Significance
The trehalose transporter in Anopheles gambiae (AgTreT1) is shown here to be important for mosquito adaptation to environmental stresses and malaria infection. As expected since AgTreT1 transports trehalose from site of synthesis in fat body to hemolymph, silencing of AgTreT1 reduces hemolymph trehalose concentration. More importantly, AgTreT1-silenced mosquitoes exhibit shorter survival under desiccation or elevated temperature, and these mosquitoes also harbor fewer parasites after an infectious bloodmeal. We conclude that AgTreT1 is critical to maintain hemolymph trehalose concentration and is a positive mediator for parasite growth. Thus AgTreT1 can be a potential target for interruption of malaria transmission.
Keywords: major hemolymph sugar, sugar transporter, malaria parasite oocysts, malaria control
Abstract
Anopheles gambiae is a major vector mosquito for Plasmodium falciparum, the deadly pathogen causing most human malaria in sub-Saharan Africa. Synthesized in the fat body, trehalose is the predominant sugar in mosquito hemolymph. It not only provides energy but also protects the mosquito against desiccation and heat stresses. Trehalose enters the mosquito hemolymph by the trehalose transporter AgTreT1. In adult female A. gambiae, AgTreT1 is predominantly expressed in the fat body. We found that AgTreT1 expression is induced by environmental stresses such as low humidity or elevated temperature. AgTreT1 RNA silencing reduces the hemolymph trehalose concentration by 40%, and the mosquitoes succumb sooner after exposure to desiccation or heat. After an infectious blood meal, AgTreT1 RNA silencing reduces the number of P. falciparum oocysts in the mosquito midgut by over 70% compared with mock-injected mosquitoes. These data reveal important roles for AgTreT1 in stress adaptation and malaria pathogen development in a major vector mosquito. Thus, AgTreT1 may be a potential target for malaria vector control.
Critical to the malaria transmission cycle, the mosquito Anopheles gambiae is a major vector for Plasmodium falciparum, the pathogen responsible for most malignant malaria in sub-Saharan Africa. In malaria endemic regions, vector mosquitoes survive harsh fluctuations of temperature and humidity (1). Mosquitoes adapt to environmental changes by adjusting expression levels of certain genes (2); however, most protective mechanisms apparently remain unknown. Recently, we characterized an aquaporin water channel from A. gambiae (AgAQP1) that is important for water homeostasis, because reduced expression protected against dehydration (3). Since water loss has profound effects on mosquito physiology, we investigated other candidate genes that may protect against environmental stress and may affect transmission of P. falciparum.
Trehalose is a nonreducing disaccharide of two glucose molecules linked by an α-α-1,1-glycosidic bond. It is abundant in insects, crustaceans, nematodes, bacteria, fungi, and plants, but not vertebrates. As the major sugar in mosquito hemolymph, trehalose is concentrated more than 10 times higher than glucose or other sugars (4). Trehalose is a versatile molecule, serving as the principal energy storage but also as a stabilizer for dry membranes and proteins due to unique chemical and physical properties—high hydration volume, lack of internal hydrogen bonds, and nonreduction (5–8).
Trehalose levels rise sharply during several stresses—desiccation (9–12), heat (13), freezing (14, 15), hyperosmolality (16), and oxidation (17). In yeast and plants, trehalose is also a signaling molecule in metabolic pathways affecting growth (18). Evidence is emerging that trehalose protects cultured cells. Increased trehalose in HEK-293 cells expressing Drosophila trehalose-phosphate synthase 1 protects the cells from hypoxic injury (17). Bovine endothelial cell line cultivated with trehalose followed by cryopreservation with trehalose in an optimized solution yielded over 80% viable cells (19). Trehalose levels in anhydrobiotic stage larvae of Polypedilum vanderplanki (sleeping chironomid) accumulate rapidly to ∼20% of the dry body mass, more than five times higher than that of larvae in fresh water (9, 20). Furthermore, a recent study has shown that injection of d-(+)-trehalose into the hemocoel of head-intact, starved cockroaches lowers the content of short neuropeptide F in hemolymph, suggesting novel roles of trehalose in regulating brain and midgut interplay in insect digestion and nutrition-associated behavior (21).
Synthesized exclusively in the fat body of mosquitoes, trehalose is transported to the circulating hemolymph for delivery to other tissues. This process involves the specific movement of trehalose across cell membranes facilitated by the trehalose transporter, TreT (9, 22). The AgTreT1 cDNA from A. gambiae is an ortholog of PvTreT1 from P. vanderplanki. Only one TreT gene is present in the A. gambiae genome, and its trehalose-transport function was characterized by heterologous expression in Xenopus oocytes (22). PvTreT1 was proposed to contribute to the dehydration resistance of P. vanderplanki larvae in vivo (9). Nevertheless, no direct evidence has supported this role of AgTreT1 in the whole vector mosquito A. gambiae.
Trehalose is a likely energy source for Plasmodium pathogens in A. gambiae mosquitoes. After ingesting an infected blood meal, Plasmodium gametocytes differentiate into male or female gametes and fuse to form ookinetes in the mosquito midgut. Mobile ookinetes then penetrate the gut lining to produce oocysts on the basal-lamina side. During the oocyst stage, malaria parasites amplify by several thousand fold, scavenging energy from the vector. Plasmodium infection has been reported to deplete sugars in vector hemolymph, suggesting that trehalose is used by parasites for rapid growth (23). Genes related to trehalose transport and metabolism may be related to the life cycle of Plasmodium spp. in mosquito vectors.
