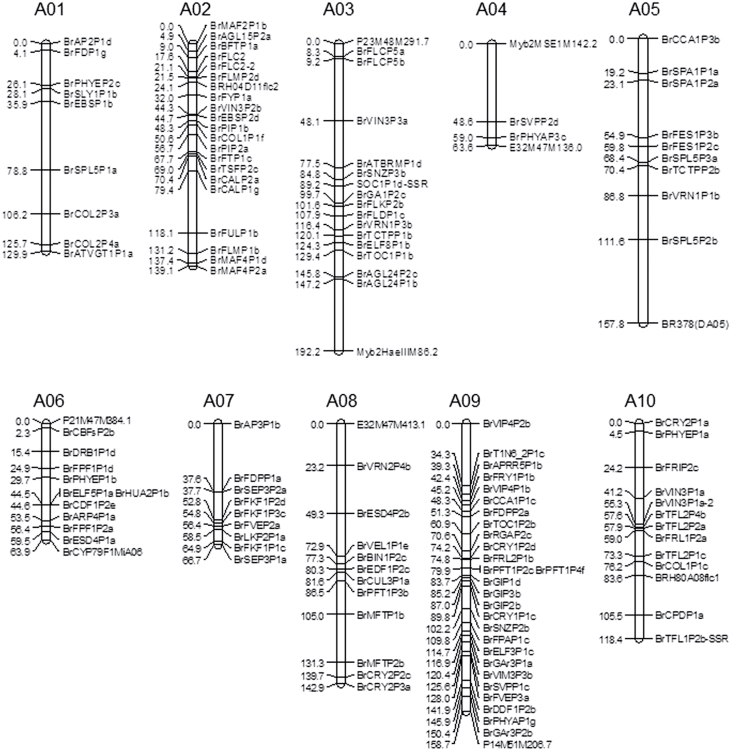
A total of 768 B. rapa genes were identified in a screen of EST, bacterial artificial chromosome (BAC), and Chinese cabbage genome sequences as homologues of 365 genes involved in flowering in Arabidopsis (Table 1; Supplementary Table S1 at JXB online). Of the Arabidopsis genes, 101 (27.7%) had three or more paralogues in B. rapa, 128 (35.1%) had two B. rapa paralogues, 136 (37.3%) had one B. rapa homologue, and for 11 (3.0%) Arabidopsis genes no homologues were identified in B. rapa. A total of 190 gene/paralogue-specific primer pairs designed to amplify B. rapa genes amplified polymorphic bands between the parents of the DH68 population (YS-143 and PC-175). These 190 polymorphic primer pairs were profiled over the DH68 mapping population, which resulted in map positions for 125 homologues of Ft genes in B. rapa, corresponding to 81 Ft genes from Arabidopsis (Fig. 1; Supplementary Table S2 at JXB online). These Ft genes were distributed over all 10 linkage groups, covering a total map length of 1233 cM. An updated genetic linkage map of DH68 contains 456 markers: 278 AFLPs, 50 SSRs, 125 Ft markers, 2 IBP (intron-based polymorphism), and 1 CAPS (cleaved amplified polymorphic sequence) (Supplementary Fig. S1 at JXB online).
Table 1.
The number of Arabidopsis orthologues encoding genes involved in flowering with ≥3, 2, 1, or 0 paralogues in B. rapaIn total, 366 Arabidopsis orthologues detected 768 B. rapa paralogues
| No. of Arabidopsis orthologues | No. of paralogues in B. rapa | Percentage |
|---|---|---|
| 101 | ≥3 | 27.6 |
| 129 | 2 | 35.3 |
| 136 | 1 | 37.3 |
| 11 | 0 | 3.0 |
Fig. 1.
The genetic map (cM) of DH68 enriched with flowering time candidate genes. The flowering time candidate gene markers are shown <Genus species, Br> <Gene name, 2–6 letter code> <Genome locus, Paralogues code> <Primer code, letter code>. The end marker of link groups is indicated by another genetic marker if the end marker is not a flowering time marker.
All 768 B. rapa genes homologous to Arabidopsis Ft genes were in silico mapped on scaffolds and chromosomes of the sequenced Chinese cabbage Chiifu genome. For 40 of the 768 Ft genes, no in silico map position could be found. The genetic map position of Ft genes in DH68 corresponded well with their in silico predicted map position in Chiifu. Only 12 genes had an inconsistent order between the genetic and physical maps, but these were still mapped to the same linkage groups (Supplementary Fig. S2 at JXB online). The relationship between the physical and genetic distance in this euchromatic sequenced part of the genome was 1 cM= ~210kb.
Flowering time QTL analysis
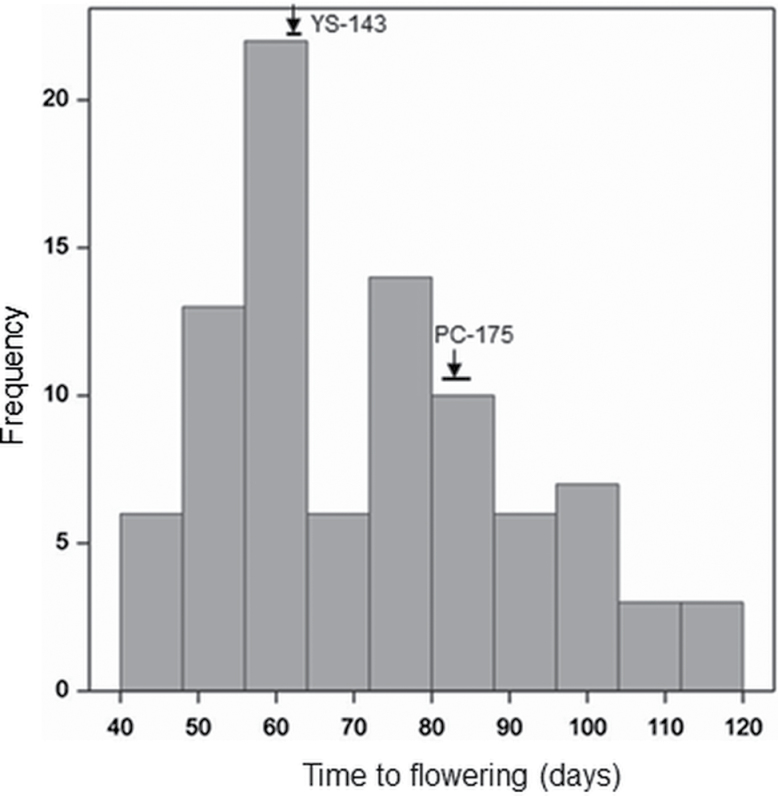
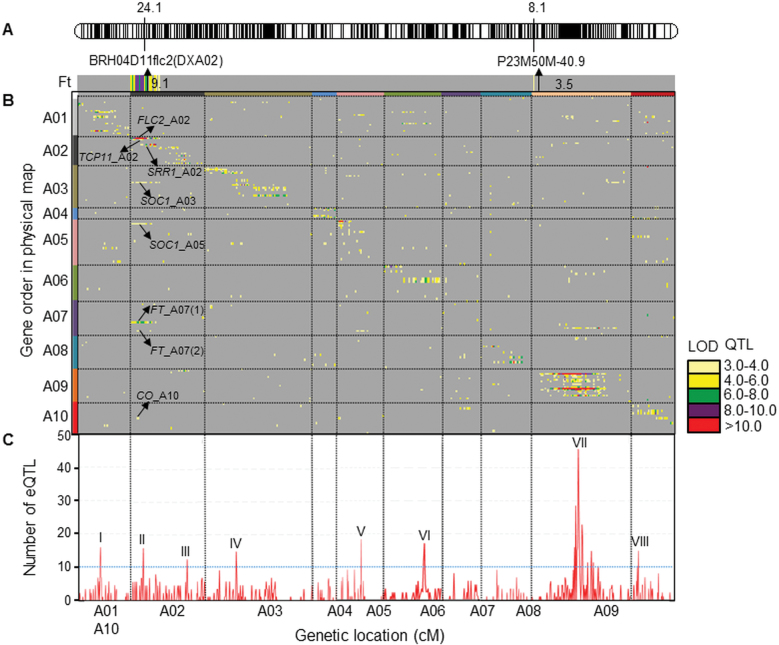
The flowering time of DH68 lines showed a distribution around the mean, which deviated from continuous as the class around 70 d had fewer DH lines (Fig. 2). QTL mapping identified two loci affecting flowering time that together explained 42.6% of the phenotypic variation (Fig. 3A). A large percentage of phenotypic variation (33.5%) was explained by a QTL on the top of A02 (24.1 cM) with a LOD value of 9.1. This QTL maps at the Ft gene BrFLC2 locus. The other flowering QTL was located on A09 with a LOD value of 3.4 (8.1 cM), accounting for 9.1% of the phenotypic variation. For both QTLs, the Yellow sarson allele decreased flowering time (Fig. 3A).
