Abstract
The present study was designed to determine whether the structural composition of the scar in middle-aged post–myocardial infraction (MI) rats is affected by the biological sex of the animals. A large MI was induced in 12-month-old male (M-MI) and female (F-MI) Sprague-Dawley rats by ligation of the left coronary artery. Four weeks after the MI, rats with transmural infarctions, greater than 50% of the left ventricular (LV) free wall, were evaluated. The extent of LV remodeling and fractional volumes of fibrillar collagen (FC), myofibroblasts, vascular smooth muscle (SM) cells, and surviving cardiac myocytes (CM) in the scars were compared between the two sexes. The left ventricle of post-MI male and female rats underwent a similar degree of remodeling as evidenced by the analogous scar thinning ratio (0.46 ± 0.02 vs. 0.42 ± 0.05) and infarct expansion index (1.06 ± 0.07 vs. 1.12 ± 0.08), respectively. Most important, the contents of major structural components of the scar revealed no evident difference between M-MI and F-MI rats (interstitial FC, 80.74 ± 2.08 vs. 82.57 ± 4.53; myofibroblasts, 9.59 ± 1.68 vs.9.56 ± 1.15; vascular SM cells, 2.27 ± 0.51 vs. 3.38 ± 0.47; and surviving CM, 3.26 ± 0.39 vs. 3.05 ± 0.38, respectively). Our data are the first to demonstrate that biological sex does not influence the structural composition of a mature scar in middle-aged post-MI rats.
Keywords: middle-aged rats, myocardial infarction, left ventricular remodeling, scar composition, sex-related differences
Sudden obstruction of coronary blood flow causes ischemic death of cardiomyocytes followed by a universal cascade of reparative events (Frangogiannis 2006) leading to infarct healing and scar formation in the heart. Nevertheless, a growing body of evidence from animal studies suggests that females exhibit a different pattern of myocardial infarction (MI) healing and left ventricular (LV) remodeling than males. For instance, post-MI female mice reveal less exaggerated inflammation and enhanced reparative fibrotic response during infarct healing, contributing to a lower rate of cardiac rupture and a lesser degree of LV remodeling in comparison with males (Cavasin et al. 2004; Gao et al. 2005; Fang et al. 2007; Wang F et al. 2007). In addition, despite comparable infarct size and LV cavity dilatation, the thickness of noninfarcted myocardium is less in post-MI female than male rats (Litwin et al. 1999), indicating a smaller increase in compensatory hypertrophy in response to post-MI cardiac remodeling in the females.
Although most of these studies were focused on earlier reparative events taking place in male and female hearts during the overlapping phases of MI healing (i.e., inflammatory, proliferative and maturation phases), surprisingly little attention has been paid to providing a comparative assessment of the structural components in a mature scar. However, it has been previously shown that the tissue composition of a mature scar is an important determinant of its mechanical properties (Connelly et al. 1985) and, thereby, can drastically influence overall ventricular performance (Dai et al. 2005; Hayakawa et al. 2003; Lichtenauer et al. 2011; Mizuno, Mickle, et al. 2005; Mizuno, Yau, et al. 2005). In recent decades, there have been a considerable number of studies demonstrating that even mature fibrotic scars are composed of the various tissue components, such as collagen fibers (Boyle and Weisman 1993; Sun et al. 1994; Sun et al. 2000; Zhang et al. 2010), elastic fibers (Mizuno, Yau, et al. 2005), myofibroblasts (Vracko and Thorning 1991; Sun and Weber 1996; Hayakawa et al. 2003; Virag and Murry 2003), viable cardiomyocytes (Fishbein et al. 1978; Kalkman et al. 1997; Virag and Murry 2003; Zhang et al. 2010), mummified dead cardiomyocytes (Boyle and Weisman 1993), and vessels (Fishbein et al. 1978; Kalkman et al. 1997; Virag and Murry 2003; Wang B et al. 2005; Zhang et al. 2010). Some of these scar components have been shown to undergo a dynamic reorganization in response to ongoing LV remodeling (Whittaker 1995; Sun and Weber 2000; Sun et al. 2002; van den Borne et al. 2010). Surprisingly, in the previous in vivo studies that used either male or female animals, only a few used quantitative methods to examine the structural composition of the post-MI scars (Boyle and Weisman 1993; Sun et al. 2000; Hayakawa et al. 2003; Virag and Murry 2003; Lichtenauer et al. 2011; Zhang et al. 2010).
Furthermore, despite the well-established fact that advanced age results in delay of infarct healing in post-MI rats (Kranz et al. 1975; Wexler 1978) and significantly increases a risk of post-MI cardiac rupture (Yang et al. 2008) as well as exaggerates adverse LV remodeling (Bujak et al. 2008) in mice, most MI experiments were done in young or young-adult animals. However, our previous studies have demonstrated that the adaptive changes in post-MI hearts of middle-aged rats (Dedkov et al. 2006; Dedkov, Zheng, et al. 2007) may provide a better correlation to the human population in which the occurrence of MI is more prevalent among middle-aged and elderly individuals of both sexes (Bairey Merz et al. 2006; Vaccarino et al. 2011).
In addition, several studies have shown that, in small rodents, such as mice and rats, significant alterations in systemic and cardiac hemodynamics occur only in hearts with large transmural MI (Michel et al. 1995; Olivetti et al. 1991; Pfeffer JM et al. 1984, 1991; Pfeffer MA et al. 1979). Therefore, only large infarcts are likely to trigger LV remodeling, leading to evident modifications in the scar structure (Hochman and Bulkley 1982). According to these observations, animals with a large transmural MI should be primarily considered for a comparative analysis of sex-related differences in the composition of the scar.
Therefore, this study addressed the hypothesis that biological sex of the animal affects the structural composition of mature scars formed in middle-aged rats in response to a large transmural MI.
Materials and Methods
All animal handling and experimental procedures were performed in accordance with the Guide for the Care and Use of Laboratory Animals published by the National Institutes of Health (NIH Publications No. 85-23, revised 1996) and approved by the University of Iowa Animal Care and Use Committee.
Animals and Experimental Model of MI
A large transmural MI was induced in 12-month-old female (F-MI, n=8) and male (M-MI, n=8) Sprague-Dawley rats (Harlan, Indianapolis, IN) under ketamine (100 mg/kg intraperitoneally [IP]) and xylazine (10 mg/kg IP) anesthesia by ligation of the left anterior descending coronary artery near its origin, as previously detailed elsewhere (Fishbein et al. 1978; Pfeffer MA et al. 1979; Boyle and Weisman 1993; Litwin et al. 1999; Dedkov et al. 2005). Following surgery, the rats were housed under climate-controlled conditions at a 12-hour light/dark cycle and provided with standard rat chow and water ad libitum.
LV Weight and Determination of Infarct Size
Four weeks after coronary ligation, a time point at which the infarcted area is completely healed (Fishbein et al. 1978; Pfeffer MA et al. 1979), the rats were anesthetized as described above and weighed, and the hearts were arrested in diastole by the infusion of 2% lidocaine hydrochloride into the left ventricle. The hearts were excised from the thorax, attached to a Langendorff apparatus, and perfusion-fixed with 4% paraformaldehyde (PFA) in phosphate-buffered saline (PBS) for 20 min at constant pressure (100 mm Hg). Then, the hearts were immersion-fixed in a fresh portion of 4% PFA solution for 24 hr at +4C. In each heart, the atria and the right ventricular free wall were removed, and the left ventricle, including the septum, was cut transversely from the apex to the base into five parallel slices of equal thickness with a blade guillotine. The LV slices and the right ventricular free wall were then briefly blotted dry with filter paper and weighed.
To determine the infarct size, all LV slices from each heart were digitized and evaluated using Image-Pro Analyzer 7.0 software (Media Cybernetics, L.P.; Silver Spring, MD), as detailed previously (Dedkov et al. 2005). Briefly, in each digitized slice, the lengths of the entire free wall and its portion occupied by the scar (both obtained at the midwall level) were measured, and the extent of the scarred area was estimated as the ratio between scar length and length of the entire free wall. Then, the mean of these ratios was calculated for each heart. Finally, the infarct size was expressed as a percentage of the LV free wall.
Tissue Sampling
Post-MI rats of both sexes were included in the final evaluation only if the size of a transmural infarct was equal to or greater than 50% of the LV free wall. According to infarct size measurements, six hearts from F-MI rats and seven hearts from M-MI rats were selected for further tissue processing. From each heart, the two midventricular LV slices were collected and embedded in paraffin, because they are most representative of the scale of LV remodeling and the extent of the transmural scar (Spadaro et al. 1980).
Histology, Immunohistochemistry, and Light and Fluorescence Microscopy
Transverse serial sections (8.0 µm thick) were cut from the paraffin-embedded LV slices and stained with hematoxylin and eosin (H&E), picrosirius red, Masson’s trichrome, and Verhoeff’s elastic tissue stains.
