Abstract
Most patients are easily liberated from mechanical ventilation (MV) following resolution of respiratory failure and a successful trial of spontaneous breathing, but about 25% of patients experience difficult weaning. MV use leads to cellular changes and weakness, which has been linked to weaning difficulties and has been labeled ventilator induced diaphragm dysfunction (VIDD). Aggravating factors in human studies with prolonged weaning include malnutrition, chronic electrolyte abnormalities, hyperglycemia, excessive resistive and elastic loads, corticosteroids, muscle relaxant exposure, sepsis and compromised cardiac function. Numerous animal studies have investigated the effects of MV on diaphragm function. Virtually all of these studies have concluded that MV use rapidly leads to VIDD and have identified cellular and molecular mechanisms of VIDD. Molecular and functional studies on the effects of MV on the human diaphragm have largely confirmed the animal results and identified potential treatment strategies. Only recently have potential VIDD treatments been tested in humans, including pharmacologic interventions and diaphragm “training”. A limited number of human studies have found that specific diaphragm training can increase respiratory muscle strength in FTW patients and facilitate weaning, but larger, multicenter trials are needed.
Keywords: ventilator weaning, ventilator induced diaphragm dysfunction, diaphragm strength training
1. Introduction
Our goals are to: 1) Describe the epidemiology of failure to wean (FTW) from mechanical ventilation (MV) in terms of the number of patients impacted and the economic burden on our healthcare system, 2) succinctly review the relationship between ventilator induced diaphragm dysfunction (VIDD), FTW and the measurement of inspiratory muscle strength in humans, 3) briefly summarize the animal literature addressing VIDD and potential prevention and treatment strategies, 4) review selected VIDD human studies, and finally 5) review the effects of respiratory muscle training/rehabilitation on diaphragm function and weaning outcome.
2. Epidemiology of failure to wean
Since its introduction in the early 1950s, positive pressure MV has become one of the hallmark treatment modalities of intensive care. Most patients are readily liberated following surgery or resolution of respiratory failure, but some patients experience difficulty and have been labeled FTW patients. FTW patients are a significant clinical and economic problem facing the American healthcare system. Many patients who experience prolonged MV have a poor one-year functional outcome and survival. Reported one-year mortality rates after prolonged MV range from 20–50% (Unroe 2010). A recent survey (Unroe et al., 2010) of 126 patients receiving long-term MV revealed that of the patients that survived for one year after MV, only 9% were able to perform activities of daily living (ADL) independently, 26% were moderately independnt with ADL and 65% were completely dependent on help with ADL. The mean 12 month medical cost per prolonged MV patient was $306,000. The number of patients requiring prolonged MV (>96 hours) in the USA is growing at about 5.5% per annum; in contrast total hospital admissions are growing at a rate of 1.1% per year (Zilberberg et al., 2008). It has been estimated that by the year 2020, approximately 605,000 patients will require MV for more than 96 hours in the United States annually and many of these patients will experience FTW. The annual cost of these hospitalizations is predicted to be $64 billion (Zilberberg and Shorr, 2008) Accordingly, identifying effective prevention and treatments strategies for FTW from MV should be considered a high priority.
The etiology of FTW is usually multifactorial and can include underlying severe respiratory disease (COPD or severe restrictive disease), heart failure, ICU complications such as sepsis, critical illness neuropathy, and pulmonary mechanical (resistance, compliance and intrinsic PEEP) or infectious complications and respiratory muscle weakness, among others (De Jonghe et al., 2007; Karakurt et al., 2011, 2012; Vassilakopoulos et al., 1998). MV sustains alveolar ventilation by inflating the lungs via positive pressure, thus functioning as “artificial breathing muscles.” MV impose variable degrees of muscular inactivity on the diaphragm (Fauroux et al., 1998), rapidly leading to atrophy and weakness. Diaphragm weakness is recognized as a major contributing factor to FTW (Purro et al., 2000; Vassilakopoulos et al., 1998). Collectively, the deleterious effects of MV on the diaphragm have been termed ventilator induced diaphragm dysfunction (VIDD), a term first coined by Vassilakopoulos and Petrof (Vassilakopoulos and Petrof, 2004).
3. Relationship between diaphragm strength and FTW in human patients
The diaphragm is the primary generator of inspiratory pressure, and its strength has been the subject of numerous studies designed to predict weaning success. Clinically, diaphragm strength is often assessed by measuring the inspiratory pressure at the endotracheal or tracheal tube with the unidirectional valve method (Caruso et al., 1999). This method is clinically practical but has low specificity, and because it is a voluntary activity, can underestimate maximal inspiratory pressure in poorly cooperative, critically ill patients (Multz et al., 1990). Furthermore, it is not specific for the diaphragm, but measures inspiratory pressure generated by all of the inspiratory muscles. More complex integrative indexes of weaning failure have not substantially improved the prediction sensitivity.
A more direct measurement of diaphragm strength uses esophageal and gastric pressure sensors to capture maximal transdiaphragmatic pressure (Pdimax), either during voluntary inspiratory efforts or with bilateral stimulation of the phrenic nerves. Although Pdimax is not practical for regular clinical use, it provides a specific measure of diaphragm strength and when coupled with phrenic stimulation, does not require active patient participation. Several authors who have studied diaphragm strength invasively have reported that patients with greater Pdimax are more likely to successfully wean than patients with weaker diaphragms (Karakurt et al., 2012) (Laghi et al., 1998; Watson et al., 2001). Additionally, FTW patients typically have elevated diaphragm EMG activity during spontaneous breathing trials, but are less efficient at converting the phrenic motor drive into inspiratory pressure or tidal volume (Liu et al., 2012; Purro et al., 2000).
4.0 Effects of MV on diaphragm function: Animal studies
Since the seminal work of Le Bourdelles (Le Bourdelles et al., 1994), approximately 57 studies have examined the effect of MV on the diaphragm in animal models. These studies have documented that controlled MV leads to VIDD in healthy young animals in remarkably short periods of time (e.g., 6–24 hours)(Gayan-Ramirez et al., 2003) (Powers et al., 2011a; Sassoon et al., 2004; Smuder et al., 2011; Supinski and Callahan, 2010). The animal studies have identified three major cellular pathways leading to VIDD: 1) ubiquitin- proteasome, 2) caspase and calpain pathways and 3) lysosomal autophagy. A thorough review of the cellular and molecular pathways contributing to VIDD is beyond the scope of this review and the reader is referred to excellent recent reviews on molecular and cellular mechanisms of VIDD for more details (Jaber et al., 2011a; Powers et al., 2011b).
Some limitations on the translation of the animal studies to human FTW patients must be acknowledged. With few exceptions, the animal work studied young animals (Criswell et al., 2003). The animals also had normal cardiopulmonary and neuromuscular function prior to MV support and were free of comorbidities during periods of MV support. Human FTW patients are usually more than 60 years of age. They often have reduced cardiopulmonary and functional capacity prior to receiving MV and frequently have serious comorbidities such as heart disease, obesity, diabetes, sarcopenia, sepsis and lung disease, which can exacerbate weaning difficulties. Because of the technical difficulty of maintaining animals on MV, few studies have extended MV support beyond 24 hours (Anzueto et al., 1997; Jaber et al., 2005), while human patients frequently receive MV support for weeks or even months. While the elegant, tightly controlled experimental animal work documents that controlled MV can rapidly induce VIDD, the discrepancies between the animals’ and human patients’ functional and health status may lead to an underestimation of the detrimental effects of MV on the human diaphragm.