In this study, we observed that AgTreT1 expression is induced by desiccation and heat. Reducing AgTreT1 expression in female A. gambiae by RNAi decreases hemolymph trehalose levels. Mosquitoes with reduced AgTreT1 levels die sooner than controls in dry or hot environments. Moreover, when AgTreT1 was silenced in A. gambiae infected with P. falciparum, significantly fewer parasite oocysts appear in the midguts than in midguts of control mosquitoes. These data suggest an important role of AgTreT1 in maintaining vector hemolymph sugar levels during desiccation and heat and reveal a unique function in P. falciparum propagation during the oocyst stage.
Results
Developmental and Tissue-Specific Expression of AgTreT1.
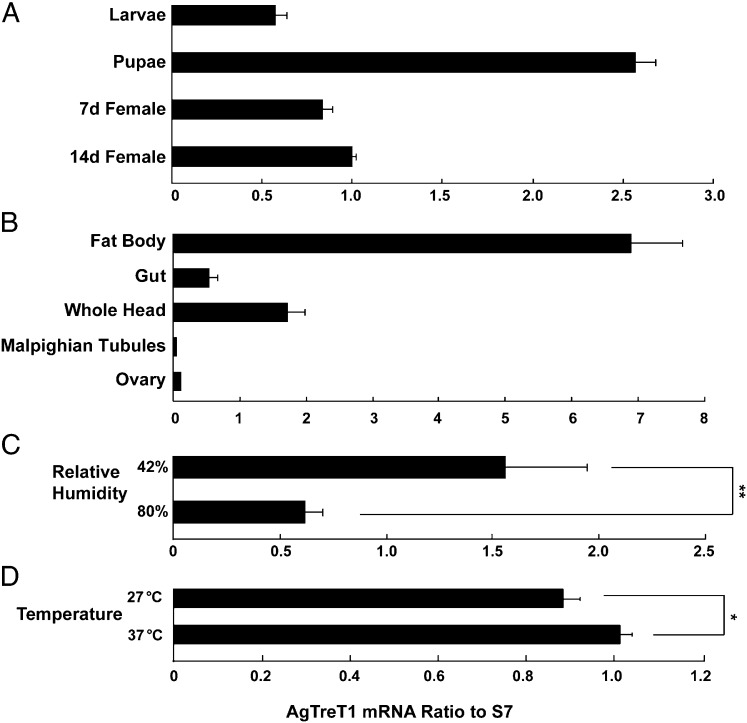
AgTreT1 expression was characterized in A. gambiae mosquitoes by quantitative RT-PCR. Total RNA was extracted from mosquitoes at different life stages. AgTreT1 mRNA expression was constitutively detected in fourth instar larvae, pupae, and 7-d- and 14-d-old adult female mosquitoes (Fig. 1A). Tissues were dissected from 7-d-old females because they are already capable of blood feeding. AgTreT1 mRNA levels were more than four times higher in fat body than in whole head, gut, Malpighian tubules, or ovary (Fig. 1B).
Fig. 1.
Expression profiles of AgTreT1. RT-qPCR revealed the expression of AgTreT1 (A) during different developmental stages—fourth instar larvae, pupae, 7-d- and 14-d-old female adults; (B) in different tissues dissected from 7-d-old female adults—fat body, whole gut, whole head, Malpighian tubules, ovary; (C) under dehydrating (42%) or normal (80%) relative humidity; and (D) at high (37 °C) or normal (27 °C) ambient temperatures. cDNA levels were normalized using the S7 ribosomal gene as the internal control. Data represent mean ± SD, *P ≤ 0.05, **P ≤ 0.01 by Student's t test.
Expression was then studied under conditions simulating environmental stresses. A. gambiae mosquitoes exposed to 42% relative humidity had AgTreT1 mRNA levels 2.5 times the control mosquitoes remaining in the normal culture, 80% relative humidity (Fig. 1C). Mosquitoes exposed to 37 °C had AgTreT1 mRNA levels slightly elevated to 1.15 times the controls remaining in the normal culture temperature, 27 °C (Fig. 1D). After stress treatments for 2 d, mosquito hemolymph was extracted and trehalose was measured. The trehalose concentration at 42% relative humidity increased to 2.6 ± 0.02 times the control level at 80% relative humidity; at 37 °C, the trehalose concentration increased to 1.9 ± 0.01 times the control at 27 °C (Fig. S1).
Maintaining Hemolymph Trehalose Concentration by AgTreT1.
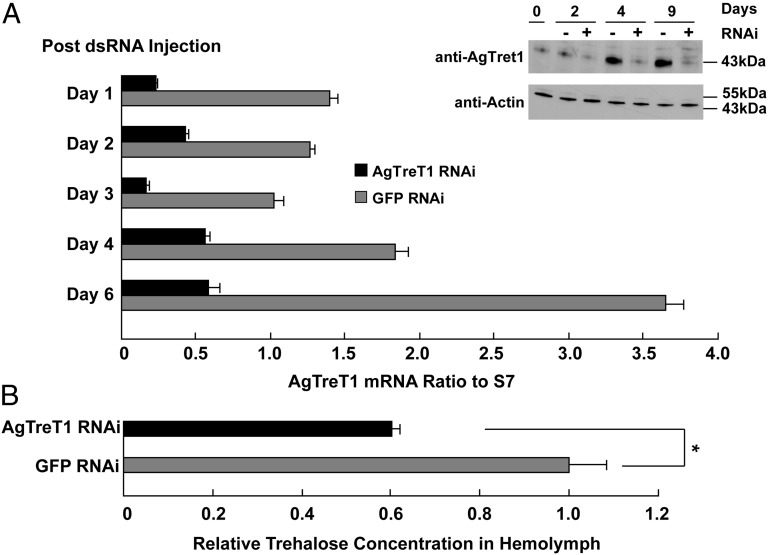
To specifically reduce expression of AgTreT1, double-stranded RNA (dsRNA) was in vitro transcribed and injected into the thorax of 4- to 6-d-old adult female mosquitoes. From day 1 to day 6 after dsRNA injection, quantitative RT-PCR showed that AgTreT1 mRNA was reduced to as low as 16% of the level in the corresponding control mosquitoes injected with GFP dsRNA (Fig. 2A). To confirm RNA silencing efficiency at the protein level, we raised a rabbit polyclonal antibody that specifically recognized the AgTreT1 band with apparent molecular weight of 43 kDa whereas preimmune serum did not (Fig. S2). Western blot showed that AgTreT1-dsRNA injection resulted in significant reduction on the protein level (Fig. 2, Inset).
