Abstract
Allograft inflammatory factor-1 (Aif-1) is a 17 kDa EF hand motif-bearing protein expressed primarily in developing spermatids and cells of monocyte/macrophage lineage. Increased Aif-1 expression has been identified in clinically important conditions, including rheumatoid arthritis, systemic sclerosis, endometriosis, and transplant-associated arteriosclerosis. Largely similar gene products arising from the same locus are known as ionized Ca2+ binding adapter-1 (Iba1), microglial response factor-1 (MRF1), and daintain; Iba1 in particular has emerged as a histologic marker of microglia and their activation in pathologic CNS conditions, including the response to facial nerve axotomy and stroke, uveitis, and experimental autoimmune neuritis and encephalomyelitis. Nevertheless, how aif-1 gene products affect cellular function is only partly understood, and the physiologic significance of these products for male fertility, immune system development, and inflammation has not been described. To permit such investigations, we generated a mouse line with targeted deletion of the coding regions of the aif-1 gene. Here we report that mice lacking Aif-1 breed well and show normal post-natal growth, but show resistance to disease in a model of collagen-induced arthritis. We anticipate that these mice will be useful for studies of Aif-1 function in a variety of immune and inflammatory disease models.
Keywords: Aif-1, Iba1, Daintain, MRF-1, spermatid, macrophage
Introduction
Aif-1, a highly conserved, 17 kDa EF hand motif-bearing phosphoprotein (Utans et al., 1995; (Imai et al., 1996), is expressed primarily by developing spermatids (Iida et al., 2001; Kohler, 2007) and cells of monocyte/macrophage lineage. Increased Aif-1 expression has been identified in infiltrating macrophages in damaged or inflamed tissues, including allografted solid organs (e.g., heart (Utans et al., 1995) and liver (Nagakawa et al., 2004)), autoimmune conditions (e.g., rheumatoid arthritis), and drug-induced disease models (e.g., kidney (Tsubata et al., 2006)). In the nervous system, Aif-1 (often referred to as Iba1 in this literature) has emerged as a histologic marker of microglia and microglial activation (Ahmed et al., 2007; Mittelbronn et al., 2001) in several pathologic conditions, including the response to facial nerve axotomy (Graeber et al., 1998), stroke (Postler et al., 2000), uveitis (Fauser et al., 2001), and experimental autoimmune encephalomyelitis (Schluesener et al., 1998).
How Aif-1 affects cellular activities is not well understood. When microglia are stimulated with colony stimulating factor-1, Aif-1 accumulates with F-actin in membrane ruffles, and overexpression studies in vitro suggest a role for this protein in cellular motility and phagocytosis (Imai and Kohsaka, 2002). It has also been suggested that Aif-1 expression in the cytoplasm of elongated spermatids contributes to the final stage of spermiogenesis (i.e., elimination of residual cytoplasm) (Iida et al., 2001), perhaps by reorganizing the actin cytoskeleton to promote residual body extrusion. Relevant intracellular signaling events may include Aif-1-mediated potentiation of a phospholipase C-gamma-dependent pathway that activates the Rac GTPase (Kanazawa et al., 2002). Exogenous or overexpressed Aif-1 induces cytokine expression, including that of IL-6 by synovial fibroblasts and mononuclear cells (Kimura et al., 2007) and IL-12 by monocyte/macrophages (Watano et al., 2001), and promotes proliferation of cells derived from inflammatory lesions (Kimura et al., 2007). Knockdown of Aif-1 with an siRNA decreased macrophage proliferation and migration, and limited activities of Akt and extracellular signal-regulated kinase 1/2 pathways, without affecting apoptosis as evaluated by caspase 3 activation (Tian et al., 2006). Manipulation of Aif-1 expression in vivo affects neointima formation after mechanical vascular injury (Sommerville et al., 2009), but the significance of Aif-1 expression in development and auto- and alloimmune inflammatory conditions is not known.
To permit such investigations, we have generated a mouse line with targeted inactivation of the aif-1 gene. Mice homozygous for the targeted allele appear grossly normal, show good viability and fertility, and have nearly normal peripheral blood cell profiles. In a collagen-induced arthritis model, they displayed significantly less paw swelling and disease activity compared to control mice. We anticipate that these mice will be useful for studies of Aif-1 function in a variety of inflammatory disease models.