4.1 Prevention Strategies: Pharmacological
Anti-oxidants have been shown to attenuate VIDD in animal models (Agten et al., 2011) (Betters et al., 2004; McClung et al., 2007; Ochala et al., 2010; Powers et al., 2011a). Long-term administration of corticosteroids has been associated with skeletal muscle and diaphragm atrophy, (Dekhuijzen et al., 1993) but some investigators have shown that short-term use of high-dose corticosteroids may offer some protection against VIDD (Maes et al., 2010; Sassoon et al., 2011). As a more comprehensive understanding of the cellular and molecular mechanisms contributing to VIDD is achieved, the prospect of developing pharmaceutical agents that can prevent or treat VIDD is promising.
4.2 Prevention Strategies: training and activity during MV support
Limited data exists examining the effects of diaphragm strength training in animals. Bisschop and coworkers examined the effects of four weeks of resistive training on diaphragm muscle fiber cross sectional area (CSA) in rats and found a 19% increase in type IIa muscle fibers with training (Bisschop et al., 1997). Smith and coworkers examined the effects of two weeks of intermittent tracheal occlusions on diaphragm muscle fiber CSA in rats and reported a 25% increase in fast, glycolytic fibers (Smith et al., 2012). Neither of these studies examined the diaphragms of trained animals after a period of MV support, but collectively the results suggest that the diaphragm muscle fibers can undergo classical strength training (hypertrophy) and presumably increased strength. Increasing diaphragm strength prior to a bout of MV support will theoretically increase the diaphragm’s functional reserve and may offer some protection against VIDD. More work examining the effects of strength training on the diaphragm is needed.
A small number of studies have examined the effects of increased diaphragm activity or training prior to and during periods of MV. Smuder and coworkers trained rats with a 10 day endurance program prior to a bout of MV support and found that training increased the antioxidant capacity in the diaphragm (Smuder et al., 2011). Endurance training protected diaphragm mitochondria against MV-induced oxidative damage and oxidative phosphorylation uncoupling. The clinical translation of this finding would require patients to be trained before undergoing MV support. It is possible to predict which patients are likely to require prolonged MV following cardiac surgery (Reddy et al., 2007). Patients at high-risk for post-operative pulmonary complications (PPC) have been shown to benefit from pre-surgical respiratory muscle training. We discuss uses of pre-operative training in Section 6.1.
Limited work has examined the effect of increased diaphragm activity during periods of MV support. Intermittent hypoxia has been used by some investigators to induce respiratory neuromuscular plasticity (Baker-Herman et al., 2004; Golder and Mitchell, 2005; Mitchell et al., 2001). While intermittent hypoxia can increase the pressure load on the diaphragm and induce neuromuscular plasticity, it is unlikely to be a useful treatment modality for human patients. Most human patients maintained on MV require hyperoxic mixtures (30–50%) to maintain arterial oxygen saturation due to the inefficiency of positive pressure ventilation and compromised cardiopulmonary function. Sassoon and coworkers used a rabbit model to examine the effect of assist-control MV on diaphragm function versus controlled MV (Sassoon et al., 2004). Controlled MV can impose a complete cessation of diaphragm electrical activity and is thought to be the most potent ventilation mode to induce VIDD (Hudson et al., 2012). Assist-control MV is more commonly used with human patients and requires the patients to generate an inspiratory effort to trigger a MV supported breath. Sassoon found that assist-control MV attenuated the loss in diaphragm contractile function compared to controlled MV. While prolonged controlled MV is rarely used in humans who will attempt weaning, this study highlighted the potential of spontaneous ventilation as a means of attenuating VIDD. Gayan-Ramirez studied the effect of intermittent spontaneous breathing activity on diaphragm force production in rats subjected to controlled MV (Gayan-Ramirez et al., 2005). In this study, 2 groups of animals received 24 hours of controlled MV; one group breathed spontaneously without MV support for five minutes every six hours. A third, control group remained sedated, but unventilated. The intermittent spontaneous breathing group maintained more diaphragm strength than the continuously ventilated group, but had reduced diaphragm strength compared to the control group (see Fig 1).
Figure 1.
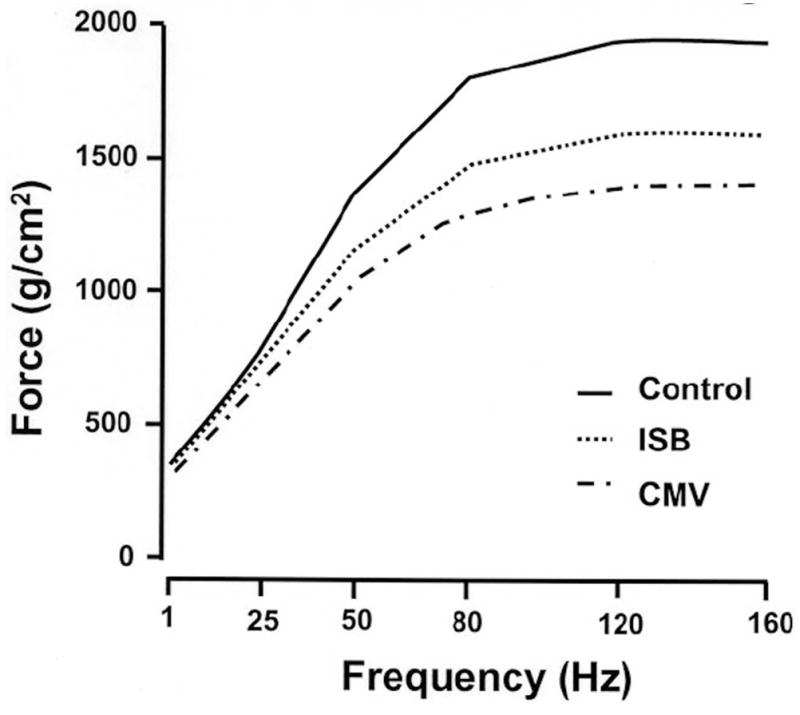
Effects of intermittent spontaneous breathing during controlled MV on diaphragm force – frequency relationship. This figure demonstrates the effects of allowing spontaneous breathing for five minutes out of every six hours on animals receiving controlled MV for 24 hours. The Control animals received no MV. The intermittent spontaneous breathing (ISB) group maintained more diaphragm force than the animals receiving continuous MV, but generated less force than the Control animals. Adapted with permission from Gayan-Ramirez, 2005.
These results have possible clinical implications: VIDD in patients could be potentially attenuated with assist modes of MV that require the patient to trigger the ventilator and with brief bouts of partial support or unsupported breathing as tolerated. The use of partially assisting modes of spontaneous ventilation, such as pressure support, makes the ventilation more acceptable to the patients and improves patient ventilator synchronicity (Tokioka et al., 1989). However patients stable on minimal ventilator settings may nevertheless develop failure when inspiratory work increases 30–60% following extubation from minimal settings (Tobin, 2012). It can be difficult to predict which patients will fail extubation from minimal MV settings, and thus short bouts of unassisted t-piece or flow-by trials may serve both to train the respiratory muscles and to identify patients who cannot maintain sufficient inspiratory motor output to breathe unassisted (Tobin, 2012). In further support of unassisted breathing trials, a recent study examining the effect of pressure support ventilation versus trach collar (fully unsupported breathing) during weaning trials in FTW patients revealed that patients weaned faster when they were required to do all of the muscular work of breathing during breathing trials (Jubran et al., 2013), compared to low levels of pressure support MV.