Fig. 2.
AgTreT1 silencing affects hemolymph trehalose levels. (A) AgTreT1 mRNA levels reduced in AgTreT1 dsRNA-injected mosquitoes to as low as 16% of controls during 6 d after injection. RNA of whole mosquitoes was used for reverse transcription and quantitative PCR, and data were normalized using the expression of S7 ribosomal gene as internal control. Black bars represent normalized mRNA level of mosquitoes injected with AgTreT1 dsRNA, and gray bars represent mosquitoes injected with GFP dsRNA as controls. (Inset) Western blot of whole female A. gambiae injected with GFP RNAi or AgTreT1 RNAi on day 0 (before injection) and day 2, 4, and 9 postinjection. (Upper) Blot against anti-AgTreT1 antibody. (Lower) Blot against anti-Actin antibody. (B) The relative concentration of hemolymph trehalose in AgTreT1 or GFP dsRNA-injected A. gambiae mosquitoes 8 d after injection. Trehalose concentrations were normalized to genomic DNA extracted from hemolymph cells. Data represent mean ± SD, *P ≤ 0.05 by Student's t test.
Phenotypic analysis was performed 8 d after injection to allow protein turnover. If AgTreT1 plays a role in the transport of trehalose from fat body to hemolymph, we expected that the reduction of AgTreT1 would decrease the trehalose concentration in hemolymph. To test this hypothesis, the body fluid of mosquitoes, which essentially contains hemolymph plasma and hemocytes, was extracted, and relative trehalose concentrations were measured. In AgTreT1 dsRNA-injected mosquitoes, hemolymph trehalose levels were reduced by 40% compared with controls (Fig. 2B), confirming a rate-limiting role of AgTreT1 in facilitating trehalose transport from fat body to hemolymph. Using the hemolymph osmolality of control mosquitoes injected with GFP dsRNA as reference, the normalized hemolymph osmolality in AgTreT1-silenced mosquitoes was reduced by 14% compared with controls (Fig. S3).
Viability of AgTreT1-Deficient Mosquitoes During Environmental Stresses.
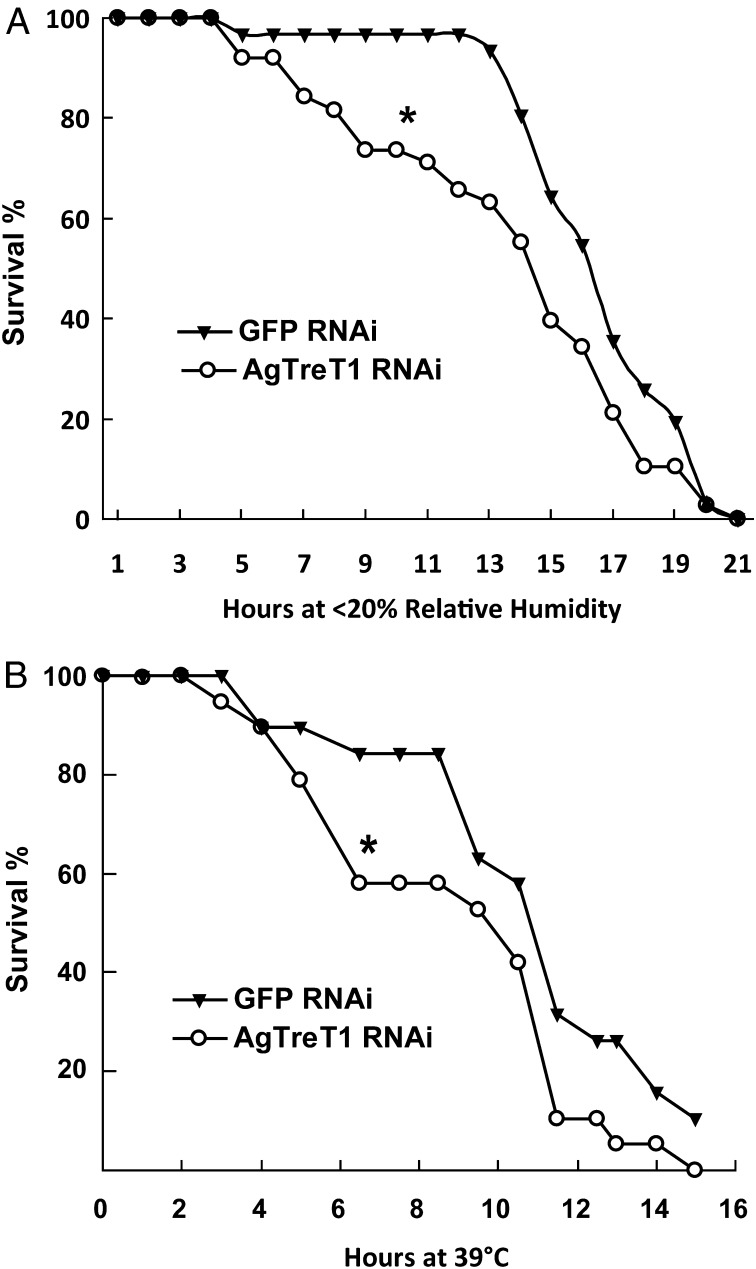
Although it is believed that trehalose serves as a protectant against various stresses in many species, direct evidence is lacking at the level of the whole organisms. Here, we reduced the trehalose concentration in A. gambiae hemolymph by silencing AgTreT1 expression with RNAi. Female A. gambiae mosquitoes were injected with AgTreT1 dsRNA, and, 8 d later, they were tested under the desiccating condition (<20% relative humidity) with comparison to control mosquitoes injected with GFP dsRNA. When incubated together, the AgTreT1 dsRNA-injected mosquitoes consistently die before control mosquitoes. A representative experiment is shown in Fig. 3A, and three repeats with similar trends are summarized in Table S1.