Results and Discussion
Targeting the aif-1 locus
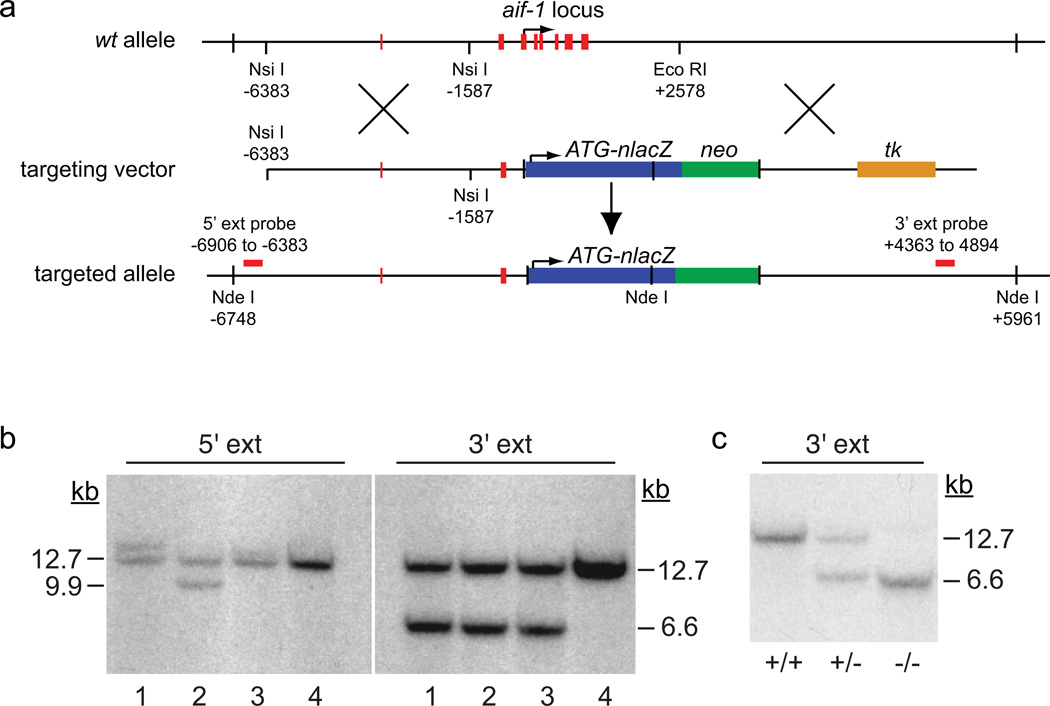
The mouse aif-1 locus lies in in the class III major compatibility complex on chromosome 17. Transcripts described in Genbank entries NM019467.2 and AK006184.1 indicate that the locus has 8 exons. Two exons encode 5’ untranslated sequences, while the other 6, which encompass the translation start and stop sites, span just 1.4 kb of the genome. To create a null allele with genetic reporter capability, we designed a targeting construct to substitute a modified lacZ gene encoding nuclear-localized β-galactosidase (Kupershmidt et al., 1999) for all aif-1 coding sequences (Figure 1a). This construct was electroporated into embryonic stem (ES) cells, and homologous recombinant clones were identified by PCR and evaluated by genomic Southern analysis using 5’ and 3’ external probes. Several clones with proper targeting were identified; in the example in Figure 1b, lane 2 shows the predicted change in Nde I restriction fragment size from 12.7 kb to 9.9 kb (5’ probe) and to 6.6 kb (3’ probe). This clone was injected into C57BL/6 blastocysts, and yielded highly chimeric founders. Germline transmission of the targeted, inactivated aif-1 allele was confirmed, and these F1 mice were crossed with C57BL/6 mice to establish the colony. Genomic Southern analysis further confirmed expected genotypes (Figure 1c). Methods for routine genotyping by PCR were established (Figure 2a). Both aif-1+/− and aif-1−/− mice of both sexes appeared grossly normal and bred well; genotyping of 130 offspring showed no significant deviation from predicted Mendelian ratios of inheritance, indicating that loss of Aif-1 has little or no effect on fertility or survival in barrier-protected, C57BL/6 mice (Table 1).
FIG. 1.
Generation of an aif-1-null allele in mice. (a) Gene targeting strategy. An arrow indicates the translation start site. nlacZ, modified β-galactosidase gene with nuclear localization signal; neo, neomycin positive selection cassette; ext, external; tk, thymidine kinase negative selection cassette. (b) Genomic Southern analysis of targeted ES cell clones. (c) Genotyping by Southern analysis of wt and aif-1-targeted mice.
