Significance
This article describes the development and validation of a novel mouse model that can be used to predict hemolytic toxicity of drugs that occurs in individuals with an enzyme deficiency known as glucose-6-phosphate dehydrogenase (G6PD) deficiency. G6PD deficiency affects more than 400 million people worldwide. In this model, nonobese diabetic/SCID mice are transfused with human RBCs from G6PD-deficient donors. Treatment with drugs known to cause hemolytic anemia in humans do not cause damage to the mouse RBCs nor to the transfused normal human RBCs; but a robust hemolytic response is observed in the mice transfused with G6PD-deficient human RBCs. The immediate impact of this model will be in advancing the development antimalarial drugs.
Abstract
Individuals with glucose 6-phosphate dehydrogenase (G6PD) deficiency are at risk for the development of hemolytic anemia when given 8-aminoquinolines (8-AQs), an important class of antimalarial/antiinfective therapeutics. However, there is no suitable animal model that can predict the clinical hemolytic potential of drugs. We developed and validated a human (hu)RBC-SCID mouse model by giving nonobese diabetic/SCID mice daily transfusions of huRBCs from G6PD-deficient donors. Treatment of SCID mice engrafted with G6PD-deficient huRBCs with primaquine, an 8-AQ, resulted in a dose-dependent selective loss of huRBCs. To validate the specificity of this model, we tested known nonhemolytic antimalarial drugs: mefloquine, chloroquine, doxycycline, and pyrimethamine. No significant loss of G6PD-deficient huRBCs was observed. Treatment with drugs known to cause hemolytic toxicity (pamaquine, sitamaquine, tafenoquine, and dapsone) resulted in loss of G6PD-deficient huRBCs comparable to primaquine. This mouse model provides an important tool to test drugs for their potential to cause hemolytic toxicity in G6PD-deficient populations.
Glucose-6-phosphate dehydrogenase (G6PD) deficiency is the most common human enzyme deficiency, with an estimated 400 million people worldwide affected by this enzymopathy (1). G6PD-deficient RBCs are uniquely sensitive to oxidative stress. Several drugs induce oxidative stress in G6PD-deficient RBCs, including the antimalarial drug primaquine (PQ), an 8-aminoquinoline (8-AQ) (2). The damaged G6PD-deficient RBCs are subsequently cleared from the peripheral circulation, resulting in “hemolytic” anemia (2). 8-AQs are the only approved drug class able to eliminate the hypnozoite stages of the malaria parasite (3), as well as stage V Plasmodium falciparum gametocytes (4, 5). These characteristics make them an ideal drug class for malaria elimination campaigns (6, 7). However, because of the hemolytic toxicity associated with PQ, this drug has sharply limited utility in public health programs for the treatment of malaria. Although new 8-AQs have been developed (8), the lack of a relevant animal model to predict hemolytic toxicity in the context of G6PD deficiency has hindered further development of this class of antimalarial drugs (2).
SCID mice have routinely been used as models of human disease (9–12). Lacking in functional immune responses, and therefore able to accept xenogenic transplants, SCID mice have been widely used as hosts for the engraftment of both normal and malignant human cells including human RBCs (huRBCs) (13, 14). Subsequent studies on the engraftment of huRBCs improved the degree and persistence of engraftment by using sublethal irradiation, chemical treatment protocols, or inclusion of denatured human serum and repeated administration of huRBCs to enhance engraftment efficiency (15–18). Recent advances in the development of immunodeficient mouse models have yielded mice with higher engraftment capacities and with minimal manipulation before engraftment. SCID mice developed on the nonobese diabetic (NOD) background (NOD/SCID) are able to support huRBCs for prolonged periods after repeated i.p. injections of huRBCs (19–21).
We report here the development of an NOD/SCID mouse model engrafted with G6PD-deficient huRBCs. Treatment with PQ and other drugs known to induce hemolytic anemia in humans produced hemolytic responses in mice engrafted with G6PD-deficient huRBCs, suggesting that this model can be used for testing drugs for the prediction of the hemolytic toxicity. Use of this model will expedite the development of safer drugs for malaria treatment and prophylaxis, as well as other antiparasitic diseases for which 8-AQs have demonstrated efficacy.
Results
Engraftment of G6PD-Deficient Human Erythrocytes into NOD/SCID Mice.
