Abstract
Background
The changes and correlations of TRAIL (TNF-related apoptosis-inducing-ligand) and CXCL8 (IL8) prior to treatment and three months following therapy as well as the corresponding Positron emission tomography (PET/CT) (SUVmax: standardized uptake maximum values) results were evaluated.
Material/Methods
The measurements were taken before and after treatment for comparison purposes. The study population comprised 29 patients with Metastatic Colorectal cancer (MCRC), undergoing PET/CT scanning prior to treatment.
Results
There were significant changes prior to treatment and three months later for sTRAIL (p=0.0080) and CXCL8 (p=0.0001)values. Generally, sTRAIL values were increasing during therapy, while a decrease was observed for CXCL8. Correlation analysis was applied to the data and revealed significant correlations for the SUVmax in the primary tumor prior to treatment and CXCL8 prior to therapy (p=0.0303). Furthermore, significant correlations were observed for the SUVmax and sTRAIL (p=0.0237) as well as CXCL8 (p=0.0002) three months after treatment initiation. CXCL8 prior to treatment was also correlated with the SUV three months after onset of treatment (p=0.0072). A significant correlation was noted for one combination of two variables, the SUVmax in the metastases and CXCL8 prior to treatment (p=0.0175). These results are supported when we group the SUVmax in the metastases following treatment into two groups with SUVmax <5 and SUVmax >5.
Conclusions
This study provides evidence that proteomics patterns of sTRAIL and CXCL8 predict tumor response und survival in MCRC patients treated with bevacizumab and within a high concordance of FDG-PET/CT findings.
Keywords: metastatic colorectal cancer (MCRC), CXCL8 (IL8), soluble TRAIL (sTRAIL), anti VEGF antibody (Bevacizumab), F-18-Deoxyglucose positron emission tomography (FDG-PET/CT), proteomics study, (SUVmax: standardized uptake maximum values)
Background
Colorectal cancer (CRC) belongs to the most common malignancies and accounts for almost 10% of all cancer deaths in the Western World. [1]. Treatment for most patients with surgery alone still remains very low and chemotherapy and/or radiotherapy are usually needed in cases of high risk stage disease [2]. The development of CRC is characterized by a sequence of events during which normal colonic epithelium gradually transforms to carcinoma tissue, in most cases via the development of colorectal adenomas [3].
Apoptosis, or programmed cell death, plays an important role in the development and maintenance of tissue homeostasis but also represents an effective mechanism by which abnormal cells, such as tumor cells, can be eliminated [4]. Apoptosis can be induced passively, through the lack of essential survival signals, or actively, through the ligand induced trimerisation of specific death receptors of the tumor necrosis factor (TNF) receptor family, such as the TNF-related apoptosis-inducing-ligand (TRAIL) receptors [5]. TRAIL is a newly identified member of this superfamily, which induces apoptosis of transformed cells but not normal cells. Like TNF and Fas ligand (FASL), TRAIL also exists physiologically in a biologically active soluble homotrimeric form, serum soluble-TRAIL; sTRAIL [6].
Several recent studies have indicated that sTRAIL is involved in the pathophysiology of different disease states such as cancer and inflammation, and defective apoptosis due to its interaction with its ligand preventing signaling for apoptosis may contribute to these diseases [7]. It has also been observed that the cytotoxic effects of antiangiogenic agents are increased in clinical phase II and III studies when these agents are combined with TRAIL-related therapies [6–8].
Although CXCL8 expression has been recently correlated with the tumor pathology of various carcinoma types, the role of CXCL8 in tumor development and metastasis is still not fully understood and often discussed controversially. Recent studies have shown that CXCL8 and its receptors CXCR1 and CXCR2 are significantly up-regulated in CRC and act as regulators of proliferation, angiogenesis, and metastasis [9–12]. Dimberg et al. reported that high plasma levels of CXCL8 tend to be correlated with distant metastasis, indicating an advanced disease stage [13].
Positron emission tomography (PET) with F-18-Deoxyglucose (FDG), a glucose analogue, is an advanced imaging technique and allows a highly sensitive whole body search for malignant foci, which are detected by their increased glucose metabolism versus benign tissues, and successful scanning has been performed in a wide variety of cancers [14,15].
