Abstract
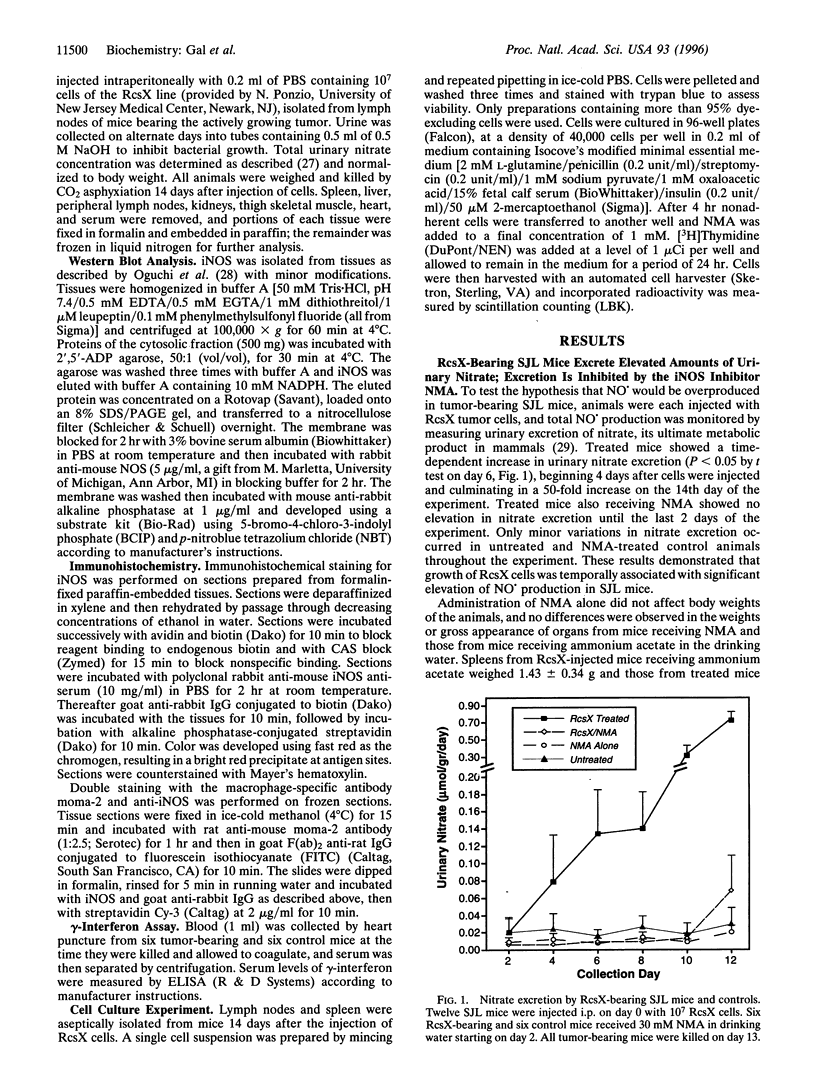
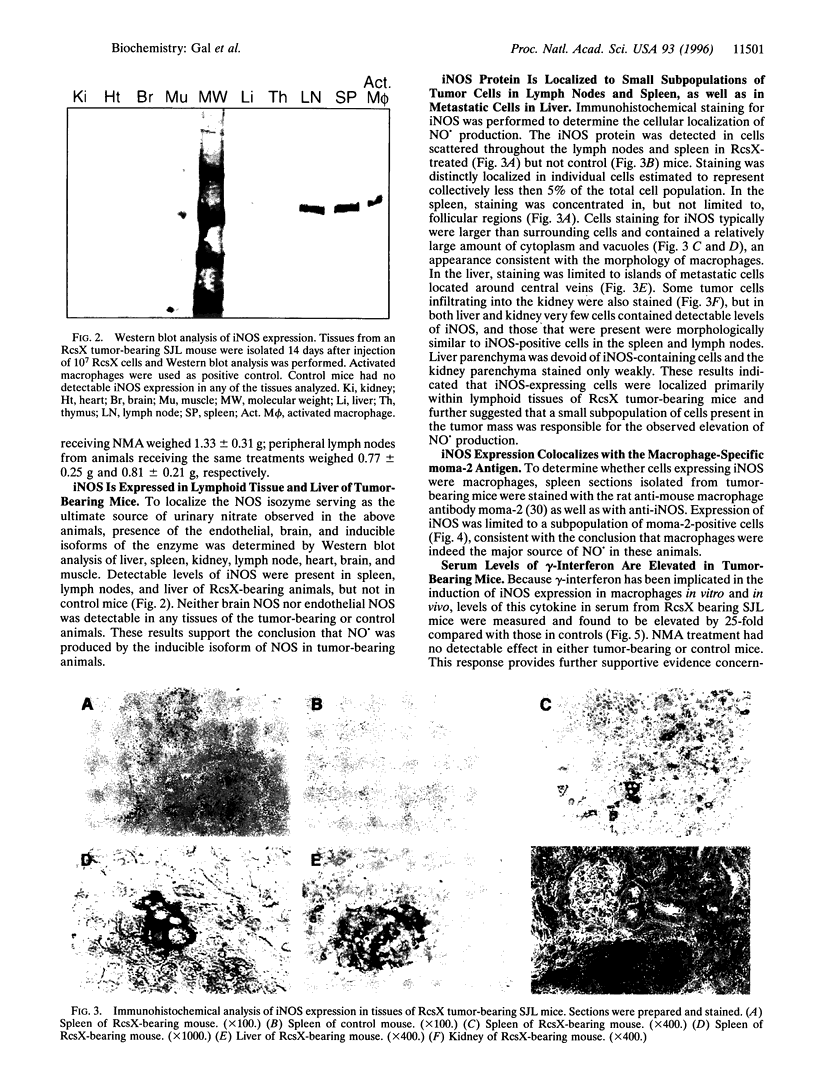
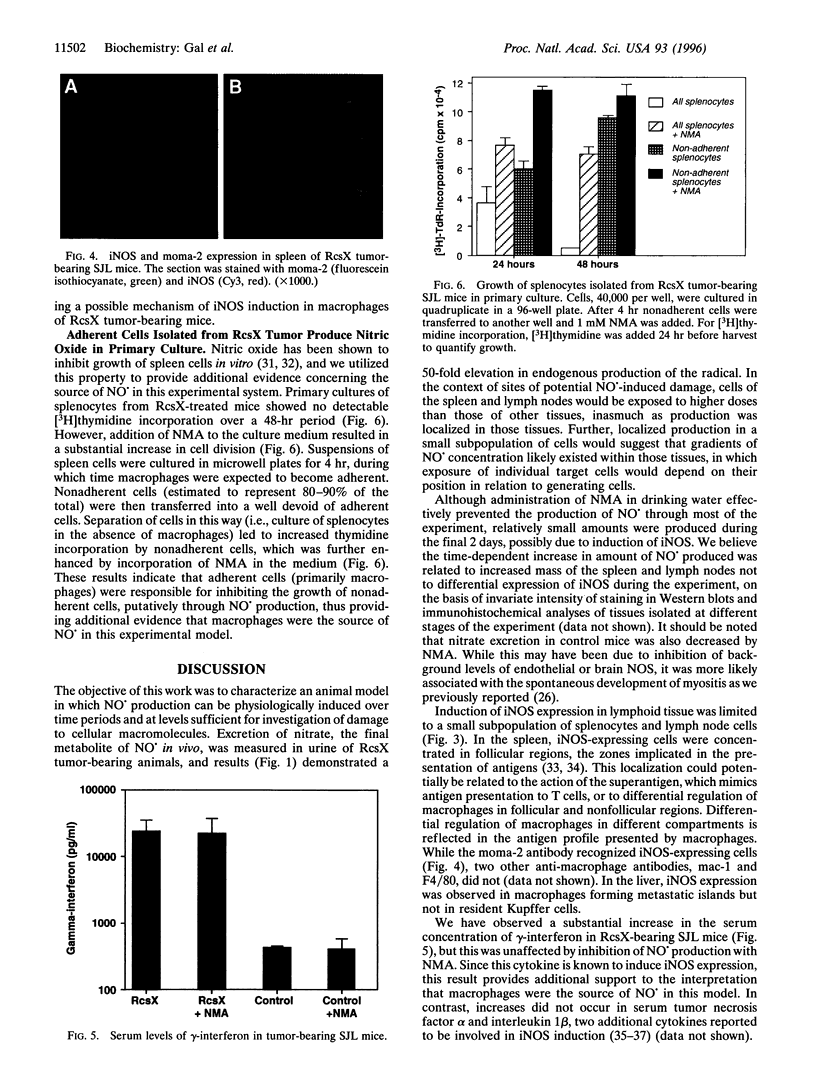
SJL mice spontaneously develop pre-B-cell lymphoma that we hypothesized might stimulate macrophages to produce nitric oxide (NO.). Transplantation of an aggressive lymphoma (RcsX) was used to induce tumor formation. Urinary nitrate excretion was measured as an index of NO. production and was found to increase 50-fold by 13 days after tumor injection. NO. production was prevented by the addition of a nitric oxide synthase (NOS) inhibitor. The expression of inducible NOS (iNOS) in various tissues was estimated by Western blot analysis and localized by immunohistochemistry. The synthase was detected in the spleen, lymph nodes, and liver of treated but not control mice. To assess whether the iNOS-staining cells were macrophages, spleen sections from ResX-bearing animals were costained with anti-iNOS antibody and the anti-macrophage antibody moma-2. Expression of iNOS was found to be limited to a subset of the macrophage population. The concentration of gamma-interferon, a cytokine known to induce NO. production by macrophages, in the serum of tumor-bearing mice, was measured and found to be elevated 25-fold above untreated mice. The ability of ResX-activated macrophages to inhibit splenocyte growth in primary culture was estimated and macrophage-derived NO. was found to inhibit cell division 10-fold. Our findings demonstrate that ResX cells stimulate NO. production by macrophages in the spleen and lymph nodes of SJL mice, and we believe this experimental model will prove useful for study of the toxicological effects of NO. under physiological conditions.
Full text
PDF

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Albina J. E., Abate J. A., Henry W. L., Jr Nitric oxide production is required for murine resident peritoneal macrophages to suppress mitogen-stimulated T cell proliferation. Role of IFN-gamma in the induction of the nitric oxide-synthesizing pathway. J Immunol. 1991 Jul 1;147(1):144–148. [PubMed] [Google Scholar]
- Albina J. E., Cui S., Mateo R. B., Reichner J. S. Nitric oxide-mediated apoptosis in murine peritoneal macrophages. J Immunol. 1993 Jun 1;150(11):5080–5085. [PubMed] [Google Scholar]