Some sections were immunostained with a monoclonal antibody against α–smooth muscle (SM) actin (1:400; clone 1A4; cat. A2547; Sigma, St. Louis, MO). The primary antibody was visualized using a peroxidase-conjugated anti–mouse secondary antibody (cat. MP-7402; ImmPress Peroxidase Reagent kit; Vector Labs, Burlingame, CA), followed by incubation with DAB substrate (cat. SK-4105; ImmPACT DAB Peroxidase Substrate kit; Vector Labs). Finally, the slides were counterstained with hematoxylin 7211 (Richard-Allan Scientific; Kalamazoo, MI). To further characterize the origin of the α-SM actin–positive cells, a subset of these sections was double immunolabeled with a Cy3-conjugated α-SM actin antibody (1:600; clone 1A4; cat. C6198; Sigma) and monoclonal anti–desmin (1:80; clone DE-U-10; cat. D1033; Sigma), monoclonal anti–cardiac actin (1:100; clone AC1-20.4.2; cat. A9357; Sigma), or rabbit anti–laminin (1:30; cat. L9393; Sigma) primary antibodies. The antibodies were visualized with the Alexa Fluor 488–conjugated goat anti–mouse (1:400; cat. A-11001; Molecular Probes, Eugene, OR) or goat anti–rabbit (1:400; cat. A-11008; Molecular Probes) secondary antibodies. The immunostained sections were mounted in ProLong Gold antifade mounting medium with DAPI to counterstain the nuclei (cat. P36931; Molecular Probes). Omission of primary antibodies served as negative controls.
The stained sections were examined under an Olympus BX53 microscope (Olympus America; Center Valley, PA). In each heart, a series of high-resolution images covering the entire left ventricle stained with H&E, picrosirius red, Masson’s trichrome, or α-SM actin was captured at ×4 magnification with an Olympus DP72 digital camera and imported into the computer. Finally, the digital assembly of the complete LV profiles was done using Adobe Photoshop CS5 software (Adobe Systems; San Jose, CA). The double- or triple-labeled fluorescence images were captured at ×60 magnification and digitally combined using Olympus cellSens Standard 1.4.1 digital imaging software (Olympus America).
Quantitative Morphometry by Digital Image Analysis
Morphometric and stereological digital analyses were performed on the reconstructed images in a blinded manner using Image-Pro Analyzer 7.0 software (Media Cybernetics; Bethesda, MD).
The H&E– and Masson’s trichrome–stained sections were used to acquire the following planimetric parameters of the left ventricle: the LV cross-sectional area (CSA) and mean diameter, the LV cavity CSA and mean cavity diameter, the average thickness of the free wall and septum, and the average scar thickness and CSA. From these measurements, the scar thinning ratio and expansion index were determined as follows: the scar thinning ratio was calculated as the ratio between the average thickness of the scar and the average thickness of the septum, whereas the expansion index (a parameter that reflects the degree of LV dilatation and scar thinning) was calculated as previously detailed (Hochman and Choo 1987; Virag and Murry 2003)—that is, (LV cavity area/LV area) × (septal wall thickness/scar thickness).
Using a composed high-resolution image of each individual scar, the fractional volumes of the following tissue components were determined: the collagen fibers in the interstitial space (interstitial collagen) and in the wall of blood vessels (vascular collagen), the surviving and mummified dead cardiac myocytes (CM), and the α-SM actin–positive cells in the wall of blood vessels (vascular SM cells) and in the interstitial space (nonvascular cells, presumably myofibroblasts). Briefly, on digital images composed from serial sections of the same left ventricle, which were stained with picrosirius red, Masson’s trichrome, or α-SM actin antibody, the total area of the scar was thoroughly outlined and measured. Then, a color image segmentation technique was applied to discretely determine the total area of scar segments occupied by the above-mentioned solid tissue components as well as by an empty space, which had been presumably occupied by soluble components of the ground substance or had been formed as a result of tissue separation during histological processing of the specimens. Subsequently, to standardize data evaluation among the scars, the area of an empty space was subtracted from the total area of the scar, and the fractional volume of each individual solid tissue component was expressed as a percentage of the remaining scar volume. Using this approach on serial sections, we determined the volume fractions of interstitial and vascular collagen on picrosirius red–stained sections (Dedkov, Zheng, et al. 2007; Whittaker et al. 1994), the volume fractions of surviving and mummified dead CM on Masson’s trichrome–stained sections (Virag and Murry 2003), and the volume fractions of myofibroblasts and vascular SM cells on α-SM actin–stained sections (Virag and Murry 2003) in each individual scar. However, after taking into consideration the structural integrity of the major tissue components and the quality of all staining procedures in each individual scar, the data related to composition of the scar structures from only five hearts per sex group were compared.
Statistical Analysis
Data are expressed as the mean ± standard error of the mean (SEM). Statistical analysis was performed using GraphPad InStat 3.05 software (GraphPad Software; La Jolla, CA). A one-tailed unpaired Student’s t-test was used to assess differences between the two groups. A probability of p≤0.05 was considered to indicate significant differences. The effect size (Cohen’s d), which expresses the difference in group means as a function of standard deviations (Cohen 1988), was calculated to estimate the extent of the difference in all comparisons between the two sex groups in the parameters determined. The computed effect sizes were interpreted according to Cohen’s convention: small effect (d = 0.2), medium effect (d = 0.5), and large effect (d = 0.8).
Results
Consistent with the objective of this study, only animals with a large transmural infarct (≥50% of the LV free wall) were selected for evaluation. The infarct size ranged from 57% to 64% in male rats and from 50% to 71% in female rats. Consequently, the mean infarct size was comparable between male and female groups of animals (Table 1).
Table 1.
Structural Parameters of the Left Ventricle in Male and Female Post-MI Hearts.
| M-MI |
F-MI |
|||
|---|---|---|---|---|
| n | 7 | 6 | p-Value | Effect Size (Cohen’s d) |
| Infarct size, % of LV free wall | 61.50 ± 1.21 | 58.21 ± 4.51 | 0.28 | 0.38 |
| BW, g | 530.86 ± 19.32 | 346.14 ± 21.01**** | <0.0001 | 3.46 |
| VW, mg | 1434.42 ± 77.06 | 1084.18 ± 16.64*** | 0.0006 | 2.56 |
| LV weight, mg | 1096.34 ± 49.36 | 863.90 ± 35.89*** | 0.001 | 2.04 |
| RV free wall weight, mg | 360.25 ± 33.12 | 259.74 ± 16.28** | 0.008 | 1.55 |
| LV weight/BW ratio, mg/g | 2.07 ± 0.07 | 2.53 ± 0.13** | 0.004 | 1.71 |
| LV weight/VW ratio, mg/mg | 0.75 ± 0.02 | 0.77 ± 0.01 | 0.19 | 0.50 |
| LV diameter, mm | 11.03 ± 0.25 | 9.88 ± 0.19** | 0.005 | 2.29 |
| LV CSA, mm2 | 96.54 ± 4.25 | 78.44 ± 3.12** | 0.007 | 2.11 |
| LV cavity diameter, mm | 7.58 ± 0.22 | 6.61 ± 0.44* | 0.03 | 1.32 |
| LV cavity CSA, mm2 | 46.38 ± 2.72 | 36.35 ± 4.52* | 0.04 | 1.57 |
| LV free wall thickness, mm | 2.29 ± 0.11 | 2.12 ± 0.21 | 0.23 | 0.48 |
| Septal wall thickness, mm | 1.77 ± 0.12 | 1.68 ± 0.11 | 0.32 | 0.32 |
| Scar thickness, mm | 0.80 ± 0.03 | 0.69 ± 0.05* | 0.03 | 1.34 |
| Scar thinning ratio | 0.46 ± 0.02 | 0.42 ± 0.05 | 0.21 | 0.53 |
| Expansion index | 1.06 ± 0.07 | 1.12 ± 0.08 | 0.28 | 0.39 |
Values are the mean ± SEM. BW, body weight; CSA, cross-sectional area; F, female; LV, left ventricle; M, male; MI, myocardial infraction; RV, right ventricle; VW, ventricular weight (LV + RV free wall). Expansion index = (LV cavity area/LV area) × (septal wall thickness/scar thickness). Scar thinning ratio = scar thickness/septal wall thickness.
p<0.05, **p<0.01, ***p<0.001, and ****p<0.0001 vs. M-MI group.