Several studies have demonstrated the added value of PET in terms of detecting recurrent CRC [16]. Furthermore, tumor angiogenesis and increased glucose metabolism may be to some extent different phenotypic expressions of a common underlying genetic process modulated by stabilization of hypoxia-inducible factors [17]. As compared to other cancer types, increased knowledge about the genetic and functional state of CRC may help to fight cancer. While one approach is based on obtaining genetic information e.g. directly from tumor tissue specimen, functional data can be retrieved e.g. from nuclear medicine imaging data. Some studies combine the approach via obtaining genetic information via gene arrays with nuclear medicine studies. The favorite tracer for functional studies is F-18-Deoxyglucose (FDG), a tracer for PET, transported and metabolized like glucose, but then trapped following the first metabolic step, the phosphorylation of intracellular FDG. Recently, PET usually applied as a combined PET and computed tomographic study, called PET-CT. Following the intravenous injection of FDG, usually whole body images are acquired to identify tumor and metastatic lesions. However, alternatively a dynamic acquisition can be performed beginning with the injection of the tracer for a time interval of 60 minutes or less. We refer to this technique as dPET-CT. The dynamic data provide the advantage to apply compartment and non-compartment models to gain detailed information from the examination. In particular, a 2-tissue compartment model is the suitable model to assess dynamic FDG data quantitatively. Overall, five parameters are provided by this model: vb, also referred to as fractional blood volume or vessel density, k1 and k2, the parameters for the transport of non-metabolized FDG into and out of the cells, and k3 as well as k4, the parameters for the phosphorylation and dephosphorylation of the intracellular FDG. Several experimental studies had been performed to assess the link of glucose transporters, hexokinases, and other genes, which may have an impact on the regulating genes of the FDG metabolism. For example, Pedersen et al. evaluated the association of the facilitated glucose transporters GLUT-1 and GLUT-3 and the expression of VEGF in two human small-cell lung cancer lines in vivo and in vitro[18]. Very limited data exist about the combination of gene expression data and functional imaging data in patients. The results in the first study demonstrate that about 50% of the total variance of the FDG kinetics is based on the effect of VEGF-A and angiopoietin-2. Interestingly, a multi-regression function, based on the 2-tissue compartment data of FDG, resulted in a correlation coefficient of r=0.75 for VEGF-A and r=0.76 for angiopoietin-2. The results direct to the potential to predict the expression of these genes using the functional data of the 2-tissue compartment model [19]. Gene arrays demand the use of tissue samples, usually obtained by surgery. Another very new approach is the determination of key parameters in blood and the combination with functional nuclear medicine data.
In our previous study, we investigated the possible role of circulating sTRAIL as a predictive marker in metastatic CRC patients’ using humanized monoclonal antibody to human vascular endothelial cell growth factor (VEGF); bevacizumab. Circulating Strail levels provide a sensitive measure of bevacizumab efficacy. In addition, post-bevacizumab treatment serum levels of sTRAIL correlate more with prognosis [20]. The role of TRAIL and another apoptotic marker (FAS/FASL) in cancer patients treated with chemotherapeutic agents has been discussed elsewhere [21]. Cancer cells transfected with CXCL8 show increased cellular proliferation, cell migration, and invasion based on functional assays [22]. Based on our previous findings, we asked the logical question whether there was any correlation between serum sTRAIL levels and the quantitative functional data of PET in MCRC patients undergoing treatment with bevacizumab. Furthermore, we also evaluated CXCL8 in the plasma of the same CRC patients. The changes and correlations of sTRAIL and CXCL8 prior to treatment and three months following therapy as well as the corresponding PET results (SUV max) were evaluated. Furthermore, the data were correlated to survival data to assess the predictive value of the parameters.
We focused in this study on sTRAIL and CXCL8 primarily. PET was performed at the same time points, so that we were able to compare the blood levels of sTRAIL and CXCL8 with the results of PET with FDG.
Material and Methods
Patients
All patients gave their informed, written consent. The study was approved by the local ethics committee and was performed in accordance with the ethical principles of the Declaration of Helsinki. The appropriate requirements were fulfilled. 29 MCRC patients (11 female and 18 male) with liver metastases were included in the study with the mean age of 67.03 years. 27.5% of the patients were previous or actual smokers. All of the patients were classified as stage IV and MCRC is mainly treated with combination of bevacizumab and irinotecan or oxaliplatin-based chemotherapy.
Laboratory methods
ELISA was done using the Diaclone sTRAIL ELISA kit (Gen-Probe, Besancon, France) according to the manufacturer’s instructions. CXCL8 was measured as pg/mL by ELISA in the serum of 29 subjects with stage-4 metastatic colon cancer who also received BevacizumAb therapy. CXCL8 levels in culture supernatants were determined using a “sandwich” ELISA with paired antibodies purchased from Endogen, Inc. (Woburn, MA, USA). 100 μL of the primary monoclonal antibody against CXCL8 (2 μg/mL) were coated on Nunc Maxisorp plates in each well. After overnight incubation at 4°C, plates were washed and blocked for 1 hour with blocking buffer (4% BSA in PBS). Plates were washed four times and 50 μL of culture supernatant or recombinant IL-8 protein at different concentrations (Endogen, Inc.) and 50 μL of biotinylated IL-8 antibody were added to each well. After 2 hours of incubation plates were washed and immunoreactivity determined using an avidin-HRP-TMB detection system (Dako Corp., Carpinteria, CA, USA). Reactions were stopped by addition of 50 μL of 0.18n H2SO4 and absorbance measured at 450 nm in an ELISA microtiter plate reader (Bio-Tek Instruments, Inc., Winooski, VT, USA). A curve of the absorbance versus the concentrations of CXCL8 in the standard wells was plotted and a nonlinear curve fit was emerged Concentrations of CXCL8 in serum samples were evaluated from the standard curve.
