Abstract
Elevations in hepatic iron content occur with aging and physiological stressors, which may promote oxidative injury to the liver. Since dysregulation of the iron regulatory hormone, hepcidin, can cause iron accumulation, our goal was to characterize the regulation of hepcidin in young (6 mo) and old (24 mo) Fischer 344 rats exposed to environmental heat stress. Liver and blood samples were taken in the control condition and after heating. Hepcidin expression did not differ between young and old rats in the control condition, despite higher levels of hepatic iron and IL-6 mRNA in the latter. Following heat stress, pSTAT3 increased in both groups, but C/EBPα and hepcidin mRNA increased only in old rats. Despite this, serum iron decreased in both age groups 2h after heat stress, suggesting hepcidin-independent hypoferremia in the young rats. The differential regulation of hepcidin between young and old rats after hyperthermia may be due to the enhanced expression of C/EBPα protein in old rats. These data support the concept of “inflammaging” and suggest that repeated exposures to stressors may contribute to the development of anemia in older individuals.
Keywords: Iron, Hypoferremia, Inflammation, IL-6, STAT3
Introduction
Exposure to extreme environmental conditions such as heat stress can result in severe pathological consequences, especially in elderly populations. After heat waves, elderly individuals suffer morbidity and mortality, and damage to internal organs such as the liver (Argaud et al., 2007; Kew et al., 1970; Semenza et al., 1996). Additionally, in rodent models, heat stress is associated with enhanced liver injury and oxidative damage in old, compared to young rats (Hall et al., 2000; Zhang et al., 2003).
The liver plays a major role in physiologic iron regulation and continuously recycles iron from transferrin and senescent erythrocytes to the bone marrow for erythropoiesis. In rodent models, aging is associated with an increase in hepatic nonheme iron (Bloomer et al., 2008; Cook and Yu, 1998; Sohal et al., 1999), the iron stored in ferritin and hemosiderin (Torrance and Bothwell, 1980). Histological sections of livers from old rats demonstrate stainable iron in nonparenchymal cells and hepatocytes, indicating iron accumulation in this organ (Bloomer et al., 2008). Moreover, whole-body iron stores (as assessed by serum ferritin) are elevated with aging in humans (Casale et al., 1981; Cook et al., 1976). In addition to the increase in iron stores with aging, both nonheme iron and labile iron (the catalytically active form) are further increased by heat stress in aged Fischer 344 rats (Bloomer et al., 2008). Heat stress is also associated with exacerbation of oxidative injury to the liver (Zhang et al., 2003), which is due in part to the fluctuations in labile iron (Bloomer et al., 2008). Due to these deleterious alterations in iron homeostasis, we were interested in determining a physiological mechanism for the increase in nonheme iron with aging and following an environmental challenge such as heat stress.
One potential mechanism for the accumulation of hepatic iron after heat stress is a blockade of the constitutive iron export from the liver. In this study, we focused on the iron regulatory hormone, hepcidin, which blocks cellular iron efflux by causing degradation of the cellular iron export protein, ferroportin (Nemeth et al., 2004b; Rivera et al., 2005a). Hepcidin is synthesized predominately by the liver (Krause et al., 2000; Park et al., 2001; Pigeon et al., 2001), and its expression is increased in parallel with increases in hepatic iron content (Courselaud et al., 2002; Pigeon et al., 2001). Under physiological conditions, increases in transferrin saturation also appear to drive hepatic hepcidin expression, leading to inhibition of intestinal iron absorption through the binding of circulating hepcidin to ferroportin (on the basolateral side of enterocytes) and diminished efflux of recycled iron from macrophages (Gao et al., 2009; Nemeth et al., 2004b).
Hepcidin expression is also induced by inflammatory stressors such as turpentine (Nicolas et al., 2002), lipopolysaccharide (LPS; (Kemna et al., 2005; Pigeon et al., 2001)), and the cytokine, interleukin-6 (IL-6; (Nemeth et al., 2004a; Nemeth et al., 2003)). Since hepcidin is induced by IL-6, it is considered a Type II acute phase protein (Moshage, 1997; Nemeth et al., 2003). The induction of hepcidin during inflammation is adaptive since hepcidin causes tissue iron retention (Rivera et al., 2005a), leading to hypoferremia (Rivera et al., 2005b); this response limits the availability of iron to pathogens. However, the effects of chronically elevated levels of hepcidin on the gut and macrophages can lead to the anemia of inflammation (Weinstein et al., 2002). The enhancement in hepcidin transcription in response to IL-6 is dependent on the activation of signal transducer and activator of transcription -3 (STAT3) by phosphorylation of its tyrosine-705 residue (Pietrangelo et al., 2007; Sakamori et al., 2010; Verga Falzacappa et al., 2007; Wrighting and Andrews, 2006). The induction of hepcidin by inflammation and the concomitant lowering of plasma iron play a pivotal role in the anemia of inflammation, and these phenomena are dependent on activation of STAT3 (Sakamori et al., 2010).
In addition to STAT3, hepcidin transcription is affected by the Smad family of transcription factors, and CCAAT/enhancer binding protein α (C/EBPα) (Babitt et al., 2007; Courselaud et al., 2002). C/EBPα mediates hepatic hepcidin transcription in response to iron loading (Courselaud et al., 2002), and there is differential regulation of C/EBPα between young and old rats after partial hepatectomy (Timchenko et al., 1998). Interestingly, C/EBPα is necessary for induction of the Type II acute phase response (Burgess-Beusse and Darlington, 1998) since it is required for normal IL-6 signaling (Mackey and Darlington, 2004). Taken together, these findings suggest that C/EBPα may also have a role in the regulation of hepcidin in old rats after hyperthermia.
The changes in iron stores with aging and in response to heat stress (Bloomer et al., 2008) prompted us to evaluate the regulation of hepcidin in these animals. Currently, little is known about the effects of aging on hepcidin expression in the liver. The few studies that do exist on hepcidin and aging have evaluated either urinary hepcidin (Ferrucci et al., 2010) or plasma hepcidin concentrations (Lee et al., 2008) in older humans (ages 63-89), without including reference groups of young individuals. Thus, there is a need to evaluate hepcidin directly in the livers of young and old animals. It is also not known whether hepcidin expression is altered in response to a physiologically relevant stressor such as environmental heat stress. We have previously reported that old rats have increased iron stores in both hepatocytes and Kupffer cells (Bloomer et al., 2008). The latter are resident macrophages of the liver, and retention of iron in these cells is a hallmark of elevated hepcidin levels (Rivera et al., 2005a). Moreover, aging is associated with a pro-inflammatory milieu, which includes increased plasma concentrations of IL-6 (Myśliwska et al., 1998). Previous studies from our laboratory have shown more severe hepatic inflammation in old rats after heat stress compared to their younger counterparts (Hall et al., 2000). Based on these observations, we first hypothesized that the hepatic iron deposition observed in old rats was due to augmented expression of hepcidin in the nonstressed state. Because heat stress elicited transient accumulation of hepatic nonheme iron in old, but not young rats (Bloomer et al., 2008), we hypothesized that heat stress would induce hepcidin expression further in old rats.
In this investigation, we report that old rats upregulate hepcidin mRNA after environmental heat stress, while young rats do not, suggesting an exaggerated acute phase response to hyperthermia in old rats. While both groups experienced hypoferremia after heat stress, only old rats induced hepcidin mRNA. This induction of hepcidin mRNA was associated with activation of STAT3 and augmented levels of C/EBPα protein, which are both positive regulators of hepcidin.
Materials and methods
Animal protocols
All animal protocols were approved by the University of Iowa Institutional Animal Care and Use Committee (ACURF #0606117). Young (6 month; ~ 360 g) and old (24 month; ~ 437 g) male Fischer 344 rats were obtained from the National Institute on Aging and housed in polyethylene cages (46 × 31 × 23 cm) with SoftZorb enrichment blend bedding (Nepco, Warrensburg, NY). Animal rooms were maintained at 24+/- 2 °C with a relative humidity of 50-55%. Food and water were provided ad libitum, and rats were housed in individual cages. The animal facility was on a 12 h light-dark cycle (0600 – 1800 hrs). To minimize duress, animals were handled daily by the investigators and familiarized to the equipment used for the heat stress experiments described below.