Fig. 2.
Frequency distributions of flowering time in DH68. Arrows and horizontal bars depict the mean ±SD of parental lines.
Fig. 3.
Summary of flowering QTLs, eQTLs, and hotspots of eQTL locations detected on the genome in B. rapa for flowering time. (A) Location of QTLs for flowering time in DH68 (YS-143×PC-175). The bar at the top represents the genetic map of the 10 linkage groups. The QTLs identified are shown as 1–LOD support intervals, the position of arrows corresponding to the maximum LOD score values. Map positions of flowering QTLs are given in centiMorgans (cM) above the genetic map, and the markers are listed below the genetic map. Upward arrows indicate the LOD score of the Ft QTLs on A02 and A09. (B) Genomic architecture of flowering time eQTLs across the chromosome of B. rapa. Chromosome borders are depicted as horizontal and vertical lines. The x-axis (cM) shows the genetic locations of markers mapped in the DH68 linkage map. The y-axis shows the location/order of probes representing candidate genes for which eQTLs were found. The displayed data are included as Supplementary Table S6 at JXB online. (C) Numbers of eQTLs in intervals of a 5 cM sliding window are given across the genome. The left y-axis shows the number of eQTLs, with the horizontal blue line showing the flowering time eQTL hotspot threshold of 10. The cut-off number of eQTLs by chance would be 10. Eight regions had a high eQTL density centring around ±0.5 cM. The eight eQTL hotspots were noted as I–VIII (arranged left to right).
eQTL analysis based on the microarray study
In order to identify genes that underlie the flowering QTLs and to construct co-expression networks that relate to flowering time, a regression analysis of the transcript abundance in the 92 DH lines was performed for the 96 557 probes that were represented on the microarray against the 456 genetic markers. In total, 28 403 probes (29.4%) were detected as significant against at least 1–36 markers with a LOD >3, corresponding to a total of 160 803 eQTLs.
From the 96 557 probes on the microarray, 1340 probes represented 610 Ft genes in B. rapa (Supplementary Tables S4, S5 at JXB online). After regression analysis, 188 out of the 1340 probes (14.0%), corresponding to 178 Ft genes in B. rapa, had one or more significant eQTLs, and within these 114 (60.6%) had a B. napus origin, 63 (33.5%) had a B. rapa origin, 10 (5.3%) had a B. oleracea origin, and only one (0.5%) had a B. carinata origin (Supplementary Table S6 at JXB online). When the genetic positions of the markers were plotted against the physical positions of the features for which eQTLs were found, an almost linear genome-wide relationship along the diagonal of the graph was observed (Fig. 3B; Supplementary Table S6 at JXB online).
eQTL analysis based on the RT-qPCR study
The expression of 17 important candidate genes for flowering time was measured by RT-qPCR, chosen as they were absent on the microarray and to verify the microarray results. Using the normalized relative abundance of transcripts, a total of 18 eQTLs for the expression level of 13 genes were mapped with significant LOD scores of at least 3 (Supplementary Fig. S3 at JXB online). Cis-acting eQTLs for three single-copy genes (ARR3_A09, FRL2_A09, and CAM1_A07) identified using the microarray were further confirmed by RT-qPCR, with higher LOD scores for RT-qPCR data (Supplementary Table S6 at JXB online). BrFLC1 had a cis eQTL on A10, while a minor trans eQTL was only detected on A09 with real-time PCR. A summary of the peaks/positions and nearest markers of eQTLs with LOD scores and explained variance is displayed in Table 2.
Table 2.
Details of eQTLs detected based on the relative transcript abundance of 11 genes obtained by RT-qPCR
| Gene | Peak of eQTL on LG (cM) | Interval (cM)a | Nearest marker | LOD score | Variance explained (%) | Regulation | Array-eQTLsb | Flowering QTLs |
|---|---|---|---|---|---|---|---|---|
| FLC1_A10.RL | (A09) 128.2 | 123.4–129.2 | P14m51m321.6 | 4.3 | 18.6 | trans | ND | – |
| (A10) 83.6 | 78.2–94.2 | BRH80A08flc1 (DA10) | 3.1 | 12.8 | cis | A10 | – | |
| FLC2_A02.RL | (A02) 24.1 | 22.5–27.2 | BRH04D11flc2 (DXA02) | 24.6 | 71.4 | cis | ND | A02 |
| FLC3_A03.RL | (A03) 29.2 | 23.4–34.5 | E37M47M128.1 | 4.9 | 22.7 | cis | A03 | – |
| FLC5_A03.RL | (A05) 54.9 | 51.1–58.9 | BrFES1P3b (XSA05) | 3.7 | 17.9 | trans | A03 | – |
| FT_A07(1).RL | (A02) 21.1 | 17.6–23.3 | BrFLC2-2 (XSA02) | 6.5 | 24.0 | trans | ND | – |
| FT_A07(2).RL | (A02) 21.1 | 17.6–23.3 | BrFLC2-2 (XSA02) | 3.4 | 16.5 | trans | ND | – |
| (A05) 81.9 | 80.5–86.0 | P14M51M121.9 | 3.0 | 12.3 | trans | ND | – | |
| SOC1_A03.RL | (A02) 48.3 | 46.7–49.9 | BrPIP1b(XSA02) | 4.7 | 17.9 | trans | ND | – |
| (A09) 128.2 | 116.9–129.2 | P14M51M321.6 | 3.6 | 13.5 | trans | ND | – | |
| SOC1_A05.RL | (A02) 36.9 | 27.2–42.9 | ENA13I (DA02) | 4.1 | 16.4 | trans | ND | - |
| CO_A10.RL | (A02) 21.5 | 16.3–29.8 | BrFLMP2d (XSA02) | 3.4 | 10.7 | trans | ND | A02 |
| (A05) 19.2 | 5.0–28.1 | BrSPA1P1a (XXA05) | 4.2 | 13.6 | trans | ND | – | |
| (A10) 76.2 | 64.2–82.2 | BrCOL1P1c (XSA10) | 4.9 | 16.1 | cis | ND | – | |
| CCA1_A05.RL | (A05) 23.1 | 22.2–32.1 | BrSPA1P2a (XSA05) | 22.1 | 69.0 | cis | ND | – |
| ARR3_A09.RL | (A09) 66.5 | 65.6–68.9 | E32M47M56.0 | 16.7 | 58.7 | cis | A09 | – |
| FRL2_A09.RL | (A09) 74.3 | 74.0–74.8 | BrCRY1P2d (XXA09) | 30.4 | 80.0 | cis | A09 | – |
| CAM1_A07.RL | (A07) 0.0 | 0.0–5.0 | BrAP3P1b (XSA07) | 3.0 | 14.6 | cis | A07 | – |
a1.0 LOD confidence interval.
