Significance
Lacking scientific understanding of the function of naps in early childhood, policy makers may curtail preschool classroom nap opportunities due to increasing curriculum demands. Here we show evidence that classroom naps support learning in preschool children by enhancing memories acquired earlier in the day as compared with equivalent intervals spent awake.
Keywords: development, education
Abstract
Despite the fact that midday naps are characteristic of early childhood, very little is understood about the structure and function of these sleep bouts. Given that sleep benefits memory in young adults, it is possible that naps serve a similar function for young children. However, children transition from biphasic to monophasic sleep patterns in early childhood, eliminating the nap from their daily sleep schedule. As such, naps may contain mostly light sleep stages and serve little function for learning and memory during this transitional age. Lacking scientific understanding of the function of naps in early childhood, policy makers may eliminate preschool classroom nap opportunities due to increasing curriculum demands. Here we show evidence that classroom naps support learning in preschool children by enhancing memories acquired earlier in the day compared with equivalent intervals spent awake. This nap benefit is greatest for children who nap habitually, regardless of age. Performance losses when nap-deprived are not recovered during subsequent overnight sleep. Physiological recordings of naps support a role of sleep spindles in memory performance. These results suggest that distributed sleep is critical in early learning; when short-term memory stores are limited, memory consolidation must take place frequently.
Preschool education provides lifelong benefits to physical and mental health (1–5). These benefits justify state-funded preschool education in states like Oklahoma and Georgia and the petition of President Barack Obama for federal funds to support preschool education for all children (www.whitehouse.gov/the-press-office/2013/01/21/inaugural-address-president-barack-obama). Recent research has focused on interventions to further enhance the outcomes from preschool education. For instance, studies of emotional training (4), nutrition education (6, 7), and dental hygiene (8) in preschools have led to enhanced curriculum-based learning in early education. However, with increased curriculum demands and taxpayer pressure, classroom nap opportunities are becoming devalued.
Recent studies in young adults have demonstrated sleep-dependent enhancements in learning. Such enhancements are thought to reflect sleep-dependent consolidation, a process by which memory storage and retrieval become more efficient (9). However, the characteristics of sleep patterns and the architecture of sleep vary dramatically throughout development. Between 3 and 5 y of age, total sleep time and time in “deep” sleep stages, slow-wave sleep (SWS) and rapid eye movement sleep (REM), decline significantly (10, 11). Moreover, in early childhood, sleep bouts are distributed across the day. Polyphasic from birth, daytime sleep bouts are reduced to a single nap early in the preschool years. Children reach the adult-like, monophasic pattern around 5 y of age, a shift primarily associated with maturational changes but also influenced by scheduling pressures (12).
Parallel to this period of abundant SWS-rich sleep and daytime napping, early childhood is typified by the dramatic increase in the acquisition of new information as a result of increased neuronal plasticity (13, 14). The maturation of the parietal and temporal cortices and a peak in experience-dependent synapse formation in the prefrontal cortex occur around the preschool age (15). Lam et al. (16) suggest that the elimination of the midday nap may be a marker of this brain maturation. Specifically, they found a negative correlation between the number of times a preschool child napped during a week and performance on a battery of cognitive assessments.
However, whether individual sleep bouts contribute to recent memories in early childhood is unknown. Counter to what is observed in young adults, performance on a procedural memory task was not improved by overnight sleep in young (6–8 y; ref. 17) or older children (7–13 y; refs. 18 and 19). Wilhelm et al. (20) suggest that the absence of procedural memory consolidation in children may be due to insufficient initial encoding; children with extended training did exhibit sleep-dependent improvements in motor skill.
Nap-dependent consolidation of declarative memories in children has not been examined. Naps are sufficient for declarative memory consolidation in young adults (21). Moreover, declarative memories have been shown to benefit from nocturnal sleep in children 6–13 y of age (17, 19, 22). Thus, naps may function to consolidate declarative memories throughout early life. However, given the transitional nature of naps during early childhood and the lack of physiological studies of naps in healthy preschool-age children, it is possible that naps do not contain the critical non-REM sleep (23) and sleep spindles (24) necessary for sleep-dependent consolidation of declarative memories.
To investigate whether in-class naps benefit declarative learning in preschool children, we measured changes in performance on a visuospatial task over a nap and an equivalent interval of wake. A visuospatial task was selected for three reasons. First, this task, like other declarative learning tasks, has been shown to engage the hippocampus (25). Hippocampal-dependent tasks are subject to neural replay during sleep, a possible mechanism underlying sleep-dependent consolidation (26). Second, visuospatial learning has been shown to benefit from overnight sleep in young adults (23). Third, the task, like the game Memory, is appealing to preschool children.
Children learned the task in the morning, and an immediate recall phase provided an initial measure of performance (Fig. 1). During the regularly scheduled nap opportunity in the early afternoon, children were either wake- or nap-promoted (within subjects; order counterbalanced), and delayed recall was subsequently tested. To examine the long-term benefit of having napped, recall was tested once more the following day (24-h recall). Additionally, we recorded polysomnography during a laboratory-based nap in a separate group of children to examine relevant nap physiology. We hypothesized that the preschool nap is sufficient for consolidation of newly learned information and that sleep-dependent changes in memory would be associated with specific physiological aspects of sleep.
