Significance
Natural killer (NK) cells are effectors of the antitumor immunity, able to kill cancer cells through the release of the cytotoxic protease granzyme B. NK-based therapies have recently emerged as promising anticancer strategies. It is well established that hypoxic microenvironment interferes with the function of antitumor immune cells and constitutes a major obstacle for cancer immunotherapies. We showed that breast cancer cells evade effective NK-mediated killing under hypoxia by activating autophagy that we have identified to be responsible for the degradation of NK-derived granzyme B. We demonstrated that blocking autophagy restored NK-mediated lysis in vitro, and facilitated breast tumor elimination by NK cells in mice. We provided evidence that targeting autophagy may pave the way to achieve more effective NK-based anticancer immunotherapy.
Keywords: hypoxic tumor microenvironment, innate immunity, breast adenocarcinoma, immunotherapy
Abstract
Recent studies demonstrated that autophagy is an important regulator of innate immune response. However, the mechanism by which autophagy regulates natural killer (NK) cell-mediated antitumor immune responses remains elusive. Here, we demonstrate that hypoxia impairs breast cancer cell susceptibility to NK-mediated lysis in vitro via the activation of autophagy. This impairment was not related to a defect in target cell recognition by NK cells but to the degradation of NK-derived granzyme B in autophagosomes of hypoxic cells. Inhibition of autophagy by targeting beclin1 (BECN1) restored granzyme B levels in hypoxic cells in vitro and induced tumor regression in vivo by facilitating NK-mediated tumor cell killing. Together, our data highlight autophagy as a mechanism underlying the resistance of hypoxic tumor cells to NK-mediated lysis. The work presented here provides a cutting-edge advance in our understanding of the mechanism by which hypoxia-induced autophagy impairs NK-mediated lysis in vitro and paves the way for the formulation of more effective NK cell-based antitumor therapies.
The major obstacle to defining an efficient immunotherapy protocol for the treatment of solid tumors is the capacity of the tumor microenvironment (TME) to inhibit the host immune response at both the local and systemic levels (1). The TME is a complex, dynamic structure composed of malignant cells and cells that influence cancer evolution, such as endothelial and immune cells (2). The TME is an important aspect of cancer biology, as it can promote neoplastic transformation, support tumor growth and invasion, protect tumors from host immunity, foster therapeutic resistance, and provide niches for dormant metastases (3). Natural killer (NK) cells are large, granular CD3−/T-cell receptor−/CD56+ lymphocytes that play a fundamental role in antitumor innate immunity (4) and participate in tumor immunosurveillance. They can kill tumor cells directly via the engagement of death receptors [Fas and TRAIL (Tumor necrosis factor-Related Apoptosis-Inducing Ligand)] and the release of perforin- and granzyme-containing granules; or indirectly via antibody-dependent cell-mediated cytotoxicity (5, 6). The role of NK cells in tumor regression was investigated by several studies showing that tumor infiltration by NK cells predicts a favorable outcome for several cancers (7–10). The activity of NK cells is regulated by the expression of activating and inhibitory receptors that together dictate the fate of target tumor cells. The function of NK cells is inhibited by the interaction between HLA class I molecules, expressed on the surface of target cells, and killer cell Ig-like receptors (KIR) and/or CD94/NKG2A, expressed on the surface of NK cells (11). The activation of NK cells is mediated by NKp46, NKp30, and NKp44, as well as CD226 and NKG2D. The up-regulation of stress-inducible proteins on the surface of cancer cells, such as MHC class I-related chain (MIC) A and B ligands and UL16-binding proteins (ULBPs), leads to the activation of NK cells through the engagement of NKG2D (12). The loss of HLA class I expression and the up-regulation of MICA/B and ULBP1 is a common characteristic of tumors (13), which makes them targets for NK-mediated lysis (14).
Accumulating data suggest that a hypoxic microenvironment protects cancer cells from the antitumor immune response by multiple overlapping mechanisms (reviewed in ref. 15). We recently reported that under hypoxic conditions, tumor cells resist antigen-specific cytotoxic T-lymphocytes (CTL)-mediated lysis in a signal transducer and activator of transcription 3 (STAT3)-dependent manner (16). Hypoxia also contributes to the reprogramming of dendritic cells (DCs) and perpetuates inflammation via the induction of a proinflammatory DC phenotype (17). Under hypoxia, tumor cells release a number of chemokines, such as endothelial monocyte-activating polypeptide II (EMAP-II) (18), vascular endothelial growth factor (VEGF) (19), endothelin 2 (20), and monocyte chemotactic protein-1 (MCP-1/CCL2) (21). The secretion of such cytokines leads to the accumulation of tumor-associated macrophages (TAM) and regulatory T-cells (Treg). TAM and Treg cells produce transforming growth factor-β and suppress the proliferation and activity of NK cells (22). In addition, it has been proposed that hypoxia contributes to the escape of prostate tumor cells from NK-mediated immunosurveillance by increasing the shedding of MIC molecules (23).