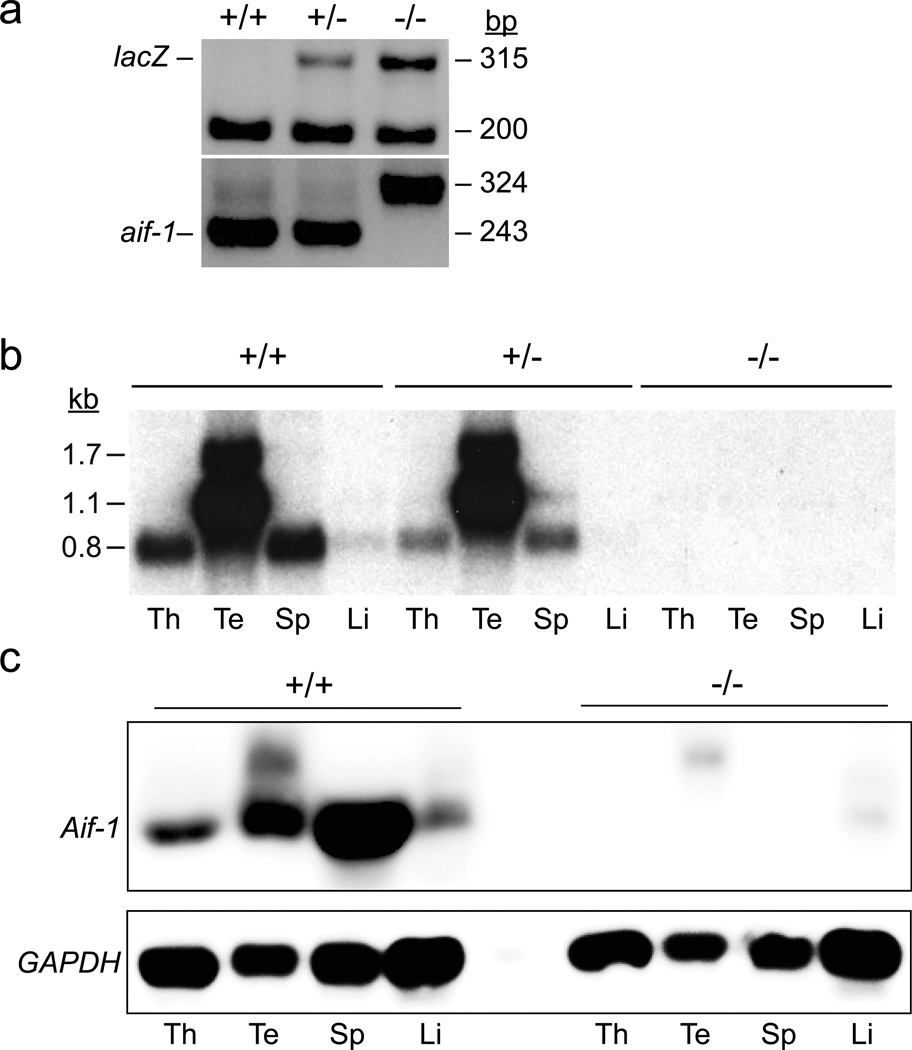
FIG. 2.
Evaluation aif-1 mRNA and protein levels in gene-targeted mice. (a) Routine genotyping, showing detection of targeted allele by PCR-based amplification of a 315 bp lacZ amplicon and loss of a 243 bp aif-1 genomic amplicon. (b) Total RNA samples (10 µg) from mice with the indicated aif-1 genotypes were assessed by Northern analysis using a radiolabeled aif-1 cDNA probe. Filters were hybridized to the probe, washed to high stringency, and evaluated by autoradiography. (c) Total protein (25 µg) was extracted from organs and evaluated by immunoblotting for Aif-1 or GAPDH, as indicated. Abbreviations: thymus (Th), testis (Te), spleen (Sp), and liver (Li).
Table 1.
genotype frequency in progeny of aif-1+/− × aif-1−/− crosses
| Genotype | heterozygous (aif-1+/−) |
homozygous (aif-1−/−) |
Total |
|---|---|---|---|
| Number expected | 65 | 65 | 130 |
| Number observeda | 60 | 70 | 130 |
| Expected Ratio (%) | 50 | 50 | 100 |
| Observed Ratio (%)b | 46.2 | 53.8 | 100 |
mice backcrossed to C57BL/6 strain and surviving to >8 weeks of age were genotyped
difference from expected Mendelian ratio was not statistically significant (by Chi-square test, P = 0.5348)
Analysis of targeting efficacy
To confirm loss of Aif-1 expression through this strategy, we euthanized wt, aif-1+/−, and aif-1−/− mice and isolated total RNA from several organs. Samples were subjected to Northern analysis using a cDNA probe encoding the entire Aif-1 open reading frame (Sibinga et al., 2002). The filter was washed to high stringency and autoradiographed. As shown in Figure 2b, robust expression of aif-1 mRNA was apparent in thymus, testis, and spleen samples from wt mice, with a faint signal also observed in liver. In the testis sample, higher molecular weight bands were observed, consistent with a previous report (Watano et al., 2001), and these signals were notably more intense than those produced by other organs. All samples from aif-1+/− mice showed decreased signal intensity relative to those from the corresponding wt tissue. Samples from aif-1−/− mice were devoid of signal, as expected based on the targeting strategy. Immunoblotting also showed loss of Aif-1 signal, although in this case, faint bands of ~22 and 17 kDa were apparent in the testis and liver samples, respectively (Figure 2c). Because our targeting strategy removed all Aif-1 coding sequences, these bands likely result from non-specific cross-reaction of the antibody, but it is possible that the 17 kDa band represents a product of the Aif-1-like gene (ENSMUSG00000001864), which is 81% similar in sequence to Aif-1.