PET CT
The study population comprised 17 of the 29 patients with MCRC, undergoing 18 FDG PET/CT scanning prior to treatment. We were able to perform a follow up PET/CT examination three months after onset of therapy in 15/17 patients. Patients were instructed to fast for at least 6 h before an injection of 18F-FDG (5 MBq/kg). Static emission images were acquired from head to thigh beginning 60 min after injection and with 2 min per bed position, on a Gemini PET/CT system (Philips). Images were reconstructed using the maximumlikelihood 3-dimensional algorithm according to standard clinical protocol: 2 iterations, relaxation parameter of 0.05, 5-mm 3-dimensional Gaussian post-filtering, and a 4×4×4 mm voxel grid sampling, and attenuation correction based on a low-dose CT scan. All images were visually interpreted by consensus between two experienced nuclear physicians and standardized uptake values (SUVmax) were calculated from the image data. Tumor volumes were determined by using an interactive, semi-automated method.
Statistical analysis
The data were evaluated using the StataMP software package, version 12.1 (StataCorp, College Station, TX, USA) on a Mac Pro 2×2.93 GHz, 2*6 Core Intel Xeon system with 24 GB RAM using Mac OS X 10.7.4 (Apple, Cupertino, CA, USA). A type I error level of p<0.05 was generally used for the statistical analysis. The Wilcoxon matched pairs signed rank test was used to assess differences in the variables prior and three months after onset of treatment. Correlation tables were calculated for all variables. Furthermore, a multivariate correlation/regression analysis was applied to the data, using the survival as the dependent variable.
Results
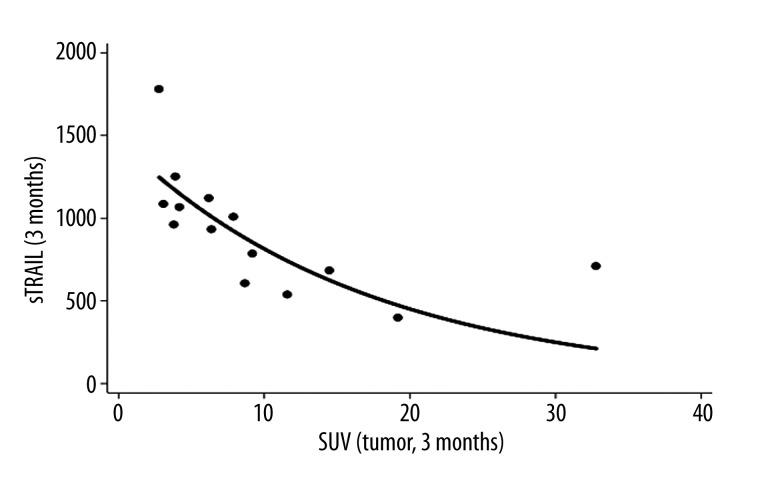
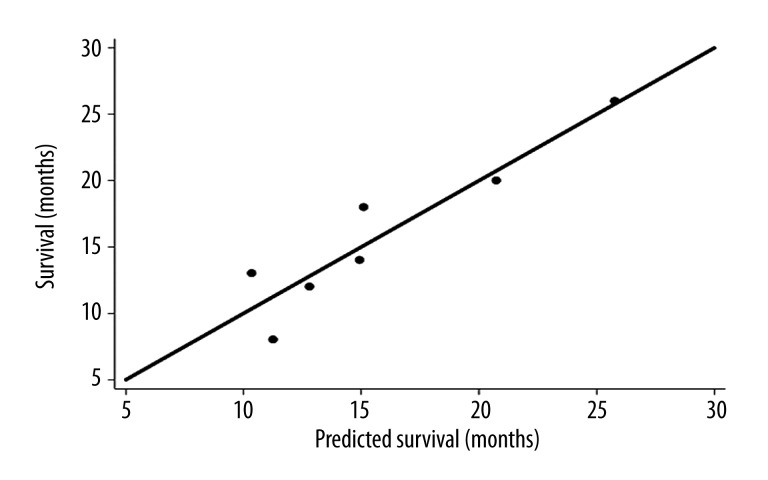
The basic statistical data are provided in Table 1. There were significant changes (p<0.05) prior to treatment and three months later for sTRAIL (p=0.0080) and CXCL8 (p=0.0001), based on the Wilcoxon matched pairs signed rank test. Generally, sTRAIL values were increasing during therapy, while a decrease was observed for CXCL8 without any significant differences for other variables. Correlation analysis revealed significant correlations for the SUVmax in the primary tumor prior to treatment and CXCL8 levels prior to therapy (r=0.5416, p=0.0303, n=16). Furthermore, significant correlations were observed for the SUVmax and sTRAIL levels(r=−0.5987, p=0.0237, n=14) (Figure 1) as well as CXCL8 levels (r=0.8304, p=0.0002, n=14) three months after treatment initiation. CXCL8 levels prior to treatment was also correlated with the SUVmax three months after onset of treatment (r=0.6618, p=0.0072, n=15). Survival data were available in seven patients. No significant correlation was noted for the correlation of survival with any of the other single variables. The highest correlations were obtained for CXCL8 levels prior and three months after onset of therapy and survival with r=0.7109 (p=0.0734) and r=0.7069 (p=0.0757), but both correlation coefficients were not statistically significant on the p<0.05 level. However, one important aspect is the predictive value of the variables, measured prior to treatment. Therefore, we evaluated also the combination of two variables and their correlation to survival. A significant correlation was noted for one combination of two variables, the SUVmax in the metastases and CXCL8 levels prior to treatment (r=0.9315, p=0.0175, n=7) (Figure 2). A multiple linear regression function was calculated, predicting survival from the SUVmax in the metastases and CXCL8 levels prior to therapy. The data show that the SUVmax in the metastases and the serum concentrations of CXCL8 levels prior to therapy are highly predictive for the survival.
