Abstract
Context
Dyspnea is one of the most distressing symptoms in cancer patients, and often worsens with breakthrough episodes on exertion. We hypothesized that fentanyl given prophylactically may alleviate breakthrough dyspnea.
Objectives
To determine the feasibility of conducting a randomized trial of subcutaneous fentanyl in cancer patients, and examine the effects of fentanyl on dyspnea, walk distance, vital signs and adverse events.
Methods
In this double-blind, randomized, controlled trial, we asked ambulatory patients with breakthrough dyspnea to perform a baseline six-minute walk test (6MWT), and then assigned them to either subcutaneous fentanyl or placebo 15 minutes before a second 6MWT. We documented the change in dyspnea numeric rating scale (NRS) score, walk distance, vital signs and adverse events between the first and second 6MWT.
Results
Twenty patients were enrolled (1:1 ratio) without attrition. Comparison between baseline and second walk showed that fentanyl was associated with significant improvements in dyspnea NRS score at the end of the 6MWT (mean [95% CI]: −1.8 [−3.2, −0.4]), dyspnea NRS score at rest 15 minutes after drug administration (−0.9 [−1.8, −0.04]), Borg Scale fatigue score at the end of the 6MWT (−1.3 [−2.4, −0.2]), 6MWT distance (+37.2m [5.8m, 68.6m]) and respiratory rate (−2.4 [−4.5, −0.3]). Non-statistically significant improvements also were observed in the placebo arm, with no difference between the two study arms. No significant adverse effects were observed.
Conclusion
Prophylactic fentanyl was safe and improved dyspnea, fatigue, walk distance and respiratory rate. We also observed a large placebo effect. Our results justify larger randomized controlled trials with higher fentanyl doses. (clinicaltrials.gov registration: NCT01515566).
Keywords: dyspnea, exercise, opioids, fentanyl, neoplasms, randomized controlled trial, prophylaxis, subcutaneous
Introduction
Dyspnea is one of the most common and distressing symptoms among cancer patients (1). Over 80% of patients who experience dyspnea report having breakthrough (or incidental) episodes, triggered by various factors such as exertion or emotional distress. Among these patients, one-third have five or more episodes per day, and the majority of these episodes last less than 10 minutes (2). Breakthrough dyspnea is particularly challenging to treat because of its transient and episodic nature.
There have only been a handful of studies on therapeutic options for breakthrough dyspnea. Several small case series have documented the efficacy of oral transmucosal fentanyl citrate and intranasal fentanyl for breakthrough dyspnea episodes (3–5). However, the only randomized controlled trial on breakthrough dyspnea comparing systemic hydromorphone, nebulized hydromorphone and nebulized saline in cancer patients found no significant difference in dyspnea relief (6). Furthermore, all of these studies involved the use of opioids for treatment rather than primary prophylaxis of breakthrough dyspnea.
Fentanyl is a rapid-acting opioid that is highly lipophilic, and is currently approved for the management of pain. When administered subcutaneously, fentanyl reaches a median peak concentration at 15 minutes (range 10–30 minutes) (7), making it a potentially attractive therapeutic agent for management of breakthrough dyspnea. An improved understanding of the efficacy of subcutaneous fentanyl given prophylactically may allow us to better manage breakthrough dyspnea and to enhance patients’ function and quality of life. In this study, we determined the feasibility of conducting a double-blind, parallel, randomized, placebo-controlled trial of subcutaneous fentanyl in cancer patients with breakthrough dyspnea. We also examined the effects of fentanyl and placebo on the intensity of dyspnea, six-minute walk distance, physiologic parameters, and adverse events.
Methods
Patients
Inclusion criteria were diagnosis of cancer, outpatient at the Supportive Care Center at M. D. Anderson Cancer Center, age 18 or older, an average intensity of breakthrough dyspnea ≥3/10 on a numeric rating scale (NRS), ability to communicate in English or Spanish, ambulatory with or without walking aid, Karnofsky Performance Status score ≥50%, and a stable dose of strong opioids with a morphine equivalent daily dose (MEDD) of between 30 mg and 580 mg. Patients with dyspnea at rest ≥7/10, supplemental oxygen >6 L/minute, delirium (Memorial Delirium Assessment Scale >13/30), allergic reaction to fentanyl, history of substance abuse, recent history of coronary artery disease, uncontrolled tachycardia or hypertension at the time of assessment were excluded. The Institutional Review Board at M. D. Anderson Cancer Center approved this study. All patients provided written informed consent.