Collectively, numerous animal studies have unequivocally shown that short (6–24 hrs) periods of MV leads to a complex cellular/biochemical response in the diaphragm culminating in oxidative stress, increased muscle fiber proteolysis and decreased contractile force. Promising, but limited data suggest that the pathways leading to VIDD can be blocked with selected antioxidant drugs, and acute high dose corticosteroids may also be beneficial in the short term. Limited data suggest that the rat diaphragm can undergo muscle fiber CSA hypertrophy in response to short term strength training, which may infer some protection against VIDD. The data also suggest that increased diaphragm activity prior to and during periods of MV may offer some protection against VIDD.
5. Effects of MV on diaphragm function: Human studies
To date, approximately 19 studies have examined the impact of MV on the structure and function of the human diaphragm, including dependent measures such as gene expression (Huang et al., 2011; Welvaart et al., 2011a), diaphragm thickness and pressure generation (Grosu et al., 2012; Hermans et al., 2010; Jaber et al., 2011b), muscle fiber CSA (Levine et al., 2008) or cellular and molecular mechanisms of VIDD (Hussain et al., 2010; Picard et al., 2012; Tang et al., 2011). The human studies have generally corroborated the animal study findings.
The rapid development of VIDD in animals and humans implies rapid changes in diaphragm gene expression. We, and others have shown that cardio-thoracic surgeries lasting 2 to 6 hours lead to gene expression changes compatible with the early stages of VIDD development (Huang et al., 2011; Welvaart et al., 2011a).
The first study examining the effects of MV on the human diaphragm muscle fiber CSA was published in 1988 (Knisely et al., 1988). Diaphragm samples from infants who were ventilated for 12 or more days before death were contrasted with samples from infants ventilated for less than 8 days before death. The results demonstrated reductions diaphragm muscle fiber CSA in the children ventilated for longer periods of time. Curiously, 20 years would pass before another study examining the effects of MV on human diaphragm muscle fiber CSA was published.
Interest in the effects of MV on the human diaphragm was reawakened in 2008 by Levine’s study documenting the rapid development of profound atrophy. In this study diaphragm muscle fiber CSA in a group of healthy subjects undergoing resection of lung tumors requiring only a few hours of MV during surgery was contrasted to a group of brain dead organ donors who had received controlled MV for an average of approximately 39 hours (Levine et al., 2008) before tissue sampling. The slow and fast fiber CSA was approximately 55% smaller in the organ donor cases compared with the control cases. The rapidity of the diaphragm CSA losses was predicted by the work completed in rodents by DeRuisseau et al, who reported that diaphragm muscle fibers atrophied approximately eight times faster than limb muscle during periods of MV support (DeRuisseau et al., 2005). A possible explanation for the different rates of atrophy in the diaphragm and limb skeletal muscle may be found in their duty cycles. Controlled MV imposes a complete cessation of activity in the diaphragm, while it is accustomed to continuous activity with a duty cycle of ~30% and rapid atrophy ensues after MV support is started. Limb muscle may be more resistant to inactivity-induced atrophy because of its intermittent activity pattern and lower duty (~5%).
Some investigators have examined the effect of MV support on human diaphragm physiological function with single muscle fiber and transdiaphragmatic pressure techniques. Welvaart measured single fiber contractile properties in the human diaphragm following two hours of surgery with MV support and reported a 35% reduction in diaphragm fiber force production (Welvaart et al., 2011b). Using repeated bilateral magnetic stimulation of the phrenic nerves and transdiaphragmatic pressure measurements, Hermans studied the natural history of diaphragmatic strength changes in 7 long-term respiratory failure patients receiving MV (Hermans et al., 2010). (Fig 2) This novel work demonstrated that the diaphragm pressure generating capacity decreased rapidly in the first 5 – 7 days of MV support, and then the rate of pressure loss slowly decreased. Jaber et al recorded serial measures of twitch tracheal airway pressure in a group of patients receiving MV (Jaber et al., 2011b). Over the course of one week of MV support, a 32% reduction in twitch tracheal airway pressure was observed. Further evidence of the rapidity with which MV can induce atrophy in the human diaphragm was presented by Grosu who studied the effects of 1 week of MV support on diaphragm thickness with sonography and reported a decrease in diaphragm thickness of 6% per day (Grosu et al., 2012). From a clinical viewpoint, the rapid decline in diaphragm strength observed in the first seven days of MV support is important, because medical lability often initially renders ICU patients unable to participate in volitional training or rehabilitation activities that might maintain diaphragm strength.
Fig 2.
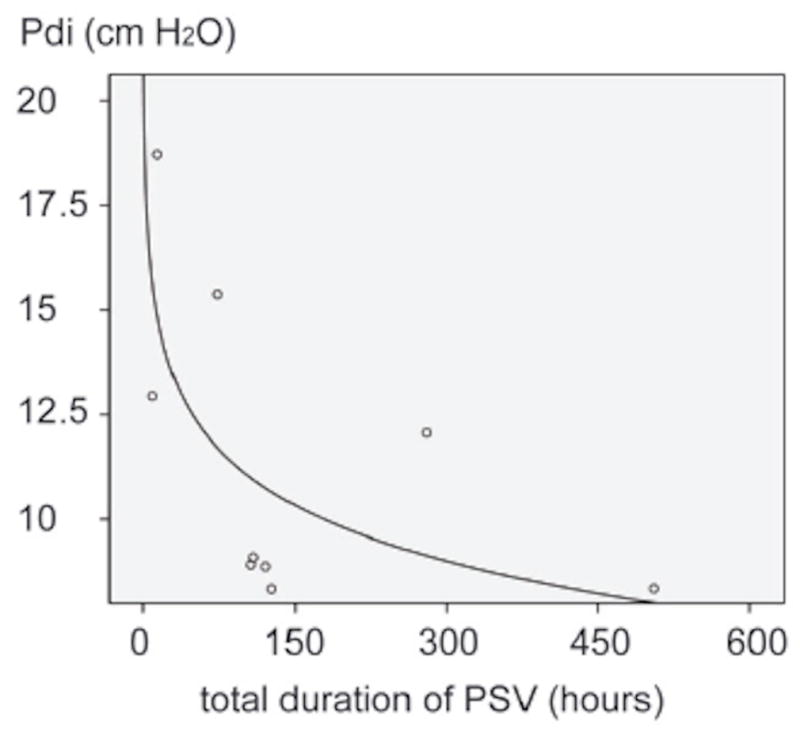
Relationship between duration of pressure support mechanical ventilation (PSV) and twitch trans diaphragmatic pressure (Pdi) generation in ventilated patients. The greatest loss in Pdi occurs in the first 5–7 days of MV and the rate of Pdi loss slows after about 7 days. Adapted from Hermans, 2010.
5.1 Prevention Strategies: Pharmacological
A single large randomized control trial showed that antioxidant supplementation (alpha-tocopherol and ascorbic acid) in the peri-operative period reduced the incidence of PPC and MV and ICU days in trauma surgery patients (Nathens et al., 2002). More work examining pharmacological means of preventing or treating VIDD in humans is needed.
