Abstract
One of the hallmarks of urinary tract infection, a serious global disease, is its tendency to recur. Uropathogenic bacteria can invade cells lining the bladder, where they form longer-term intracellular reservoirs shielded from antibiotics, re-emerging at a later date to initiate flare-ups. In these cases, only lengthy systemic antibiotic treatment can eradicate all the reservoirs. Yet, long courses of antibiotics are not ideal, as they can lead to side effects and an increase in antibiotic resistance. Moreover, most antibiotics lose some potency by the time they reach the bladder, and many cannot permeate cells, so they cannot access intracellular reservoirs. Here, using coaxial electrohydrodynamic forming, we developed novel core–shell capsules containing antibiotics as a prototype for a future product that could be infused directly into the bladder. Gentamicin was encapsulated in a polymeric carrier (polymethylsilsesquioxane) and these capsules killed Enterococcus faecalis, a common chronic uropathogen, in vitro in a dose-responsive, slow-release manner. Capsules containing a fluorescent tracer dye in place of gentamicin penetrated human bladder cells and released their dye cargo with no apparent toxicity, confirming their ability to successfully permeate cells. These results suggest that such antibiotic capsules could prove useful in the treatment of recalcitrant UTI.
Keywords: urinary tract infection, drug delivery, microbiology
1. Introduction
Urinary tract infection (UTI) is a serious condition and one of the most common infectious diseases worldwide [1]. Up to a half of all women will experience one or more UTI in their lifetime, with 10–15% suffering from recurrent infections [2]. UTI is particularly prevalent among the elderly, being a major cause of a diverse collection of distressing lower urinary tract symptoms (LUTS) including urgency, frequency and incontinence, as well as other problems such as mental confusion and imbalance leading to falls, and very serious complications including pyelonephritis and septicaemia [1,2]. As our global population ages, this disease burden will only worsen [3].
While acute UTI can often be successfully treated with a short course of oral antibiotics, a significant subset of infections occurs below routine diagnostic thresholds and become chronic and resistant to treatment. The bacteria that cause UTI are opportunistic pathogens, entering the bladder via the urethra and attaching to its luminal surface, a specialized epithelial cell layer known as the urothelium [4]. Studies over the last decade have determined that Escherichia coli, the species most commonly associated with acute UTI, can abandon its normal free-living lifestyle and demonstrate an intriguing ability to physically invade the urothelium, taking up residence in the cytoplasm of apical cells and initiating longer-term intracellular colonization of the tissue. Shielded from the host immune system and from antibiotics in the bladder lumen, such bacterial reservoirs can persist and later emerge to initiate a fresh round of acute infection [4]. Recent studies have shown that UTI is also associated with other species of chronic, invasive bacteria besides E. coli, including Enterococcus faecalis [5–7].
Long-term antibiotic treatment of chronic UTI, when needed, is inefficient because the concentration of systemic drug needed to achieve an effective dose in the bladder is extremely high, and crucially, because many antibiotics cannot penetrate the bladder urothelium to eradicate intracellular reservoirs. Conventional antibiotics merely keep planktonic (extracellular) infection at bay until the bladder epithelium turns over (a process that may take many months), during which patient compliance, antibiotic resistance, drug expense and side effects are all problematic. Although some systemic antibiotics may counter uropathogens via a systemic haematogenous (blood to bladder) route of entry, our clinical experience is that the oral route is not efficient enough to cure many recalcitrant cases of chronic UTI. Thus, there is a great need for improved delivery systems to achieve the high concentrations of antibiotics needed to counter chronic infection in a localized manner and in a modality that allows the drug to pass through the urothelial cell membranes.
The aim of this study was to assess the efficacy of encapsulating high doses of a common antibiotic in polymeric microspheres, in a formulation suitable for infusion directly into the bladder by a routine clinical procedure (such as a urinary catheter) to achieve a controlled-release formulation against uropathogenic bacteria. We therefore explored the use of coaxial electrohydrodynamic forming (CEHDF) to prepare such capsules with a tailored release profile. In this forming system, two different liquid solutions simultaneously flow through a device containing two concentrically aligned, coaxial needles, under the influence of an electric field that focuses the liquid streams into a fine jet. When the jet breaks up, near monodisperse capsule formation results. The coaxially aligned needle device facilitates the simultaneous co-flow of gases, liquids or suspensions, which allows the formation of encapsulated structures [8]. This technique is advantageous in that sensitive pharmaceutical ingredients can be encapsulated safely under ambient conditions without the use of surfactants, harsh solvents or high shear stress [9,10]. By regulating various processing parameters, capsule size and morphology can be precisely controlled [11]. In just a single step, one can achieve high encapsulation efficiency and multiple component encapsulation. The process is considered low cost and durable, and multi-layered particles can be produced to specified dimensions with an excellent degree of uniformity (from 200 nm to 100 μm with less than 1% variation) and dose control, at the rate of more than 109–1017 per minute per nozzle [12,13].