Fig. 3.
Survival of mosquitoes with silenced AgTreT1 expression under environmental stresses. Female A. gambiae mosquitoes were incubated overnight in (A) dehydrating (<20% relative humidity) or (B) high-temperature environment (39 °C). The reduction of AgTreT1 was confirmed by RT-qPCR and Western blot. Closed triangles represent percentages of living mosquitoes injected with GFP dsRNA at specified time points, and open circles represent percentages of living mosquitoes injected with AgTreT1 dsRNA. Mosquitoes with reduced AgTreT1 expression succumbed sooner than controls under desiccation (A) and heat stresses (B). *P ≤ 0.05 by the log-rank test in survival analysis. Each plot is a representative of three different experiments with similar trends. Sample sizes and P values of three repeats are summarized in Table S1.
High ambient temperatures at and above 39 °C are not uncommon in malaria-endemic regions, especially during dry seasons in Africa. Female A. gambiae mosquitoes were injected with either AgTreT1 dsRNA or GFP dsRNA. Eight days later, both groups were tested for their ability to survive heat (39 °C), which is well above the normal laboratory rearing temperature (27 °C). Relative humidity was maintained at 80% for both groups. At 39 °C, AgTreT1 dsRNA-injected mosquitoes consistently die before control GFP dsRNA-injected mosquitoes. A representative survival plot is shown in Fig. 3B, and three experiments with similar trends are summarized in Table S1. To confirm that mosquitoes died of heat but not simply due to the reduction of AgTreT1, we incubated gene-silenced mosquitoes at 27 °C on day six to day seven postinjection, and we did not observe significant mosquito death (Fig. S4).
Malaria Oocysts in AgTreT1-Deficient Mosquitoes.
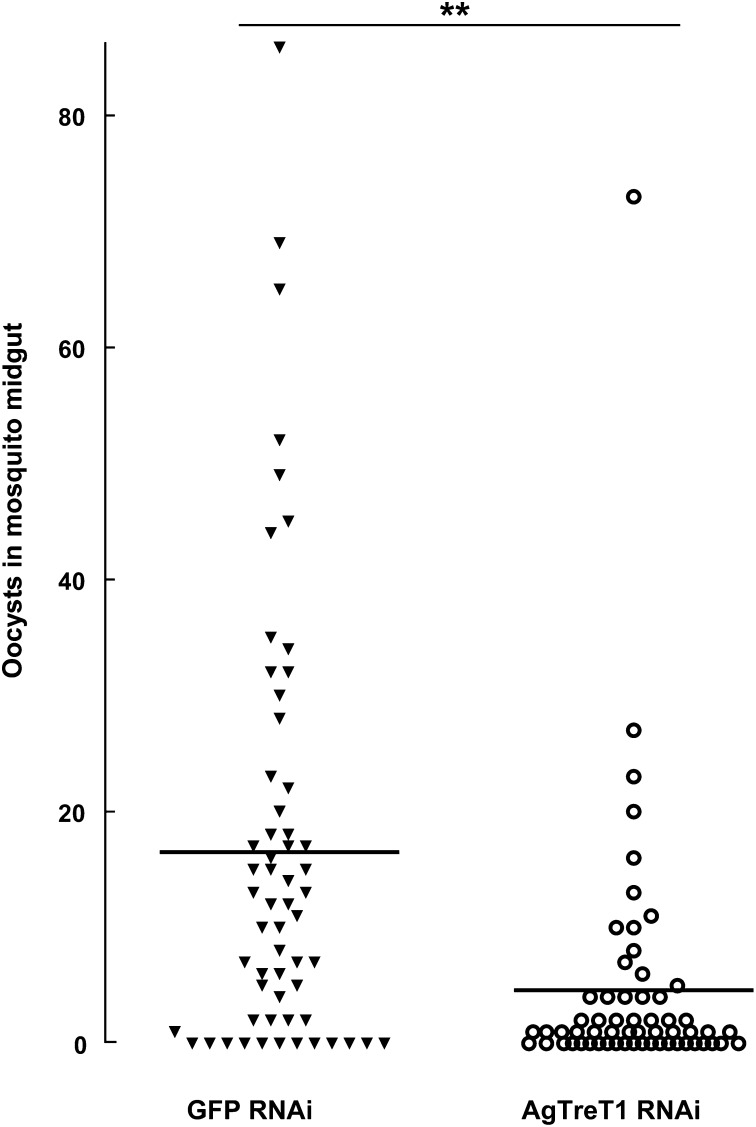
Although direct evidence is still lacking, rodent parasite Plasmodium berghei is believed to use trehalose for growth and development in Anopheles stephensi mosquitoes (22). In this study, we investigated whether the growth of human malaria parasite P. falciparum is affected by trehalose levels in its natural vector A. gambiae. Five-day-old A. gambiae females were infected by P. falciparum GFP-3D7 strain through membrane feeding. AgTreT1 expression was silenced by RNAi 1 d after infection. Eight days after P. falciparum infection, mosquito midguts were dissected, and GFP-3D7 oocysts were counted under a fluorescence microscope. A representative plot was chosen from three similar experiments (Fig. 4) revealing the mean number of oocysts in AgTreT1-deficient mosquitoes (4.6) to be significantly less than in control mosquitoes (16.5). Results from three experiments are summarized in Table S2. The rate of uninfected mosquitoes was lower in GFP-RNAi controls (31.1%) than in AgTreT1-RNAi mosquitoes (43.5%), indicating lower infection prevalence in AgTreT1-reduced mosquitoes.