Table 1.
The demographics of the study group (stage 4 metastatic colorectal cancer patients).
| Patients no. | Patients (age/gender) | sTRAIL (p.t.) (pg/ml) | sTRAIL (3 m.) (pg/ml) | CXCL8 (p.t.) (pg/ml) | CXCL8 (3 m.) (pg/ml) | SUVmax (tumor., p.t.) | SUVmax (met., p.t.) | SUVmax (tumor., 3 m.) | SUVmax (met., 3 m.) | Survival (months) |
|---|---|---|---|---|---|---|---|---|---|---|
| 1 | 67M | 501.5814 | 397.5194 | 43.096544 | 40.699789 | 16.1 | 3.5 | 19.2 | 5.9 | 12 |
| 2 | 72M | 849.7316 | 1006.6056 | 412.445 | 36.523.568 | 14.4 | 9.9 | 7.9 | 3.2 | |
| 3 | 73M | 1443.125 | 931.02785 | 67.122246 | 46.139845 | 4.2 | 1.9 | 6.4 | 11.5 | 13 |
| 4 | 58M | 653.0687 | 1251.5044 | 41.898184 | 40.611835 | 3.9 | 0.5 | 3.9 | 2.2 | |
| 5 | 62F | 897.8619 | 1084.5968 | 116.31139 | 58.322049 | 6.8 | 4.2 | 3.1 | 1.2 | |
| 6 | 67M | 388.2827 | 959.6922 | 53.073329 | 42.162316 | 5.3 | 7.6 | 3.8 | 4.3 | 20 |
| 7 | 69M | 498.2957 | 1066.6089 | 87.220271 | 36.069397 | 5.1 | 4.1 | 4.2 | 3.3 | 14 |
| 8 | 57F | 938.5702 | 783.93101 | 99.236221 | 80.325302 | 2.5 | 2 | 9.2 | 5.8 | 8 |
| 9 | 73M | 785.4201 | 537.69815 | 200.36118 | 67.928442 | 23.7 | 3.1 | 11.6 | 2 | |
| 10 | 65M | 680.6913 | 708.49874 | 579.51812 | 427.29427 | 17 | 3.9 | 32.8 | 3.6 | |
| 11 | 64M | 740.9074 | 682.15036 | 61.691115 | 49.881125 | 6.1 | 4.5 | 14.5 | 2.8 | 26 |
| 12 | 68F | 682.1504 | 1120.4555 | 127.15772 | 43.096544 | 4.4 | 2.7 | 6.2 | 8.8 | |
| 13 | 75M | 815.2683 | 1779.1667 | 43.699789 | 32.151945 | 4.2 | 1.3 | 2.8 | 1.1 | |
| 14 | 49M | 233.4931 | 604.14496 | 129.21215 | 67.895966 | 9.1 | 3 | 8.7 | 6.5 | |
| 15 | 69F | 739.4297 | 45.52577 | 31.47115 | 5.7 | 4 | 6.5 | 5.2 | ||
| 16 | 71F | 726.1497 | ||||||||
| 17 | 68M | 591.3107 | ||||||||
| 18 | 66M | 897.8619 | 1983.099 | 7.9 | 2.1 | |||||
| 19 | 69F | 1105.535 | 717.316 | 45.52577 | 41.12468 | |||||
| 20 | 72M | 698.2333 | 264.253 | 123.4909 | ||||||
| 21 | 79M | 692.3781 | 810.7833 | 128.4658 | 46.75663 | |||||
| 22 | 65F | 667.5829 | 992.8774 | 192.2149 | 35.50152 | |||||
| 23 | 69M | 1054.406 | 1220.911 | 54.37399 | 49.25081 | |||||
| 24 | 68F | 908.41 | 2301.356 | 43.69979 | 42.96439 | |||||
| 25 | 61F | 479.595 | 463.228 | |||||||
| 26 | 66F | 418.774 | 2037.491 | 184.2637 | 123.4976 | |||||
| 27 | 62M | 406.794 | 1721.683 | 18 | ||||||
| 28 | 71M | 609.8651 | 822.7488 | 46.13985 | 26.13247 | 13.3 | 4.3 | |||
| 29 | 69M | 622.1554 | 1274.654 |
Figure 1.
