Abstract
Background
Cytolethal distending toxin (CDT) is the only known virulence factor found in H. hepaticus, the cause of chronic typhlocolitis and hepatitis leading to colonic and hepatocellular carcinomas in mice. Interaction of the tripartite polypeptide CdtA, CdtB and CdtC subunits produced by H. hepaticus CDT (HhepCDT) causes cell cycle arrest and apoptotic death of cultured cells; however, the contribution of individual subunit to these processes has not been investigated.
Materials and Methods
The temporal relationship between cell cycle and apoptotic death of human epithelial HeLa and INT407 cells intoxicated with HhepCDT holotoxin or reconstituted recombinant HhepCDT was compared by flow cytometry. The genotoxic activity of individual and combinations of recombinant HhepCDT protein subunits or increasing concentrations of individual recombinant HhepCDT protein subunits transfected into HeLa cells were assessed at 72 hours post-treatment by flow cytometry.
Results
Similar time course of HhepCDT-induced G2/M cell cycle arrest and apoptotic death was found with both cell lines which reached a maximum at 72 hours. The presence of all three HhepCDT subunits was required for maximum cell cycle arrest and apoptosis of both cell lines. Transfection of HeLa cells with HhepCdtB, but not with HhepCdtA or HhepCdtC resulted in a dose-dependent G2/M arrest and apoptotic death.
Conclusion
All three subunits of HhepCDT are required for maximum epithelial cell cycle arrest and progression to apoptotic death, and HhepCdtB subunit alone is necessary and sufficient for epithelial cell genotoxicity.
INTRODUCTION
Cytolethal distending toxin (CDT) is a bacterial genotoxin found in at least two dozen Gram-negative bacteria of the Gamma and Epsilon classes of Proteobacteria (1). In nearly all bacteria that produce CDT, an operon consisting of cdtA, cdtB and cdtC genes, respectively encoding the corresponding tripartite polypeptide complex of subunits CdtA, CdtB, and CdtC is present (1–3). Exceptions to this paradigm are the Salmonella enterica subsp. enterica serotypes including S. Typhi, S. Paratyphi A and several other serotypes harboring a CDT pathogenicity islet encoding a CdtB subunit without corresponding cdtA and cdtC genes (4, 5).
It is generally accepted that CDT is an AB two-component bacterial toxin, in which the “A” component represented by CdtB is the biologically active genotoxin, and the “B” component represented by CdtA and CdtC is responsible for interaction with host cell lipid raft membrane microdomains and intracellular delivery of CdtB (6, 7). Although the CdtB subunit displays conserved structural and functional homologies to members of the phosphodiesterase family including mammalian deoxyribonuclease I (DNaseI) (8–11), the in vitro nuclease activity of CdtB is several folds less than that of mammalian DNaseI (8, 12–15). As such, CDT is the only member of a novel class of AB toxins that exhibits DNase activity and translocates to the nucleus of eukaryotic cells where it exerts genotoxic damage. However, on the basis of divergent genotoxic requirements for host cell and tissue intoxication that correlates with structural heterogeneity, the unifying concept that CDTs produced by various bacteria interact with host cells by using similar binding, uptake and intracellular pathways has been challenged (16). Consequently, a host pathogen interaction model based on individual pathogen in vivo infection microenvironment might be required.
The prototype for enterohepatic Helicobacter species (EHS), H. hepaticus colonizes the lower intestinal and hepatobiliary tracts of susceptible mouse strains and genetically engineered laboratory mice where it causes persistent typhlocolitis and hepatitis leading to colon and hepatocellular carcinomas (17–24). Although CDT is the only known virulence factor found in H. hepaticus (25), its role in the pathogenesis of infection and disease remains incompletely understood (26, 27). We previously showed that H. hepaticus CDT (HhepCDT) causes apoptotic cell death of cultured human intestinal epithelial INT407 cells by activation of the intrinsic (mitochondrial) pathway (28), a finding consistent with induction of apoptosis in hepatocytes and biliary epithelial cells of laboratory mice experimentally infected with H. hepaticus (22, 23, 29–31). Moreover, experimental infection of interleukin-10-deficient C57BL/6 mice with wild-type and a CDT-deficient isogenic H. hepaticus mutant has revealed a critical role for HhepCDT in persistent intestinal colonization leading to development of typhlocolitis associated with modulation of the host immune response (22–24). More importantly, HhepCDT-induced modulation of host adaptive immunity to promote persistent intestinal colonization has been demonstrated in outbred Swiss Webster mice further confirming the importance of this virulence factor in an immunocompetent host (32).
Besides H. hepaticus, several EHS produce CDT including other important intestinal pathogens of laboratory mice such as H. bilis and H. mastomyrinus, a novel EHS, H. marmotae found in the liver of woodchucks, feces of cats and liver and intestine of prairie dogs, but also several newly emerging intestinal pathogens of human beings such as H. cinaedi, H. winghamensis, H. canis, H. bilis and H. pullorum (1, 33, 34). In addition to EHS, several clinically important human and animal bacterial pathogens produce CDT including the foodborne and waterborne intestinal pathogens Campylobacter jejuni (CjejCDT), several pathotypes of Escherichia coli (EcolCDT) and Shigella species, the periodontopathogenic Aggregatibacter actinomycetemcomitans (AactCDT), and the venereal pathogen Haemophilus ducreyi (HducCDT). Irrespective of the bacterial species of origin, CDT-induces genotoxicity by causing double-stranded DNA breaks resulting in activation of the ATM-dependent DNA damage response (DDR) leading to arrest of the cell cycle and apoptotic death of a wide range of eukaryotic cells in vitro (1–3).
Analysis of eukaryotic cell cycle is commonly achieved by monitoring of the nuclear DNA content by flow cytometry following staining with propidium iodide (PI), a fluorogenic compound that binds stoichiometrically to nucleic acids (35). Because one of the earliest indication of eukaryotic cell apoptosis is the translocation of the membrane phospholipid phosphatidylserine (PS) from the inner to the outer leaflet of the plasma membrane, and PS binds to annexin V, a high affinity PS binding protein, staining of cells with fluorescein isothiocyanate (FITC)-conjugated annexin V can be used to quantitatively assess apoptotic cell death by flow cytometry (36, 37). When combined with PI staining, dual analysis of quadrant dot plots allows simultaneous classification of individual cells to early (annexin V positive and PI negative) or late (annexin V and PI positive) phase of apoptosis, necrosis (annexin V negative and PI positive) or viable (neither annexin V nor PI positive).
CDT-induced alterations of host cell homeostasis likely contributes to infection and disease caused by H. hepaticus. Although HhepCDT holotoxin obtained from whole-cell lysate and cloned HhepCdtABC have been shown to cause cell cycle arrest of human epithelial HeLa (33, 38), rat fibroblast Rat2 (39), and mouse liver C3H/An (27) cell lines and apoptosis of human intestinal INT407 (28) cell line in vitro, the temporal relationship between cell cycle arrest and apoptotic cell death and the contribution of individual HhepCDT subunits to genotoxicity have not been characterized. Given that (i) simultaneous examination of cell cycle and apoptosis parameters has only been examined with AactCDT interaction with lymphocytes (40, 41) and EcolCdtB-V variant with human brain microvascular endothelial cells (42), (ii) H. hepaticus colonizes the intestinal and hepatobiliary tract, and (iii) the molecular contribution of individual HhepCDT subunits to genotoxicity has not been determined previously, the objective of the present study were to assess the contribution of individual and combinations of HhepCDT subunits to genotoxicity of target intestinal epithelial cells. Because CDT produced by the prototype EHS, H. hepaticus shares a high degree of structural and functional homologies with Campylobacter jejuni, a clinically important human and animal bacterial pathogen that colonizes the same intestinal niche, and together have a CDT that is distinct from that produced by other bacterial pathogens (43), findings from the present study provide a molecular basis for intestinal epithelial cell interaction and intoxication within the context of a relevant in vivo infection microenvironment for these pathogens. We hypothesized that all three HhepCDT protein subunits are required for maximum genotoxicity and HhepCdtB is required for human epithelial cell genotoxicity in vitro. First, we assessed the temporal relationship between cell cycle and apoptosis of human epithelial HeLa and INT407 cells intoxicated with HhepCDT holotoxin or reconstituted recombinant HhepCDT. Next, the contribution of individual and combinations of HhepCDT subunits to genotoxicity were characterized in detail. Simultaneous quantitative assessment of HhepCDT-induced intoxication revealed a similar time course for G2/M cell cycle arrest and apoptotic death with both cell lines reaching a maximum within 72 hours. The present data further confirm the contribution of HhepCDT to epithelial cell death and support the concept that all three HhepCDT subunits are required for genotoxicity in vitro. Although previous data indicating that CdtB is necessary and sufficient for induction of cell cycle arrest of cultured SV40-transformed simian kidney fibroblast-like COS-1 cell line and rat embryo fibroblast REF 52 cell line with C. jejuni (8) and human T-lymphocytes with A. actinomycetemcomitans (44), the present data confirm this requirement for H. hepaticus and extends these observations to induction of apoptotic death of cultured human intestinal epithelial cells.