Polymethylsilsesquioxane (PMSQ) is a hybrid polymeric material that has received significant attention as a drug carrier. PMSQ is stable, biocompatible, biodurable and non-toxic with superior chemical and physical stability during drug release, making it suitable for medical applications [14].
In this report, we assess a novel, PMSQ-based core–shell capsule designed for slow release of an antibiotic to investigate the future suitability of this general approach for intrabladder treatment of UTI.
2. Results and discussion
First, using a CEHDF system (figure 1a), we produced polymeric capsules containing gentamicin sulfate, a common antibiotic known for its stability, along with control capsules containing only polymer (see the electronic supplementary material). Briefly, 14 wt% of PMSQ solution was pumped through the outer needle while the inner needle was fed with 30 mg ml−1 of gentamicin (figure 1a) at flow rates of 650 and 150 μl min−1, respectively. A stable ‘cone-jet’ was achieved following the application of an electric field, at a voltage of 5–7 kV, which ultimately led to the formation of polymeric capsules. Control capsules were prepared the same way except only the outer needle was used to introduce polymer alone. We studied the resulting capsules using standard electron microscopy (EM; see the electronic supplementary material). As shown by scanning EM, the average size of the drug capsule (figure 1b) and control capsules (figure 1c) were 850 ± 100 nm and 450 ± 50 nm, respectively (n = 100 in both cases, where n is the number of particles counted). This increase in overall size in the former is consistent with the successful addition of the drug, which would ultimately lead to a dense core forming inside the polymeric carrier. Indeed, transmission EM micrographs show evidence of these two layers in the drug version only (see figure 1b,c insets).
Figure 1.
(a) Schematic of the CEHDF experimental set-up using a two-needle coaxial device for the preparation of polymeric capsules. Scanning EM reveals the size and morphology of the drug-containing (b) and control (c) capsules; scale bars, 5 μm. Insets on top right of each show a representative capsule by transmission EM; scale bars, 500 nm.
To test the capsules in an antimicrobial assay, we assessed the ability of the drug capsules to kill the common uropathogen E. faecalis in an in vitro assay (see the electronic supplementary material for details). We chose this species because it is the most invasive bacteria seen in our patient group [6]. Briefly, fresh liquid cultures of bacteria were challenged with a dilution series of the drug capsules, compared with a similar series of control capsules and with injection-grade unencapsulated gentamicin solution used in clinical practice. We also included a dilution of the unencapsulated drug corresponding to the much lower concentration estimated to occur in the bladder after systemic injection according to the manufacturer's pharmacological information. At various times post-inoculation, an aliquot of cultures was removed to enumerate living bacteria. As shown, the drug capsules exhibited a sustained release of activity over a number of days (figure 2a), in a dose-responsive manner (figure 2b). The release profile of the capsules is consistent with the intended diffusion of the drug from the polymeric capsules over time, a feature that should prolong antimicrobial activity in a treatment situation. Of note, the drug capsules killed bacteria to a similar degree and kinetics as did the dose of free gentamicin at typical bladder concentrations.
Figure 2.
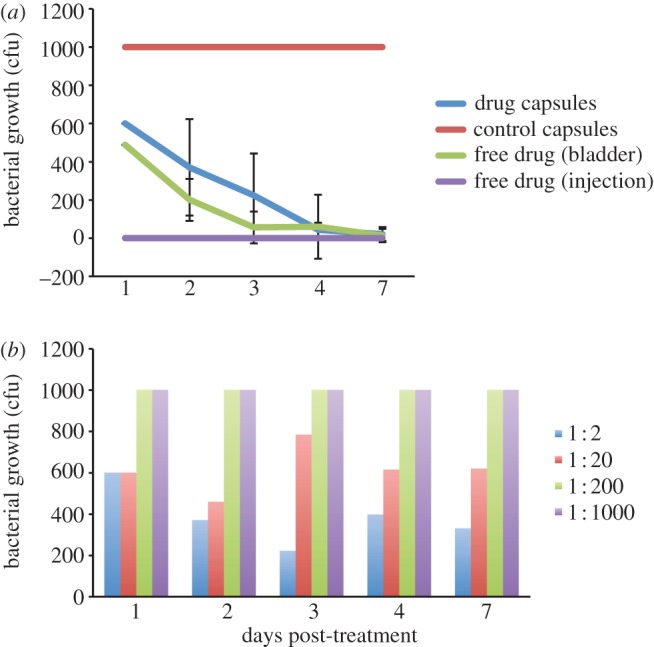
Antimicrobial activity of the various interventions against E. faecalis (see colour legend) in broth culture as a function of days of continuous treatment, as measured by colony-forming units growing on a defined segment of agar. (a) Drug capsules and control capsules were used in an undiluted formulation; the free drug was tested at two concentrations; (1) the very high dose present in the original systemic injection solution (‘injection’), and (2) at the lower concentration typical and realistic for that achieved in the bladder after systemic injection (‘bladder’). The mean of a representative experiment performed in triplicate is shown; bars indicate standard deviation. Note that there was no deviation in the control capsules (because growth was saturated) nor in the free drug (‘injection’), which was zero in all replicates. (b) Dose response of drug capsules in various dilutions as shown in the colour legend; the axes are the same as that for (a).