Animals were exposed to a two-heat stress protocol; the rationale for this protocol and the use of rodents as an experimental model have been described in detail elsewhere (Bloomer et al., 2008; Haak et al., 2009; Hubbard et al., 1976). Briefly, two bouts of heat stress were utilized in order to mimic conditions that elderly humans would experience with repeated environmental challenge (e.g. during a heat wave). Initial experiments in the laboratory (Hall et al., 2000) established that the two-heat stress protocol resulted in exaggerated inflammation and oxidative stress in the livers of old, compared to young rats, whereas a single heating did not result in cellular dysfunction. While a preconditioning effect of the first heating cannot be ruled out, the two-heat stress protocol was utilized here to more accurately model what humans would experience during multiple exposures to hyperthermia. Rats were exposed to two bouts of heat stress separated by 24 hours; the heating bout began at 1000 hrs, which was 4 h after the beginning of the light cycle. During each heat stress, core temperature (Tc) was elevated from ~37°C to 41°C degrees over a period of one hour. Tc was then maintained at 41°C for an additional 30 minutes thereafter. Tc was monitored continuously with a colonic thermistor probe inserted 6-7 cm into the colon, to which animals were familiarized on at least two occasions before the first heat stress. To increase Tc, a heat lamp was utilized; adjusting the height of the lamp and turning it on and off ensured a gradual and consistent increase in Tc at a rate of ~0.06°C/min. After the first bout of heat stress, rats were returned to their cages and allowed to cool passively. Approximately 22.5 hours after the first heat stress, animals were exposed to a second, identical heat stress. In each heating bout, animals were conscious and unrestrained. This experimental model does not cause heat stroke, but does elicit symptoms that have been reported in the clinical literature such as liver injury (Hall et al., 2000; Kew et al., 1970). Liver tissue was harvested either immediately after (0 h), or at 2 and 24 hours after the second heat stress in separate groups of rats. These time points were chosen to coincide with previous studies showing early increases in oxidative stress (Zhang et al., 2003) and hepatic iron (Bloomer et al., 2008), followed by a return of these parameters to nonstressed conditions (24 h timepoint). Redox environment, oxidative injury, histological inflammation, and subcellular damage in this model have been characterized extensively in previous studies (Bloomer et al., 2008; Haak et al., 2009; Hall et al., 2000; Oberley et al., 2008; Zhang et al., 2004; Zhang et al., 2003). At the indicated times, animals were given an overdose of pentobarbital sodium, and the livers were removed quickly and rinsed in phosphate buffered saline (PBS), then snap-frozen in liquid nitrogen. Blood samples were taken by cardiac puncture using a heparinized 18-gauge needle. Samples were centrifuged and plasma was snap-frozen in liquid nitrogen for later analysis. Nonheated animals served as controls and underwent sham heating protocols at similar times as the heated animals. Liver and serum samples were from animals used in a previous study (Bloomer et al., 2008).
Hepatic nonheme iron content
Frozen liver tissue was assayed for hepatic nonheme iron content (HIC) spectrophotometrically according to the method of Torrance and Bothwell (Torrance and Bothwell, 1980), as described previously (Bloomer et al., 2008). Iron values were originally reported in a previous study (Bloomer et al., 2008) and are repeated in Table 1 to assist with interpretation of the results.
TABLE 1.
Hepatic iron contents (μg/g dry weight)
| Con | 0 h | 2 h | 24 h | |
|---|---|---|---|---|
| Young | 392 ± 14.5 | 455 ± 33.8 | 444 ± 39.9 | 422 ± 25.4 |
| Old | 845 ± 31.4* | 1152 ± 55.7*† | 1049 ± 106* | 983 ± 90.3* |
Notes: Numbers indicate time in hours after heat stress; Con=nonheated controls. Data are expressed as means +/- SEM; n=7-9 animals per group.
Significant difference between age groups at a given time point;
significant effect of heat stress within an age group.
Real time quantitative PCR
Quantitation of gene expression was performed using Sybr Green Real-Time PCR reagents (Applied Biosystems, Foster City, CA) and the ABI Prism 7700 Sequence Detection System. Total RNA was isolated from liver samples by the Trizol method (Invitrogen Life Science Technologies, Carlsbad, CA) and quantified spectrophotometrically. RNA was reverse transcribed to cDNA using Super Script First-Strand Synthesis System for RT-PCR (Invitrogen, # 11904-018). The real time reaction mixture contained 40 ng cDNA, primers (hepcidin, 300 nM; 18S, 30 nM), and 1X Sybr Green (Applied Biosystems, #4364344). Forty cycles of PCR were utilized. The primers used for rat hepcidin PCR were: sense: 5’-TGTCTCCTGCTTCTCCTCCT-3’; antisense: 5’-GTTGGTGTCTCGCTTCCTTC-3’. Each sample was also assayed for 18S expression using TaqMan 18S ribosomal RNA control reagents (Applied Biosystems, Foster City, CA) and expression of the gene of interest was normalized to 18S expression. To quantitate target gene mRNA expression, the comparative Ct method was utilized and results are expressed as 2-ΔΔCt as described previously (Brown et al., 2007). Nonheated animals in each age group served as the reference control and samples were run in triplicate on each plate.
Hepatic IL-6 mRNA expression
Total liver RNA was isolated with the RNeasy midi kit from Qiagen. Approximately 200 mg of liver tissue was used for homogenization, and the purity of the resulting RNA was confirmed by the 260-280 nm ratio using a spectrophotometer. The one step RT-PCR kit from Qiagen was utilized, to which 2 ug RNA, and the appropriate primers (IL-6: 0.2uM, GAPDH: 0.06 uM) were added. The primer sequences utilized were the following: IL-6 sense: 5’-GCCCTTCAGGAACAGCTATG-3’; antisense: 5’-CATTGGAAGTTGGGGTAGGA-3’; GAPDH sense: 5’-TGTCAACGGATTTGGCCGTATTGGC-3’; antisense: 5’GAAGACGCCAGTAGACTCCACGAC-3’. The cDNA products were separated on 2% agarose gels, and bands were quantified using the GelPro system. The densities of IL-6 bands were normalized to GAPDH, and results are expressed as this ratio.
Plasma measures
Plasma IL-6 was determined using a commercially available kit (Thermo Scientific/Pierce, Rockford, IL), according to the manufacturer’s instructions. Serum iron was analyzed by the University of Iowa Clinical Pathology Laboratory using standard techniques based on the ferrozine assay.
Immunoblotting
Liver samples were homogenized in RIPA buffer (50 mM Tris, 150 mM NaCl, 0.25% sodium deoxycholate, 1% triton-X, 1 mM EDTA, 1 mM Na3VO4), with protease/phosphatase inhibitor cocktail (HALT #78440, Thermo Scientific, Rockford, IL). Protein samples (50 μg) were separated on freshly poured 12% tris-glycine gels, then transferred to nitrocellulose membranes. Membranes were incubated overnight at 4°C with primary antibodies, washed, then probed with secondary antibodies for one hour. Anti-C/EBPα (Santa Cruz Biotechnology, Santa Cruz, CA, sc-365318) was used at a 1:500 dilution with anti-mouse secondary antibody used at a 1:4000 dilution (Santa Cruz, sc-2031). Anti-phospho-Stat-3-Tyr705 (Cell Signaling Technologies, Beverly, MA # 9145) was used at a 1:2000 dilution with anti-rabbit secondary (Santa Cruz, sc-2030) antibody used at a 1:4000 dilution. Anti-STAT3 (Cell Signaling Technologies # 9132) was used at a 1:2000 dilution with anti-rabbit secondary (sc-2030) also at a 1:4000 dilution. Anti-HFE (Santa Cruz, sc-18806) was used at a 1:200 dilution with anti-goat secondary antibody (Santa Cruz, sc-2020) at a 1:1000 dilution. Anti-TFR2 (Santa Cruz, sc-48747) was used at a 1:500 dilution with anti-rabbit secondary antibody (Santa Cruz, sc-2030) at a 1:4000 dilution. Super Signal West Pico Chemiluminescent Substrate (Thermo Scientific/Pierce, Rockford, IL) was utilized to develop membranes. Protein bands were visualized using the ChemiDoc system (Bio Rad, Hercules, CA) and densitometry was carried out using the Image Lab program (Bio Rad, Hercules, CA).
