Abstract
Aim
Characterize clinical factors related to nocturia and sleep disruption in PD using polysomnography (PSG).
Methods
Sixty-three PD patients were recruited regardless of sleep or voiding complaints from a university-based movement disorders clinic for a 48-hour inpatient PSG protocol. Nocturia frequency and bother related to urinary symptoms were assessed using the International Prostate Symptom Score (IPSS) and were corroborated by measurements of PSG-defined sleep made immediately preceding and subsequent to each in-lab voiding episode. PSG measures included whole-night total sleep time (TST), sleep efficiency (SE), Apnea/Hypopnea Index (AHI), and time to PSG-defined sleep following nocturia episodes. Differences between groups were assessed using Mantel-Haenszel chi-square, t-tests, or Wilcoxon signed rank tests. Linear regression was used to assess factors associated with reported nocturia frequency.
Results
Sixty patients completed the IPSS. Thirty-seven (61%) reported at least 2 nocturia episodes nightly; those individuals demonstrated lower PSG-defined SE (p =.01) and TST (p =.02) than patients with 0-1 episodes. Participants reporting 2-3 episodes of nocturia with high bother on the IPSS (n=12) demonstrated lower whole-night TST (280.5 ± 116.1 min vs. 372.5 ± 58.7 min, p=0.03) and worse SE (59.2% ± 22.7 vs. 75.9% ± 11.2, p=0.04) when compared to participants with 2-3 episodes of nocturia with low bother (n=13).
Conclusions
These results verify objectively that PD patients with nocturia have poor sleep. Furthermore, among individuals with comparable levels of reported nocturia, higher bother is associated with poorer sleep as defined on PSG.
Keywords: nocturia, bother, polysomnography, sleep efficiency, Parkinson disease
Introduction
As Parkinson disease (PD) progresses, non-motor symptoms such as voiding dysfunction and disturbed sleep are more predictive of poor health-related quality of life (HRQOL) than are motor symptoms (1). Nocturia is the most common urinary symptom in PD, occurring in 60-80% of patients, and is among the most bothersome (2; 3). The negative impact of nocturia on sleep may be related to the total number of nocturnal episodes, yet in the non-PD population, there is evidence that the construct of ‘bother’ associated with nocturia may be explanatory for decreased HRQOL (4; 5). Some of the relationship between nocturia and the bother it causes unexplained by the number of voids might be explained by specific parameters of sleep (6; 7). Indeed, one previous study in community-dwelling older adults suggested that nocturia and nocturia accompanied by problems returning to sleep were two distinct conditions (8), and a second study suggested that a subjective feeling of morning sleepiness or difficulty falling back asleep might be related to increased bother (9). However, no objective evidence exists on this issue in PD patients or any other population.
The present study of voiding and sleep in PD is unique because patients underwent an in-laboratory 48-hour polysomnography (PSG) evaluation examining sleep before and after each voiding episode. The study was not designed to examine lower urinary tract function or urine production in PD patients and therefore did not collect data on post-void residuals, total 24-hour urine production, or voided volumes. However, this study did allow a precise and detailed analysis of objectively measured (PSG) sleep immediately prior and subsequent to each nocturnal voiding episode.
PD patients were recruited regardless of voiding or sleep complaints. No studies have characterized clinical factors related to nocturia in PD using PSG, although a recent epidemiologic study performed in a general population suggested that nocturia was associated with poorer quality sleep monitored with PSG (10). Unfortunately that single-night, in-home study did not examine sleep correlates immediately before and after voiding episodes. Understanding the precise relationship between objectively measured sleep and nocturia is important to develop clinical intervention trials addressing these non-motor symptoms with the overall goal of improving HRQOL and function in PD.
Materials and Methods
Subjects
These results represent a secondary analysis of idiopathic PD patients (n=63) from a university-based movement disorders clinic (recruited from March 2007 through June 2011 inclusive) who were participating in a study evaluating sleep across a range of parkinsonian conditions. The diagnosis of idiopathic PD was confirmed by a neurologist with additional fellowship training in movement disorders. All participants underwent evaluation using a 48-hour in-patient PSG protocol. Study procedures were approved by the Emory University Institutional Review Board (Atlanta, GA, USA) and participants provided written, informed consent. Patients were administered the Unified Parkinson’s Disease Rating Scale (UPDRS) by a board-certified neurologist; for those patients taking dopaminergics this was administered while they were taking their usual home medications.