6. Effects of respiratory muscle activity/rehabilitation on diaphragm function and weaning outcome in humans
6.1 Respiratory Muscle training prior to MV
Pre-operative specific inspiratory muscle training (IMT) has been shown to reduce post-operative diaphragm weakness and enhance clinical outcomes. Hulzebos et al examined the effect of pre-operative IMT on PPC in a randomized control trial (Hulzebos et al., 2006a). 279 patients at high risk for PPC following coronary artery bypass surgery were randomly allocated to control and IMT groups. Patients performed IMT for 2 weeks prior to surgery (30% of maximal inspiratory pressure for 20 minutes daily) and the incidence of PPC and duration of hospitalization were examined. The IMT group had a lower incidence of PPC and shorter hospital stays. Pre-operative IMT has also been effective for patients with pulmonary co-morbidities who undergo cardiac, vascular and abdominal surgeries. Training was associated with gains in pre-operative inspiratory strength up to 25% (Hulzebos et al., 2006a; Nomori et al., 1994; Weiner et al., 1998), significant decreases in the rate of PPCs, and faster post-operative weaning (Hulzebos et al., 2006b; Weiner et al., 1998). While thoraco-abdominal surgeries can acutely reduce maximal inspiratory pressure generation up to 50% (Chetta et al., 2006), pre-operative IMT appears to attenuate this loss of strength (Kulkarni et al., 2010). Notably, pre-operative relaxation exercises or incentive spirometry alone do not significantly improve strength and length-of-stay (Hulzebos et al., 2006a; Kulkarni et al., 2010), suggesting specific IMT may induce direct neuromuscular remodeling. More work is needed to characterize the potential benefits of pre-surgical conditioning on the remodeling of the respiratory muscles.
6.2 Inspiratory muscle training during MV
Several authors have examined functional changes in the human diaphragm following MV. Ayas published a case study on the effect of hemidiaphragm stimulation in a patient with a high cervical spinal cord injury who was ventilated with bilateral phrenic pacemakers, and one of the pacing wire sites became infected requiring resumption of full time MV support (Ayas et al., 1999). Pacing was stopped and the patient was maintained on standard MV for eight months before resuming bilateral phrenic pacing. The remaining functional phrenic nerve pacer was used 30 minutes/day and before resumption of bilateral, full-time phrenic pacing, the thickness (via ultrasound) and inspiratory function of both hemidiaphragms were studied. The hemidiaphragm that was paced daily over eight months did not atrophy and was able to generate the same tidal volume observed before initiating fulltime MV support. After eight months of inactivity, the un-paced hemidiaphragm’s thickness decreased by approximately 50% and it could generate only 35% of the inspired tidal volume it did before stopping routine pacing.
Since the diaphragm and accessory respiratory muscles appear susceptible to atrophy and hypertrophy with changes in activation and loading, IMT is a biologically plausible treatment for FTW patients. Several authors have published case studies documenting the use of various IMT activities to improve muscle performance and reported weaning outcomes. One of the first studies to successfully use IMT in FTW patients used isocapnic hypernea as a training mode (Belman, 1981). The regime was impractical for clinical use, due to the complex equipment required to maintain a stable end tidal CO2 pressure, but these results helped to establish that FTW patients could tolerate short bouts of IMT. Other investigators followed the rationale for specific, voluntary exercises with uncontrolled case reports. They found that patients tolerated IMT, and many patients who had previously failed to wean were liberated from MV (Aldrich and Karpel, 1985; Aldrich and Uhrlass, 1987; Tan et al., 1992). Some of these studies used inspiratory resistance devices placed on the end of the endotracheal or tracheal tubes to provide the training stimulus and training was typically conducted for 10 – 15 minutes, twice a day with uncontrolled airflows and breathing frequencies (Aldrich and Karpel, 1985; Aldrich et al., 1989).
From a muscle rehabilitation standpoint, the use of a resistive device with uncontrolled flow rates to impose a strength training load on the inspiratory muscles has several problems (Reid and Samrai, 1995). First, the pressure generated across a resistive load is proportional to the inspiratory airflow. Inspiring with a slower flow therefore requires less pressure generation by the inspiratory muscles. In practice, some patients learn to adopt low inspiratory flow rates to reduce the inspiratory work associated with resistive-loading and reduce their perceived effort (Jederlinic et al., 1984). While the patients may feel more comfortable with reduced inspiratory airflows and pressure, the lowered muscle tension needed to generate low flow reduces the muscle strength-training stimulus. Additionally, the use of two 15-minute training bouts with uncontrolled breathing frequencies means that the patients were completing endurance training bouts rather than strength training bouts.
Many of the problems with using resistive training devices were alleviated with the introduction of spring-loaded threshold devices adapted for IMT. An inspiratory threshold valve requires the patient to generate sufficient inspiratory pressure to compress a spring, open a poppet valve and allow inspiratory airflow. Threshold valves can be set to open at a specific, repeatable pressures and generally do not present any resistive loading over the inspiratory flow rates typically achieved by FTW patients (Gosselink et al., 1996). Threshold inspiratory trainers provide a quantified, repeatable pressure overload to the inspiratory muscles that is not dependent upon the inspiratory flow. In the last 10 years, several investigators have used inspiratory threshold devices to train FTW patients and have reported good results, which included improved inspiratory pressure generation and weaning from MV. Although the majority of these studies were uncontrolled case reports (Bissett et al., 2012; Chang et al., 2005; Martin et al., 2002) (Sprague and Hopkins, 2003), they offered proof-of-concept that specific inspiratory muscle strength training (IMST) is feasible, safe and could improve the inspiratory muscle strength.
In a recent controlled trial, our group examined the effectiveness of IMST on weaning outcome in long-term FTW patients (Martin et al., 2011). In this study, patients had been supported by MV for an average of 6.5 weeks and had failed multiple weaning attempts with usual clinical care. Patients were randomly allocated to Usual care/SHAM training and an IMST group used threshold-training devices with a high pressure, low repetition training program (4 sets of 6–10 inspiratory efforts daily, 5 days/week at the maximal pressure tolerated). After approximately 2 weeks of treatment, the IMST group improved maximal inspiratory pressure measured at the tracheal tube by approximately 35%, and 71% of the IMST patients were weaned. The Usual care group’s maximal inspiratory pressure increased 6% and 47% of the usual care subjects were weaned. The differences in maximal inspiratory pressure and weaning outcome between the IMST and Usual care groups were significant, P <0.05.
In a randomized trial, Cader and coworkers examined the effects of IMST on elderly FTW patients and found that training increased maximal inspiratory pressure, led to a slower, larger tidal volume breathing pattern during unsupported breathing trials and significantly reduced the time to weaning (Cader et al., 2010).
While these randomized IMST trials are encouraging that a clinically practical IMST program can improve inspiratory muscle strength and weaning outcome, the sample sizes were small, and they were conducted at a single sites. Larger, multicenter trials are needed.
Not all researchers found training to be effective at improving maximal inspiratory pressure. Caruso examined the effect of increasing the inspiratory pressure trigger on a ventilator as a means of imposing a threshold pressure overload on the inspiratory muscles in ventilated patients (Caruso et al., 2005). Training was conducted in a progressive manner for 5–30 minutes each day, with the ventilator pressure trigger set to 10–40% of the patient’s maximal inspiratory pressure. The duration of the weaning period was contrasted with usual care patients. The training regimen did not increase maximal inspiratory pressure or decrease the duration of MV support. Since the patients received full MV assistance after reaching the ventilator’s trigger pressure, it is possible that a more sustained muscle contraction is required to induce diaphragm plasticity.
