Abstract
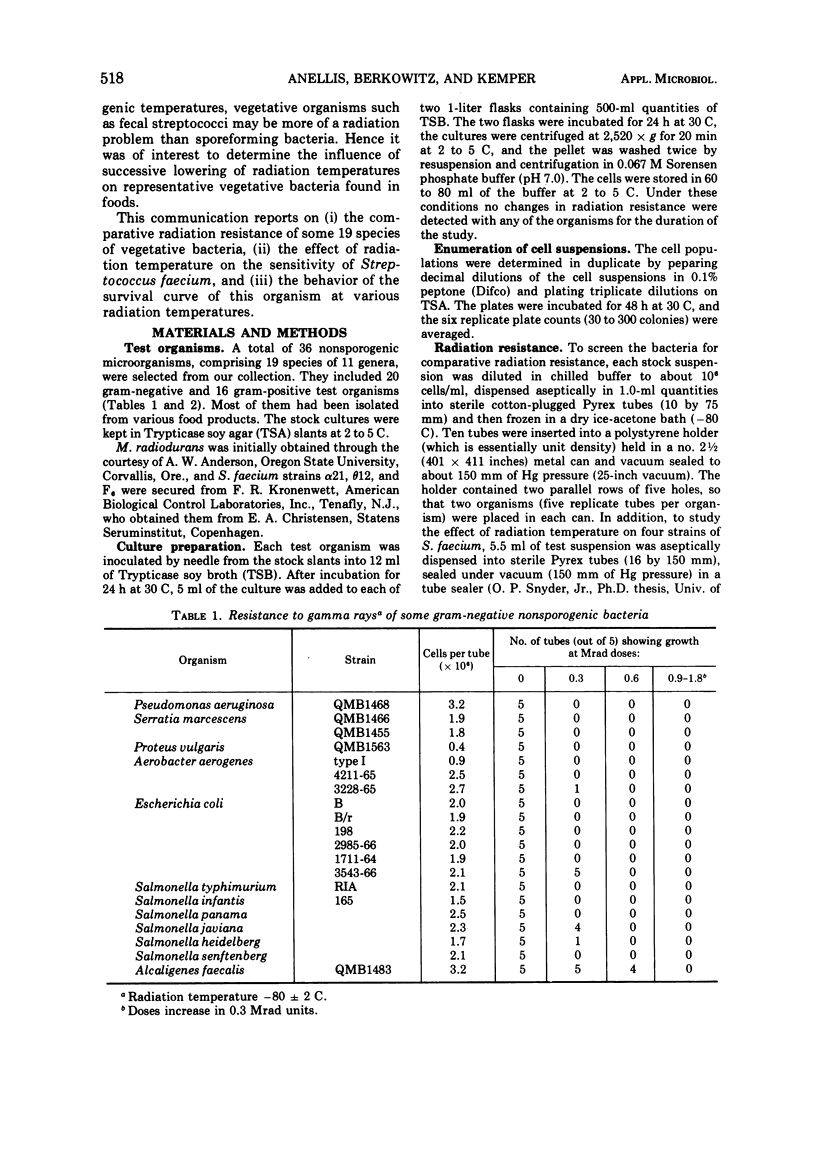
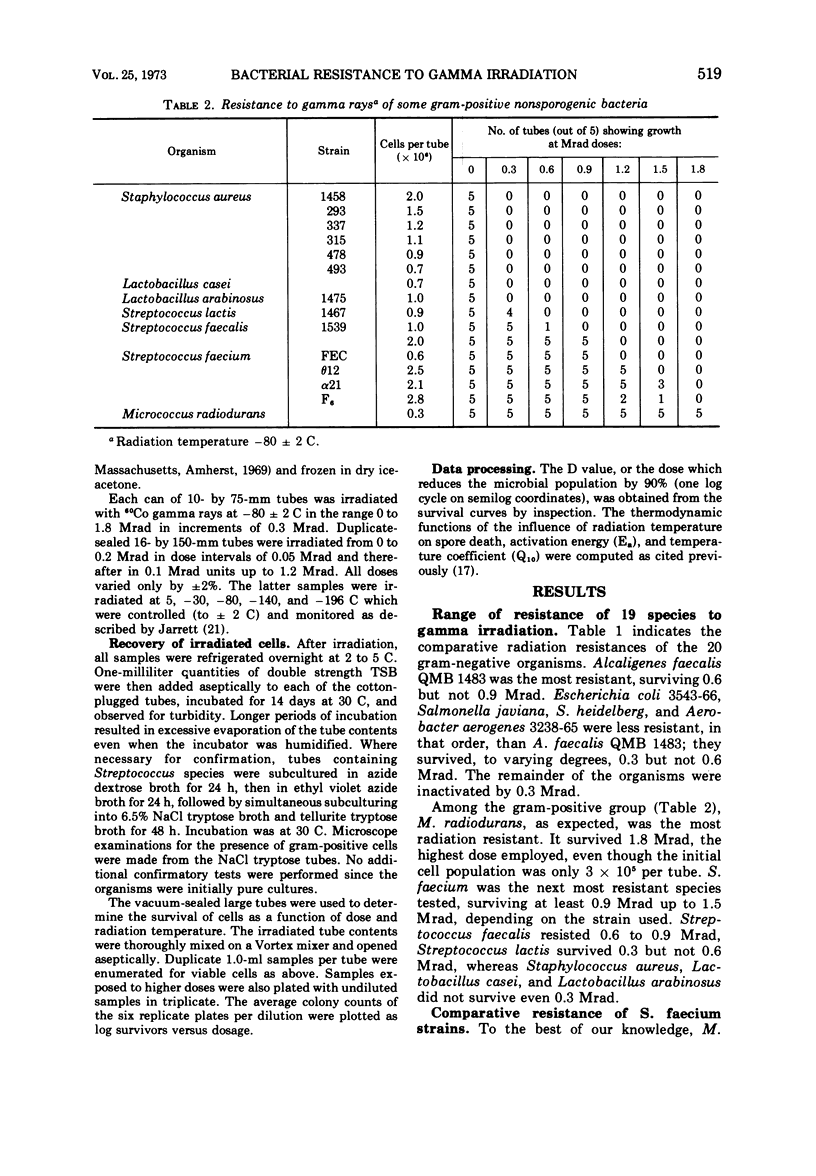
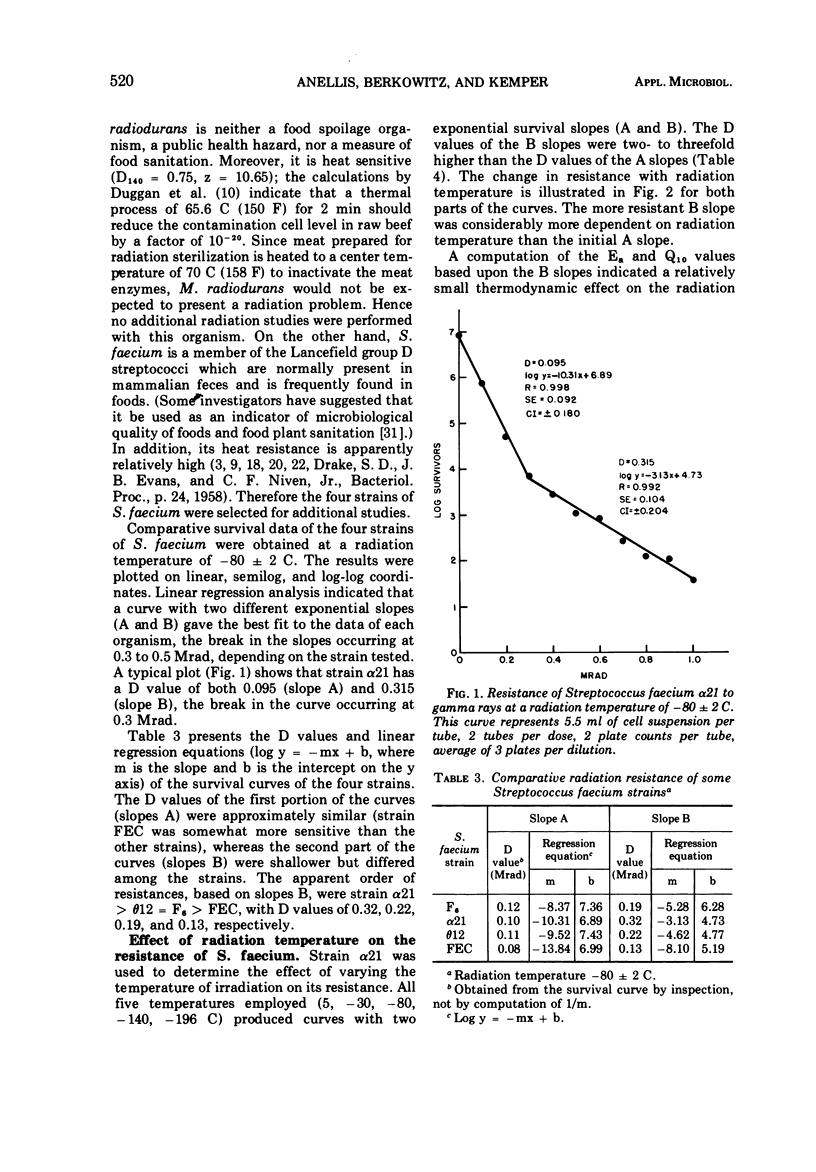
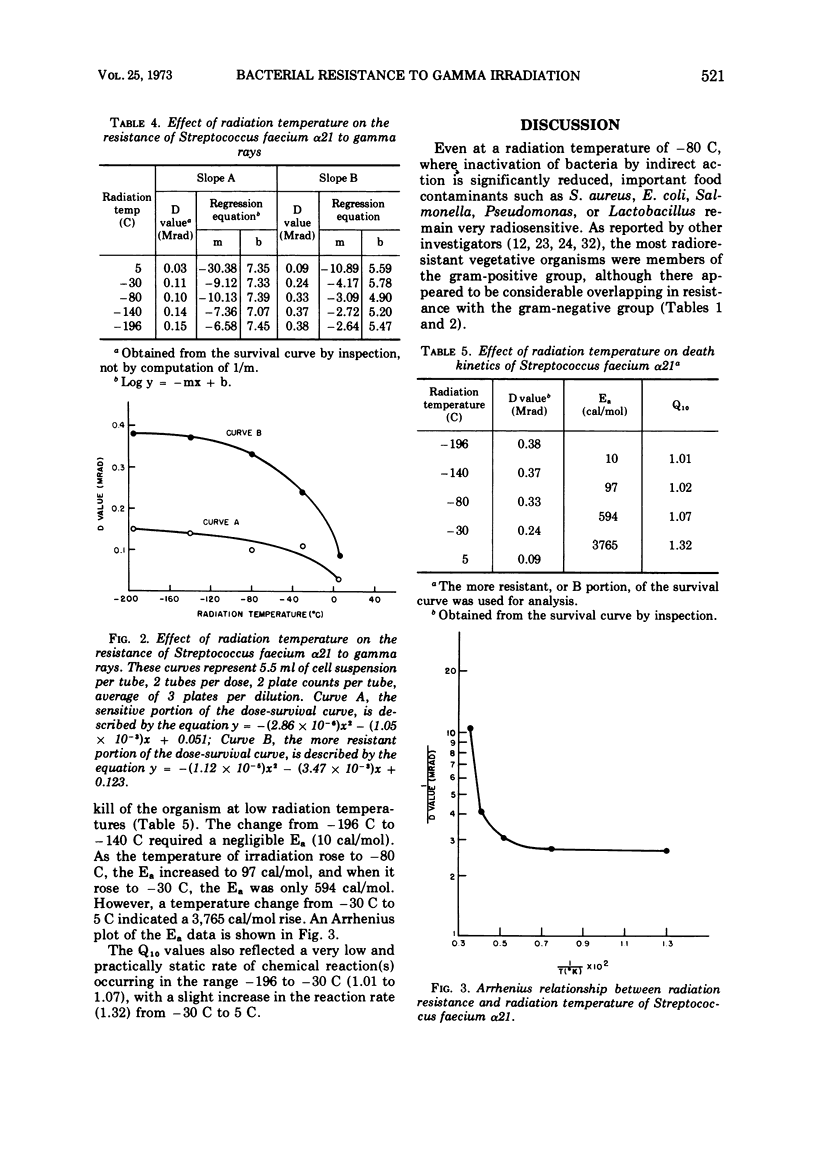
A total of 36 microorganisms, comprising 19 species of 11 genera, were screened for radiation resistance with 60Co gamma rays at a radiation temperatore of -80 ± 2 C in phosphate buffer (pH 7.0) under vacuum. Micrococcus radiodurans was the most resistant organism. An initial population of 2.8 × 105 cells per dose of this species survived 2.4 but not 2.7 Mrad. Of the remaining 18 species with initial populations of about 106 cells per dose, Streptococcus faecium survived 0.9 to 1.5 Mrad, depending on the strain tested. S. faecalis QM survived 0.9 but not 1.2 Mrad. S. faecalis 1539 and Alcaligenes faecallis survived 0.6 but not 0.9 Mrad. Three species of Salmonella, one strain each of Escherichia coli, Streptococcus lactis, and Aerobacter aerogenes survived 0.3 but not 0.6 Mrad. The remaining 22 bacteria did not survive 0.3 Mrad, the lowest dose tested. Detailed survival curve determinations for four strains of S. faecium, the most resistant of the test bacteria of public health significance, indicated the following order of resistance at -80 C: α21 > θ12 = F6 > FEC. Each strain produced two exponential survival curves with different slopes, the breaks occurring at 0.3 to 0.5 Mrad. The