- Alleva D. G., Burger C. J., Elgert K. D. Tumor-induced regulation of suppressor macrophage nitric oxide and TNF-alpha production. Role of tumor-derived IL-10, TGF-beta, and prostaglandin E2. J Immunol. 1994 Aug 15;153(4):1674–1686. [PubMed] [Google Scholar]
- Ankarcrona M., Dypbukt J. M., Brüne B., Nicotera P. Interleukin-1 beta-induced nitric oxide production activates apoptosis in pancreatic RINm5F cells. Exp Cell Res. 1994 Jul;213(1):172–177. doi: 10.1006/excr.1994.1187. [DOI] [PubMed] [Google Scholar]
- Arnon R. Experimental allergic encephalomyelitis--susceptibility and suppression. Immunol Rev. 1981;55:5–30. doi: 10.1111/j.1600-065x.1981.tb00337.x. [DOI] [PubMed] [Google Scholar]
- Beckmann J. S., Ye Y. Z., Anderson P. G., Chen J., Accavitti M. A., Tarpey M. M., White C. R. Extensive nitration of protein tyrosines in human atherosclerosis detected by immunohistochemistry. Biol Chem Hoppe Seyler. 1994 Feb;375(2):81–88. doi: 10.1515/bchm3.1994.375.2.81. [DOI] [PubMed] [Google Scholar]
- Berisha H. I., Pakbaz H., Absood A., Said S. I. Nitric oxide as a mediator of oxidant lung injury due to paraquat. Proc Natl Acad Sci U S A. 1994 Aug 2;91(16):7445–7449. doi: 10.1073/pnas.91.16.7445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen L. L., Adams J. C., Steinman R. M. Anatomy of germinal centers in mouse spleen, with special reference to "follicular dendritic cells". J Cell Biol. 1978 Apr;77(1):148–164. doi: 10.1083/jcb.77.1.148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cross A. H., Misko T. P., Lin R. F., Hickey W. F., Trotter J. L., Tilton R. G. Aminoguanidine, an inhibitor of inducible nitric oxide synthase, ameliorates experimental autoimmune encephalomyelitis in SJL mice. J Clin Invest. 1994 Jun;93(6):2684–2690. doi: 10.1172/JCI117282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Denham S., Rowland I. J. Inhibition of the reactive proliferation of lymphocytes by activated macrophages: the role of nitric oxide. Clin Exp Immunol. 1992 Jan;87(1):157–162. doi: 10.1111/j.1365-2249.1992.tb06430.