Abstract
Background
Despite the proliferation of health information technology (IT) interventions, descriptions of the unique considerations for conducting randomized trials of health IT interventions intended for patient use are lacking.
Purpose
Our purpose is to evaluate Pocket PATH® (Personal Assistant for Tracking Health), a novel health IT intervention, as an exemplar of how to address issues that may be unique to a randomized controlled trial to evaluate health IT intended for patient use.
Methods
An overview of the study protocol is presented. Unique considerations for health IT intervention trials and strategies to maintain equipoise, monitor data safety and intervention fidelity, and keep pace with changing technology during such trials are described.
Lessons Learned
The sovereignty granted to technology, the rapid pace of changes in technology, ubiquitous use in health care and obligation to maintain the safety of research participants challenge researchers to address these issues in ways that maintain the integrity of intervention trials designed to evaluate the impact of health IT interventions intended for patient use.
Conclusions
Our experience evaluating the efficacy of Pocket PATH may provide practical guidance to investigators about how to comply with established procedures for conducting randomized controlled trials and include strategies to address the unique issues associated with the evaluation of health IT for patient use.
Keywords: Pocket PATH® (Personal Assistant for Tracking Health), health information technology (health IT), randomized, controlled trials (RCT), consumer health technology, trial of intervention efficacy
Introduction
Health information technology (IT) represents one of the ‘transforming’ advancements that impact the way patients manage their health information, communicate with healthcare providers, and support the goal for patients to be informed, active participants in their health care (1–3). The recent proliferation of health IT interventions intended for patient use and the growing recognition of the importance of rigorous trials to evaluate such interventions (4), revealed several substantial issues that can arise in such trials. A description of the unique considerations for conducting randomized trials of health IT interventions intended for patient use is therefore warranted and timely.
Maintaining equipoise
The ethical basis for medical research involving assignment of participants to different interventions requires investigators to maintain equipoise during the conduct of the trial (5,6). Equipoise refers to a state of genuine uncertainty regarding the comparative therapeutic merits of different arms of an intervention trial (7). Evidence suggests that even when consent forms include clear statements about equipoise, the majority of participants remain unaware that the best intervention is in fact unknown (5, 8–10). Participants often hold a preference for one intervention over another based on personal values and interest (11, 12), suggesting that equipoise may not be fully understood (7).
While a full discourse of culture and technology is beyond the scope of this paper, it is in this context that the issue of maintaining equipoise between technological and non-technological interventions is situated (13) and is thus an important consideration for health IT researchers. In our society, technology often is granted sovereignty (14). The success of technology in providing convenience, comfort and speed has led our society to view non-technological alternatives as naturally inferior. Technology often is equated with progress and promise and, as such, implies that technology is better than traditional or conventional approaches that are considered old-fashioned and outdated (14, 15). In trials where participants are assigned randomly to different interventions, at least one of which is technology-based, it is important to provide assurance that none of the participants, including those randomized to a standard care control group, are being harmed by having a chance at receiving an inferior therapy.
Monitoring data and safety
While investigators conducting research on human beings are obligated to guard the safety and well being of study participants, data and safety monitoring (DSM) procedures may or may not be required depending on the complexity or risks involved in the study. According to the codes of ethics developed to address the obligations of researchers employing electronic data collection and communication via the Internet and other health technologies, (16–19) researchers need to establish procedures to monitor the health indicators collected as outcome data of a health IT intervention so that worrisome condition changes are detected and patient safety is ensured.
Maintaining intervention consistency amidst the changing pace of technology
A hallmark of intervention trials is achievement of internal validity of the intervention, (i.e., keeping the intervention constant over the course of a trial). Such consistency is necessary for drawing accurate conclusions about the relationship between the intervention and the effects that are examined in the study. However, achieving intervention consistency may be particularly challenging in the case of health IT interventions when IT itself is an integral part of the intervention, not merely a mode of intervention delivery. The speed of technology development clearly outpaces the timeline required to develop and conduct a typical full-scale intervention trial (20). During the same study period, new technologies and applications are constantly being developed and entering the technological landscape. New developments may be problematic because the health IT interventions under study may rely on technology platforms and applications that are either merely outdated, at best, or, at worst, completely obsolete by the end of the trial period. Conversely, the inability to use the newer technologies and hardware that become available during the trial is also problematic, since the latest technologies are often simpler for designers to program and more intuitive for users. Furthermore, new and independent technologies targeting the same health outcomes may be introduced to the market and thus available to the participants during the course of the study.
Riley and others (21–24) have suggested strategies for conducting trials of health IT interventions in order to keep pace with technology development, including such strategies as focusing on testing the core components of the health IT intervention rather than a specific delivery platform, leveraging a variety of technologies to deliver the health IT intervention during the trial, shortening follow-up periods to accommodate changes in technology, evaluating proximate outcomes and model or simulated more distal outcomes, or using alternative designs in order to identify combinations of intervention components that optimize outcomes.