Study Design
This double-blind, randomized, parallel, placebo-controlled trial was designed to examine study feasibility and the effect of subcutaneous fentanyl on dyspnea in a before-after comparison. We also included a placebo arm to examine the magnitude of placebo effect. Patients were screened and approached by our research staff for this trial at the Supportive Care Outpatient Clinic. Using a computer that generated a randomization scheme by minimization, the study pharmacist randomly assigned patients in a 1:1 ratio to receive either a single dose of fentanyl or placebo (normal saline) given subcutaneously.
Enrolled patients were asked to perform a baseline six-minute walk test (6MWT) without any medications. This was followed by a rest period during which they were asked about their level of dyspnea every five minutes for up to one hour. When their dyspnea level was less than or equal to baseline dyspnea +1, they were given a single dose of subcutaneous fentanyl or placebo followed by a second 6MWT 15 minutes later. This time interval was chosen based on pharmacokinetic data demonstrating a median peak concentration at 15 minutes (range 10–30 minutes) (7).
Study Interventions
Fentanyl (50 mcg/ml, Baxter Healthcare Corp., Deerfield, Illinois) and placebo (0.9% sodium chloride, Hospira Inc., Lake Forest, Illinois) were prepared by the M. D. Anderson Cancer Center pharmacy. Allocation was concealed by using a secured website that was only accessible to the study pharmacist after patient enrollment. Both the patient and research staff conducting the study assessments were blinded to the study intervention and the randomization sequence.
For patients randomized to the fentanyl arm, a parenteral fentanyl dose was selected using the following sliding scale: 30 mcg, 50 mcg, 80 mcg, 130 mcg, 210 mcg and 350 mcg of subcutaneous fentanyl for MEDD of 30–49 mg/day, 50–79 mg/day, 80–129 mg/day, 130–209 mg/day, 210–349 mg/day and 350–580 mg/day, respectively. The fentanyl dose was designed to be 15–25% of the MEDD, which was based on the rescue opioids for breakthrough pain (8). We used a proportional approach instead of a titration approach to determine the prophylactic dose of subcutaneous fentanyl because it is less cumbersome. Fentanyl was administered subcutaneously by a research nurse. When the study medication was greater than 2 ml, we used a butterfly needle to administer the medication, followed by a 2 ml saline flush. Patients randomized to the placebo arm received preservative free 0.9% normal saline prepared in a syringe identical in appearance and volume to the fentanyl product they would otherwise receive.
Study Assessments and Endpoints
At baseline, we collected information on patient characteristics (age, sex, cancer diagnosis, comorbidities and medication use) and the Cancer Dyspnea Scale (CDS), a validated 12-item questionnaire that assesses the quality of dyspnea over the past few days (9, 10). Each item has a score between one and five, with a total score of up to 60, and subscores for sense of effort, anxiety, and discomfort.
Our primary outcome was retention rate, defined as the percentage of subjects able to complete the study. Secondary outcomes included changes in dyspnea, walk test distance and physiologic changes between the first walk test and second walk test for each intervention.
Six-minute walk tests were carried out following guidelines from the American Thoracic Society (11). The intensity of dyspnea “now” at 0, 1, 2, 3, 4, 5 and 6 minutes of each walk test were assessed using a validated NRS ranging from 0 (“no shortness of breath”) to 10 (“worst possible shortness of breath”) (9, 12, 13). The NRS was used to assess dyspnea every five minutes during the rest period. We measured the distance walked every minute. Fatigue also was assessed before and after each walk using the Borg scale as per guidelines (11), and may be related to dyspnea and/or fentanyl.
We also documented physiologic variables such as heart rate, respiratory rate, blood pressure, and oxygen saturation (Alaris® SpO2 module-8200 series oximeter, Alaris Medical Systems, San Diego, CA, USA) immediately before and after each walk test. Adverse effects such as dizziness, drowsiness, nausea, pruritus and pain with needle injection were assessed prior to medication administration and after the second walk test using an 11-point NRS, with 0 being absent and 10 denoting worst possible. After completion of each intervention, we asked patients about their change in dyspnea (better/same/worse) using the Global Symptom Evaluation (14, 15).
Statistical Analysis
Our primary objective was to determine the feasibility of conducting a randomized trial of subcutaneous fentanyl and placebo. We hypothesized that more than 65% of the enrolled patients would complete the process of randomization and the two walk tests. The planned enrollment of 20 patients was powered to detect a 65% completion rate with a 95% confidence interval (CI) between 45% and 85%. For the secondary objective, 10 patients per arm provides 80% power to detect an effect size as small as one within arms with a two-tailed α of 0.05. This study is not powered for a direct comparison between fentanyl and placebo.