Exclusion criteria
Exclusion criteria included serious medical co-morbidities such as a history of stroke, myocardial infarction, congestive heart failure, active cancer, severe obstructive pulmonary disease, or asthma. PD participants with a history of dementia or a neurosurgical intervention for PD were also excluded.
Measurements
Sleep Outcomes
Participants underwent a 48-hour intensive in-patient protocol conducted in a relatively sequestered, sound-attenuated sleep laboratory located within Wesley Woods Hospital at Emory University School of Medicine. Full details of the protocol are described elsewhere (11). PSGs were performed with the Embla Flaga A10 digital recording system (Medcare Corporation, Buffalo, NY) that employs standard gold cup electrodes for recording of electronencephalography (EEG), electrooculography (EOG), and electromyography (EMG) for sleep staging and determination of sleep architecture. We also recorded respiratory airflow and effort with nasal pressure transducer and respiratory belts and oximetry with a non-invasive finger probe. All recordings were scored visually by expert scorers with high inter-rater reliability(12). For each night, we calculated Total Sleep Time (TST) (in minutes), Sleep Efficiency (SE) (percent of time spent in bed asleep), and the Apnea-Hypopnea Index (AHI) (the number of breathing events per hour of sleep).
During lab nights, patients were instructed to use the bathroom as frequently as they do when at home. The presence of a dedicated overnight technologist assigned to monitor the patient’s sleep with PSG, as well as with continuous infra-red video and audio monitoring, allowed this to be accomplished relatively easily. Patients merely indicated to the technologist, who monitored the patient from an adjacent room, when they wished to use the bathroom during the night, much as they would when at home. The technologist then entered the patient’s bedroom, unplugged electrodes from the terminal box, and allowed the patient to arise unencumbered to use the nearby bathroom. All patients were ambulatory; none customarily used a bedpan, urinal, or bedside commode. In this study, our definition of an in-lab nocturia episode meets the strictest definition (13): a) the initial nocturia episode had to occur after the formal initiation of the sleep period (lights were turned out) and some PSG-defined sleep had to occur prior to that first requested void; and b) the final nocturia episode of the night must have been followed by some PSG-defined sleep. To restate, none of the final nocturia episodes reported upon here represented the termination of sleep on the lab night. To further characterize the consequences of the voiding episode for sleep disruption, we also analyzed more specifically 60 minutes immediately subsequent to each nocturia episode. Not all voids resulted in a return to sleep within those initial 60 minutes, but in all cases (including all final voids for the night) there was a return to PSG-defined sleep. Based on preliminary analyses of our data demonstrating evidence of laboratory adaptation effects from night 1 to night 2, we limited analyses to only night 2 data (14).
Nocturia
Sleep diaries were completed by participants for a week prior to the PSG evaluation to collect data on sleep and nocturia in the home environment. The validated International Prostate Symptom Score (IPSS) was used to assess self-reported nocturia and overall bother related to urinary symptoms (15; 16). The IPSS has been used in research studies of both men and women with PD to assess the prevalence of urinary symptoms (2). The nocturia frequency variable used the participant’s response to IPSS question 7: During the last month or so, how many times do you most typically get up to urinate from the time you get into bed at night until the time you get up in the morning? Response categories were: 0 times, 1 time, 2 times, 3 times, 4 times, 5 or more times. Note that this definition of nocturia differs slightly from that used in the ICS definition (13), which requires the intention to return to sleep and that some sleep has occurred prior to the initial voiding episode of the night. Nocturia occurring at least two times per night has been shown to be clinically significant because of effects on bother and HRQOL (5). Table I and Figure 1 define participant groups with low bother versus high bother based on the response categories to the global bother question, (#8) on the IPSS (16). This methodology is similar to our previously published study in men with nocturia without PD (9).
Table I.