Extent of Structural LV Remodeling Is Similar in Male and Female Post-MI Hearts
The LV structural parameters are shown in Table 1. The values for external and internal LV dimensions and the LV weights were significantly lowe in female than in male rats. These findings are consistent with the fact that the male rats were markedly (39%) heavier than their age-matched female counterparts (p≤0.001, d = 3.46). Such a profound difference in body weight caused females to have a 23% greater LV weight and body weight ratio than males (p≤0.01, d = 1.71). However, despite this sex-related difference in LV size, the average wall thickness and LV weight to ventricular weight ratio remained comparable between the two groups, suggesting a similar degree of compensatory LV hypertrophy. Most important, although the scars in the female hearts were evidently thinner than in those in male hearts on average by 13% (p≤0.05, d = 1.34), the scar thinning ratio and expansion index were not different between the male and female rats, indicating the analogous scale of LV remodeling.
The Content and Distribution of Major Structural Components of LV Scar Do Not Differ in Male and Female Post-MI Hearts
The relative contents of the major scar components are shown in Table 2. The quantitative assessment of the scars revealed that in female rats, the area of the scar was 60% smaller than that in male rats (p≤0.01, d = 2.31). However, this difference is a consequence of thinner scars in the former (Table 1). Most important, despite the apparent difference in the scar area, both groups of rats did not reveal detectable sex-related distinctions among the fractional volumes and distribution of major structural components, such as fibrillar collagen (Fig. 1A), myofibroblasts (Fig. 2A), and surviving CM (Fig. 3A).
Table 2.
Structural Components of the LV Scar in Male and Female Post-MI Hearts.
| Scar CSA, mm2 | Interstitial Collagen, % | Vascular Collagen, % | Myofibroblasts, % | Vascular SM Cells, % | Surviving CM, % | Mummified Dead CM, % | |
|---|---|---|---|---|---|---|---|
| M-MI (n=5) | 12.69 ± 0.88 | 80.74 ± 2.08 | 0.52 ± 0.17 | 9.59 ± 1.68 | 2.27 ± 0.51 | 3.26 ± 0.39 | 3.34 ± 0.80 |
| F-MI (n=5) | 8.20 ± 0.86** | 82.57 ± 4.53 | 0.97 ± 0.26 | 9.56 ± 1.15 | 3.38 ± 0.47 | 3.05 ± 0.38 | 1.88 ± 1.09 |
| p-Value | 0.004 | 0.34 | 0.08 | 0.50 | 0.08 | 0.36 | 0.15 |
| Effect size (Cohen’s d) | 2.31 | 0.25 | 0.97 | 0.007 | 1.04 | 0.25 | 0.70 |
Values are the mean ± SEM. CM, cardiac myocytes; CSA, cross-sectional area; F, female; M, male; MI, myocardial infraction; SM, smooth muscle.
p<0.01 vs. M-MI group.
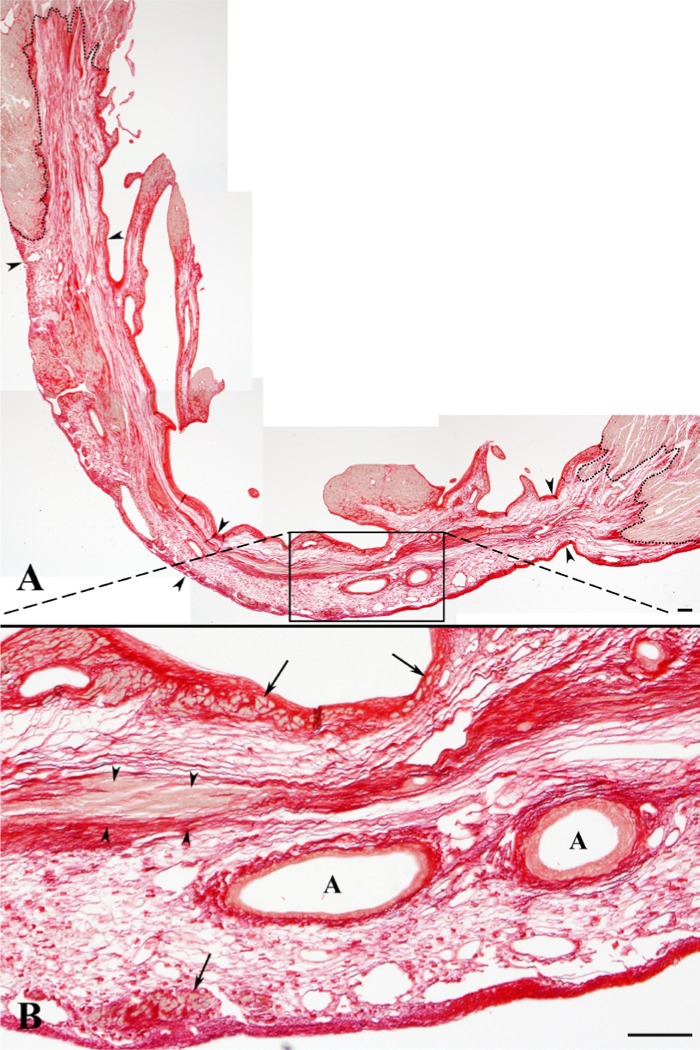
Figure 1.
Distribution of fibrillar collagen (red) visualized in a representative scar with picrosirius red stain. (A) Depicts the entire left ventricle scar, presented from an assembly (collage) of five images obtained with a magnification of 4X. Low-power view of an entire scar demonstrating its transmural portion (arrowheads) and well-demarcated, irregular borders (dotted lines). (B) High-power view of the area (outlined by a black box in A) displaying the arrangement of collagen fibers in loose (areolar) and dense, irregular connective tissue. In all scars, densely packed collagen fibers were noticed along the endocardial and epicardial surfaces, including subendocardial and subepicardial regions with surviving cardiac myocytes (CM) (arrows), in areas around the residual coronary arteries (A), and in the midwall layer where they occasionally embedded the remnants of mummified dead CM (arrowheads). On the other hand, loosely packed collagen fibers were dispersed through the rest of the scar. Scale bars are 100 µm.
Figure 2.
Distribution of α–smooth muscle (SM) actin–positive (+) cells (dark brown) detected in a representative scar with an anti–α-SM actin antibody, followed by visualization with DAB. (A) Depicts the entire left ventricle scar, presented from an assembly (collage) of five images obtained with a magnification of 4X. Low-power view of an entire scar demonstrating its transmural portion (arrowheads) and well-delineated, irregular borders (dotted lines). (B) High-power view of the area (outlined by a black box in A) displaying the arrangement of α-SM actin+ cells. In all scars, α-SM actin+ cells were detected in two separate structural compartments: vascular and non-vascular (interstitial). The α-SM actin+ cells associated with the wall of vessels (arrowheads), including large arteries (A) and veins (V), were designated as vascular SM cells. On the other hand, the α-SM actin+ cells seen in the interstitial space of subendocardial and subepicardial regions as well as in the midwall layer (arrows) were recognized as myofibroblasts. Note that surviving cardiac myocytes (M) seen in close proximity to a large coronary vein showed no immunoreaction for α-SM actin. Scale bars are 100 µm.
Figure 3.
Distribution of surviving and mummified dead cardiac myocytes (CM) visualized in a representative scar with Masson’s trichrome stain. (A) Depicts the entire left ventricle scar, presented from an assembly (collage) of four images obtained with a magnification of 4X. Low-power view of an entire scar demonstrating its transmural portion (arrowheads) and well-outlined, irregular borders (dotted lines). (B) High-power view of the area (outlined by a black box in A) displaying the arrangement of surviving CM (dark red) in subendocardial and subepicardial layers (arrows) and showing the presence of residual mummified muscle cells in the midwall layer (arrowheads). Note the presence of surviving CM in close proximity to a large vein (V) but not an artery (A). (A, B) In the upper right region, two small artifacts from the staining procedure were removed during imaging processing. Scale bars are 100 µm.
A thorough morphological analysis of the scars in both sexes demonstrated that fibrillar collagen was the major scar tissue component, generally organized into three distinguishable layers of densely packed fibers located in subepicardial, midwall, and subendocardial regions; in contrast, the rest of the scars were filled by loosely packed collagen fibers (Figs. 1B and 4A: arrows).
Figure 4.
High-power view of the deposition sites (A–C) and the quantitative content (D) of interstitial and vascular collagen fibers (red) in the scar stained with picrosirius red stain. (A) Densely packed interstitial collagen fibers surrounding myofibroblasts (arrowheads) and surviving cardiac myocytes (CM) (asterisks) in the subendocardial layer are located between an endocardial surface (a dotted line) and loosely packed interstitial collagen fibers (arrows). (B) Densely packed interstitial collagen fibers enfold the area of mummified dead CM (a dotted line), with some collagen deposition in between the degenerated muscle fibers (arrowheads). (C) Residual coronary artery (A) demonstrating a well-defined demarcation border between the arterial wall (a dotted line) and densely packed interstitial collagen fibers enclosing myofibroblasts (arrowheads) in the midwall layer. All collagen fibers detected around vascular smooth muscle cells (asterisks) within the arterial wall border are counted as vascular collagen. (D) Fractional volume of interstitial and vascular collagen fibers in the scar. Scale bars are 20 µm. Values are the mean ± SEM.