We summarized the baseline demographics using descriptive statistics, including means, standard deviations (SDs), ranges, 95% CIs and frequencies. To determine the effect size, we calculated the mean difference between the first and second walk test and the 95% CI for dyspnea, fatigue, walk distance and physiologic variables. Intention-to-treat analysis was conducted to compare the fentanyl and placebo arms using the two-sample t-test for normally distributed variables (i.e., dyspnea, fatigue, walk distance and vital signs) and the Mann-Whitney test for non-parametric variables (i.e., adverse effects). For these exploratory analyses, a two-sided P-value of <0.05 was considered to be statistically significant. The Statistical Analysis System (SAS version 9.2, SAS Institute Inc., Cary, NC) was used for statistical analysis.
Results
Patient Characteristics
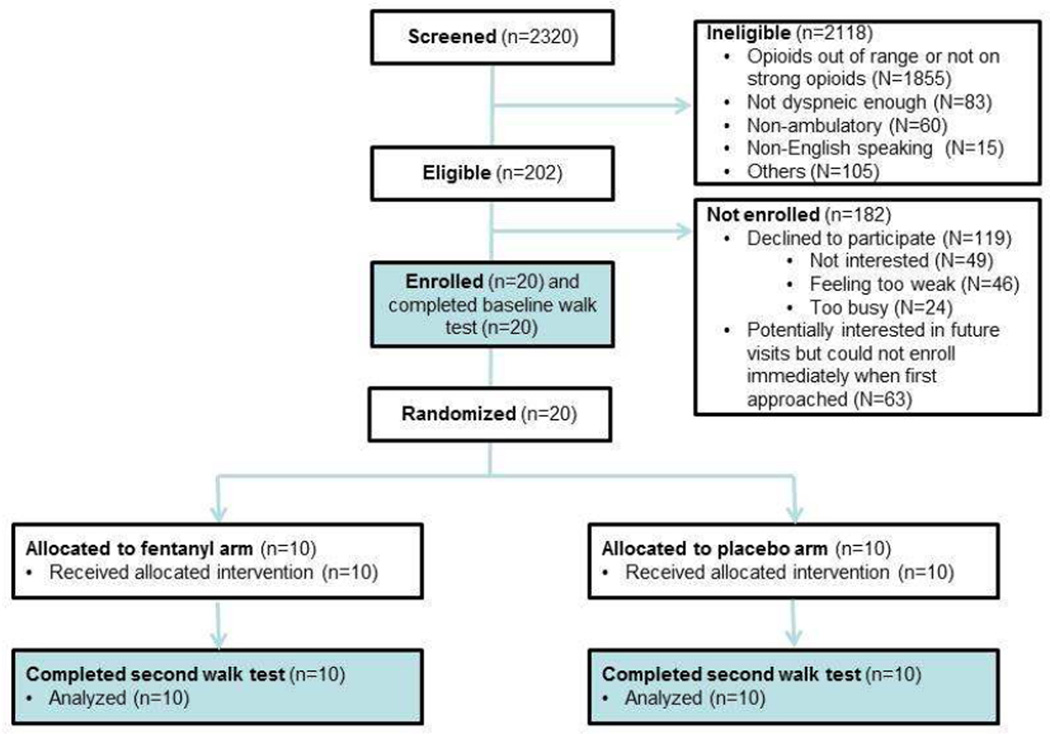
Fig. 1 depicts the study flow chart. Among the 2230 patients screened, 202 (9%) were eligible. Twenty (10%) of the eligible patients were enrolled into this study. The patients’ baseline characteristics are shown in Table 1. The baseline level of dyspnea was higher in the placebo group compared with the fentanyl group as measured by Edmonton Symptom Assessment System, dyspnea NRS and Cancer Dyspnea Scale.
Figure 1. CONSORT diagram.
Table 1.