Bother assessment and response
| Question | Response categories | Groups |
|---|---|---|
|
| ||
| If you were to spend the rest of your life with your urinary problem the way it is now, how would you feel about that?14 | Delighted (1) | LOW Bother |
| Pleased (2) | ||
| Mostly satisfied (3) | ||
|
| ||
| Mixed (about equally satisfied and dissatisfied) (4) | Intermediate Bother | |
|
| ||
| Mostly dissatisfied (5) | HIGH Bother | |
| Unhappy (6) | ||
| Terrible (7) | ||
Figure 1. Participants in study and the comparison groups (LOW and HIGH bother).
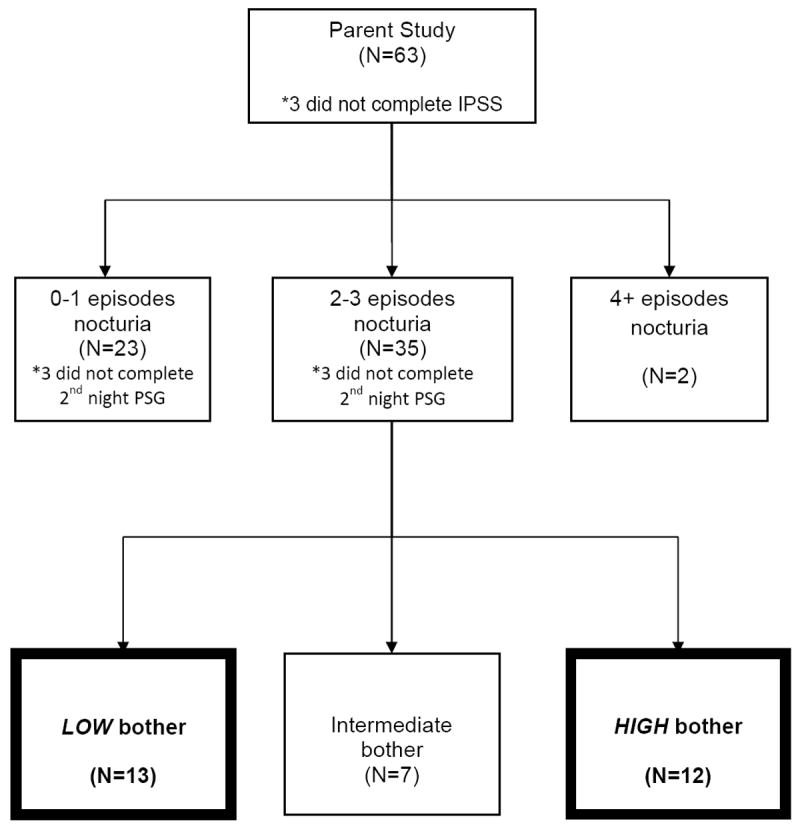
IPSS: International Prostate Symptom Score
PSG: Polysomnography
Additional Clinical Factors
Body mass index was calculated based on patient self-report of height and weight. Orthostatic blood pressure measurement for each patient in the protocol was tested a mean number of 13.4 times throughout the daytime hours and after meals. Following American Academy of Neurology guidelines, we defined orthostasis as a drop in systolic blood pressure of 20 mm Hg or greater or a drop in diastolic blood pressure of 10 mm Hg or greater, after 3 minutes of standing. Diabetes mellitus and hypertension diagnoses were assigned based upon review of current medications. Either oral hypoglycemic drugs or insulin use constituted a diagnosis of diabetes. Dopaminergic medications were converted to daily levodopa equivalents, pergolide equivalents (dopamine agonists), or total levodopa equivalents (combined including COMT inhibitors and extended release preparations of levodopa) using formulae provided elsewhere (17; 18). Medications that could affect lower urinary tract symptoms were categorized as either antimuscarinics, antiparkinsonian medications with significant anticholinergic properties (amantadine, trihexyphenidyl, benztropine), or alpha-blockers used for benign prostatic enlargement in men. Within each category, these medications were assessed as present or absent. Only one participant was prescribed a medication in all three categories and only two participants were prescribed a medication in two of the three categories.
Statistical Analysis
Because data were normally distributed, t-tests were used to evaluate differences between groups related to nocturia frequency. Mantel-Haenszel chi square tests were used for categorical values. Linear regression was used to assess factors associated with frequency of nocturia. The Wilcoxon rank sum test was used for the analysis related to bother from urinary symptoms because of the small sample size. Statistical analyses were performed using SAS 9.2 (SAS Institute, Cary, NC).