Monitoring intervention fidelity for health IT
The importance of customizing the intervention fidelity evaluation plan to the unique components of each intervention has been established, as have the challenges associated with evaluating fidelity of complex interventions (25). Because implementation of most health IT interventions involves a dynamic interplay between the participant and a technological application or interface and can vary at any stage of the process (delivery, receipt, acceptance, and intention to use), models of intervention fidelity which focus exclusively on intervention delivery are deemed inadequate for evaluating the fidelity of health IT interventions. An assessment of human factors, such as receipt and acceptance known to influence user adoption and intended behavior change (26–30) also should be included in monitoring to ensure that the health IT intervention is implemented as planned.
We describe a randomized controlled trial (RCT) designed to evaluate the effects of Pocket PATH® (Personal Assistant for Tracking Health), a novel health IT intervention to promote self-care agency (the capability and willingness to engage actively in self-care behaviors), the performance of self-care behaviors and ultimately transplant-related health outcomes among patients with chronic illness to illustrate how we addressed the issues unique to trials of health IT intended for patient use. Because our trial tests Pocket PATH in a specific patient population (recipients of lung transplants), we also provide a brief overview of this population and the relevance of health IT to it.
Methods
Health IT and lung transplantation
Barely 84% of lung transplant recipients (LTRs) survive the first year after transplantation, and only 64% and 53% of LTRs are alive by 3 and 5 years, respectively (31). LTRs experience more transplant-related complications, higher health resource utilization, and higher mortality than recipients of other solid organs (32). Prevention and detection of early complications is known to reduce the likelihood of future impairments in lung function and, therefore, morbidity and mortality (33). However, no RCT has tested health IT interventions designed to promote self-care and improve health after lung transplantation.
The features of Pocket PATH were designed to support patients’ self-care agency and performance of self-care behaviors, such as adhering to the medical regimen, performing self-monitoring and communicating condition changes to clinicians after lung transplantation. These behaviors are known to promote better health outcomes for persons with a variety of chronic illnesses; thus health IT interventions designed to support self-care behaviors among different patient populations are likely to include similar features although the details of the regimens and particular health indicators may differ among types of chronic illnesses. Therefore, the issues associated with conducting a RCT of Pocket PATH are likely to be generalizable to other IT interventions with similar goals.
Pocket PATH provides the LTR a Smartphone with customized programs (see Figure 1) for recording vital signs, symptoms and values from laboratory assays, graphical displays of changes in those values over time, and automatic generation of feedback messages to provide decision-support for LTRs about when and what to report to their transplant providers (see Table 1).
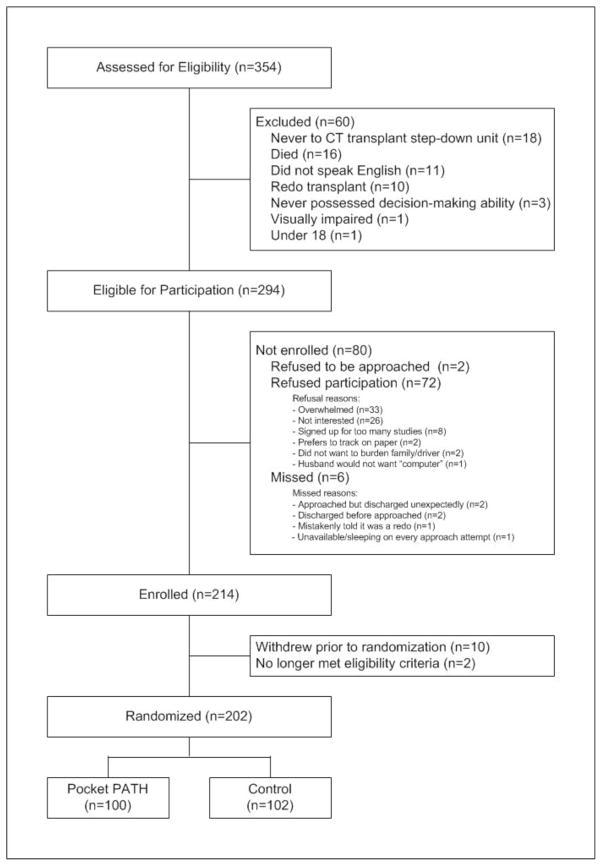
Figure 1.
Flow Diagram of Recruitment and Randomization in the Pocket PATH Trial
Table 1.
Features of Pocket PATH® Intervention to Support Self-Care Behaviors
Features to Support Self-Monitoring
|
Based on the success of Pocket PATH in promoting self-care and quality of life in the early post-transplant period (34), a full–scale, RCT to compare the efficacy of Pocket PATH for promoting self-care agency (the capability and willingness to engage in self-care behaviors), self-care behaviors and hence improving transplant-related health during the first 12 months after lung transplantation relative to standard care is nearing completion.
Design and setting
The Pocket PATH trial uses an RCT design with repeated-measures. The RCT is conducted in association with University of Pittsburgh Medical Center Cardiothoracic Transplant Program and is approved by the University of Pittsburgh Institutional Review Board.
