Abstract
Solitary chemosensory cells in the non-neuronal epithelium of the anterior nasal cavity have bitter taste cell-like molecular characteristics and are involved in the detection of noxious substances. Here, we demonstrate that Pou2f3/Skn-1a, which is necessary for generation of sweet, umami, and bitter taste cells, is also necessary for the generation or differentiation of solitary chemosensory cells.
Keywords: transcription factor, Pou2f3, solitary chemosensory cell, differentiation, taste cell
Solitary chemosensory cells (SCCs) are epithelial sensory cells observed in vertebrates.1–3) They are distributed on the body surface in fish and in the respiratory epithelia in mammals. Although little is known about fish SCCs,4) knowledge of mammalian SCCs has been accumulating in the past several years. In mammals, SCCs express bitter receptors (Tas2rs), gustducin, phospholipase C-β2 (PLC-β2), and transient receptor potential channel M5 (TRPM5) and detect noxious substances such as bitter chemicals and acyl-homoserine lactone quorum-sensing molecules.2,5) Activated SCCs transmit chemical information to the trigeminal neurons via synapses and regulate respiratory responses to hazardous chemicals.2,5,6) Thus, SCCs function as a gatekeeper of biophylactic reaction(s), similar to the function of taste cells eliciting aversive behaviors. SCCs also express a sweet and umami receptor component (Tas1r3) enigmatically, but its function in SCCs is unknown.7) Like other epithelial chemosensory cells, such as taste cells, SCCs undergo continuous turnover.8) However, the mechanisms of development and differentiation of SCCs remain unclear.
Pou2f3, a POU homeodomain transcription factor also known as Skn-1a, is involved in the generation of sweet, umami, and bitter taste cells (so-called type II taste cells).9) Its loss-of-function mutation expanded the sour taste cell population at the expense of sweet, umami, and bitter taste cells. Thus, Pou2f3/Skn-1a functions as an early determinant of the differentiation to these taste cells, and there should be terminal selectors regulating the terminal differentiation to respective sweet, umami, and bitter taste cells.9)
Here we show that Pou2f3/Skn-1a is necessary for generation or functional differentiation of SCCs. The commonalities between SCCs and bitter cells, including intracellular signaling molecules and physiological functions to detect noxious substances, suggested that a common mechanism underlies the differentiation of these chemosensory cells. Intriguingly, SCCs co-express Tas1r3 and Tas2rs, while Tas1rs and Tas2rs are expressed in different subsets of taste cells in taste buds. This further suggests a possibility that the mechanism of differentiation of SCCs would be the same as the early differentiation of the precursors prior to the terminal differentiation to bitter, sweet, and umami cells, which requires Pou2f3/Skn-1a. Therefore, we first investigated the expression of Pou2f3/Skn-1a mRNA in the anterior nasal cavity by in situ hybridization analysis. In this research, we used 1- to 2-month-old male C57BL6/J and Pou2f3/Skn-1a-knockout mice. Preparation of mouse tissues, materials, and methods were as described previously.7,10) All animal experiments were approved by the Institutional Animal Care and Use Committee of Monell Chemical Senses Center and Tokyo Institute of Technology.
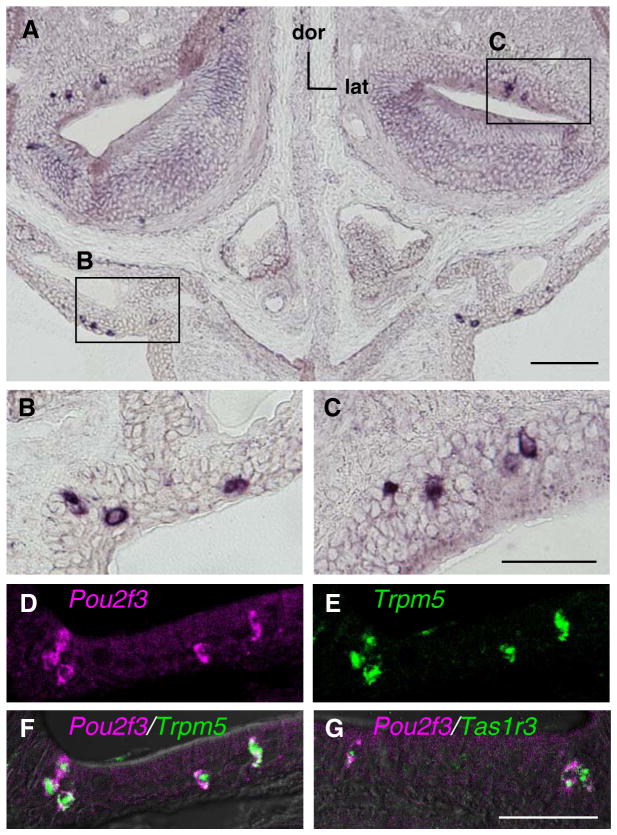
In situ hybridization analysis showed that the scattered signals of Pou2f3/Skn-1a mRNA were detected in the epithelial cells of the mouse anterior nasal cavity (Fig. 1A). These signals were observed in a small population of cells in the non-neuronal epithelial cell layer of the vomeronasal organ and respiratory epithelium (Fig. 1B and C). The distribution and frequency of Pou2f3/Skn-1a mRNA signals were similar to those of the SCCs.2,7) Then, we carried out double-label in situ hybridization analysis to examine whether Pou2f3/Skn-1a is expressed in the SCCs. The signals of Pou2f3/Skn-1a mRNA overlapped with those of Trpm5 and Tas1r3 mRNAs, markers for the SCCs in the anterior nasal cavity7) (Fig. 1D–G). These results clearly show the expression of Pou2f3/Skn-1a in the SCCs.