b ND indicates that the gene was not represented on the Cogenics Microarray.
Genome distribution of eQTLs
When expression profiles of eQTLs of the microarray (188 Ft probes/genes with eQTLs) and RT-qPCR (13 Ft probes/genes with eQTLs) were combined, a total of 201 Ft genes/probes revealed a total of 1145 significant marker–probe associations (919 from the microarray and 226 from RT-qPCR) against all 456 genetic markers (Table 3). Based on their genetic map position or physical map position relative to markers with genetic map positions, 58 genes/probes had cis eQTLs on the chromosome where they physically mapped, 86 genes/probes were trans regulated by loci on different chromosomes, while 40 genes/probes were both cis and trans regulated, and for 17 genes/probes it was unknown whether they were cis or trans regulated (Supplementary Table S6 at JXB online). To visualize eQTL hotspots, the density of eQTLs was calculated in a 5 cM sliding window (Fig. 3C). Eight eQTL hotspots I–VIII are distributed over seven linkage groups with 11–40 eQTLs/hotspots, regulating the transcription of Ft genes. Two of the eight hotspots were on A02, with 14 (24.1 cM on A02) and 11 (110.1 cM on A02) eQTLs. The largest hotspot (VII) had 40 eQTLs at 74.2 cM on A09. All these hotspots may contain key regulators: genes controlling the expression of many other genes.
Table 3.
Number and proportions of significant eQTLs (marker–probe associations) with different LOD scores in different linkage groups
| LOD level | 3–4 | 4–6 | 6–8 | 8–10 | >10 | Total | Percentage |
|---|---|---|---|---|---|---|---|
| A01 | 50 | 38 | 8 | 4 | 2 | 102 | 8.9 |
| A02 | 81 | 44 | 14 | 8 | 10 | 157 | 13.7 |
| A03 | 87 | 59 | 11 | 2 | 0 | 159 | 13.9 |
| A04 | 20 | 14 | 4 | 1 | 0 | 39 | 3.4 |
| A05 | 47 | 20 | 1 | 1 | 4 | 73 | 6.4 |
| A06 | 50 | 26 | 8 | 1 | 0 | 85 | 7.4 |
| A07 | 25 | 14 | 0 | 0 | 0 | 39 | 3.4 |
| A08 | 25 | 17 | 7 | 0 | 2 | 51 | 4.5 |
| A09 | 163 | 100 | 24 | 17 | 52 | 356 | 31.1 |
| A10 | 38 | 38 | 5 | 1 | 2 | 84 | 7.3 |
| Total eQTLs | 586 | 370 | 82 | 35 | 72 | 1145 |
Co-localization analysis of eQTLs and flowering QTLs
Subsequently, a co-localization analysis was performed between flowering QTLs and eQTLs (Fig. 3; Supplementary Fig. S2 at JXB online). A strong cis eQTL for FLC2_A02 on A02 at 24.1 cM (95% confidence interval) with a LOD of 25, explaining 71.4% of the BrFLC2 transcript variability, co-localized with the major flowering QTL on A02 (Supplementary Fig. S2 at JXB online) with very similar shapes of the LOD plots (Supplementary Fig. S3 at JXB online). The YS-143 BrFLC2 allele was associated with both decreased BrFLC2 transcript abundance and decreased flowering time.
At this FLC2_A02 locus, several trans eQTLs were identified for CO_A10, FT_A07(1), FT_A07(2), SOC1_A03, and SOC1_A05, and cis eQTLs for SRR1_A02 and TCP11_A02 (Supplementary Fig. S3; Supplementary Table S6 at JXB online). As the confidence intervals co-localized with that of the FLC2_A02 cis eQTL, BrFLC2 seems a major regulator for the expression of these genes and for flowering time in B. rapa.
At A09, a minor flowering QTL maps; no clear candidate Ft genes can be assigned as cis eQTLs map proximal to this flowering QTL. At chromosome A10, another BrFLC paralogue maps: FLC1_A10.RL with a cis eQTL. However, at this locus, no QTL for flowering time is identified (Supplementary Table S6 at JXB online).
