Significance
Human ovaries hold follicles containing oocytes. When follicles mature, they release eggs for fertilization. Patients with primary ovarian insufficiency develop menopausal symptoms at less than 40 y of age. They have few remaining follicles and their only chance for bearing a baby is through egg donation. Kawamura et al. demonstrated that Hippo and Akt signaling pathways regulate follicle growth. Using an in vitro activation approach, they first removed ovaries from infertile patients, followed by fragmentation to disrupt Hippo signaling and drug treatment to stimulate Akt signaling. After grafting ovarian tissues back to patients, they found rapid follicle growth in some patients and successfully retrieved mature eggs. After in vitro fertilization and embryo transfer, a live birth is now reported.
Keywords: ovary, aging, YAP, CCN2, PTEN
Abstract
Primary ovarian insufficiency (POI) and polycystic ovarian syndrome are ovarian diseases causing infertility. Although there is no effective treatment for POI, therapies for polycystic ovarian syndrome include ovarian wedge resection or laser drilling to induce follicle growth. Underlying mechanisms for these disruptive procedures are unclear. Here, we explored the role of the conserved Hippo signaling pathway that serves to maintain optimal size across organs and species. We found that fragmentation of murine ovaries promoted actin polymerization and disrupted ovarian Hippo signaling, leading to increased expression of downstream growth factors, promotion of follicle growth, and the generation of mature oocytes. In addition to elucidating mechanisms underlying follicle growth elicited by ovarian damage, we further demonstrated additive follicle growth when ovarian fragmentation was combined with Akt stimulator treatments. We then extended results to treatment of infertility in POI patients via disruption of Hippo signaling by fragmenting ovaries followed by Akt stimulator treatment and autografting. We successfully promoted follicle growth, retrieved mature oocytes, and performed in vitro fertilization. Following embryo transfer, a healthy baby was delivered. The ovarian fragmentation–in vitro activation approach is not only valuable for treating infertility of POI patients but could also be useful for middle-aged infertile women, cancer patients undergoing sterilizing treatments, and other conditions of diminished ovarian reserve.
Between 5% and 10% of reproductive-age women are infertile due to polycystic ovarian syndrome (PCOS) (1), whereas 1% of them suffer from infertility due to primary ovarian insufficiency (POI) (2, 3). They are infertile due to aberrant follicle growth. As early as the 1930s, ovarian wedge resection (4) was used for PCOS treatment to induce follicle growth, followed by recent success based on ovarian “drilling” by diathermy or laser (5). In addition, ovarian cortices are routinely fragmented to allow better freezing and grafting for fertility preservation in cancer patients who underwent sterilizing treatment (6). Subsequent autotransplantation of ovarian fragments is associated with spontaneous follicle growth. Underlying mechanisms for these disruptive procedures to promote follicle growth are, however, unclear.
The Hippo signaling pathway is essential to maintain optimal organ size and is conserved in all metazoan animals (7–9). Hippo signaling consists of several negative growth regulators acting in a kinase cascade that ultimately phosphorylates and inactivates key Hippo signaling effectors, Yes-associated protein(YAP)/transcriptional coactivator with PDZ-binding motif(TAZ). When Hippo signaling is disrupted, decreases in YAP phosphorylation increase nuclear levels of YAP. YAP acts in concert with TEAD transcriptional factors to increase downstream CCN growth factors and baculoviral inhibitors of apoptosis repeat containing (BIRC) apoptosis inhibitors (7). CCN proteins, in turn, stimulate cell growth, survival, and proliferation (10).
Using a murine model, we now demonstrated the promotion of follicle growth following ovarian fragmentation and allo-transplantation. Ovarian fragmentation increased actin polymerization, decreased phospho-YAP (pYAP) levels, increased nuclear localization of YAP, as well as enhanced expression of CCN growth factors and BIRC apoptosis inhibitors. Fragmentation-induced follicle growth was partially blocked by CCN2 antibodies and verteporfin, a small molecule that inhibits interactions of YAP with TEAD transcriptional factors (11).
Studies using phosphatase and tensin homolog deleted from chromosome 10 (PTEN) deletion mice indicated the stimulatory roles of Akt signaling in the development of primordial (12) and secondary follicles (13). Our earlier report demonstrated the ability of Akt stimulators to activate dormant primordial follicles (14). We now demonstrated additive increases in follicle growth when ovarian fragments containing secondary and smaller follicles were treated with Akt stimulators. Using this in vitro activation (IVA) method for infertility treatment of POI patients, we successfully promoted the growth of residual follicles in autografts and report a viable birth following oocyte retrieval and in vitro fertilization (IVF)–embryo transfer.
Results
Ovarian Fragmentation Promoted Follicle Growth.