Results
Of the 63 participants with PD, 60 completed the IPSS and constitute the sample for this analysis (65% male with an average age of 63 ± 9.7, range 32-83). The mean UPDRS motor score was 17.1 ± 8.4. The proportion of blood pressure measurements with any evidence of orthostasis was 11.6%.
Almost all of the PD participants (93%) reported at least one episode of nocturia and 62% had at least two episodes of nocturia on the IPSS. Those with 2 or more episodes of nocturia were more likely to have a diagnosis of hypertension versus those with 0-1 episodes of nocturia, but were similar with respect to mean BMI and presence of diabetes mellitus. Increased motor symptom severity was associated with increased frequency of nocturia (linear regression adjusted for age and gender: p-value = 0.03). Dosages of dopaminergic medications (equivalents for levodopa alone, dopamine agonists alone, or total dopaminergic dose, expressed as total levodopa equivalents) did not differ between those with or without clinically significant nocturia (Table II). Cognition as measured by the Mini-Mental State Exam (19) was not related to nocturia frequency (Table II). While PSG-measured SE was poor for the entire sample, it was significantly worse in those with 2 or more episodes of nocturia per night compared to those with less frequent nocturia (Table II).
Table II.
Characteristics of study participants with Parkinson disease and self-reported nocturia (0-1 episode nightly vs. 2 or more nightly)
| Variable* | Self-Reported Nocturia (0-1 episode) | Self-reported Nocturia (2 or more episodes) | p-value** |
|---|---|---|---|
| CLINICAL CHARACTERISTICS | |||
| Age (years) | N=23 | N=37 | |
| 63.1 ± 7.7 | 63.6 ± 9.8 | 0.8 | |
|
| |||
| Gender (% male) | 12 (52%) | 27 (73%) | 0.1 |
|
| |||
| BMI (kg/m2) | N=23 | N=37 | |
| 26.5 ± 6.0 | 28.3 ± 4.4 | 0.2 | |
|
| |||
| Hypertension | N=23 | N=37 | |
| 3 (13%) | 19 (51%) | 0.003 | |
|
| |||
| Diabetes mellitus | N=23 | N=37 | |
| 1 (4%) | 4 (10%) | 0.4 | |
|
| |||
| MMSE | N=23 | N=37 | |
| 28.6 ± 1.5 | 28.5 ± 1.9 | 0.9 | |
|
| |||
| PARKINSON DISEASE CHARACTERISTICS | |||
| UPDRS (Part III) | N=21 | N=30 | |
| 14.5 ± 6.4 | 19.7 ± 9.1 | 0.03 | |
|
| |||
| Hoehn & Yahr | N=23 | N=37 | |
| 2.2 ± 0.5 | 2.1 ± 0.6 | 0.5 | |
|
| |||
| Total daily levodopa dose (mg)† | N=23 | N=37 | |
| 359 ± 320 | 350 ± 379 | 0.9 | |
|
| |||
| Total daily dopamine agonist dose (mg)‡ | N=23 | N=37 | |
| 1.3 ± 1.5 | 1.5 ± 1.6 | 0.6 | |
|
| |||
| Total daily levodopa equivalents (mg) | N=23 | N=37 | |
| 450 ± 366 | 459 ± 401 | 0.9 | |
|
| |||
| POLYSOMNOGRAPHY | |||
| Nighttime trips to the bathroom during PSG | N=20 | N=34 | |
| 1.0 ± 1.0 | 1.76 ± 0.9 | 0.007 | |
|
| |||
| Sleep Efficiency (%) | N=20 | N=34 | |
| 77.8 ± 11.8 | 66.6 ± 19.6 | 0.01 | |
|
| |||
| Total Sleep Time (minutes) | N=20 | N=34 | |
| 380.1 ± 46.5 | 321.0 ± 103.7 | 0.02 | |
|
| |||
| Apnea-Hypopnea Index | N=20 | N=34 | |
| 5.1 ± 6.1 | 6.3 ± 8.3 | 0.5 | |
Unless indicated values represent mean ± SD
p-value: T-test, chi-square, or Mantel-Haenszel chi-square
UPDRS Part III: Unified Parkinson Disease Rating Scale motor score
MMSE: Mini-Mental State Exam
Corrected for sustained release forms of levodopa
Pergolide equivalent dose
Figure 1 indicates that, based on the IPSS classification, high bother occurred in 12 and low bother occurred in 13 of those individuals with 2-3 episodes of nocturia. Bother was uncommon among the 23 patients with 0-1 nocturia episodes, with only 3 patients reporting high bother. Because of our interest in finding PSG- and other correlates of bother, we focused the remainder of our analyses on the aforementioned 25 cases. PSG-defined sleep measures clearly differentiated those patients with and without bother (Table III). Those with high bother related to urinary symptoms had lower SE and