Specific aims
The primary aims of the Pocket PATH trial are to: 1) compare the effects of Pocket PATH versus standard care on self-care agency and self-care behaviors at 2, 6, and 12 months post-discharge after lung transplantation, and 2) compare the effects of Pocket PATH versus standard care on transplant-related health (post-transplant complications, re-hospitalizations, and health related quality of life) at 2, 6, and 12 months post-discharge after lung transplantation. A secondary aim of the study is to explore intervention fidelity, patient acceptance and adoption of the Pocket PATH intervention to support the processes of self-care after lung transplantation.
Sample
Eligible participants include adult LTRs who are recovering on the cardio-thoracic transplant step-down unit prior to hospital discharge and are able to read and speak English. LTRs excluded had received a prior organ transplant (to avoid experiential effects) or were experiencing a condition that precluded discharge from the hospital or limited their ability to be actively involved in their post-transplant care. The targeted sample size of 202 was set to provide 80% power to detect an effect size of 0.45 (34) for the performance of self-care behaviors at a two-tailed significance level of 0.05 using a t-test for independent samples.
After baseline measures were collected and discharge was imminent, recipients were assigned randomly to either the Pocket PATH intervention group or the standard care control group. Recruitment and randomization for the study were completed in December 2011 (see eFigure 1).
Trial arms
Control group: Standard care
Standard preparation of LTRs for discharge and transition to home included a one-on-one, educational session delivered by the transplant nurse coordinator of the clinical team prior to hospital discharge. During the session, “Need to Know” topics (e.g., self-monitoring and medication taking) were reviewed with each LTR and typically a family caregiver(s). A home reference binder was provided to each recipient that included paper-and-pencil “Log Sheets” and instructions to record daily health indicators (e.g., pulse, blood pressure, temperature, spirometry, and symptoms). LTRs also were given written instructions describing parameters for “when to call” the transplant team if changes in these health indicators occurred. After discharge, a referral for at least one home visit was arranged. All LTRs were scheduled to return to the transplant center for routine follow-up evaluations within the first week, then monthly for the first three months, at least quarterly for the first year, and more frequently as needed. The transplant nurse documented the delivery of each component of the discharge educational session in the recipient’s medical record.
Intervention Group: Pocket PATH intervention in addition to standard care
The core components of the Pocket PATH intervention, goals and the corresponding interventionist behaviors are shown in Table 2. Components included defining self-care agency (i.e., what it means for patients actively to participate in their care), the importance of performing recommended self-care behaviors and the role of Pocket PATH in promoting higher levels of patient activation and self-care. LTRs also were provided the Pocket PATH device and trained to use its features and custom programs, including return demonstrations of competency using the device for self-monitoring, adhering to the regimen and decision-support about reporting condition changes to the transplant providers. The training session lasted approximately 30 minutes and was delivered by one of two trained study interventionists. Following training, recipients were instructed to use the device and its programs to track health indicators and to report changes in their conditions according to the coordinator’s discharge instructions. Recipients were given a Pocket PATH User Support Manual and a toll-free number to call for help with technical problems with the device that was answered by research staff members during regular business hours. In the event that recipients were not able to perform the tasks after training, remedial training was provided on an individual basis. Because the device was intended to assist with self-care behaviors, data recorded on it were logged and graphed for each recipient to view (see Figure 2). The device was programmed to generate automatic decision support messages reminding recipients to report changes to the transplant clinicians whenever health indicators reached the threshold for reporting changes described in the standard discharge instructions. Data were not shared directly with clinicians; instead the data were uploaded automatically to the research project’s data base daily via secure, cellular connection.Throughout the training session and subsequent contacts, trainers stressed to LTRs, both orally and in the training materials, that the transplant team maintained responsibility for managing all clinical care and that changes in clinical data should be reported to the transplant care providers. Whenever an LTR contacted the research staff about a clinical issue, the research team redirected him/her to a member of the clinical transplant team.
Table 2.
Core Components, Measurable Goals and Interventionists’ Behaviors
| Core Component Goal |
Interventionists’ Behaviors |
|---|---|
|
I. Introduction & Preparation Goal: patient at ease and prepared for learning |
|
| |
| |
|
II. Discussion of Activation & Self-Care Goal: patient understands the importance of being an activated patient and performing recommended self- care activities |
|
| |
| |
|
III. Explanation of Pocket PATH Goal: patient understands the role of technology in promoting higher levels of patient activation |
|
|
IV: Demonstration Goal: patient engages in hands-on practice using Pocket PATH features |
|
| |
| |
| |
| |
|
V. Evaluation of patient competence Goal: patient demonstrates competence in using Pocket PATH features and draws linkages between use and improved patient activation and self-care |
|
Figure 2.