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Farias-Eisner R., Sherman M. P., Aeberhard E., Chaudhuri G. Nitric oxide is an important mediator for tumoricidal activity in vivo. Proc Natl Acad Sci U S A. 1994 Sep 27;91(20):9407–9411. doi: 10.1073/pnas.91.20.9407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hibbs J. B., Jr, Taintor R. R., Vavrin Z., Rachlin E. M. Nitric oxide: a cytotoxic activated macrophage effector molecule. Biochem Biophys Res Commun. 1988 Nov 30;157(1):87–94. doi: 10.1016/s0006-291x(88)80015-9. [DOI] [PubMed] [Google Scholar]
- Katz I. R., Chapman-Alexander J., Jacobson E. B., Lerman S. P., Thorbecke G. J. Growth of SJL/J-derived transplantable reticulum cell sarcoma as related to its ability to induce T-cell proliferation in the host. III. Studies on thymectomized and congenitally athymic SJL mice. Cell Immunol. 1981 Nov 15;65(1):84–92. doi: 10.1016/0008-8749(81)90054-x. [DOI] [PubMed] [Google Scholar]
- Kaur H., Halliwell B. Evidence for nitric oxide-mediated oxidative damage in chronic inflammation. Nitrotyrosine in serum and synovial fluid from rheumatoid patients. FEBS Lett. 1994 Aug 15;350(1):9–12. doi: 10.1016/0014-5793(94)00722-5. [DOI] [PubMed] [Google Scholar]
- Kraal G., Rep M., Janse M. Macrophages in T and B cell compartments and other tissue macrophages recognized by monoclonal antibody MOMA-2. An immunohistochemical study. Scand J Immunol. 1987 Dec;26(6):653–661. doi: 10.1111/j.1365-3083.1987.tb02301.x. [DOI] [PubMed] [Google Scholar]
- Lasky J. L., Ponzio N. M., Thorbecke G. J. Characterization and growth factor requirements of SJL lymphomas. I. Development of a B cell growth factor-dependent in vitro cell line, cRCS-X. J Immunol. 1988 Jan 15;140(2):679–687. [PubMed] [Google Scholar]
- Leaf C. D., Wishnok J. S., Tannenbaum S. R. L-arginine is a precursor for nitrate biosynthesis in humans. Biochem Biophys Res Commun. 1989 Sep 15;163(2):1032–1037. doi: 10.1016/0006-291x(89)92325-5. [DOI] [PubMed] [Google Scholar]
- Lejeune P., Lagadec P., Onier N., Pinard D., Ohshima H., Jeannin J. F. Nitric oxide involvement in tumor-induced immunosuppression. J Immunol. 1994 May 15;152(10):5077–5083. [PubMed] [Google Scholar]
- Lerman S. P., Carswell E. A., Chapman J., Thorbecke G. J. Properties of reticulum cell sarcomas in SJL/J mice. III. Promotion of tumor growth in irradiated mice by normal lymphoid cells. Cell Immunol. 1976 Apr;23(1):53–67. doi: 10.1016/0008-8749(76)90171-4. [DOI] [PubMed] [Google Scholar]