MATERIALS AND METHODS
Bacterial Strains and Growth Conditions
Helicobacter hepaticus mouse strain 3B1/Hh-1 (ATCC 51449T, Manassas, VA;(19) was propagated on trypticase soy agar supplemented with 5% (vol/vol) sheep blood (TSAB; Remel, Lenexa, KS, USA) and incubated at 37°C under microaerobic conditions (Mitsubishi Gas Chemical Co. Inc., New York, NY, USA). Chemically competent Escherichia coli (One Shot TOP10 and BL21 Star [DE3] strains; Invitrogen, Carlsbad, CA, USA) were grown on Luria-Bertani (LB) agar plates at 37°C under aerobic conditions. Transformants were selected by plating onto LB agar containing 30 μg/mL (w/v) of kanamycin (Sigma Chemical Co., St. Louis, MO, USA).
Preparation of HhepCDT Holotoxin
Helicobacter hepaticus grown on TSAB for 2 to 3 days were harvested by washing the surface growth with phosphate-buffered saline (PBS) (pH 7.2) and centrifugation at 800 X g for 20 minutes at 4°C. The pellet was suspended in 1.0 mL of ice-cold PBS and lysed by sonication as previously described (35). After removing intact bacteria and cell debris by centrifugation at 6000 X g for 20 minutes at 4°C, the supernatant was filtered through 0.22 μm filters and stored at −20°C until needed.
Expression and Purification of H. hepaticus CdtA, CdtB and CdtC Subunit Fusion Proteins
Oligonucleotide primers were designed (Table 1) to amplify the full length H. hepaticus cdtA, cdtB and cdtC genes without the N-terminal signal sequence (GenBank accession no. AF163667). For directional cloning into the His6-tagged vector pET-28a (+) (Novagen, Madison, WI, USA) 5′-EcoRI and 3′-XhoI overhangs were included in the respective primers. After PCR amplification, purified products were digested with appropriate restriction enzymes before cloning into the vector and transformation into E. coli TOP10, expression and purification of individual His6-tagged fusion protein as described previously (12). The purity of each fusion protein was confirmed by sodium dodecyl sulfate polyacrylamide gel electrophoresis (SDS-PAGE) and immunoblot analysis with a mouse monoclonal anti-His6 immunoglobulin (Ig) G1 antibody (Novagen) (12). The amino acid sequence of each purified CDT fusion protein was verified by mass spectrometry (University of Nebraska-Lincoln Mass Spectrometry Core Facility).
Table 1.
Primers for expression of recombinant Helicobacter hepaticus CDT fusion protein subunits.
| Primers | Sequence (5′–3′) | Location in Cdt gene (bp) | PCR products size (bp) |
|---|---|---|---|
|
| |||
| cdtAF | CGGAATTCACACCAAAGGTTCATCAGCC† | 54–696 | 642 |
| cdtAR | CGGCTCGAGTTATTTAATGAAAAAAATAGG | ||
|
| |||
| cdtBF | CGGAATTCAATCTTGAAGATTATAGAG | 770–1536 | 766 |
| cdtBR | CCGCTCGAGCTAAAATCGTCCAAAATGC | ||
|
| |||
| cdtCF | CGGAATTCGGTGGTCCTGAAGATTTACCAG | 1610–2098 | 488 |
| cdtCF | CCGCTCGAGTTAAGGATTAATAGTTTTTGC | ||
underlined sequences indicate restriction sites (5′-EcoRI and 3′-XhoI) for cloning into pET28(+) vector.
Cell Culture Conditions
The human cervical epithelial carcinoma HeLa cell line (ATCC CCL-2) and intestinal epithelial INT407 cell line (ATCC CCL-6) were maintained in Eagle’s minimum essential medium (MEK; Sigma) supplemented with 10% fetal bovine serum (Atlanta Biologicals, Norcross, GA, USA) and gentamicin (50 μg/mL, Sigma) at 37°C in a humidified atmosphere of 5% carbon dioxide in air as previously described (35). Approximately 3 × 105 HeLa or INT407 cells seeded into 25 cm2 flasks (Costar, Cambridge, MA) containing 5 mL of medium were allowed to adhere for 24 hours followed by changing the medium to MEM supplemented with 1% fetal bovine serum overnight before adding HhepCDT. For transfection experiments, approximately 1 × 105 HeLa cells were seeded into each well of 6-well plates (Techno Plastic Products TD, Trasadingen, Switzerland). After changing the medium to MEM supplemented with 1% fetal bovine serum overnight, the cells were transfected with different concentrations of individual recombinant HhepCDT fusion protein subunits.
Intoxication of Cultured Human Epithelial Cells
Intoxication of HeLa or INT407 cells was carried out by adding either 50 μg of HhepCDT holotoxin or individual and combinations of reconstituted recombinant HhepCDT fusion protein subunits as described previously (45, 46). For recombinant HhepCDT fusion protein subunits, 20 μg of each individually purified protein were mixed alone or together in different combinations in 100 μL of PBS (pH 7.4), incubated for 1 hour at 30°C before adding reconstituted CDT complexes to triplicate 25 cm2 flasks containing HeLa or INT407 cells in 5 mL of medium (12 μg final total protein concentration) and further incubation for the indicated time. For transfection experiments, individual His6-tagged recombinant HhepCDT fusion protein subunits were delivered into triplicate wells of 6-well plates containing HeLa cells using the Chariot protein transfection reagent as described by the manufacturer (Active Motif, Carlsbad, CA). Briefly, individual recombinant HhepCDT fusion protein subunits were diluted in 100 μl of PBS to approximately 4, 8 and 20 μg/ml final concentrations, mixed with 10 μl of Chariot reagent and incubated at room temperature for 30 minutes to form Chariot-CDT protein complexes. The Chariot-CDT protein complexes were added to 50–60% confluent cell monolayers in serum free medium and incubated for 2 hours at 37°C in a humidified atmosphere of 5% carbon dioxide in air, followed by further incubation for 72 hours in growth medium.
Determinations of Cell Cycle and Apoptosis by Flow Cytometry
Simultaneous quantitative assessment of cell cycle and apoptosis of control and HhepCDT intoxicated HeLa and INT407 was accomplished by using dual-labeling flow cytometry analysis. At indicated times post-incubation, cells in an individual well were harvested by treatment with 0.05 % (w/v) trypsin-EDTA (Invitrogen), and divided equally into two aliquots for determination of number of cells at each phase of the cell cycle or FITC-conjugated annexin V binding by flow cytometry analysis (20, 47). Briefly, CDT-treated cells were harvested and incubated with 5 μL of FITC-Annexin-V (Southern Biotechnology Associates Inc. Birmingham, AL) and PI (50 μg/mL) for 15 minutes in the dark in a binding buffer containing 10 mM HEPES pH 7.4, 140 mM NaCl, 2.5 mM CaCl2 and 0.1% bovine serum albumin, and the percentages of 1 × 104 gated cells were categorized as early or late apoptotic, necrotic or viable (47, 48). In some experiments, intoxicated HeLa and INT407 cells were prepared for flow cytometry analysis as described previously (35, 49). Harvested cells were treated with 70% ethanol for 5 minutes at −20°C, rehydrated with 1.0 mL of PBS at room temperature for 15 minutes, and after centrifugation, the cells were re-suspended in PI staining buffer (100 mM Tris pH 7.4, 150 mM NaCl, 1 mM CaCl2, 0.5 mM MgCl2, 0.1% Triton X-100, 0.02 mg/ml RNase A and 3 μg/mL PI). The percentages of 1.0 × 104 cells at each stage of the cell cycle were quantitated based on excitation at 488 nm and emission at 630 nm (FACScan Flow Cytometer, Becton Dickinson, San Jose, CA, USA). The data was analyzed using Cell Quest and ModFit LT software (Becton Dickinson).
Data Analysis
The associations between the presence of HhepCDT and G2/M phase arrest or apoptosis were determined by using the Student t-test. The significance of association was tested at α-value of 0.05. Flow cytometry analysis data for HeLa and INT407 cells in G2/M phase were expressed as mean percent of HeLa cells or INT407 cells with 4N DNA contents ± standard deviations.