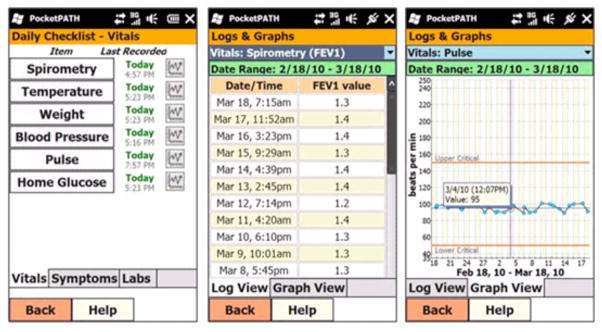
Selected Screen Shots of the Pocket PATH® Graphic User Interface
Measures
Outcome measures
Self-care behaviors (performing self-monitoring, adhering to the regimen and communicating changes in clinical condition), and transplant-related health outcomes (complications, re-hospitalizations, psychological distress, and health-related quality of life) were assessed at 2 months, 6 months, and 12 months post-hospital discharge. These intervals were selected because: a) they coincided with return visits for routine post-transplant evaluations, b) we detected effects by 2 months post-intervention in the pilot RCT (30), c) studies have shown that patients’ orientation toward sharing responsibility with their health care providers is malleable, with intervention effects seen as early as 8 weeks (35, 36), and d) variation in transplant-related health outcomes are evident as early as 6 weeks post-transplant (36, 37). Patient characteristics that may account for variation in intervention effects (socio-demographic characteristics, self-care agency, quality of relationship with primary lay caregiver, and health locus of control beliefs) were assessed at baseline prior to randomization.
Health IT measures
Four measures of technology acceptance (38, 39), based on concepts of the Technology Acceptance Model (TAM), (40, 41) were assessed: Perceived Ease of Use, Perceived Usefulness, Attitudes Toward Using, and Intention to Use the Technology. The range of scores for the four technology acceptance measures is 1–7; lower scores reflect higher levels of technology acceptance. Consistent with the developers’ recommendations, they were administered by a trained data collector (non-interventionist) after the Pocket PATH intervention had been delivered and the LTR had demonstrated receipt but before they had experience using Pocket PATH independently. Evidence that these technology acceptance measures predict a substantial portion of the use and acceptance of technologies abounds (42–48). The measures are standardized, reliable (38), and valid (49) and have been used across different settings. The After Scenario Questionnaire (ASQ), (50) a three-item, reliable and valid questionnaire of satisfaction with training to use a technology, was administered by a trained data collector (non-interventionist) after completion of the TAM scales. The ASQ assesses user satisfaction with the ease of task completion, the amount of time it took to complete a task, and the adequacy of support information (such as a user manual or help screens) during the training session. Items are rated on a seven-point Likert-type, scale (1 = Strongly Agree, to 7 = Strongly Disagree); lower scores reflect higher levels of satisfaction with technology training.
Technology adoption
Data were collected for utilization of Pocket PATH based on time-stamped data uploaded from each device over the course of the study. Patterns of usage were examined for changes over time. We limited this evaluation to data related to participants’ technology acceptance and satisfaction with technology training for several reasons: 1) satisfaction and acceptance were assessed early in the study period prior to participants’ independent use of Pocket PATH, 2) final data regarding Pocket PATH adoption and its impact on self-care and transplant-related health for the 12-month study period for all participants is not yet available, 3) there is insufficient power to draw conclusions about adoption and impact of Pocket PATH on self-care and health outcomes until all participants have completed the trial, and 4) our purpose is to raise awareness of issues and considerations for conducting trials of health IT intended for patient use.
Results
Participants’ scores for the measures of health IT demonstrated high levels of acceptance of the device by those assigned to receive it and satisfaction with the Pocket PATH training session (see Table 3). Scores close to 1 indicate greater acceptance (TAM) and greater satisfaction with training (TAQ).
Table 3.
Mean and Standard Deviation for Health IT Measures (TAM and ASQ), IT Intervention Arm Only
| Technology Acceptance Model (TAM) | Mean Score | Standard Deviation |
|---|---|---|
| Perceived Ease of Use | 1.44 | .67 |
| Perceived Usefulness | 1.40 | .57 |
| Attitudes Toward Using Technology | 1.34 | .53 |
| Intention to Use the technology | 1.28 | .80 |
| After Scenario Questionnaire (ASQ)** | 1.25 | .52 |
N=65; TAM measures were added to the battery of instruments after the start of study. Possible scores for the TAM measures ranged from 1–7 with lower scores indicating greater acceptance.
N=93; 6 participants did not complete the ASQ. Scoring was based on a 7-point scale; lower scores indicate greater satisfaction.
Lessons Learned
Maintaining equipoise between technology-based interventions and control conditions
When the Pocket PATH trial was getting underway, both transplant clinicians and potential study participants expressed preconceptions about the superiority of the health IT intervention compared to standard care. Our research team employed several strategies to promote equipoise throughout the Pocket PATH trial. We intentionally referred to the trial as The Study Comparing Methods for Tracking Health at Home when communicating with the transplant clinicians, potential study participants, and the Institutional Review Board. Terms such as Pocket PATH and references to Smartphone or technology were explicitly omitted from the trial title, from conversations about the study, and on the consent form. Because the nurses and other members of the transplant team served as liaisons to identify LTRs who met the eligibility criteria, educational sessions about the study and the need to maintain equipoise were provided to members of the clinical lung transplant surgical team; informational flyers were posted in the staff lounges to increase awareness of the study and the need for equipoise. The consent materials included explicit statements regarding the uncertainty of intervention benefits and, hence, the need to conduct the trial. Whenever a LTR or clinician expressed an opinion about the superiority of the Pocket PATH intervention, he/she was reminded that the integrity of the trial depended on suspending beliefs about the superiority of technology.