- Nakano K., Cinader B. Accelerated age-dependent decline in the T suppressor capacity of SJL mice. Eur J Immunol. 1980 Apr;10(4):309–316. doi: 10.1002/eji.1830100416. [DOI] [PubMed] [Google Scholar]
- Nakauchi H., Osada H., Yagita H., Okumura K. Molecular evidence that SJL reticulum cell sarcomas are derived from pre-B cell. Clonal rearrangement of heavy chain but not of light chain immunoglobulin genes. J Immunol. 1987 Oct 15;139(8):2803–2809. [PubMed] [Google Scholar]
- Nathan C., Xie Q. W. Nitric oxide synthases: roles, tolls, and controls. Cell. 1994 Sep 23;78(6):915–918. doi: 10.1016/0092-8674(94)90266-6. [DOI] [PubMed] [Google Scholar]
- Oguchi S., Iida S., Adachi H., Oshima H., Esumi H. Induction of Ca2+/calmodulin-dependent NO synthase in various organs of rats by Propionibacterium acnes and lipopolysaccharide treatment. FEBS Lett. 1992 Aug 10;308(1):22–25. doi: 10.1016/0014-5793(92)81041-j. [DOI] [PubMed] [Google Scholar]
- Pick E., Keisari Y. A simple colorimetric method for the measurement of hydrogen peroxide produced by cells in culture. J Immunol Methods. 1980;38(1-2):161–170. doi: 10.1016/0022-1759(80)90340-3. [DOI] [PubMed] [Google Scholar]
- Ponzio N. M., Hayama T., Nagler C., Katz I. R., Hoffmann M. K., Gilbert K., Vilcek J., Thorbecke G. J. Ia-restricted interaction of normal lymphoid cells and SJL lymphoma (reticulum cell sarcoma) leading to lymphokine production. II. Rapid production of antibody-enhancing factor, interleukin 2, and immune interferon. J Natl Cancer Inst. 1984 Feb;72(2):311–320. [PubMed] [Google Scholar]
- Schmidt H. H., Lohmann S. M., Walter U. The nitric oxide and cGMP signal transduction system: regulation and mechanism of action. Biochim Biophys Acta. 1993 Aug 18;1178(2):153–175. doi: 10.1016/0167-4889(93)90006-b. [DOI] [PubMed] [Google Scholar]
- Schmidt H. H., Walter U. NO at work. Cell. 1994 Sep 23;78(6):919–925. doi: 10.1016/0092-8674(94)90267-4. [DOI] [PubMed] [Google Scholar]
- Schuman E. M., Madison D. V. Locally distributed synaptic potentiation in the hippocampus. Science. 1994 Jan 28;263(5146):532–536. doi: 10.1126/science.8290963. [DOI] [PubMed] [Google Scholar]