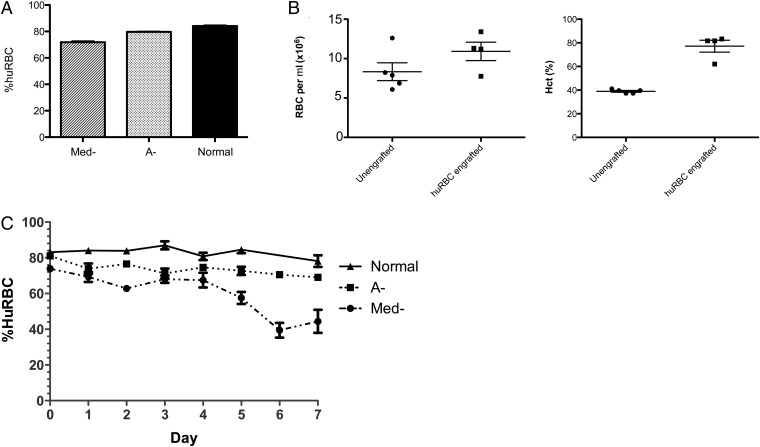
A number of genetic variants resulting in loss of G6PD activity have been described (22). Depending on the genetic variant, drug-induced hemolysis may be mild and self-limiting as observed in individuals with the African variant (A-) or severe and life threatening as described in individuals with the Mediterranean variant (Med-) (23). To determine whether huRBCs from G6PD-deficient donors could be successfully engrafted into NOD/SCID mice, RBCs from A- or Med- donors or donors with normal G6PD activity were injected i.p. daily for 14 d (herein referred to as A- mice, Med- mice, or normal huRBC SCID mice, respectively). After 14 d, the percentage of huRBCs was assessed by flow cytometry after staining of blood cells with an FITC-conjugated anti-human glycophorin A (CD235a) mAb. Normal huRBC SCID mice engrafted at a level of 84.1% ± 0.7% huRBCs (mean ± SEM, n = 40) after 14 d (Fig. 1A). Med- and A- huRBC SCID mice engraftment efficiencies were both significantly lower, with 72.8% ± 1.1% (n = 99) and 77.7% ± 0.8% (n = 242) huRBCs at 14 d, respectively (P < 0.0001). As a control, we evaluated the effects of huRBC engraftment on the hematocrit and peripheral RBC concentration (mouse and human) after 14 d of engraftment (Fig. 1B). There was increased hematocrit and peripheral RBC concentration in huRBC-engrafted SCID mice compared with unengrafted control NOD/SCID mice. Unengrafted NOD/SCID mice had a mean peripheral RBC concentration of 8.3 × 106, within the normal range for murine peripheral RBC concentration (e.g., 6.4 × 106 to 9.4 × 106 RBC/μL), whereas huRBC-engrafted mice were slightly higher, with 1.1 × 107 RBCs/mL.
Fig. 1.
Engraftment of Med-, A-, and normal huRBCs in NOD/SCID mice. (A) Mice were engrafted with huRBCs from Med-, A-, and normal donors, n = 99, 242, and 40, respectively in 5, 12, and 2 separate experiments. Mice received 5 × 109 red blood cells i.p. for 14 d and were assessed for levels of huRBCs at day 14 by FACS staining using an anti-CD235a FITC-conjugated monoclonal antibody. The graph shows the engraftment efficiency after 14 d in Med-, A-, and normal mice. Values are % mean huRBCs ± SEM. (B) Comparison of total RBCs and hematocrit in huRBC SCID mice. The number of RBCs per μL of blood was determined, as well as the hematocrit in NOD/SCID mice (unengrafted) and in NOD/SCID given huRBCs for 14 d (n = 5 and 4, respectively). Dots represent individual mice. (C) Stability of engrafted Med-, A-, and normal huRBCs in NOD/SCID mice was assessed over 7 d after the last injection of huRBCs. n = 18, 43, and 10 mice from Med-, A-, and normal huRBC SCID mice from 5, 12, and 2 independent experiments, respectively. Data points shown are the mean ± SEM.
We next assessed the stability of the engrafted huRBCs over time (Fig. 1C). After the 14 d of engraftment, circulating huRBCs were measured daily for 7 d, and the percent huRBCs in peripheral blood of mice was assessed by flow cytometry. Mice engrafted with G6PD normal huRBCs maintained relatively stable levels of huRBCs over time, with only 6.1% average decline by day 7 after last huRBC injection. A- huRBC mice retained 85.2% of initial measured huRBC values by day 7, but this was not significantly different from normal huRBC SCID mice. In contrast, the Med- mice showed a significantly steeper loss in huRBCs over time relative to both A- and normal huRBC mice (P < 0.05, one-way ANOVA, Kruskal-Wallis test), likely reflecting the greater fragility of the Med- G6PD-deficient huRBCs (24).
PQ Treatment Induces Selective Hemolysis in NOD/SCID Mice Engrafted with A- and Med- G6PD-Deficient huRBCs but Not in Mice Engrafted with Normal huRBCs.
To assess the hemolytic capacity of PQ, NOD/SCID mice were engrafted with RBCs from a normal, an A-, or a Med- donor. For the initial evaluations of PQ effects, mice were treated with PQ given i.p. at 12.5 mg/kg per day after 14 d of engraftment. PQ was administered i.p. in two divided doses (8 h apart) for 7 d. A vehicle control group (e.g., mice treated with PBS) was included for each experiment. The percent huRBCs and murine reticulocytes levels were assessed daily during the 7-d treatment period by flow cytometry.
As shown in Fig. 2A, PQ treatment results in loss of huRBCs in both the A- and Med- huRBC SCID mice but not in normal huRBC SCID mice. The kinetics of decline in huRBCs is more rapid in the Med- huRBC SCID mice compared with the A- huRBC SCID mice, with almost 100% of huRBCs removed from circulation at day 5 vs. day 7 for Med- huRBC vs. A- huRBC, respectively. However, in both groups the decline in huRBCs begins after 3 d of PQ treatment and results in significant and profound loss of the circulating huRBCs.
Fig. 2.