Identification of Ft gene modules
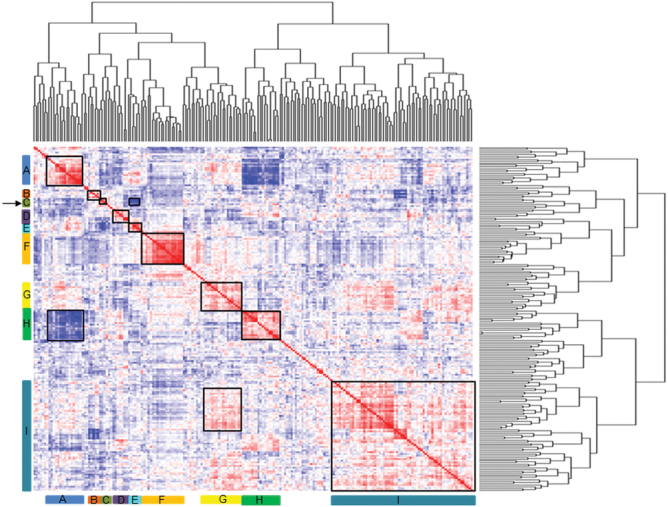
To analyse the functional relevance of the gene expression signatures to the phenotypic traits, a correlation matrix was calculated based on expression values in log2 scale of 198 candidate Ft genes with eQTLs and the flowering time trait (days). The candidate Ft genes were classified into 11 functional pathways based on information available in the literature, and annotation: gibberellin (17), floral meristems (28), vernalization (30), autonomous (5), red/far red light signalling (16), photoperiod/circadian clock (51; these two classes were combined, consisting of nine circadian clock genes, 22 photoperiod genes, and 20 genes that were classified into both these pathways; Supplementary Fig. S4K at JXB online), regulation of transcription (2), light signalling (14), floral integrators (4), development (18), and unknown functional pathways (13). The heat map (Fig. 4; Supplementary Table 7 at JXB online) shows clear clusters of genes with correlated expression. For ease of description, these clusters were numbered from block A to block I, with I being a very large block in which smaller clusters with higher correlations can be distinguished. In block A, the transcript abundance of 15 genes mostly belonging the photoperiod/circadian clock functional pathway was significantly positively correlated among pairs of transcripts, and these were negatively correlated with block H, with 17 genes, nine from the photoperiod/circadian pathway and the remaining from five different functional pathways. Block B consists of only five genes that belong to as many different functional pathways, with negative correlation to block E genes. In the small block C, the vernalization pathway gene FLC2_A02 and the circadian clock gene TCP11_A02 were positively correlated with flowering time. Importantly, block C was significantly negatively correlated with block E that includes the vernalization pathway gene FLC1_A10, the floral development gene pistillata PI_A02, and the floral integrators SOC1_A05, SOC1_A03, FT_A07(1), and FT_A07(2). Block D is a small group of genes from several functional pathways. Block G contains genes from different functional pathways, with correlations between genes from other blocks, especially block I. Block H mainly consists of genes from the daylength/circadian pathways, with many negative correlations to genes from block A. The very large block I consists of 64 genes that belong to many different functional pathways. A cluster of genes can be identified with genes all mapping on A09, with, among others, correlations to the BrCDF and BrGI genes from the daylength/circadian pathways. As several blocks contain co-regulated genes that belong to different functional pathways, there is clear evidence for cross-talk between these pathways.
Fig. 4.
Correlation heat map of gene expression data using microarray and RT-qPCR, and flowering time phenotype (Ft, in days). Pearson’s correlation was calculated among 199 genes and the Ft phenotype to show the co-expression pattern of genes in the heat map. The agglomerative hierarchical cluster is shown alongside the heat map to illustrate grouping patterns and Ft phenotypes. Colour key indicates the correlation higher than absolute value 0.3 between genes: blue, negative correlation; red, positive correlation. The flowering time trait is located in block C, as indicated by the arrow. The displayed data are included as Supplementary Table S7 at JXB online.
Co-regulation network of Ft genes and flowering time
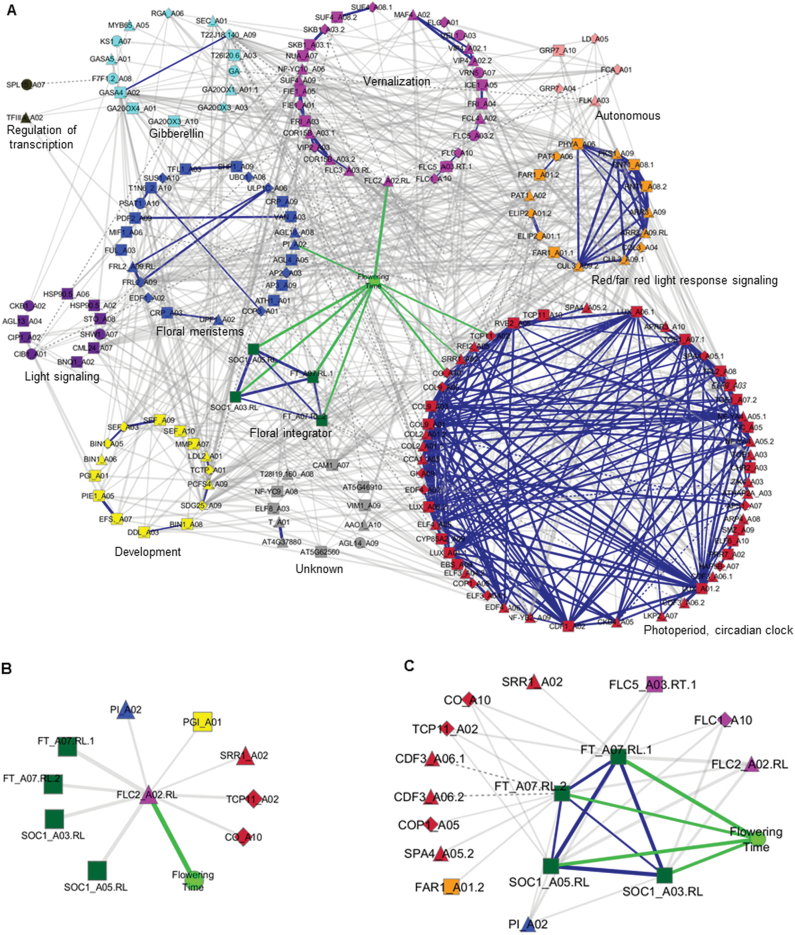
To elucidate further how gene expression is regulated in leaves of DH68 lines and to identify genes/alleles underlying the flowering time QTLs, a co-regulation network was constructed. For this, a Pearson’s correlation matrix calculated based on LOD scores of 198 Ft genes with eQTLs and flowering time data of 2008 over all DH68 lines, was superimposed onto Cytoscape (a Pearson’s correlation was performed). The aim was to construct a co-regulation network in order to visualize correlations of the flowering time phenotype with Ft genes, grouped according to their functional pathways, and also to look at correlations among genes both within and between functional pathways and flowering time (Fig. 5A; Supplementary Table S8 at JXB online). FLC2_A02.RL had the highest correlation (r=0.89) with the flowering time phenotype. Of particular interest are four major floral integrator genes, namely FT_A07.RL.1, FT_A07.RL.2, SOC1_A03.RL, and SOC1_A05.RL, that are significantly correlated to each other, but also to the flowering time phenotype and FLC2_A02.RL. Similarly, the photoperiod/circadian clock genes (CO_A10, TCP11_A02, and SRR1_A02) and the floral meristem gene PI_A02 are significantly correlated with the flowering time phenotype (Fig. 5A).
Fig. 5.
Co-regulation expression network of flowering time genes and flowering time in Brassica rapa. (A) Whole network visualization of correlation of all 197 flowering genes and flowering time phenotype. Genes were arranged in circles according to 11 different flowering time pathways. (B) A co-regulation subnetwork was extracted from the whole network focused on FLC2_A02.RL. (C) A co-regulation subnetwork was extracted from the whole network focused on the floral integrator pathway genes, which are all correlated to the flowering time trait. The correlation coefficients were calculated by using the LOD score values of 197 genes after eQTL mapping and of flowering time phenotype (Ft, in days) to visualize the co-regulation of flowering time pathway genes and phenotypic traits. The 197 Ft genes were from 11 functional pathways known for flowering time regulation. Only significant correlation coefficient values (r |>| 0.3) at a P-value of 0.05 were shown as edges in the network. The vertices of the network indicate genes or phenotype, and edges represent significant correlation. The grey coloured edges indicate the correlation coefficient between genes from different pathways, blue colour edges the correlation coefficient between genes within a pathway, and green colour edges the correlation coefficient between genes and flowering time phenotype. The wider the edge width, the higher the correlation coefficient. The solid lines indicate positive correlation and dotted lines indicate negative correlation. Shapes of the nodes indicate cis/trans regulation of genes (triangle, cis; square, trans; diamond, cis/trans regulation; and circle, unknown regulation), and a green coloured round-shaped node indicates the flowering time phenotypic trait. The displayed data are included as Supplementary Table S8 at JXB online.