Most significantly, the areas with densely packed collagen fibers often contained surviving CM (Figs. 1B: arrows, 3B: arrows, 4A: asterisks, 6A, B: asterisks, and 7A, B: asterisks) or mummified dead CM (Figs. 1B: arrowheads, 3B: arrowheads, 4B and 6E: asterisks), as well as myofibroblasts (Figs. 4A, C: arrowheads, 6A–D: arrowheads, and 8A, B: arrows) and some residual coronary arteries (Figs. 1B, 3B, 4C, and 5). Accordingly, the same areas were the principal locations for α-SM actin–containing myofibroblasts (Figs. 2B: arrows and 6A–D: arrowheads) and vascular SM cells (Figs. 2B: arrowheads, 5A and 7D: arrowheads). The locations of vascular SM cells were evidently restricted to the wall of arterial vessels, demonstrating a great variety of sizes (Fig. 2B: arrowheads). In some large-resistance vessels, the vascular SM cells had undergone marked centripetal growth, resembling the neointimal formation (Fig. 5), which occasionally almost obliterated the vessel lumen.
Figure 6.
High-power view of the sites of myofibroblasts (A–E) and the quantitative content (F) of myofibroblasts and vascular smooth muscle (SM) cells in the scar. (A) Myofibroblasts (arrowheads) in the subendocardial layer are located just beneath an endocardial surface (a dotted line) within dense, irregular connective tissue that also contains α-SM actin–negative cardiac myocytes (CM) (asterisks). (B) A similar region as in A stained with Verhoeff’s elastic tissue stain: myofibroblasts (arrowheads), surrounded by elastic fibers (black), are located in the subendocardial layer just beneath the endocardial surface (a dotted line). Note that surviving CM (asterisks), enclosed within dense, irregular connective tissue (arrows), have no association with elastic fibers. (C) The compact bundle of myofibroblasts (arrowheads) located in the midwall layer demonstrates obvious distinction from dense, irregular connective tissue (a dotted line) that contains α-SM actin–negative fibroblasts (arrows). (D) A similar region as in C stained with Masson’s trichrome: myofibroblasts (arrowheads) form a bundle in the midwall layer that is well demarcated (a dotted line) from dense, irregular connective tissue containing fibroblasts (arrows). (E) Myofibroblasts (arrowheads) invading the area of α-SM actin–negative dead cardiac myocytes (asterisks) are located in the middle of myofibroblasts bundle (a dotted line). (F) Fractional volume of myofibroblasts and vascular SM cells in the scar. Scale bars are 20 µm. Values are the mean ± SEM.
Figure 7.
High-power view of the sites of surviving cardiac myocytes (CM) (A–E) and the quantitative content (F) of surviving and mummified dead CM in the scar. (A) Hematoxylin and eosin (H&E)–stained section demonstrating surviving CM (asterisks) engulfed in dense, irregular connective tissue of the subendocardial layer and bordered by a bundle of subendocardial myofibroblasts (arrows) from above and the capillary reach (arrowheads) layer of loose connective tissue from below. (B) A similar region as in A stained with Masson’s trichrome showing surviving CM (asterisks) embedded in dense, irregular connective tissue of the subendocardial layer (blue) just beneath the bundle of subendocardial myofibroblasts (arrows). Note that, in contrast to the myofibroblasts, CM are located in close proximity to capillary-like microvessels containing erythrocytes (arrowheads). (C) Picrosirius red-stained section revealing surviving CM (asterisks) around the former coronary vein (V), in contrast to the former thick-walled (arrowheads) coronary artery (A). Note that each CM is enveloped in a densely packed layer of collagen fibers (red). (D) The same region as in C stained for α–smooth muscle (SM) actin demonstrating that CM (asterisks) survive along a thin-walled vein (V) without vascular SM cells (arrows), whereas an artery (A) with a thick, SM cell–containing wall (asterisks), has no association with live CM. (E) H&E–stained section showing a large group of surviving CM (red) surrounding a large thin-walled vein (arrowheads) in the subepicardial layer. Note that, in contrast to avascular dense connective tissue seen just beneath an epicardial surface (pink), CM are surrounded by capillary-reach (asterisks), loose connective tissue. (F) Fractional volume of surviving and mummified dead CM in the scar. A dotted line in A, C, D, and E indicates the epicardial surface. Scale bars are 20 µm (A, B, E) and 50 µm (C, D). Values are the mean ± SEM.
Figure 8.
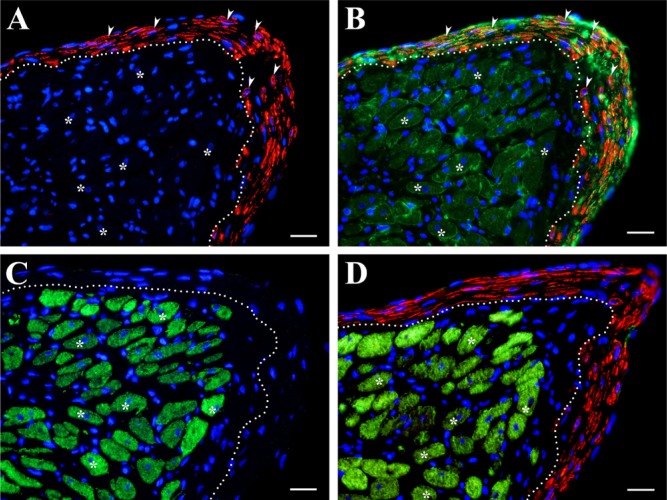
High-power view of the same site of myofibroblasts and surviving cardiac myocytes (CM) in the subendocardial layer of the scar, acquired from serial sections immunostained using a multilabeling fluorescence technique. (A) Myofibroblasts (red and arrowheads) positively labeled for α–smooth muscle (SM) actin are located just beneath an endocardial surface and above the area with surviving CM (a dotted line). (B) Myofibroblasts (red and arrowheads indicating the same cells as in A) positively labeled for α-SM actin and outlined by laminin immunostaining (green) showed clear delineation (a dotted line) from the area below that containing α-SM actin–negative, laminin-outlined CM (green and asterisks). (C) Surviving CM (green and asterisks) positively labeled for cardiac actin are located just beneath the layer of subendocardial myofibroblasts. Note that the layer of myofibroblasts demarcated by a dotted line showed no positive immunolabeling for cardiac actin. (D) Surviving CM (green and asterisks) positively labeled for desmin showed no immunoreaction for α-SM actin. On the contrary, the layer of α-SM actin–positive myofibroblasts (red) demarcated by a dotted line remained desmin negative. Note that, in all images (A–D), the asterisks identified the location of the same surviving CM, whereas DAPI-counterstained nuclei of all cells are in blue. Scale bars are 20 µm.
Figure 5.