Fig. 4.
P. falciparum oocysts in AgTreT1-silenced mosquitoes. Female A. gambiae mosquitoes were infected with P. falciparum GFP-3D7 parasites, and dsRNA targeting AgTreT1 or GFP was injected into mosquito thorax on the next day. Oocyst numbers in dissected mosquito midguts were counted 8 d postinfection. Closed triangles represent oocyst numbers in individual mosquitoes injected with GFP dsRNA, and open circles represent oocysts in mosquitoes injected with AgTreT1 dsRNA in a representative experiment. Horizontal lines indicate mean values. **P ≤ 0.01 by the Mann–Whitney U test. Three experiments with similar trends are summarized in Table S2.
Discussion
Trehalose Protection Against Desiccation and Heat.
In this study, we found that trehalose, an abundant osmolite in A. gambiae hemolymph, protects the mosquito against desiccation and heat. Synthesized in the fat body, trehalose is transported into the hemolymph by the specific transporter AgTreT1. We found high AgTreT1 transcript levels in fat body and whole head, the latter of which is presumably due to adherent fat body (Fig. 1B). Moreover, AgTreT1 mRNA levels and hemolymph trehalose concentrations increase during desiccation and heat (Fig. 1 C and D and Fig. S1), suggesting protective roles of trehalose transported through AgTreT1. Silencing AgTreT1 reduces hemolymph trehalose concentration and lowers hemolymph osmolality (Fig. 2B and Fig. S3), accelerating water evaporation due to increased vapor pressure. Based on Raoult's law, increasing trehalose concentration should do the opposite.
An intriguing, but not exclusive, mechanism is that trehalose also serves as a stabilizer for proteins and biomembrane structures in vivo. This unique property of trehalose, compared with other disaccharides, has been studied with purified proteins and cultured cells in vitro. Oral administration of trehalose was even applied in a mouse model of Huntington disease (8, 24, 25). Dryness and high temperature cause similar damage, such as denaturation of proteins and disruption of membranes. Certain other genes, such as heat shock proteins, are also activated by both stresses in many organisms (26).
Nevertheless, other evidence suggests that mechanisms protecting against desiccation may be distinct from mechanisms protecting against heat. For example, Cronobacter sakazakii, an emerging food-borne pathogen, shows different tolerance toward antibiotics when exposed to desiccation or heat (27). In another example, expression of Anhydrin and LEA-1 peptide stabilizers in the nematode Aphelenchus avenae are markedly up-regulated by dehydration but not by heat (11). In our study, AgTreT1 mediated trehalose level appears to protect A. gambiae against both dryness and high temperature because gene silencing leads to decreased mosquito viability under both stresses (Fig. 3). The protection mechanism is likely to use the same trehalose metabolism and transport pathway. We remain mindful that trehalose may not be the only factor in the protection machinery. Other protective agents and their roles in different types of stresses warrant further investigation in vector mosquitoes.
Unlike the closed circulation system of vertebrates, mosquito hemolymph flows in direct contact with internal organs, immersing them in trehalose. Protection against dehydration mediated by a trehalose transporter has been verified by transfection of PvTreT1 from P. vanderplanki larvae, which improved the desiccation tolerance of cultured Chinese hamster ovary cells in the presence of trehalose (28).
Here, we provide evidence for a protective role for AgTreT1 in whole mosquitoes. Silencing AgTreT1 expression decreases transport of trehalose from fat body to hemolymph. Trehalose movement from hemolymph to internal organs is likely limited by the trehalose concentration in hemolymph, possibly by the low levels of AgTreT1 expression in those organs. We observed an up-regulation of AgTreT1 transcript and increased hemolymph trehalose concentration when mosquitoes were in low relative humidity or high temperature. Similar mRNA induction of the TreT ortholog in P. vanderplanki was reported during desiccation (9). Thus, multiple lines of research suggest that trehalose transport determines hemolymph trehalose concentration, thereby protecting living organisms in adverse environmental conditions.
AgTreT1 Expression and P. falciparum Growth.
It is believed that Plasmodium spp. propagation in mosquitoes consumes nutrients from the vector. Hemolymph sugars, predominantly trehalose, are decreased after Plasmodium infection (23). However, molecular understanding of how trehalose is used by the parasite is still not clear. One possibility is that the parasite scavenges and metabolizes trehalose directly whereas another is that trehalose is hydrolyzed to glucose by either the vector or the parasite and the glucose is then taken up by the parasite.
Based on sequence analysis, the enzymes responsible for trehalose metabolism are present in A. gambiae—including putative trehalose synthase, trehalase, and the trehalose transporter. So far, orthologs of these genes have not been identified in Plasmodium spp. A hexose transporter was cloned from P. falciparum, but targeted gene deletion proved lethal during the erythrocytic stages of the parasite (29). Therefore, it is likely that P. falciparum propagation during vector stages depletes glucose from the mosquito by scavenging uptake into the parasite cell through membrane transporters. Trehalose, as the energy storage of mosquito, is mobilized to compensate for the glucose depletion. Because trehalose is the major blood sugar in mosquitoes, reductions due to AgTreT1 silencing would deplete the energy source for parasite growth, resulting in fewer parasite oocysts that eventually survived the energy scavenge.