Treatment with PQ induces hemolysis in G6PD-deficient huRBC NOD/SCID mice. Human RBCs from A-, Med- G6PD-deficient or G6PD normal donors were engrafted i.p. into NOD/SCID mice. Mice were then treated for 7 d with 12.5 mg/kg per day of PQ or with vehicle control as a split dose, 8 h apart. Blood was assessed for the percentage of huRBCs and muRetics by FACS every 24 h after first treatment dose through termination of experiment at 7 d. Results are for four to five mice per group. The kinetics of (A) huRBC and (B) muRetic levels are shown. (C) Spleen and liver weight were also assessed at the termination of experiment and are presented as normalized data relative to total body weight.
Reticulocyte levels in the peripheral circulatory system are maintained within a tight range (∼1% of total RBC) by complex feedback systems controlling the erythropoietic process. During an acute loss of RBCs, the proportion of reticulocytes in the peripheral blood rises dramatically as a compensatory mechanism for the acute loss of blood, suggesting that an increase in reticulocytes levels could be a secondary marker for hemolytic toxicity. Consistent with the observations of a decline in huRBCs, increases in murine reticulocytes (muRetic) were seen in both Med- and A- huRBC SCID mice treated with PQ, with a final mean level of muRetics of 9.1–11.4% of total RBC by day 7 (Fig. 2B). The mean percent muRetic levels remained below 1% in normal huRBC SCID mice treated with PQ, consistent with the lack of hemolytic effect observed. Interestingly, muRetic levels showed a biphasic increase, with the first elevation observed after 2 d of treatment and a second and larger increase starting at day 5 (Med- huRBC SCID mice) or day 6 (A- huRBC SCID mice) after treatment. Body, spleen, and liver weights were also determined at the termination of the experiment (i.e., at day 7 of PQ treatment) (Fig. 2C). Consistent with a role for the spleen in destruction of damaged RBCs (25), we observed significant increases in spleen weight in Med- and A- huRBC SCID mice treated with PQ (P = 0.016 and P = 0.026, respectively) but not in normal huRBC SCID mice treated with PQ (P = 0.09). Liver weights and body weights were not affected in any group of mice.
Oral Administration of PQ Induces a Dose-Dependent Hemolytic Response in A- huRBC NOD/SCID Mice.
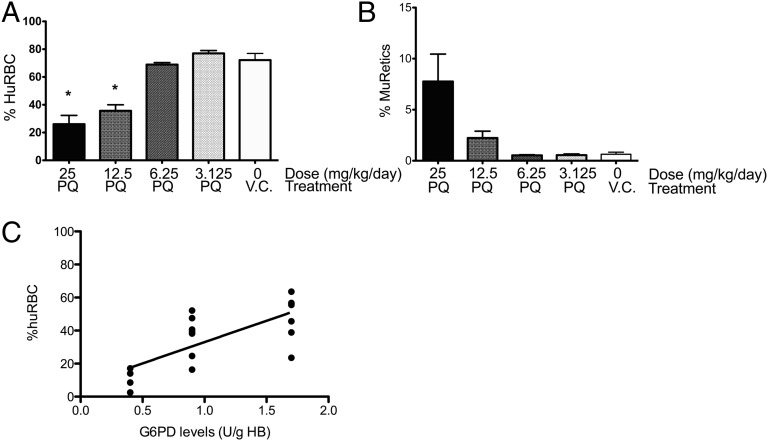
Because PQ is administered orally in humans, we wanted to determine whether PQ given by oral gavage (p.o.) would also induce loss of huRBC in G6PD-deficient huRBC SCID mice. In addition, we also wanted to determine whether there was a PQ dose dependence. A- huRBC SCID mice were treated with 25, 12.5, 6.25, and 3.125 mg/kg of PQ given p.o. once per day for 7 d, and at the end of treatment the percentage of huRBCs and muRetics was assessed (Fig. 3 A and B). Both the 25 and 12.5 mg/kg per day oral doses induced significant losses of huRBCs and increases in muRetics by day 7 of treatment compared with the vehicle control (P < 0.0001 and P = 0.0035, respectively), but this effect was absent or minimal at the 6.25 and 3.125 mg/kg per day oral doses. Thus, oral administration of PQ induces a dose-related loss of huRBCs in A- huRBC SCID mice, with a concomitant increase in muRetics.
Fig. 3.
Evaluation of dose of PQ and G6PD levels on hemolytic response. A- huRBC SCID mice were treated with PQ p.o. daily for 7 d with indicated doses of PQ (25, 12.5, 6.25, or 3.125 mg/kg per day) or given PBS as a vehicle control and assessed for percentage huRBCs (A) and muRetics (B) at day 7 by FACS analysis. Each treatment group consisted of 4 mice. *Statistically significant differences (one-way ANOVA with Bonferroni posttest, P < 0.001). (C) G6PD levels were measured at start of engraftment and correlated with percentage of huRBCs at 7 d after treatment with PQ (25 mg/kg per day, p.o., 7 d) using Spearman rank correlation (P = 0.0005, r = 0.7373). Data pooled from three experiments with three donors. Each dot represents an individual mouse.
Initial Levels of G6PD Deficiency Predict Hemolytic Response in A- huRBC NOD/SCID Mice.
A- G6PD deficiency results in variable levels of G6PD in huRBCs. Levels of G6PD were measured in each blood sample before engraftment. To determine whether there was a correlation between initial G6PD levels and response to PQ, we performed a correlation analysis of G6PD levels with the degree of huRBC loss at day 7 after treatment with 25 mg/kg per day p.o. A significant correlation was observed, with lower initial levels of G6PD predicting a greater loss of huRBC after treatment with PQ (Fig. 3C), which is consistent with studies demonstrating that Med- G6PD-deficient populations are more sensitive to drug-induced hemolytic anemia (2).