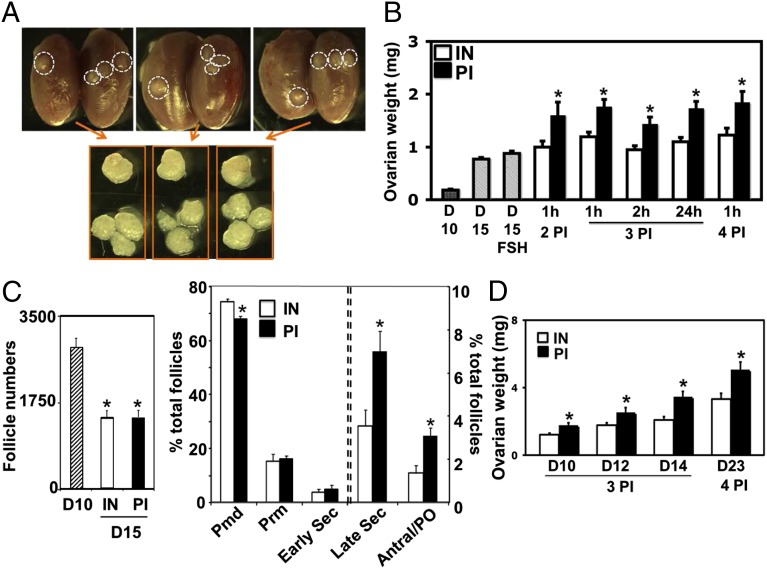
We fragmented ovaries from juvenile (day 10) mice containing secondary and smaller follicles, followed by allo-transplantation under kidney capsules of adult hosts. As shown in Fig. 1A, major increases in graft sizes were evident after cutting ovaries into three pieces and grafting for 5 d compared with paired intact ovaries. Graft weights increased after cutting ovaries into 2–4 pieces or incubating fragments for up to 24 h before grafting (Fig. 1B). Histological analyses (Fig. S1A) and follicle counting of grafts (Fig. 1C and Fig. S1B) indicated a loss of total follicles following fragmentation/grafting. However, major increases in the percentage of late secondary and antral/preovulatory follicles were evident, accompanied by decreases in primordial follicles (Fig. 1C). Compared with day 10 ovaries, the grafting procedure led to decreases in absolute number of primordial, primary, and early secondary follicles (Fig. S1B). Furthermore, cutting/grafting of ovaries from older mice, including those containing early antral follicles from day 23 animals, also increased graft weights (Fig. 1D).
Fig. 1.
Ovarian fragmentation and grafting promoted follicle growth in mice. Paired ovaries from juvenile mice were grafted into kidneys of adult ovariectomized mice (intact, IN; pieces, PI). Hosts were injected with FSH daily for 5 d before graft retrieval. (A) Morphology of paired ovarian grafts with or without fragmentation into three pieces. (A, Upper) Grafts inside kidney capsules. (A, Lower) Isolated paired grafts. (B) Weights of paired ovaries following fragmentation into 2–4 pieces from day 10 (D10) mice and incubated for 1–24 h before grafting. Ovarian weights before grafting (D10) and at 5 d after grafting with (D15 FSH) or without FSH treatment (D15) served as controls; n = 8–22. (C) Follicle dynamics before and after grafting of intact and fragmented (three pieces) ovaries from day 10 mice. (C, Left) Total follicle numbers. (C, Right) Follicle dynamics; n = 5. Pmd, primordial; Prm, primary; Sec, secondary; PO, preovulatory. (D) Weights of paired ovaries from mice at different ages following fragmentation into 3–4 pieces and grafting. Mean ± SEM; *P < 0.05; n = 8–22.
After grafting for 5 d, hosts received an ovulating dose of human chorionic gonadotropin (hCG). As shown in Fig. S1C, numbers of oocytes retrieved from fragmented grafts per ovary were 3.1-fold of those from intact grafts, accompanied by increased percentages of mature oocytes. Mature oocytes retrieved from fragmented grafts were fertilized and their development to early embryos was comparable to controls. After embryo transfer, healthy pups were delivered (Fig. S1D). Similar to mouse studies, fragmentation/autotransplantation of ovaries from rats also increased graft weights (Fig. S1 E and F).
Ovarian Fragmentation Increased Actin Polymerization and Disrupted Hippo Signaling.
Real-time RT-PCR and immunoblotting analyses (Fig. S2 A and B) indicated the expression of transcripts and proteins for key Hippo signaling genes in ovaries of juvenile mice. Also, immunohistochemical staining of ovaries from adult mice (Fig. S2C) indicated the expression of MST1/2, salvador (SAV)1, large tumor suppressor 1/2 (LATS1/2), and TAZ mainly in the cytoplasm of granulosa cells, theca cells, and oocytes of follicles at all sizes but at lower levels in the corpus luteum.
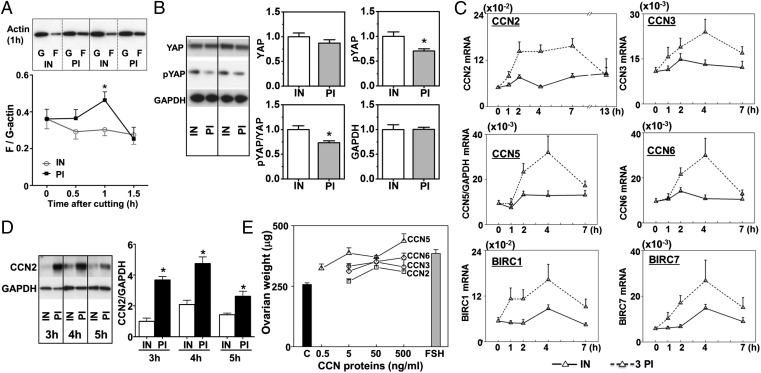
Polymerization of globular actin (G-actin) to the filamentous form (F-actin) is important for cell shape maintenance and locomotion. Recent genome-wide RNAi screening demonstrated that induction of extra F-actin formation disrupted Hippo signaling and induced overgrowth in Drosophila imaginal discs and human HeLa cells (15, 16). As shown in Fig. 2A, a transient increase in ratios of F-actin to G-actin was detected at 1 h after ovarian fragmentation. The Hippo signaling kinase cascade phosphorylates YAP to promote its cytoplasmic localization and degradation, thus decreasing its transcriptional actions. When Hippo signaling is disrupted, decreases in pYAP increase nuclear YAP levels (17). After ovarian fragmentation and incubation for 1 h, decreases in pYAP levels and pYAP to total YAP ratios were evident (Fig. 2B), suggesting Hippo signaling disruption. In intact ovaries from day 10 mice, immunohistochemical staining indicated that YAP was localized in the cytoplasm of granulosa cells in most follicles at primary and secondary stages (Fig. S2D). At 4 h after fragmentation, nuclear staining of YAP was found in granulosa cells of primary and secondary follicles.
