Abstract
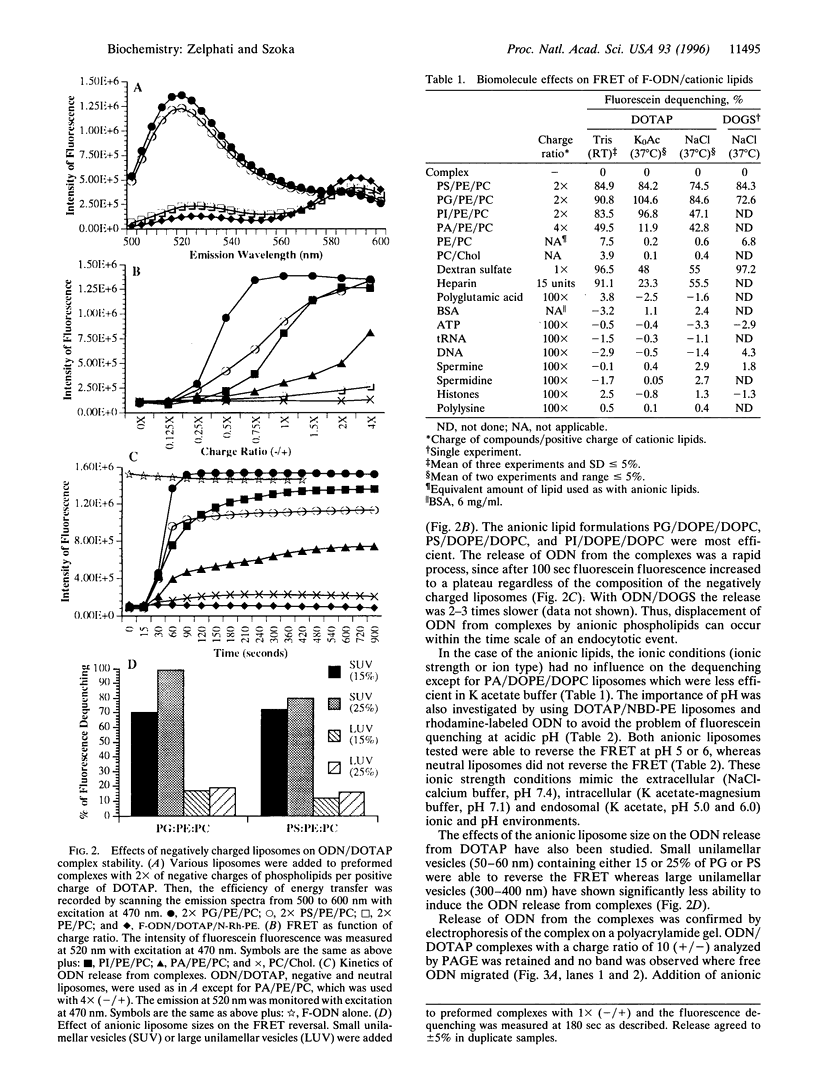
We propose a mechanism for oligonucleotide (ODN) release from cationic lipid complexes in cells that accounts for various observations on cationic lipid-nucleic acid-cell interactions. Fluorescent confocal microscopy of cells treated with rhodamine-labeled cationic liposome/ fluorescein-labeled ODN (F-ODN) complexes show the F-ODN separates from the lipid after internalization and enters the nucleus leaving the fluorescent lipid in cytoplasmic structures. ODN displacement from the complex was studied by fluorescent resonance energy transfer. Anionic liposome compositions (e.g., phosphatidylserine) that mimic the cytoplasmic facing monolayer of the cell membrane released ODN from the complex at about a 1:1 (-/+) charge ratio. Release was independent of ionic strength and pH. Physical separation of the F-ODN from monovalent and multivalent cationic lipids was confirmed by gel electrophoresis. Fluid but not solid phase anionic liposomes are required, whereas the physical state of the cationic lipids does not effect the release. Water soluble molecules with a high negative linear charge density, dextran sulfate, or heparin also release ODN. However, ATP, spermidine, spermine, tRNA, DNA, polyglutamic acid, polylysine, bovine serum albumin, or histone did not release ODN, even at 100-fold charge excess (-/+). Based upon these results, we propose that the complex, after internalization by endocytosis, induces flip-flop of anionic lipids from the cytoplasmic facing monolayer. Anionic lipids laterally diffuse into the complex and form a charged neutralized ion-pair with the cationic lipids. This leads to displacement of the ODN from the cationic lipid and its release into the cytoplasm.