D values (doses which reduce the microbial population by 90%) of the more resistant cell fractions were two- to three-fold higher than the more sensitive cell fraction. The resistance of strain α21 was determined at different radiation temperature (+5, -30, -80, -140, -196 C). The D value-radiation temperature relationship followed a quadratic equation. Computations of Ea and Q10 values (activation energy and temperature coefficient, respectively) showed a very small thermodynamic effect on radiation death. An Arrhenius evaluation of the temperature effect on cell kill indicated that there was no simple physicochemical mechanism which might explain the change in D value as a function of temperature.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- CHRISTENSEN E. A., HOLM N. W. INACTIVATION OF DRIED BACTERIA AND BACTERIAL SPORES BY MEANS OF IONIZING RADIATION. Acta Pathol Microbiol Scand. 1964;60:253–264. doi: 10.1111/apm.1964.60.2.253. [DOI] [PubMed] [Google Scholar]
- CHRISTENSEN E. A., KJEMS E. THE RADIATION RESISTANCE OF SUBSTRAINS FROM STREPTOCOCCUS FAECIUM SELECTED AFTER IRRADIATION OF TWO DIFFERENT STRAINS. Acta Pathol Microbiol Scand. 1965;63:281–290. doi: 10.1111/apm.1965.63.2.281. [DOI] [PubMed] [Google Scholar]
- CHRISTENSEN E. A. RADIATION RESISTANCE OF ENTEROCOCCI DRIED IN AIR. Acta Pathol Microbiol Scand. 1964;61:483–486. doi: 10.1111/apm.1964.61.3.483. [DOI] [PubMed] [Google Scholar]