Baseline Patient Characteristics
| Placebo (n=10) | Fentanyl (n=10) | All patients (N=20) | |
|---|---|---|---|
| n (%)a | n (%)a | n (%)a | |
| Average age (range) | 54 (30–73) | 55 (27–75) | 55 (27–75) |
| Female sex | 5 (50) | 6 (60) | 11 (55) |
| Race | |||
| Caucasian | 6 (60) | 8 (80) | 14 (70) |
| Black | 2 (20) | 1 (10) | 3 (15) |
| Hispanic | 2 (20) | 1 (10) | 3 (15) |
| Education | |||
| High school or less | 4 (40) | 2 (20) | 6 (30) |
| College | 5 (50) | 5 (50) | 10 (50) |
| Advanced degree | 1 (10) | 3 (30) | 4 (20) |
| Cancer type | |||
| Breast | 2 (20) | 3 (30) | 5 (25) |
| Gastrointestinal | 1 (10) | 0 (0) | 1 (5) |
| Genitourinary | 3 (30) | 0 (0) | 3 (15) |
| Gynecologic | 0 (0) | 2 (20) | 2 (10) |
| Lung | 3 (30) | 1 (10) | 4 (20) |
| Sarcoma | 1 (10) | 4 (40) | 5 (25) |
| Cancer stage | |||
| I-II | 1 (10) | 2 (20) | 3 (15) |
| III | 3 (30) | 1 (10) | 4 (20) |
| IV | 6 (60) | 7 (70) | 13 (65) |
| Cancer Dyspnea Scale, mean (SD) | |||
| Effort | 9 (6) | 4 (3) | 7 (5) |
| Anxiety | 6 (5) | 2 (2) | 4 (4) |
| Discomfort | 3 (3) | 2 (2) | 3 (3) |
| Total | 35 (10) | 28 (5) | 31 (8) |
| Average dyspnea NRS during breakthrough episodes over the last week, mean (SD) | 5.4 (1.5) | 4.3 (2.1) | 4.9 (1.9) |
| Edmonton Symptom Assessment Scale, mean (SD) | |||
| Pain | 5 (3) | 3 (2) | 4 (3) |
| Fatigue | 6 (3) | 5 (2) | 5 (2) |
| Nausea | 3 (3) | 1 (2) | 2 (3) |
| Depression | 2 (2) | 3 (4) | 3 (3) |
| Anxiety | 4 (4) | 3 (3) | 4 (3) |
| Drowsiness | 4 (4) | 3 (3) | 4 (3) |
| Decreased appetite | 5 (3) | 4 (3) | 4 (3) |
| Decreased well being | 6 (3) | 5 (3) | 5 (3) |
| Dyspnea | 5 (3) | 3 (3) | 4 (3) |
| Decreased sleep | 5 (3) | 5 (3) | 5 (3) |
| Potential contributors of dyspnea | |||
| Pulmonary parenchymal lesions | 5 (50) | 7 (70) | 12 (60) |
| Pleural effusion | 0 | 1 (10) | 1 (5) |
| Other | 5 (50) | 2 (20) | 7 (35) |
| Comorbidities | |||
| COPD | 0 (0) | 1 (10) | 1 (5) |
| Heart failure | 0 (0) | 0 (0) | 0 (0) |
| Asthma | 1 (10) | 2 (20) | 3 (15) |
| Bronchiectasis | 0 (0) | 0 (0) | 0 (0) |
| Concurrent therapies | |||
| Opioids | 10 (100) | 10 (100) | 20 (100) |
| Bronchodilators | 1 (10) | 0 | 1 (5) |
| Steroids | 1 (10) | 0 | 1 (5) |
| Supplemental oxygen | 0 | 1 (10) | 1 (5) |
| Morphine equivalent daily doses, median (interquartile range) in mg | 140 (44–263) | 103 (60–146) | 103 (54–188) |
| Karnofsky Performance Status, mean (SD) | 79 (9) | 80 (8) | 80 (8) |
COPD = chronic obstructive pulmonary disease; ECOG = Eastern Oncology Cooperative Group; NRS = numeric rating scale; SD = standard deviation
Unless otherwise specified.
Study Feasibility
Patients were recruited between July 10, 2012 and December 12, 2012. We enrolled 20 patients over five months with a 100% completion rate and no loss to follow-up. All patients walked for the full six minutes during both tests. After the first walk, 12 patients returned to their baseline level of dyspnea within five minutes of rest, five within 10 minutes, and three within 15 minutes.