Fig. 2.
Fragmentation of murine ovaries increased actin polymerization, disrupted Hippo signaling, and increased CCN growth factors and apoptosis inhibitors. (A) Ovarian fragmentation increased F-actin levels. Paired ovaries from day 10 mice were cut into three pieces or kept intact before immunoblotting analyses of F- and G-actin levels (Upper). (A, Lower) F- to G-actin ratios; n = 6–11. (B) Ovarian fragmentation decreased pYAP levels and pYAP to total YAP ratios. Paired ovaries with or without cutting were incubated for 1 h, followed by immunoblotting. (B, Left) Representative immunoblots. (B, Right) Ratios of different antigens; n = 8 pairs. (C) Ovarian fragmentation increased expression of CCN growth factors and BIRC apoptosis inhibitors. Paired ovaries with or without cutting were incubated for 1 h with subsequent grafting before analyses of transcript levels normalized by GAPDH. Intact ovaries, solid lines; pooled three pieces, dashed lines; n = 10–15. (D) Ovarian fragmentation increased CCN2 proteins. Paired ovaries with or without cutting were incubated for 3–5 h before immunoblotting. (D, Upper) Representative blots. (D, Lower) Quantitative analyses; n = 3–5. (E) Treatment with CCN2, 3, 5, and 6 increased ovarian explant weights. Explants from day 10 mice were cultured with different CCN growth factors for 4 d before weighing; n = 5–6. Mean ± SEM; *P < 0.05. IN, intact; PI, pieces.
Disruption of Hippo signaling leads to increased expression of downstream CCN growth factors and BIRC apoptosis inhibitors (7, 8). As shown in Fig. 2C, ovarian fragmentation and subsequent grafting increased transcript levels for several CCN growth factors (CCN2, 3, 5, and 6) and apoptosis inhibitors (BIRC1 and 7) in fragmented ovaries. Similar changes were found following continuous culture without grafting (Fig. S3A). Immunoblotting of highly expressed CCN2 demonstrated increased CCN2 proteins in fragmented ovaries (Fig. 2D). Real-time RT-PCR analyses showed fragmentation-induced increases in CCN2 transcripts in somatic cells, but not oocytes (Fig. S3B). The ability of CCN proteins to promote ovarian growth was further demonstrated by dose-dependent increases in ovarian explant weights after culturing with CCN2, 3, 5, and 6 (Fig. 2E). Analyses of follicle dynamics indicated the ability of CCN factors to promote the development of primary follicles to the late secondary stage in ovarian explants (Fig. S3C), underscoring the role of CCN proteins as ovarian growth factors.
Roles of Hippo Signaling and CCN2 in Fragmentation-Induced Follicle Growth.
YAP has no transcriptional activity and its actions are dependent on downstream transcriptional factors. Recent drug library screening identified a small molecule verteporfin, capable of inhibiting YAP association with TEAD transcriptional factors and suppressing YAP-induced liver overgrowth (11). Because fragmentation-induced CCN and BIRC changes were transient, we injected day 10 mice for 3 h with verteporfin before obtaining ovaries for fragmentation. As shown in Fig. S4A, pretreatment with verteporfin blocked fragmentation-induced increases in CCN2 transcripts without affecting those for anti-Müllerian hormone, a secondary follicle marker. In contrast to graft weight increases found between intact and fragmented ovarian pairs from vehicle-pretreated animals, no significant changes in graft weights were found between intact and fragmented pairs after pretreatment with verteporfin (Fig. S4B). Follicle counting of grafts indicated no loss of total follicles with verteporfin pretreatment (Fig. S4C). In contrast, verteporfin pretreatment prevented fragmentation-induced increases in late secondary follicles, with smaller suppression of antral/preovulatory follicles. We further incubated ovarian fragments with CCN2 antibodies for 18 h before grafting. Neutralization of endogenous CCN2 suppressed fragmentation-induced graft weight gain by 75% (Fig. S4D). These findings underscore the role of Hippo signaling in fragmentation-induced follicle growth.
Additive Effects of Hippo Signaling Disruption and Akt Stimulation on Secondary Follicle Growth.
In addition to the stimulatory role of Akt signaling in primordial follicle development (12, 14), conditional deletion of the PTEN gene in granulosa cells of secondary follicles also promoted follicle growth (13). We isolated secondary follicles from juvenile mice and demonstrated the ability of Akt stimulating drugs (PTEN inhibitor and PI3K activator) to promote secondary follicle growth (Fig. 3A). We further tested combined effects of Akt stimulating drugs and Hippo signaling disruption on ovarian graft growth. Using ovaries obtained from day 10 mice containing secondary and smaller follicles, we found additive increases in ovarian graft weights when fragmented ovaries were incubated with Akt stimulating drugs followed by grafting (Fig. 3B). Counting of follicles indicated increases in late secondary and antral/preovulatory follicles induced by fragmentation and Akt stimulation (Fig. 3C and Fig. S5).
Fig. 3.