Employing appropriate safety monitoring procedures
Data from the Pocket PATH devices were uploaded automatically to the project Web site in order to track patients’ utilization of the device and its features, but these data were not shared automatically with the treating clinicians. We considered it our responsibility to monitor data that may have indicated an unsafe change a patient’s clinical condition and outlined steps for handling worrisome values. If during the process of monitoring participants’ use of Pocket PATH the research team became aware of worrisome values, these data could not be ignored. We developed a protocol for the research team to follow when critical values were uploaded to ensure participant safety while keeping threats to study integrity to a minimum. The aim was to follow a series of steps from the least to most intrusive to ensure that the patient was safe (e.g., reviewing the electronic medical record to see whether a critical value had been entered erroneously or the values had returned to normal) before resorting to contacting the patient or clinical team directly to avoid influencing the outcomes of interest. Since the purpose of the trial was to determine the impact of technology-enabled interventions on self-care and thus patient outcomes, the protocol specified that only if the more conservative actions were ineffective and the project staff judged the threat to be critical would a clinical provider be notified. If such an intervention were necessary, it would be duly recorded for analysis purposes. While it was important to include clinician notification in the protocol, in the end, this step was not necessary during the entire Pocket PATH trial.
In studies such as this one, unexpected risks may be detected more readily when the responsibility for safety monitoring rests with a single member of the research team who can perform close monitoring of individual participant data; therefore the project director who had the clinical experience to detect unanticipated changes in participant conditions was responsible for: a) reviewing uploaded data every 72 hours to ensure that changes were detected in a timely manner and 2) following the established protocol for handling any critical values. Our monitoring plan also included a mechanism for objective review and oversight of the process. On a quarterly basis, de-identified data were reviewed by a clinical expert in lung transplantation who assessed whether all critical values had been detected and handled appropriately according to our protocol. The expert also was poised to review the impact of notifying clinicians of critical values had this final protocol-defined action been warranted. A full description of our monitoring plan and recommendations for developing monitoring procedures for other trials involving health IT have been published (51). The monitoring strategies included risk-monitoring procedures, automatic surveillance to detect critical values and procedures for timely and appropriate action to ensure participant safety (52). Data recorded by participants in the standard care group were not available to the research team until participants shared their health logs with their transplant clinicians during their regularly scheduled return clinic visits to the transplant center which coincided with our data assessment time points.
Maintaining intervention consistency amidst the changing pace of technology
Some health IT trials may be better able than others to maintain intervention consistency while keeping pace with technological developments; the trial of the Pocket PATH intervention exemplifies strategies for dealing with changes beyond the control of the trial designers. The Pocket PATH software originally was developed for the iPAQ [HP iPAQ hx2755 Pocket PC; Hewlett Packard; Windows mobile V. 2003 SE]. Although at the time the iPAQ was among the most versatile pocket personal computers, it relied on modem connectivity to upload data remotely. By the time the RCT was ready to begin, cellular connectivity became available so we abandoned the iPAQ in favor of a Smartphone. We decided to provide LTR Smartphones (e.g., Tilt, HTC, Windows Mobile Professional 6) loaded with the Pocket PATH programs. To avoid having to re-write the graphic user interface programs, our choices were limited to phones that used Windows Mobile with similar screen dimensions. As new versions of.NET and OpenNETCF (the frameworks used for developing the programs) were released, we migrated to the newer versions because they added more features and simpler ways of doing things but also because the older versions no longer were supported. Migrations almost always led to compatibility problems with what we had already built, thus requiring adjustments to the source code. Each migration required testing to ensure Pocket PATH worked as well for users as before the changes.
Ultimately, as often occurs, as new device models were introduced, the older Tilt models were no longer available for purchase, so we had to change models [Pure, HTC, Windows Mobile Professional 6.5]. Migrating from iPAQ to Smartphones presented challenges, especially to adapt the graphing functionality to work with any screen resolution. However, since the Tilt and Pure phones used different versions of Windows Mobile than the iPAQ, to keep the user interface and features consistent, our software engineer had to ensure that the Pocket PATH programs worked seamlessly across different versions of Windows Mobile. Even upgrading from version 6 to 6.5 caused problems as some things were not backwards compatible. Uploading mechanisms had to be modified between the Tilt and Pure versions because v6.5 of Windows Mobile added certain restrictions to internet connectivity from within applications. Additionally, while we were able to maintain consistency with our original intervention, every time we were forced to switch to a different mobile device we had to update our training manuals and educational materials to match the newer model, adding cost and effort to an already expensive intervention trial. After changing platforms three times, we purchased enough Pure models to complete the study rather than having to change phone platforms again.