To determine how the capsules would behave in the presence of cultured human bladder cells, and in particular to assess their ability to permeate cells, we prepared capsules loaded with fluorescein isothiocyanate (FITC), a green fluorescent tracker dye, in place of gentamicin (figure 3a, inset lower left; see the electronic supplementary material for details). We incubated these capsules with adherent cultures of T24, a human bladder carcinoma cell line. At various times post-treatment, samples were removed, fixed and stained for microscopy to assess green fluorescence as well as the membrane and nuclei of the cells (see the electronic supplementary material for details). These experiments show that bladder cells specifically took up the capsules, and FITC cargo was released inside cells as early as 2 h post-delivery, as evidenced by intense green staining of those cells (figure 3a shows a representative culture at 12 h post-treatment). This staining cannot merely represent free FITC released prematurely into the culture medium, as in this case all cells would be faintly fluorescent, as opposed to the observed strong, specific green staining in isolated cells. Moreover, the nuclei and morphology of positive cells appeared healthy, and the overall growth in treated and untreated wells was similar, suggesting that the capsules were not unduly toxic to human cells.
Figure 3.

(a) Confocal micrograph of human bladder cells in culture dosed with FITC-loaded microcapsules (green) for 12 h; the cell labelled with a yellow arrow has taken up the cargo; intact capsules indicated with white arrows. A membrane stain (wheat germ agglutinin, red) highlights the boundaries of the cells, whereas the blue stain (4′,6-diamidino-2-phenylindole (DAPI)) shows the cell nucleus. Greyscale images to the right show the individual channels of the same image separately. Scale bar, 10 μm. The inset image, lower left, shows an epifluorescence microscopy image of the FITC-containing capsules in solution prior to the experiment; scale bar, 2 μm. (b) Epifluorescent images of shed bladder epithelial cells derived from urine of an infected patient, subsequently dosed with FITC-loaded microcapsules (green) for 2 h; the cells labelled with a yellow arrow have taken up capsules; cells labelled with blue arrows have not, indicating specific uptake. The blue stain (DAPI) shows the cell nucleus. Greyscale images to the right show the individual channels of the same image separately. Scale bar, 10 μm.
T24 cells are a model of the human bladder, but their cancerous state and adaptation to tissue culture means that they are not a perfect mimic. Therefore, we wanted to inspect capsule uptake in a more physiologically relevant setting. We obtained ethical approval and patient consent to sample urine from patients suffering from LUTS infections. Patients with UTI shed infected bladder epithelial cells into their urine as part of an immune response to infection [5,15], so we tested the capsules on these cells (see electronic supplementary material for details). Briefly, we challenged the shed cells in liquid culture with a suspension of the FITC-containing capsules, and after incubation, we fixed and stained the cells for microscopy similar to the above. As shown in figure 3b, we saw specific uptake and release of FITC cargo in patient cells, indicating that the delivery is successful in bladder cells derived from UTI patients. At the moment, the mechanism of cell penetration is unknown; further cell biological investigations are underway to elucidate the processes involved.
3. Conclusion
These pilot data show the feasibility of loading an antibiotic into a non-cell-toxic microcapsule that can both penetrate bladder cells and provide slow release of the drug that retains its bactericidal properties. The flexibility of CEHDF means that such capsules could be further optimized to exhibit a variety of desired parameters. The capsules could be introduced directly into the bladder via a catheter in a straightforward clinical outpatient procedure to treat UTI in high, localized doses, eradicating any intracellular reservoirs. This would offer a good solution to the current problems faced with curing recalcitrant UTI via high-dose, systemic antibiotic delivery and might ultimately replace long-term systemic antibiotic use. Given that the antibiotic components would already have regulatory approval, and that the polymer used for the shell is already considered safe in vivo, we predict that, after satisfying the normal licensing protocols, clinical use of an optimized version of these capsules in future would be a feasible and attractive goal.
Acknowledgements
We thank Roshne Patel and David Holland for technical assistance, and the members of our group for helpful discussion.