NOD/SCID Mice Engrafted with G6PD A- huRBCs Are Sensitive to Other Hemolytic 8-AQ Drugs but Not to Nonhemolytic Quinolines.
To assess the specificity of our huRBC SCID mouse model, we tested three additional 8-AQ drugs known to be hemolytic, along with others known to be free from hemolytic toxicity. Thus, A- huRBC SCID mice were treated with pamaquine, one of the first synthetic antimalarial drugs to be introduced clinically (2), with sitamaquine, another 8-AQ analog that has been under development for the treatment of leishmaniasis (26, 27), and with tafenoquine, currently under development for malaria prophylaxis (28). Like PQ, pamaquine, sitamaquine, and tafenoquine cause hemolysis in individuals with G6PD deficiency (26, 27). A- huRBC SCID mice were treated with pamaquine (50 mg/kg or 75 mg/kg) or with sitamaquine (40 mg/kg) p.o. once daily for 7 d (Fig. 4). The pamaquine doses of 75 and 50 mg/kg per day and sitamaquine dose of 40 mg/kg per day were selected on the basis of the clinical dose regimen relative to PQ. Tafenoquine (2.5 mg/kg p.o. once daily for 3 d) was also tested using a dose regimen shown to be effective for causal prophylaxis in mice. The percent loss of huRBCs was calculated at the end of 7 d of treatment (relative to percentage of huRBCs at start of treatment). Pamaquine, sitamaquine, and tafenoquine treatment resulted in loss of huRBC in A- G6PD mice at levels similar to PQ-treated mice but significantly different from vehicle control-treated mice (one-way ANOVA, P < 0.0001).
Fig. 4.
Qualification of the G6PD-deficient huRBC SCID mouse model with other antimalarial drugs. (A) A- huRBC SCID mice were treated p.o. with PQ (25 mg/kg per day for 7 d), vehicle control (PBS for 7 d), pamaquine (75 or 50 mg/kg per day for 7 d), sitamaquine (40 mg/kg per day for 7 d), tafenoquine (2.5 mg/kg per day for 3 d), chloroquine (25 mg/kg per day for 7 d), mefloquine (40 mg/kg per day for 3 d), or doxycycline (60 mg/kg per day for 7 d). The percentage of huRBCs as measured by flow cytometry at day 7 of treatment was subtracted from the percentage of huRBCs at start of treatment and then divided by percentage of huRBCs at start of treatment to determine the percent loss of huRBCs. n = 15 for PQ and vehicle control, n = 4 for other groups. (B) A- huRBC SCID mice were treated p.o. with PQ (25 mg/kg per day for 3 d), dapsone (20 mg/kg per day for 3 d), pyrimethamine (50 mg/kg per day for 3 d), or vehicle control. Percentages of huRBCs were assessed at posttreatment day 0, day 4, and day 7.
We next tested two other antimalarial drugs with the basic quinoline structure but that are known to be free of hemolytic potential clinically: chloroquine, a 4-aminoquinoline, and mefloquine, a quinoline methanol. An additional structurally unrelated drug, doxycycline, was also used. Doses were selected on the basis of doses known to produce efficacy in mouse blood stage malaria infection. HuRBC levels in chloroquine-, mefloquine-, and doxycycline-treated A- huRBC mice were similar to those seen in the vehicle controls (Fig. 4A). These results corroborate that the loss of huRBCs we observe in G6PD-deficient huRBC SCID mice was observed with 8-AQ drugs known to have hemolytic toxicity in G6PD individuals and was not a nonspecific response to quinoline antimalarial drugs. Finally, in a separate experiment we tested a known hemolytic drug, dapsone—which is not an 8-AQ but is known to cause hemolysis in both G6PD-deficient and normal individuals (29)—along with a known nonhemolytic drug used in malaria chemotherapy, pyrimethamine. As seen in Fig. 4B, dapsone induced loss of huRBCs similar to PQ, whereas pyrimethamine had no effect. These data suggest that this model reliably reflects known human red cell toxicity in G6PD-deficient subjects.
Discussion
Primaquine, an 8-AQ, is an important antimalarial drug because treatment with PQ can eradicate the liver stage of Plasmodium vivax (6) and the stage V P. falciparum gametocytes (7). However, PQ treatment causes hemolytic anemia in G6PD-deficient individuals (4, 27, 30), a major drawback for its application in malaria elimination campaigns. Development of nonhemolytic 8-AQ antimalarials (or at least 8-AQ derivatives with an improved therapeutic window) is therefore critical so that this class of compounds can achieve more widespread use for the treatment and elimination of malaria. A major limitation in drug development has been the lack of a suitable animal model to test potential hemolytic toxicity of novel drugs. In this article we describe the development of a humanized NOD/SCID mouse model containing circulating G6PD-deficient human RBCs that reproduces drug-induced hemolytic toxicity and thus can be used for the testing and validation of the hemolytic capacity of new 8-AQ analogs and other drugs with potential hemolytic toxicity.