Changes in Dyspnea, Fatigue, Walk Distance and Physiologic Variables
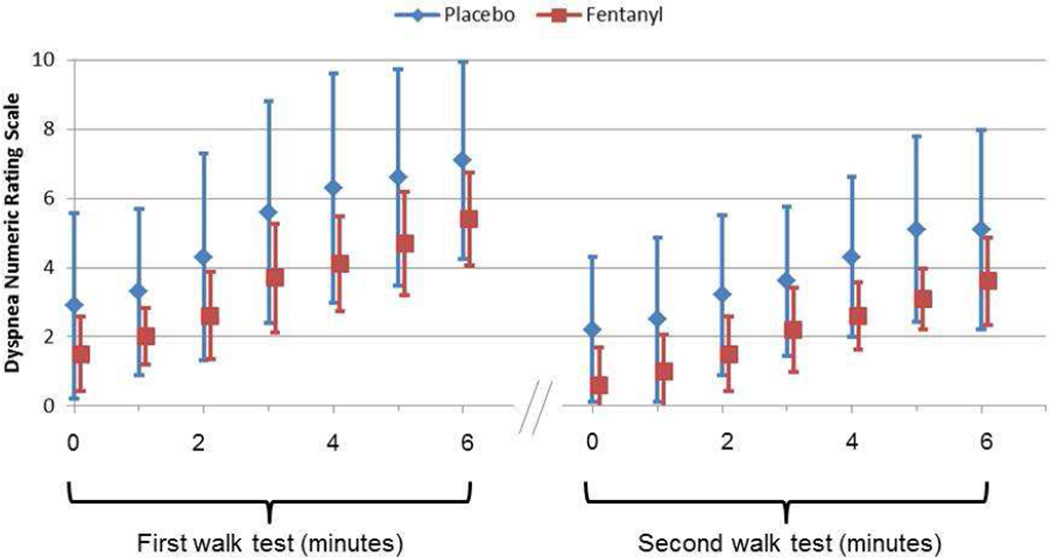
As shown in Table 2, subcutaneous fentanyl was associated with significant improvements in dyspnea NRS score at the end of the 6MWT (mean −1.8; 95% CI −3.2, −0.4; Fig. 2); dyspnea NRS score at rest before the 6MWT (mean −0.9; 95% CI −1.8, −0.04; Fig. 2); Borg Scale fatigue score at the end of the 6MWT (mean −1.3; 95% CI −2.4, −0.2); 6MWT distance (mean 37.2 m; 95% CI 5.8 m, 68.6 m); and respiratory rate (mean −2.4; 95% CI −4.5, −0.3).
Table 2.
Change in Dyspnea, Fatigue, Walk Distance and Physiologic Variables Between First and Second Walk Tests With Fentanyl and Placebo
| Baseline Walk Test |
Second Walk Test |
Difference Between First and Second Walk Tests |
|
|---|---|---|---|
| Variable | Mean (SD) | Mean (SD) | Mean change (95% CI)a |
| Dyspnea numeric rating scale | |||
| Placebo: 0 minutes | 2.9 (2.7) | 2.2 (2.1) | −0.7 (−1.5, 0.1) |
| Fentanyl: 0 minutesb | 1.5 (1.1) | 0.6 (1.1) | −0.9 (−1.8, −0.04) |
| Placebo: 6 minutes | 7.1 (2.8) | 5.1 (2.9) | −2 (−4, 0.02) |
| Fentanyl: 6 minutes | 5.4 (1.3) | 3.6 (1.3) | −1.8 (−3.2, −0.4) |
| Walk distance at 6 minutes | |||
| Placebo: 6 minutes | 399 (86.4) | 417.9 (89.3) | 18.9 (−10.4, 48.2) |
| Fentanyl: 6 minutes | 397.7 (98.1) | 434.9 (95.4) | 37.2 (5.8, 68.6) |
| Borg Scale Fatigue | |||
| Placebo: 0 minutes | 3.8 (3.1) | 2.7 (3.5) | −1.1 (−1.8, −0.4) |
| Fentanyl: 0 minutesb | 2.4 (1.6) | 1.3 (1.4) | −1.1 (−1.9, −0.3) |
| Placebo: 6 minutes | 4.6 (3.2) | 2.8 (3.3) | −1.9 (−4, 0.3) |
| Fentanyl: 6 minutes | 3.5 (1) | 2.2 (1.3) | −1.3 (−2.4, −0.2) |
| Respiratory rate | |||
| Placebo: 0 minutes | 18.6 (1.3) | 18.6 (1.6) | 0 (−1, 1) |
| Fentanyl: 0 minutesb | 18.8 (3.3) | 18.2 (1.8) | −0.6 (−3.5, 2.3) |
| Placebo: 6 minutes | 24.6 (5.7) | 23.4 (3.9) | −1.2 (−4.6, 2.2) |
| Fentanyl: 6 minutes | 23.4 (2.7) | 21 (2.9) | −2.4 (−4.5, −0.3) |
| Oxygen saturation | |||
| Placebo: 0 minutes | 95 (2.2) | 96.2 (1.9) | 1.2 (−0.6, 3) |
| Fentanyl: 0 minutesb | 97.1 (2.2) | 96.5 (2.6) | −0.6 (−1.6, 0.4) |
| Placebo: 6 minutes | 95.3 (3.1) | 96.1 (1.9) | 0.8 (−1.6, 3.2) |
| Fentanyl: 6 minutes | 98 (2.3) | 96.8 (2.4) | −1.2 (−2.7, 0.3) |
CI = confidence interval; SD = standard deviation.