In spite of essential modifications, we believe we were able to maintain consistency in the user-interface and functionality so that each LTR in the intervention arm had a comparable experience with Pocket PATH. However, some health IT interventions may be linked inextricably to certain technologies, applications or interfaces and less able to be modified to keep pace with newly introduced technologies and applications. Depending on the health IT intervention under study, investigators may be forced to maintain intervention consistency over the course of the trial at the risk of evaluating an intervention that is outdated and possibly obsolete by the end of the trial.
Maintaining intervention fidelity for health IT interventions
A variety of models of intervention fidelity exist, but we adopted the following definition: the degree to which the intervention implementation process is an effective realization of the intervention as planned (53). We developed an intervention fidelity framework to guide the development of a multi-component plan to evaluate intervention fidelity for the Pocket PATH trial. A full description of the components of the framework, how each is measured and how the data regarding fidelity is used to test the relationships purported in the model and to draw conclusions about the consistency, validity, and effectiveness of the Pocket PATH intervention have been published (54). The concepts included in the model of intervention fidelity for health IT interventions go beyond delivery (the extent to which the intervention is delivered as intended) and receipt (the extent to which the intervention is received as intended) to include the concept of technology acceptance (the extent to which the subject has positive perceptions, attitudes, and intention to use the intervention). Although these concepts are believed to apply to fidelity of all health IT interventions, the information that is monitored and the types of data available are intervention specific (34, 35, 45). We believe that this multi-dimensional view of intervention fidelity allows for a better understanding of the role that technology acceptance plays in the adoption of health IT interventions and thus enactment of the behaviors the interventions are intended to promote.
Discussion and Conclusion
In this study we used a novel health IT application to maximize the contribution of LTRs themselves in preventing and detecting post-transplant complications. Health IT interventions may be particularly appropriate for organ-transplant recipients because most live outside the local region of the transplant center and no other personal computer support is required in the home. All LTRs were expected to perform the self-care activities supported by Pocket PATH; ratings of technology acceptance by participants in the IT intervention arm were high, but it is as yet unknown whether the use of this health IT device will prove to be superior to standard care in promoting self-care behaviors and transplant-related health outcomes. Data analysis of the trial outcome measures was underway at the time of acceptance of this paper for publication.
The protocol to evaluate the efficacy of Pocket PATH provides practical guidance to investigators who wish to evaluate other health IT interventions, particularly those designed to promote self-care behaviors among patients with other chronic illness. Issues of compliance with established procedures for conducting RCTs and strategies to address the issues uniquely associated with the evaluation of health IT intended for patient use in the Pocket PATH trial included maintaining equipoise (i.e. uncertainty regarding the outcome of the study), ensuring an appropriate level of safety monitoring surveillance, ensuring the validity and integrity of trial data, customizing the plan for monitoring intervention fidelity (i.e., including all potentially variable aspects of intervention fidelity, including delivery, receipt, acceptance, and intention to use), and adopting strategies to keep pace with technology change.
Acknowledgments
This work was supported by the National Institutes of Health, National Institute of Nursing Research [NR010711, DeVito Dabbs, PI]
Footnotes
Conflict of Interest Statement
None Declared
ClinicalTrials.gov identifier: NCT00818025
References
- 1.Cayton H. The flat-pack patient? Creating health together. Patient Educ Couns. 2006;62:288–90. doi: 10.1016/j.pec.2006.06.016. [DOI] [PubMed] [Google Scholar]
- 2.Dimick C. The empowered patient: Preparing for a new patient interaction. J AHIMA. 2010;81:26–31. [PubMed] [Google Scholar]
- 3.Jimison HB, Sher PP. Advances in presenting health information to patients. In: Chapman G, Sonnenbreg F, editors. Decision-Making in health care: Theory, psychology & applications. Cambridge: 2000. pp. 334–61. [Google Scholar]