Full text
PDF




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Behr J. P., Demeneix B., Loeffler J. P., Perez-Mutul J. Efficient gene transfer into mammalian primary endocrine cells with lipopolyamine-coated DNA. Proc Natl Acad Sci U S A. 1989 Sep;86(18):6982–6986. doi: 10.1073/pnas.86.18.6982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bennett C. F., Chiang M. Y., Chan H., Shoemaker J. E., Mirabelli C. K. Cationic lipids enhance cellular uptake and activity of phosphorothioate antisense oligonucleotides. Mol Pharmacol. 1992 Jun;41(6):1023–1033. [PubMed] [Google Scholar]
- Casu B. Structure and biological activity of heparin. Adv Carbohydr Chem Biochem. 1985;43:51–134. doi: 10.1016/s0065-2318(08)60067-0. [DOI] [PubMed] [Google Scholar]
- Chiang M. Y., Chan H., Zounes M. A., Freier S. M., Lima W. F., Bennett C. F. Antisense oligonucleotides inhibit intercellular adhesion molecule 1 expression by two distinct mechanisms. J Biol Chem. 1991 Sep 25;266(27):18162–18171. [PubMed] [Google Scholar]
- Devaux P. F. Protein involvement in transmembrane lipid asymmetry. Annu Rev Biophys Biomol Struct. 1992;21:417–439. doi: 10.1146/annurev.bb.21.060192.002221. [DOI] [PubMed] [Google Scholar]
- Düzgüneş N., Goldstein J. A., Friend D. S., Felgner P. L. Fusion of liposomes containing a novel cationic lipid, N-[2,3-(dioleyloxy)propyl]-N,N,N-trimethylammonium: induction by multivalent anions and asymmetric fusion with acidic phospholipid vesicles. Biochemistry. 1989 Nov 14;28(23):9179–9184. doi: 10.1021/bi00449a033. [DOI] [PubMed] [Google Scholar]
- Fattal E., Nir S., Parente R. A., Szoka F. C., Jr Pore-forming peptides induce rapid phospholipid flip-flop in membranes. Biochemistry. 1994 May 31;33(21):6721–6731. doi: 10.1021/bi00187a044. [DOI] [PubMed] [Google Scholar]
- Felgner P. L., Gadek T. R., Holm M., Roman R., Chan H. W., Wenz M., Northrop J. P., Ringold G. M., Danielsen M. Lipofection: a highly efficient, lipid-mediated DNA-transfection procedure. Proc Natl Acad Sci U S A. 1987 Nov;84(21):7413–7417. doi: 10.1073/pnas.84.21.7413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Felgner P. L., Ringold G. M. Cationic liposome-mediated transfection. Nature. 1989 Jan 26;337(6205):387–388. doi: 10.1038/337387a0. [DOI] [PubMed] [Google Scholar]
- Gao X., Huang L. Cytoplasmic expression of a reporter gene by co-delivery of T7 RNA polymerase and T7 promoter sequence with cationic liposomes. Nucleic Acids Res. 1993 Jun 25;21(12):2867–2872. doi: 10.1093/nar/21.12.2867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kõiv A., Kinnunen P. K. Binding of DNA to liposomes containing different derivatives of sphingosine. Chem Phys Lipids. 1994 Jun 24;72(1):77–86. doi: 10.1016/0009-3084(94)90018-3. [DOI] [PubMed] [Google Scholar]
- Lappalainen K., Urtti A., Söderling E., Jäskeläinen I., Syrjänen K., Syrjänen S. Cationic liposomes improve stability and intracellular delivery of antisense oligonucleotides into CaSki cells. Biochim Biophys Acta. 1994 Dec 30;1196(2):201–208. doi: 10.1016/0005-2736(94)00224-x. [DOI] [PubMed] [Google Scholar]
- Legendre J. Y., Szoka F. C., Jr Delivery of plasmid DNA into mammalian cell lines using pH-sensitive liposomes: comparison with cationic liposomes. Pharm Res. 1992 Oct;9(10):1235–1242. doi: 10.1023/a:1015836829670. [DOI] [PubMed] [Google Scholar]
- Malone R. W., Felgner P. L., Verma I. M. Cationic liposome-mediated RNA transfection. Proc Natl Acad Sci U S A. 1989 Aug;86(16):6077–6081. doi: 10.1073/pnas.86.16.6077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rose J. K., Buonocore L., Whitt M. A. A new cationic liposome reagent mediating nearly quantitative transfection of animal cells. Biotechniques. 1991 Apr;10(4):520–525. [PubMed] [Google Scholar]
- Sixou S., Szoka F. C., Jr, Green G. A., Giusti B., Zon G., Chin D. J. Intracellular oligonucleotide hybridization detected by fluorescence resonance energy transfer (FRET). Nucleic Acids Res. 1994 Feb 25;22(4):662–668. doi: 10.1093/nar/22.4.662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vidal M., Hoekstra D. In vitro fusion of reticulocyte endocytic vesicles with liposomes. J Biol Chem. 1995 Jul 28;270(30):17823–17829. doi: 10.1074/jbc.270.30.17823. [DOI] [PubMed] [Google Scholar]
- Wrobel I., Collins D. Fusion of cationic liposomes with mammalian cells occurs after endocytosis. Biochim Biophys Acta. 1995 May 4;1235(2):296–304. doi: 10.1016/0005-2736(95)80017-a. [DOI] [PubMed] [Google Scholar]
- Yaroslavov A. A., Kul'kov V. E., Polinsky A. S., Baibakov B. A., Kabanov V. A. A polycation causes migration of negatively charged phospholipids from the inner to outer leaflet of the liposomal membrane. FEBS Lett. 1994 Feb 28;340(1-2):121–123. doi: 10.1016/0014-5793(94)80185-1. [DOI] [PubMed] [Google Scholar]
- Zabner J., Fasbender A. J., Moninger T., Poellinger K. A., Welsh M. J. Cellular and molecular barriers to gene transfer by a cationic lipid. J Biol Chem. 1995 Aug 11;270(32):18997–19007. doi: 10.1074/jbc.270.32.18997. [DOI] [PubMed] [Google Scholar]
- Zhou X., Huang L. DNA transfection mediated by cationic liposomes containing lipopolylysine: characterization and mechanism of action. Biochim Biophys Acta. 1994 Jan 19;1189(2):195–203. doi: 10.1016/0005-2736(94)90066-3. [DOI] [PubMed] [Google Scholar]
- Zon G., Geiser T. G. Phosphorothioate oligonucleotides: chemistry, purification, analysis, scale-up and future directions. Anticancer Drug Des. 1991 Dec;6(6):539–568. [PubMed] [Google Scholar]