In Fig. 5B, FLC2_A02.RL is depicted as a central node with strong correlation to the flowering time phenotype, and nine genes which also are intercorrelated; these genes are the floral integrators SOC1_A05.RL, SOC1_A03.RL, FT_A07.RL.1, and FT_A07.RL.2, the floral meristem identity gene PI_A02, and development and the photoperiod/circadian clock pathway genes PGI_A01, SRR1_A02, TCP11_A02, and CO_A10. These data clearly point to BrFLC2 as a candidate gene for the major flowering QTL and key regulator of these nine Ft genes. The total network clearly shows that the genes in the photoperiod/circadian pathway are strongly correlated, while expression of genes in the red/far red response pathways also show strong intracorrelation. However, this is much less the case for all other functional pathways, for example genes in the vernalization pathway (Fig. 5A).
In order to better visualize interpathway correlations, every functional cluster inside the general gene network was extracted. This results in a presentation of the genes involved in these functional pathways with their correlations to genes from other pathways, resulting in a multitude of interactions and their connections to the flowering time phenotype (Fig. 5; Supplementary Fig. S4A–K at JXB online). For instance, based on the floral integrator functional pathway, a core subnetwork was revealed that connected to 12 nodes, containing three vernalization genes (FLC1_A10.RL, FLC2_A02.RL, and FLC5_A03. RL.1), seven photoperiod/circadian clock pathway genes (SRR1_A02, CO_A10, TCP11_A02, CDF3_A06.1, CDF3_A06.2, COP1_A05, and SPA4_A05.2), one red/far red light response signalling gene FAR1_A01.2, and one floral meristem gene PI_A02. All these genes were positively correlated, while the circadian clock genes CDF3_A06.1 and CDF3_A06.2 were significantly negatively correlated with FT_A07.RL.2 (Fig. 5C). Interestingly, looking at the genes in the unknown functional pathway, CAM1_A07 was clearly correlated to many genes from seven functional pathways and seems to be a major regulator (Supplementary Fig. S4J at JXB online). In Arabidopsis, CAM1 encodes a calmodulin that is involved in thigmomorphogenesis (Chehab et al., 2009). All of these subnetworks overlapped significantly with one another, illustrating the intercorrelation between many genes and pathways which all lead to the regulation of the flowering time phenotype in B. rapa.
Differential expression of duplicated Ft genes
In order to understand the role of Ft genes that are duplicated in B. rapa, experiments were carried out to investigate whether paralogues of the same Arabidopsis genes had common trans-regulators. From the 201 Ft genes with eQTLs, 88 (44%) had ≥2 paralogues corresponding to 36 Arabidopsis orthologous Ft genes (Supplementary Table S9 at JXB online). From this set of genes, eight sets of paralogues had common trans eQTLs, while 16 had different trans eQTLs. The other 12 gene sets mapped cis to linked positions, and not to syntenic positions in the different subgenomes, and were not further analysed. The BrFLC2 and BrFLC3 genes were cis regulated, while BrFLC1 was both cis and trans regulated, and BrFLC5 was trans regulated.
Discussion
The combined use of molecular markers, and phenotypic and gene expression data generated from a segregating population, offers the opportunity to detect phenotypic and expression QTLs from which co-expression networks can be generated that represent important components of flowering time regulation in B. rapa. In this study, a B. rapa genetic map with 456 markers was constructed (Supplementary Fig. S1 at JXB online), including 125 flowering time candidate gene markers, which are used as bridge markers between the genetic DH68 map and the Chinese cabbage physical map, which makes eQTL studies possible, as positional information allows determination of whether eQTLs are cis or trans regulated. The goal is to identify co-location between flowering time QTLs and eQTLs.
It is concluded that genes involved in flowering time regulation are preferentially maintained in B. rapa, as 27.7% of Arabidopsis Ft genes had ≥3 B. rapa paralogues/duplicated Ft genes, while for only 11 (3.0%) of all Arabidopsis genes were no homologues identified in B. rapa. In B. rapa, genome triplication followed by genome fractionation may have expanded gene families that underlie environmental adaptability, as observed in other polyploid species. Another explanation is the gene balance hypothesis, which states that genes encoding proteins that work in complexes are also less fractionated. As many Ft genes are transcription factors, that either work in complexes or are parts of the same modules, this gene balance hypothesis may also explain the high numbers of paralogues retained since polyploidization (Birchler and Veitia, 2010). In a recent publication, Lou et al. (2012) performed a detailed analysis of the circadian genes, a subset of the genes described herein, and concluded that circadian clock genes are preferentially retained relative to their neighbouring genes, a set of randomly chosen genes, and a set of housekeeping genes. They conclude that preferential retention of these circadian genes is consistent with the gene dosage hypothesis, which predicts preferential retention of highly networked or dose-sensitive genes.
When comparing the genetic order of the 125 Ft markers on the DH68 genetic map with the order of these markers based on the Chiifu genome sequence, there was in general a good fit, with few exceptions (Supplementary Fig. S2 at JXB online). At present, it cannot be concluded whether the inconsistency in order of a few markers between the genetic and physical map is because of incorrect map positions, or whether this is caused by genome rearrangements that differentiate the reference genome Chiifu and the two parental genotypes used in this cross.
Flowering time data are presented for the DH68 lines, progeny from a cross between Yellow sarson (YS-143) and Pak choi (PC-175). In total, two genomic regions with flowering QTLs were identified in this study, the two flowering QTLs were also reported in previous studies (Lou et al., 2007; Li et al., 2009; Zhao et al., 2010). Co-location of flowering QTLs on A02 with the Ft gene BrFLC2 is the first indication that allelic variation in this gene is the cause for the flowering QTLs. Additional evidence is provided by co-localization of the eQTL for BrFLC2 with the major flowering QTL on A02 (Fig. 3).
In Arabidopsis, FLC acts as a dosage-dependent repressor of flowering, whose expression is down-regulated by vernalization (Michaels and Amasino, 1999). Evidence accumulated that the FLC homologues in Brasscia species act similarly to AtFLC and play a central role in the repression of flowering time (Schranz et al., 2002; Lou et al., 2007; Li et al., 2009; Zhao et al., 2010). Zhao et al. (2010) measured BrFLC2 expression during plant development after varying periods of vernalization and demonstrated that seedling vernalization decreases BrFLC2 expression in a dosage-dependent matter, and that this effect is most evident at the seedling stage, where flowering time is already established. The BrFLC paralogue BrFLC1 was mapped on A10, with an FLC1_A10.RL cis eQTL (83.6 cM, LOD=2.8 by microarray and LOD=3.1 by RT-qPCR), but at this locus no QTL for flowering time is identified in this study. However, in the study of Lou et al. (2007), using the reciprocal cross, a flowering QTL at this locus was identified in August 2005 only (2.7 LOD value, 18% explained variation). Li et al. (2009) mapped QTLs for bolting, budding, lobe depth ratio, and flowering time that co-localized with the BrFLC1 locus in an F2 mapping population from a cross between ‘Yellow sarson’ and ‘Osome’, a leafy vegetable. Allelic variation in BrFLC1 also had an effect on flowering time when a Chinese cabbage collection was studied, caused by a splicing site variation in BrFLC1 alleles (Yuan et al., 2009). For the flowering QTL on A09, no clear candidate gene could be predicted among the genetic or in silico mapped B. rapa homologues of A. thaliana Ft genes. However, in this study, the possibility cannot be excluded that novel genes in B. rapa, possibly orthologues of A. thaliana genes without a role in regulating flowering time, play a role in regulating flowering time in B. rapa. It is possible that this A09 QTL can be explained by B. rapa genes that have no homology to A. thaliana flowering genes.