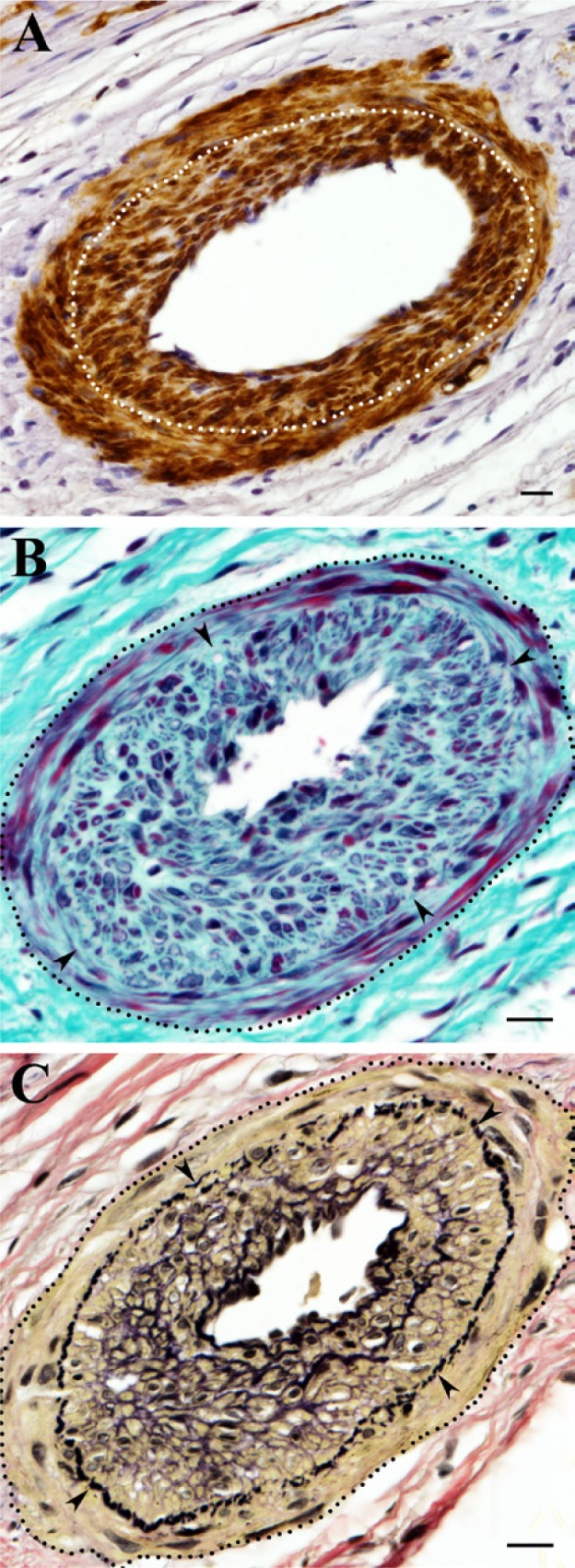
The arrangement of vascular smooth muscle (SM) cells within the wall of a residual coronary artery that underwent hyperplastic transformation in the scar. (A) Vascular SM cells positively stained for α-SM actin (dark brown) demonstrate two distinct layers (separated by a dotted line) in the arterial wall: circumferential (external) and centripetal (internal), which resembles the hyperplastic neointimal formation. (B) A similar arterial vessel as in A stained with Masson’s trichrome stain showing an apparent distinction between circumferential and centripetal layers (arrowheads) of vascular SM cells (dark red) as well as the well-defined vascular border (a dotted line) surrounded by dense, irregular connective tissue (blue). Note that, in the area of neointimal growth, most vascular SM cells are embedded in the thick layers of the extracellular matrix. (C) The same vessel as in B (a dotted line) stained with Verhoeff’s elastic tissue stain demonstrating the centripetal growth of vascular SM cells (yellow) within neointima formation beneath the internal elastic lamina (arrowheads). Note that neointimal SM cells in contrast to vascular SM cells of the circumferential layer are often surrounded by elastic fibers (black). Scale bars are 20 µm.
At the same time, the distinct spatial location, characteristic spindle-shaped morphological appearance, and positive reaction to α-SM actin made myofibroblasts easily distinguishable from surviving CM (Figs. 4A, C: arrowheads, 6A: arrowheads, 7A, B: arrows, and 8A, B: arrowheads), mummified dead CM (Fig. 6E), and fibroblasts (Fig. 6C, D: arrowheads). Furthermore, the absence of both desmin and cardiac actin expressions in α-SM actin–containing myofibroblasts, which was a noticeable feature of surviving CM (Fig. 8C,D), confirmed the clear distinction between these two cell populations.
In contrast to myofibroblasts that were surrounded by mainly “avascular” dense connective tissue, the neighboring surviving CM, located in subendocardial or subepicardial layers (Figs. 6A, B: asterisks and 8C, D), were often seen in association with the well-developed network of capillary-like microvessels (Fig. 7A, B: arrowheads and E: asterisks). Although the noticeable groups of surviving CM were rarely detected in the midwall layers, with the exception of some areas near the scar border, the large clusters of live CM could be frequently seen in close proximity to large veins deep in the scar tissue (Fig. 7C, D: asterisks and E).
Despite a high degree of similarity between the sexes, some minor discrepancies were noted with regard to the contents of vascular collagen (Fig. 4D) and vascular SM cells (Fig. 6F) as well as mummified dead CM (Fig. 7F) in male and female scars. For instance, compared with males (Table 2), LV scars of female rats had a slightly higher content of vascular collagen (p=0.08, d = 0.97) and vascular SM cells (p=0.08, d = 1.04) but a smaller volume fraction of mummified dead CM (p=0.15, d = 0.70).
Discussion
The key findings of this study were as follows: 1) the pattern of structural LV remodeling induced by a large transmural MI remained analogous between the sexes despite the fact that the left ventricle of the female middle-aged rats was significantly smaller than that of males, and 2) the volume fractions of major structural components were nearly identical in the mature LV scars of male and female middle-aged rats, although the female scars revealed significantly smaller volume and thickness compared with the scars in male counterparts.
Sex-Related Differences in LV Remodeling and Scar Formation
Despite a growing number of clinical observations demonstrating sex-related differences in the cardiac adaptation to postischemic injury (Kwon et al. 2009; Ostadal et al. 2009; Vaccarino 2010; Vaccarino et al. 2011), the experimental findings related to sex-specific alterations during post-MI remodeling and especially scar formation remained obscure. Most of these data were from experiments either exploring the earlier stages of post-MI healing in mice (Cavasin et al. 2004; Gao et al. 2005; Fang et al. 2007; Wang F et al. 2007) or analyzing cardiac remodeling and LV functional performance during the chronic post-MI phases in rats (Pfeffer MA et al. 1979; Litwin et al. 1999; Jain et al. 2002). Surprisingly, all of these studies were done on young or young-adult animals. However, young or even adult rodents are not suitable as models of myocardial infarction in humans, because myocardial infarctions occur primarily in middle-aged and older individuals (Bairey Merz et al. 2006). The importance of using older animals in MI experiments, especially in the case of small rodents, has been compelled by the findings of studies published earlier by our group (Christensen et al. 2009; Dedkov et al. 2005; Dedkov, Zheng, et al. 2007; Zhang et al. 2010) and other laboratories (Kranz et al. 1975; Wexler 1978; Raya et al. 1997; Bujak et al. 2008; Yang et al. 2008), which demonstrated the existence of significant age-associated differences in reparative responses during myocardial wound healing and LV remodeling. Accordingly, we exclusively used middle-aged rats of both sexes in all experiments described in this article. Another important factor that we considered in this study was the size of the MI. A great body of evidence demonstrates that rats can easily tolerate a small- and moderate-sized MI with no detectable alterations in cardiac performance, global LV geometry, and hemodynamics (Pfeffer MA et al. 1979; Olivetti et al. 1991), whereas a large MI has always triggered progressive LV remodeling and impairment in LV function (Michel et al. 1995; Olivetti et al. 1991; Pfeffer JM et al. 1984, 1991; Pfeffer MA et al. 1979). In the latter case, the process of ventricular reorganization and scar formation would certainly be affected by a higher level of circulating neurohumoral factors, which have been shown to be proportional to the degree of cardiac functional insufficiency and, hence, the size of the MI (Michel et al. 1995). Considering this, we performed sex-related comparisons only between the rats with comparably large infarcts. One of the important findings of our study is that despite a significant difference in heart size, the left ventricles of male and female middle-aged rats demonstrated a nearly identical pattern of global structural remodeling 4 weeks after a similarly large MI, although some regional differences were evident. These observations are consistent with the previous study by Jain and colleagues (2002), who demonstrated that despite the smaller chamber diameter and thinner septal and free walls, the left ventricle of Dahl salt-resistant female rats showed relatively similar global changes 5 weeks after a large MI compared with male counterparts. In contrast, the data from an earlier study, involving male and female Sprague-Dawley rats with a large MI, have reported a significant sex-related difference in chamber geometry 6 weeks after MI (Litwin et al. 1999). Considering that hearts with a large MI are more prone to undergoing continuing LV remodeling (Pfeffer JM et al. 1991), such discrepancy among the findings in these studies can primarily be attributed to the variations in the duration of the post-MI period. Hence, in our study, we focused on a period of 4 weeks because scar formation is usually completed between the third and fourth week after infarction (Boyle and Weisman 1993; Fishbein et al. 1978), and during this period of post-MI healing, there are no detectable changes in external LV dimensions (Roberts et al. 1984) that are characteristic of compensated rather than adverse LV remodeling (Pfeffer MA and Braunwald 1990). Accordingly, we assume that, during the compensatory phase of post-MI healing, the left ventricles of male and female middle-aged rats with a similarly large MI followed the analogous sex-independent pattern of global LV remodeling due to the corresponding neurohumoral reaction (Michel et al. 1995) and comparable alterations in systemic hemodynamic and LV loading conditions (Olivetti et al. 1991; Pfeffer JM et al. 1984, 1991). Although it is feasible to argue that the reparative processes in middle-aged (12-month-old) female rats could be affected by a low estrogen level (Nass et al. 1984; Rubin et al. 1994), the sex hormone–independent nature of chronic LV modifications during post-MI cardiac remodeling has been established in estrogen-deficient young female rats (Hugel et al. 1999) and mice (Cavasin et al. 2003).