Protein expression of pSTAT3 was normalized to total STAT3 levels and the results are expressed as this ratio. The term, STAT3 activation, is defined as the ratio of phosphorylated STAT3 to total STAT3 protein. Exposure times varied significantly between pSTAT3 (30 min) and STAT3 (30-90 sec). In initial experiments, we observed that both beta actin and GAPDH increased significantly with age; thus, they were not valid loading controls in this model. Moreover, there was a trend for an increase in GAPDH in the old group immediately (0h) following heat stress (data not shown). Therefore, after exposure for the protein of interest, all other membranes were stained with Ponceau S staining solution (0.2% Ponceau S w/v in 5% acetic acid) to confirm equal loading and transfer, and protein expression was normalized to the density of a consistent band on the Ponceau-stained membrane (~ 40kDa). Results were further normalized to the young, nonheated group, which was given a value of 1. Each sample was run in duplicate.
Statistical analysis
All data for a particular parameter were analyzed first by a 2-factor ANOVA. Where appropriate, Tukey’s post-hoc test was used to determine significant effects of heat stress within an age group. Additionally, when initially indicated by the ANOVA, T-tests (for independent samples) were used to determine significant differences between young and old rats within a particular time point. A p-value of less than 0.05 was considered statistically significant.
Results
Core temperatures during heat stress
Core temperatures were monitored continuously and recorded every five minutes in young and old rats under nonheated conditions and during hyperthermia. Each age group experienced an identical heating rate during each bout of hyperthermia (Fig. 1).
Figure 1.
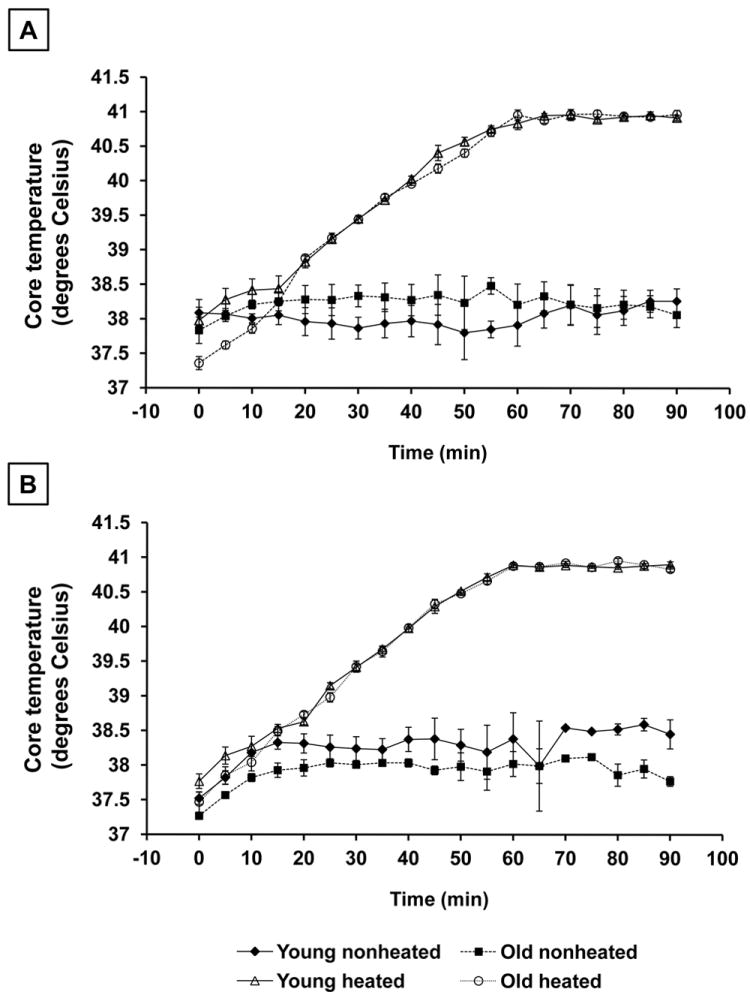
Core temperature (Tc) recordings of young and old rats during nonheated conditions and during each heating bout. Tc was monitored continuously, and temperatures were recorded every 5 minutes during the heating bout. A: Tc during the first heating bout. B: Tc during the second heating bout. Results are expressed as means +/- SEM, (n=3 young and old animals in the nonheated groups; n=6-7 young and old animals in the heated groups).
Hepatic iron content and hepcidin expression
In the control condition, there was no difference in hepatic hepcidin expression between young and old rats despite the fact that hepatic iron concentrations (HIC) were more than two-fold higher in the old rats (Table 1, Fig. 2). A further increase in hepatic iron in the old rats immediately (0 h) following heat stress preceded a doubling of hepatic hepcidin transcript at 2 h after stress. The increase in hepcidin expression in the old rats was transient and returned to control levels at 24 h after hyperthermia. Young rats did not experience significant changes in hepcidin expression or hepatic iron concentration at any time point after heat stress (Fig. 2).
Figure 2.
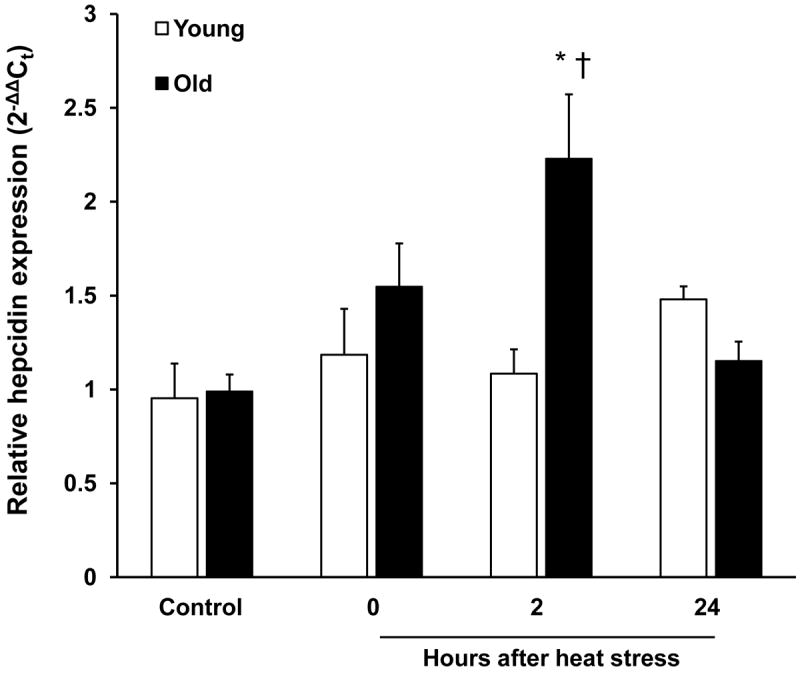
Environmental heat stress is associated with induction of hepatic hepcidin in old rats. Real-time quantitative PCR analysis of hepatic hepcidin mRNA expression in young and old rats under control conditions and at the indicated times after heat stress. Results were normalized to 18S expression using the 2-ΔΔCt method. Results are expressed as means +SEM, n = 5-9 young and old animals under control conditions and at each time point after heat stress. * Significant difference between young and old animals within a time point. † Significant effect of heat stress within an age group.
Plasma iron
Plasma iron concentrations were significantly greater in old rats in the control condition. Two hours after heat stress, plasma iron concentrations decreased in both groups, and the old rats tended to remain hypoferremic 24 hours after heat stress (p=0.09). In contrast, plasma iron rebounded in the young rats at 24 hours after heat stress and was increased compared to control concentrations (Fig. 3).
Figure 3.