Statistically significant values are in boldface.
The moment just prior to the six-minute walk test, 15 minutes after fentanyl/placebo administration.
Figure 2. Average dyspnea numeric rating scale scores during the first and second 6-minute walk tests.
The error bars represent standard deviations.
For the placebo arm, we also observed non-statistically significant trends toward improvement in dyspnea NRS score at the end of the 6MWT (mean −2; 95% CI −4, 0.02); dyspnea NRS score at rest before the 6MWT (mean −0.7; 95% CI −1.5, 0.1); Borg Scale fatigue score at the end of the 6MWT (mean −1.9; 95% CI −4, −0.3); 6MWT distance (mean 18.9 m; 95% CI −10.4 m, 48.2 m); and respiratory rate (mean −2.4; 95% CI −4.5, −0.3).
Heart rate, systolic and diastolic blood pressure and oxygen saturation did not differ between baseline and the second 6MWT in both study groups. Comparison between fentanyl and placebo revealed no statistically significant differences in any of the outcome measures.
Adverse Effects
Both fentanyl and placebo were well tolerated. Fentanyl was associated with a higher level of pain with needle injection as compared with placebo (median 2 vs. 0, P=0.01) (Table 3).
Table 3.
Adverse Effects of Fentanyl and Placebo
| Adverse Effect | Fentanyl Median (IQR) | Placebo Median (IQR) | P-valuea |
|---|---|---|---|
| Dizziness | 0 (0–0) | 0 (0–0.75) | 0.29 |
| Drowsiness | 0 (−0.75–0) | 0 (−3.25–0) | 0.48 |
| Nausea | 0 (0–0) | 0 (0–0) | 0.32 |
| Pruritus | 0 (0–0) | 0 (0–0) | 0.15 |
| Pain with subcutaneous injection | 2 (0.25–6.75) | 0 (0–0) | 0.01 |
The Mann-Whitney test was used for comparison between fentanyl and placebo.
Global Symptom Evaluation
Among the 10 patients who received fentanyl, six rated their dyspnea as better with the study intervention in the second walk as compared to the first walk, three rated it as the same, and one rated it as worse. For the placebo group, eight, one and one patients perceived their dyspnea as better, the same and worse, respectively. The difference between study interventions was not statistically significant (P=0.78, Fisher’s exact test).
Discussion
Our primary objective was to determine the feasibility of conducting a randomized controlled trial of subcutaneous fentanyl for breakthrough dyspnea in cancer patients. Our fast enrollment and high completion rates strongly suggest that this study design is feasible, which may be helpful for other investigators studying breakthrough dyspnea. Our secondary objective was to examine the effect of subcutaneous fentanyl on various patient-reported outcomes, function, physiologic variables and adverse events. We found that prophylactic fentanyl was safe, and reduced dyspnea while enhancing physiologic parameters and activity level. This study was not powered for a direct comparison between fentanyl and placebo, and we detected a large placebo effect. Given that fentanyl was well tolerated, our results justify adequately powered randomized controlled trials with high fentanyl doses to examine the role of prophylactic opioid administration for breakthrough dyspnea.
Because breakthrough episodes occur quickly and resolve within minutes (Fig. 2), a prophylaxis strategy such as the one tested in this study may be more practical than secondary prevention. We found that the 6MWT reliably induce dyspnea in cancer patients. Furthermore, by having patients do two walks, we were able to obtain an effect size by intra-individual comparison, thus minimizing the inter-individual variability. A crossover trial would have further minimized inter-individual variability between study arms, but would have require a washout period. This would have extended this study to two days, making it logistically more difficult.