The interaction between malaria parasites and vector mosquitoes has drawn much attention for possible interventions to prevent transmission of this pathogen (30). Most current studies on vectors are focused on mosquito immune genes. Expression levels of certain genes involved in the mosquito immune response correlate with Plasmodium spp. propagation in the vector. For example, targeted silencing of AgSTAT-A reduced the number of early oocysts in mosquito midgut, and TEP1 silencing increased the number of developing parasites (31, 32). In our study, silencing AgTreT1 expression significantly decreases infection intensity during oocyst stage (Fig. 4) and decreases infection prevalence. This reduction is important for malaria control because, in the field, even a single oocyst probably results in an infective mosquito. The reduction of intensity and prevalence likely occurs by inhibiting P. falciparum propagation and limiting the carbon and energy sources for parasite growth. Whether AgTreT1 or trehalose is directly related to mosquito immunity is unclear. Trehalose concentration in hemolymph may be related to multiple physiological processes in mosquito vectors.
Together, these studies have established that AgTreT1 is a factor in mosquito adaptation to dehydration and heat. Furthermore, AgTreT1 is also a positive mediator of P. falciparum development as gene silencing reduced parasite load in the mosquito midgut. These findings improve the understanding of malaria parasite development in the mosquito vector and provide a previously undescribed potential target for interrupting malaria transmission.
Materials and Methods
Mosquitoes, Routine Molecular Biology, RNAi, Antibodies, and Western Blot.
All experiments used methods and guidelines approved by the Animal Ethical Care Committee of Johns Hopkins University in compliance with US guidelines for the use of animals in research. AgTreT1 mRNA sequence was obtained from National Center for Biotechnology Information GenBank (XM_315568) and www.VectorBase.org (gene no. AGAP005563). Detailed methods on mosquito (Keele strain) rearing, RNA extraction, reverse transcription, quantitative PCR, relative humidity determination, RNA interference, Western blots, and desiccation assay were described in our previous paper (3). Primers designed for AgTreT1 cloning, qPCR, and RNAi are listed in Table S3. A rabbit polyclonal antibody was commercially raised against the AgTreT1 sequence at positions 243–256 (RGRKADVEPELKGI) and purified by affinity chromatography (GenScript). Rabbit anti-Actin antibody (Sigma; A2066) was used for loading control.
Hemolymph Extraction, Osmolality, and Trehalose Measurements.
Seven days after dsRNA injection, mosquitoes were anesthetized on ice, and 1–1.5 μL of PBS was injected into the thorax using a fine glass needle. The needle was then withdrawn from the thorax and inserted into the abdomen to harvest perfused hemolymph. Approximately 10–15 μL of hemolymph from 10 mosquitoes injected with AgTreT1 or GFP dsRNA were pooled for each measurement. Osmolality was measured with a VAPRO pressure osmometer 5520 (Wesco Inc.) with the manufacturer’s guidance.
In the trehalose measurement, extracted hemolymph was aliquoted into three parts. The first aliquot was used to measure total glucose after trehalase treatment. To hydrolyze hemolymph trehalose into glucose, a 40-μL reaction containing 0.2 μL of hemolymph and 10 mU of porcine trehalase (Sigma; T8778) in 50 mM Mes (2-[N-morpholino] ethanesulfonic acid) at pH 6.7 was incubated at 37 °C for 1 h. The second aliquot was used to measure the glucose concentration without trehalase treatment. The reaction system was the same as the first part except that trehalase was omitted. Then, the glucose concentrations of these two aliquots were determined with the Glucose HK Kit (Sigma; GAHK-20) In each 55-μL glucose test, 5 μL of the trehalase treatment product was used. The readouts at OD340nm were within linear range. Trehalose concentrations were calculated by the following equation:
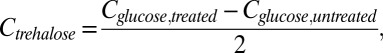 |
where Cglucose,untreated is the basal glucose concentration, Cglucose,treated is the total glucose concentration treated by trehalase, and Ctrehalose is the trehalose concentration in the hemolymph. d-(+)-trehalose dihydrate (Sigm; T3663) and d-(+)-Glucose (Sigma; G8270) were positive controls. Experiments were repeated four times. The third aliquot was used to determine the relative level of genomic DNA in AgTreT1-silenced extract compared with control extract. The measured trehalose concentrations in AgTreT1 RNAi mosquitoes or controls were normalized to the amount of genomic DNA extracted with the DNeasy Blood and Tissue Kit (Qiagen; 69504). qPCR was performed to measure the relative level of ribosomal gene S7 in hemolymph extracts. To verify the specificity, melting curves of qPCR were examined, and the correct sizes of amplicons were confirmed by electrophoresis. Normalized trehalose concentrations of AgTreT1-silenced and control hemolymph were subject to the Mann–Whitney U test with software GRAPHPAD PRISM 5.
Desiccation and Heat Assays.
Four hundred nanograms of dsRNA was injected into the thorax of mosquitoes, and the mosquitoes were cultivated at 17 °C and 80% relative humidity to recover until the desiccation assay as described (3) or the heat assay in this paragraph. Both assays were repeated three times with similar results. The median survival is 6–8.5 h in the presence of desiccant and ∼17 h in the absence of desiccant (3). In the heat assay, ∼20 mosquitoes injected with AgTreT1 or GFP dsRNA were put in cardboard cups and cultivated at 17 °C for optimal recovery. On day 6 after dsRNA injection, the cups were transferred to 27 °C and incubated for 16 h for acclimation. The heat assays were carried out on day 7 after dsRNA injection. Mosquitoes were placed in a Forma Environmental Chamber (Model 3851; Thermo Electron Corp.) at 39 °C and 80% relative humidity. Mosquito viability was monitored every 1–1.5 h. Survival analyses and log-rank tests were performed as described (3).