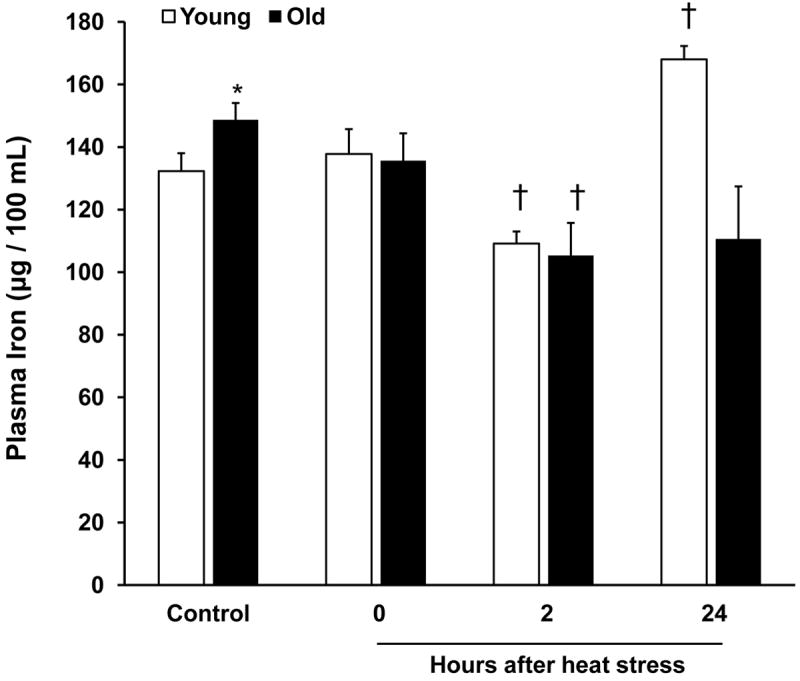
Hypoferremia in young and old rats after heat stress. Plasma iron concentrations were assayed in young and old rats under control conditions and at the indicated times after heat stress. Results are presented as means + SEM, n = 5-10 young and old animals under control conditions and at each time point after heat stress (except for the plasma iron concentration of old rats at 24 h, where n=3). * Significant difference between young and old animals within a time point. † Significant effect of heat stress within an age group.
IL-6 expression and STAT3 activation
To determine a potential mechanism for the induction of hepcidin in old rats, we analyzed hepatic IL-6 mRNA expression via semi-quantitative RT-PCR. In the control condition, IL-6 was higher in old, compared to young rats. Immediately (0h) after heat stress, IL-6 transcripts increased in both young and old rats. While IL-6 expression declined to control values in the old rats at 2 h, it remained elevated in the young rats. At 24h after heat stress, IL-6 mRNA levels declined to nonheated values in young rats, but in old rats, IL-6 expression was lower than control values (Fig. 4A). Because there were differences in IL-6 expression between young and old rats in the control condition, we determined the fold induction of IL-6 after heat stress. Fold induction of IL-6 was similar between age groups at 0h; however, at 2 and 24 h after heat stress fold induction of IL-6 was significantly lower in old, compared to young rats (Fig 4B).
Figure 4.
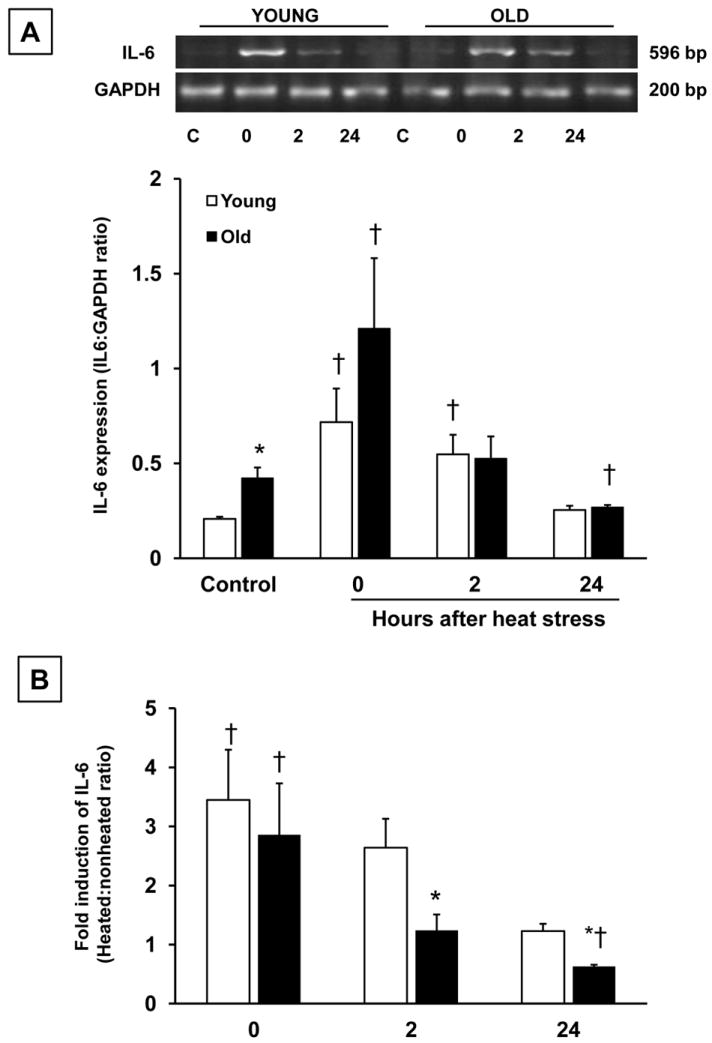
Heat stress increases hepatic expression of IL-6 mRNA in young and old rats. Semi-quantitative RT-PCR analysis of IL-6 mRNA expression in young and old rats under nonheated conditions, and at the indicated times after heat stress. A. Relative IL-6 mRNA expression normalized to GAPDH. B. Fold induction of IL-6 after heat stress in young and old rats at 0, 2 and 24 h after heating. IL-6 to GAPDH ratios from each heated time point were normalized to nonheated values within each age group. Results are expressed as means +SEM, n = 4-5 young and old animals under control conditions and at each time point after heat stress. * Significant difference between young and old animals within a time point. † Significant effect of heat stress within an age group.
Plasma IL-6 levels were below the limit of detection of the assay (31 pg/mL) for both young and old rats in the control condition. Immediately after heat stress (0 h), plasma IL-6 increased to 46 pg/mL (+/- 5.8 pg/mL, SEM) in the old animals (p=0.061). Plasma IL-6 returned to control levels in the old rats at 2 and 24 h after heating. In the young rats, plasma IL-6 concentrations remained below the lower limit of detection at all time points (data not shown).
Under control conditions, pSTAT3 was barely detectable by immunoblotting in young and old rats. Immediately (0 h) after heat stress, both age groups demonstrated an increase in the phosphorylation of STAT3. In both young and old rats, pSTAT3 levels declined at 2 h after heat stress and became undetectable by 24 h following heating (Fig 5).
Figure 5.
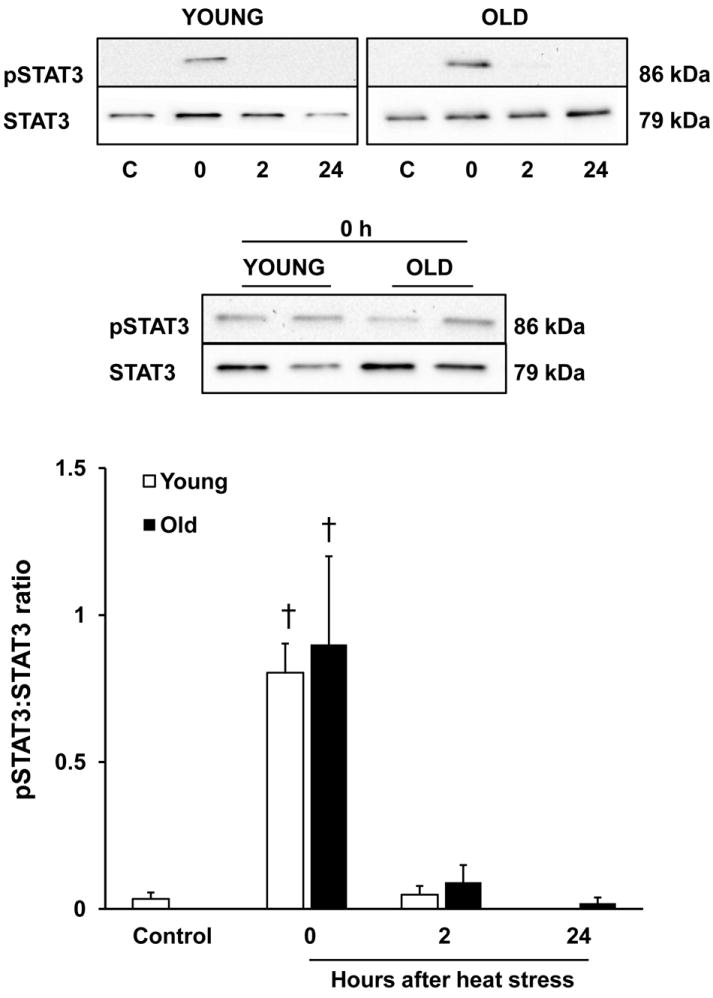
Hyperthermia elicits STAT3 activation in young and old rats. Immunoblot analysis of phosphorylated STAT-3 (Tyr-705) in the livers of young and old rats under control conditions and at the indicated times after a two-heat stress protocol. Top panel: representative immunoblots of pSTAT3 and STAT3 for each age group. Middle panel: samples from young and old rats at 0 h compared on the same blot. Bottom panel: results from quantitation of densitometry values, normalized as the ratio of pSTAT3 to STAT3. Results are presented as means + SEM, n = 7-9 young and old animals under control conditions and at each time point after heat stress. † Significant effect of heat stress within an age group.