Correlation of the SUVmax in the tumor and sTRAIL three months after onset of therapy.
Figure 2.
Multivariate correlation analysis with the SUVmax in the metastases and CXCL8 prior to treatment as independent variables and the survival as the dependent variable. The scatter plot demonstrates, that the two variables are highly predictive for the overall survival. The line reflects the line of identity.
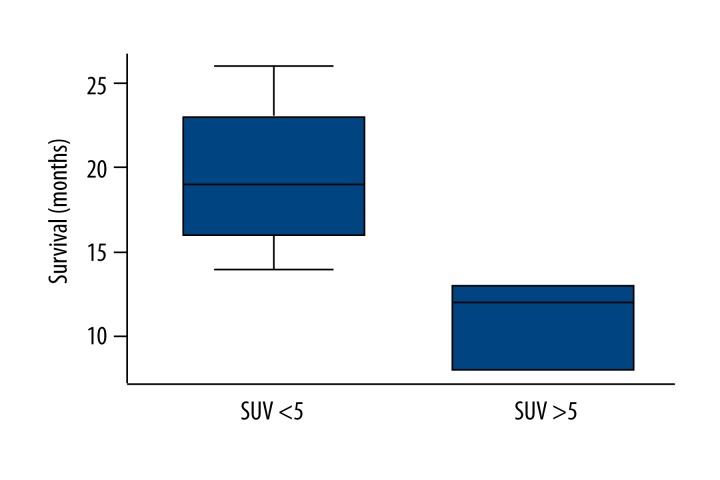
These results are supported when we group the SUVmax in the metastases following treatment into two groups with SUVmax <5 and SUVmax >5. There is a significant difference for both groups regarding overall survival, with a lower survival associated with SUVmax exceeding 5 (Figure 3). In some studies the value of SUVmax differences is emphasized. However, we noted no significant correlations for the survival and the differences of the SUVmaxs, sTRAIL, and CXCL8 prior therapy and three months after onset of treatment (Table 2).
Figure 3.
Box plot of the SUVmax in the metastases three months after onset of treatment and the survival. A limit of SUV=5 was predictive for a short or long survival.
Table 2.
Descriptive statistics of all data.
| Statistical parameter | sTRAIL (p.t.) | sTRAIL (3 m.) | CXCL8 (p.t.) | CXCL8 (3 m.) | SUVmax (tumor, p.t.) | SUV max (tumor, 3 m.) | SUV max (met., p.t.) | SUV max (met., 3 m.) | Survival (months) |
|---|---|---|---|---|---|---|---|---|---|
| Mean | 714.72 | 1058.62 | 127.19 | 68.06 | 8.81 | 9.39 | 3.68 | 4.49 | 15.86 |
| Median | 692.38 | 976.29 | 87.22 | 43.10 | 6.10 | 6.50 | 3.50 | 3.60 | 14.00 |
| SD | 246.41 | 526.79 | 129.33 | 85.03 | 5.95 | 7.91 | 2.26 | 2.89 | 5.96 |
| Number of data | 29 | 26 | 23 | 21 | 17 | 15 | 17 | 13 | 7 |
SD – standard deviation; 3 m. – 3 months after onset of treatment; p.t. – prior therapy; met. – metastases).
Discussion
Angiogenesis is a universal requirement for the growth of solid tumors beyond the limitations of oxygen diffusion from the existing vasculature [23]. Inhibition of angiogenesis has proven to be beneficial in multiple types of malignancies, including colon cancer [24]. VEGF-A is one of the major regulators of both physiological and pathological angiogenesis. Bevacizumab is a humanized monoclonal antibody directed against vascular endothelial growth factor [25]. Even though thousands of patients have been enrolled in randomized clinical trials of bevacizumab, only few translational researches are focused on biomarkers to the response or resistance to combined therapies [26]. Treatment strategies incorporating bevacizumab have demonstrated efficacy in metastatic colorectal cancer (MCRC) [27,28]. Most recent studies indicate potential predictive value for proteomics patterns of sTRAIL and CXCL8 in MCRC patients [20,24]. Moreover in cancer research, several studies have shown that both the membrane bound TRAIL and sTRAIL can induce apoptosis in a wide variety of tumor types by activating death receptors [20,29]. sTRAIL is also used as a positive marker for apoptosis. Another study with human glioblastoma cells has indicated that TRAIL inhibits angiogenesis stimulated by VEGF expression [30]. A significant increase of sTRAIL concentrations following treatment was observed in our study. It can be assumed that, this is based on a general effect of the chemotherapy in these patients inducing apoptosis. Perik et al. Evaluated sTRAIL and other apoptosis associated proteins in 34 patients with breast tumors and 12 healthy control persons [31]. The authors found significant differences for the control group and the breast cancer group regarding the serum levels of apoptotic proteins. However, no correlation of the markers with the individual survival of the tumor patients was demonstrated. We had survival data available in seven patients, but did not find a single correlation of survival with any other of the variables. The highest single pair correlations were obtained not for sTRAIL but for CXCL8, but this was statistically not significant with p<0.05. Recent studies suggesting that alternative angiogenic factors might be potentially involved in resistance to anti-VEGF treatment [32–34]. Sustained tumor angiogenesis could occur through VEGF-independent mechanisms, thus indicating that these angiogenic factors may serve as predictors of bevacizumab efficacy. As in a previous study, increased CXCL8 protein levels, a potent VEGF-independent pro-angiogenic