Effect of Biological Sex on the Structural Composition of a Scar
Although a universal sequence of reparative events triggered by an acute MI has been thoroughly investigated for decades (Frangogiannis 2006), evidence of sex-related differences in scar composition during inflammatory and proliferative phases of post-MI healing in mice has been documented only recently (Cavasin et al. 2004; Gao et al. 2005; Fang et al. 2007; Wang F et al. 2007). Surprisingly, these studies did not investigate the possible sex-specific modifications during post-MI scar maturation, although Sun and colleagues (Sun and Weber 2000; Sun et al. 2002) previously revealed the highly dynamic pattern of scar transformation in rats, particularly during the later phases of myocardial healing. Accordingly, our study is the first to report a sex-specific comparison of the structural composition of the mature scar in middle-aged post-MI rats.
Despite the fact that post-MI scars are often composed of a variety of structural components (collagen and elastic fibers, myofibroblasts, surviving and dead CM, residual and newly formed vessels), there are few studies demonstrating the quantitative data with regard to more than one scar component (Boyle and Weisman 1993; Lichtenauer et al. 2011; Virag and Murry 2003). Hence, our study is unique because it has evaluated the contents of several major scar components at once and, more importantly, the study has been done in the context of potential sex-specific differences. To our surprise, we found no evident sex-specific distinctions in fractional volumes among all major structural components of 4-week-old scars (namely, fibrillar collagen, surviving and mummified dead CM, myofibroblasts, and vascular SM cells) in middle-aged post-MI rats. We also compared the spatial distribution of these components in each scar to further validate this observation. Our finding of a nearly identical three-layered organization of densely packed fibrillar collagen merged by loose connective tissue within the scars of middle-aged rats is consistent with the observations reported earlier in post-MI dogs (Whittaker et al. 1989) and rats (Sun et al. 1994; Zhang et al. 2010). Considering that the left ventricles in male and female rats had undergone a similar extent of post-MI remodeling, the relatively analogous distribution of fibrillar collagen within the scars may to some extent reflect the similar regional directions of tensile force (Holmes et al. 1997) applied to the scar by adjacent hypertrophied CM (Vracko et al. 1988; Vracko et al. 1989). The same tensile forces might also be responsible for the distribution of nonvascular spindle-shaped α-SM actin–positive cells, presumably myofibroblasts, within the areas primarily corresponding to those layers of densely packed fibrillar collagen, as seen in Figs. 2B (arrows) and 6A–D (arrowheads) of our study and in reports previously published by others on mice (Virag and Murry 2003), rats (Sun and Weber 1996), dogs (Frangogiannis et al. 2000), and humans (Willems et al. 1994). Such spatial codistribution, however, could be a consequence of myofibroblast involvement in collagen deposition (Cleutjens et al. 1995), as suggested by Sun and colleagues (Sun and Weber 1996; Sun et al. 2000).
On the other hand, a separate large group of α-SM actin–positive cells seen in the scars of middle-aged rats of both sexes was primarily restricted to the walls of arteries and arterioles, representing the vascular SM cells (Vracko and Thorning 1991; Sun et al. 2002; Dobaczewski et al. 2004). A similar finding has been reported in a number of studies on post-MI dogs (Kramer et al. 1998; Dobaczewski et al. 2004) and rats (Kalkman et al. 1997; Sun and Weber 2000). In agreement with these studies, we also noticed that, in some large arteries, the vascular SM cells had contributed to the neointimal growth that narrows the vessel lumen (Dobaczewski et al. 2004; Kalkman et al. 1997; Sun et al. 2002). Considering that the portion of vascular SM cells among all α-SM actin–positive cells was greater in female rats than in males (26% vs. 20%, respectively), it appears that the inward growth of vascular SM cells within the wall of residual arteries is greater in female than in male scars. This finding implies that the remodeling process of large-resistance vessels in female scars might be influenced by a different mitogenic milieu than that in male scars. One possibility is that the greater number of macrophages detected in healing post-MI scars of female animals during an inflammatory phase (Cavasin et al. 2004) could underlie current observations, because macrophages facilitate inward remodeling in arterial vessels experiencing a reduction in blood flow (Bakker et al. 2008). Such an assumption is also supported by the fact that the slightly higher fractional volume of mummified dead CM seen in post-MI scars of middle-aged male rats (as compared with females) may be a result of reduced macrophage response during MI healing similar to that observed in rats after inhibition of immune response with high doses of corticosteroids (Kloner et al. 1978; Mannisi et al. 1987) or in monocyte chemotactic protein 1–deficient mice showing an impeded phagocytic response (Dewald et al. 2005).
Despite a likely different phagocytic response between the sexes, an extent of long-term CM survival did not differ in the large transmural scars of male and female middle-aged rats. Our observations concerning the spared clusters of surviving CM in subendocardial and subepicardial areas of scars, as well as around the residual large veins, are in agreement with the previous findings on young rats (Boyle and Weisman 1993; Fishbein et al. 1978; Kalkman et al. 1997; Sun et al. 1994; Zhang et al. 2010) and mice (Virag and Murry 2003). The sex-independent distribution of surviving CM within the scars caused by a similarly high level of coronary artery occlusion is rather a result of the virtually identical branching pattern of major coronary arteries in the heart of small rodents (Clauss et al. 2006; Dedkov, Thomas, et al. 2007) that would restrict the long-term CM salvaging only to the areas with an adequate diffusion for oxygen and nutrients (Wang B et al. 2005; Zhang et al. 2010).
Study Limitations
The current study investigated sex-related differences only in mature scars at one time point during a compensated phase of post-MI remodeling. Although it is unlikely, it is possible that the structural composition of the scar could be further modified by the continuing heart transformation during an adverse phase of cardiac remodeling. Furthermore, because we have selected rats only with a large MI for a final evaluation, the impact of the moderate- or small-size infarcts on the scar composition remains unknown. In addition, we focused only on the structural components that provide most of the tensile strength to the scar, leaving undetermined the contents of other important scar components, such as the ground substance, elastic fibers, and microvasculature. Furthermore, the presented data did not differentiate the content of vascular SM cells between newly formed and residual vessels, and lacked explicit information regarding the precise nature of nonvascular α-SM actin–containing cells, which were all recognized as myofibroblasts.
Conclusions
Taken together, our data demonstrate that, despite a significant difference in size between the two sexes, the left ventricles of male and female rats undergo a similar degree of structural remodeling in response to a comparably large transmural MI. Moreover, because the structural composition of the mature LV scars reveals no apparent sex-related differences among the middle-aged rats, which experienced an MI of the same size, it is plausible to speculate that scar maturation occurs in a sex-independent manner.
Acknowledgments
We thank Lance P. Christensen for performing all surgical procedures, Alice O’Connor for help with histological techniques, and Dr. Min-Kyung Jung for assistance with statistical analysis.
Footnotes
Declaration of Conflicting Interests: The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding: The author(s) disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: This study was supported by National Institute of Health grant R01-HL-62587 (RJT).