C/EBPa expression
Hepatic iron is increased in old rats (Bloomer et al., 2008; Cook and Yu, 1998; Sohal et al., 1999), and because C/EBPα is induced by iron, and further mediates the transcription of hepcidin (Courselaud et al., 2002), we were interested in characterizing protein levels of C/EBPα in this model. Steady-state levels of C/EBPα were ~ 1.5-fold higher in old rats compared to young in the nonheated condition. After heat stress, there was a rapid elevation in C/EBPα in old rats only, which became significant at 2 h. This approximate 2-fold elevation in C/EBPα was sustained for at least 24 h after heat stress (Fig. 6).
Figure 6.
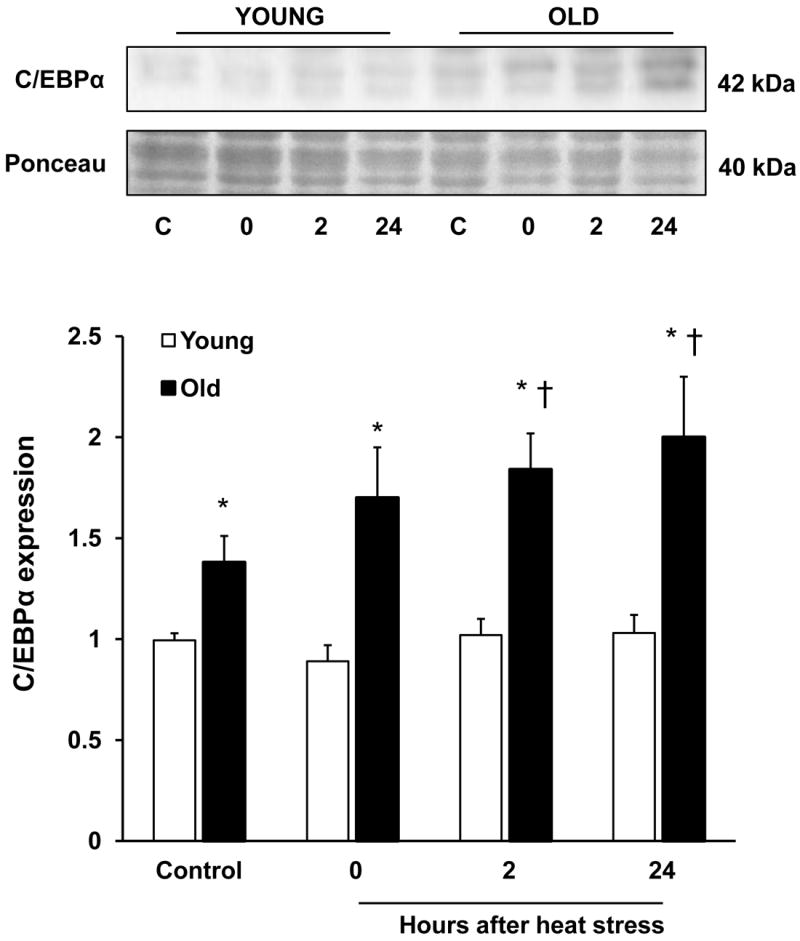
Aging and heat stress are associated with increased C/EBPα protein expression. Top panel: representative immunoblots of the transcription factor, C/EBPα (top) and Ponceau stain (bottom) in the livers of young and old rats. Bottom panel: quantitation of C/EBPα expression, normalized to the Ponceau stain. Results are presented as means + SE, n = 7-9 young and old animals under control conditions and at each time point after heat stress. *Significant difference between young and old animals within a time point. † Significant effect of heat stress within an age group.
Expression of HFE and TFR2 proteins
Hepcidin is also controlled by a signaling cascade initiated by the interaction of the hemochromatosis protein, Hfe, and transferrin receptor-2 (TFR2; (Gao et al., 2010; Gao et al., 2009; Goswami and Andrews, 2006; Kawabata et al., 2005; Wallace et al., 2007). Moreover, TFR2 can participate in cellular iron uptake (Waheed et al., 2008). Thus, we considered the possibility that changes in Hfe and TFR2 protein levels affected the hyperthermia-induced increase in hepcidin expression in our model. Heat stress did not affect protein levels of either Hfe or TFR2 in either age group (Fig. S1); however, aging was associated with decreased expression of Hfe protein in the control condition (Fig S1A). There was no difference in TFR2 expression between young and old animals (Fig S1B).
Discussion
Due to the deleterious role of iron in age-related pathologies (Bloomer et al., 2008; Xu et al., 2010), as well as the increased prevalence of anemia with aging (Makipour et al., 2008), it is crucial to develop a better understanding of the changes in iron regulation with aging in vivo. To our knowledge, this is the first study to evaluate age-related changes in hepatic hepcidin mRNA expression in a whole animal model. Moreover, we show an elevation in hepcidin mRNA with a physiologically relevant stressor in old rats, which was associated with activation of STAT3 and augmented expression of C/EBPα. The induction of hepcidin in vivo after environmental heat stress is a novel observation and confirms previous studies showing that hepcidin is upregulated in response to cellular stress in vitro (Oliveira et al., 2009; Vecchi et al., 2009).
There were several unique and unexpected findings in this investigation. First, despite higher liver nonheme iron content, and increased expression of IL-6 mRNA and C/EBPα protein in old rats in the control condition, hepcidin mRNA levels were similar between young and old rats. We have previously shown that storage iron is increased in both the hepatocytes and Kupffer cells in the livers of old rats under control conditions (Bloomer et al., 2008). Since iron deposition in macrophages is a classic feature of hepcidin action (Rivera et al., 2005a), we initially hypothesized that hepcidin mRNA would be elevated in old rats. The reason that this was not observed is unclear. It is possible that there is a gradual desensitization to iron, IL-6 and/or C/EBPα over time. However, we cannot exclude the possibility that there are increases in hepcidin at the protein level with aging. To our knowledge, a validated, commercially available antibody for rat hepcidin is not currently available. Furthermore, concerns have been raised regarding the specificity of immunoassays for the biologically active form of hepcidin protein (Macdougall et al., 2010).
Despite the lack of upregulation of hepcidin mRNA under control conditions, we have shown that old rats induce hepcidin mRNA after heat stress. In pursuing a mechanism for this observation, we evaluated several cellular pathways that have been shown to regulate hepcidin expression. For example, the interaction of transferrin receptor-2 (TFR2) and Hfe regulates hepcidin expression (Gao et al., 2010; Gao et al., 2009; Goswami and Andrews, 2006; Kawabata et al., 2005; Wallace et al., 2007). On the plasma membrane, Hfe associates with transferrin receptor-1 (TFR1); the binding of TFR1 to plasma transferrin causes Hfe to dissociate from TFR1 and subsequently bind to TFR2 (Goswami and Andrews, 2006). The interaction of Hfe and TFR2 then activates a signaling pathway that results in increased expression of hepcidin. In this study, we evaluated protein levels of both TFR2 and Hfe and found that neither were altered by heat stress in either age group. However, we found a significant reduction in Hfe expression with aging. This difference might be attributable to the increased hepatic iron content in old rats, since Hfe protein expression is lower in chronically iron-treated rats compared to controls (Bloomer and Brown, unpublished observations).