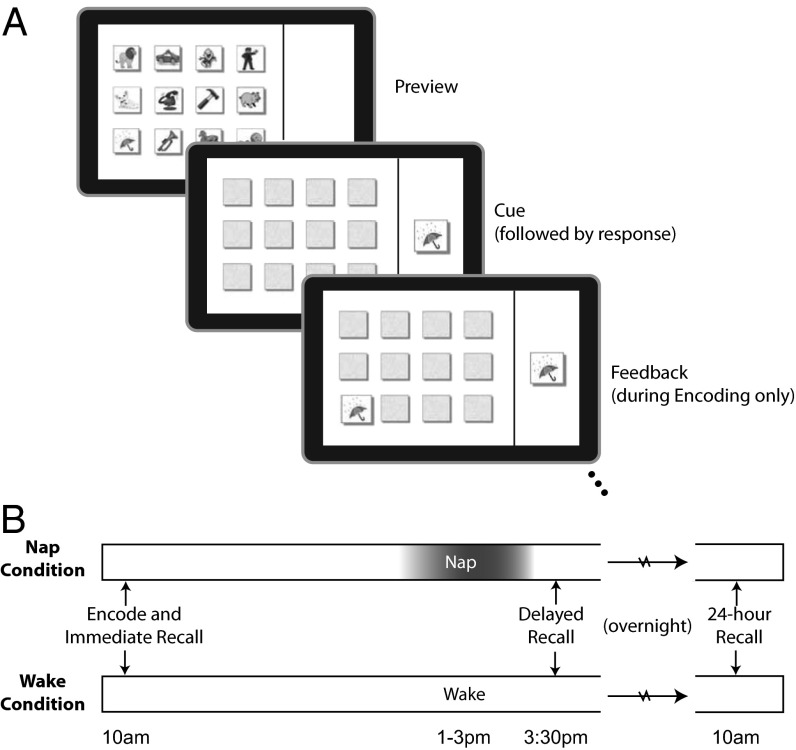
Fig. 1.
(A) Preschool children learned the spatial locations of 9 or 12 items on a grid. Following a brief preview, one item was presented on the right side of the screen, and children were asked to locate the item by pointing to its position. During the encoding phase, feedback was provided. This phase continued until performance reached 75% accuracy. (B) Immediately following the encoding phase, immediate recall was tested. Children again responded as to the location of each item, but feedback was not provided. During the afternoon nap opportunity, children were either nap- or wake-promoted, and delayed recall was tested shortly afterward. Recall was tested once again the following morning (“24-hour Recall”).
Results
Forty children [31 female; mean (M) = 49.76 mo, SD = 8.23, and range = 36–67 mo], who completed both the nap- and wake-promoted conditions in their preschool classroom, were included in the analysis. Average nap length, as documented by experimenters present in the classroom, was 77.71 min (SD = 18.75 min).
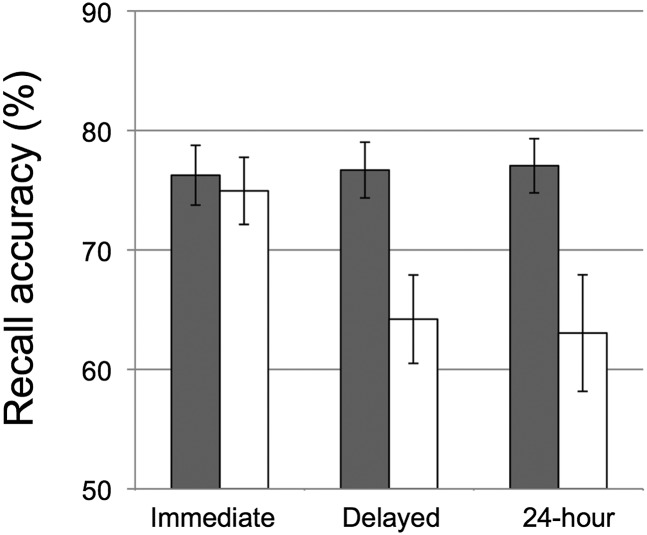
Children performed similarly at baseline (immediate recall) in both conditions [t(39) = 0.351, P = 0.728]. However, delayed recall was significantly greater following the nap than after equivalent time awake [t(38) = 2.837, P = 0.007; Fig. 2]. As a further control for potential differences in baseline performance, a difference score, or the change in accuracy across the nap/wake period (delayed minus immediate recall), was calculated. Here too we see that significantly more items were forgotten following wake than following the nap [t(38) = 2.457, P = 0.019], showing a clear nap benefit on memory retention.
Fig. 2.
Recall accuracy was tested immediately following encoding (“Immediate”), following the nap opportunity (“Delayed”), and again the following day (“24-hour”) across two conditions: a nap-promoted condition (gray bars) and wake-promoted condition (white bars). Error bars represent ±1 SE.
Optimal performance following the nap is unlikely to be associated with reduced fatigue or enhanced attention relative to the wake condition for two reasons. First, child-rated sleepiness did not differ for the nap and wake conditions [t(15) = 0.719, P = 0.48], and experimenter-rated sleepiness of the child was actually greater following the nap compared with wake [t(18) = 4.87, P < 0.001]. More significantly, performance in the nap condition remained superior when recall was again probed the following morning, after nighttime recovery sleep for the wake condition [t(22) = 2.824, P = 0.01; Fig. 2]. This latter result also suggests the long-term benefits of the nap on memory consolidation.