Effect of aif-1 inactivation on spleen weight and hematologic indices
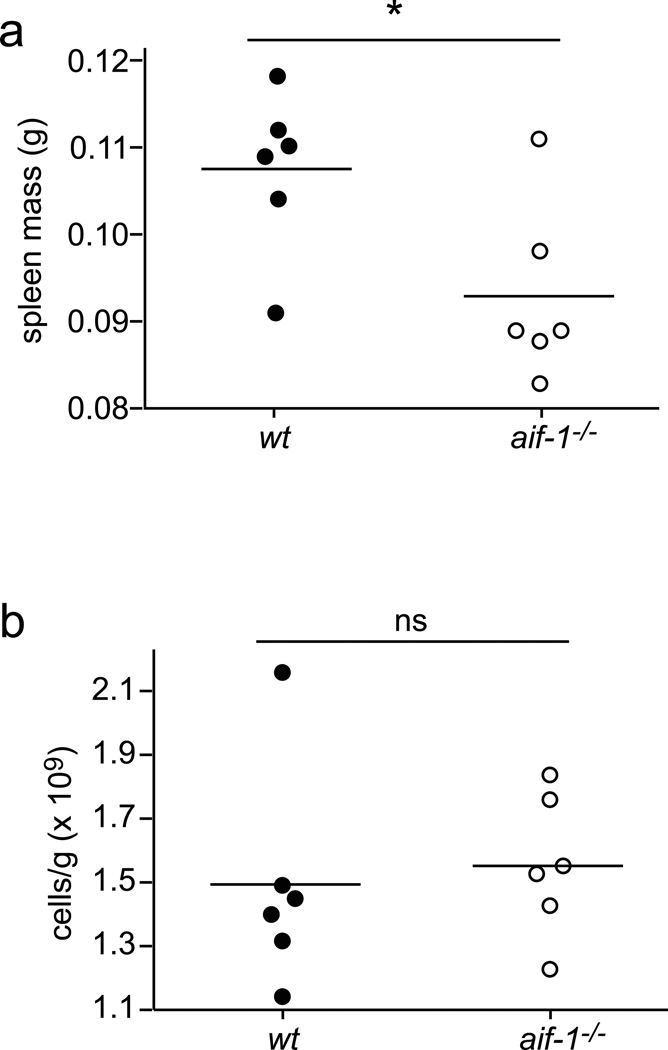
On necropsy, spleens from aif-1−/− mice appeared smaller than those of wt littermates. On average, Aif-1-deficient spleens weighed less, while splenic cell density (cell number per gram of tissue) was similar to wt (Figure 3). We also evaluated peripheral blood components. While erythrocyte numbers, hemoglobin, and hematocrit concentrations from Aif-1-deficient mice were all slightly lower than those in wt mice, these individual differences did not reach statistical significance (Table 2). On the other hand, mice lacking Aif-1 had ~10% fewer circulating platelets compared to their wt counterparts. The cellular basis for these modest differences in hematopoietic cell populations is not known at present.
FIG. 3.
Spleen weight and cell density. (a) Spleens from wt and Aif-1-deficient mice were weighed. *, P < .05. (b) cells per gram of spleen tissue, as measured by hemocytometer. ns, difference not significant.
Table 2.