RESULTS
Expression and Purification of Recombinant HhepCdtA, CdtB and CdtC Fusion Proteins
Purified recombinant His6-tagged HhepCDT fusion protein subunits had the expected molecular masses by SDS-PAGE electrophoresis; approximately 28-kDa for CdtA, 32-kDa for CdtB and 23-kDa for CdtC. The identity of each protein was confirmed by immunoblot analysis with anti-His6 antibody (data not shown) and amino acid sequence determination by mass spectrometry. The expression and purification of individual fusion proteins ensured the absence of cross-contamination in subsequent experiments.
HhepCDT-induced Cell Cycle Arrest and Apoptotic Death
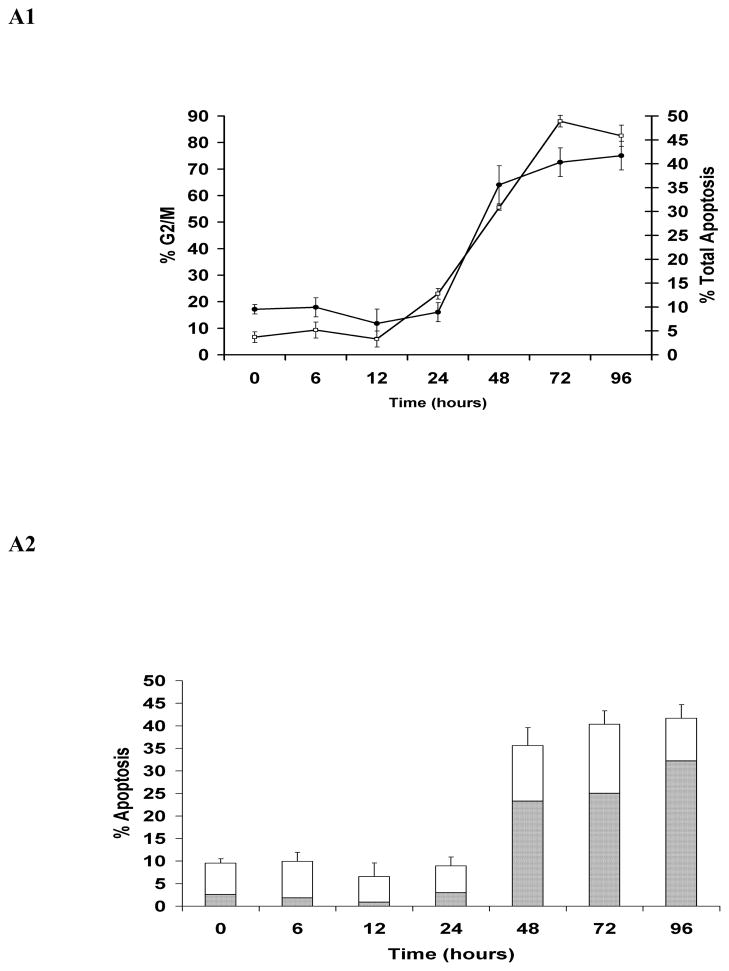
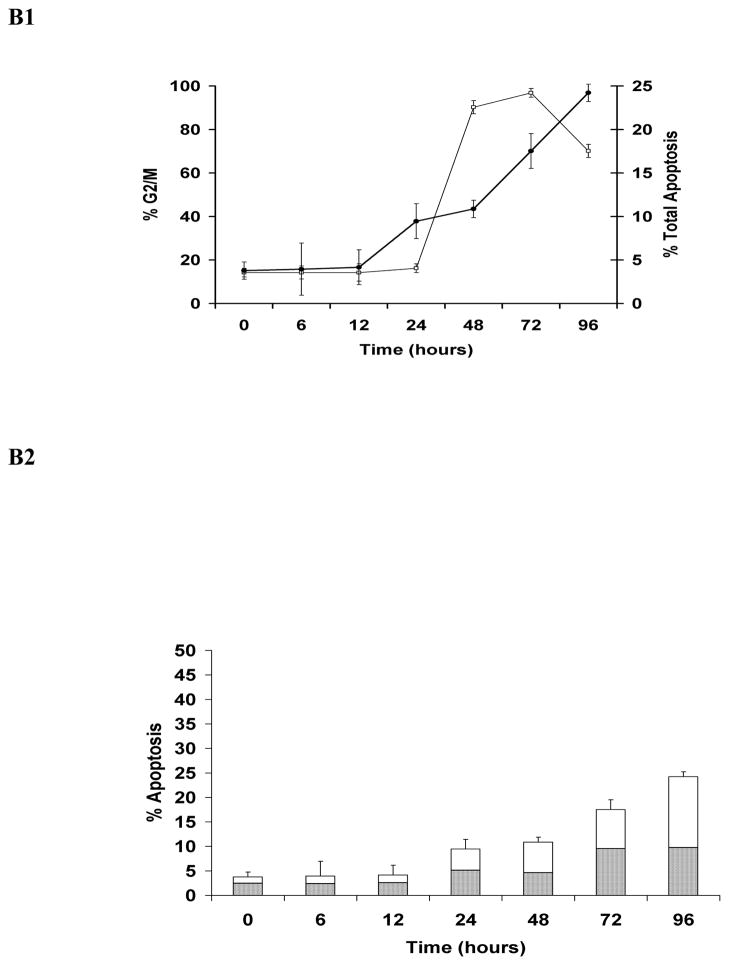
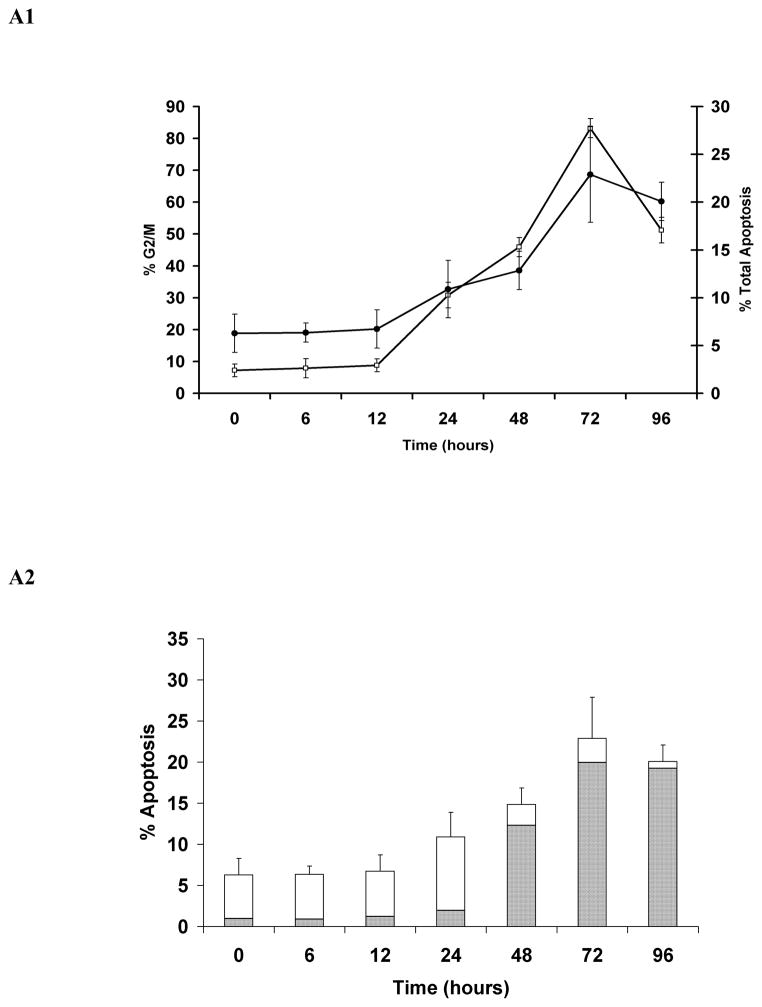
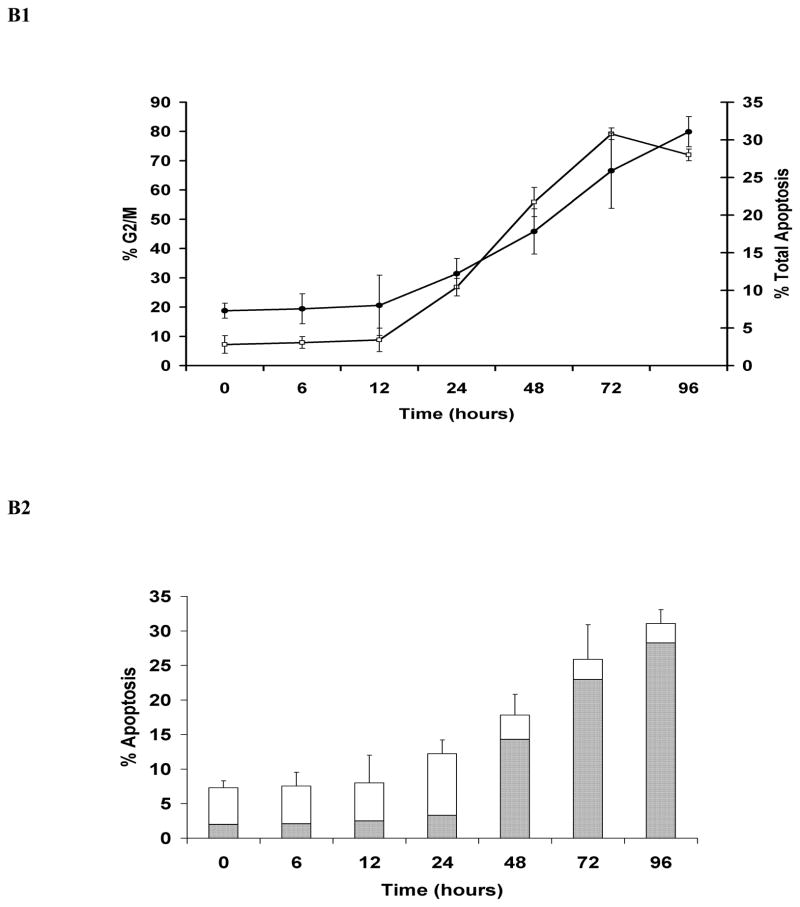
Simultaneous quantitative assessment of HhepCDT-induced intoxication revealed a similar time course for G2/M cell cycle arrest and apoptotic death with both HeLa and INT407 cells reaching a maximum within 72 hours. While HeLa cells treated with HhepCDT holotoxin showed significant arrest at the G2/M phase of the cell cycle between 12 and 24 hours post-treatment (12 hours, 5.9% ± 3%; 24 hours, 23.0% ± 2%; P<0.0001; Fig. 1A1), apoptotic cell death increased significantly between 24 and 48 hours post-treatment (24 hours, 8.9% ± 2%; 48 hours, 35.6% ± 3%; P<0.0001; Fig. 1A2). Similarly, treatment of HeLa cells with reconstituted recombinant HhepCDT displayed significant G2/M cell cycle arrest between 12 and 24 hours post-treatment (12 hours, 8.8% ± 2%; 24 hours, 30.8% ± 4%; P<0.0001; Fig. 2A1), with significant apoptotic cell death following a similar time course (Fig. 2A2). Similar intoxication kinetics was seen with INT407 cells treated with HhepCDT holotoxin (Figs. 1B1 and 1B2) or reconstituted recombinant HhepCDT (Figs. 2B1 and 2B2). With both cell lines, induction of greater percentages of cell cycle arrest than apoptotic death with HhepCDT suggested that the majority of the cells undergoing cell cycle arrest underwent apoptotic death; however, a subset of intoxicated cells might be undergoing premature senescence.
Fig. 1. Kinetics of HeLa and INT407 cell cycle arrest and apoptosis induced by Helicobacter hepaticus CDT holotoxin.
Simultaneous quantitative flow cytometry analysis of HeLa (A) and INT407 (B) G2/M cell cycle arrest (open squares) and apoptosis (solid circles in A1 and B1; solid bars in A2 and white bars in B2 represent early and late apoptosis, respectively) over 96 hours post-treatment with HhepCDT holotoxin (50 μg/mL final total protein concentration). Each point and bar represents mean percent and standard deviation of 1.0 × 104 cells in three independent experiments.