Greater change in accuracy across the nap trended toward a positive correlation with nap duration (r = 0.304, P = 0.058) but was not predicted by age (r = −0.037, P = 0.84). To avoid ceiling performance, a 9-item grid was used for children <44 mo old; a 12-item grid was used for older children. To assess whether the number of items encoded (9 vs. 12) influenced the change in performance over the nap, we conducted an ANOVA with condition (nap vs. wake) as a within-subjects measure and items (9 vs. 12) as a between-subjects factor. Condition remained significant [F(1, 36) = 6.256, P = 0.017], whereas the main effect of items [F(1, 36) = 0.034, P = 0.854] and the interaction of condition × items [F(1, 36) = 0.506, P = 0.481] were not significant.
Nap habituality was also examined with respect to the nap benefit. This measure was derived from caregiver reports of nap frequency. We defined habitually napping children (n = 17) as children who napped 5 d or more per week on average, and nonhabitually napping children (n = 10) were defined as children who napped fewer than 2 d/wk. The benefit of the nap relative to wake was greatest for children who napped habitually [t(16) = 2.561, P = 0.021]. Performance of nonhabitually napping children did not benefit from an intervening nap [t(9) = 0.347, P = 0.736]. Such effects cannot be attributed to differences in immediate recall, which did not differ for habitually (M = 73.5, SD = 13.19) and nonhabitually [M = 72.9, SD = 12.97; F(1,25) = 0.012, P = 0.913] napping children. As seen in Fig. 3, habitually napping children tended to have greater memory decay over wake than the nonhabitually napping children, whereas changes during the nap intervals were similar.
Fig. 3.
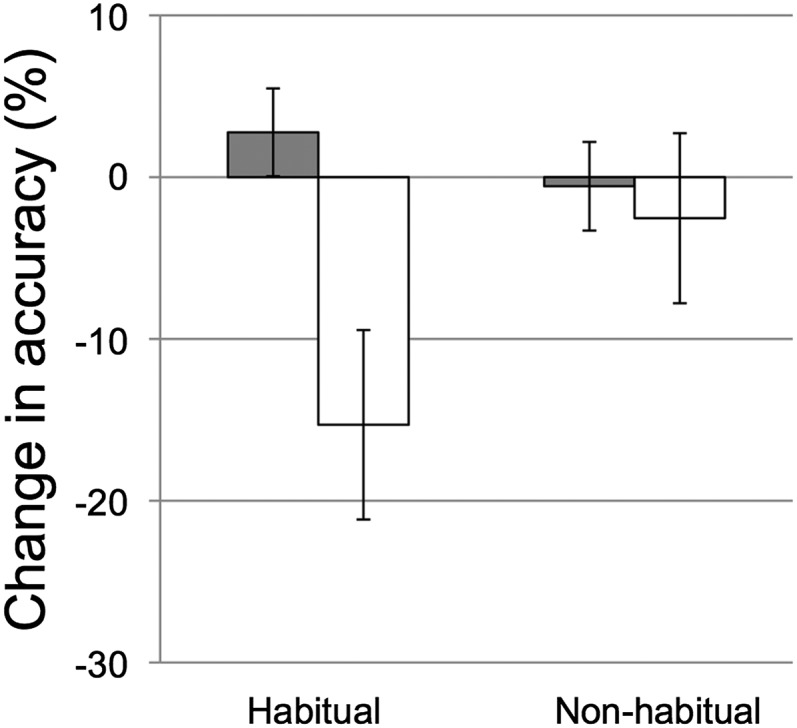
Change in recall accuracy (delayed recall minus immediate recall) across the nap (gray bars) and wake (white bars) intervals for those who took five to seven naps per week (“Habitual”) and those who took zero to two naps per week (“Non-habitual”). Error bars represent ±1 SE.
From these results, it may be argued that sleep plays merely a passive role, protecting memories from interference from waking activities (27). This explanation seems unlikely given that in both conditions, 3 h of wake passed before the ∼1 h nap. Thus, decay would be expected in both conditions. Rather, in the time between immediate and delayed recall, there was no change in recall in the nap condition [t(38) = 0.351, P = 0.727]. As such, we posit that the nap may restore the memory following waking interference, as recently suggested (28). To examine whether memories were consolidated over the nap, we recruited a group of 14 additional children (eight females; M = 49.83 mo, SD = 10.17, and range = 33–66 mo) who completed the nap condition in the sleep laboratory, with polysomnography recorded during the nap.