- CHRISTENSEN E. A., SEHESTED K. RADIATION RESISTANCE OF STREPTOCOCCUS FAECIUM AND SPORES OF BACILLUS SUBTILIS DRIED IN VARIOUS MEDIA. Acta Pathol Microbiol Scand. 1964;62:448–458. doi: 10.1111/apm.1964.62.3.448. [DOI] [PubMed] [Google Scholar]
- ERDMAN I. E., THATCHER F. S., MACQUEEN K. F. Studies on the irradiation of microorganisms in relation to food preservation. I. The comparative sensitivities of specific bacteria of public health significance. Can J Microbiol. 1961 Apr;7:199–205. doi: 10.1139/m61-026. [DOI] [PubMed] [Google Scholar]
- ERDMAN I. E., THATCHER F. S., MACQUEEN K. F. Studies on the irradiation of microorganisms in relation to food preservation. II. Irradiation resistant mutants. Can J Microbiol. 1961 Apr;7:207–215. doi: 10.1139/m61-027. [DOI] [PubMed] [Google Scholar]
- GRECZ N., SNYDER O. P., WALKER A. A., ANELLIS A. EFFECT OF TEMPERATURE OF LIQUID NITROGEN ON RADIATION RESISTANCE OF SPORES OF CLOSTRIDIUM BOTULINUM. Appl Microbiol. 1965 Jul;13:527–536. doi: 10.1128/am.13.4.527-536.1965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grecz N., Walker A. A., Anellis A., Berkowitz D. Effect of irradiation temperature in the range--196 to 95C on the resistance of spores of Clostridium botulinum 33A in cooked beef. Can J Microbiol. 1971 Feb;17(2):135–142. doi: 10.1139/m71-024. [DOI] [PubMed] [Google Scholar]