An additional consideration for the clinical use of IMST in FTW patients is the potential for inducing damage in muscle that may have undergone ultrastructural damage due to MV support. Time-dependent myofibrillar damage has been reported in the diaphragms of brain-dead organ donors who had received controlled MV for 24 – 249 hours (Jaber et al., 2011b). Some patients may be at higher risk, such as patients with pre-existing diaphragm dysfunction due to COPD and dynamic hyperinflation (Ottenheijm et al., 2007). Orozco-Levi showed that patients with COPD were more susceptible to diaphragm muscle damage when they underwent high intensity inspiratory loads to task failure, but limited damage was seen in age-matched control subjects (Orozco-Levi et al., 2001). These findings suggest that patients who chronically operate with a diaphragm at a mechanical disadvantage may be more susceptible to injury. Because of the risk of damaging already fragile muscle, good clinical practice dictates that any rehabilitation effort to improve diaphragm strength in FTW patients should start at low pressures and gradually increase as the muscle adapts to training.
7. Summary
MV is one of the most important treatment modalities in intensive care, and recent advances have improved the interaction between patient and the ventilator but MV support can lead to VIDD and FTW in some patients. The number of patients experiencing FTW is rapidly growing; these patients account for enormous medical costs and they have poor clinical outcomes. Numerous animal studies have clearly shown the MV use rapidly leads to VIDD. Animal studies have found that anti-oxidants and physical activity can block or attenuate VIDD induced by short-term MV use. Limited human work examining VIDD has largely corroborated the animal results, but more work investigating cellular and functional changes in the human diaphragm following MV support and IMST are needed. Limited data indicates that FTW patients benefit from specific IMST, leading to reduced MV time and improved weaning rate, but these studies have used small sample sizes and were conducted at single sites. Large, multicenter trials examining IMST against a routine protocolized approach to weaning are needed.
Highlights.
Mechanical ventilation rapidly leads to diaphragm dysfunction in animals and humans.
Failure to wean patients accumulate enormous healthcare costs.
The number of failure to wean patients is growing rapidly.
Diaphragm strength training may facilitate weaning from mechanical ventilation.
Acknowledgments
Supported by James & Esther King Biomedical Research Program, State of Florida Biomedical Grant 09KW-07, (ADM and AG) and NIH K12HD055929 (BKS). The funding agencies provided support to the authors, but provided no input on the content of the publication.
Footnotes
Disclosure Statement: Drs. Martin and Gabrielli along with the University of Florida hold a US patent addressing the modification of clinical mechanical ventilators to provide specific inspiratory muscle strength training to ventilated patients.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Agten A, Maes K, Smuder A, Powers SK, Decramer M, Gayan-Ramirez G. N-Acetylcysteine protects the rat diaphragm from the decreased contractility associated with controlled mechanical ventilation. Crit Care Med. 2011:777–782. doi: 10.1097/CCM.0b013e318206cca9. [DOI] [PubMed] [Google Scholar]
- Aldrich TK, Karpel JP. Inspiratory muscle resistive training in respiratory failure. Am Rev Respir Dis. 1985;131:461–462. doi: 10.1164/arrd.1985.131.3.461. [DOI] [PubMed] [Google Scholar]
- Aldrich TK, Karpel JP, Uhrlass RM, Sparapani MA, Eramo D, Ferranti R. Weaning from mechanical ventilation: adjunctive use of inspiratory muscle resistive training. Crit Care Med. 1989;17:143–147. [PubMed] [Google Scholar]
- Aldrich TK, Uhrlass RM. Weaning from mechanical ventilation: successful use of modified inspiratory resistive training in muscular dystrophy. Crit Care Med. 1987;15:247–249. [PubMed] [Google Scholar]
- Anzueto A, Peters JI, Tobin MJ, de los Santos R, Seidenfeld JJ, Moore G, Cox WJ, Coalson JJ. Effects of prolonged controlled mechanical ventilation on diaphragmatic function in healthy adult baboons. Crit Care Med. 1997;25:1187–1190. doi: 10.1097/00003246-199707000-00021. [DOI] [PubMed] [Google Scholar]
- Ayas NT, McCool FD, Gore R, Lieberman SL, Brown R. Prevention of human diaphragm atrophy with short periods of electrical stimulation. Am J Respir Crit Care Med. 1999;159:2018–2020. doi: 10.1164/ajrccm.159.6.9806147. [DOI] [PubMed] [Google Scholar]
- Baker-Herman TL, Fuller DD, Bavis RW, Zabka AG, Golder FJ, Doperalski NJ, Johnson RA, Watters JJ, Mitchell GS. BDNF is necessary and sufficient for spinal respiratory plasticity following intermittent hypoxia. Nat Neurosci. 2004;7:48–55. doi: 10.1038/nn1166. [DOI] [PubMed] [Google Scholar]
- Belman MJ. Respiratory failure treated by ventilatory muscle training (VMT). A report of two cases. Eur J Respir Dis. 1981;62:391–395. [PubMed] [Google Scholar]
- Betters JL, Criswell DS, Shanely RA, Van Gammeren D, Falk D, Deruisseau KC, Deering M, Yimlamai T, Powers SK. Trolox attenuates mechanical ventilation-induced diaphragmatic dysfunction and proteolysis. Am J Respir Crit Care Med. 2004;170:1179–1184. doi: 10.1164/rccm.200407-939OC. [DOI] [PubMed] [Google Scholar]
- Bisschop A, Gayan-Ramirez G, Rollier H, Gosselink R, Dom R, de Bock V, Decramer M. Intermittent inspiratory muscle training induces fiber hypertrophy in rat diaphragm. Am J Respir Crit Care Med. 1997;155:1583–1589. doi: 10.1164/ajrccm.155.5.9154861. [DOI] [PubMed] [Google Scholar]
- Bissett B, Leditschke IA, Green M. Specific inspiratory muscle training is safe in selected patients who are ventilator-dependent: a case series. Intensive Crit Care Nurs. 2012;28:98–104. doi: 10.1016/j.iccn.2012.01.003. [DOI] [PubMed] [Google Scholar]
- Cader SA, Vale RG, Castro JC, Bacelar SC, Biehl C, Gomes MC, Cabrer WE, Dantas EH. Inspiratory muscle training improves maximal inspiratory pressure and may assist weaning in older intubated patients: a randomised trial. Journal of physiotherapy. 2010;56:171–177. doi: 10.1016/s1836-9553(10)70022-9. [DOI] [PubMed] [Google Scholar]
- Caruso P, Denari SD, Ruiz SA, Bernal KG, Manfrin GM, Friedrich C, Deheinzelin D. Inspiratory muscle training is ineffective in mechanically ventilated critically ill patients. Clinics. 2005;60:479–484. doi: 10.1590/s1807-59322005000600009. [DOI] [PubMed] [Google Scholar]