Fig. 1.
Expression of Pou2f3/Skn-1a in the SCCs in the Nasal Epithelia.
Single-label (A–C) and double-label (D–G) in situ hybridization of Pou2f3/Skn-1a and genes expressed in SCCs in coronal sections of mouse anterior nose. dor, dorsal; lat, lateral. B and C, Magnified views of the Pou2f3/Skn-1a mRNA signals in the respiratory epithelia (B) and non-neuronal epithelial cell layer of the vomeronasal organ (C). D-G, Signals of Pou2f3/Skn-1a mRNA (magenta) were observed in cells expressing Trpm5 (green, E and F) and Tas1r3 (green, G) in the anterior nasal epithelia. Scale bars, 100 μm in A and 50 μm in C and G.
To examine the impact of Pou2f3/Skn-1a on the SCCs, we examined the expression of genes in Pou2f3/Skn-1a-knockout mice. We detected the signals for Tas1r3, Tas2r105, Tas2r108, Gnat3 (gustducin), Plcb2, and Trpm5 mRNAs in the nasal epithelium of wild-type mice, all of which should be expressed in the SCCs2,7) (Fig. 2, top), but no signal of these mRNAs was observed in the Pou2f3/Skn-1a-knockout mice (Fig. 2, bottom). These results suggest that Pou2f3/Skn-1a is necessary for the generation or differentiation of the SCCs in the anterior nasal cavity. Sweet, umami, and bitter taste cells were absent in the taste buds of Pou2f3/Skn-1a-knockout mice.9) Pou2f3/Skn-1a is presumably involved in the generation of the SCCs in the anterior nasal cavity.
Fig. 2.
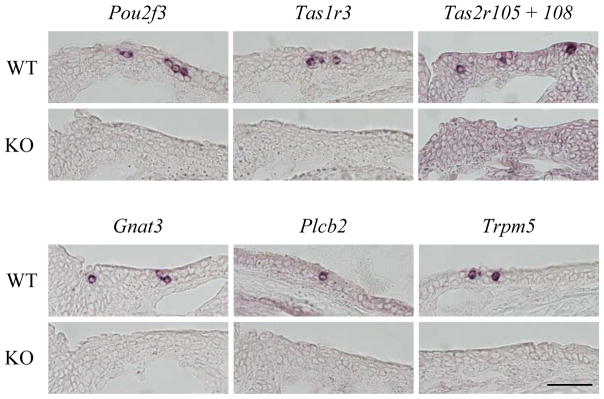
Loss of the Expression of SCC Signaling Genes in Pou2f3/Skn-1a-Knockout Mice.
In situ hybridization of signaling molecules in SCCs on sections of wild-type (WT, top) and Pou2f3/Skn-1a-knockout (KO, bottom) mice was carried out to examine the impact of Pou2f3/Skn-1a on SCCs. The mRNA signals of Pou2f3/Skn-1a, Tas1r3, mixed probes of Tas2r105 and Tas2r108, Gnat3, Plcb2, and Trpm5 observed in wild-type mice was completely absent in the Pou2f3/Skn-1a-knockout mice. Scale bar, 50 μm.