less TST than those with a similar number of reported nocturia episodes and low bother. The AHI was similar. We analyzed PSG data from 42 distinct nocturia episodes among these 25 patients (data from 4 additional episodes were excluded because no sleep occurred prior to first nocturnal void [n = 2] or the patient did not return to sleep after the final nocturnal void [n = 2]). Nocturia frequency during PSG did not differentiate these groups, but patients reporting high bother had a trend toward longer sleep latency following nocturia episodes in the laboratory relative to those with low bother (p=0.08). Prior to the first nocturia episode during PSG, participants with low bother averaged 205.5 ± 90.2 minutes of sleep while those with high bother averaged 140.9 ± 99.9 minutes. However, these values were not statistically different (p=0.15). Clinical characteristics such as the proportion of persons with diabetes or hypertension, average dopaminergic equivalents, UPDRS motor scores or the use of medications affecting the bladder, did not differentiate the groups.
Table III.
Sleep characteristics (from PSG and sleep diary) among PD participants with 2-3 episodes of self-reported nocturia based on classification by bother related to urinary symptoms
| Variable | Bother (No) | Bother (Yes) | p-value* |
|---|---|---|---|
| POLYSOMNOGRAPHY | N=13 | N=12 | |
| Sleep efficiency (%) | 75.9 ± 11.9 | 59.2 ± 22.7 | 0.04 |
| Total sleep time (minutes) | 372.5 ± 58.7 | 280.5 ± 116.1 | 0.03 |
| Apnea-Hypopnea Index | 4.7 ± 3.8 | 6.2 ± 9.8 | 0.6 |
| Hypoxic burden** | 5.2 ± 6.2 | 1.7 ± 2.1 | 0.08 |
| Nocturia episodes during PSG | 1.5 ± 0.9 | 1.9 ± 1.2 | 0.3 |
| Sleep efficiency in first hour after nocturia episode (%)† | 63.9 ± 26.1 | 51.4 ± 34.1 | 0.3 |
| Time to return to sleep after nocturia episode (minutes)† | 8.7 ± 10.6 | 22.5 ± 21.9 | 0.08 |
| SLEEP DIARY (at least 5 days)‡ | |||
| Percent of awakenings due to nocturia | 74.4 ± 18.9 | 81.0 ± 34.0 | 0.60 |
| Avg number of nighttime awakenings | 2.9 ± 1.7 | 2.8 ± 0.7 | 0.75 |
Values represent mean ± standard deviation
p-value: Wilcoxon rank sum test
Minutes of total sleep time spent with SaO2 < 90%
Sleep efficiency and time to return to sleep after awakening secondary to nocturia are based on the results of 11 participants in each bother group who had nocturia during the second night of PSG monitoring.
Sleep diaries were available for 13 individuals in the low bother group and 11 individuals in the high bother group.
Discussion
This study provides critical insights into factors related to nocturia and disrupted sleep by objectively measuring sleep among a well-defined patient cohort, in this case with PD. Clinically significant nocturia and disturbed sleep (as measured by PSG) were both highly prevalent in this sample even though participants were recruited regardless of sleep or voiding complaints. As is typical of PD patients (20), participants had poor sleep generally, but a higher frequency of nocturia was associated with significantly worse sleep when measured objectively by in-laboratory PSG. Additionally, those with high bother related to nocturia demonstrated worse sleep generally and a trend toward poorer sleep specifically during the hour after getting up to void at night in the laboratory. The latter observation has been noted previously via self-reports in community-dwelling older adults with nocturia (8), but has never before been validated in any population using PSG. Interestingly, although some evidence has suggested associations between sleep apnea and nocturia in non-PD patients (21), our AHI data did not suggest sleep apnea was a frequent explanation of the nocturia experienced by these participants. We view this as compatible with recent work suggesting sleep apnea is not a major cause of disrupted sleep in PD(22).