Our results showing an increase in hepcidin mRNA and C/EBPα after heat stress demonstrate that old rats respond appropriately to an acute phase stimulus by inducing hepcidin. The increase in C/EBPα in old, but not young rats after heat stress parallels previous findings in a partial hepatectomy model (Timchenko et al., 1998). Since STAT3 activation also increased after heat stress in old rats, it is likely that the combination of STAT3 signaling plus augmented levels of C/EBPα led to enhanced hepcidin expression, whereas it did not in young rats. Indeed, the hepcidin promoter region contains both C/EBP and STAT3 binding sites, with one region containing overlap for these transcription factors (Courselaud et al., 2002). The lack of induction of hepcidin in young rats may be attributed to the lack of an increase in hepatic iron, and the absence of an increase in C/EBPα.
In both young and old rats, hepatic STAT3 became phosphorylated after heat stress, which coincided with an increase in IL-6 mRNA. In the young rats, STAT3 activation was transient, despite elevated expression of IL-6 at 2 h, which may suggest a rapid adaptation of the IL-6 receptor and/or STAT3 to IL-6. Given that plasma IL-6 concentrations were below the limit of detection in young animals, it is possible that IL-6 levels increase locally within the liver, mediating an autocrine or paracrine response. Although the presumption that local IL-6 levels increase in the livers of young animals requires confirmation, the finding that the young animals had higher fold induction of IL-6 at 2 and 24 hours and that they do not experience severe liver damage after heating (Hall et al., 2000), leads us to speculate that IL-6 has a protective role in this model. IL-6 is elevated in other models of hyperthermia (Leon et al., 2006; Welc et al., 2012), and its induction protects against liver injury (Camargo et al., 1997; Hong et al., 2002; Klein et al., 2005). In old rats, IL-6 mRNA expression was higher than young rats in the nonheated condition, which is consistent with the theory of “inflammaging” (Franceschi et al., 2007; Myśliwska et al., 1998). While old rats displayed an elevation in hepatic IL-6 mRNA after heating, this response quickly diminished, and IL-6 mRNA expression fell to below control values at 24 h after heating. The reason for this decline is unclear, but may have implications for hepatoprotection in old rats after heat stress.
In this study, we have shown that environmental heat stress is associated with rapid hypoferremia in both age groups, despite the lack of induction of hepcidin in the young rats. An increase in hepcidin typically leads to hypoferremia by causing degradation of ferroportin in macrophages and enterocytes (Nemeth et al., 2004b). In the old rats, hepcidin mRNA was upregulated at two hours after heat stress, and animals were already hypoferremic at this timepoint. While it is unclear whether the upregulation in hepcidin mRNA at 2 h can account for the hypoferremia at the same time point, the upregulation in hepcidin mRNA may explain the trend toward hypoferremia at 24 h in the old rats. In the young rats, we observed hypoferremia after heating with no corresponding change in hepcidin mRNA levels. Thus, our data suggest that the early changes in plasma iron in this model are independent of changes in hepcidin expression, which is consistent with other studies (Constante et al., 2006; Laftah et al., 2006; Layoun et al., 2012).
The development of hypoferremia in young rats was unexpected, considering that they do not experience a significant increase in hepatic iron after heat stress. It is possible that serum iron is directed to other iron-storing organs such as the spleen (Layoun et al., 2012) or bone marrow (Cook et al., 1973) following hyperthermia in the young animals. Mobilization of iron from extrahepatic storage sites back into the circulation may account for the rebound in plasma iron observed in young rats at 24 h, given that hepatic iron content in these animals were similar to control conditions. These changes in plasma iron warrant further inquiry into the physiologic destinations of iron after a stressor in both young and old rats.
Overall, we have demonstrated that environmental heat stress stimulated hepcidin mRNA expression in old rats, which was associated with increased levels of C/EBPα protein. Based on the time course in which the increase in hepatic iron preceded the subsequent induction of hepcidin mRNA after heat stress in the old rats, our results suggest that the induction of hepcidin is a consequence of the transient accumulation of hepatic iron, rather than a causal factor. The dissociation between hepcidin mRNA levels and plasma iron concentrations invite future studies to determine the mechanism of hypoferremia in this model. Nevertheless, our results clearly show that heat stress was associated with an exaggerated acute phase response in old rats, which included a lowering of plasma iron concentrations. These observations suggest that repeated exposures to stressors may contribute to the development of anemia in aged individuals, and highlight the importance of further investigations into the responses of elderly populations to environmental stressors.
Supplementary Material
Supplemental Figure: Hfe and TFR2 expression in young and old rats. Immunoblot analysis of Hfe (Panel A) and TFR2 (Panel B) in the livers of young and old rats under control conditions and at the indicated times after a two-heat stress protocol. Results are presented as means + SE, n = 7-9 young and old animals under control conditions and at each time point after heat stress. *Significant difference between young and old animals within a time point.
Acknowledgments
The authors thank Kimberly Broadhurst, Elizabeth Cramer, Mindy Drake, Frederick Liaboe, M. Meleah Mathahs, and Stephanie Shaw Ross for expert technical assistance.
Footnotes
Conflicts of interest
The authors declare no financial conflicts of interest.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Argaud L, Ferry T, Le Q, Marfisi A, Ciorba D, Achache P, Ducluzeau R, Robert D. Short- and long-term outcomes of heatstroke following the 2003 heat wave in lyon, france. Arch Int Med. 2007;167(20):2177–2183. doi: 10.1001/archinte.167.20.ioi70147. [DOI] [PubMed] [Google Scholar]
- Babitt JL, Huang FW, Xia Y, Sidis Y, Andrews NC, Lin HY. Modulation of bone morphogenetic protein signaling in vivo regulates systemic iron balance. J Clin Invest. 2007;117(7):1933–1939. doi: 10.1172/JCI31342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bloomer SA, Brown KE, Buettner GR, Kregel KC. Dysregulation of hepatic iron with aging: Implications for heat stress-induced oxidative liver injury. Am J Physiol - Reg Int Comp Physiol. 2008;294(4):R1165–R1174. doi: 10.1152/ajpregu.00719.2007. [DOI] [PubMed] [Google Scholar]
- Brown KE, Broadhurst KA, Mathahs MM, Weydert J. Differential expression of stress-inducible proteins in chronic hepatic iron overload. Toxicol Appl Pharm. 2007;223(2):180–186. doi: 10.1016/j.taap.2007.05.011. [DOI] [PubMed] [Google Scholar]
- Burgess-Beusse BL, Darlington GJ. C/ebpα is critical for the neonatal acute-phase response to inflammation. Mol Cell Biol. 1998;18(12):7269–7277. doi: 10.1128/mcb.18.12.7269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Camargo CA, Madden JF, Gao W, Selvan RS, Clavien P. Interleukin-6 protects liver against warm ischemia/reperfusion injury and promotes hepatocyte proliferation in the rodent. Hepatology. 1997;26(6):1513–1520. doi: 10.1002/hep.510260619. [DOI] [PubMed] [Google Scholar]