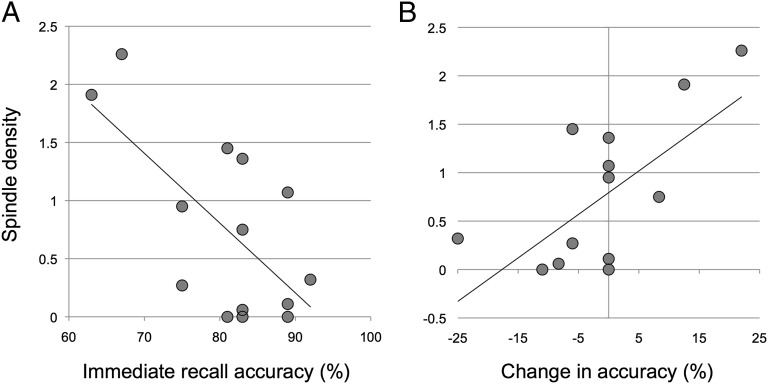
Mean length of the laboratory-recorded nap was similar to that of the classroom naps (M = 73.83 min, SD = 19.92). Naps contained little (n = 4; 1–10 min) to no (n = 10) REM sleep. Rather, naps were largely composed of non-REM stage 2 and SWS (i.e., non-REM stage 3; Table 1). There was a significant negative correlation between sleep spindle density and immediate (baseline) recall performance (r = −0.665, P = 0.010; Fig. 4A), consistent with recent reports that spindle activity has a negative association with IQ (29) and specific aspects of cognitive performance (30) in children. Sleep spindle density was also positively correlated with the change in memory performance across the nap period (r = 0.647, P = 0.012), such that a greater magnitude of sleep benefit was associated with greater density of sleep spindles during the nap. Given a significant correlation between immediate recall and the change in memory over the nap (r = −0.707, P = 0.005), to further disentangle the spindle-related baseline difference in immediate recall, we examined an adjusted measure of the change in performance over the nap. Specifically, the adjusted change in recall provides a measure of the change in memory from immediate to delayed recall as a percent of immediate recall [(delayed − immediate)/immediate]. Here, too, we found a significant correlation between the adjusted change in recall over the nap and spindle density (r = 0.693, P = 0.006; Fig. 4B). This relationship cannot be attributed to potential age-related differences in sleep physiology because the relationship between spindle density and age was not significant (r = −0.077, P = 0.792). However, it should be noted that the adjusted change also correlated with immediate recall (r = −0.740, P = 0.002), suggesting that these may not be completely disentangled. Other correlations between sleep-dependent performance changes and physiological measures of sleep (e.g., percent time in stage 2 or 3 non-REM sleep, spindle frequency, and spindle amplitude) did not survive correction for multiple comparisons (α = 0.01; all P values > 0.08).
Table 1.
Descriptive variables for polysomnography-recorded naps in 14 children
| Sleep variable | Mean (SD) |
| Time in bed, min | 96.15 (20.69) |
| Total sleep time, min | 73.83 (19.92) |
| Sleep latency, min | 14.39 (10.11) |
| Non-REM stage 1, % | 7.94 (4.39) |
| Non-REM stage 2, % | 42.09 (14.90) |
| Non-REM stage 3, % | 46.19 (17.15) |
| REM, % | 1.32 (3.03) |
| Spindle density, spindles per minute of non-REM stage 2 sleep | 0.96 (0.74) |
| Spindle frequency, Hz | 11.84 (0.98) |
| Spindle amplitude, μV | 67.87 (15.63) |
Fig. 4.
Sleep spindle density (spindles per minute of non-REM stage 2 sleep) associations with (A) immediate recall accuracy and (B) the change in recall accuracy from the immediate to delayed recall phase.
Given that enrollment criteria for the laboratory-based study required that the child be likely to nap in the laboratory, many (n = 7) were habitually napping (five to seven naps per week), and only one was classified as nonhabitually napping. Six napped often (three to four naps per week). Although this prohibited a comparison of nap physiology for habitually and nonhabitually napping children, we compared nap physiology and performance for habitually napping children (n = 7) with that of children who napped nonhabitually or often (n = 7). Groups did not differ in nap length, sleep onset latency, wake after sleep onset, or percent of the nap in stage 2 or 3 non-REM or REM (all P values > 0.24). Those that napped habitually had a lower percent of the nap spent in stage 1 non-REM sleep [t(12) = −3.096, P = 0.009] and lower sleep efficiency [time spent asleep divided by time in bed after sleep onset; t(12) = 3.377, P = 0.006]. However, this difference did not yield any performance difference across groups [change in accuracy across naps for habitually versus nonhabitually to often napping; t(12) = −0.095, P = 0.926].
Discussion
The present results illustrate a benefit of midday naps on learning in the preschool classroom. Following a nap, children recalled 10% more of the spatial locations than when they had been kept awake during the nap opportunity. This effect cannot be attributed to differences in alertness or inattention due to nap deprivation because the nap benefit remained the following day, after overnight sleep, which should equate these factors across the two conditions. Thus, the negative effects of nap deprivation on memory consolidation cannot be reversed with overnight sleep.
By examining the physiology of the intervening sleep interval, sleep-dependent changes in recall were specifically associated with sleep spindle density. Such an association would not be expected if the nap merely protected the memory from waking interference (31, 32). Moreover, if the reduced interference during sleep only provided an enhanced opportunity for memory consolidation (as opposed to being a process unique to sleep), one would expect a correlation between the change in memory over the nap and time spent asleep, particularly SWS in which long-term potentiation may be inhibited (32, 33). We did not find such an association, nor did the length of non-REM stage 2 correlate with sleep-dependent changes in memory. Rather, changes in performance over sleep were specifically associated with spindle density.
Although observed spindle frequencies (mean = 11.84 Hz) were in the range of slow spindles defined for adults (34), they were identified at central electrodes in non-REM stage 2 sleep, characteristic of fast spindles in adults. However, a clear justification for segmentation of fast versus slow spindles has not been identified for this age range. Nonetheless, slow and fast spindles have been associated with hippocampal reactivation and plasticity (34–38), and studies in young adults have found an association between spindles and learning (24, 39, 40). Likewise, we posit that in preschool children, spindles may mark hippocampal–neocortical interactions, a process underlying the stabilization and consolidation of the memory.