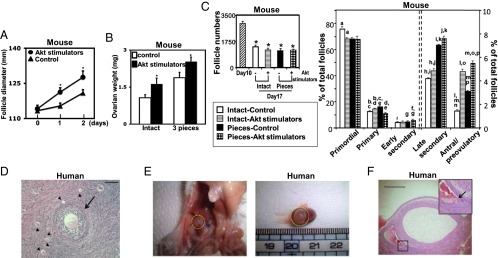
Additive effects of Hippo signaling disruption and Akt stimulation promoted secondary follicle growth. (A) Secondary follicles were isolated from juvenile mice and cultured with Akt stimulators; n = 30. (B) Additive increases in graft weights following ovarian fragmentation and/or Akt stimulation. Paired ovaries from juvenile mice were fragmented and incubated with or without Akt stimulators for 2 d followed by allo-transplantation for 5 d before graft weight determination; n = 8–10. (C) Follicle dynamics before and after grafting of intact and fragmented murine ovaries with or without treatment with Akt stimulators. (C, Left) Total follicle numbers. (C, Right) Follicle dynamics; n = 4. Same letter symbols indicate significant differences (P < 0.05). (D–F) Vitrified human cortical strips were thawed and fragmented into cubes before treatment with Akt stimulators for 2 d followed by grafting into immune-deficient mice for 4 wk. (D) Cortical strips before grafting. Arrow, a secondary follicle; arrowheads, primordial/primary follicles. (Scale bar, 100 μm.) (E) A kidney graft in situ (Left) and after isolation (Right), showing an antral follicle. (F) Histology of two large antral follicles with the side view of one showing an oocyte at the germinal vesicle stage (arrow). (Scale bar, 1 mm.) Mean ± SEM; *P < 0.05.
We obtained human ovarian cortical cubes containing secondary and smaller follicles. RT-PCR analyses demonstrated the expression of key Hippo signaling genes (Fig. S6A), whereas immunohistochemical analyses showed the expression of SAV1, LATS1/2, YAP, and TAZ in granulosa cells, theca cells, and oocytes of primordial to secondary follicles (Fig. S6B). We then thawed cryopreserved human ovarian cortical strips (1–2 mm thickness and 1 × 1 cm) and cut them into small cubes (1–2 mm2) before incubation. Real-time RT-PCR analyses indicated time-dependent increases in transcript levels for CCN2, 3, 5, and 6 (Fig. S6C). Higher CCN growth factor expression was found in ovarian cubes after further fragmentation from strips, suggesting fragmentation-induced disruption of Hippo signaling. We then cut human cortical strips containing secondary and smaller follicles (Fig. 3D) and incubated them with Akt stimulators before xenografting into immune-deficient mice. Within 4 wk, antral follicles were detected, demonstrating rapid follicle growth (Fig. 3 E and F).
Hippo Signaling Disruption and Akt Stimulation as Infertility Treatment.
In patients with POI, also known as premature ovarian failure, early exhaustion of ovarian function is evident due to genetic, immunological, iatrogenic, or other causes (2). POI is characterized by amenorrhea and elevated serum FSH before 40 y of age. Patients are infertile due to a lack of follicle growth and ovulation; oocyte donation is the only treatment option.
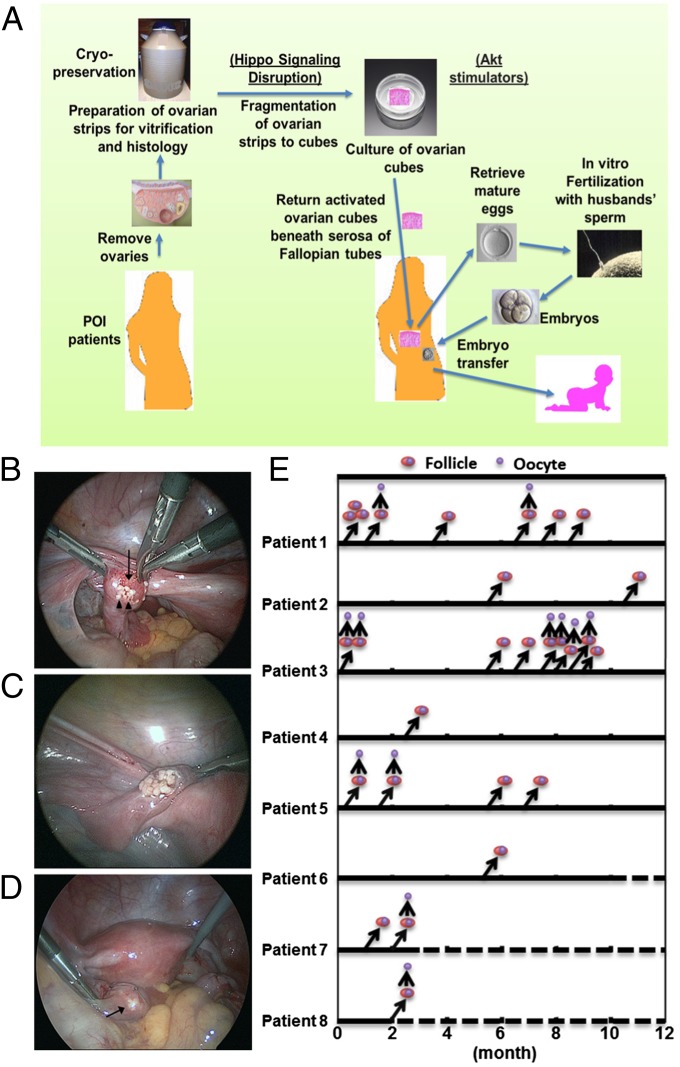
We obtained ovaries from POI patients for IVA based on Hippo signaling disruption and Akt stimulation, followed by autotransplantation and IVF–embryo transfer (Fig. 4A). Using laparoscopic surgery, ovaries were removed from 27 POI patients (37.3 ± 5.8 y of age; duration of amenorrhea, 6.8 ± 2.1 y), cut into strips (1–2 mm thickness and 1 × 1 cm), and vitrified (18). Randomly selected pieces were used for histological analyses, and ovaries from 13 of 27 patients contained residual follicles. Before autografting, frozen ovarian strips were thawed and fragmented into ∼100 cubes of 1–2 mm2, followed by treatment with Akt stimulating drugs for 2 d. Forty to 80 ovarian cubes each were then autotransplanted beneath the serosa of Fallopian tubes (Fig. 4 B–D; Movie S1).