References
- Bairey Merz CN, Shaw LJ, Reis SE, Bittner V, Kelsey SF, Olson M, Johnson BD, Pepine CJ, Mankad S, Sharaf BL, et al. 2006. Insights from the NHLBI-sponsored Women’s Ischemia Syndrome Evaluation (WISE) study: Part II. Gender differences in presentation, diagnosis, and outcome with regard to gender-based pathophysiology of atherosclerosis and macrovascular and microvascular coronary disease. J Am Coll Cardiol. 47:S21–S29 [DOI] [PubMed] [Google Scholar]
- Bakker EN, Matlung HL, Bonta P, de Vries CJ, van Rooijen N, Vanbavel E. 2008. Blood flow–dependent arterial remodelling is facilitated by inflammation but directed by vascular tone. Cardiovasc Res. 78:341–348 [DOI] [PubMed] [Google Scholar]
- Boyle MP, Weisman HF. 1993. Limitation of infarct expansion and ventricular remodeling by late reperfusion: study of time course and mechanism in a rat model. Circulation. 88:2872–2883 [DOI] [PubMed] [Google Scholar]
- Bujak M, Kweon HJ, Chatila K, Li N, Taffet G, Frangogiannis NG. 2008. Aging-related defects are associated with adverse cardiac remodeling in a mouse model of reperfused myocardial infarction. J Am Coll Cardiol. 51:1384–1392 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cavasin MA, Sankey SS, Yu AL, Menon S, Yang XP. 2003. Estrogen and testosterone have opposing effects on chronic cardiac remodeling and function in mice with myocardial infarction. Am J Physiol Heart Circ Physiol. 284:H1560–H1569 [DOI] [PubMed] [Google Scholar]
- Cavasin MA, Tao Z, Menon S, Yang XP. 2004. Gender differences in cardiac function during early remodeling after acute myocardial infarction in mice. Life Sci. 75:2181–2192 [DOI] [PubMed] [Google Scholar]
- Christensen LP, Zhang RL, Zheng W, Campanelli JJ, Dedkov EI, Weiss RM, Tomanek RJ. 2009. Postmyocardial infarction remodeling and coronary reserve: effects of ivabradine and beta blockade therapy. Am J Physiol Heart Circ Physiol. 297:H322–H330 [DOI] [PubMed] [Google Scholar]
- Clauss SB, Walker DL, Kirby ML, Schimel D, Lo CW. 2006. Patterning of coronary arteries in wildtype and connexin43 knockout mice. Dev Dyn. 235:2786–2794 [DOI] [PubMed] [Google Scholar]
- Cleutjens JP, Verluyten MJ, Smiths JF, Daemen MJ. 1995. Collagen remodeling after myocardial infarction in the rat heart. Am J Pathol. 147:325–338 [PMC free article] [PubMed] [Google Scholar]
- Cohen J. 1988. Statistical power analysis for the behavioral sciences. 2nd ed. Hillsdale, NJ: Lawrence Erlbaum [Google Scholar]
- Connelly CM, Vogel WM, Wiegner AW, Osmers EL, Bing OH, Kloner RA, Dunn-Lanchantin DM, Franzblau C, Apstein CS. 1985. Effects of reperfusion after coronary artery occlusion on post-infarction scar tissue. Circ Res. 57:562–577 [DOI] [PubMed] [Google Scholar]
- Dai W, Wold LE, Dow JS, Kloner RA. 2005. Thickening of the infarcted wall by collagen injection improves left ventricular function in rats: a novel approach to preserve cardiac function after myocardial infarction. J Am Coll Cardiol. 46:714–719 [DOI] [PubMed] [Google Scholar]
- Dedkov EI, Christensen LP, Weiss RM, Tomanek RJ. 2005. Reduction of heart rate by chronic beta1-adrenoceptor blockade promotes growth of arterioles and preserves coronary perfusion reserve in postinfarcted heart. Am J Physiol Heart Circ Physiol. 288:H2684–H2693 [DOI] [PubMed] [Google Scholar]
- Dedkov EI, Thomas MT, Sonka M, Yang F, Chittenden TW, Rhodes JM, Simons M, Ritman EL, Tomanek RJ. 2007. Synectin/syndecan-4 regulate coronary arteriolar growth during development. Dev Dyn. 236:2004–2010 [DOI] [PubMed] [Google Scholar]
- Dedkov EI, Zheng W, Christensen LP, Weiss RM, Mahlberg-Gaudin F, Tomanek RJ. 2007. Preservation of coronary reserve by ivabradine-induced reduction in heart rate in infarcted rats is associated with decrease in perivascular collagen. Am J Physiol Heart Circ Physiol. 293:H590–H598 [DOI] [PubMed] [Google Scholar]
- Dedkov EI, Zheng W, Tomanek RJ. 2006. Compensatory growth of coronary arterioles in postinfarcted heart: regional differences in DNA synthesis and growth factor/receptor expression patterns. Am J Physiol Heart Circ Physiol. 291:H1686–H1693 [DOI] [PubMed] [Google Scholar]
- Dewald O, Zymek P, Winkelmann K, Koerting A, Ren G, Abou-Khamis T, Michael LH, Rollins BJ, Entman ML, Frangogiannis NG. 2005. CCL2/monocyte chemoattractant protein-1 regulates inflammatory responses critical to healing myocardial infarcts. Circ Res. 96:881–889 [DOI] [PubMed] [Google Scholar]
- Dobaczewski M, Akrivakis S, Nasser K, Michael LH, Entman ML, Frangogiannis NG. 2004. Vascular mural cells in healing canine myocardial infarcts. J Histochem Cytochem. 52:1019–1029 [DOI] [PubMed] [Google Scholar]
- Fang L, Gao XM, Moore XL, Kiriazis H, Su Y, Ming Z, Lim YL, Dart AM, Du XJ. 2007. Differences in inflammation, MMP activation and collagen damage account for gender difference in murine cardiac rupture following myocardial infarction. J Mol Cell Cardiol. 43:535–544 [DOI] [PubMed] [Google Scholar]
- Fishbein MC, Maclean D, Maroko PR. 1978. Experimental myocardial infarction in the rat: qualitative and quantitative changes during pathologic evolution. Am J Pathol. 90:57–70 [PMC free article] [PubMed] [Google Scholar]
- Frangogiannis NG. 2006. The mechanistic basis of infarct healing. Antioxid Redox Signal. 8:1907–1939 [DOI] [PubMed] [Google Scholar]
- Frangogiannis NG, Michael LH, Entman ML. 2000. Myofibroblasts in reperfused myocardial infarcts express the embryonic form of smooth muscle myosin heavy chain (SMemb). Cardiovasc Res. 48:89–100 [DOI] [PubMed] [Google Scholar]
- Gao XM, Xu Q, Kiriazis H, Dart AM, Du XJ. 2005. Mouse model of post-infarct ventricular rupture: time course, strain- and gender-dependency, tensile strength, and histopathology. Cardiovasc Res. 65:469–477 [DOI] [PubMed] [Google Scholar]
- Hayakawa K, Takemura G, Kanoh M, Li Y, Koda M, Kawase Y, Maruyama R, Okada H, Minatoguchi S, Fujiwara T, et al. 2003. Inhibition of granulation tissue cell apoptosis during the subacute stage of myocardial infarction improves cardiac remodeling and dysfunction at the chronic stage. Circulation. 108:104–109 [DOI] [PubMed] [Google Scholar]
- Hochman JS, Bulkley BH. 1982. Expansion of acute myocardial infarction: an experimental study. Circulation. 65:1446–1450 [DOI] [PubMed] [Google Scholar]
- Hochman JS, Choo H. 1987. Limitation of myocardial infarct expansion by reperfusion independent of myocardial salvage. Circulation. 75:299–306 [DOI] [PubMed] [Google Scholar]
- Holmes JW, Nunez JA, Covell JW. 1997. Functional implications of myocardial scar structure. Am J Physiol. 272:H2123–H2130 [DOI] [PubMed] [Google Scholar]
- Hugel S, Reincke M, Stromer H, Winning J, Horn M, Dienesch C, Mora P, Schmidt HH, Allolio B, Neubauer S. 1999. Evidence against a role of physiological concentrations of estrogen in post–myocardial infarction remodeling. J Am Coll Cardiol. 34:1427–1434 [DOI] [PubMed] [Google Scholar]
- Jain M, Liao R, Podesser BK, Ngoy S, Apstein CS, Eberli FR. 2002. Influence of gender on the response to hemodynamic overload after myocardial infarction. Am J Physiol Heart Circ Physiol. 283:H2544–H2550 [DOI] [PubMed] [Google Scholar]
- Kalkman EA, van Haren P, Saxena PR, Schoemaker RG. 1997. Regionally different vascular response to vasoactive substances in the remodelled infarcted rat heart: aberrant vasculature in the infarct scar. J Mol Cell Cardiol. 29:1487–1497 [DOI] [PubMed] [Google Scholar]
- Kloner RA, Fishbein MC, Lew H, Maroko PR, Braunwald E. 1978. Mummification of the infarcted myocardium by high dose corticosteroids. Circulation. 57:56–63 [DOI] [PubMed] [Google Scholar]
- Kramer MF, Kinscherf R, Aidonidis I, Metz J. 1998. Occurrence of a terminal vascularisation after experimental myocardial infarction. Cell Tissue Res. 291:97–105 [DOI] [PubMed] [Google Scholar]
- Kranz D, Hecht A, Fuhrmann I. 1975. The influence of age on the wound healing of experimental myocardial infarction in rats. Exp Pathol (Jena). 11:107–114 [DOI] [PubMed] [Google Scholar]