Given the correlation between immediate recall and over-nap changes in performance, we cannot rule out the possibility that the association between spindles and sleep-dependent changes in performance reflects the association between sleep spindles and general cognitive abilities. In adults, sleep spindles are positively correlated with IQ (41), whereas in children, there is a negative association between spindle activity and IQ (29) and other cognitive measures (30). Consistent with this literature, using immediate recall as a proxy for cognitive abilities, we found a negative correlation between spindle density and immediate recall. However, studies in young adults suggest that sleep spindles play both a trait-like and state-like role in memory and consolidation (42). It is likely that the small sample size of the laboratory-based study prohibited a similar dissociation here.
Sleep conveyed the greatest benefit on learning for children who regularly nap. Likewise, young adults who habitually nap (defined as one or more nap per week) show performance improvements on a procedural learning task (a cup and ball game) following a 20-min nap, whereas those that do not nap habitually (two or fewer naps per month) have performance decrements following the nap (43). Moreover, sleep-dependent improvements for these young adults were also associated with sleep spindle density but only for those who nap habitually. These authors suggest that higher power in the spindles and across other frequency bands (alpha and delta) for those that nap habitually may be indicative of more efficient naps. Likewise, in our study, it is possible that children who napped habitually had more efficient naps resulting in greater consolidation. Our laboratory-based sample was largely composed of habitually napping children, prohibiting comparison of sleep physiology for those who nap habitually and nonhabitually. However, given that over-nap changes were similar for the habitually and nonhabitually napping children (with differences instead in over-wake performance; Fig. 3), we posit that a more likely explanation is that memories are less susceptible to decay over wake for those children who no longer regularly nap. In other words, memories may still be physiologically processed when nonhabitually napping children sleep midday, but this boost has less impact given less decay of the memory over wake. Such an explanation is consistent with the suggestion that the transition from biphasic to monophasic sleep may coincide with brain maturation (16). In the less mature brain, memories in short-term (hippocampal) stores may be more susceptible to interference from additional encoding requiring more frequent consolidation. With brain maturation, the capacity for memories in short-term memory storage may be increased, thus decreasing the need for frequent consolidation.
Collectively, these data suggest that midday naps in the preschool classroom support the academic goals of early education. Although curriculum demands for preschool classrooms are increasing, the benefit of the sleep on learning warrants preservation of the nap opportunity. Moreover, techniques for enhancing preschool naps should be investigated. Currently, preschools largely lack guidelines around the structure of the nap, and nap promotion tools are underused and unstudied. Finally, it is worth considering whether naps may be a target for assisting children with learning delays. Protecting the nap opportunity for these children may be critical, and nap promotion may enhance learning from interventions aimed at improving cognitive abilities.
Methods
Seventy-seven preschool children (50 female; M = 46.8 mo, SD = 8.91, and range = 36–67 mo) were initially recruited from six preschool classrooms. Children were required to have normal or corrected-to-normal vision and no present or past diagnosis of disordered sleep. Children were excluded from analysis if they were unable to complete the nap (n = 10) or wake condition (n = 1) or failed to complete the task (n = 4). Additionally, although we attempted to avoid ceiling performance by adjusting the number of items encoded and training to criterion (described below), participants (n = 22) were removed from the analysis if they had ceiling performance (immediate recall = 100%). No participants had floor performance (all immediate recall > 22%).
In the morning (∼10:00 AM), children briefly previewed a matrix of squares each with a cartoon image of a common noun (e.g., umbrella, policeman, or cat). A 9-item grid was used for children <44 mo old; a 12-item grid was used for older children. After previewing the items for 30 s, the images were virtually “flipped over” (leaving a matrix of cards, each with an identical pattern; Fig. 1A). Subsequently, an image appeared on the right of the screen, and the child was to point to the corresponding hidden image in the matrix. During this encoding phase, the image in the selected location would be revealed, providing the participant with feedback regarding their responses. After all items were presented once, if performance was not at criterion (7 of 9 items or 9 of 12 items), all items were presented again. This process was repeated until the performance criterion was reached. The average number of presentations to reach this criterion was 2.20 for the 9-item grid and 2.09 for the 12-item grid. Following encoding, memory for the spatial location of the images was probed in the immediate recall phase (Fig. 1B). During immediate recall, children located each item once, and no feedback was given, thus providing an accurate measure of memory without additional learning opportunities.
After immediate recall, children went about their regular classroom routine (outside time and lunch) until the regularly scheduled classroom nap opportunity, ∼1:00–3:00 PM. Enrolled children were either wake- or nap-promoted (within subjects; order counterbalanced; conditions separated by 1–3 wk). In both conditions, the room was darkened and quiet, and children remained on their individual cots/mats as per the typical classroom naptime routine. In the wake condition, children were given quiet activities as needed to encourage wakefulness. In the nap condition, children were encouraged to nap with verbal encouragement and soothing per typical classroom techniques (e.g., back and foot rubbing). Experimenters recorded nap onset and wake time (and any midnap waking) for all enrolled children. After the nap opportunity, delayed recall, identical to immediate recall, was tested (Fig. 1B). At delayed recall, experimenters and children also separately rated child sleepiness on a modified visual sleepiness scale (44). The following morning, memory was probed in the 24-h recall phase. Due to absences or scheduling conflicts, only 23 children completed this probe for both conditions.