Fig. 2. Kinetics of HeLa and INT407 cell cycle arrest and apoptosis induced by reconstituted recombinant Helicobacter hepaticus CDT.
Simultaneous quantitative flow cytometry analysis of HeLa (A) and INT407 (B) G2/M cell cycle arrest (open squares) and apoptosis (solid circles in A1 and B1; solid bars in A2 and white bars in B2 represent early and late apoptosis, respectively) over 96 hours post-treatment with reconstituted recombinant HhepCDT (12 μg/mL final total protein concentraton). Each point and bar represents mean percent and standard deviation of 1.0 × 104 cells in three independent experiments.
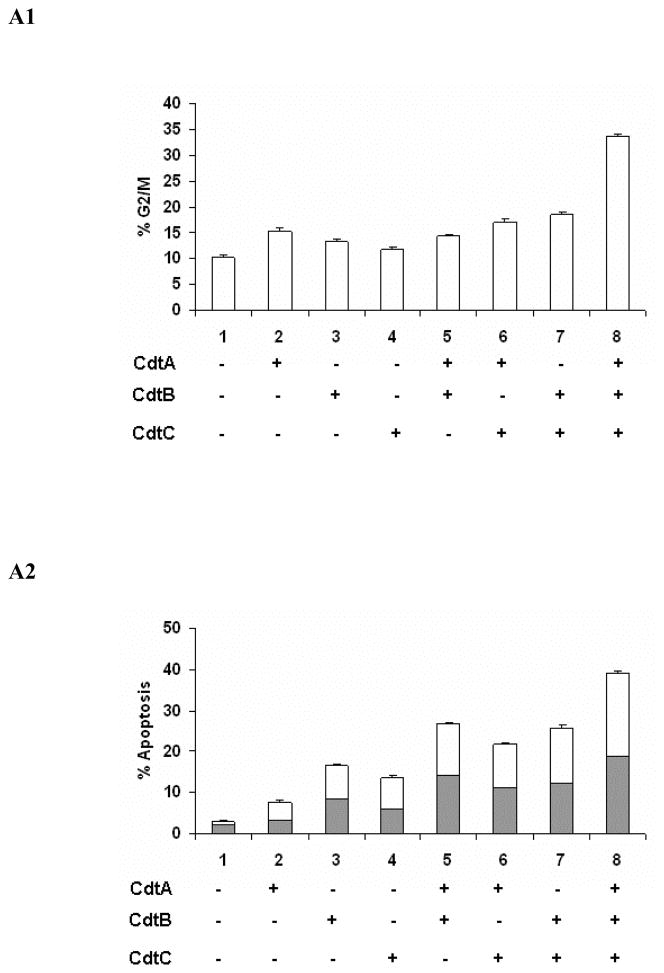
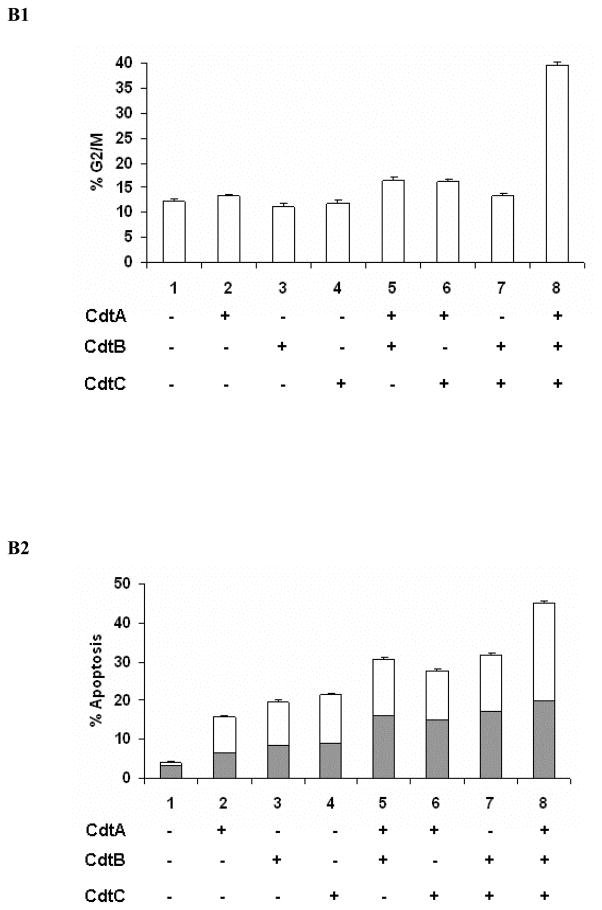
All Three HhepCDT Protein Subunits are Required for Maximum Genotoxicity
To gain further insight into the relationship between cell cycle arrest and apoptotic cell death we assessed HeLa and INT407 cells for cell cycle and apoptosis following treatment with individual and combinations of reconstituted recombinant HhepCDT fusion protein subunits. While HeLa (Figs. 3A1 and 3A2) and INT407 (Figs. 3B1 and 3B2) cells treated with individual recombinant HhepCDT fusion proteins displayed background levels genotoxicity, cells treated with HhepCDT consisting of all three reconstituted fusion protein subunits displayed maximum levels of G2/M cell cycle arrest (33.6 % ± 0.6%; control 10.3 % ± 0.3%, P<.0001) and apoptotic death (39.2% ± 0.5%; control 3.0% ± 0.4%, P< .0001). Interestingly, intermediate levels of apoptosis, but not cell cycle arrest were found with HeLa (26.8% ± 0.5%; control 3.0% ± 0.4%; P<.0001) and INT407 (30.8% ± 0.5%; control 4.1% ± 0.2%; P<.0001) cells treated with di-protein combination consisting of HhepCdtAB. Similarly, HeLa cells treated with di-protein combinations consisting of HhepCdtBC (25.8 ± 0.4%, P<.0001) and HhepCdtAC (21.7 ± 0.4%, P<.0001) displayed intermediate levels of apoptosis. Similar levels of apoptosis were seen with intoxicated INT407 cells (Figs. 3B1 and 3B2). In parallel control experiments, treatment of each cell line with irrelevant recombinant HhepPutA protein, expressed and purified by using the same expression vector and protocol (50) did not induce significant cell cycle arrest or apoptosis confirming the specificity of the recombinant HhepCDT-induced genotoxicity. Taken together the data further demonstrated the requirement for all three HhepCDT subunits in order to achieve maximum cell cycle arrest and apoptosis of HeLa and INT407 cells in vitro, and suggested significant but lower levels of apoptotic cell death with di-protein subunit combinations.