- 4.Jüni PAD, Egger M. Systematic reviews in health care: Assessing the quality of controlled clinical trials. BMJ. 2001;323:42–6. doi: 10.1136/bmj.323.7303.42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Gallo C, Perrone F, De Placido S, Giusti C. Informed versus randomised consent to clinical trials. Lancet. 1995;346(8982):1060–4. doi: 10.1016/s0140-6736(95)91741-1. [DOI] [PubMed] [Google Scholar]
- 6.Madsen SM, Holm S, Davidsen B, Munkholm P, Schlichting P, Riis P. Ethical aspects of clinical trials: the attitudes of participants in two non-cancer trials. J Intern Med. 2000;248(6):463–74. doi: 10.1046/j.1365-2796.2000.00755.x. [DOI] [PubMed] [Google Scholar]
- 7.Freedman B. Equipoise and the ethics of clinical research. N Engl J Med. 1987;317(3):141–5. doi: 10.1056/NEJM198707163170304. [DOI] [PubMed] [Google Scholar]
- 8.White DR, Muss HB, Michielutte R, Cooper MR, Jackson DV, Richards F, et al. Informed consent: patient information forms in chemotherapy trials. Am J Clin Oncol. 1984;7(2):183–90. [PubMed] [Google Scholar]
- 9.Ellis PM, Dowsett SM, Butow PN, Tattersall MHN. Attitudes to randomized clinical trials amongst out-patients attending a medical oncology clinic. Health Expect. 1999;2(1):33–43. doi: 10.1046/j.1369-6513.1999.00028.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Cassileth BR, Lusk EJ, Miller DS, Hurwitz S. Attitudes toward clinical trials among patients and the public. JAMA. 1982;248(8):968–70. [PubMed] [Google Scholar]
- 11.Kerr CEP, Robinson EJ, Lilford RJ, Edwards SJL, Braunholtz DA, Stevens AJ. The impact of describing clinical trial treatments as new or standard. Patient Educ Couns. 2004;53(1):107–13. doi: 10.1016/S0738-3991(03)00124-1. [DOI] [PubMed] [Google Scholar]
- 12.McPherson K. Incorporating patient preferences into clinical trials: Information about patients’ preference must be obtained first. BMJ. 1998;317(7150):78. [PMC free article] [PubMed] [Google Scholar]
- 13.Pacey A. The Culture of Technology. Boston: MIT Press; 1983. [Google Scholar]
- 14.Postman N. Technopoly: The surrender of culture to technology. New York: Knopf; 1993. [Google Scholar]
- 15.Reiser SJ. Medicine and the reign of technology. New York: Cambridge University Press; 1978. [Google Scholar]
- 16.Communications of the ACM. 1993. ACM code of ethics and professional conduct; p. 94. [Google Scholar]
- 17.Association AP. Ethical principles of psychologists and code of conduct 1992. 2012 Apr 17; Available from: http://www.apa.org/ethics/code/index.aspx.
- 18.Reseachers AoI, AoIR Ethics Working Committee. Ethical decision making and internet research. Association of Internet Reseachers (AoIR); 2002. [Google Scholar]
- 19.Frankel M, Siang S. Ethical and legal aspects of human subjects research on the internet: A report of a workshop June 10–11, 1999. Publ Am Assoc Adv Sci. 1999 [Google Scholar]
- 20.Riley W. Evaluation of mHealth. National Heart, Lung, and Blood Institute IOM mPreventViolence Meeting. 2011 Dec 9; [Google Scholar]
- 21.Riley W, Rivera D, Atienza A, Nilsen W, Allison S, Mermelstein R. Health behavior models in the age of mobile interventions: Are our theories up to the task? Transl Behav Med. 2011;1(1):53–71. doi: 10.1007/s13142-011-0021-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Collins LM, Murphy SA, Strecher V. The multiphase optimization strategy (MOST) and the sequential multiple assignment randomized trial (SMART): New methods for more potent eHealth interventions. Am J Prev Med. 2007;32(5 Suppl):S112–8. doi: 10.1016/j.amepre.2007.01.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Collins LM, Dziak JJ, Li R. Design of experiments with multiple independent variables: A resource management perspective on complete and reduced factorial designs. Psychol Methods. 2009;14(3):202–24. doi: 10.1037/a0015826. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Murphy SA. An experimental design for the development of adaptive treatment strategies. Stat Med. 2005;24(10):1455–81. doi: 10.1002/sim.2022. [DOI] [PubMed] [Google Scholar]
- 25.Song M-K, Happ MB, Sandelowski M. Development of a tool to assess fidelity to a psycho-educational intervention. J Adv Nurs. 2010;66(3):673–82. doi: 10.1111/j.1365-2648.2009.05216.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Moore GC, Benbasat I. Development of an instrument to measure the perceptions of adopting an information technology innovation. Inform Syst Res. 1991;2(3):192–222. [Google Scholar]
- 27.Rogers EM. Diffusion of Innovations. New York: The Free Press; 1983. [Google Scholar]
- 28.Tornatzky LG, Klein KJ. Innovation characteristics and innovation adoption-implementation: A meta-analysis of findings. IEEE Trans Eng Manage. 1982;EM-29(1):28–45. [Google Scholar]
- 29.Ajzen I, Fishbein M. Understanding attitudes and predicting social behavior. Engewood Cliffs, NJ: Prentice-Hall; 1980. [Google Scholar]
- 30.Fishbein M, Ajzen I. Belief, attitude, intention and behaviour: An introduction to theory and research. Reading, MA: Addison-Wesley; 1975. [Google Scholar]