hematologic indices
| wt | aif-1−/− | P value | |
|---|---|---|---|
| WBCa | 6.12±0.75 | 7.98±0.85 | .132 |
| %Neutrophil | 20.64±1.13 | 21.69±1.08 | .519 |
| %Lymphocyte | 75.80±1.27 | 74.81±1.33 | .601 |
| %Monocyte | 3.35±0.24 | 3.12±0.33 | .586 |
| %Eosinophil | 0.11±0.04 | 0.31±0.11 | .121 |
| %Basophil | 0.09±0.03 | 0.08±0.02 | .832 |
| RBCb | 10.11±0.20 | 9.67±0.17 | .129 |
| Hemoglobinc | 15.05±0.29 | 14.30±0.23 | .066 |
| Hematocrit (%) | 41.70±0.81 | 39.95±0.66 | .126 |
| Plateletd | 0.794±0.017 | 0.715±0.025 | .027* |
WBC, white blood cell count in thousands per cubic millimeter
RBC, red blood count in millions per cubic millimeter
Hemoglobin in g/100 milliliter blood
platelet count in millions per cubic millimeter
n = 6 for each genotype
Effect of aif-1 inactivation on autoimmune disease
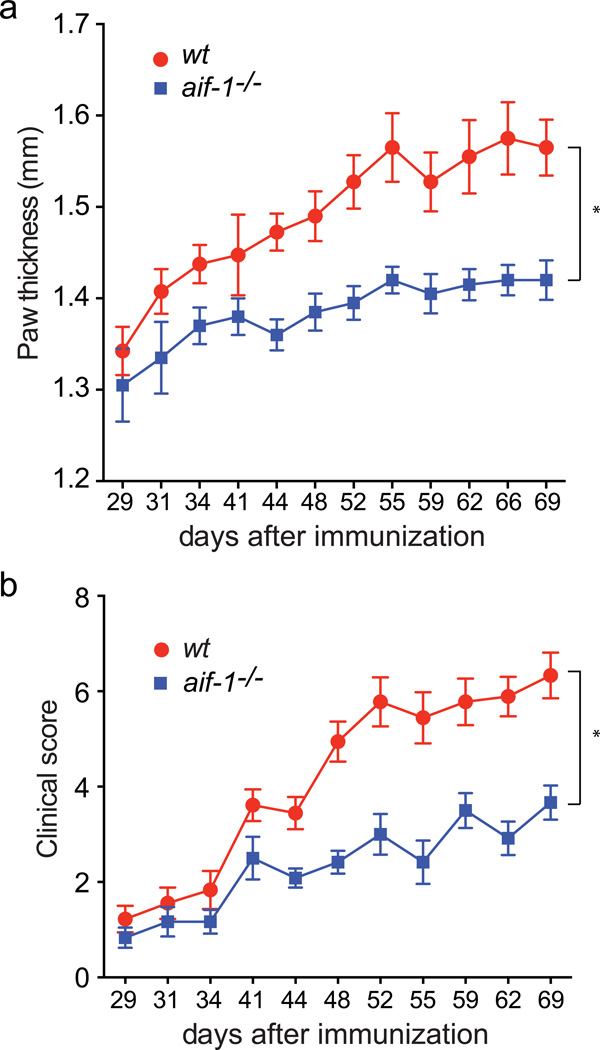
Increased Aif-1 expression, infiltration of Aif-1-expressing cells, and single nucleotide polymorphisms in the aif-1 locus have been linked to multiple autoimmune diseases, notably rheumatoid arthritis (Kimura et al., 2007); (Pawlik et al., 2012), but whether Aif-1 has an essential role in the pathogenesis of such processes is not clear. For initial evaluation of how Aif-1-deficiency affects inflammatory disease progression, we studied 12 week-old male wt control and aif-1-targeted mice, immunizing them by intradermal injection of chicken collagen type II (CII) emulsified in complete Freund’s adjuvant (Inglis et al., 2008). The animals were examined every few days to evaluate disease activity as reflected in measured paw thickness and overall clinical score, as described (Inglis et al., 2008). We saw thickening and erythema in the paws, but not in knee or ankle joints, consistent with the milder level of disease seen in C57BL/6 compared with DBA/1 mice (Inglis et al., 2007). In both genotypes, these indices increased during the period from 29 to 69 days after immunization, but in mice that lacked Aif-1, both paw thickening and disease scores were significantly attenuated relative to control mice (Figure 4).
FIG. 4.
Effect of aif-1 inactivation in a collagen-induced arthritis model. Wt (n = 9) and aif-1−/− (n = 6) mice were immunized with chicken collagen, as described in Methods. (a) Paw diameter was measured from 29 to 69 days after immunization using a microcaliper. (b) Disease activity was scored during the same time period, as described in methods. *, P<.0001 for genotype-dependent difference in paw thickness or disease activity.
In summary, these studies demonstrate the successful generation, by homologous recombination, of a null allele for the aif-1 gene in mice. The effect of global loss of Aif-1 expression on mouse development has not been previously described. Although aif-1 mRNA is normally expressed strongly in the testis (Figure 2b), mice lacking one or both copies of the gene proved to have good fertility and viability. Decreased spleen weight and lower peripheral blood platelet counts in aif-1-null animals indicate that loss of Aif-1 has modest effects on hematologic development; the precise cellular basis of these effects remains to be determined. Several prior reports have implicated Aif-1 in the pathogenesis of multiple inflammatory and immunologic conditions, but the functional significance of Aif-1 in these diseases and disease models has not been previously tested in vivo. Our initial studies using a model of autoimmune arthritis support the idea that Aif-1 promotes disease activity, and demonstrate the utility of this new transgenic mouse line. We anticipate that the line will be useful in characterizing how Aif-1 contributes to a variety of other inflammatory diseases.