Figure 3 shows an overview of eQTLs and flowering QTLs. In Supplementary Fig. S2 at JXB online, the genetic and physical maps are aligned with the Ft genes without genetic map positions placed in intervals between mapped flanking markers; this facilitates the identification of cis eQTLs and genes underlying eQTLs and flowering QTLs. A clear diagonal is visualized, which demonstrates that the expression of many genes is cis regulated. As described in other studies (Schadt et al., 2008; Holloway et al., 2011; Jiang et al., 2011), generally cis eQTLs exert stronger effects than trans eQTLs. In this study, cis eQTLs have LOD scores ranging from 3.0 to 30.4 (average 5.4, median 4.2), while trans eQTLs range from 3.0 to 58.6 (average 4.3, median 3.5). Forty-eight Ft genes (represented by 103 features) had eQTLs that mapped to A09; both cis and trans regulated (Supplementary Table S6 at JXB online). This implies that the expression of many (Ft candidate) genes (103 features out of 768 features, 13.4%) is genetically regulated by trans-acting regulators on A09. Remarkably, only one minor flowering QTL is detected on A09, proximal to the QTL hotspot (Table 3; Supplementary Fig. S3C at JXB online). It is also remarkable that from the eight flowering time eQTL hotspots, only the one on A02 co-localizes with a major flowering time QTL, while for the other loci it has to be concluded that regulatory hotspots for expression differences in Ft genes do not affect the flowering time itself in this experiment. It was investigated whether these flowering time eQTL hotspots co-localize with flowering time QTLs identified in other experiments using the same or other populations. This was not the case for DH68, but for its reciprocal cross DH38 (PC-175×YS-143) and for DH30 (Japanese vegetable turnip×common parent YS-143), flowering QTLs were detected on corresponding positions on A01, A03, A06, and A10; however, flowering QTLs have also been detected on A05 and A09 without eQTL hotspots (Lou et al., 2007).
In several cases, paralogues of the same genes, such as BrSOC1, BrFT, but also BrFLC, are regulated by the same trans-acting factors (Supplementary Tables S7, S9 at JXB online). Overall, however, only 33% (eight) of the paralogue sets of genes had common trans eQTLs, while 67% (16) had different trans eQTLs in this study.
Flowering time in days, gene expression profiles of leaves from 5-week-old DH lines, and physical and genetic map positions of Ft genes were jointly analysed. This led to the detection of two QTLs for flowering time, with one major flowering QTL on A02 mapping at the BrFLC2 locus, which points to a central role for BrFLC2 in regulating flowering time, as there is a major cis eQTL for BrFLC2, trans eQTLs for floral integrator genes BrFT and BrSOC1 and the daylength/circadian rhythm genes BrCO, and cis eQTLs for BrSRR1 and BrTCP11 (Fig. 5A).
The MADS domain transcription factor suppressor of overexpression of constans (SOC1) is a floral integrator. Several MADS domain proteins, including SOC1 heterodimers, are able to bind SOC1 regulatory sequences, while SOCI also binds to many flowering time regulatory and floral homeotic genes, which also bind to the SOC1 regulatory sequences. Data of Immink et al. (2012) proved that SOC1 constitutes a major hub in the regulatory networks underlying both floral timing and flower development. The TCP11 protein is found to interact with different components of the core circadian clock, while SRR1 plays an important role in phytochrome B light signalling and is also required for the normal clock function (Giraud et al., 2010; Staiger et al., 2013). Mutations or allelic variation in the circadian clock genes can also alter flowering time (Fornara et al., 2010). This is through the fact that circadian clock genes ensure that CO transcription peaks late in the day, which is enhanced by GI under long-day conditions. If CO transcription shifts to earlier in the day, CO is activated not only in long days but also in short days, which leads to earlier FT transcription and flowering under short-day conditions. Arabidopsis SRR1 mutants had only subtle early flowering phenotypes in long days, but flowered much earlier in short days compared with the wild type. Experiments under different daylength regimes for DH68 would be interesting to compare gene co-expression networks.
The ultimate proof for the role of BrFLC2 in flowering time regulation is expression of the PC-175 late allele in YS-143, and to assess flowering time phenotype. Brassica rapa is, however, extremely recalcitrant to transformation, and even though transgenic Chinese cabbage has been reported, no reports of transgenic oil types are published (Jung et al., 2012; Park et al., 2012). Allelic variation of the BrFLC2 gene was determined by sequencing both parental (PC-175 and YS-143) alleles. In the YS-143 allele, a deletion of 56bp was detected at the exon 4 (12bp) and intron 4 (44bp) junctions (Supplementary Fig. S5 at JXB online). The PC-175 sequence did not have this deletion and was identical to the reference Chinese cabbage sequence in this region. Wu et al. (2012) sequenced BrFLC2 alleles from Yellow sarson (accession L143), three Chinese cabbage genotypes, Rapid cycling (accession L144), Mizuna, Neep greens, Turnip, and Caixin, and identified a discontinuous 57bp insertion/deletion (InDel) across exon 4 and intron 4 in Yellow sarson resulting in a non-functional allele (Wu et al., 2012). This deletion was absent in all other vegetable accessions they tested and present in many of the tested oil types, and homozygous in the three Yellow sarson accessions tested, but absent in the other accessions they tested. This deletion in BrFLC2 may be the cause of the major flowering time QTL on A02, and may mask allelic differences of other BrFLC paralogues.
In conclusion, the data presented show that genes involved in flowering time are preferentially maintained in B. rapa, as the percentage of Arabidopsis Ft genes with ≥3 B. rapa paralogues was three times more (27.6%) than this percentage for all Arabidopsis genes (10.2%). The combined genetic analysis of flowering time and gene expression profiles in leaves from 5-week-old non-flowering DH lines from a cross between an oil type and a leafy type B. rapa led to identification of BrFLC2 as a candidate gene for a major flowering QTL on A02, and as a trans-regulator of important floral integrator genes BrSOC1 and BrFT, and key daylength/circadian rhythm genes BrCO, BrSRR1, and BrTCP11. This showed that orthologues of A. thaliana Ft genes play similar roles in B. rapa, and that for the genetic analysis of flowering time in B. rapa, Arabidopsis is a good model system.
Supplementary data
Supplementary data are available at JXB online.