Fig. 4.
Ovarian fragmentation/Akt stimulation followed by autografting promoted follicle growth in POI patients to generate mature oocytes for IVF–embryo transfer, pregnancy, and delivery. (A) Under laparoscopic surgery, ovaries were removed and cut into strips. Ovarian strips from POI patients were vitrified. After thawing, strips were fragmented into 1–2 mm2 cubes, before treatment with Akt stimulators. Two days later, cubes were autografted under laparoscopic surgery beneath serosa of Fallopian tubes. Follicle growth was monitored via transvaginal ultrasound and serum estrogen levels. After detection of antral follicles, patients were treated with FSH followed by hCG when preovulatory follicles were found. Mature oocytes were then retrieved and fertilized with the husband’s sperm in vitro before cyropreservation of four-cell stage embryos. Patients then received hormonal treatments to prepare the endometrium for implantation followed by transferring of thawed embryos. (B) Transplantation of ovarian cubes beneath the serosa of Fallopian tubes. Arrow, fallopian tube; arrowheads, cubes. (C) Multiple cubes were put beneath serosa. (D) Serosa after grafting. Ovarian cubes are visible beneath serosa (arrow). (E) Detection of preovulatory follicles in grafts for oocyte retrieval. Following ultrasound monitoring, follicle growth was found in eight patients. After follicles reached the antral stage (>5 mm in diameter, right upward arrows), patients were treated with FSH followed by hCG for egg retrieval (upward arrows). Double circles represent preovulatory follicles, whereas single circles represent retrieved oocytes. Dashed lines depict ongoing observation.
Following weekly or biweekly transvaginal ultrasound monitoring, together with serum estrogen measurement, follicle growth was found in eight patients (Fig. 4E), all of them belonging to those with histological signs of residual follicles. After follicles reached the antral stage (>5 mm in diameter; Fig. 4E, right upward arrows), patients were treated daily with FSH, followed by an injection of hCG when follicles reached >16 mm in diameter. Thirty-six hours later, egg retrieval was performed (Fig. 4E, upward arrows) under transvaginal ultrasound. Similar to normal menstrual cycles, usually only one follicle emerged from the growing pool to reach the preovulatory stage. Although earlier data indicated that 6 mo are needed for the development of human primordial follicles to the preovulatory stage (14, 19), all eight patients developed preovulatory follicles (∼2 cm) in less than 6 mo of grafting, with some of them (patients 1, 3, 5, and 7) developing preovulatory follicles within 3 wk, indicating rapid growth of secondary follicles. In contrast, preovulatory follicles developed after 6 mo of grafting were likely derived from primordial follicles.
Mature oocytes were successfully retrieved from five patients for intracytoplasmic sperm injection (ICSI) using the husband’s sperm. When embryos reached the four-cell stage, they were cryopreserved. For patient 1, one of two embryos was transferred. However, no pregnancy occurred. For patient 5, one of two embryos was transferred and pregnancy was diagnosed based on elevated serum hCG levels.
Patient 3 reached menarche at 11 y of age with regular menses. At 23 y of age, she experienced irregular cycles and became amenorrhea at 25 y of age with elevated FSH levels (>40 m international unit (mIU)/mL). Despite diverse testing including chromosome analysis, her pathogenesis was unknown. At 29 y of age, her ovaries were removed for fragmentation and Akt drug treatment. After monitoring of follicle growth and obtaining four four-cell embryos developed from six oocytes, two embryos were transferred and a successful singleton pregnancy was established. Consistent with reported safety of short-term treatment with Akt stimulators to activate primordial follicles in mice (20), a healthy baby (male; birth weight, 3,254 g; and Apgar score, 9 at 1 min/10 at 5 min) was delivered at 37 wk and 2 d of pregnancy. Physical features of the baby are normal, together with normal placenta and umbilical cord. No abnormal growth was detected in the transplanted site of the Fallopian tubes.
Discussion
Findings across multiple organ systems and model organisms have implicated Hippo signaling in the maintenance of organ sizes (7–9). However, our results uniquely document a role for Hippo signaling in mammalian ovaries. Our data indicate that ovarian fragmentation increased actin polymerization and disrupted Hippo signaling by decreasing pYAP levels together with increased nuclear localization of YAP, leading to increased expression of CCN growth factors and BIRC apoptosis inhibitors. Secreted CCN2 and related factors promoted follicle growth after transplantation (Fig. S7).