Materials and Methods
Gene targeting
The standing committee of the Albert Einstein Institute for Animal Studies approved these studies, and the mouse model will be shared with the research community after manuscript publication. A 4.2 kb EcoRI-EcoRI fragment containing the known coding sequences of the aif-1 locus was obtained from a clone isolated from a 129SvJ mouse genomic library in the Lambda Fix II phage (Stratagene); this fragment was recloned into a PGK-tk/pBluescript targeting vector backbone. The Aif-1 coding sequences were excised and replaced by a modified lacZ gene encoding nuclear-localized β-galactosidase and a neomycin cassette (Kupershmidt et al., 1999). The 5’ arm of the construct was extended by ligating a 4.8 kb NsiI-NsiI aif-1 genomic fragment into the Nsi I site 1.67 kb 5’ to the Aif-1 translation start site. The final construct was confirmed by restriction digestion and nucleotide sequencing across all junctions. The construct was linearized and electroporated into 129SvEv mouse ES cells, which were cultured in the presence of neomycin for positive selection and ganciclovir for negative selection. Genomic DNA was prepared from ES cells using 100 mM Tris pH 8.5, 0.2 M NaCl, 5 mM EDTA, 0.2% SDS, and 0.1 mg/ml Proteinase K. Individual clones were screened for targeting of the aif-1 locus by PCR with Redtaq polymerase (Sigma), forward primer PGK polyA (AACCAAATTAAGGGCCAGCT), and reverse primer aif-1 3’ flanking (ATCCCTCGGAGAGAGAAAGG); cycling conditions were 94°C×30 s, 64°×30 s, and 72°×90 s for a total of 36 cycles.
Genomic Southern analysis
Genomic DNA isolated from mouse tails was extracted with phenol-chloroform prior to overnight restriction enzyme digestion, electrophoresed through 0.8% agarose gels, and transferred to GeneScreen Plus (PerkinElmer) nylon filters. DNA probes were labeled with 32P-dCTP using the Prime-It RmT Random Primer Labeling Kit (Stratagene), hybridized to filters, and processed as described (Sibinga et al., 2002).
Mouse genotyping
Routine genotyping to identify the intact aif-1 gene was performed by PCR of genomic DNA obtained from tail biopsies using primers (forward, TGGGCAAGAGATCTGCCATCTTGA, and reverse, AAGAGACACAGGCATCAGGGACAA) to amplify a 243 bp fragment spanning the 3’ most exon-intron junction. Touchdown cycling conditions were used, decreasing the annealing temperature by 0.5° per cycle from 64–58°C over 12 cycles, followed by 25 cycles with an annealing temperature of 58°C. The targeted allele was identified by multiplex PCR using lacZ-specific (Jackson Labs IMR39 and IMR40) and PCR control (Jackson Labs IMR 15 and IMR16) primers; this reaction used standard PCR cycling with a 60°C annealing temperature.
Northern analysis
Total RNA was extracted from mouse organs using Trizol (Invitrogen). Samples (10 µg) were fractionated on 1.2% formaldehyde-agarose gels and transferred to BrightStar Plus (Ambion) nylon filters, which were then hybridized at 68°C for 2 h to the random-primed, 32P-labeled mouse aif-1 cDNA probe (106 cpm/ml) in QuikHyb solution (Stratagene) (Sibinga et al., 2002). The filters were washed in 30 mM sodium chloride, 3 mM sodium citrate, and 0.1% SDS at 55°C and autoradiographed with Kodak Biomax MS film for 8 h at −80°C.
Immunoblotting
Total proteins were extracted from wt and aif-1−/− mice, separated by SDS-polyacrylamide gel electrophoresis, transferred to Immobilon-P membrane (Millipore), probed with a polyclonal antibody against Aif-1 (1:250, Wako), and evaluated by chemiluminescent detection (Pierce).
Collagen-induced arthritis model
The targeted aif-1 allele was backcrossed at least 8 generations onto a C57BL/6 mouse genetic background. To induce arthritis, wt and aif-1−/− mice (age 12–14 weeks, 3–6 per genotype) were immunized with CII using a standard protocol (Inglis et al., 2008). Briefly, CII (4 mg/ml, MD Bioproducts) was dissolved overnight in 0.1 M acetic acid and mixed with equal parts of Complete Freund’s Adjuvant containing 5 mg/ml of Mycobacterium Tuberculosis H37Ra (Difco). All mice were immunized with a total of 200 µg of this emulsion injected intradermally at the base of the tail and at a second site slightly anterior to the first. Mice were boosted with 100 µg CII in Incomplete Freund’s Adjuvant 14 days after the primary immunization. Starting 20 days after initial immunization, paw diameters of the hind and fore limbs were measured using microcalipers (Kroeplin), and these measures were averaged. Scores for the two genotypes across 8 time points were evaluated using a two-way analysis of variance, with a Bonferroni correction and significance set at 0.05 (Prism 6 software, Graphpad). Disease activity was scored by evaluating each limb on a 0–3 scale, with 0 being normal and 3 referring to swelling of the entire paw with erythema and ankylosis, as described (Inglis et al., 2008).