In this study, we found that Pou2f3/Skn-1a is essential for the generation or differentiation of SCCs as well as bitter taste cells.9) However, unlike bitter taste cells, SCCs co-express Tas1r3 with Tas2rs and have synapses.2, 7) It will be interesting to compare bitter cells and SCCs to identify mechanisms responsible for these differences. Taste cell-like chemosensory cells have been found in other tissues.11–14) Comparative analyses across these cells may reveal crucial mechanisms for generating a variety of closely related chemosensory cells.
Acknowledgments
We thank Dr. Keiko Abe for critical reading of the manuscript. This work was supported in part by NIH grants (DC011143 to I.M. and DC00882 to A.A.B.), Monell Institutional Fund (I.M.), and a Grant-in-Aid for Scientific Research (20570208 to J.H.). Histochemical analysis was performed at the Monell Histology and Cellular Localization Core, which is supported, in part, by funding from NIH Core Grant P30DC011735 (to Robert F. Margolskee, Monell Chemical Senses Center). M.O. is a Japan Society for the Promotion of Science Postdoctoral Fellow for Research Abroad.
Abbreviations
- SCC
solitary chemosensory cell
- PLC-β2
phospholipase C-β2
- TRPM5
transient receptor potential channel M5
References
- 1.Finger TE. Brain Behav Evol. 1997;50:234–243. doi: 10.1159/000113337. [DOI] [PubMed] [Google Scholar]
- 2.Finger TE, Böttger B, Hansen A, Anderson KT, Alimohammadi H, Silver WL. Proc Natl Acad Sci USA. 2003;100:8981–8986. doi: 10.1073/pnas.1531172100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Kotrschal K. Trends Ecol Evol. 1996;11:110–114. doi: 10.1016/0169-5347(96)81088-3. [DOI] [PubMed] [Google Scholar]
- 4.Ohmoto M, Okada S, Nakamura S, Abe K, Matsumoto I. J Comp Neurol. 2011;519:1616–1629. doi: 10.1002/cne.22589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Tizzano M, Gulbransen BD, Vandenbeuch A, Clapp TR, Herman JP, Sibhatu HM, Churchill ME, Silver WL, Kinnamon SC, Finger TE. Proc Natl Acad Sci USA. 2010;107:3210–3215. doi: 10.1073/pnas.0911934107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Ogura T, Krosnowski K, Zhang L, Bekkerman M, Lin W. PLoS ONE. 2010;5:e11924. doi: 10.1371/journal.pone.0011924. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ohmoto M, Matsumoto I, Yasuoka A, Yoshihara Y, Abe K. Mol Cell Neurosci. 2008;38:505–517. doi: 10.1016/j.mcn.2008.04.011. [DOI] [PubMed] [Google Scholar]
- 8.Gulbransen BD, Finger TE. J Neurocytol. 2005;34:117–122. doi: 10.1007/s11068-005-5051-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Matsumoto I, Ohmoto M, Narukawa M, Yoshihara Y, Abe K. Nat Neurosci. 2011;14:685–687. doi: 10.1038/nn.2820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Taruno A, Vingtdeux V, Ohmoto M, Ma Z, Dvoryanchikov G, Li A, Adrien L, Zhao H, Leung S, Abernethy M, Koppel J, Davies P, Civan MM, Chaudhari N, Matsumoto I, Hellekant G, Tordoff MG, Marambaud P, Foskett JK. Nature. 2013;495:223–226. doi: 10.1038/nature11906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Krasteva G, Canning BJ, Hartmann P, Veres TZ, Papadakis T, Muhlfeld C, Schliecker K, Tallini YN, Braun A, Hackstein H, Baal N, Weihe E, Schutz B, Kotlikoff M, Ibanez-Tallon I, Kummer W. Proc Natl Acad Sci USA. 2011;108:9478–9483. doi: 10.1073/pnas.1019418108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Deshpande DA, Wang WC, McIlmoyle EL, Robinett KS, Schillinger RM, An SS, Sham JS, Liggett SB. Nat Med. 2010;16:1299–1304. doi: 10.1038/nm.2237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Eberle JA, Richter P, Widmayer P, Chubanov V, Gudermann T, Breer H. Front Physiol. 2013;4:58. doi: 10.3389/fphys.2013.00058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Margolskee RF, Dyer J, Kokrashvili Z, Salmon KS, Ilegems E, Daly K, Maillet EL, Ninomiya Y, Mosinger B, Shirazi-Beechey SP. Proc Natl Acad Sci USA. 2007;104:15075–15080. doi: 10.1073/pnas.0706678104. [DOI] [PMC free article] [PubMed] [Google Scholar]