Our preliminary findings suggest that prophylactic fentanyl may reduce dyspnea, fatigue and respiratory rate while improving walk distance. Dyspnea immediately before the 6MWT also decreased with fentanyl. This was assessed 15 minutes after the fentanyl dose, suggesting that fentanyl is effective for dyspnea at rest as well. A single opioid dose equivalent to 15–25% of the MEDD was well tolerated in our cohort of opioid-tolerant patients. At the highest dose of fentanyl, we administered 7 ml (350 mcg) of study medication subcutaneously without complications. The safety profile of fentanyl supports that higher doses can be tested in future trials. The half-life of subcutaneous fentanyl is 10 hours (range 5.5–16.4 hours) in humans (17), making it an appropriate choice even for patients exerting for long periods. Future studies also should examine the therapeutic effects of other opioids.
We included the placebo arm to estimate the placebo effect size. Notably, the change in dyspnea, fatigue, respiratory rate and walk distance were comparable in magnitude between placebo and subcutaneous fentanyl, although these effects were not statistically significant in the placebo group. The large placebo effect may be a result of obsequiousness bias, reporting bias, interviewer bias and period/training effect (16). Indeed, a placebo effect is common in supportive care studies, and the encouragement provided by research staff throughout the study may have contributed to a positive outcome (18). The use of subcutaneous injection also may contribute to the placebo effect. Furthermore, the placebo group had a higher baseline level of dyspnea compared with the fentanyl group. A higher level of baseline symptom burden has previously been shown by our group and others to be associated with a larger placebo effect (19). Future study designs should consider standardization of the patient encounter experience and stratification of baseline dyspnea level.
A direct comparison between fentanyl and placebo revealed no between-group differences. However, it should be emphasized that this study was designed to examine within-arm effect sizes only and was underpowered (i.e., power=20%) to detect a one-point difference in dyspnea between arms. The observed SD for the dyspnea NRS was 1.3 for fentanyl and 2.9 for placebo. To detect a one-point difference in dyspnea NRS scores between fentanyl and placebo using a two-sided, two-sample Satterthwaite t-test, assuming 80% power and α=0.05, a study would need 81 patients per arm.
Although this preliminary study cannot support the routine use of fentanyl for breakthrough dyspnea, it suggests that the study design is feasible and that the therapeutic roles of fentanyl and placebo need to be further examined in future randomized controlled trials. Given the considerable variation in baseline dyspnea level, future studies should stratify by baseline intensity to minimize imbalance between groups. Higher doses of fentanyl could potentially provide a greater benefit over placebo and need to be tested.
This study has several limitations. The small sample size means that our findings are susceptible to random errors and regression to the mean. We also conducted multiple statistical tests for secondary outcomes as an exploratory analysis. However, we provided 95% CIs for the effect size instead of P-values. Finally, the use of subcutaneous fentanyl requires training and may not be feasible for everyday use. Further research using rapid onset fentanyl through the transmucosal and intranasal routes may be helpful.
Conclusions
Prophylactic fentanyl was safe and improved dyspnea, fatigue, walk distance and respiratory rate. We also observed a large placebo effect. Our results justify larger placebo-controlled, randomized, controlled trials to examine the effect of fentanyl at higher doses.
Acknowledgments
Dr. Bruera is supported in part by National Institutes of Health grants RO1NR010162-01A1, RO1CA122292-01, and RO1CA124481-01. Dr. Hui is supported in part by an institutional startup grant (#18075582). This study also was supported by the M. D. Anderson Cancer Center Support Grant (CA 016672). The funding sources were not involved in the conduct of the study or development of the submission.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Disclosures
The authors declare no conflicts of interest.