The optimum engraftment protocol for the human red cells into the NOD/SCID mice results in high relative huRBC levels—approximately 80% of total red cells—that are stably maintained for at least 7 d with cells from a G6PD normal donor. With an A- type G6PD-deficient donor, stability is a bit lower but still relatively comparable to normal huRBCs. However, with the Med- deficiency, stability is much reduced, likely reflecting the almost complete lack of G6PD activity and resultant marked depletion of cellular NADPH and reduced glutathione. These would compromise red cell oxidative defenses and reduce cellular metabolic competency.
PQ-induced hemolytic anemia in G6PD individuals is not immediate but occurs after several days of treatment (31). Clinical indicators of hemolytic anemia in humans include decreased hemoglobin concentration, low hematocrit levels, low haptoglobin, reticulocytosis, elevations in unconjugated bilirubin and lactate dehydrogenase, and Heinz body formation (2, 32, 33). In our model, drug-induced hemolysis was evaluated using two primary parameters: loss of human RBCs and induction of murine reticulocyte production. These were measured using sensitive and quantitative flow cytometry-based assays. We observed both loss of huRBCs and an increase in circulating mouse reticulocytes in our model after PQ treatment, regardless of route of administration. The decline in huRBCs was not observed until after 3 d of PQ treatment and mirrors the kinetics in huRBC loss reported by Beutler et al. (31) in early studies of PQ drug trials in human volunteers that were G6PD-deficient. The delay is thought to be due to the accumulation of red cell damage over time with recycling of redox-active metabolites. The red cell loss is likely “extravascular,” in that it results from the removal of damaged RBCs from the circulation by tissue (e.g., splenic) macrophages via phagocytosis; this phenomenon was demonstrated in an in vitro erythrophagocytosis assay (34). In the present studies, posttreatment spleen and liver weights were also evaluated as indirect/secondary markers of hemolysis. The major organ of red cell sequestration and removal is the spleen (34). The degree of red cell damage and removal determines the size of spleen; high RBC clearance results in larger spleen size. Consistent with this model, we observed increases in spleen size and no effect on liver size in A- and Med- huRBC NOD/SCID mice given doses of PQ that induced loss of huRBCs.
We observed increases in murine reticulocytes in both A- and Med- huRBC NOD/SCID mice, also consistent with PQ-induced hemolysis. During severe and rapid hemolysis of RBCs, the bone marrow increases the rate of RBC production, resulting in significantly higher than normal reticulocyte numbers in the peripheral blood; reticulocytes normally account for less than 1% of total red blood cells but after hemolysis may comprise up to 15% (35). Interestingly, we observed a biphasic increase in murine reticulocytes, with an early lower response observed at 2 to 3 d when huRBCs first declined and then a second larger increase by day 7, greater than 10% murine reticulocytes in Med- huRBC mice. These observations would suggest a switch from bone marrow to extramedullary eyrythropoesis, which can occur under conditions of oxygen stress.
The effects of the PQ were specific to NOD/SCID mice engrafted with A- or Med- G6PD-deficient blood; NOD/SCID mice engrafted with G6PD-normal blood did not exhibit hemolysis. As expected, the more severe hemolysis after PQ treatment occurred in the Med- huRBC SCID mice compared with A- huRBC SCID mice, reflecting the differences in susceptibility to oxidative stress of RBC and greater fragility (as seen by poorer engraftment and reduced stability after engraftment) in Med- erythrocytes.
Med- G6PD-deficient humans given PQ also show an exaggerated hemolytic response (23). These findings suggest that the hemolytic effect of PQ is specific to G6PD-deficient huRBCs and that the more severe the G6PD deficiency (e.g., Med-), the more rapid the loss of huRBCs after PQ treatment. The correlation between G6PD levels and loss of huRBC observed in this study (Fig. 3C) is also consistent with this model.
Our model is therefore an appealing screening tool for the hemolytic potential of drugs at the preclinical stage. We further validated the model by demonstrating that two other 8-AQ derivatives (pamaquine and sitamaquine), as well as dapsone, a non-8-AQ, all known to produce clinical hemolysis, also induced hemolysis in our model; further, drugs known not to have hemolytic potential (mefloquine, chloroquine, pyrimethamine, and doxycycline) showed no activity, thus suggesting that this model can be used to evaluate potential human hemolytic toxicity.
The clinical picture for hemolysis in malaria patients treated with PQ may be considerably more complicated, because the parasite-induced effects in red blood cells also result in oxidative stress, and this could conceivably render cells more susceptible to PQ-induced hemolysis. The G6PD-deficient erythrocytes are considered relatively resistant to the malaria infection, predictably owing to preexisting oxidative stress (36). The P. falciparum infection in antioxidant-blunted G6PD-deficient RBCs results in a remarkable increase in expression of enzymes associated with antioxidant defense (37).
Taken together, these findings demonstrate the development of a G6PD-deficient huRBC SCID mouse model that can be used to evaluate hemolytic toxicity of new drugs for the treatment of malaria in the G6PD-deficient populations. Use of this model to identify nonhemolytic 8-AQ analogs, 8-AQs that have less hemolytic effect, and coadministration of 8-AQ plus partner drugs that would protect huRBCs from the hemolytic effect of 8-AQ will also afford a more robust and comprehensive approach to reduce the hemolytic liability associated with 8-AQs in G6PD-deficient individuals and aid in the campaigns to eliminate malaria.
Materials and Methods
Mice.