It is becoming clear that most ovarian follicles are constrained to growth under physiological conditions due to local Hippo signaling. Consistent with the role of Hippo signaling genes in restraining ovarian follicle growth, specific deletion of SAV1 or MST1/2 genes in hepatocytes resulted in enlarged livers (21, 22). Likewise, conditional deletion of SAV1 led to enlarged hearts (23). Hippo signaling is also critical for tissue regeneration and expansion of tissue-specific progenitor cells (17). For the ovary, LATS1-null female mice exhibited a POI phenotype (24), whereas LATS1 regulates the transcriptional activity of FOXL2, a gene mutated in some POI patients (25) (Fig. S7, boxed). Genome-wide association studies also implicated YAP as a susceptibility gene for PCOS (26), whereas deletion of CCN2/CTGF in ovarian granulosa cells in mice led to subfertility and aberrant follicle development (27) Also, genome-wide analyses identified changes in gene copy numbers for BIRC1 in POI patients (28).
F-actin formation in the stress fiber is required for the disruption of Hippo signaling and nuclear YAP accumulation (29). F-actin probably functions as a scaffold for Hippo signaling components because Hippo signaling genes MST1/2, merlin, and Amot all bind to actin (30). The upstream diaphanous (DIAPH) genes accelerate actin nucleation and suppress actin depolymerization. Of interest, disruption of the DIAPH2 coding region was found in a POI family (31), whereas genome-wide association studies identified DIAPH2 (32) and DIAPH3 (33) as candidate genes in regulating follicle reserve and menopause (Fig. S7).
Intestinal damage using dextran sodium sulfate decreases pYAP to total YAP ratios in regenerating crypts (34). Also, CCN1/CYR61 was induced in proximal straight tubules following ischemic reperfusion injury of the kidney (35). In the obstructed bladder, expression of CCN2/CTGF and CCN1/CYR61 were also induced (36). Disruption of Hippo signaling following actin polymerization likely represents a general mechanism in regulating tissue damage and remodeling, linking mechanical alterations of structural components to intracellular signaling.
Changes in actin polymerization and downstream events induced by ovarian fragmentation were transient in nature, and increases in CCN2/3/5/6 transcript levels occurred even when frozen human ovarian strips were fragmented after thawing. CCN growth factors and apoptosis inhibitors likely induce additional downstream changes, including the PI3K–target of rapamycin (TOR) signaling pathway (37), to promote follicle growth. Although vascularization changes during grafting cannot be ruled out, treatment with CCN2 antibodies or verteporfin partially suppressed fragmentation-induced increases in graft weights, underscoring the role of Hippo signaling.
Mechanical tension associated with the rigid sclerotic capsules in some PCOS ovaries could lead to arrested follicle development. Ovarian wedge resection (4, 38) or drilling by diathermy/laser (5) in PCOS patients results in follicle growth and comparable live birth rate compared with the popular gonadotropin treatment. Our studies suggest that damage incurred by cutting or drilling PCOS ovaries could enhance actin polymerization and disrupt Hippo signaling to promote follicle growth. Local administration of actin polymerization drugs or CCN growth factors could provide new treatments for PCOS patients and minimize follicle loss associated with ovarian damage.
Conditional deletion of the PTEN gene in granulosa cells of secondary follicles in mice promoted follicle growth (13). We demonstrated additive increases in ovarian graft weights and follicle growth following Hippo signaling disruption (fragmentation) and Akt stimulation (treatment with PTEN inhibitors and PI3K activators). Using the present IVA protocol, rapid growth of human secondary follicles to the antral stage was found in immune-deficient mice. Although the exact stage of residual follicles in individual POI ovaries is unclear, we generated preovulatory follicles from several patients in a few weeks.
The present approach represents a possible unique infertility therapy for POI patients. This paper is a report of birth after ovarian vitrification and IVA/grafting to promote follicle growth. Our data indicated that less than half of our POI patients contained residual follicles and 62% of them responded to the therapy by showing follicle growth in grafts. Because few ovarian strips are needed for autotransplantation, we are now removing one ovary from patients and recommending continuation when residual follicles are detected based on histology. Although a healthy baby was born, more studies are needed to ensure the safety of the present IVA procedure. Because POI represents a disease with heterogeneous etiologies and >50% of our POI patients do not have residual follicles, it is important to note that patients without follicles will not respond to the present IVA treatment.
POI patients have intermittent and unpredictable ovarian functions. Although 5–10% of POI patients in reported studies have a chance to conceive, only a 1.5% pregnancy rate was found in controlled trials (39). Studies of a cohort of 358 young POI patients (26.6 ± 7.9 y of age at time of diagnosis) indicated a spontaneous pregnancy rate of 4.4% during 13 y of observation (40). In our 27 older POI patients (37.3 ± 5.8 y of age), the amenorrhea duration is 6.8 ± 2.1 y with no spontaneous pregnancy. In contrast to the rare spontaneous pregnancy found in some POI patients, the present approach represents a systematic activation of residual follicles and monitoring of follicle growth. Our detection of preovulatory follicles in eight out of 27 POI patients during <1 y of observation and successful derivation of embryos from five patients suggested that the eventual success rate could be as high as 30% (8/27) after repeated autografting and optimization of follicle monitoring and oocyte retrieval. Although five patients with histological signs of residual follicles did not respond to the present treatment, we are initiating second grafting because only fragments from selective strips were grafted.
Variable local Hippo signaling could lead to protracted preovulatory follicle development after 6 mo of grafting. In addition to POI, the present approach could be useful for fertility preservation in cancer patients undergoing sterilizing treatments and other conditions of diminished ovarian reserve. Although menopause occurs at 51 y of age, many middle-aged women between 40 and 45 y of age suffer from aging-associated infertility. Because their ovaries still contain secondary and smaller follicles (41), our approach should be effective. Without overcoming age- or environment-related increases in genetic defects in oocytes, the present approach provides more mature oocytes for embryonic development.