factor, significantly associated with lower relapse rate in ovarian cancer patients treated with bevacizumab and cyclophosphamide [35]. Gabellini et al. investigated the role of CXCL8 and its receptors in melanoma cell lines [36]. The authors noted an increase of both, angiogenesis and cell proliferation, by CXCL8 and the interaction with their receptors. The data support the aspect, that CXCL8 is important for tumor progression. We found significant changes in CXCL8 prior and three months after onset of treatment, giving evidence for a good therapeutic effect. However, no significant correlation with survival was noted. FDG-PET/CT scanning has been successfully performed in a wide variety of cancers including the CRC [37,38]. FDG is primarily dependent on the expression of glucose transporters to get into the cells and the activities of hexokinases. Most of the intracellular FDG is trapped in the phosphorylated form for a certain time. Another parameter is the fractional blood volume, also named as vessel density. A sufficient fractional blood volume is needed so that FDG can be arrive via the blood at the tumor and be available for transport into the cells. However, the glucose transporters are not independent genes but their expression is modulated by other genes. It is already known that the FDG kinetics is modulated by angiogenesis and proliferation associates genes. In colorectal tumors it was reported that VEGF-A and angiopoietin-2 contribute to approximately 50% of the overall variance of the FDG kinetics. Furthermore, k1, the compartment parameter for the FDG transport into the cells, is modulated by cdk2, a gene related to the cell cycle [39]. In tumors with an SUV >12 also cyclin-D2 has an impact on the uptake. These data suggested that PET with FDG can be helpful in CRC to assess the therapeutic effects of treatment. No investigation has been conducted on the relation between FDG-PET/CT findings, treatment response and serum levels of any biomarkers to the best of our knowledge. We investigated the correlation between serum sTRAIL and CXCL8 levels and SUVmax n the detection of treatment efficacy by FDG-PET/CT in bevacizumab treated stage-IV CRC patients. In our study, while there is a high correlation between CXCL8 level 3 months after bevacizumab and SUVmax values, posttreatment (3 months after) sTRAIL level is negatively correlated with SUVmax values and much more significant in SUVmax values of lymph node metastases. This might be expected because of the aggressiveness and the low concentration of cancer cells. Another reason might be of it is well known that the degree of differentiation of primary and metastatic lesions with gastrointestinal cancers can be very different. Overall, it can be assumed that the higher tracer uptake in metastases give evidence for a higher rate of angiogenesis and proliferation in these lesions. Further studies are needed to assess the aggressiveness of tumor and metastatic lesions and their correlation to biomarkers and FDG kinetics. Interestingly, the combination of CXCL8 and the SUVmax in the metastases prior to treatment was highly predictive for the overall survival. However, the number of seven patients is limited, but the results are statistically significant. CXCL8 and the FDG kinetics seem to be sensitive parameters for the biological parameters of malignant lesions. Our study, the measures of the latter may prove to be useful surrogate markers to monitor efficacy of treatment in patients suffering from this disease.
Conclusions
This study provides evidence that proteomics patterns of sTRAIL and CXCL8 predict tumor response und survival in MCRC patients treated with bevacizumab and within a high concordance of FDG-PET/CT findings. Although the association found in our study is biologically plausible, biomarker-embedded translational trials and FDG-PET/CT follow-ups are warranted to validate these findings. The high correlation between CXCL8 and FDG uptake in metastases prior to treatment and survival directed to a promising approach to individualize treatment of patients. Our observations rather reflect the need to investigate and clarify the proper TRAILs for their use in the efficiency of anti-VEGF treatment.
Acknowledgements
We thank all participating patients and volunteers. Metin Erkiliç, Harun Süslü, Gizem Esra Genc.
Footnotes
Declaration of interest
All authors declare that they have no conflict of interest.