Data accessibility
All data are reported within this paper and the electronic supplementary material.
Funding statement
We acknowledge the UCL Crucible funding scheme and the EPSRC (EP/J01334X/1) for supporting this work.
References
- 1.Foxman B. 2002. Epidemiology of urinary tract infections: incidence, morbidity, and economic costs. Am. J. Med. 113 (Suppl. 1A), 5S–13S. ( 10.1016/S0002-9343(02)01054-9) [DOI] [PubMed] [Google Scholar]
- 2.Hooton TM. 2001. Recurrent urinary tract infection in women. Intl. J. Antimicrob. Agents 17, 259–268. ( 10.1016/S0924-8579(00)00350-2) [DOI] [PubMed] [Google Scholar]
- 3.Irwin DE, Kopp ZS, Agatep B, Milsom I, Abrams P. 2011. Worldwide prevalence estimates of lower urinary tract symptoms, overactive bladder, urinary incontinence and bladder outlet obstruction. BJUI 108, 1132–1138. ( 10.1111/j.1464-410X.2010.09993.x) [DOI] [PubMed] [Google Scholar]
- 4.Hunstad DA, Justice SS. 2010. Intracellular lifestyles and immune evasion strategies of uropathogenic Escherichia coli. Annu. Rev. Microbiol. 64, 203–221. ( 10.1146/annurev.micro.112408.134258) [DOI] [PubMed] [Google Scholar]
- 5.Horsley H, Holland D, Tuz M, Malone-Lee J, Sathiananthamoorthy S, Kelsey M, Kupelian A, Rohn JL. Submitted. Enterococcus faecalis subverts and invades the host urothelium in patients with chronic urinary tract infection. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Khasriya R, Sathiananthamoorthy S, Ismail S, Kelsey M, Wilson M, Rohn JL, Malone-Lee J. 2013. Spectrum of bacterial colonization associated with urothelial cells from patients with chronic lower urinary tract symptoms. J. Clin. Microbiol. 51, 2054–2062. ( 10.1128/JCM.03314-12) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Szabados F, Kleine B, Anders A, Kaase M, Sakinc T, Schmitz I, Gatermann S. 2008. Staphylococcus saprophyticus ATCC 15305 is internalized into human urinary bladder carcinoma cell line 5637. FEMS Micro Lett. 285, 163–169. ( 10.1111/j.1574-6968.2008.01218.x) [DOI] [PubMed] [Google Scholar]
- 8.Farook U, Stride E, Edirisinghe MJ. 2009. Preparation of suspensions of phospholipid-coated microbubbles by coaxial electrohydrodynamic atomization. J. R. Soc. Interface 6, 271–277. ( 10.1098/rsif.2008.0225) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Pareta R, Edirisinghe MJ. 2006. A novel method for the preparation of biodegradable microspheres for protein drug delivery. J. R. Soc. Interface 3, 573–582. ( 10.1098/rsif.2006.0120) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Xie J, Ng WJ, Lee LY, Wang CH. 2008. Encapsulation of protein drugs in biodegradable microparticles by co-axial electrospray. J. Colloid Interface Sci. 317, 469–476. ( 10.1016/j.jcis.2007.09.082) [DOI] [PubMed] [Google Scholar]
- 11.Enayati M, Chang M-W, Bragman F, Edirisinghe M, Stride E. 2011. Electrohydrodynamic preparation of particles, capsules and bubbles for biomedical engineering applications. Colloids Surf. A: Physicochem. Eng. Aspects 382, 154–164. ( 10.1016/j.colsurfa.2010.11.038) [DOI] [Google Scholar]
- 12.Chang MW, Stride E, Edirisinghe M. 2010. Controlling the thickness of hollow polymeric microspheres prepared by electrohydrodynamic atomization. J. R. Soc. Interface 7(Suppl. 4), S451–S460. ( 10.1098/rsif.2010.0092.focus) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Labbaf S, Deb S, Cama G, Stride E, Edirisinghe M. 2013. Preparation of multicompartment sub-micron particles using a triple-needle electrohydrodynamic device. J. Colloid Interface Sci. 409, 245–254. ( 10.1016/j.jcis.2013.07.033) [DOI] [PubMed] [Google Scholar]
- 14.Abbasi F, Mirzadeh H, Katbab A-A. 2001. Modification of polysiloxane polymers for biomedical applications: a review. Polym. Int. 50, 1279–1287. ( 10.1002/pi.783) [DOI] [Google Scholar]
- 15.Rosen DA. 2007. Detection of intracellular bacterial communities in human urinary tract infection. PLoS Med. 4, e329 ( 10.1371/journal.pmed.0040329) [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
All data are reported within this paper and the electronic supplementary material.