- Caruso P, Friedrich C, Denari SD, Ruiz SA, Deheinzelin D. The unidirectional valve is the best method to determine maximal inspiratory pressure during weaning. Chest. 1999;115:1096–1101. doi: 10.1378/chest.115.4.1096. [DOI] [PubMed] [Google Scholar]
- Chang AT, Boots RJ, Henderson R, Paratz JD, Hodges PW. Case report: inspiratory muscle training in chronic critically ill patients--a report of two cases. Physiother Res Int. 2005;10:222–226. doi: 10.1002/pri.14. [DOI] [PubMed] [Google Scholar]
- Chetta A, Tzani P, Marangio E, Carbognani P, Bobbio A, Olivieri D. Respiratory effects of surgery and pulmonary function testing in the preoperative evaluation. Acta Biomed. 2006;77:69–74. [PubMed] [Google Scholar]
- Criswell DS, Shanely RA, Betters JJ, McKenzie MJ, Sellman JE, Van Gammeren DL, Powers SK. Cumulative effects of aging and mechanical ventilation on in vitro diaphragm function. Chest. 2003;124:2302–2308. doi: 10.1378/chest.124.6.2302. [DOI] [PubMed] [Google Scholar]
- De Jonghe B, Bastuji-Garin S, Durand MC, Malissin I, Rodrigues P, Cerf C, Outin H, Sharshar T. Respiratory weakness is associated with limb weakness and delayed weaning in critical illness. Crit Care Med. 2007;35:2007–2015. doi: 10.1097/01.ccm.0000281450.01881.d8. [DOI] [PubMed] [Google Scholar]
- Dekhuijzen PN, Gayan-Ramirez G, de Bock V, Dom R, Decramer M. Triamcinolone and prednisolone affect contractile properties and histopathology of rat diaphragm differently. J Clin Invest. 1993;92:1534–1542. doi: 10.1172/JCI116732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DeRuisseau KC, Shanely RA, Akunuri N, Hamilton MT, Van Gammeren D, Zergeroglu AM, McKenzie M, Powers SK. Diaphragm unloading via controlled mechanical ventilation alters the gene expression profile. Am J Respir Crit Care Med. 2005;172:1267–1275. doi: 10.1164/rccm.200503-403OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fauroux B, Isabey D, Desmarais G, Brochard L, Harf A, Lofaso F. Nonchemical influence of inspiratory pressure support on inspiratory activity in humans. J Appl Physiol. 1998;85:2169–2175. doi: 10.1152/jappl.1998.85.6.2169. [DOI] [PubMed] [Google Scholar]
- Gayan-Ramirez G, de Paepe K, Cadot P, Decramer M. Detrimental effects of short-term mechanical ventilation on diaphragm function and IGF-I mRNA in rats. Intensive Care Med. 2003;29:825–833. doi: 10.1007/s00134-003-1688-0. [DOI] [PubMed] [Google Scholar]
- Gayan-Ramirez G, Testelmans D, Maes K, Racz GZ, Cadot P, Zador E, Wuytack F, Decramer M. Intermittent spontaneous breathing protects the rat diaphragm from mechanical ventilation effects. Crit Care Med. 2005;33:2804–2809. doi: 10.1097/01.ccm.0000191250.32988.a3. [DOI] [PubMed] [Google Scholar]
- Golder FJ, Mitchell GS. Spinal synaptic enhancement with acute intermittent hypoxia improves respiratory function after chronic cervical spinal cord injury. J Neurosci. 2005;25:2925–2932. doi: 10.1523/JNEUROSCI.0148-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gosselink R, Wagenaar RC, Decramer M. Reliability of a commercially available threshold loading device in healthy subjects and in patients with chronic obstructive pulmonary disease. Thorax. 1996;51:601–605. doi: 10.1136/thx.51.6.601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grosu HB, Lee YI, Lee J, Eden E, Eikermann M, Rose K. Diaphragm Muscle Thinning in Mechanically Ventilated Patients. Chest. 2012;142:1455–1460. doi: 10.1378/chest.11-1638. [DOI] [PubMed] [Google Scholar]
- Hermans G, Agten A, Testelmans D, Decramer M, Gayan-Ramirez G. Increased duration of mechanical ventilation is associated with decreased diaphragmatic force: a prospective observational study. Crit Care. 2010;14:R127. doi: 10.1186/cc9094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang TT, Deoghare HV, Smith BK, Beaver TM, Baker HV, Mehinto AC, Martin AD. Gene expression changes in the human diaphragm after cardiothoracic surgery. J Thorac Cardiovasc Surg. 2011;142:1214–1222. 1222, e1211–1220. doi: 10.1016/j.jtcvs.2011.02.025. [DOI] [PubMed] [Google Scholar]
- Hudson MB, Smuder AJ, Nelson WB, Bruells CS, Levine S, Powers SK. Both high level pressure support ventilation and controlled mechanical ventilation induce diaphragm dysfunction and atrophy. Crit Care Med. 2012;40:1254–1260. doi: 10.1097/CCM.0b013e31823c8cc9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hulzebos EH, Helders PJ, Favie NJ, De Bie RA, Brutel de la Riviere A, Van Meeteren NL. Preoperative intensive inspiratory muscle training to prevent postoperative pulmonary complications in high-risk patients undergoing CABG surgery: a randomized clinical trial. JAMA. 2006a;296:1851–1857. doi: 10.1001/jama.296.15.1851. [DOI] [PubMed] [Google Scholar]
- Hulzebos EH, van Meeteren NL, van den Buijs BJ, de Bie RA, Brutel de la Riviere A, Helders PJ. Feasibility of preoperative inspiratory muscle training in patients undergoing coronary artery bypass surgery with a high risk of postoperative pulmonary complications: a randomized controlled pilot study. Clin Rehabil. 2006b;20:949–959. doi: 10.1177/0269215506070691. [DOI] [PubMed] [Google Scholar]
- Hussain SN, Mofarrahi M, Sigala I, Kim HC, Vassilakopoulos T, Maltais F, Bellenis I, Chaturvedi R, Gottfried SB, Metrakos P, Danialou G, Matecki S, Jaber S, Petrof BJ, Goldberg P. Mechanical ventilation-induced diaphragm disuse in humans triggers autophagy. Am J Respir Crit Care Med. 2010;182:1377–1386. doi: 10.1164/rccm.201002-0234OC. [DOI] [PubMed] [Google Scholar]
- Jaber S, Jung B, Matecki S, Petrof BJ. Clinical review: Ventilator-induced diaphragmatic dysfunction - human studies confirm animal model findings! Crit Care. 2011a;15:206. doi: 10.1186/cc10023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jaber S, Petrof BJ, Jung B, Chanques G, Berthet JP, Rabuel C, Bouyabrine H, Courouble P, Koechlin-Ramonatxo C, Sebbane M, Similowski T, Scheuermann V, Mebazaa A, Capdevila X, Mornet D, Mercier J, Lacampagne A, Philips A, Matecki S. Rapidly progressive diaphragmatic weakness and injury during mechanical ventilation in humans. Am J Respir Crit Care Med. 2011b;183:364–371. doi: 10.1164/rccm.201004-0670OC. [DOI] [PubMed] [Google Scholar]
- Jaber S, Sebbane M, Koechlin C, Hayot M, Capdevila X, Eledjam JJ, Prefaut C, Ramonatxo M, Matecki S. Effects of short vs. prolonged mechanical ventilation on antioxidant systems in piglet diaphragm. Intensive Care Med. 2005;31:1427–1433. doi: 10.1007/s00134-005-2694-1. [DOI] [PubMed] [Google Scholar]