References
- 1.Tishelman C, Petersson LM, Degner LF, Sprangers MA. Symptom prevalence, intensity, and distress in patients with inoperable lung cancer in relation to time of death. J Clin Oncol. 2007;25:5381–5389. doi: 10.1200/JCO.2006.08.7874. [DOI] [PubMed] [Google Scholar]
- 2.Reddy SK, Parsons HA, Elsayem A, Palmer JL, Bruera E. Characteristics and correlates of dyspnea in patients with advanced cancer. J Palliat Med. 2009;12:29–36. doi: 10.1089/jpm.2008.0158. [DOI] [PubMed] [Google Scholar]
- 3.Benitez-Rosario MA, Martin AS, Feria M. Oral transmucosal fentanyl citrate in the management of dyspnea crises in cancer patients. J Pain Symptom Manage. 2005;30:395–397. doi: 10.1016/j.jpainsymman.2005.10.002. [DOI] [PubMed] [Google Scholar]
- 4.Sitte T, Bausewein C. Intranasal fentanyl for episodic breathlessness. J Pain Symptom Manage. 2008;36:e3–e6. doi: 10.1016/j.jpainsymman.2008.08.002. [DOI] [PubMed] [Google Scholar]
- 5.Gauna AA, Kang SK, Triano ML, Swatko ER, Vanston VJ. Oral transmucosal fentanyl citrate for dyspnea in terminally ill patients: an observational case series. J Palliat Med. 2008;11:643–648. doi: 10.1089/jpm.2007.0161. [DOI] [PubMed] [Google Scholar]
- 6.Charles MA, Reymond L, Israel F. Relief of incident dyspnea in palliative cancer patients: a pilot, randomized, controlled trial comparing nebulized hydromorphone, systemic hydromorphone, and nebulized saline. J Pain Symptom Manage. 2008;36:29–38. doi: 10.1016/j.jpainsymman.2007.08.016. [DOI] [PubMed] [Google Scholar]
- 7.Capper SJ, Loo S, Geue JP, et al. Pharmacokinetics of fentanyl after subcutaneous administration in volunteers. Eur J Anaesthesiol. 2010;27:241–246. doi: 10.1097/EJA.0b013e328331a361. [DOI] [PubMed] [Google Scholar]
- 8.Bruera E, MacEachern T, Ripamonti C, Hanson J. Subcutaneous morphine for dyspnea in cancer patients. Ann Intern Med. 1993;119:906–907. doi: 10.7326/0003-4819-119-9-199311010-00007. [DOI] [PubMed] [Google Scholar]
- 9.Dorman S, Byrne A, Edwards A. Which measurement scales should we use to measure breathlessness in palliative care? A systematic review. Palliat Med. 2007;21:177–191. doi: 10.1177/0269216307076398. [DOI] [PubMed] [Google Scholar]
- 10.Tanaka K, Akechi T, Okuyama T, Nishiwaki Y, Uchitomi Y. Development and validation of the Cancer Dyspnoea Scale: a multidimensional, brief, self-rating scale. Br J Cancer. 2000;82:800–805. doi: 10.1054/bjoc.1999.1002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.ATS Committee on Proficiency Standards for Clinical Pulmonary Function Laboratories. ATS statement: guidelines for the six-minute walk test. Am J Respir Crit Care Med. 2002;166:111–117. doi: 10.1164/ajrccm.166.1.at1102. [DOI] [PubMed] [Google Scholar]
- 12.Gift AG, Narsavage G. Validity of the numeric rating scale as a measure of dyspnea. Am J Crit Care. 1998;7:200–204. [PubMed] [Google Scholar]
- 13.Hui D, Morgado M, Vidal M, et al. Dyspnea in hospitalized advanced cancer patients: subjective and physiologic correlates. J Palliat Med. 2013;16:274–280. doi: 10.1089/jpm.2012.0364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Redelmeier DA, Guyatt GH, Goldstein RS. Assessing the minimal important difference in symptoms: a comparison of two techniques. J Clin Epidemiol. 1996;49:1215–1219. doi: 10.1016/s0895-4356(96)00206-5. [DOI] [PubMed] [Google Scholar]
- 15.Guyatt GH, Feeny DH, Patrick DL. Measuring health-related quality of life. Ann Intern Med. 1993;118:622–629. doi: 10.7326/0003-4819-118-8-199304150-00009. [DOI] [PubMed] [Google Scholar]
- 16.Cook C. Mode of administration bias. J Man Manip Ther. 2010;18:61–63. doi: 10.1179/106698110X12640740712617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Capper SJ, Loo S, Geue JP, et al. Pharmacokinetics of fentanyl after subcutaneous administration in volunteers. Eur J Anaesthesiol. 2010;27:241–246. doi: 10.1097/EJA.0b013e328331a361. [DOI] [PubMed] [Google Scholar]
- 18.Bruera E, Yennurajalingam S, Perez-Cruz PE, et al. Methylphenidate (MP) and nursing telephone intervention (NTI) for cancer-related fatigue (CRF) in advanced cancer patients: a double-blind randomized phase II trial; Paper presented at the 48th American Society of Clinical Oncology (ASCO) Annual Meeting; Chicago, Illinois, USA. 2012. [Google Scholar]
- 19.de la Cruz M, Hui D, Parsons HA, Bruera E. Placebo and nocebo effects in randomized double-blind clinical trials of agents for the therapy for fatigue in patients with advanced cancer. Cancer. 2010;116:766–774. doi: 10.1002/cncr.24751. [DOI] [PMC free article] [PubMed] [Google Scholar]