Fig. 3. All three Helicobacter hepaticus CDT subunits are required for maximum HeLa and INT407 cell cycle arrest and apoptotic cell death.
Quantitative flow cytometry analysis of HeLa (A) and INT407 (B) G2/M cell cycle arrest (A1 and B1 open bars) and apoptosis (solid bars and white bars in A2 and B2 represent early and late apoptosis, respectively) 72 hours post-treatment with individual and combinations of recombinant HhepCDT fusion protein subunits (12 μg/mL final total protein concentration). Each bar represents mean percent and standard deviation of 1.0 × 104 cells in three independent experiments.
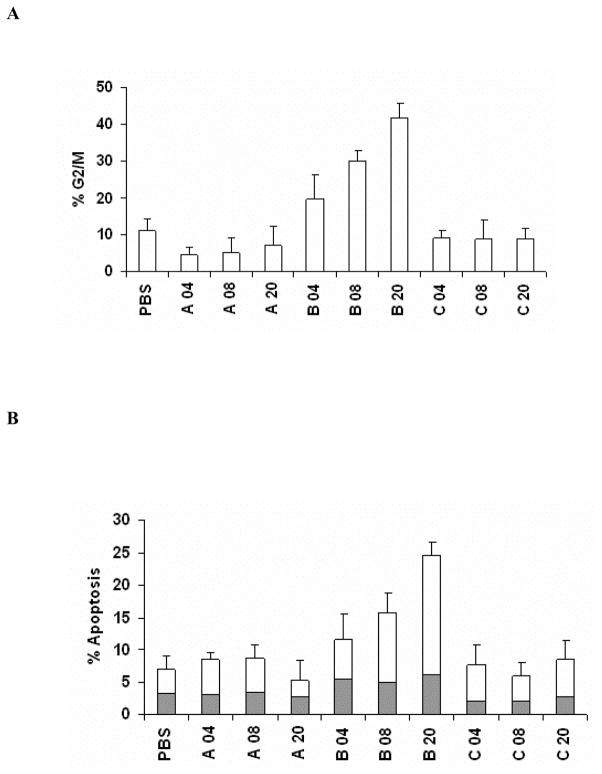
H. hepaticus CdtB is Necessary and Sufficient for Genotoxicity
To assess the contribution of individual HhepCDT subunits to genotoxicity, HeLa cells were transfected with increasing concentrations from 4 up to 20 μg/mL of either His6-tagged CdtA, CdtB or CdtC. Transfection of HeLa cells with CdtA or CdtC did not result in significant G2/M arrest or apoptosis (Fig. 4A and 4B). By contrast, transfection of HeLa cells with HhepCdtB subunit alone resulted in dose-dependent cell cycle arrest (Fig. 4A) and apoptosis (Fig. 4B). At 72 hours post-transfection of HeLa cells with HhepCdtB, increasing percentages of cells arrested at the G2/M phase of the cell cycle from 19.5% ± 7% with 4 μg/mL to 30.0% ± 3% with 8 μg/mL to 41.7% ± 4% (PBS, 11.1% ± 3%; P<.0001) with 20 μg/mL (Fig. 4A) were seen. Similarly, the percentages of HeLa cells in early and late phases of apoptotic cell death steadily increased with increasing concentrations of HhepCdtB from 11.6% ± 4% with 4 μg/mL to 15.8% ± 2% with 8 μg/mL to 24.5% ± 2% with 20 μg/mL (PBS 7% ± 2%; P<.0001; Fig. 4B). Taken together, these findings demonstrate for the first time that HhepCdtB subunit is necessary and sufficient for both epithelial cell cycle arrest and apoptotic death.
Fig. 4. Helicobacter hepaticus CdtB alone is necessary and sufficient for HeLa cell cycle arrest and apoptotic cell death.
Quantitative flow cytometry analysis of HeLa G2/M cell cycle arrest (A open bars) and apoptosis (solid bars and white bars in B represent early and late apoptosis, respectively) 72 h post-transfection with increasing concentrations from 4, 8 and 20 μg/mL of recombinant HhepCDT fusion protein subunits. Each bar represents mean percent and standard deviation of 1.0 × 104 cells in three independent experiments.
DISCUSSION
The CDTs produced by H. hepaticus as well as by other EHS and several Gram negative bacterial pathogens cause cell cycle arrest and apoptotic death of eukaryotic cells in vitro (1–3, 33, 38). However, the relationship between HhepCDT-induced cell cycle arrest and apoptosis has not been investigated in detail. In the present study, simultaneous analysis of the same cell preparation for cell cycle and apoptosis parameters allowed detailed assessment of the kinetics of each genotoxin-induced process. We found that HeLa and INT407 cells intoxicated with either HhepCDT holotoxin or reconstituted recombinant HhepCDT displayed similar time course for G2/M cell cycle arrest and apoptotic death reaching a maximum within 72 hours. These findings are in agreement with previous observations demonstrating apoptosis as a consequence of cell cycle arrest in mitogen activated human peripheral blood mononuclear cells (40) or human T-cell leukemia Jurkat cell line treated with recombinant AactCDT (41), and human brain microvascular endothelial cells treated with E. coli CdtB-V variant (42). These kinetics of cell cycle arrest progressing to apoptotic cell death are consistent with our previous report indicating an ATM-dependent DDR of INT407 cells intoxicated with reconstituted recombinant HhepCDT (28). In these experiments, phosphorylation of Histone 2AX (γH2AX) was upregulated within 24 hours after HhepCDT treatment, and this was followed first by an increase in the pro-apoptotic Bax to anti-apoptotic Bcl2 protein ratio between 24 and 48 hours, and then, by a gradual cytoplasmic release of mitochondrial cytochrome c correlating with maximum caspase 3/7 activation by 72 hours post-treatment. With both cell lines, induction of greater percentages of cell cycle arrest than apoptotic cell death with HhepCDT suggested that the majority of the cells undergoing cell cycle arrest underwent apoptotic death; however, a subset of intoxicated cells might undergo premature senescence.
Next, we examined the contribution of individual and combinations of HhepCDT protein subunits to induction of HeLa and INT407 cell genotoxicity. Consistent with previous observations, we found a requirement for the presence of all three HhepCDT subunits for induction of maximum cell cycle arrest of both cell lines (14, 45, 51–58). Interestingly, di-protein combinations of HhepCDT subunits exerted significant, albeit lower levels of apoptosis, but not cell cycle arrest with both cell lines. This lack of induction of significant cell cycle arrest is consistent with previous observations using di-protein combinations of CjejCDT with INT407 cells (6), HducCDT with human epithelial HEp-2 cells (55), AactCDT with Chinese hamster ovary (CHO) cells (14), and EcolCDT with fibroblast-like Cos-7 cells (56). However, our data contrast from other studies indicating significant cell cycle arrest of HeLa cells treated with di-protein combinations of CjejCdtBC (45) or human T-cell leukemia Jurkat cells treated with di-protein combinations containing AactCdtB as one of the components (52, 53). These apparent discrepancies might be attributed to different experimental conditions including different recombinant fusion protein constructs, target cells and treatment procedures. This suggestion is supported by demonstration of induction of intermediate G2/M cell cycle arrest and reduced cell viability of human epithelial HepG2 cells treated with AactCdtBC di-protein combination (57).