Figure S1. An updated genetic linkage map constructed with 125 candidate Ft gene target markers (GT) based on the DH68 population (YS-143×PC-175).
Figure S2. Ft genes as anchors between the genetic map (YS-143×PC-175) (cM) and physical map (Mb).
Figure S3. The relative abundance of 13 candidate Ft gene transcripts using RT-qPCR, plotted at 1 cM distances along all linkage groups.
Figure S4. Extraction of functional clusters from the general gene network.
Figure S5. BrFLC2 nucleotide sequence (DNA) analyses within exon 4–exon 5 and comparison with Arabidopsis thaliana (cDNA) and the reference genome Chiifu BrFLC2.
Table S1. Selected flowering time genes.
Table S2. The sequence-informative markers of the flowering time genes mapped on a genetic linkage map.
Table S3. Primers for real-time RT-PCR.
Table S4. Annotation of the flowering time genes used in the microarray.
Table S5. Annotation of the sequence of flowering time genes in the microarray.
Table S6. Detailed information for eQTLs and pQTLs identified in this study.
Table S7. Detailed co-expression correlation values.
Table S8. The LOD correlation matrix of eQTLs.
Table S9. eQTL location of differentially duplicated paralogues/probes for the Ft gene set (2–8 copies).
Table S10. List of the physical order all Ft candidate genes and eQTLs mapped on their genetic locations.
Acknowledgements
We thank René Smulders and Yiqian Fu for critical feedback on the manuscript, Xiaoming Song for calculation of the eQTL numbers, and Jihua Chen for his advice concerning the figures. This work was supported by the Funding PSA program ‘Genomics of developmental and nutritional traits in Brassica rapa’ [grant no. 08-PSA-BD-02 to GB]; by the National Program on Key Basic Research Projects, the 973 Program [grant no. 2012CB113900 to XH]; by the National Natural Science Foundation of China [grant no. 31171976 to JZ]; and the Hebei Science Found for Distinguished Young Scholars [grant no. C2013204118 to JZ].
References
- Alonso-Blanco C, Aarts MGM, Bentsink L, Keurentjes JJB, Reymond M, Vreugdenhil D, Koornneef M. 2009. What has natural variation taught us about plant development, physiology, and adaptation? The Plant Cell 21, 1877–1896 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Andres F, Coupland G. 2012. The genetic basis of flowering responses to seasonal cues. Nature Reviews Genetics 13, 627–639 [DOI] [PubMed] [Google Scholar]
- Beek JG, Verkerk R, Zabel P, Lindhout P. 1992. Mapping strategy for resistance genes in tomato based on RFLPs between cultivars: Cf9 (resistance to Cladosporium fulvum) on chromosome 1. Theoretical and Applied Genetics 84, 106–112 [DOI] [PubMed] [Google Scholar]
- Birchler JA, Veitia RA. 2010. The gene balance hypothesis: implications for gene regulation, quantitative traits and evolution. New Phytologist 186, 54–62 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bonnema G, Carpio DPD, Zhao JJ. 2011. Diversity analysis and molecular taxonomy of Brassica vegetable crops. In: Kole C, Sadowski J, eds, Genetics, genomics and breeding of crop plants. Enfield, USA: Science Publishers, 81–124 [Google Scholar]
- Brachi B, Faure N, Horton M, Flahauw E, Vazquez A, Nordborg M, Bergelson J, Cuguen J, Roux F. 2010. Linkage and association mapping of Arabidopsis thaliana flowering time in nature. PLOS Genetics 6, e1000940. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chehab EW, Eich E, Braam J. 2009. Thigmomorphogenesis: a complex plant response to mechano-stimulation. Journal of Experimental Botany 60, 43–56 [DOI] [PubMed] [Google Scholar]
- Cheng F, Wu J, Fang L, Sun S, Liu B, Lin K, Bonnema G, Wang X. 2012. Biased gene fractionation and dominant gene expression among the subgenomes of Brassica rapa . PLoS One 7, e36442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Bodt S, Maere S, Van de Peer Y. 2005. Genome duplication and the origin of angiosperms. Trends in Ecology and Evolution 20, 591–597 [DOI] [PubMed] [Google Scholar]
- Flagel LE, Wendel JF. 2009. Gene duplication and evolutionary novelty in plants. New Phytologist 183, 557–564 [DOI] [PubMed] [Google Scholar]
- Fornara F, de Montaigu A, Coupland G. 2010. SnapShot: control of flowering in Arabidopsis . Cell 141, 550–550 [DOI] [PubMed] [Google Scholar]
- Fu J, Jansen RC. 2006. Optimal design and analysis of genetic studies on gene expression. Genetics 172, 1993–1999 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Giraud E, Ng S, Carrie C, Duncan O, Low J, Lee CP, van Aken O, Harvey Millar A, Murcha M, Whelan J. 2010. TCP transcription factors link the regulation of genes encoding mitochondrial proteins with the circadian clock in Arabidopsis thaliana . The Plant Cell 22, 3921–3934 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holloway B, Luck S, Beatty M, Rafalski JA, Li B. 2011. Genome-wide expression quantitative trait loci (eQTL) analysis in maize. BMC Genomics 12, 336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ihaka R, Gentleman R. 1996. R: a language for data analysis and graphics. Journal of Computational and Graphical Statistics 5, 299–314 [Google Scholar]
- Immink RGH, Posé D, Ferrario S, et al. 2012. Characterization of SOC1’s central role in flowering by the identification of its upstream and downstream regulators. Plant Physiology 160, 433–449 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jansen RC, Nap JP. 2001. Genetical genomics: the added value from segregation. Trends in Genetics 17, 388–391 [DOI] [PubMed] [Google Scholar]
- Jiang N, Wang M, Jia T, Wang L, Leach L, Hackett C, Marshall D, Luo Z. 2011. A robust statistical method for association-based eQTL analysis. PLoS One 6, e23192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jung YJ, Lee SY, Moon YS, Kang KK. 2012. Enhanced resistance to bacterial and fungal pathogens by overexpression of a human cathelicidin antimicrobial peptide (hCAP18/ LL-37) in Chinese cabbage. Plant Biotechnology Reports 6, 39–46 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keurentjes JJB, Fu J, Terpstra IR, Garcia JM, Van Den, Ackerveken G, Snoek LB, Peeters AJM, Vreugdenhil D, Koornneef M, Jansen RC. 2007. Regulatory network construction in Arabidopsis by using genome-wide gene expression quantitative trait loci. Proceedings of the National Academy of Sciences, USA 104, 1708–1713 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim SY, Park BS, Kwon SJ, et al. 2007. Arabidopsis and Brassica rapa by the overexpression of FLOWERING LOCUS C (FLC) homologs isolated from Chinese cabbage (Brassica rapa L. ssp. pekinensis). Plant Cell Reports 26, 327–336 [DOI] [PubMed] [Google Scholar]
- Li F, Kitashiba H, Inaba K, Nishio T. 2009. A Brassica rapa linkage map of EST-based SNP markers for identification of candidate genes controlling flowering time and leaf morphological traits. DNA Research 16, 311–323 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Livak KJ, Schmittgen TD. 2001. Analysis of relative gene expression data using real-time quantitative PCR and the 2-ΔΔCT method. Methods 25, 402–408 [DOI] [PubMed] [Google Scholar]