- Jederlinic P, Muspratt JA, Miller MJ. Inspiratory muscle training in clinical practice. Physiologic conditioning or habituation to suffocation? Chest. 1984;86:870–873. doi: 10.1378/chest.86.6.870. [DOI] [PubMed] [Google Scholar]
- Jubran A, Grant BJ, Duffner LA, Collins EG, Lanuza DM, Hoffman LA, Tobin MJ. Effect of pressure support vs unassisted breathing through a tracheostomy collar on weaning duration in patients requiring prolonged mechanical ventilation: a randomized trial. JAMA. 2013;309:671–677. doi: 10.1001/jama.2013.159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karakurt Z, Fanfulla F, Ceriana P, Carlucci A, Grassi M, Colombo R, Karakurt S, Nava S. Physiologic determinants of prolonged mechanical ventilation in patients after major surgery. J Crit Care. 2011 doi: 10.1016/j.jcrc.2011.08.009. [DOI] [PubMed] [Google Scholar]
- Karakurt Z, Fanfulla F, Ceriana P, Carlucci A, Grassi M, Colombo R, Karakurt S, Nava S. Physiologic determinants of prolonged mechanical ventilation in patients after major surgery. J Crit Care. 2012;27:221 e229–216. doi: 10.1016/j.jcrc.2011.08.009. [DOI] [PubMed] [Google Scholar]
- Knisely AS, Leal SM, Singer DB. Abnormalities of diaphragmatic muscle in neonates with ventilated lungs. J Pediatr. 1988;113:1074–1077. doi: 10.1016/s0022-3476(88)80585-7. [DOI] [PubMed] [Google Scholar]
- Kulkarni SR, Fletcher E, McConnell AK, Poskitt KR, Whyman MR. Pre-operative inspiratory muscle training preserves postoperative inspiratory muscle strength following major abdominal surgery - a randomised pilot study. Ann R Coll Surg Engl. 2010;92:700–707. doi: 10.1308/003588410X12771863936648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laghi F, Topeli A, Tobin MJ. Does resistive loading decrease diaphragmatic contractility before task failure? J Appl Physiol. 1998;85:1103–1112. doi: 10.1152/jappl.1998.85.3.1103. [DOI] [PubMed] [Google Scholar]
- Le Bourdelles G, Viires N, Boczkowski J, Seta N, Pavlovic D, Aubier M. Effects of mechanical ventilation on diaphragmatic contractile properties in rats. Am J Respir Crit Care Med. 1994;149:1539–1544. doi: 10.1164/ajrccm.149.6.8004310. [DOI] [PubMed] [Google Scholar]
- Levine S, Nguyen T, Taylor N, Friscia ME, Budak MT, Rothenberg P, Zhu J, Sachdeva R, Sonnad S, Kaiser LR, Rubinstein NA, Powers SK, Shrager JB. Rapid disuse atrophy of diaphragm fibers in mechanically ventilated humans. N Engl J Med. 2008;358:1327–1335. doi: 10.1056/NEJMoa070447. [DOI] [PubMed] [Google Scholar]
- Liu L, Liu H, Yang Y, Huang Y, Liu S, Beck J, Slutsky AS, Sinderby C, Qiu H. Neuro-ventilatory efficiency and extubation readiness in critically ill patients. Crit Care. 2012;16:R143. doi: 10.1186/cc11451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maes K, Agten A, Smuder A, Powers SK, Decramer M, Gayan-Ramirez G. Corticosteroid effects on ventilator-induced diaphragm dysfunction in anesthetized rats depend on the dose administered. Respiratory research. 2010;11:178. doi: 10.1186/1465-9921-11-178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martin AD, Davenport PD, Franceschi AC, Harman E. Use of inspiratory muscle strength training to facilitate ventilator weaning: a series of 10 consecutive patients. Chest. 2002;122:192–196. doi: 10.1378/chest.122.1.192. [DOI] [PubMed] [Google Scholar]
- Martin AD, Smith BK, Davenport P, Harman E, Gonzalez-Rothi RJ, Baz M, Layon AJ, Banner M, Caruso LJ, Deoghare H, Huang TT, Gabrielli A. Inspiratory muscle strength training improves weaning outcome in failure to wean patients: a randomized trial. Critical Care. 2011;15:R84. doi: 10.1186/cc10081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McClung JM, Kavazis AN, Whidden MA, DeRuisseau KC, Falk DJ, Criswell DS, Powers SK. Antioxidant administration attenuates mechanical ventilation-induced rat diaphragm muscle atrophy independent of protein kinase B (PKB Akt) signalling. The Journal of physiology. 2007;585:203–215. doi: 10.1113/jphysiol.2007.141119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mitchell GS, Baker TL, Nanda SA, Fuller DD, Zabka AG, Hodgeman BA, Bavis RW, Mack KJ, Olson EB., Jr Invited review: Intermittent hypoxia and respiratory plasticity. J Appl Physiol. 2001;90:2466–2475. doi: 10.1152/jappl.2001.90.6.2466. [DOI] [PubMed] [Google Scholar]
- Multz AS, Aldrich TK, Prezant DJ, Karpel JP, Hendler JM. Maximal inspiratory pressure is not a reliable test of inspiratory muscle strength in mechanically ventilated patients. Am Rev Respir Dis. 1990;142:529–532. doi: 10.1164/ajrccm/142.3.529. [DOI] [PubMed] [Google Scholar]
- Nathens AB, Neff MJ, Jurkovich GJ, Klotz P, Farver K, Ruzinski JT, Radella F, Garcia I, Maier RV. Randomized, prospective trial of antioxidant supplementation in critically ill surgical patients. Ann Surg. 2002;236:814–822. doi: 10.1097/00000658-200212000-00014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nomori H, Kobayashi R, Fuyuno G, Morinaga S, Yashima H. Preoperative respiratory muscle training. Assessment in thoracic surgery patients with special reference to postoperative pulmonary complications. Chest. 1994;105:1782–1788. doi: 10.1378/chest.105.6.1782. [DOI] [PubMed] [Google Scholar]
- Ochala J, Radell PJ, Eriksson LI, Larsson L. EMD 57033 partially reverses ventilator-induced diaphragm muscle fibre calcium desensitisation. Pflugers Arch. 2010;459:475–483. doi: 10.1007/s00424-009-0744-1. [DOI] [PubMed] [Google Scholar]
- Orozco-Levi M, Lloreta J, Minguella J, Serrano S, Broquetas JM, Gea J. Injury of the human diaphragm associated with exertion and chronic obstructive pulmonary disease. Am J Respir Crit Care Med. 2001;164:1734–1739. doi: 10.1164/ajrccm.164.9.2011150. [DOI] [PubMed] [Google Scholar]
- Ottenheijm CA, Heunks LM, Dekhuijzen PN. Diaphragm muscle fiber dysfunction in chronic obstructive pulmonary disease: toward a pathophysiological concept. Am J Respir Crit Care Med. 2007;175:1233–1240. doi: 10.1164/rccm.200701-020PP. [DOI] [PubMed] [Google Scholar]
- Picard M, Jung B, Liang F, Azuelos I, Hussain S, Goldberg P, Godin R, Danialou G, Chaturvedi R, Rygiel K, Matecki S, Jaber S, Des Rosiers C, Karpati G, Ferri L, Burelle Y, Turnbull DM, Taivassalo T, Petrof BJ. Mitochondrial Dysfunction and Lipid Accumulation in the Human Diaphragm during Mechanical Ventilation. Am J Respir Crit Care Med. 2012:1140–1149. doi: 10.1164/rccm.201206-0982OC. [DOI] [PubMed] [Google Scholar]