Induction of apoptotic cell death has been well documented with various target cells intoxicated with holotoxin produced by HducCDT (59–61), AactCDT (40, 41, 62–66), CjejCDT (67) and certain EcolCdtB-V (42); however, the contribution of individual CDT subunit produced by bacteria in general and by H. hepaticus in particular to induction of apoptotic cell death has not been investigated previously. We found that apoptotic death of HeLa and INT407 cells was maximal only when all three HhepCDT subunits were present. This data is the first conclusive evidence for any CDT that all three subunits are required for induction of maximum apoptotic cell death. Although treatment of HeLa and INT407 cells with HhepCDT di-protein combinations did not result in significant cell cycle arrest, intermediate levels of apoptotic cell death with di-protein combinations suggested potential alternate pathway for apoptotic activation. On the basis of distinct clustering of bacterial CdtA and CdtC subunit amino acid sequences and correlation with specific target cell membrane biomolecular compositions and intracellular toxin transport pathways, variations in intrinsic contribution of individual CDT subunits might account for variable genotoxicity with di-protein subunits (43, 68).
Characterization of genotoxic activity of individual HhepCDT fusion protein subunits by using HeLa cell protein transfection revealed a dose-dependent G2/M arrest and apoptotic cell death with HhCdtB, but not with HhCdtA and HhCdtC subunits. This data further confirmed that HhepCdtB is necessary and sufficient for genotoxicity, a finding consistent with induction of genotoxicity in INT407 cells following microinjection of purified CjejCdtB subunit (8) or following transfection of AactCdtB protein subunit into human epithelial Ca9-22 cells (62) or electroporation of EcolCdtB into simian Cos-7 cells (56). Although the mechanism of eukaryotic cell uptake with the Chariot transfection system is unknown, a dose-dependent nuclear translocation and genotoxic damage was found. Further studies using this technology should provide a greater understanding of intracellular trafficking and mechanism of cellular genotoxicity of individual CDT protein subunits.
In conclusion, the present study demonstrated sequential G2/M arrest and apoptotic cell death of human epithelial HeLa and INT407cells by HhepCDT. Since H. hepaticus is the prototype for EHS, the data suggest a similar mechanism for CDT-induced genotoxicity with other EHS.
Acknowledgments
We thank ShiHong Gill in the School of Veterinary Medicine and Biomedical Sciences at the University of Nebraska-Lincoln for her excellent technical assistance with flow cytometry. This study was funded in part by the NIH/NIAID grant RO3 AI059532 to GED.
References
- 1.Jinadasa RN, Bloom SE, Weiss RS, Duhamel GE. Cytolethal distending toxin: a conserved bacterial genotoxin that blocks cell cycle progression, leading to apoptosis of a broad range of mammalian cell lineages. Microbiology. Jul;157(Pt 7):1851–75. doi: 10.1099/mic.0.049536-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Guerra L, Cortes-Bratti X, Guidi R, Frisan T. The biology of the cytolethal distending toxins. Toxins (Basel) Mar;3(3):172–90. doi: 10.3390/toxins3030172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Smith JL, Bayles DO. The contribution of cytolethal distending toxin to bacterial pathogenesis. Critical Reviews in Microbiology. 2006 Oct-Dec;32(4):227–48. doi: 10.1080/10408410601023557. [DOI] [PubMed] [Google Scholar]
- 4.den Bakker HC, Moreno Switt AI, Govoni G, Cummings CA, Ranieri ML, Degoricija L, et al. Genome sequencing reveals diversification of virulence factor content and possible host adaptation in distinct subpopulations of Salmonella enterica. BMC Genomics. 12:425. doi: 10.1186/1471-2164-12-425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Haghjoo E, Galan JE. Salmonella typhi encodes a functional cytolethal distending toxin that is delivered into host cells by a bacterial-internalization pathway. Proceedings of the National Academy of Sciences of the United States of America. 2004 Mar 30;101(13):4614–9. doi: 10.1073/pnas.0400932101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Lara-Tejero M, Galan JE. CdtA, CdtB, and CdtC form a tripartite complex that is required for cytolethal distending toxin activity. Infection and Immunity. 2001 Jul;69(7):4358–65. doi: 10.1128/IAI.69.7.4358-4365.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Boesze-Battaglia K, Besack D, McKay T, Zekavat A, Otis L, Jordan-Sciutto K, et al. Cholesterol-rich membrane microdomains mediate cell cycle arrest induced by Actinobacillus actinomycetemcomitans cytolethal-distending toxin. Cellular Microbiology. 2006 May;8(5):823–36. doi: 10.1111/j.1462-5822.2005.00669.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Lara-Tejero M, Galan JE. A bacterial toxin that controls cell cycle progression as a deoxyribonuclease I-like protein. Science. 2000 Oct 13;290(5490):354–7. doi: 10.1126/science.290.5490.354. [DOI] [PubMed] [Google Scholar]
- 9.Nesic D, Hsu Y, Stebbins CE. Assembly and function of a bacterial genotoxin. Nature. 2004 May 27;429(6990):429–33. doi: 10.1038/nature02532. [DOI] [PubMed] [Google Scholar]
- 10.Yamada T, Komoto J, Saiki K, Konishi K, Takusagawa F. Variation of loop sequence alters stability of cytolethal distending toxin (CDT): crystal structure of CDT from Actinobacillus actinomycetemcomitans. Protein Science: a publication of the Protein Society. 2006 Feb;15(2):362–72. doi: 10.1110/ps.051790506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Hu X, Stebbins CE. Dynamics and assembly of the cytolethal distending toxin. Proteins. 2006 Dec 1;65(4):843–55. doi: 10.1002/prot.21167. [DOI] [PubMed] [Google Scholar]
- 12.Dassanayake RP, Griep MA, Duhamel GE. The cytolethal distending toxin B sub-unit of Helicobacter hepaticus is a Ca2+- and Mg2+-dependent neutral nuclease. FEMS Microbiol Lett. 2005 Oct 15;251(2):219–25. doi: 10.1016/j.femsle.2005.08.005. [DOI] [PubMed] [Google Scholar]
- 13.Elwell CA, Dreyfus LA. DNase I homologous residues in CdtB are critical for cytolethal distending toxin-mediated cell cycle arrest. Molecular Microbiology. 2000 Aug;37(4):952–63. doi: 10.1046/j.1365-2958.2000.02070.x. [DOI] [PubMed] [Google Scholar]
- 14.Mao X, DiRienzo JM. Functional studies of the recombinant subunits of a cytolethal distending holotoxin. Cellular Microbiology. 2002 Apr;4(4):245–55. doi: 10.1046/j.1462-5822.2002.00186.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Li L, Sharipo A, Chaves-Olarte E, Masucci MG, Levitsky V, Thelestam M, et al. The Haemophilus ducreyi cytolethal distending toxin activates sensors of DNA damage and repair complexes in proliferating and non-proliferating cells. Cellular Microbiology. 2002 Feb;4(2):87–99. doi: 10.1046/j.1462-5822.2002.00174.x. [DOI] [PubMed] [Google Scholar]
- 16.Gargi A, Reno M, Blanke SR. Bacterial toxin modulation of the eukaryotic cell cycle: are all cytolethal distending toxins created equally? Frontiers in cellular and infection microbiology. 2012;2:124. doi: 10.3389/fcimb.2012.00124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Pellicano R, Menard A, Rizzetto M, Megraud F. Helicobacter species and liver diseases: association or causation? Lancet Infect Dis. 2008 Apr;8(4):254–60. doi: 10.1016/S1473-3099(08)70066-5. [DOI] [PubMed] [Google Scholar]
- 18.Ward JM, Fox JG, Anver MR, Haines DC, George CV, Collins MJ, Jr, et al. Chronic active hepatitis and associated liver tumors in mice caused by a persistent bacterial infection with a novel Helicobacter species. J Natl Cancer Inst. 1994 Aug 17;86(16):1222–7. doi: 10.1093/jnci/86.16.1222. [DOI] [PubMed] [Google Scholar]