A recent study utilizing ambulatory PSG monitoring in a large population of community-dwelling middle-aged and older adults demonstrated worse sleep among those with nocturia compared to those with no nocturia (10). Although our study complements these findings, our data are also somewhat different. That study (10) used neither the IPSS nor the ICS definition of nocturia and based its findings entirely on a global, retrospective report of monthly frequency of awakening to go to the bathroom. Additionally, the investigators relied upon a single night of home-recorded sleep and did not report voids on the recording night. Thus, PSG-defined sleep characteristics were based upon total night recording and not events subsequent to each voiding episode. In contrast to the use of ambulatory PSG, the use of in-laboratory PSG in our study allowed us to carefully evaluate characteristics of sleep both preceding and subsequent to observed voiding episodes during the lab night. Additionally, our data speak directly to the important issue of ‘bother’ associated with urinary symptoms, in this case in patients with PD. Because PSG-measured sleep differentiated those reporting high or low bother, our data provide the first crucial objective validation for this widely embraced construct in urology.
As has been demonstrated in other studies of PD, reported nocturia frequency was associated with increased motor symptom severity (23; 24), with the statistically significant difference exceeding the minimum clinically important difference of five points on the UPDRS motor scale (25). However, UPDRS motor score and dopaminergic medication dose equivalents did not differentiate those with high or low bother. These results suggest that the associations between bother and poor sleep are probably not due to suboptimal treatment for PD.
Nocturia is associated with multiple comorbid conditions that may coexist within a single individual. Nocturia in PD may be related to PD-specific conditions, but our results also highlight the prevalence of hypertension, which has previously been associated with nocturia in community-dwelling adults (10). The number of persons with diabetes mellitus did not differ significantly, but overall only 5 participants had this diagnosis. These results have implications for treatment as multicomponent strategies targeting multiple potential risk factors may lead to overall better outcomes than single intervention approaches.
This study has some limitations. First, the IPSS bother score used in this study queried global bother related to all urinary symptoms and not nocturia specifically. Second, as mentioned previously, the IPSS definition of self-reported nocturia differs from the ICS definition because some sleep prior to first nocturia episode and sleep after the final nocturia episode are stipulated by the latter but not the former. By studying patients in the sleep laboratory, we were able to apply the more rigorous ICS criterion objectively. We now have demonstrated conclusively that valid measures of human sleep are indeed disturbed specifically by nocturia events. Third, some participants did not complete the IPSS or the 2nd night PSG evaluation; however, these numbers were small and represented about 10% of the sample. Fourth, we did not utilize 24-hour frequency-volume charts to assess for nocturnal polyuria, which could have added additional data regarding clinical factors related to nocturia in PD. Fifth, we were able to observe patients using in-lab PSG for only two nights. Future studies, perhaps using ambulatory equipment examining the sleep of patients in their own homes, would provide even more relevant data on this important, but generally overlooked aspect of sleep in PD patients.
Conclusion
By objectively measuring sleep with PSG and self-reported nocturia frequency and bother from urinary symptoms using a validated questionnaire, this study uses a novel approach to evaluate these burdensome non-motor symptoms in PD. The relationship between nocturia and sleep is multifactorial in PD; however, these results suggest not only are sleep disruption and nocturia common, but bother related to nocturia is reflected objectively when measured by PSG. Further study, including more detailed urological evaluation with functional measures of lower urinary tract function, is warranted to determine if interventions for sleep provide an independent therapeutic target among PD patients with nocturia.
Acknowledgments
Funding sources: Supported by NINDS RO1 NS-050595 and PHS Grant UL1 RR025008 and KL2 RR025009 (Dr. Trotti) from the Clinical and Translational Science Award program, National Institutes of Health, National Center for Research Resources. Dr. Vaughan is supported by a VA Rehabilitation R&D career development award (1IK2RX000747-01) and the John A. Hartford Foundation.
Role of sponsors: The funding sources had no role in design and conduct of the study; collection, management, analysis, and interpretation of the data; and preparation, review, or approval of the manuscript. The authors’ work was independent of the funders.