Caregivers completed the Child Sleep Habits Questionnaire (45) to assess the child’s sleep habits and screen against sleep disorders. Habitually napping children (n = 17) were defined as children who napped 5 d or more per week on average, and nonhabitually napping children (n = 10) were defined as children who napped fewer than 2 d/wk.
All procedures were approved by the University of Massachusetts, Amherst Institutional Review Board. As such, informed parental consent and child assent were obtained before testing commenced.
Polysomnography.
To examine whether sleep-dependent changes in memory were associated with a specific sleep process, we recorded polysomnography during a laboratory-based nap in an additional group of children (n = 14; eight females; M = 49.83 mo, SD = 10.17, and range = 33–66 mo). Enrollment criteria, in addition to those described above, required that the child be likely to nap in the laboratory. Consistent with this, seven children were reported to nap habitually (five to seven naps per week), and six napped often (three to four naps per week). Only one child did not nap habitually (zero to two naps per week). Following the encoding and immediate recall phases (∼1:00 PM), an AURA PSG ambulatory system (Grass Products, Natus Technologies) with a montage including two electrooculogram, two chin electromyogram, and five cortical electroencephalogram leads (F3, F4, C3, and C4; referenced to Cz) was applied. Data were scored for sleep stages according to the revised American Academy of Sleep Medicine manual (46). Sleep spindles were visually detected at C3 by a trained physiologist using TWin PSG Clinical Software (Grass Products, Natus Technologies), and spindle onsets and offsets were marked. Spindle onsets and offsets were subsequently verified by a second trained physiologist. Measurements of peak amplitude and peak frequency of the visually identified spindles were determined with an in-house MATLAB (r2009b) routine based on the method and definitions described by Wamsley et al. (47). Specifically, EEG data were filtered from 0.5 to 35 Hz. The maximum voltage achieved between spindle onset and offset was considered the peak spindle amplitude. To measure the peak spindle frequency, the spindle frequencies were decomposed by applying a fast Fourier transform. The peak spindle frequency was defined as the spectral peak in the range from 9 to 15 Hz. Although the spindle frequency range is often defined to start between 10 and 12 Hz, we opt to start at 9 Hz, consistent with Mölle et al. (48), given the lower alpha range for this age group compared with adults (49) and evidence that the sigma peak frequency is likewise lower for 2–5 y compared with 8–20 y (50).
Statistical Analyses.
Two-tailed paired-samples t tests were used to determine differences in immediate, delayed, and 24-h recall accuracies and the difference score (delayed minus immediate recall) between the nap and wake conditions. Separate analyses were conducted for habitually and nonhabitually napping groups. To determine the association between the change in performance across the nap (difference score) and nap length, spindle density (number of spindles per minute of non-REM stage 2), and other physiological variables, Pearson correlation coefficients were used. The Sidak correction for multiple comparisons was used such that P values must be below α = 0.01 to be considered statistically significant.
Acknowledgments
This work was supported in part by National Institutes of Health Grant R01 HL111695 (to R.M.C.S.) and a Commonwealth College Honors Research Grant (to K.D.).
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission.
See Commentary on page 17171.
References
- 1.Behrman RE. The Future of Children. Vol 5. Princeton, NJ: Princeton-Brookings; 1995. [Google Scholar]
- 2.Muennig P, Schweinhart L, Montie J, Neidell M. Effects of a prekindergarten educational intervention on adult health: 37-year follow-up results of a randomized controlled trial. Am J Public Health. 2009;99(8):1431–1437. doi: 10.2105/AJPH.2008.148353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Palfrey JS, et al. The Brookline Early Education Project: A 25-year follow-up study of a family-centered early health and development intervention. Pediatrics. 2005;116(1):144–152. doi: 10.1542/peds.2004-2515. [DOI] [PubMed] [Google Scholar]
- 4.Ramey CT, et al. Persistent effects of early childhood education on high-risk children and their mothers. Appl Dev Sci. 2000;4(1):2–14. [Google Scholar]
- 5.Ramey CT, Ramey SL. Early learning and school readiness: Can early intervention make a difference. Merrill Palmer Q. 2004;50(4):471–491. [Google Scholar]
- 6.Hu C, et al. Evaluation of a kindergarten-based nutrition education intervention for pre-school children in China. Public Health Nutr. 2010;13(2):253–260. doi: 10.1017/S1368980009990814. [DOI] [PubMed] [Google Scholar]