- 19.Fox JG, Dewhirst FE, Tully JG, Paster BJ, Yan L, Taylor NS, et al. Helicobacter hepaticus sp. nov., a microaerophilic bacterium isolated from livers and intestinal mucosal scrapings from mice. J Clin Microbiol. 1994 May;32(5):1238–45. doi: 10.1128/jcm.32.5.1238-1245.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Solnick JV, Schauer DB. Emergence of diverse Helicobacter species in the pathogenesis of gastric and enterohepatic diseases. Clin Microbiol Rev. 2001 Jan;14(1):59–97. doi: 10.1128/CMR.14.1.59-97.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Nagamine CM, Sohn JJ, Rickman BH, Rogers AB, Fox JG, Schauer DB. Helicobacter hepaticus infection promotes colon tumorigenesis in the BALB/c-Rag2(−/−) Apc(Min/+) mouse. Infect Immun. 2008 Jun;76(6):2758–66. doi: 10.1128/IAI.01604-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Ge Z, Rogers AB, Feng Y, Lee A, Xu S, Taylor NS, et al. Bacterial cytolethal distending toxin promotes the development of dysplasia in a model of microbially induced hepatocarcinogenesis. Cellular Microbiology. 2007 Aug;9(8):2070–80. doi: 10.1111/j.1462-5822.2007.00939.x. [DOI] [PubMed] [Google Scholar]
- 23.Pratt JS, Sachen KL, Wood HD, Eaton KA, Young VB. Modulation of host immune responses by the cytolethal distending toxin of Helicobacter hepaticus. Infection and Immunity. 2006 Aug;74(8):4496–504. doi: 10.1128/IAI.00503-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Young VB, Knox KA, Pratt JS, Cortez JS, Mansfield LS, Rogers AB, et al. In vitro and in vivo characterization of Helicobacter hepaticus cytolethal distending toxin mutants. Infection and Immunity. 2004 May;72(5):2521–7. doi: 10.1128/IAI.72.5.2521-2527.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Suerbaum S, Josenhans C, Sterzenbach T, Drescher B, Brandt P, Bell M, et al. The complete genome sequence of the carcinogenic bacterium Helicobacter hepaticus. Proceedings of the National Academy of Sciences of the United States of America. 2003 Jun 24;100(13):7901–6. doi: 10.1073/pnas.1332093100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Nougayrede JP, Taieb F, De Rycke J, Oswald E. Cyclomodulins: bacterial effectors that modulate the eukaryotic cell cycle. Trends in Microbiology. 2005 Mar;13(3):103–10. doi: 10.1016/j.tim.2005.01.002. [DOI] [PubMed] [Google Scholar]
- 27.Avenaud P, Castroviejo M, Claret S, Rosenbaum J, Megraud F, Menard A. Expression and activity of the cytolethal distending toxin of Helicobacter hepaticus. Biochemical and Biophysical Research Communications. 2004 Jun 4;318(3):739–45. doi: 10.1016/j.bbrc.2004.04.089. [DOI] [PubMed] [Google Scholar]
- 28.Liyanage NP, Manthey KC, Dassanayake RP, Kuszynski CA, Oakley GG, Duhamel GE. Helicobacter hepaticus cytolethal distending toxin causes cell death in intestinal epithelial cells via mitochondrial apoptotic pathway. Helicobacter. 2010 Apr;15(2):98–107. doi: 10.1111/j.1523-5378.2010.00749.x. [DOI] [PubMed] [Google Scholar]
- 29.Nyska A, Maronpot RR, Eldridge SR, Haseman JK, Hailey JR. Alteration in cell kinetics in control B6C3F1 mice infected with Helicobacter hepaticus. Toxicol Pathol. 1997 Nov-Dec;25(6):591–6. doi: 10.1177/019262339702500609. [DOI] [PubMed] [Google Scholar]
- 30.Ihrig M, Schrenzel MD, Fox JG. Differential susceptibility to hepatic inflammation and proliferation in AXB recombinant inbred mice chronically infected with Helicobacter hepaticus. Am J Pathol. 1999 Aug;155(2):571–82. doi: 10.1016/S0002-9440(10)65152-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Hailey JR, Haseman JK, Bucher JR, Radovsky AE, Malarkey DE, Miller RT, et al. Impact of Helicobacter hepaticus infection in B6C3F1 mice from twelve National Toxicology Program two-year carcinogenesis studies. Toxicol Pathol. 1998 Sep-Oct;26(5):602–11. doi: 10.1177/019262339802600503. [DOI] [PubMed] [Google Scholar]
- 32.Ge Z, Feng Y, Whary MT, Nambiar PR, Xu S, Ng V, et al. Cytolethal distending toxin is essential for Helicobacter hepaticus colonization in outbred Swiss Webster mice. Infection and Immunity. 2005 Jun;73(6):3559–67. doi: 10.1128/IAI.73.6.3559-3567.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Chien CC, Taylor NS, Ge Z, Schauer DB, Young VB, Fox JG. Identification of cdtB homologues and cytolethal distending toxin activity in enterohepatic Helicobacter spp. Journal of Medical Microbiology. 2000 Jun;49(6):525–34. doi: 10.1099/0022-1317-49-6-525. [DOI] [PubMed] [Google Scholar]
- 34.Hansen R, Thomson JM, Fox JG, El-Omar EM, Hold GL. Could Helicobacter organisms cause inflammatory bowel disease? FEMS Immunol Med Microbiol. Feb;61(1):1–14. doi: 10.1111/j.1574-695X.2010.00744.x. [DOI] [PubMed] [Google Scholar]
- 35.Dassanayake RP, Zhou Y, Hinkley S, Stryker CJ, Plauche G, Borda JT, et al. Characterization of cytolethal distending toxin of Campylobacter species isolated from captive macaque monkeys. J Clin Microbiol. 2005 Feb;43(2):641–9. doi: 10.1128/JCM.43.2.641-649.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Fink SL, Cookson BT. Apoptosis, pyroptosis, and necrosis: mechanistic description of dead and dying eukaryotic cells. Infect Immun. 2005 Apr;73(4):1907–16. doi: 10.1128/IAI.73.4.1907-1916.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Martin SJ, Reutelingsperger CP, McGahon AJ, Rader JA, van Schie RC, LaFace DM, et al. Early redistribution of plasma membrane phosphatidylserine is a general feature of apoptosis regardless of the initiating stimulus: inhibition by overexpression of Bcl-2 and Abl. J Exp Med. 1995 Nov 1;182(5):1545–56. doi: 10.1084/jem.182.5.1545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Young VB, Knox KA, Schauer DB. Cytolethal distending toxin sequence and activity in the enterohepatic pathogen Helicobacter hepaticus. Infection and Immunity. 2000 Jan;68(1):184–91. doi: 10.1128/iai.68.1.184-191.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Guidi R, Guerra L, Levi L, Stenerlow B, Fox JG, Josenhans C, et al. Chronic exposure to the cytolethal distending toxins of Gram-negative bacteria promotes genomic instability and altered DNA damage response. Cellular Microbiology. 2013 Jan;15(1):98–113. doi: 10.1111/cmi.12034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Shenker BJ, Hoffmaster RH, Zekavat A, Yamaguchi N, Lally ET, Demuth DR. Induction of apoptosis in human T cells by Actinobacillus actinomycetemcomitans cytolethal distending toxin is a consequence of G2 arrest of the cell cycle. Journal of Immunology. 2001 Jul 1;167(1):435–41. doi: 10.4049/jimmunol.167.1.435. [DOI] [PubMed] [Google Scholar]
- 41.Shenker BJ, Demuth DR, Zekavat A. Exposure of lymphocytes to high doses of Actinobacillus actinomycetemcomitans cytolethal distending toxin induces rapid onset of apoptosis-mediated DNA fragmentation. Infection and Immunity. 2006 Apr;74(4):2080–92. doi: 10.1128/IAI.74.4.2080-2092.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Bielaszewska M, Sinha B, Kuczius T, Karch H. Cytolethal distending toxin from Shiga toxin-producing Escherichia coli O157 causes irreversible G2/M arrest, inhibition of proliferation, and death of human endothelial cells. Infection and Immunity. 2005 Jan;73(1):552–62. doi: 10.1128/IAI.73.1.552-562.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Eshraghi A, Maldonado-Arocho FJ, Gargi A, Cardwell MM, Prouty MG, Blanke SR, et al. Cytolethal distending toxin family members are differentially affected by alterations in host glycans and membrane cholesterol. The Journal of Biological Chemistry. 2010 Jun 11;285(24):18199–207. doi: 10.1074/jbc.M110.112912. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Shenker BJ, Hoffmaster RH, McKay TL, Demuth DR. Expression of the cytolethal distending toxin (Cdt) operon in Actinobacillus actinomycetemcomitans: evidence that the CdtB protein is responsible for G2 arrest of the cell cycle in human T cells. Journal of Immunology. 2000 Sep 1;165(5):2612–8. doi: 10.4049/jimmunol.165.5.2612. [DOI] [PubMed] [Google Scholar]