- Lou P, Wu J, Cheng F, Cressman LG, Wang X, Robertson McClung C. 2012. Preferential retention of circadian clock genes during diploidization following whole genome triplication in Brassica rapa . The Plant Cell 24, 2415–2426 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lou P, Zhao J, Kim JS, et al. 2007. Quantitative trait loci for flowering time and morphological traits in multiple populations of Brassica rapa . Journal of Experimental Botany 58, 4005–4016 [DOI] [PubMed] [Google Scholar]
- Michaels SD, Amasino RM. 1999. FLOWERING LOCUS C encodes a novel MADS domain protein that acts as a repressor of flowering. The Plant Cell 11, 949–956 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Michaels SD, He Y, Scortecci KC, Amasino RM. 2003. Attenuation of FLOWERING LOCUS C activity as a mechanism for the evolution of summer-annual flowering behavior in Arabidopsis . Proceedings of the National Academy of Sciences, USA 100, 10102–10107 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Montgomery J, Wittwer CT, Palais R, Zhou L. 2007. Simultaneous mutation scanning and genotyping by high-resolution DNA melting analysis. Nature Protocols 2, 59–66 [DOI] [PubMed] [Google Scholar]
- Okazaki K, Sakamoto K, Kikuchi R, Saito A, Togashi E, Kuginuki Y, Matsumoto S, Hirai M. 2007. Mapping and characterization of FLC homologs and QTL analysis of flowering time in Brassica oleracea . Theoretical and Applied Genetics 114, 595–608 [DOI] [PubMed] [Google Scholar]
- Østergaard L, King GJ. 2008. Standardized gene nomenclature for the Brassica genus. Plant Methods 4, 1–10 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Park YH, Choi C, Park EM, Kim HS, Park HJ, Bae SC, Ahn I, Kim MG, Park SR, Hwang DJ. 2012. Over-expression of rice leucine-rich repeat protein results in activation of defense response, thereby enhancing resistance to bacterial soft rot in Chinese cabbage. Plant Cell Reports 31, 1845–1850 [DOI] [PubMed] [Google Scholar]
- Park JY, Koo DH, Hong CP, et al. 2005. Physical mapping and microsynteny of Brassica rapa ssp. pekinensis genome corresponding to a 222 kbp gene-rich region of Arabidopsis chromosome 4 and partially duplicated on chromosome 5. Molecular Genetics and Genomics 274, 579–588 [DOI] [PubMed] [Google Scholar]
- Roux F, Touzet P, Cuguen J, Le Corre V. 2006. How to be early flowering: an evolutionary perspective. Trends in Plant Science 11, 375–381 [DOI] [PubMed] [Google Scholar]
- Sankoff D, Zheng C, Zhu Q. 2010. The collapse of gene complement following whole genome duplication. BMC Genomics 11, 313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schadt EE, Molony C, Chudin E, et al. 2008. Mapping the genetic architecture of gene expression in human liver. PLoS Biology 6, 1020–1032 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schranz ME, Quijada P, Sung SB, Lukens L, Amasino R, Osborn TC. 2002. Characterization and effects of the replicated flowering time gene FLC in Brassica rapa . Genetics 162, 1457–1468 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shannon P, Markiel A, Ozier O, Baliga NS, Wang JT, Ramage D, Amin N, Schwikowski B, Ideker T. 2003. Cytoscape: a software environment for integrated models of biomolecular interaction networks. Genome Research 13, 2498–2504 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Staiger D, Allenbach L, Salathia N, Fiechter V, Davis SJ, Millar AJ, Chory J, Fankhauser C. 2003. The arabidopsis SRR1 gene mediates phyB signaling and is required for normal circadian clock function. Genes and Development 17, 256–268 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tadege M, Sheldon CC, Helliwell CA, Stoutjesdijk P, Dennis ES, Peacock WJ. 2001. Control of flowering time by FLC orthologues in Brassica napus . The Plant Journal 28, 545–553 [DOI] [PubMed] [Google Scholar]
- Tang H, Woodhouse MR, Cheng F, Schnable JC, Pedersen BS, Conant G, Wang X, Freeling M, Pires JC. 2012. Altered patterns of fractionation and exon deletions in Brassica rapa support a two-step model of paleohexaploidy. Genetics 190, 1563–1574 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trick M, Cheung F, Drou N, Fraser F, Lobenhofer EK, Hurban P, Magusin A, Town CD, Bancroft I. 2009. A newly-developed community Microarray resource for transcriptome profiling in Brassica species enables the confirmation of Brassica-specific expressed sequences. BMC Plant Biology 9, 50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Ooijen JW. 2004. MapQTL® 5, software for the mapping of quantitative trait loci in experimental populations. Wageningen, The Netherlands [Google Scholar]
- Voorrips RE. 2002. MapChart: software for the graphical presentation of linkage maps and QTLs. Journal of Heredity 93, 77–78 [DOI] [PubMed] [Google Scholar]
- Wang J, Long Y, Wu B, Liu J, Jiang C, Shi L, Zhao J, King GJ, Meng J. 2009. The evolution of Brassica napus FLOWERING LOCUST paralogues in the context of inverted chromosomal duplication blocks. BMC Evolutionary Biology 9, 271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang X, Wang H, Wang J, et al. 2011. The genome of the mesopolyploid crop species Brassica rapa . Nature Genetics 43, 1035–1039 [DOI] [PubMed] [Google Scholar]
- Wu J, Wei K, Cheng F, Li S, Wang Q, Zhao J, Bonnema G, Wang X. 2012. A naturally occurring InDel variation in BraA.FLC.b (BrFLC2) associated with flowering time variation in Brassica rapa . BMC Plant Biology 12, 151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yuan Y, Wu J, Sun R, Zhang X, Xu D, Bonnema G, Wang X. 2009. A naturally occurring splicing site mutation in the Brassica rapa FLC1 gene is associated with variation in flowering time. Journal of Experimental Botany 60, 1299–1308 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang X, Cal AJ, Borevitz JO. 2011. Genetic architecture of regulatory variation in Arabidopsis thaliana . Genome Research 21, 725–733 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao J, Wang X, Deng B, Lou P, Wu J, Sun R, Xu Z, Vromans J, Koornneef M, Bonnema G. 2005. Genetic relationships within Brassica rapa as inferred from AFLP fingerprints. Theoretical and Applied Genetics 110, 1301–1314 [DOI] [PubMed] [Google Scholar]
- Zhao J, Kulkarni V, Liu N, Pino Del Carpio D, Bucher J, Bonnema G. 2010. BrFLC2 (FLOWERING LOCUS C) as a candidate gene for a vernalization response QTL in Brassica rapa . Journal of Experimental Botany 61, 1817–1825 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zou X, Suppanz I, Raman H, Hou J, Wang J, Long Y, Jung C, Meng J. 2012. Comparative analysis of FLC homologues in Brassicaceae provides insight into their role in the evolution of oilseed rape . PLoS One 7, e45751. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.