Eight- to nine-week-old female NOD.CB17-Prkdcscid/J mice (herein referred to as NOD/SCID mice) were purchased from the Jackson Laboratories. Mice were maintained on sterile food and water in a specific pathogen-free animal facility at Upstate Medical University and were routinely acclimated for 1 wk in the facility before initiation of experimental procedures. Softened food was provided in sterile water in the cage during drug treatment as part of standard of care. All animals were treated according to Upstate Medical University’s Committee for the Humane Use of Animals and in compliance with the Animal Welfare Act and other federal statutes and regulations relating to animals and experiments involving animals and adhere to the principles stated in the Guide for the Care and Use of Laboratory Animals (38).
Collection and Processing of Donor Blood.
G6PD-deficient and G6PD-normal volunteer blood donors were recruited through the Walter Reed Army Institute of Research (WRAIR), Silver Spring, MD, under approved institutional review board protocols. The donor blood samples were analyzed for G6PD levels using the Kinetic method (Quest Diagnostics) and confirmed by genomic sequencing for G6PD deficiency. Donor G6PD levels were 0–0.1 IU/g HgB for the Med- donors (n = 2) and 0.4–1.9 IU/g HgB for the A- donors (n = 8). Genomic DNA was isolated from whole blood using Blood Mini kits (Qiagen) according to the manufacturer’s protocol. Specific PCR reactions were performed to isolate exons 3–5 that correspond to the common A/A- allele found in patients of African descent, or exon 6 that corresponds to the Mediterranean mutant allele (39). PCR products were checked for size on agarose gels and purified using a Qiagen PCR Purification kit and sequenced on both forward and reverse strands across the regions of interest to verify presence of mutant allele. Up to 450 mL of leukocyte-depleted whole blood was obtained by venous puncture in citrate phosphate dextrose adenine anticoagulant. Blood was transported overnight in a controlled temperature-cooling chamber to Upstate Medical University. Whole blood was centrifuged at 900 × g for 40 min at room temperature (RT). Plasma was carefully aspirated and stored at 4 °C. RBCs were resuspended in RPMI 1640 (HyClone Laboratories) and washed twice at 900 × g for 20 min each at RT. Plasma-free RBCs were reconstituted in RPMI 1640 with 25% autologous plasma at a concentration of 5 × 109 RBC/mL. RBC were aliquoted into 14 equal proportions and stored at 4 °C.
Engraftment of RBCs into NOD/SCID Mice.
NOD/SCID mice were injected i.p. once daily with a 1-mL suspension of RBCs for 14 d. An aliquot of RBCs was prewarmed to 37 °C before injection. Engraftment levels were determined at the end of 14 d. Approximately 5 μL of tail snip blood was collected into 100 μL of sterile heparin-PBS (10 U sodium heparin per mL). Using V-bottomed 96-well plates, 2 × 105 RBCs were stained for 30 min at 4 °C in the dark with phycoerythrin (PE)-conjugated anti-human CD235a antibody (Abcam) in staining buffer (0.5% BSA in PBS). Cells were washed twice and reconstituted in staining buffer for assessment by flow cytometry. Baseline levels of muRetics were also determined. RBCs (2 × 105) were stained with an FITC-labeled anti-mouse CD71 (eBioscience), a reticulocyte marker, and a PE-labeled anti-TER119 (Invitrogen) monoclonal antibody for mouse erythroid cell detection. Cells were acquired on a Guava EasyCyte Plus flow cytometer (Millipore). Analysis of the flow data was done using FlowJo software (TreeStar).
Drug Treatment.
Mice with peripheral huRBC levels greater than 60% were randomized for drug treatment, with 4 to 5 mice per group assigned. Drugs were provided by the Division of Experimental Therapeutics, WRAIR, or by Swiss Tropical and Public Health Initiative and were reconstituted in appropriate vehicle (PBS, hydroxyethyl cellulose-Tween, or Tween-EtOH). Tail-snip blood was collected on days 2, 4, 5, 6, and 7 of treatment for the determination of huRBC and muRetic levels. On the last day of treatment, mice were killed 1 hour after last dose and were assessed for hematocrit levels and body, spleen, and liver weights. Liver and spleen weights were calculated as a percent of total body weight for each mouse to account for variability in animal weights.
Statistics.
Statistical analysis was performed using GraphPad Prism (GraphPad Software). Mann-Whitney or one-way ANOVA with Bonferonni correction at 95% confidence intervals was used to determine differences between groups as appropriate.
Acknowledgments
We thank Caroline Othoro, Marino Mauro, Julie Ritchie, and Nancy Fiore for excellent technical assistance; and Jeff Friedman (The Scripps Research Institute) for genotyping the G6PD-deficient donors. This work was supported by grants from the US Army Medical Research and Materiel Command, via Awards W81XWH-07-2-0095 and WX81XWH-10-2-0059 to the University of Mississippi (to L.A.W.). Funding was also provided by the Medicines for Malaria Venture (to R.R.).
Footnotes
The authors declare no conflict of interest.
This work was presented in abstract form at the 59th Annual Meeting of the American Society of Tropical Medicine and Hygiene, Atlanta, GA, November 3–7, 2010.
This article is a PNAS Direct Submission.