- 45.Lee RB, Hassane DC, Cottle DL, Pickett CL. Interactions of Campylobacter jejuni cytolethal distending toxin subunits CdtA and CdtC with HeLa cells. Infection and Immunity. 2003 Sep;71(9):4883–90. doi: 10.1128/IAI.71.9.4883-4890.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Elwell C, Chao K, Patel K, Dreyfus L. Escherichia coli CdtB mediates cytolethal distending toxin cell cycle arrest. Infection and Immunity. 2001 May;69(5):3418–22. doi: 10.1128/IAI.69.5.3418-3422.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Shounan Y, Feng X, O’Connell PJ. Apoptosis detection by annexin V binding: a novel method for the quantitation of cell-mediated cytotoxicity. J Immunol Methods. 1998 Aug 1;217(1–2):61–70. doi: 10.1016/s0022-1759(98)00090-8. [DOI] [PubMed] [Google Scholar]
- 48.Vermes I, Haanen C, Steffens-Nakken H, Reutelingsperger C. A novel assay for apoptosis. Flow cytometric detection of phosphatidylserine expression on early apoptotic cells using fluorescein labelled Annexin V. J Immunol Methods. 1995 Jul 17;184(1):39–51. doi: 10.1016/0022-1759(95)00072-i. [DOI] [PubMed] [Google Scholar]
- 49.Cortes-Bratti X, Chaves-Olarte E, Lagergard T, Thelestam M. The cytolethal distending toxin from the chancroid bacterium Haemophilus ducreyi induces cell-cycle arrest in the G2 phase. The Journal of Clinical Investigation. 1999 Jan;103(1):107–15. doi: 10.1172/JCI3831. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Krishnan N, Doster AR, Duhamel GE, Becker DF. Characterization of a Helicobacter hepaticus putA mutant strain in host colonization and oxidative stress. Infection and Immunity. 2008 Jul;76(7):3037–44. doi: 10.1128/IAI.01737-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Lara-Tejero M, Galán JE. CdtA, CdtB, and CdtC form a tripartite complex that is required for cytolethal distending toxin activity. Infection and Immunity. 2001;69(7):4358–65. doi: 10.1128/IAI.69.7.4358-4365.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Shenker BJ, Besack D, McKay T, Pankoski L, Zekavat A, Demuth DR. Actinobacillus actinomycetemcomitans cytolethal distending toxin (Cdt): evidence that the holotoxin is composed of three subunits: CdtA, CdtB, and CdtC. Journal of Immunology. 2004 Jan 1;172(1):410–7. doi: 10.4049/jimmunol.172.1.410. [DOI] [PubMed] [Google Scholar]
- 53.Shenker BJ, Besack D, McKay T, Pankoski L, Zekavat A, Demuth DR. Induction of cell cycle arrest in lymphocytes by Actinobacillus actinomycetemcomitans cytolethal distending toxin requires three subunits for maximum activity. Journal of Immunology. 2005 Feb 15;174(4):2228–34. doi: 10.4049/jimmunol.174.4.2228. [DOI] [PubMed] [Google Scholar]
- 54.Frisk A, Lebens M, Johansson C, Ahmed H, Svensson L, Ahlman K, et al. The role of different protein components from the Haemophilus ducreyi cytolethal distending toxin in the generation of cell toxicity. Microbial Pathogenesis. 2001 Jun;30(6):313–24. doi: 10.1006/mpat.2000.0436. [DOI] [PubMed] [Google Scholar]
- 55.Wising C, Svensson LA, Ahmed HJ, Sundaeus V, Ahlman K, Jonsson IM, et al. Toxicity and immunogenicity of purified Haemophilus ducreyi cytolethal distending toxin in a rabbit model. Microbial Pathogenesis. 2002 Aug;33(2):49–62. doi: 10.1006/mpat.2002.0516. [DOI] [PubMed] [Google Scholar]
- 56.McSweeney LA, Dreyfus LA. Nuclear localization of the Escherichia coli cytolethal distending toxin CdtB subunit. Cellular Microbiology. 2004 May;6(5):447–58. doi: 10.1111/j.1462-5822.2004.00373.x. [DOI] [PubMed] [Google Scholar]
- 57.Akifusa S, Poole S, Lewthwaite J, Henderson B, Nair SP. Recombinant Actinobacillus actinomycetemcomitans cytolethal distending toxin proteins are required to interact to inhibit human cell cycle progression and to stimulate human leukocyte cytokine synthesis. Infection and Immunity. 2001 Sep;69(9):5925–30. doi: 10.1128/IAI.69.9.5925-5930.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Lewis DA, Stevens MK, Latimer JL, Ward CK, Deng K, Blick R, et al. Characterization of Haemophilus ducreyi cdtA, cdtB, and cdtC mutants in in vitro and in vivo systems. Infection and Immunity. 2001 Sep;69(9):5626–34. doi: 10.1128/IAI.69.9.5626-5634.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Gelfanova V, Hansen EJ, Spinola SM. Cytolethal distending toxin of Haemophilus ducreyi induces apoptotic death of Jurkat T cells. Infection and Immunity. 1999 Dec;67(12):6394–402. doi: 10.1128/iai.67.12.6394-6402.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Xu T, Lundqvist A, Ahmed HJ, Eriksson K, Yang Y, Lagergard T. Interactions of Haemophilus ducreyi and purified cytolethal distending toxin with human monocyte-derived dendritic cells, macrophages and CD4+ T cells. Microbes and Infection / Institut Pasteur. 2004 Nov;6(13):1171–81. doi: 10.1016/j.micinf.2004.07.003. [DOI] [PubMed] [Google Scholar]
- 61.Wising C, Azem J, Zetterberg M, Svensson LA, Ahlman K, Lagergard T. Induction of apoptosis/necrosis in various human cell lineages by Haemophilus ducreyi cytolethal distending toxin. Toxicon: official journal of the International Society on Toxinology. 2005 May;45(6):767–76. doi: 10.1016/j.toxicon.2005.01.016. [DOI] [PubMed] [Google Scholar]
- 62.Yamamoto K, Tominaga K, Sukedai M, Okinaga T, Iwanaga K, Nishihara T, et al. Delivery of cytolethal distending toxin B induces cell cycle arrest and apoptosis in gingival squamous cell carcinoma in vitro. European Journal of Oral Sciences. 2004 Oct;112(5):445–51. doi: 10.1111/j.1600-0722.2004.00157.x. [DOI] [PubMed] [Google Scholar]
- 63.Nalbant A, Chen C, Wang Y, Zadeh HH. Induction of T-cell apoptosis by Actinobacillus actinomycetemcomitans mutants with deletion of ltxA and cdtABC genes: possible activity of GroEL-like molecule. Oral Microbiology and Immunology. 2003 Dec;18(6):339–49. doi: 10.1046/j.0902-0055.2003.00082.x. [DOI] [PubMed] [Google Scholar]
- 64.Ohara M, Hayashi T, Kusunoki Y, Miyauchi M, Takata T, Sugai M. Caspase-2 and caspase-7 are involved in cytolethal distending toxin-induced apoptosis in Jurkat and MOLT-4 T-cell lines. Infection and Immunity. 2004 Feb;72(2):871–9. doi: 10.1128/IAI.72.2.871-879.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Ohara M, Hayashi T, Kusunoki Y, Nakachi K, Fujiwara T, Komatsuzawa H, et al. Cytolethal distending toxin induces caspase-dependent and -independent cell death in MOLT-4 cells. Infection and Immunity. 2008 Oct;76(10):4783–91. doi: 10.1128/IAI.01612-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Rabin SD, Flitton JG, Demuth DR. Aggregatibacter actinomycetemcomitans cytolethal distending toxin induces apoptosis in nonproliferating macrophages by a phosphatase-independent mechanism. Infection and Immunity. 2009 Aug;77(8):3161–9. doi: 10.1128/IAI.01227-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Hickey TE, Majam G, Guerry P. Intracellular survival of Campylobacter jejuni in human monocytic cells and induction of apoptotic death by cytholethal distending toxin. Infection and Immunity. 2005 Aug;73(8):5194–7. doi: 10.1128/IAI.73.8.5194-5197.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Gargi A, Tamilselvam B, Powers B, Prouty MG, Lincecum T, Eshraghi A, et al. Cellular interactions of the cytolethal distending toxins from Escherichia coli and Haemophilus ducreyi. The Journal of Biological Chemistry. 2013 Jan 10; doi: 10.1074/jbc.M112.448118. [DOI] [PMC free article] [PubMed] [Google Scholar]