- Powers SK, Hudson MB, Nelson WB, Talbert EE, Min K, Szeto HH, Kavazis AN, Smuder AJ. Mitochondria-targeted antioxidants protect against mechanical ventilation-induced diaphragm weakness. Crit Care Med. 2011a;39:1749–1759. doi: 10.1097/CCM.0b013e3182190b62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Powers SK, Smuder AJ, Criswell DS. Mechanistic links between oxidative stress and disuse muscle atrophy. Antioxidants & redox signaling. 2011b;15:2519–2528. doi: 10.1089/ars.2011.3973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Purro A, Appendini L, De Gaetano A, Gudjonsdottir M, Donner CF, Rossi A. Physiologic determinants of ventilator dependence in long-term mechanically ventilated patients. Am J Respir Crit Care Med. 2000;161:1115–1123. doi: 10.1164/ajrccm.161.4.9812160. [DOI] [PubMed] [Google Scholar]
- Reddy SL, Grayson AD, Griffiths EM, Pullan DM, Rashid A. Logistic risk model for prolonged ventilation after adult cardiac surgery. Ann Thorac Surg. 2007;84:528–536. doi: 10.1016/j.athoracsur.2007.04.002. [DOI] [PubMed] [Google Scholar]
- Reid WD, Samrai B. Respiratory muscle training for patients with chronic obstructive pulmonary disease. Phys Ther. 1995;75:996–1005. doi: 10.1093/ptj/75.11.996. [DOI] [PubMed] [Google Scholar]
- Sassoon CS, Zhu E, Caiozzo VJ. Assist-control mechanical ventilation attenuates ventilator-induced diaphragmatic dysfunction. Am J Respir Crit Care Med. 2004;170:626–632. doi: 10.1164/rccm.200401-042OC. [DOI] [PubMed] [Google Scholar]
- Sassoon CS, Zhu E, Fang L, Ramar K, Jiao GY, Caiozzo VJ. Interactive effects of corticosteroid and mechanical ventilation on diaphragm muscle function. Muscle Nerve. 2011;43:103–111. doi: 10.1002/mus.21821. [DOI] [PubMed] [Google Scholar]
- Smith B, Martin AD, Vandenborne K, Darragh BDPWD. Chronic Intrinsic Transient Tracheal Occlusion Elicits Diaphragmatic Muscle Fiber Remodeling in Conscious Rodents. PloS one. 2012;7:e49264. doi: 10.1371/journal.pone.0049264. doi:49210.41371/journal.pone.0049264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smuder AJ, Min K, Hudson MB, Kavazis AN, Kwon OS, Nelson WB, Powers SK. Endurance exercise attenuates ventilator-induced diaphragm dysfunction. J Appl Physiol. 2011:501–510. doi: 10.1152/japplphysiol.01086.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sprague SS, Hopkins PD. Use of inspiratory strength training to wean six patients who were ventilator-dependent. Phys Ther. 2003;83:171–181. [PubMed] [Google Scholar]
- Supinski GS, Callahan LA. Calpain activation contributes to endotoxin-induced diaphragmatic dysfunction. Am J Respir Cell Mol Biol. 2010;42:80–87. doi: 10.1165/rcmb.2008-0275OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tan S, Duara S, Silva Neto G, Afework M, Gerhardt T, Bancalari E. The effects of respiratory training with inspiratory flow resistive loads in premature infants. Pediatr Res. 1992;31:613–618. doi: 10.1203/00006450-199206000-00015. [DOI] [PubMed] [Google Scholar]
- Tang H, Lee M, Budak MT, Pietras N, Hittinger S, Vu M, Khuong A, Hoang CD, Hussain SN, Levine S, Shrager JB. Intrinsic apoptosis in mechanically ventilated human diaphragm: linkage to a novel Fos/FoxO1/Stat3-Bim axis. FASEB J. 2011;25:2921–2936. doi: 10.1096/fj.11-183798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tobin MJ. Extubation and the myth of “minimal ventilator settings”. Am J Respir Crit Care Med. 2012;185:349–350. doi: 10.1164/rccm.201201-0050ED. [DOI] [PubMed] [Google Scholar]
- Tokioka H, Saito S, Kosaka F. Comparison of pressure support ventilation and assist control ventilation in patients with acute respiratory failure. Intensive Care Med. 1989;15:364–367. doi: 10.1007/BF00261494. [DOI] [PubMed] [Google Scholar]
- Unroe M, Kahn JM, Carson SS, Govert JA, Martinu T, Sathy SJ, Clay AS, Chia J, Gray A, Tulsky JA, Cox CE. One-year trajectories of care and resource utilization for recipients of prolonged mechanical ventilation: a cohort study. Ann Intern Med. 2010;153:167–175. doi: 10.1059/0003-4819-153-3-201008030-00007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vassilakopoulos T, Petrof BJ. Ventilator-induced diaphragmatic dysfunction. Am J Respir Crit Care Med. 2004;169:336–341. doi: 10.1164/rccm.200304-489CP. [DOI] [PubMed] [Google Scholar]
- Vassilakopoulos T, Zakynthinos S, Roussos C. The tension-time index and the frequency/tidal volume ratio are the major pathophysiologic determinants of weaning failure and success. Am J Respir Crit Care Med. 1998;158:378–385. doi: 10.1164/ajrccm.158.2.9710084. [DOI] [PubMed] [Google Scholar]
- Watson AC, Hughes PD, Louise Harris M, Hart N, Ware RJ, Wendon J, Green M, Moxham J. Measurement of twitch transdiaphragmatic, esophageal, and endotracheal tube pressure with bilateral anterolateral magnetic phrenic nerve stimulation in patients in the intensive care unit. Crit Care Med. 2001;29:1325–1331. doi: 10.1097/00003246-200107000-00005. [DOI] [PubMed] [Google Scholar]
- Weiner P, Zeidan F, Zamir D, Pelled B, Waizman J, Beckerman M, Weiner M. Prophylactic inspiratory muscle training in patients undergoing coronary artery bypass graft. World J Surg. 1998;22:427–431. doi: 10.1007/s002689900410. [DOI] [PubMed] [Google Scholar]
- Welvaart WN, Paul MA, Kuster DW, van Wieringen W, Rustenburg F, Stienen GJ, Vonk-Noordegraaf A, Ottenheijm CA. Gene expression profile in the diaphragm following contractile inactivity during thoracic surgery. Int J Physiol Pathophysiol Pharmacol. 2011a;3:167–175. [PMC free article] [PubMed] [Google Scholar]
- Welvaart WN, Paul MA, Stienen GJ, van Hees HW, Loer SA, Bouwman R, Niessen H, de Man FS, Witt CC, Granzier H, Vonk-Noordegraaf A, Ottenheijm CA. Selective diaphragm muscle weakness after contractile inactivity during thoracic surgery. Ann Surg. 2011b;254:1044–1049. doi: 10.1097/SLA.0b013e318232e75b. [DOI] [PubMed] [Google Scholar]
- Zilberberg MD, de Wit M, Pirone JR, Shorr AF. Growth in adult prolonged acute mechanical ventilation: Implications for healthcare delivery*. Crit Care Med. 2008:1451–1455. doi: 10.1097/CCM.0b013e3181691a49. [DOI] [PubMed] [Google Scholar]
- Zilberberg MD, Shorr AF. Prolonged acute mechanical ventilation and hospital bed utilization in 2020 in the United States: implications for budgets, plant and personnel planning. BMC health services research. 2008;8:242. doi: 10.1186/1472-6963-8-242. [DOI] [PMC free article] [PubMed] [Google Scholar]