Source of support: Departmental sources
References
- 1.Valentini V, Coco C, Gambacorta MA, et al. Evidence and research perspectives for surgeons in the European Rectal Cancer Consensus Conference (EURECA-CC2) Acta Chir Iugosl. 2010;57(3):9–16. doi: 10.2298/aci1003009v. [DOI] [PubMed] [Google Scholar]
- 2.Weitz J, Koch M, Debus J, et al. Colorectal cancer. Lancet. 2005;365:153–65. doi: 10.1016/S0140-6736(05)17706-X. [DOI] [PubMed] [Google Scholar]
- 3.Jass JR, Whitehall VL, Young J, et al. Emerging concepts in colorectal neoplasia. Gastroenterology. 2002;123:862–76. doi: 10.1053/gast.2002.35392. [DOI] [PubMed] [Google Scholar]
- 4.Steinbach G, Lynch PM, Phillips RK, et al. The effect of celecoxib, a cyclooxygenase-2 inhibitor, in familial adenomatous polyposis. N Engl J Med. 2000;342:1946–52. doi: 10.1056/NEJM200006293422603. [DOI] [PubMed] [Google Scholar]
- 5.Opferman JT, Korsmeyer SJ. Apoptosis in the development and maintenance of the immune system. Nat Immunol. 2003;4(5):410–15. doi: 10.1038/ni0503-410. [DOI] [PubMed] [Google Scholar]
- 6.Holland PM. Targeting Apo2L/TRAIL receptors by soluble Apo2L/TRAIL. Cancer Lett. 2013;332(2):156–62. doi: 10.1016/j.canlet.2010.11.001. [DOI] [PubMed] [Google Scholar]
- 7.Wandinger KP, Lünemann JD, Wengert O, et al. TNF related apoptosis inducing ligand (TRAIL) as a potential response marker for interferon-beta treatment in multiple sclerosis. Lancet. 2003;361(9374):2036–43. doi: 10.1016/S0140-6736(03)13641-0. [DOI] [PubMed] [Google Scholar]
- 8.Ding W, Cai T, Zhu H, et al. Synergistic antitumor effect of TRAIL in combination with sunitinib in vitro and in vivo. Cancer Lett. 2010;293:158–66. doi: 10.1016/j.canlet.2010.01.005. [DOI] [PubMed] [Google Scholar]
- 9.Kargi A, Yalcin AD, Erin N, et al. IL8 and Serum Soluble TRAIL Levels Following Anti- VEGF Monoclonal Antibody Treatment in Patients With Metastatic Colon Cancer. Clin Lab. 2012;58(5–6):501–5. Erratum in: Clin Lab, 2012; 58(9–10):1103–7. [PubMed] [Google Scholar]
- 10.Varney ML, Singh S, Li A, et al. Small molecule antagonists for CXCR2 and CXCR1 inhibit human colon cancer liver metastases. Cancer Lett. 2011;300(2):180–88. doi: 10.1016/j.canlet.2010.10.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Oladipo O, Conlon S, O’Grady A, et al. The expression and prognostic impact of CXC-chemokines in stage II and III colorectal cancer epithelial and stromal tissue. Br J Cancer. 2011;104(3):480–87. doi: 10.1038/sj.bjc.6606055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Chen Y, Shi M, Yu GZ, et al. Interleukin-8, a promising predictor for prognosis of pancreatic cancer. World J Gastroenterol. 2012;18(10):1123–29. doi: 10.3748/wjg.v18.i10.1123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Dimberg J, Ström K, Löfgren S, et al. DNA promoter methylation status and protein expression of interleukin-8 in human colorectal adenocarcinomas. Int J Colorectal Dis. 2012;27(6):709–14. doi: 10.1007/s00384-011-1367-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Delbeke D, Vitola JV, Sandler MP, et al. Staging recurrent metastatic colorectal carcinoma with PET. J Nucl Med. 1997;38:1120–96. [PubMed] [Google Scholar]
- 15.Strauss LG, Conti PS. The applications of PET in clinical oncology. J Nucl Med. 1991;32:623–48. [PubMed] [Google Scholar]
- 16.Schiepers C, Penninckx F, De Vadder N, et al. Contribution of PET in the diagnosis of recurrent colorectal cancer: comparison with conventional imaging. Eur J Surg Oncol. 1995;21:517–22. doi: 10.1016/s0748-7983(95)97046-0. [DOI] [PubMed] [Google Scholar]
- 17.Semenza GL. HIF-1 and tumor progression: pathophysiology and therapeutics. Trends Mol Med. 2002;8(4 Suppl):S62–67. doi: 10.1016/s1471-4914(02)02317-1. [DOI] [PubMed] [Google Scholar]
- 18.Pedersen MW, Holm S, Lund EL, et al. Coregulation of glucose uptake and vascular endothelial growth factor (VEGF) in two smallcell lung cancer (SCLC) sublines in vivo and in vitro. Neoplasia. 2001;3(1):80–87. doi: 10.1038/sj.neo.7900133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Strauss LG, Koczan D, Klippel S, et al. Impact of angiogenesis-related gene expression on the tracer kinetics of 18F-FDG in colorectal tumors. J Nucl Med. 2008;49:1238–44. doi: 10.2967/jnumed.108.051599. [DOI] [PubMed] [Google Scholar]