P. falciparum Infection and Oocyst Determination.
Five-day-old A. gambiae female adults were infected with a P. falciparum GFP-3D7 strain (33) by membrane feeding. The gametocytemia in each independent infection experiment was 0.2–0.3%. On day 1 after infected-blood feeding, fully-engorged females were anesthetized on ice, separated from the unfed mosquitoes, and used for dsRNA injection. Mosquito viability was monitored after injection with no significant difference between the AgTreT1 or GFP dsRNA injected groups. Eight days after blood feeding, mosquito midguts were dissected, and GFP-3D7 oocysts were counted under a fluorescence microscope (Leica DM 2500). At least 30 mosquitoes were used in experimental or control group in each experiment. The assay was repeated three times. The Mann–Whitney U test was performed with software GRAPHPAD PRISM 5.
Supplementary Material
Acknowledgments
We thank Dr. Marcelo Jacobs-Lorena for critically reading the manuscript. We appreciate Drs. Takashi Okuda and Takahiro Kikawada from the National Institute of Agrobiological Sciences in Japan for providing PvTret1 antibody and helpful discussions. We are also grateful to the Insectary, Parasitology, and Gene Array Core Facilities at the Johns Hopkins Malaria Research Institute (JHMRI) for help in data collection and supply of materials. Lastly, the Keele strain of A. gambiae mosquitoes used in this study is established by Drs. Hilary Hurd and Paul Eggleston from Keele University. This study was supported by National Institutes of Health Grants R01 HL48268 and U19 AI089680, a pilot grant from JHMRI and the Bloomberg Philanthropies (to P.A. and K.L.), the Jiangsu Health International Exchange Program in China, National Natural Science Foundation of China Grant 31201893, Natural Science Foundation of Jiangsu Province Grant BK2011164, and Jiangsu Health Science Projects X201110 and X200736.
Footnotes
The authors declare no conflict of interest.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1316709110/-/DCSupplemental.
References
- 1.Bernstein AS, Myers SS. Climate change and children’s health. Curr Opin Pediatr. 2011;23(2):221–226. doi: 10.1097/MOP.0b013e3283444c89. [DOI] [PubMed] [Google Scholar]
- 2.Zhang Q, Denlinger DL. Elevated couch potato transcripts associated with adult diapause in the mosquito Culex pipiens. J Insect Physiol. 2011;57(5):620–627. doi: 10.1016/j.jinsphys.2011.02.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Liu K, Tsujimoto H, Cha SJ, Agre P, Rasgon JL. Aquaporin water channel AgAQP1 in the malaria vector mosquito Anopheles gambiae during blood feeding and humidity adaptation. Proc Natl Acad Sci USA. 2011;108(15):6062–6066. doi: 10.1073/pnas.1102629108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Becker A, Schlöder P, Steele JE, Wegener G. The regulation of trehalose metabolism in insects. Experientia. 1996;52(5):433–439. doi: 10.1007/BF01919312. [DOI] [PubMed] [Google Scholar]
- 5. Jain NK, Roy I (2010) Trehalose and protein stability. Curr Protoc Protein Sci Chap 4, Unit 4.9, 10.1002/0471140864.ps0409s59. [DOI] [PubMed] [Google Scholar]
- 6.Crowe JH. Trehalose as a “chemical chaperone”: Fact and fantasy. Adv Exp Med Biol. 2007;594:143–158. doi: 10.1007/978-0-387-39975-1_13. [DOI] [PubMed] [Google Scholar]
- 7.Crowe JH, Hoekstra FA, Crowe LM. Anhydrobiosis. Annu Rev Physiol. 1992;54:579–599. doi: 10.1146/annurev.ph.54.030192.003051. [DOI] [PubMed] [Google Scholar]
- 8.Kaushik JK, Bhat R. Why is trehalose an exceptional protein stabilizer? An analysis of the thermal stability of proteins in the presence of the compatible osmolyte trehalose. J Biol Chem. 2003;278(29):26458–26465. doi: 10.1074/jbc.M300815200. [DOI] [PubMed] [Google Scholar]
- 9.Kikawada T, et al. Trehalose transporter 1, a facilitated and high-capacity trehalose transporter, allows exogenous trehalose uptake into cells. Proc Natl Acad Sci USA. 2007;104(28):11585–11590. doi: 10.1073/pnas.0702538104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Benoit JB, et al. Mechanisms to reduce dehydration stress in larvae of the Antarctic midge, Belgica antarctica. J Insect Physiol. 2007;53(7):656–667. doi: 10.1016/j.jinsphys.2007.04.006. [DOI] [PubMed] [Google Scholar]
- 11.Browne JA, et al. Dehydration-specific induction of hydrophilic protein genes in the anhydrobiotic nematode Aphelenchus avenae. Eukaryot Cell. 2004;3(4):966–975. doi: 10.1128/EC.3.4.966-975.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Crowe LM, Crowe JH. Anhydrobiosis: A strategy for survival. Adv Space Res. 1992;12(4):239–247. doi: 10.1016/0273-1177(92)90178-z. [DOI] [PubMed] [Google Scholar]
- 13.Singer MA, Lindquist S. Thermotolerance in Saccharomyces cerevisiae: The Yin and Yang of trehalose. Trends Biotechnol. 1998;16(11):460–468. doi: 10.1016/s0167-7799(98)01251-7. [DOI] [PubMed] [Google Scholar]