Statistical methods
Paw measurements and disease scores are shown as mean ± standard error (SE). Statistical significance was determined by Student’s t test (two-tailed), multiple analysis of variance (ANOVA) with Bonferroni correction, or Chi-square test, as appropriate, with significance accepted for P <.05. The analyses were conducted with GraphPad Prism.
Acknowledgements
We thank Jennifer Hadix for excellent technical assistance in early stages of this project.
Contract grant sponsor: US National Institutes of Health (NIH); Contract grant number: HL67944
Footnotes
I.C. and P.C. contributed equally to this work.
References
- Ahmed Z, Shaw G, Sharma VP, Yang C, McGowan E, Dickson DW. Actin-binding proteins coronin-1a and IBA-1 are effective microglial markers for immunohistochemistry. J Histochem Cytochem. 2007;55:687–700. doi: 10.1369/jhc.6A7156.2007. [DOI] [PubMed] [Google Scholar]
- Fauser S, Nguyen TD, Bekure K, Schluesener HJ, Meyermann R. Differential activation of microglial cells in local and remote areas of IRBP1169-1191-induced rat uveitis. Acta Neuropathol. 2001;101:565–571. doi: 10.1007/s004010000319. [DOI] [PubMed] [Google Scholar]
- Graeber MB, Lopez-Redondo F, Ikoma E, Ishikawa M, Imai Y, Nakajima K, Kreutzberg GW, Kohsaka S. The microglia/macrophage response in the neonatal rat facial nucleus following axotomy. Brain Research. 1998;813:241–253. doi: 10.1016/s0006-8993(98)00859-2. [DOI] [PubMed] [Google Scholar]
- Iida H, Doiguchi M, Yamashita H, Sugimachi S, Ichinose J, Mori T, Shibata Y. Spermatid-specific expression of Iba1, an ionized calcium binding adapter molecule-1, in rat testis. Biology of Reproduction. 2001;64:1138–1146. doi: 10.1095/biolreprod64.4.1138. [DOI] [PubMed] [Google Scholar]
- Imai Y, Ibata I, Ito D, Ohsawa K, Kohsaka S. A novel gene iba1 in the major histocompatibility complex class III region encoding an EF hand protein expressed in a monocytic lineage. Biochem Biophys Res Commun. 1996;224:855–862. doi: 10.1006/bbrc.1996.1112. [DOI] [PubMed] [Google Scholar]
- Imai Y, Kohsaka S. Intracellular signaling in M-CSF-induced microglia activation: role of Iba1. Glia. 2002;40:164–174. doi: 10.1002/glia.10149. [DOI] [PubMed] [Google Scholar]
- Inglis JJ, Criado G, Medghalchi M, Andrews M, Sandison A, Feldmann M, Williams RO. Collagen-induced arthritis in C57BL/6 mice is associated with a robust and sustained T-cell response to type II collagen. Arthritis Res Ther. 2007;9:R113. doi: 10.1186/ar2319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Inglis JJ, Simelyte E, McCann FE, Criado G, Williams RO. Protocol for the induction of arthritis in C57BL/6 mice. Nat Protoc. 2008;3:612–618. doi: 10.1038/nprot.2008.19. [DOI] [PubMed] [Google Scholar]
- Kanazawa H, Ohsawa K, Sasaki Y, Kohsaka S, Imai Y. Macrophage/microglia-specific protein Iba1 enhances membrane ruffling and Rac activation via phospholipase C-gamma -dependent pathway. J Biol Chem. 2002;277:20026–20032. doi: 10.1074/jbc.M109218200. [DOI] [PubMed] [Google Scholar]
- Kimura M, Kawahito Y, Obayashi H, Ohta M, Hara H, Adachi T, Tokunaga D, Hojo T, Hamaguchi M, Omoto A, Ishino H, Wada M, Kohno M, Tsubouchi Y, Yoshikawa T. A critical role for allograft inflammatory factor-1 in the pathogenesis of rheumatoid arthritis. J Immunol. 2007;178:3316–3322. doi: 10.4049/jimmunol.178.5.3316. [DOI] [PubMed] [Google Scholar]
- Kohler C. Allograft inflammatory factor-1/Ionized calcium-binding adapter molecule 1 is specifically expressed by most subpopulations of macrophages and spermatids in testis. Cell Tissue Res. 2007;330:291–302. doi: 10.1007/s00441-007-0474-7. [DOI] [PubMed] [Google Scholar]