- 20.Bisgin A, Kargi A, Yalcin AD, et al. Increased sTRAIL levels were correlated with patient survival in BevacizumAb treated metastatic colon cancer patients. BMC Cancer. 2012;12:58. doi: 10.1186/1471-2407-12-58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Granci V, Bibeau F, Kramar A, et al. Prognostic significance of TRAIL-R1 and TRAIL-R3 expression in metastatic colorectal carcinomas. Eur J Cancer. 2008;44:2312–18. doi: 10.1016/j.ejca.2008.06.042. [DOI] [PubMed] [Google Scholar]
- 22.Ning Y, Manegold PC, Hong YK, et al. Interleukin-8 is associated with proliferation, migration, angiogenesis and chemosensitivity in vitro and in vivo in colon cancer cell line models. Int J Cancer. 2011;128(9):2038–49. doi: 10.1002/ijc.25562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Carmeliet P. Angiogenesis in health and disease. Nat Med. 2003;9:653–60. doi: 10.1038/nm0603-653. [DOI] [PubMed] [Google Scholar]
- 24.Gerger A, Labonte M, Lenz HJ. Molecular predictors of response to antiangiogenesis therapies. Cancer J. 2011;17:134–41. doi: 10.1097/PPO.0b013e318212db3c. [DOI] [PubMed] [Google Scholar]
- 25.Jubb AM, Harris AL. Biomarkers to predict the clinical efficacy of bevacizumab in cancer. Lancet Oncol. 2010;11:1172–83. doi: 10.1016/S1470-2045(10)70232-1. [DOI] [PubMed] [Google Scholar]
- 26.Hayes DF. Bevacizumab treatment for solid tumors: boon or bust? JAMA. 2011;305:506–8. doi: 10.1001/jama.2011.57. [DOI] [PubMed] [Google Scholar]
- 27.Cunningham D, Atkin W, Lenz HJ, et al. Colorectal cancer. Lancet. 2010;375:1030–47. doi: 10.1016/S0140-6736(10)60353-4. [DOI] [PubMed] [Google Scholar]
- 28.Welch S, Spithoff K, Rumble RB, Maroun J. Bevacizumab combined with chemotherapy for patients with advanced colorectal cancer: a systematic review. Ann Oncol. 2010;21:1152–62. doi: 10.1093/annonc/mdp533. [DOI] [PubMed] [Google Scholar]
- 29.Shi J, Zheng D, Liu Y, et al. Overexpression of soluble TRAIL induces apoptosis in human lung adenocarcinoma and inhibits growth of tumor xenografts in nude mice. Cancer Res. 2005;65(5):1687–92. doi: 10.1158/0008-5472.CAN-04-2749. Erratum in: Cancer Res, 2005; 65(9):3966. [DOI] [PubMed] [Google Scholar]
- 30.Cantarella G, Risuglia N, Dell’eva R, et al. TRAIL inhibits angiogenesis stimulated by VEGF expression in human glioblastoma cells. Br J Cancer. 2006;94(10):1428–35. doi: 10.1038/sj.bjc.6603092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Perik PJ, Van der Graaf WT, De Vries EG, et al. Circulating apoptotic proteins were increased in long-term disease-free breast cancer survivors. Acta Oncol. 2006;45(2):175–83. doi: 10.1080/02841860500482225. [DOI] [PubMed] [Google Scholar]
- 32.Ferrara N. Pathways mediating VEGF-independent tumor angiogenesis. Cytokine Growth Factor Rev. 2010;21:21–26. doi: 10.1016/j.cytogfr.2009.11.003. [DOI] [PubMed] [Google Scholar]
- 33.Waugh DJ, Wilson C. The interleukin-8 pathway in cancer. Clin Cancer Res. 2008;14:6735–41. doi: 10.1158/1078-0432.CCR-07-4843. [DOI] [PubMed] [Google Scholar]
- 34.Cao Y, Zhong W, Sun Y. Improvement of antiangiogenic cancer therapy by understanding the mechanisms of angiogenic factor interplay and drug resistance. Semin Cancer Biol. 2009;19:338–43. doi: 10.1016/j.semcancer.2009.05.001. [DOI] [PubMed] [Google Scholar]
- 35.Schultheis AM, Lurje G, Rhodes KE, et al. Polymorphisms and clinical outcome in recurrent ovarian cancer treated with cyclophosphamide and bevacizumab. Clin Cancer Res. 2008;14:7554–63. doi: 10.1158/1078-0432.CCR-08-0351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Gabellini C, Trisciuoglio D, Desideri M, et al. Functional activity of CXCL8 receptors, CXCR1 and CXCR2, on human malignant melanoma progression. Eur J Cancer. 2009;45(14):2618–27. doi: 10.1016/j.ejca.2009.07.007. [DOI] [PubMed] [Google Scholar]
- 37.Huebner RH, Park KC, Shepherd JE, et al. A meta-analysis of the literature for whole-body FDG-PET detection of recurrent colorectal cancer. J Nucl Med. 2000;41:1177–89. [PubMed] [Google Scholar]
- 38.Delbeke D, Vitola JV, Sandler MP, et al. Staging recurrent metastatic colorectal carcinoma with PET. J Nucl Med. 1997;38:1120–96. [PubMed] [Google Scholar]
- 39.Strauss LG, Koczan D, Klippel S, et al. Impact of cell-proliferation-associated gene expression on 2-deoxy-2-(18)f]fluoro-D-glucose (FDG) kinetics as measured by dynamic positron emission tomography (dPET) in colorectal tumors. Mol Imaging Biol. 2011;13(6):1290–300. doi: 10.1007/s11307-010-0465-z. [DOI] [PubMed] [Google Scholar]