References
- 1.Gallagher DA, Lees AJ, Schrag A. What are the most important nonmotor symptoms in patients with Parkinson’s disease and are we missing them? Mov Disord. 2010;25:2493–2500. doi: 10.1002/mds.23394. [DOI] [PubMed] [Google Scholar]
- 2.Winge K, Skau A-M, Stimpel H, Nielsen KK, Werdelin L. Prevalence of bladder dysfunction in Parkinsons disease. Neurourol Urodyn. 2006;25:116–122. doi: 10.1002/nau.20193. [DOI] [PubMed] [Google Scholar]
- 3.Martinez-Martin P, Schapira AHV, Stocchi F, Sethi K, Odin P, MacPhee G, Brown RG, Naidu Y, Clayton L, Abe K, Tsuboi Y, MacMahon D, Barone P, Rabey M, Bonuccelli U, Forbes A, Breen K, Tluk S, Olanow CW, Thomas S, Rye D, Hand A, Williams AJ, Ondo W, Chaudhuri KR. Prevalence of nonmotor symptoms in Parkinson’s disease in an international setting; Study using nonmotor symptoms questionnaire in 545 patients. Mov Disord. 2007;22:1623–1629. doi: 10.1002/mds.21586. [DOI] [PubMed] [Google Scholar]
- 4.Coyne KS, Zhou Z, Bhattacharyya SK, Thompson CL, Dhawan R, Versi E. The prevalence of nocturia and its effect on health-related quality of life and sleep in a community sample in the USA. BJU Int. 2003;92:948–954. doi: 10.1111/j.1464-410x.2003.04527.x. [DOI] [PubMed] [Google Scholar]
- 5.Tikkinen KA, Johnson TM, 2nd, Tammela TL, Sintonen H, Haukka J, Huhtala H, Auvinen A. Nocturia frequency, bother, and quality of life: how often is too often? A population-based study in Finland. Euro Urol. 2010;57:488–496. doi: 10.1016/j.eururo.2009.03.080. [DOI] [PubMed] [Google Scholar]
- 6.Yoshimura K, Kamoto T, Oka Y, Tsukamoto T, Oshiro K, Suzukamo Y, Kinukawa N, Ogawa O. Differences between bothersome and non-bothersome night-time frequency. Neurourol Urodyn. 2007;26:1014–1019. doi: 10.1002/nau.20451. [DOI] [PubMed] [Google Scholar]
- 7.Yu H-J, Chen F-Y, Huang P-C, Chen TH-H, Chie W-C, Liu C-Y. Impact of nocturia on symptom-specific quality of life among community-dwelling adults aged 40 years and older. Urology. 2006;67:713–718. doi: 10.1016/j.urology.2005.10.054. [DOI] [PubMed] [Google Scholar]
- 8.Endeshaw Y. Correlates of Self-Reported Nocturia Among Community-Dwelling Older Adults. J Geron Ser A: Biol Sci Med Sci. 2009;64A:142–148. doi: 10.1093/gerona/gln009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Vaughan CP, Eisenstein R, Bliwise D, Endeshaw Y, Nagamia Z, Wolf RA, Johnson TM., 2nd Self-rated sleep characteristics and bother from nocturia. Int J Clin Pract. 2012;66:369–373. doi: 10.1111/j.1742-1241.2011.02868.x. [DOI] [PubMed] [Google Scholar]
- 10.Parthasarathy S, Fitzgerald M, Goodwin JL, Unruh M, Guerra S, Quan SF. Nocturia, Sleep-Disordered Breathing, and Cardiovascular Morbidity in a Community-Based Cohort. PLoS ONE. 2012;7:e30969. doi: 10.1371/journal.pone.0030969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Bliwise DL, Trotti LM, Wilson AG, Greer SA, Wood-Siverio C, Juncos JJ, Factor SA, Freeman A, Rye DB. Daytime alertness in Parkinson’s disease: Potentially dose-dependent, divergent effects by drug class. Mov Disord. 2012;27:1118–1124. doi: 10.1002/mds.25082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Bliwise DL, Williams ML, Irbe D, Ansari FP, Rye DB. Inter-rater reliability for identification of REM sleep in Parkinson’s disease. Sleep. 2000;23:671–676. [PubMed] [Google Scholar]