- Kupershmidt S, Yang T, Anderson ME, Wessels A, Niswender KD, Magnuson MA, Roden DM. Replacement by homologous recombination of the minK gene with lacZ reveals restriction of minK expression to the mouse cardiac conduction system. Circ Res. 1999;84:146–152. doi: 10.1161/01.res.84.2.146. [DOI] [PubMed] [Google Scholar]
- Mittelbronn M, Dietz K, Schluesener HJ, Meyermann R. Local distribution of microglia in the normal adult human central nervous system differs by up to one order of magnitude. Acta Neuropathol (Berl) 2001;101:249–255. doi: 10.1007/s004010000284. [DOI] [PubMed] [Google Scholar]
- Nagakawa Y, Nomoto S, Kato Y, Montgomery RA, Williams GM, Klein AS, Sun Z. Over-expression of AIF-1 in liver allografts and peripheral blood correlates with acute rejection after transplantation in rats. Am J Transplant. 2004;4:1949–1957. doi: 10.1111/j.1600-6143.2004.00621.x. [DOI] [PubMed] [Google Scholar]
- Pawlik A, Kurzawski M, Dziedziejko V, Safranow K, Paczkowska E, Maslinski W, Drozdzik M, Gawronska-Szklarz B. Allograft inflammatory factor-1 gene polymorphisms in patients with rheumatoid arthritis. Genet Test Mol Biomarkers. 2012;16:341–345. doi: 10.1089/gtmb.2011.0201. [DOI] [PubMed] [Google Scholar]
- Postler E, Rimner A, Beschorner R, Schluesener HJ, Meyermann R. Allograft-inflammatory-factor-1 is upregulated in microglial cells in human cerebral infarctions. J Neuroimmunol. 2000;104:85–91. doi: 10.1016/s0165-5728(99)00222-2. [DOI] [PubMed] [Google Scholar]
- Schluesener HJ, Seid K, Kretzschmar J, Meyermann R. Allograft-inflammatory factor-1 in rat experimental autoimmune encephalomyelitis, neuritis, and uveitis: expression by activated macrophages and microglial cells. Glia. 1998;24:244–251. doi: 10.1002/(sici)1098-1136(199810)24:2<244::aid-glia9>3.0.co;2-3. [DOI] [PubMed] [Google Scholar]
- Sibinga NES, Feinberg MW, Yang H, Werner F, Jain MK. Macrophage-restricted and interferon gamma-inducible expression of the allograft inflammatory factor-1 gene requires Pu.1. J Biol Chem. 2002;277:16202–16210. doi: 10.1074/jbc.M200935200. [DOI] [PubMed] [Google Scholar]
- Sommerville LJ, Xing C, Kelemen SE, Eguchi S, Autieri MV. Inhibition of allograft inflammatory factor-1 expression reduces development of neointimal hyperplasia and p38 kinase activity. Cardiovasc Res. 2009;81:206–215. doi: 10.1093/cvr/cvn242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tian Y, Kelemen SE, Autieri MV. Inhibition of AIF-1 expression by constitutive siRNA expression reduces macrophage migration, proliferation, and signal transduction initiated by atherogenic stimuli. Am J Physiol Cell Physiol. 2006;290:C1083–C1091. doi: 10.1152/ajpcell.00381.2005. [DOI] [PubMed] [Google Scholar]
- Tsubata Y, Sakatsume M, Ogawa A, Alchi B, Kaneko Y, Kuroda T, Kawachi H, Narita I, Yamamoto T, Gejyo F. Expression of allograft inflammatory factor-1 in kidneys: A novel molecular component of podocyte. Kidney Int. 2006;70:1948–1954. doi: 10.1038/sj.ki.5001941. [DOI] [PubMed] [Google Scholar]
- Utans U, Arceci RJ, Yamashita Y, Russell ME. Cloning and characterization of allograft inflammatory factor-1: a novel macrophage factor identified in rat cardiac allografts with chronic rejection. J Clin Invest. 1995;95:2954–2962. doi: 10.1172/JCI118003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Watano K, Iwabuchi K, Fujii S, Ishimori N, Mitsuhashi S, Ato M, Kitabatake A, Onoe K. Allograft inflammatory factor-1 augments production of interleukin-6, -10 and-12 by a mouse macrophage line. Immunology. 2001;104:307–316. doi: 10.1046/j.1365-2567.2001.01301.x. [DOI] [PMC free article] [PubMed] [Google Scholar]