References
- 1.Nkhoma ET, Poole C, Vannappagari V, Hall SA, Beutler E. The global prevalence of glucose-6-phosphate dehydrogenase deficiency: A systematic review and meta-analysis. Blood Cells Mol Dis. 2009;42(3):267–278. doi: 10.1016/j.bcmd.2008.12.005. [DOI] [PubMed] [Google Scholar]
- 2.Beutler E, Duparc S. G6PD Deficiency Working Group Glucose-6-phosphate dehydrogenase deficiency and antimalarial drug development. Am J Trop Med Hyg. 2007;77(4):779–789. [PubMed] [Google Scholar]
- 3.Baird JK, Hoffman SL. Primaquine therapy for malaria. Clin Infect Dis. 2004;39(9):1336–1345. doi: 10.1086/424663. [DOI] [PubMed] [Google Scholar]
- 4.Brueckner RP, Ohrt C, Baird JK, Milhous WK. 8-Aminoquinolines. In: Rosenthal P, editor. Anti-malarial Chemotherapy: Mechanisms of Action, Resistance, and New Directions in Drug Discovery. Totowa, NJ: Humana; 2001. [Google Scholar]
- 5.White NJ. The role of anti-malarial drugs in eliminating malaria. Malar J. 2008;7(Suppl 1):S8. doi: 10.1186/1475-2875-7-S1-S8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Wells TN, Burrows JN, Baird JK. Targeting the hypnozoite reservoir of Plasmodium vivax: The hidden obstacle to malaria elimination. Trends Parasitol. 2010;26(3):145–151. doi: 10.1016/j.pt.2009.12.005. [DOI] [PubMed] [Google Scholar]
- 7.White NJ. Primaquine to prevent transmission of falciparum malaria. Lancet Infect Dis. 2013;13(2):175–181. doi: 10.1016/S1473-3099(12)70198-6. [DOI] [PubMed] [Google Scholar]
- 8.Shanks GD, et al. A new primaquine analogue, tafenoquine (WR 238605), for prophylaxis against Plasmodium falciparum malaria. Clin Infect Dis. 2001;33(12):1968–1974. doi: 10.1086/324081. [DOI] [PubMed] [Google Scholar]
- 9.Bosma MJ, Carroll AM. The SCID mouse mutant: Definition, characterization, and potential uses. Annu Rev Immunol. 1991;9:323–350. doi: 10.1146/annurev.iy.09.040191.001543. [DOI] [PubMed] [Google Scholar]
- 10.Shultz LD, Ishikawa F, Greiner DL. Humanized mice in translational biomedical research. Nat Rev Immunol. 2007;7(2):118–130. doi: 10.1038/nri2017. [DOI] [PubMed] [Google Scholar]
- 11.Davis PH, Stanley SL., Jr Breaking the species barrier: Use of SCID mouse-human chimeras for the study of human infectious diseases. Cell Microbiol. 2003;5(12):849–860. doi: 10.1046/j.1462-5822.2003.00321.x. [DOI] [PubMed] [Google Scholar]
- 12.Thomsen M, Yacoub-Youssef H, Marcheix B. Reconstitution of a human immune system in immunodeficient mice: Models of human alloreaction in vivo. Tissue Antigens. 2005;66(2):73–82. doi: 10.1111/j.1399-0039.2005.00409.x. [DOI] [PubMed] [Google Scholar]
- 13.Moore JM, Kumar N, Shultz LD, Rajan TV. Maintenance of the human malarial parasite, Plasmodium falciparum, in SCID mice and transmission of gametocytes to mosquitoes. J Exp Med. 1995;181(6):2265–2270. doi: 10.1084/jem.181.6.2265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ishihara C, et al. Transfusion with xenogeneic erythrocytes into SCID mice and their clearance from the circulation. J Vet Med Sci. 1994;56(6):1149–1154. doi: 10.1292/jvms.56.1149. [DOI] [PubMed] [Google Scholar]
- 15.Moreno A, Badell E, Van Rooijen N, Druilhe P. Human malaria in immunocompromised mice: New in vivo model for chemotherapy studies. Antimicrob Agents Chemother. 2001;45(6):1847–1853. doi: 10.1128/AAC.45.6.1847-1853.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Tsuji M, Ishihara C, Arai S, Hiratai R, Azuma I. Establishment of a SCID mouse model having circulating human red blood cells and a possible growth of Plasmodium falciparum in the mouse. Vaccine. 1995;13(15):1389–1392. doi: 10.1016/0264-410x(95)00081-b. [DOI] [PubMed] [Google Scholar]
- 17.Badell E, et al. Human malaria in immunocompromised mice: An in vivo model to study defense mechanisms against Plasmodium falciparum. J Exp Med. 2000;192(11):1653–1660. doi: 10.1084/jem.192.11.1653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Badell E, Pasquetto V, Eling W, Thomas A, Druilhe P. Human Plasmodium liver stages in SCID mice: A feasible model? Parasitol Today. 1995;11(5):169–171. doi: 10.1016/0169-4758(95)80147-2. [DOI] [PubMed] [Google Scholar]
- 19.Rajan TV, Moore JM, Shultz LD. Immunodeficient mice as hosts for hemoparasitic infections. Parasitol Today. 1996;12(12):479–485. doi: 10.1016/s0169-4758(96)10066-1. [DOI] [PubMed] [Google Scholar]