- 13.van Kerrebroeck P, Abrams P, Chaikin D, Donovan J, Fonda D, Jackson S, Jennum P, Johnson T, Lose G, Mattiasson A, Robertson G, Weiss J. The standardisation of terminology in nocturia: Report from the standardisation sub-committee of the International Continence Society. Neurourol Urodyn. 2002;21:179–183. doi: 10.1002/nau.10053. [DOI] [PubMed] [Google Scholar]
- 14.Agnew HW, Webb WB, Williams RL. The first night effect: an EEG study of sleep. Psychophysiology. 1966;2:263–266. doi: 10.1111/j.1469-8986.1966.tb02650.x. [DOI] [PubMed] [Google Scholar]
- 15.Barry MJ, Fowler FJ, Jr, O’Leary MP, Bruskewitz RC, Holtgrewe HL, Mebust WK, Cockett AT. The American Urological Association symptom index for benign prostatic hyperplasia. The Measurement Committee of the American Urological Association. J Urol. 1992;148:1549–1557. doi: 10.1016/s0022-5347(17)36966-5. [DOI] [PubMed] [Google Scholar]
- 16.O’Leary MP. Validity of the “Bother Score” in the evaluation and treatment of symptomatic benign prostatic hyperplasia. Rev Urol. 2005;7:1–10. [PMC free article] [PubMed] [Google Scholar]
- 17.Hobson DE, Lang AE, Martin WRW, Razmy A, Rivest J, Fleming J. Excessive Daytime Sleepiness and Sudden-Onset Sleep in Parkinson Disease. JAMA. 2002;287:455–463. doi: 10.1001/jama.287.4.455. [DOI] [PubMed] [Google Scholar]
- 18.Grosset KA, Grosset DG. Proposed dose equivalence for rapid switching between dopamine agonists in Parkinson’s disease. Clin Therapeut. 2006;28:1063–1064. doi: 10.1016/j.clinthera.2006.06.016. [DOI] [PubMed] [Google Scholar]
- 19.Folstein MF, Folstein SE, McHugh PR. “Mini-mental state” : A practical method for grading the cognitive state of patients for the clinician. J Psych Res. 1975;12:189–198. doi: 10.1016/0022-3956(75)90026-6. [DOI] [PubMed] [Google Scholar]
- 20.Bliwise DL, Trotti LM, Rye DB. Movement disorders specific to sleep and sleep in waking movement disorders. In: Watts RL, Standaert DG, Obeso JA, editors. Movement Disorders. New York: McGraw Hill; 2012. pp. 935–974. [Google Scholar]
- 21.Endeshaw YW, Johnson TM, Kutner MH, Ouslander JG, Bliwise DL. Sleep-Disordered Breathing and Nocturia in Older Adults. J Am Geriatr Soc. 2004;52:957–960. doi: 10.1111/j.1532-5415.2004.52264.x. [DOI] [PubMed] [Google Scholar]
- 22.Cochen De Cock V, Abouda M, Leu S, Oudiette D, Roze E, Vidailhet M, Similowski T, Arnulf I. Is obstructive sleep apnea a problem in Parkinson’s disease? Sleep Med. 2010;11:247–252. doi: 10.1016/j.sleep.2009.05.008. [DOI] [PubMed] [Google Scholar]
- 23.Young A, Horne M, Churchward T, Freezer N, Holmes P, Ho M. Comparison of sleep disturbance in mild versus severe Parkinson’s disease. Sleep. 2002;25:573–577. [PubMed] [Google Scholar]
- 24.Dhawan V, Dhoat S, Williams AJ, DiMarco A, Pal S, Forbes A, Tobías A, Martinez-Martin P, Chaudhuri KR. The range and nature of sleep dysfunction in untreated Parkinson’s disease (PD). A comparative controlled clinical study using the Parkinson’s disease sleep scale and selective polysomnography. J Neurol Sci. 2006;248:158–162. doi: 10.1016/j.jns.2006.05.004. [DOI] [PubMed] [Google Scholar]
- 25.Schrag A, Sampaio C, Counsell N, Poewe W. Minimal clinically important change on the unified Parkinson’s disease rating scale. Mov Disord. 2006;21:1200–1207. doi: 10.1002/mds.20914. [DOI] [PubMed] [Google Scholar]