- Casale G, Bonora C, Migliavacca A, Zurita IE, de Nicola P. Serum ferritin and ageing. Age Ageing. 1981;10(2):119–122. doi: 10.1093/ageing/10.2.119. [DOI] [PubMed] [Google Scholar]
- Constante M, Jiang W, Wang D, Raymond V-A, Bilodeau M, Santos MM. Distinct requirements for hfe in basal and induced hepcidin levels in iron overload and inflammation. Am J Physiol - Gastrointest Liver Physiol. 2006;291(2):G229–G237. doi: 10.1152/ajpgi.00092.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cook CI, Yu BP. Iron accumulation in aging: Modulation by dietary restriction. Mech Age Dev. 1998;102:1–13. doi: 10.1016/s0047-6374(98)00005-0. [DOI] [PubMed] [Google Scholar]
- Cook J, Finch C, Smith N. Evaluation of the iron status of a population. Blood. 1976;48(3):449–455. [PubMed] [Google Scholar]
- Cook J, Hershko C, Finch C. Storage iron kinetics v: Iron exchange in the rat. Br J Haematol. 1973;25(695-706) doi: 10.1111/j.1365-2141.1973.tb01782.x. [DOI] [PubMed] [Google Scholar]
- Courselaud B, Pigeon C, Inoue Y, Inoue J, Gonzalez FJ, Leroyer P, Gilot D, Boudjema K, Guguen-Guillouzo C, Brissot P, Loréal O, Ilyin G. C/ebpα regulates hepatic transcription of hepcidin, an antimicrobial peptide and regulator of iron metabolism. J Biol Chem. 2002;277(43):41163–41170. doi: 10.1074/jbc.M202653200. [DOI] [PubMed] [Google Scholar]
- Ferrucci L, Semba RD, Guralnik JM, Ershler WB, Bandinelli S, Patel KV, Sun K, Woodman RC, Andrews NC, Cotter RJ, Ganz T, Nemeth E, Longo DL. Proinflammatory state, hepcidin, and anemia in older persons. Blood. 2010;115(18):3810–3816. doi: 10.1182/blood-2009-02-201087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Franceschi C, Capri M, Monti D, Giunta S, Olivieri F, Sevini F, Panourgia MP, Invidia L, Celani L, Scurti M, Cevenini E, Castellani GC, Salvioli S. Inflammaging and anti-inflammaging: A systemic perspective on aging and longevity emerged from studies in humans. Mech Age Dev. 2007;128(1):92–105. doi: 10.1016/j.mad.2006.11.016. [DOI] [PubMed] [Google Scholar]
- Gao J, Chen J, De Domenico I, Koeller DM, Harding CO, Fleming RE, Koeberl DD, Enns CA. Hepatocyte-targeted hfe and tfr2 control hepcidin expression in mice. Blood. 2010;115(16):3374–3381. doi: 10.1182/blood-2009-09-245209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gao J, Chen J, Kramer M, Tsukamoto H, Zhang A-S, Enns CA. Interaction of the hereditary hemochromatosis protein hfe with transferrin receptor 2 is required for transferrin-induced hepcidin expression. Cell Metab. 2009;9(3):217–227. doi: 10.1016/j.cmet.2009.01.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goswami T, Andrews NC. Hereditary hemochromatosis protein, hfe, interaction with transferrin receptor 2 suggests a molecular mechanism for mammalian iron sensing. J Biol Chem. 2006;281(39):28494–28498. doi: 10.1074/jbc.C600197200. [DOI] [PubMed] [Google Scholar]
- Haak JL, Buettner GR, Spitz DR, Kregel KC. Aging augments mitochondrial susceptibility to heat stress. Am J Physiol - Reg Int Comp Physiol. 2009;296(3):R812–R820. doi: 10.1152/ajpregu.90708.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hall DM, Xu L, Drake VJ, Oberley LW, Oberley TD, Moseley PL, Kregel KC. Aging reduces adaptive capacity and stress protein expression in the liver after heat stress. J Appl Physiol. 2000;89(2):749–759. doi: 10.1152/jappl.2000.89.2.749. [DOI] [PubMed] [Google Scholar]
- Hong F, Kim W, Tian Z, Jaruga B, Ishac E, Shen X, Gao B. Elevated interleukin-6 during ethanol consumption acts as a potential endogenous protective cytokine against ethanol-induced apoptosis in the liver: Involvement of induction of bcl-2 and bcl-x(l) proteins. Oncogene. 2002;21(1):32–43. doi: 10.1038/sj.onc.1205016. [DOI] [PubMed] [Google Scholar]
- Hubbard R, Matthew W, Linduska J, Curtis F, Bowers W, Leav I, Mager M. The laboratory rat as a model for hyperthermic syndromes in humans. Am J Physiol. 1976;231(4):1119–1123. doi: 10.1152/ajplegacy.1976.231.4.1119. [DOI] [PubMed] [Google Scholar]
- Kawabata H, Fleming RE, Gui D, Moon SY, Saitoh T, O’Kelly J, Umehara Y, Wano Y, Said JW, Koeffler HP. Expression of hepcidin is down-regulated in tfr2 mutant mice manifesting a phenotype of hereditary hemochromatosis. Blood. 2005;105(1):376–381. doi: 10.1182/blood-2004-04-1416. [DOI] [PubMed] [Google Scholar]
- Kemna E, Pickkers P, Nemeth E, van der Hoeven H, Swinkels D. Time-course analysis of hepcidin, serum iron, and plasma cytokine levels in humans injected with LPS. Blood. 2005;106(5):1864–1866. doi: 10.1182/blood-2005-03-1159. [DOI] [PubMed] [Google Scholar]
- Kew M, Bersohn I, Seftel H, Kent G. Liver damage in heatstroke. Am J Med. 1970;49:192–202. doi: 10.1016/s0002-9343(70)80075-4. [DOI] [PubMed] [Google Scholar]
- Klein C, Wustefeld T, Assmus U, Roskams T, Rose-John S, ller M, Manns MP, Ernst M, Trautwein C. The il-6–gp130–stat3 pathway in hepatocytes triggers liver protection in t cell–mediated liver injury. J Clin Invest. 2005;115(4):860–869. doi: 10.1172/JCI200523640. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krause A, Neitz S, Mägert H-J, Schulz A, Forssmann W-G, Schulz-Knappe P, Adermann K. Leap-1, a novel highly disulfide-bonded human peptide, exhibits antimicrobial activity. FEBS Lett. 2000;480(2–3):147–150. doi: 10.1016/s0014-5793(00)01920-7. [DOI] [PubMed] [Google Scholar]
- Laftah AH, Sharma N, Brookes MJ, McKie AT, Simpson RJ, Iqbal TH, Tselepis C. Tumour necrosis factor α causes hypoferraemia and reduced intestinal iron absorption in mice. Biochem J. 2006;397(1):61–67. doi: 10.1042/bj20060215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Layoun A, Huang H, Calvé A, Santos MM. Toll-like receptor signal adaptor protein myd88 is required for sustained endotoxin-induced acute hypoferremic response in mice. Am J Pathol. 2012;180(6):2340–2350. doi: 10.1016/j.ajpath.2012.01.046. [DOI] [PubMed] [Google Scholar]
- Lee P, Gelbart T, Waalen J, Beutler E. The anemia of ageing is not associated with increased plasma hepcidin levels. Blood Cell Mol Dis. 2008;41(3):252–254. doi: 10.1016/j.bcmd.2008.06.005. [DOI] [PubMed] [Google Scholar]
- Leon LR, Blaha MD, DuBose DA. Time course of cytokine, corticosterone, and tissue injury responses in mice during heat strain recovery. Journal of Applied Physiology. 2006;100(4):1400–1409. doi: 10.1152/japplphysiol.01040.2005. [DOI] [PubMed] [Google Scholar]
- Macdougall IC, Malyszko J, Hider RC, Bansal SS. Current status of the measurement of blood hepcidin levels in chronic kidney disease. Clin J Am Soc Neph. 2010;5(9):1681–1689. doi: 10.2215/CJN.05990809. [DOI] [PubMed] [Google Scholar]
- Mackey SL, Darlington GJ. CCAAT enhancer-binding protein α is required for interleukin-6 receptor α signaling in newborn hepatocytes. J Biol Chem. 2004;279(16):16206–16213. doi: 10.1074/jbc.M400737200. [DOI] [PubMed] [Google Scholar]
- Makipour S, Kanapuru B, Ershler WB. Unexplained anemia in the elderly. Sem Hematol. 2008;45(4):250–254. doi: 10.1053/j.seminhematol.2008.06.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moshage H. Cytokines and the hepatic acute phase response. J Pathol. 1997;181:257–266. doi: 10.1002/(SICI)1096-9896(199703)181:3<257::AID-PATH756>3.0.CO;2-U. [DOI] [PubMed] [Google Scholar]
- Myśliwska J, Bryl E, Foerster J, Myśliwski A. Increase of interleukin 6 and decrease of interleukin 2 production during the ageing process are influenced by the health status. Mech Age Dev. 1998;100(3):313–328. doi: 10.1016/s0047-6374(97)00154-1. [DOI] [PubMed] [Google Scholar]