- 31.Stehlik J, Edwards LB, Kucheryavaya AY, Benden C. The registry of the International Society for Heart and Lung Transplantation: Twenty-eighth adult heart transplant report--2011. J Heart Lung Transplant. 2011;30(10):1078–9. doi: 10.1016/j.healun.2011.08.003. [DOI] [PubMed] [Google Scholar]
- 32.OPTN. OPTN / SRTR 2010 Annual data report. Department of Health and Human Services, Health Resources and Services Administration, Healthcare Systems Bureau, Division of Transplantation; 2011. [accessed 20 Feb 2012]. http://www.srtr.org/annual_reports/2010/ [Google Scholar]
- 33.Alvarez A, Lama R, Algar J, Santos F, Briceno J, Aranda JL, et al. Predicting mortality after lung transplantation. Transplant Proc. 2001;33:1630–1. doi: 10.1016/s0041-1345(00)02621-x. [DOI] [PubMed] [Google Scholar]
- 34.DeVito Dabbs AJ, Dew MA, Myers B, et al. Evaluation of a hand-held, computer-based intervention to promote early self-care behaviors after lung transplant. Clin Transplant. 2009;23:537–45. doi: 10.1111/j.1399-0012.2009.00992.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Greenfield S, Kaplan S, Ware JE. Expanding patient involvement in care: Effects on patient outcomes. Ann Intern Med. 1985;102:520–8. doi: 10.7326/0003-4819-102-4-520. [DOI] [PubMed] [Google Scholar]
- 36.Sabati N, Snyder M, Edin-Stibbe C, Lindgren B, Finkelstein S. Facilitators and barriers to adherence with home monitoring using electronic spirometry. AACN Clin Issues. 2001;12(2):178–85. doi: 10.1097/00044067-200105000-00002. [DOI] [PubMed] [Google Scholar]
- 37.De Vito Dabbs A, Johnson B, Wardzinski W, Iacono A. Development and evaluation of the electronic version of the questionnaire for lung transplant patients (e-QLTP) Prog Transplant. 2007;17(1):29–35. doi: 10.1177/152692480701700104. [DOI] [PubMed] [Google Scholar]
- 38.Davis FD. Perceived usefulness, perceived ease of use, and user acceptance of information technology. MIS Quart. 1989;13(3):319–40. [Google Scholar]
- 39.Davis FD, Bagozzi RP, Warshaw PR. User acceptance of computer technology: A comparison of two theoretical models. Manage Sci. 1989;35(8):982–1003. [Google Scholar]
- 40.Venkatesh V, Davis FD. A theoretical extension of the technology acceptance model: Four longitudinal field studies. Manage Sci. 2000;46(2):186–204. [Google Scholar]
- 41.Venkatesh V, Morris MG, Davis GB, Davis FD. User acceptance of information technology: Toward a unified view. MIS Quart. 2003;27(3):425–78. [Google Scholar]
- 42.Bagozzi RP. The legacy of the Technology Acceptance Model and a proposal for a paradigm shift. J Assoc Inf Syst. 2007;8(4):244–54. [Google Scholar]
- 43.Holden RJ, Karsh B. The Technology Acceptance Model: Its past and its future in health care. J Biomed Inform. 2010;43:159–72. doi: 10.1016/j.jbi.2009.07.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.King WR, He J. A meta-analysis of the technology acceptance model. Inform & Manage. 2006;43(6):740–55. [Google Scholar]
- 45.Lee Y, Kozar K, Larsen K. The technology acceptance model: Past, present, and future. Commun AIS. 2003;12(1):752–80. [Google Scholar]
- 46.Legris P, Ingham J, Collerette P. Why do people use technology? A critical review of the technology acceptance model. Inform Manage. 2003;40(3):191–204. [Google Scholar]
- 47.Ma Q, Liu L. The technology acceptance model: A meta-analysis of empirical findings. JOEUC. 2004;16(1):59–72. [Google Scholar]
- 48.Yousafzai S, Foxall G, Pallister J. Technology acceptance: a meta-analysis of the TAM: Part 2. J Model Manag. 2007;2(3):281–304. [Google Scholar]
- 49.Poelmans S, Wessa P, Milis K, Bloemen E, Doom EC. Usability and acceptance of e-learning in statistics education; International Conference of Education, Research and Innovation 2008 (ICERI 2008); 2008. pp. 1–10. [Google Scholar]
- 50.Lewis JR. IBM computer usability satisfaction questionnaires: Psychometric evaluation and instructions for use. Int J Hum-Comput Int. 1995;7(1):57–78. [Google Scholar]
- 51.Kovach KA, Aubrecht JA, Dew MA, Myers BA, DeVito Dabbs AJ. Data safety and monitoring for research involving remote health monitoring. Telemed eHealth. 2011;17(7):574–9. doi: 10.1089/tmj.2010.0219. Epub. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Conley E, Owens D, Luzio S, Subramanian M, Ali A, Hardisty A, et al. Simultaneous trend analysis for evaluating outcomes in patient-centred health monitoring services. Health Care Manag Sci. 2008;11(2):152–66. doi: 10.1007/s10729-008-9061-z. [DOI] [PubMed] [Google Scholar]
- 53.Carroll KM, Nich C, Sifry RI, Nuro KF, Frankforter TL, Ball SA, et al. A general system for evaluating therapist adherence and competence in psychotherapy research in addictions. Drug Alcohol Depend. 2000;57(3):225–38. doi: 10.1016/s0376-8716(99)00049-6. [DOI] [PubMed] [Google Scholar]
- 54.DeVito Dabbs AJ, Song MK, Hawkins RP, Aubrecht JA, Kovach KA, Terhorst L, et al. An intervention fidelity framework for technology-based behavioral interventions. Nurs Res. 2011;60(5):340–7. doi: 10.1097/NNR.0b013e31822cc87d. [DOI] [PMC free article] [PubMed] [Google Scholar]