- KOH W. Y., MOREHOUSE C. T., CHANDLER V. L. Relative resistances of micro-organisms to cathode rays. I. Nonsporeforming bacteria. Appl Microbiol. 1956 May;4(3):143–146. doi: 10.1128/am.4.3.143-146.1956. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kniewallner K., Prändl O. Versuche zur Verminderung der Hitzeresistenz von Mikroorganismen. Wien Tierarztl Monatsschr. 1970 Oct;57(10):330–337. [PubMed] [Google Scholar]
- Lewis N. F., Alur M. D., Kumta U. S. Radiation sensitivity of fish microflora. Indian J Exp Biol. 1971 Jan;9(1):45–47. [PubMed] [Google Scholar]
- Ley F. J., Winsley B., Harbord P., Keall A., Summers T. Radiation sterilization: microbiological findings from subprocess dose treatment of disposable plastic syringes. J Appl Bacteriol. 1972 Mar;35(1):53–61. doi: 10.1111/j.1365-2672.1972.tb03673.x. [DOI] [PubMed] [Google Scholar]
- NIVEN C. F., Jr Microbiological aspects of radiation preservation of food. Annu Rev Microbiol. 1958;12:507–524. doi: 10.1146/annurev.mi.12.100158.002451. [DOI] [PubMed] [Google Scholar]
- TARPLEY W., ILAVSKY J., MANOWITZ B., HORRIGAN R. V. Radiation sterilization. I. The effect of high energy gamma radiation from kilocurie radioactive sources on bacteria. J Bacteriol. 1953 Mar;65(3):305–309. doi: 10.1128/jb.65.3.305-309.1953. [DOI] [PMC free article] [PubMed] [Google Scholar]