- Kwon DH, Halley CM, Popovic ZB, Carrigan TP, Zysek V, Setser R, Schoenhagen P, Flamm SD, Starling RC, Desai MY. 2009. Gender differences in survival in patients with severe left ventricular dysfunction despite similar extent of myocardial scar measured on cardiac magnetic resonance. Eur J Heart Fail. 11:937–944 [DOI] [PubMed] [Google Scholar]
- Lichtenauer M, Mildner M, Baumgartner A, Hasun M, Werba G, Beer L, Altmann P, Roth G, Gyongyosi M, Podesser BK, et al. 2011. Intravenous and intramyocardial injection of apoptotic white blood cell suspensions prevents ventricular remodelling by increasing elastin expression in cardiac scar tissue after myocardial infarction. Basic Res Cardiol. 106:645–655 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Litwin SE, Katz SE, Litwin CM, Morgan JP, Douglas PS. 1999. Gender differences in postinfarction left ventricular remodeling. Cardiology. 91:173–183 [DOI] [PubMed] [Google Scholar]
- Mannisi JA, Weisman HF, Bush DE, Dudeck P, Healy B. 1987. Steroid administration after myocardial infarction promotes early infarct expansion: a study in the rat. J Clin Invest. 79:1431–1439 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Michel JB, Nicolletti A, Arnal JF. 1995. Left ventricular remodelling following experimental myocardial infarction. Eur Heart J. 16(Suppl I):49–57 [DOI] [PubMed] [Google Scholar]
- Mizuno T, Mickle DA, Kiani CG, Li RK. 2005. Overexpression of elastin fragments in infarcted myocardium attenuates scar expansion and heart dysfunction. Am J Physiol Heart Circ Physiol. 288:H2819–H2827 [DOI] [PubMed] [Google Scholar]
- Mizuno T, Yau TM, Weisel RD, Kiani CG, Li RK. 2005. Elastin stabilizes an infarct and preserves ventricular function. Circulation. 112:I81–I88 [DOI] [PubMed] [Google Scholar]
- Nass TE, LaPolt PS, Judd HL, Lu JK. 1984. Alterations in ovarian steroid and gonadotrophin secretion preceding the cessation of regular oestrous cycles in ageing female rats. J Endocrinol. 100:43–50 [DOI] [PubMed] [Google Scholar]
- Olivetti G, Capasso JM, Meggs LG, Sonnenblick EH, Anversa P. 1991. Cellular basis of chronic ventricular remodeling after myocardial infarction in rats. Circ Res. 68:856–869 [DOI] [PubMed] [Google Scholar]
- Ostadal B, Netuka I, Maly J, Besik J, Ostadalova I. 2009. Gender differences in cardiac ischemic injury and protection—experimental aspects. Exp Biol Med (Maywood). 234:1011–1019 [DOI] [PubMed] [Google Scholar]
- Pfeffer JM, Pfeffer MA, Fletcher PJ, Braunwald E. 1984. Ventricular performance in rats with myocardial infarction and failure. Am J Med. 76:99–103 [DOI] [PubMed] [Google Scholar]
- Pfeffer JM, Pfeffer MA, Fletcher PJ, Braunwald E. 1991. Progressive ventricular remodeling in rat with myocardial infarction. Am J Physiol. 260:H1406–H1414 [DOI] [PubMed] [Google Scholar]
- Pfeffer MA, Braunwald E. 1990. Ventricular remodeling after myocardial infarction: experimental observations and clinical implications. Circulation. 81:1161–1172 [DOI] [PubMed] [Google Scholar]
- Pfeffer MA, Pfeffer JM, Fishbein MC, Fletcher PJ, Spadaro J, Kloner RA, Braunwald E. 1979. Myocardial infarct size and ventricular function in rats. Circ Res. 44:503–512 [DOI] [PubMed] [Google Scholar]
- Raya TE, Gaballa M, Anderson P, Goldman S. 1997. Left ventricular function and remodeling after myocardial infarction in aging rats. Am J Physiol. 273:H2652–H2658 [DOI] [PubMed] [Google Scholar]
- Roberts CS, Maclean D, Maroko P, Kloner RA. 1984. Early and late remodeling of the left ventricle after acute myocardial infarction. Am J Cardiol. 54:407–410 [DOI] [PubMed] [Google Scholar]
- Rubin BS, Lee CE, King JC. 1994. A reduced proportion of luteinizing hormone (LH)–releasing hormone neurons express Fos protein during the preovulatory or steroid-induced LH surge in middle-aged rats. Biol Reprod. 51:1264–1272 [DOI] [PubMed] [Google Scholar]
- Spadaro J, Fishbein MC, Hare C, Pfeffer MA, Maroko PR. 1980. Characterization of myocardial infarcts in the rat. Arch Pathol Lab Med. 104:179–183 [PubMed] [Google Scholar]
- Sun Y, Cleutjens JP, Diaz-Arias AA, Weber KT. 1994. Cardiac angiotensin converting enzyme and myocardial fibrosis in the rat. Cardiovasc Res. 28:1423–1432 [DOI] [PubMed] [Google Scholar]
- Sun Y, Kiani MF, Postlethwaite AE, Weber KT. 2002. Infarct scar as living tissue. Basic Res Cardiol. 97:343–347 [DOI] [PubMed] [Google Scholar]
- Sun Y, Weber KT. 1996. Angiotensin converting enzyme and myofibroblasts during tissue repair in the rat heart. J Mol Cell Cardiol. 28:851–858 [DOI] [PubMed] [Google Scholar]
- Sun Y, Weber KT. 2000. Infarct scar: a dynamic tissue. Cardiovasc Res. 46:250–256 [DOI] [PubMed] [Google Scholar]
- Sun Y, Zhang JQ, Zhang J, Lamparter S. 2000. Cardiac remodeling by fibrous tissue after infarction in rats. J Lab Clin Med. 135:316–323 [DOI] [PubMed] [Google Scholar]
- Vaccarino V. 2010. Ischemic heart disease in women: many questions, few facts. Circ Cardiovasc Qual Outcomes. 3:111–115 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vaccarino V, Badimon L, Corti R, de Wit C, Dorobantu M, Hall A, Koller A, Marzilli M, Pries A, Bugiardini R. 2011. Ischaemic heart disease in women: are there sex differences in pathophysiology and risk factors? Position paper from the working group on coronary pathophysiology and microcirculation of the European Society of Cardiology. Cardiovasc Res. 90:9–17 [DOI] [PMC free article] [PubMed] [Google Scholar]
- van den Borne SW, Diez J, Blankesteijn WM, Verjans J, Hofstra L, Narula J. 2010. Myocardial remodeling after infarction: the role of myofibroblasts. Nat Rev Cardiol. 7:30–37 [DOI] [PubMed] [Google Scholar]
- Virag JI, Murry CE. 2003. Myofibroblast and endothelial cell proliferation during murine myocardial infarct repair. Am J Pathol. 163:2433–2440 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vracko R, Thorning D. 1991. Contractile cells in rat myocardial scar tissue. Lab Invest. 65:214–227 [PubMed] [Google Scholar]
- Vracko R, Thorning D, Frederickson RG. 1989. Connective tissue cells in healing rat myocardium. A study of cell reactions in rhythmically contracting environment. Am J Pathol. 134:993–1006 [PMC free article] [PubMed] [Google Scholar]
- Vracko R, Thorning D, Frederickson RG, Cunningham D. 1988. Myocyte reactions at the borders of injured and healing rat myocardium. Lab Invest. 59:104–114 [PubMed] [Google Scholar]
- Wang B, Ansari R, Sun Y, Postlethwaite AE, Weber KT, Kiani MF. 2005. The scar neovasculature after myocardial infarction in rats. Am J Physiol Heart Circ Physiol. 289:H108–H113 [DOI] [PubMed] [Google Scholar]
- Wang F, Keimig T, He Q, Ding J, Zhang Z, Pourabdollah-Nejad S, Yang XP. 2007. Augmented healing process in female mice with acute myocardial infarction. Gend Med. 4:230–247 [DOI] [PubMed] [Google Scholar]
- Wexler BC. 1978. Myocardial infarction in young vs old male rats: pathophysiologic changes. Am Heart J. 96:70–80 [DOI] [PubMed] [Google Scholar]
- Whittaker P. 1995. Unravelling the mysteries of collagen and cicatrix after myocardial infarction. Cardiovasc Res. 29:758–762 [PubMed] [Google Scholar]
- Whittaker P, Boughner DR, Kloner RA. 1989. Analysis of healing after myocardial infarction using polarized light microscopy. Am J Pathol. 134:879–893 [PMC free article] [PubMed] [Google Scholar]
- Whittaker P, Kloner RA, Boughner DR, Pickering JG. 1994. Quantitative assessment of myocardial collagen with picrosirius red staining and circularly polarized light. Basic Res Cardiol. 89:397–410 [DOI] [PubMed] [Google Scholar]
- Willems IE, Havenith MG, De Mey JG, Daemen MJ. 1994. The alpha–smooth muscle actin-positive cells in healing human myocardial scars. Am J Pathol. 145:868–875 [PMC free article] [PubMed] [Google Scholar]
- Yang Y, Ma Y, Han W, Li J, Xiang Y, Liu F, Ma X, Zhang J, Fu Z, Su YD, et al. 2008. Age-related differences in postinfarct left ventricular rupture and remodeling. Am J Physiol Heart Circ Physiol. 294:H1815–H1822 [DOI] [PubMed] [Google Scholar]
- Zhang RL, Christensen LP, Tomanek RJ. 2010. Chronic heart rate reduction facilitates cardiomyocyte survival after myocardial infarction. Anat Rec (Hoboken). 293:839–848 [DOI] [PubMed] [Google Scholar]