- Stavnezer J., Lasky J. L., Ponzio N. M., Scheid M. P., Thorbecke G. J. Reticulum cell sarcomas of SJL mice have rearranged immunoglobulin heavy and light chain genes. Eur J Immunol. 1989 Jun;19(6):1063–1069. doi: 10.1002/eji.1830190616. [DOI] [PubMed] [Google Scholar]
- Tamir S., deRojas-Walker T., Gal A., Weller A. H., Li X., Fox J. G., Wogan G. N., Tannenbaum S. R. Nitric oxide production in relation to spontaneous B-cell lymphoma and myositis in SJL mice. Cancer Res. 1995 Oct 1;55(19):4391–4397. [PubMed] [Google Scholar]
- Tao X., Stout R. D. T cell-mediated cognate signaling of nitric oxide production by macrophages. Requirements for macrophage activation by plasma membranes isolated from T cells. Eur J Immunol. 1993 Nov;23(11):2916–2921. doi: 10.1002/eji.1830231128. [DOI] [PubMed] [Google Scholar]
- Tsiagbe V. K., Thorbecke G. J. Paraproteins and primary lymphoma in SJL mice. I. Individuality of idiotypes on paraproteins. Cell Immunol. 1990 Sep;129(2):494–502. doi: 10.1016/0008-8749(90)90223-e. [DOI] [PubMed] [Google Scholar]
- Tsiagbe V. K., Yoshimoto T., Asakawa J., Cho S. Y., Meruelo D., Thorbecke G. J. Linkage of superantigen-like stimulation of syngeneic T cells in a mouse model of follicular center B cell lymphoma to transcription of endogenous mammary tumor virus. EMBO J. 1993 Jun;12(6):2313–2320. doi: 10.1002/j.1460-2075.1993.tb05885.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wei X. Q., Charles I. G., Smith A., Ure J., Feng G. J., Huang F. P., Xu D., Muller W., Moncada S., Liew F. Y. Altered immune responses in mice lacking inducible nitric oxide synthase. Nature. 1995 Jun 1;375(6530):408–411. doi: 10.1038/375408a0. [DOI] [PubMed] [Google Scholar]
- Weinberg J. B., Granger D. L., Pisetsky D. S., Seldin M. F., Misukonis M. A., Mason S. N., Pippen A. M., Ruiz P., Wood E. R., Gilkeson G. S. The role of nitric oxide in the pathogenesis of spontaneous murine autoimmune disease: increased nitric oxide production and nitric oxide synthase expression in MRL-lpr/lpr mice, and reduction of spontaneous glomerulonephritis and arthritis by orally administered NG-monomethyl-L-arginine. J Exp Med. 1994 Feb 1;179(2):651–660. doi: 10.1084/jem.179.2.651. [DOI] [PMC free article] [PubMed] [Google Scholar]