- 14.Aguilera J, Randez-Gil F, Prieto JA. Cold response in Saccharomyces cerevisiae: New functions for old mechanisms. FEMS Microbiol Rev. 2007;31(3):327–341. doi: 10.1111/j.1574-6976.2007.00066.x. [DOI] [PubMed] [Google Scholar]
- 15.Wharton DA. Cold tolerance of New Zealand alpine insects. J Insect Physiol. 2011;57(8):1090–1095. doi: 10.1016/j.jinsphys.2011.03.004. [DOI] [PubMed] [Google Scholar]
- 16.Wood JM, et al. Osmosensing and osmoregulatory compatible solute accumulation by bacteria. Comp Biochem Physiol A Mol Integr Physiol. 2001;130(3):437–460. doi: 10.1016/s1095-6433(01)00442-1. [DOI] [PubMed] [Google Scholar]
- 17.Chen Q, Behar KL, Xu T, Fan C, Haddad GG. Expression of Drosophila trehalose-phosphate synthase in HEK-293 cells increases hypoxia tolerance. J Biol Chem. 2003;278(49):49113–49118. doi: 10.1074/jbc.M308652200. [DOI] [PubMed] [Google Scholar]
- 18.Elbein AD, Pan YT, Pastuszak I, Carroll D. New insights on trehalose: A multifunctional molecule. Glycobiology. 2003;13(4):17R–27R. doi: 10.1093/glycob/cwg047. [DOI] [PubMed] [Google Scholar]
- 19.Campbell LH, Brockbank KG. Culturing with trehalose produces viable endothelial cells after cryopreservation. Cryobiology. 2012;64(3):240–244. doi: 10.1016/j.cryobiol.2012.02.006. [DOI] [PubMed] [Google Scholar]
- 20.Kikawada T, Minakawa N, Watanabe M, Okuda T. Factors Inducing Successful Anhydrobiosis in the African Chironomid Polypedilum vanderplanki: Significance of the Larval Tubular Nest. Integr Comp Biol. 2005;45(5):710–714. doi: 10.1093/icb/45.5.710. [DOI] [PubMed] [Google Scholar]
- 21.Mikani A, Wang QS, Takeda M. Brain-midgut short neuropeptide F mechanism that inhibits digestive activity of the American cockroach, Periplaneta americana upon starvation. Peptides. 2012;34(1):135–144. doi: 10.1016/j.peptides.2011.10.028. [DOI] [PubMed] [Google Scholar]
- 22.Kanamori Y, et al. The trehalose transporter 1 gene sequence is conserved in insects and encodes proteins with different kinetic properties involved in trehalose import into peripheral tissues. Insect Biochem Mol Biol. 2010;40(1):30–37. doi: 10.1016/j.ibmb.2009.12.006. [DOI] [PubMed] [Google Scholar]
- 23.Mack SR, Samuels S, Vanderberg JP. Hemolymph of Anopheles stephensi from noninfected and Plasmodium berghei-infected mosquitoes. 3. Carbohydrates. J Parasitol. 1979;65(2):217–221. [PubMed] [Google Scholar]
- 24.Zancan P, Sola-Penna M. Trehalose and glycerol stabilize and renature yeast inorganic pyrophosphatase inactivated by very high temperatures. Arch Biochem Biophys. 2005;444(1):52–60. doi: 10.1016/j.abb.2005.09.014. [DOI] [PubMed] [Google Scholar]
- 25.Jain NK, Roy I. Effect of trehalose on protein structure. Protein Sci. 2009;18(1):24–36. doi: 10.1002/pro.3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Laksanalamai P, Robb FT. Small heat shock proteins from extremophiles: A review. Extremophiles. 2004;8(1):1–11. doi: 10.1007/s00792-003-0362-3. [DOI] [PubMed] [Google Scholar]
- 27.Al-Nabulsi AA, et al. Impact of environmental stress desiccation, acidity, alkalinity, heat or cold on antibiotic susceptibility of Cronobacter sakazakii. Int J Food Microbiol. 2011;146(2):137–143. doi: 10.1016/j.ijfoodmicro.2011.02.013. [DOI] [PubMed] [Google Scholar]
- 28.Chakraborty N, et al. Trehalose transporter from African chironomid larvae improves desiccation tolerance of Chinese hamster ovary cells. Cryobiology. 2012;64(2):91–96. doi: 10.1016/j.cryobiol.2011.11.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Slavic K, et al. Life cycle studies of the hexose transporter of Plasmodium species and genetic validation of their essentiality. Mol Microbiol. 2010;75(6):1402–1413. doi: 10.1111/j.1365-2958.2010.07060.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Karunamoorthi K. Vector control: A cornerstone in the malaria elimination campaign. Clin Microbiol Infect. 2011;17(11):1608–1616. doi: 10.1111/j.1469-0691.2011.03664.x. [DOI] [PubMed] [Google Scholar]
- 31.Gupta L, et al. The STAT pathway mediates late-phase immunity against Plasmodium in the mosquito Anopheles gambiae. Cell Host Microbe. 2009;5(5):498–507. doi: 10.1016/j.chom.2009.04.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Blandin S, et al. Complement-like protein TEP1 is a determinant of vectorial capacity in the malaria vector Anopheles gambiae. Cell. 2004;116(5):661–670. doi: 10.1016/s0092-8674(04)00173-4. [DOI] [PubMed] [Google Scholar]
- 33.Talman AM, Blagborough AM, Sinden RE. A Plasmodium falciparum strain expressing GFP throughout the parasite’s life-cycle. PLoS ONE. 2010;5(2):e9156. doi: 10.1371/journal.pone.0009156. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.