- 20.Ishihara C, et al. Erythrocyte-replaced mouse model for Haemoparasite studies: Comparison of NOD/shi-scid and C.B-17/Jcl-scid mouse upon acceptability of human erythrocytes. J Vet Med Sci. 2003;65(8):831–837. doi: 10.1292/jvms.65.831. [DOI] [PubMed] [Google Scholar]
- 21.Angulo-Barturen I, et al. A murine model of falciparum-malaria by in vivo selection of competent strains in non-myelodepleted mice engrafted with human erythrocytes. PLoS ONE. 2008;3(5):e2252. doi: 10.1371/journal.pone.0002252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Vulliamy T, Beutler E, Luzzatto L. Variants of glucose-6-phosphate dehydrogenase are due to missense mutations spread throughout the coding region of the gene. Hum Mutat. 1993;2(3):159–167. doi: 10.1002/humu.1380020302. [DOI] [PubMed] [Google Scholar]
- 23.Clyde DF. Clinical problems associated with the use of primaquine as a tissue schizontocidal and gametocytocidal drug. Bull World Health Organ. 1981;59(3):391–395. [PMC free article] [PubMed] [Google Scholar]
- 24.Beutler E. G6PD: Population genetics and clinical manifestations. Blood Rev. 1996;10(1):45–52. doi: 10.1016/s0268-960x(96)90019-3. [DOI] [PubMed] [Google Scholar]
- 25.Beutler E. Drug-induced hemolytic anemia. Pharmacol Rev. 1969;21(1):73–103. [PubMed] [Google Scholar]
- 26.Loiseau PM, Cojean S, Schrével J. Sitamaquine as a putative antileishmanial drug candidate: From the mechanism of action to the risk of drug resistance. Parasite. 2011;18(2):115–119. doi: 10.1051/parasite/2011182115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Tekwani BL, Walker LA. 8-Aminoquinolines: Future role as antiprotozoal drugs. Curr Opin Infect Dis. 2006;19(6):623–631. doi: 10.1097/QCO.0b013e328010b848. [DOI] [PubMed] [Google Scholar]
- 28.Elmes NJ, Nasveld PE, Kitchener SJ, Kocisko DA, Edstein MD. The efficacy and tolerability of three different regimens of tafenoquine versus primaquine for post-exposure prophylaxis of Plasmodium vivax malaria in the Southwest Pacific. Trans R Soc Trop Med Hyg. 2008;102(11):1095–1101. doi: 10.1016/j.trstmh.2008.04.024. [DOI] [PubMed] [Google Scholar]
- 29.Olteanu H, et al. High prevalence of Dapsone-induced oxidant hemolysis in North American SCT recipients without glucose-6-phosphate-dehydrogenase deficiency. Bone Marrow Transplant. 2012;47(3):399–403. doi: 10.1038/bmt.2011.83. [DOI] [PubMed] [Google Scholar]
- 30.Vale N, Moreira R, Gomes P. Primaquine revisited six decades after its discovery. Eur J Med Chem. 2009;44(3):937–953. doi: 10.1016/j.ejmech.2008.08.011. [DOI] [PubMed] [Google Scholar]
- 31.Beutler E. The hemolytic effect of primaquine and related compounds: A review. Blood. 1959;14(2):103–139. [PubMed] [Google Scholar]
- 32.Cappellini MD, Fiorelli G. Glucose-6-phosphate dehydrogenase deficiency. Lancet. 2008;371(9606):64–74. doi: 10.1016/S0140-6736(08)60073-2. [DOI] [PubMed] [Google Scholar]
- 33.Luzzatto L. Glucose 6-phosphate dehydrogenase deficiency: From genotype to phenotype. Haematologica. 2006;91(10):1303–1306. [PubMed] [Google Scholar]
- 34.Bowman ZS, Jollow DJ, McMillan DC. Primaquine-induced hemolytic anemia: Role of splenic macrophages in the fate of 5-hydroxyprimaquine-treated rat erythrocytes. J Pharmacol Exp Ther. 2005;315(3):980–986. doi: 10.1124/jpet.105.090407. [DOI] [PubMed] [Google Scholar]
- 35.Greene S. G6PD deficiency as protection against falciparum malaria: An epidemiologic critique of populations and experimental studies. Yearb Phys Anthropol. 1993 ed, Steegman A (Wiley-Liss, NY), pp 153–178. [Google Scholar]
- 36.Manganelli G, Masullo U, Passarelli S, Filosa S. Glucose-6-phosphate dehydrogenase deficiency: Disadvantages and possible benefits. Cardiovasc Hematol Disord Drug Targets. 2013;13(1):73–82. doi: 10.2174/1871529x11313010008. [DOI] [PubMed] [Google Scholar]
- 37.Akide-Ndunge OB, et al. Co-ordinated stage-dependent enhancement of Plasmodium falciparum antioxidant enzymes and heat shock protein expression in parasites growing in oxidatively stressed or G6PD-deficient red blood cells. Malar J. 2009;8:113. doi: 10.1186/1475-2875-8-113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Anonymous . Guide for the Care and Use of Laboratory Animals (National Academy of Sciences) Washington, DC: National Academies Press; 2010. [Google Scholar]
- 39.Bulliamy T, Luzzatto L, Hirono A, Beutler E. Hematologically important mutations: Glucose-6-phosphate dehydrogenase. Blood Cells Mol Dis. 1997;23(2):302–313. doi: 10.1006/bcmd.1997.0147. [DOI] [PubMed] [Google Scholar]