- Nemeth E, Rivera S, Gabayan V, Keller C, Taudorf S, Pedersen BK, Ganz T. Il-6 mediates hypoferremia of inflammation by inducing the synthesis of the iron regulatory hormone hepcidin. J Clin Invest. 2004a;113(9):1271–1276. doi: 10.1172/JCI20945. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nemeth E, Tuttle MS, Powelson J, Vaughn MB, Donovan A, Ward DM, Ganz T, Kaplan J. Hepcidin regulates cellular iron efflux by binding to ferroportin and inducing its internalization. Science. 2004b;306(5704):2090–2093. doi: 10.1126/science.1104742. [DOI] [PubMed] [Google Scholar]
- Nemeth E, Valore EV, Territo M, Schiller G, Lichtenstein A, Ganz T. Hepcidin, a putative mediator of anemia of inflammation, is a type ii acute-phase protein. Blood. 2003;101:2461–2463. doi: 10.1182/blood-2002-10-3235. [DOI] [PubMed] [Google Scholar]
- Nicolas G, Chauvet C, Viatte L, Danan JL, Bigard X, Devaux I, Beaumont C, Kahn A, Vaulont S. The gene encoding the iron regulatory peptide hepcidin is regulated by anemia, hypoxia, and inflammation. J Clin Invest. 2002;110(7):1037–1044. doi: 10.1172/JCI15686. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oberley TD, Swanlund JM, Zhang HJ, Kregel KC. Aging results in increased autophagy of mitochondria and protein nitration in rat hepatocytes following heat stress. J Histochem Cytochem. 2008;56(6):615–627. doi: 10.1369/jhc.2008.950873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oliveira SJ, Pinto JP, Picarote G, Costa VM, Carvalho F, Rangel M, de Sousa M, de Almeida SF. ER stress-inducible factor CHOP affects the expression of hepcidin by modulating C/EBPα activity. PLOS ONE. 2009;4(8):e6618. doi: 10.1371/journal.pone.0006618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Park CH, Valore EV, Waring AJ, Ganz T. Hepcidin, a urinary antimicrobial peptide synthesized in the liver. J Biol Chem. 2001;276(11):7806–7810. doi: 10.1074/jbc.M008922200. [DOI] [PubMed] [Google Scholar]
- Pietrangelo A, Dierssen U, Valli L, Garuti C, Rump A, Corradini E, Ernst M, Klein C, Trautwein C. Stat3 is required for IL-6-gp130–dependent activation of hepcidin in vivo. Gastroenterology. 2007;132(1):294–300. doi: 10.1053/j.gastro.2006.10.018. [DOI] [PubMed] [Google Scholar]
- Pigeon C, Ilyin G, Courselaud B, Leroyer P, Turlin B, Brissot P, Loréal O. A new mouse liver-specific gene, encoding a protein homologous to human antimicrobial peptide hepcidin, is overexpressed during iron overload. J Biol Chem. 2001;276(11):7811–7819. doi: 10.1074/jbc.M008923200. [DOI] [PubMed] [Google Scholar]
- Rivera S, Liu L, Nemeth E, Gabayan V, Sorensen OE, Ganz T. Hepcidin excess induces the sequestration of iron and exacerbates tumor-associated anemia. Blood. 2005a;105(4):1797–1802. doi: 10.1182/blood-2004-08-3375. [DOI] [PubMed] [Google Scholar]
- Rivera S, Nemeth E, Gabayan V, Lopez MA, Farshidi D, Ganz T. Synthetic hepcidin causes rapid dose-dependent hypoferremia and is concentrated in ferroportin-containing organs. Blood. 2005b;106(6):2196–2199. doi: 10.1182/blood-2005-04-1766. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sakamori R, Takehara T, Tatsumi T, Shigekawa M, Hikita H, Hiramatsu N, Kanto T, Hayashi N. Stat3 signaling within hepatocytes is required for anemia of inflammation in vivo. J Gastroenterol. 2010;45(2):244–248. doi: 10.1007/s00535-009-0159-y. [DOI] [PubMed] [Google Scholar]
- Semenza JC, Rubin CH, Falter KH, Selanikio JD, Flanders WD, Howe HL, Wilhelm JL. Heat-related deaths during the july 1995 heat wave in chicago. New Eng J Med. 1996;335(2):84–90. doi: 10.1056/NEJM199607113350203. [DOI] [PubMed] [Google Scholar]
- Sohal RS, Wennberg-Kirch E, Jaiswal K, Kwong LK, Forster MJ. Effect of age and caloric restriction on bleomycin-chelatable and nonheme iron in different tissues of c57bl/6 mice. Free Rad Biol Med. 1999;27(3/4):287–293. doi: 10.1016/s0891-5849(99)00052-0. [DOI] [PubMed] [Google Scholar]
- Timchenko NA, Wilde M, Kosai K-I, Heydari A, Bilyeu TA, Finegold MJ, Mohamedali K, Richardson A, Darlington GJ. Regenerating livers of old rats contain high levels of c/ebpα that correlate with altered expression of cell cycle associated proteins. Nuc Acids Res. 1998;26(13):3293–3299. doi: 10.1093/nar/26.13.3293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Torrance JD, Bothwell TH. Tissue iron stores. In: Cook JD, editor. Iron Methods in hematology. New York: Churchill Livingstone; 1980. pp. 90–115. [Google Scholar]
- Vecchi C, Montosi G, Zhang K, Lamberti I, Duncan SA, Kaufman RJ, Pietrangelo A. ER stress controls iron metabolism through induction of hepcidin. Science. 2009;325(5942):877–880. doi: 10.1126/science.1176639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Verga Falzacappa MV, Vujic Spasic M, Kessler R, Stolte J, Hentze MW, Muckenthaler MU. Stat3 mediates hepatic hepcidin expression and its inflammatory stimulation. Blood. 2007;109(1):353–358. doi: 10.1182/blood-2006-07-033969. [DOI] [PubMed] [Google Scholar]
- Waheed A, Britton RS, Grubb JH, Sly WS, Fleming RE. Hfe association with transferrin receptor 2 increases cellular uptake of transferrin-bound iron. Arch Biochem Biophys. 2008;474(1):193–197. doi: 10.1016/j.abb.2008.02.041. [DOI] [PubMed] [Google Scholar]
- Wallace DF, Summerville L, Subramaniam VN. Targeted disruption of the hepatic transferrin receptor 2 gene in mice leads to iron overload. Gastroenterology. 2007;132(1):301–310. doi: 10.1053/j.gastro.2006.11.028. [DOI] [PubMed] [Google Scholar]
- Weinstein DA, Roy CN, Fleming MD, Loda MF, Wolfsdorf JI, Andrews NC. Inappropriate expression of hepcidin is associated with iron refractory anemia: Implications for the anemia of chronic disease. Blood. 2002;100(10):3776–3781. doi: 10.1182/blood-2002-04-1260. [DOI] [PubMed] [Google Scholar]
- Welc SS, Phillips NA, Oca-Cossio J, Wallet SM, Chen DL, Clanton TL. Hyperthermia increases interleukin-6 in mouse skeletal muscle. Am J Physiol - Cell Physiol. 2012;303(4):C455–C466. doi: 10.1152/ajpcell.00028.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wrighting DM, Andrews NC. Interleukin-6 induces hepcidin expression through STAT3. Blood. 2006;108(9):3204–3209. doi: 10.1182/blood-2006-06-027631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu J, Marzetti E, Seo AY, Kim J-S, Prolla TA, Leeuwenburgh C. The emerging role of iron dyshomeostasis in the mitochondrial decay of aging. Mech Age Dev. 2010;131(7–8):487–493. doi: 10.1016/j.mad.2010.04.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang HJ, Doctrow SR, Xu L, Oberley LW, Beecher B, Morrison J, Oberley TD, Kregel KC. Redox modulation of the liver with chronic antioxidant enzyme mimetic treatment prevents age-related oxidative damage associated with environmental stress. FASEB J. 2004;18(13):1547–1549. doi: 10.1096/fj.04-1629fje. [DOI] [PubMed] [Google Scholar]
- Zhang HJ, Xu L, Drake VJ, Oberley LW, Kregel KC. Heat-induced liver injury in old rats is associated with exaggerated oxidative stress and altered transcription factor activation. FASEB J. 2003;17(15):2293–2295. doi: 10.1096/fj.03-0139fje. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental Figure: Hfe and TFR2 expression in young and old rats. Immunoblot analysis of Hfe (Panel A) and TFR2 (Panel B) in the livers of young and old rats under control conditions and at the indicated times after a two-heat stress protocol. Results are presented as means + SE, n = 7-9 young and old animals under control conditions and at each time point after heat stress. *Significant difference between young and old animals within a time point.