Methods
Animals, ovarian fragmentation/grafting, ovarian explant and follicle cultures, actin measurement, RT-PCR analyses, and immunostaining/blotting are provided in SI Methods. Also, included are patient treatments and human/animal subject approval. In addition, a movie of human grafting is included (Movie S1).
Supplementary Material
Acknowledgments
We thank M. Hoshina, K. Tarumi, N. Takahashi, and S. Tsukamoto for technical assistance. No federal funds were used for the human IVF work. This work was supported by funds from the National Institutes of Health National Institute of Child Health and Human Development (U54 HD068158 as part of the Specialized Cooperative Centers Program in Reproduction and Infertility Research, to A.J.H.) and California Institute of Regenerative Medicine Grant RB2-01553 (to A.J.H.). K.K. is supported by Grant-In-Aid for Scientific Research (24390376, 23013004, and 24659722) and funds from Uehara Memorial Foundation, Naito Foundation, Terumo Life Science Foundation, Astellas Foundation, and Mochida Memorial Foundation.
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1312830110/-/DCSupplemental.
References
- 1.Broekmans FJ, et al. PCOS according to the Rotterdam consensus criteria: Change in prevalence among WHO-II anovulation and association with metabolic factors. BJOG. 2006;113(10):1210–1217. doi: 10.1111/j.1471-0528.2006.01008.x. [DOI] [PubMed] [Google Scholar]
- 2.Nelson LM. Clinical practice. Primary ovarian insufficiency. N Engl J Med. 2009;360(6):606–614. doi: 10.1056/NEJMcp0808697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.De Vos M, Devroey P, Fauser BC. Primary ovarian insufficiency. Lancet. 2010;376(9744):911–921. doi: 10.1016/S0140-6736(10)60355-8. [DOI] [PubMed] [Google Scholar]
- 4.Stein IF, Leventhal ML. Amenorrhea associated with bilateral polycystic ovaries. Am J Obstet Gynecol. 1935;29:181. [Google Scholar]
- 5.Farquhar C, Brown J, Marjoribanks J. Laparoscopic drilling by diathermy or laser for ovulation induction in anovulatory polycystic ovary syndrome. Cochrane Database Syst Rev. 2012;6:CD001122. doi: 10.1002/14651858.CD001122.pub4. [DOI] [PubMed] [Google Scholar]
- 6.Donnez J, et al. Children born after autotransplantation of cryopreserved ovarian tissue. A review of 13 live births. Ann Med. 2011;43(6):437–450. doi: 10.3109/07853890.2010.546807. [DOI] [PubMed] [Google Scholar]
- 7.Pan D. Hippo signaling in organ size control. Genes Dev. 2007;21(8):886–897. doi: 10.1101/gad.1536007. [DOI] [PubMed] [Google Scholar]
- 8.Halder G, Johnson RL. Hippo signaling: Growth control and beyond. Development. 2011;138(1):9–22. doi: 10.1242/dev.045500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Hergovich A. Mammalian Hippo signalling: A kinase network regulated by protein-protein interactions. Biochem Soc Trans. 2012;40(1):124–128. doi: 10.1042/BST20110619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Holbourn KP, Acharya KR, Perbal B. The CCN family of proteins: Structure-function relationships. Trends Biochem Sci. 2008;33(10):461–473. doi: 10.1016/j.tibs.2008.07.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Liu-Chittenden Y, et al. Genetic and pharmacological disruption of the TEAD-YAP complex suppresses the oncogenic activity of YAP. Genes Dev. 2012;26(12):1300–1305. doi: 10.1101/gad.192856.112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Reddy P, et al. Oocyte-specific deletion of Pten causes premature activation of the primordial follicle pool. Science. 2008;319(5863):611–613. doi: 10.1126/science.1152257. [DOI] [PubMed] [Google Scholar]
- 13.Fan HY, Liu Z, Cahill N, Richards JS. Targeted disruption of Pten in ovarian granulosa cells enhances ovulation and extends the life span of luteal cells. Mol Endocrinol. 2008;22(9):2128–2140. doi: 10.1210/me.2008-0095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Li J, et al. Activation of dormant ovarian follicles to generate mature eggs. Proc Natl Acad Sci USA. 2010;107(22):10280–10284. doi: 10.1073/pnas.1001198107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Sansores-Garcia L, et al. Modulating F-actin organization induces organ growth by affecting the Hippo pathway. EMBO J. 2011;30(12):2325–2335. doi: 10.1038/emboj.2011.157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Fernández BG, et al. Actin-Capping Protein and the Hippo pathway regulate F-actin and tissue growth in Drosophila. Development. 2011;138(11):2337–2346. doi: 10.1242/dev.063545. [DOI] [PubMed] [Google Scholar]
- 17.Zhao B, Tumaneng K, Guan KL. The Hippo pathway in organ size control, tissue regeneration and stem cell self-renewal. Nat Cell Biol. 2011;13(8):877–883. doi: 10.1038/ncb2303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Suzuki N, et al. Assessment of long-term function of heterotopic transplants of vitrified ovarian tissue in cynomolgus monkeys. Hum Reprod. 2012;27(8):2420–2429. doi: 10.1093/humrep/des178. [DOI] [PubMed] [Google Scholar]
- 19.McGee EA, Hsueh AJ. Initial and cyclic recruitment of ovarian follicles. Endocr Rev. 2000;21(2):200–214. doi: 10.1210/edrv.21.2.0394. [DOI] [PubMed] [Google Scholar]