- 7.Sweitzer SJ, et al. Lunch is in the bag: Increasing fruits, vegetables, and whole grains in sack lunches of preschool-aged children. J Am Diet Assoc. 2010;110(7):1058–1064. doi: 10.1016/j.jada.2010.04.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Jackson RJ, et al. The effects of a supervised toothbrushing programme on the caries increment of primary school children, initially aged 5-6 years. Caries Res. 2005;39(2):108–115. doi: 10.1159/000083155. [DOI] [PubMed] [Google Scholar]
- 9.Stickgold R, Walker MP. Sleep-dependent memory consolidation and reconsolidation. Sleep Med. 2007;8(4):331–343. doi: 10.1016/j.sleep.2007.03.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ohayon MM, Carskadon MA, Guilleminault C, Vitiello MV. Meta-analysis of quantitative sleep parameters from childhood to old age in healthy individuals: Developing normative sleep values across the human lifespan. Sleep. 2004;27(7):1255–1273. doi: 10.1093/sleep/27.7.1255. [DOI] [PubMed] [Google Scholar]
- 11.Montgomery-Downs HE, O’Brien LM, Gulliver TE, Gozal D. Polysomnographic characteristics in normal preschool and early school-aged children. Pediatrics. 2006;117(3):741–753. doi: 10.1542/peds.2005-1067. [DOI] [PubMed] [Google Scholar]
- 12.Weissbluth M. Naps in children: 6 months-7 years. Sleep. 1995;18(2):82–87. doi: 10.1093/sleep/18.2.82. [DOI] [PubMed] [Google Scholar]
- 13.Goldman-Rakic PS. Development of cortical circuitry and cognitive function. Child Dev. 1987;58(3):601–622. [PubMed] [Google Scholar]
- 14.Lenroot RK, Giedd JN. Brain development in children and adolescents: Insights from anatomical magnetic resonance imaging. Neurosci Biobehav Rev. 2006;30(6):718–729. doi: 10.1016/j.neubiorev.2006.06.001. [DOI] [PubMed] [Google Scholar]
- 15.Casey BJ, Tottenham N, Liston C, Durston S. Imaging the developing brain: What have we learned about cognitive development? Trends Cogn Sci. 2005;9(3):104–110. doi: 10.1016/j.tics.2005.01.011. [DOI] [PubMed] [Google Scholar]
- 16.Lam JC, Mahone EM, Mason T, Scharf SM. The effects of napping on cognitive function in preschoolers. J Dev Behav Pediatr. 2011;32(2):90–97. doi: 10.1097/DBP.0b013e318207ecc7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Wilhelm I, Diekelmann S, Born J. Sleep in children improves memory performance on declarative but not procedural tasks. Learn Mem. 2008;15(5):373–377. doi: 10.1101/lm.803708. [DOI] [PubMed] [Google Scholar]
- 18.Fischer S, Wilhelm I, Born J. Developmental differences in sleep’s role for implicit off-line learning: Comparing children with adults. J Cogn Neurosci. 2007;19(2):214–227. doi: 10.1162/jocn.2007.19.2.214. [DOI] [PubMed] [Google Scholar]
- 19.Prehn-Kristensen A, et al. Sleep in children enhances preferentially emotional declarative but not procedural memories. J Exp Child Psychol. 2009;104(1):132–139. doi: 10.1016/j.jecp.2009.01.005. [DOI] [PubMed] [Google Scholar]
- 20.Wilhelm I, Metzkow-Mészàros M, Knapp S, Born J. Sleep-dependent consolidation of procedural motor memories in children and adults: The pre-sleep level of performance matters. Dev Sci. 2012;15(4):506–515. doi: 10.1111/j.1467-7687.2012.01146.x. [DOI] [PubMed] [Google Scholar]
- 21.Tucker MA, et al. A daytime nap containing solely non-REM sleep enhances declarative but not procedural memory. Neurobiol Learn Mem. 2006;86(2):241–247. doi: 10.1016/j.nlm.2006.03.005. [DOI] [PubMed] [Google Scholar]
- 22.Backhaus J, Hoeckesfeld R, Born J, Hohagen F, Junghanns K. Immediate as well as delayed post learning sleep but not wakefulness enhances declarative memory consolidation in children. Neurobiol Learn Mem. 2008;89(1):76–80. doi: 10.1016/j.nlm.2007.08.010. [DOI] [PubMed] [Google Scholar]
- 23.Rasch B, Büchel C, Gais S, Born J. Odor cues during slow-wave sleep prompt declarative memory consolidation. Science. 2007;315(5817):1426–1429. doi: 10.1126/science.1138581. [DOI] [PubMed] [Google Scholar]
- 24.Schabus M, et al. Sleep spindles and their significance for declarative memory consolidation. Sleep. 2004;27(8):1479–1485. doi: 10.1093/sleep/27.7.1479. [DOI] [PubMed] [Google Scholar]
- 25.Postma A, Kessels RP, van Asselen M. How the brain remembers and forgets where things are: The neurocognition of object-location memory. Neurosci Biobehav Rev. 2008;32(8):1339–1345. doi: 10.1016/j.neubiorev.2008.05.001. [DOI] [PubMed] [Google Scholar]
- 26.O’Neill J, Pleydell-Bouverie B, Dupret D, Csicsvari J. Play it again: Reactivation of waking experience and memory. Trends Neurosci. 2010;33(5):220–229. doi: 10.1016/j.tins.2010.01.006. [DOI] [PubMed] [Google Scholar]
- 27.Ellenbogen JM, Hulbert JC, Stickgold R, Dinges DF, Thompson-Schill SL. Interfering with theories of sleep and memory: Sleep, declarative memory, and associative interference. Curr Biol. 2006;16(13):1290–1294. doi: 10.1016/j.cub.2006.05.024. [DOI] [PubMed] [Google Scholar]