- 20.Adhikari D, et al. The safe use of a PTEN inhibitor for the activation of dormant mouse primordial follicles and generation of fertilizable eggs. PLoS ONE. 2012;7(6):e39034. doi: 10.1371/journal.pone.0039034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Lu L, et al. Hippo signaling is a potent in vivo growth and tumor suppressor pathway in the mammalian liver. Proc Natl Acad Sci USA. 2010;107(4):1437–1442. doi: 10.1073/pnas.0911427107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Lee KP, et al. The Hippo-Salvador pathway restrains hepatic oval cell proliferation, liver size, and liver tumorigenesis. Proc Natl Acad Sci USA. 2010;107(18):8248–8253. doi: 10.1073/pnas.0912203107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Heallen T, et al. Hippo pathway inhibits Wnt signaling to restrain cardiomyocyte proliferation and heart size. Science. 2011;332(6028):458–461. doi: 10.1126/science.1199010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.St John MA, et al. Mice deficient of Lats1 develop soft-tissue sarcomas, ovarian tumours and pituitary dysfunction. Nat Genet. 1999;21(2):182–186. doi: 10.1038/5965. [DOI] [PubMed] [Google Scholar]
- 25.Pisarska MD, Kuo FT, Bentsi-Barnes IK, Khan S, Barlow GM. LATS1 phosphorylates forkhead L2 and regulates its transcriptional activity. Am J Physiol Endocrinol Metab. 2010;299(1):E101–E109. doi: 10.1152/ajpendo.00534.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Li T, et al. Identification of YAP1 as a novel susceptibility gene for polycystic ovary syndrome. J Med Genet. 2012;49(4):254–257. doi: 10.1136/jmedgenet-2011-100727. [DOI] [PubMed] [Google Scholar]
- 27.Nagashima T, et al. Connective tissue growth factor is required for normal follicle development and ovulation. Mol Endocrinol. 2011;25(10):1740–1759. doi: 10.1210/me.2011-1045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Aboura A, et al. Array comparative genomic hybridization profiling analysis reveals deoxyribonucleic acid copy number variations associated with premature ovarian failure. J Clin Endocrinol Metab. 2009;94(11):4540–4546. doi: 10.1210/jc.2009-0186. [DOI] [PubMed] [Google Scholar]
- 29.Wada K, Itoga K, Okano T, Yonemura S, Sasaki H. Hippo pathway regulation by cell morphology and stress fibers. Development. 2011;138(18):3907–3914. doi: 10.1242/dev.070987. [DOI] [PubMed] [Google Scholar]
- 30.Boggiano JC, Fehon RG. Growth control by committee: Intercellular junctions, cell polarity, and the cytoskeleton regulate Hippo signaling. Dev Cell. 2012;22(4):695–702. doi: 10.1016/j.devcel.2012.03.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Bione S, et al. A human homologue of the Drosophila melanogaster diaphanous gene is disrupted in a patient with premature ovarian failure: Evidence for conserved function in oogenesis and implications for human sterility. Am J Hum Genet. 1998;62(3):533–541. doi: 10.1086/301761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.He C, et al. A large-scale candidate gene association study of age at menarche and age at natural menopause. Hum Genet. 2010;128(5):515–527. doi: 10.1007/s00439-010-0878-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Schuh-Huerta SM, et al. Genetic variants and environmental factors associated with hormonal markers of ovarian reserve in Caucasian and African American women. Hum Reprod. 2012;27(2):594–608. doi: 10.1093/humrep/der391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Cai J, et al. The Hippo signaling pathway restricts the oncogenic potential of an intestinal regeneration program. Genes Dev. 2010;24(21):2383–2388. doi: 10.1101/gad.1978810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Muramatsu Y, et al. Early detection of cysteine rich protein 61 (CYR61, CCN1) in urine following renal ischemic reperfusion injury. Kidney Int. 2002;62(5):1601–1610. doi: 10.1046/j.1523-1755.2002.00633.x. [DOI] [PubMed] [Google Scholar]
- 36.Chaqour B, et al. Cyr61 and CTGF are molecular markers of bladder wall remodeling after outlet obstruction. Am J Physiol Endocrinol Metab. 2002;283(4):E765–E774. doi: 10.1152/ajpendo.00131.2002. [DOI] [PubMed] [Google Scholar]
- 37.Tumaneng K, et al. YAP mediates crosstalk between the Hippo and PI(3)K–TOR pathways by suppressing PTEN via miR-29. Nat Cell Biol. 2012;14(12):1322–1329. doi: 10.1038/ncb2615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Lunde O, Djøseland O, Grøttum P. Polycystic ovarian syndrome: A follow-up study on fertility and menstrual pattern in 149 patients 15-25 years after ovarian wedge resection. Hum Reprod. 2001;16(7):1479–1485. doi: 10.1093/humrep/16.7.1479. [DOI] [PubMed] [Google Scholar]
- 39.van Kasteren YM, Schoemaker J. Premature ovarian failure: A systematic review on therapeutic interventions to restore ovarian function and achieve pregnancy. Hum Reprod Update. 1999;5(5):483–492. doi: 10.1093/humupd/5.5.483. [DOI] [PubMed] [Google Scholar]
- 40.Bidet M, et al. Resumption of ovarian function and pregnancies in 358 patients with premature ovarian failure. J Clin Endocrinol Metab. 2011;96(12):3864–3872. doi: 10.1210/jc.2011-1038. [DOI] [PubMed] [Google Scholar]
- 41.Broekmans FJ, Soules MR, Fauser BC. Ovarian aging: Mechanisms and clinical consequences. Endocr Rev. 2009;30(5):465–493. doi: 10.1210/er.2009-0006. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.