- 28.Brawn TP, Nusbaum HC, Margoliash D. Sleep consolidation of interfering auditory memories in starlings. Psychol Sci. 2013;24(4):439–447. doi: 10.1177/0956797612457391. [DOI] [PubMed] [Google Scholar]
- 29.Geiger A, et al. The sleep EEG as a marker of intellectual ability in school age children. Sleep. 2011;34(2):181–189. doi: 10.1093/sleep/34.2.181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Chatburn A, et al. Sleep spindle activity and cognitive performance in healthy children. Sleep. 2013;36(2):237–243. doi: 10.5665/sleep.2380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Ellenbogen JM, Payne JD, Stickgold R. The role of sleep in declarative memory consolidation: Passive, permissive, active or none? Curr Opin Neurobiol. 2006;16(6):716–722. doi: 10.1016/j.conb.2006.10.006. [DOI] [PubMed] [Google Scholar]
- 32.Mednick SC, Cai DJ, Shuman T, Anagnostaras S, Wixted JT. An opportunistic theory of cellular and systems consolidation. Trends Neurosci. 2011;34(10):504–514. doi: 10.1016/j.tins.2011.06.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Wixted JT. The psychology and neuroscience of forgetting. Annu Rev Psychol. 2004;55:235–269. doi: 10.1146/annurev.psych.55.090902.141555. [DOI] [PubMed] [Google Scholar]
- 34.De Gennaro L, Ferrara M. Sleep spindles: An overview. Sleep Med Rev. 2003;7(5):423–440. doi: 10.1053/smrv.2002.0252. [DOI] [PubMed] [Google Scholar]
- 35.Marshall L, Helgadóttir H, Mölle M, Born J. Boosting slow oscillations during sleep potentiates memory. Nature. 2006;444(7119):610–613. doi: 10.1038/nature05278. [DOI] [PubMed] [Google Scholar]
- 36.Rosanova M, Ulrich D. Pattern-specific associative long-term potentiation induced by a sleep spindle-related spike train. J Neurosci. 2005;25(41):9398–9405. doi: 10.1523/JNEUROSCI.2149-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Schabus M, et al. Hemodynamic cerebral correlates of sleep spindles during human non-rapid eye movement sleep. Proc Natl Acad Sci USA. 2007;104(32):13164–13169. doi: 10.1073/pnas.0703084104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Inostroza M, Born J. Sleep for preserving and transforming episodic memory. Annu Rev Neurosci. 2013;36:79–102. doi: 10.1146/annurev-neuro-062012-170429. [DOI] [PubMed] [Google Scholar]
- 39.Gais S, Mölle M, Helms K, Born J. Learning-dependent increases in sleep spindle density. J Neurosci. 2002;22(15):6830–6834. doi: 10.1523/JNEUROSCI.22-15-06830.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Schmidt C, et al. Encoding difficulty promotes postlearning changes in sleep spindle activity during napping. J Neurosci. 2006;26(35):8976–8982. doi: 10.1523/JNEUROSCI.2464-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Fogel SM, Nader R, Cote KA, Smith CT. Sleep spindles and learning potential. Behav Neurosci. 2007;121(1):1–10. doi: 10.1037/0735-7044.121.1.1. [DOI] [PubMed] [Google Scholar]
- 42.Schabus M, et al. Interindividual sleep spindle differences and their relation to learning-related enhancements. Brain Res. 2008;1191:127–135. doi: 10.1016/j.brainres.2007.10.106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Milner CE, Fogel SM, Cote KA. Habitual napping moderates motor performance improvements following a short daytime nap. Biol Psychol. 2006;73(2):141–156. doi: 10.1016/j.biopsycho.2006.01.015. [DOI] [PubMed] [Google Scholar]
- 44.Maldonado CC, Bentley AJ, Mitchell D. A pictorial sleepiness scale based on cartoon faces. Sleep. 2004;27(3):541–548. doi: 10.1093/sleep/27.3.541. [DOI] [PubMed] [Google Scholar]
- 45.Owens JA, Spirito A, McGuinn M. The Children’s Sleep Habits Questionnaire (CSHQ): Psychometric properties of a survey instrument for school-aged children. Sleep. 2000;23(8):1043–1051. [PubMed] [Google Scholar]
- 46.AASM . The AASM Manual for the Scoring of Sleep and Associated Events: Rules, Terminology, and Technical Specifications. Westchester, IL: Am Acad of Sleep Med; 2007. [Google Scholar]
- 47.Wamsley EJ, et al. Reduced sleep spindles and spindle coherence in schizophrenia: Mechanisms of impaired memory consolidation? Biol Psychiatry. 2012;71(2):154–161. doi: 10.1016/j.biopsych.2011.08.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Mölle M, Bergmann TO, Marshall L, Born J. Fast and slow spindles during the sleep slow oscillation: Disparate coalescence and engagement in memory processing. Sleep. 2011;34(10):1411–1421. doi: 10.5665/SLEEP.1290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Marshall PJ, Bar-Haim Y, Fox NA. Development of the EEG from 5 months to 4 years of age. Clin Neurophysiol. 2002;113(8):1199–1208. doi: 10.1016/s1388-2457(02)00163-3. [DOI] [PubMed] [Google Scholar]
- 50.Kurth S, et al. Mapping of cortical activity in the first two decades of life: A high-density sleep electroencephalogram study. J Neurosci. 2010;30(40):13211–13219. doi: 10.1523/JNEUROSCI.2532-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]