It is well established that the adaptation of cancer cells to hypoxic stress can also occur through the activation of autophagy, which is a process responsible for the degradation and recycling of long-lived proteins and cytoplasmic organelles in well-characterized structures named autophagosomes. Recently, the role of autophagy was expanded to include immunological functions, including interactions with the immune system in the TME (16, 24, 25). Although these studies highlight autophagy as an important player in the regulation of cancer immunity, no data are currently available that address the mechanisms by which autophagy regulates NK-dependent immune responses during hypoxia.
While clinical trials using NK-based immunotherapy have shown promising results for the treatment of hematological malignancies, solid tumors are frequently resistant to this therapy. Understanding the molecular mechanisms that underlie tumor escape from NK-mediated surveillance remains an issue of major interest. In this report, we demonstrate that hypoxia reduces breast cancer cell susceptibility to NK-mediated lysis by a mechanism involving the activation of autophagy and the subsequent degradation of NK-derived granzyme B (GzmB) in vitro. We provide in vivo evidence that blocking autophagy in hypoxic tumors induces tumor regression by facilitating NK-mediated elimination. This study highlights autophagy as a unique mechanism of tumor cell resistance to NK-mediated lysis.
Results
Hypoxia-Induced Autophagy in Breast Cancer Cells Decreases Susceptibility to NK-Mediated Lysis.
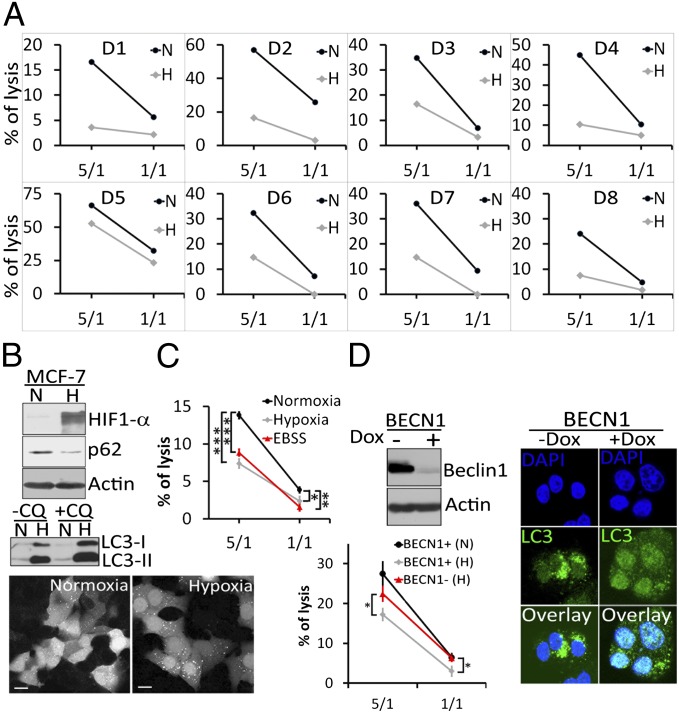
We investigated whether hypoxia impairs NK-mediated lysis of MCF-7 breast adenocarcinoma cells. Using NK cells isolated from the peripheral blood monocellular cells (PBMC) of eight healthy donors (D1 to D8), we demonstrated (Fig. 1A) that despite the interindividual variability of NK cell cytolytic potential, the percentage of hypoxic (H) MCF-7 cell lysis by NK cells was decreased compared with that of normoxic (N) MCF-7 cells in all cases.
Fig. 1.
Hypoxia-induced autophagy decreases the susceptibility of breast cancer cells to NK-mediated lysis in vitro. (A) Cytotoxicity assays were performed in duplicate using NK cells isolated from eight healthy donors (D1 to D8) at 5/1 or 1/1 E/T (Effector/Target) ratios on normoxic (N) or hypoxic (H) MCF-7 cells. Cell death was assessed by flow cytometry using TO-PRO-3. (B, Upper) Cells were cultured under normoxia (N) or hypoxia (H) conditions for 16 h. The expression levels of hypoxia inducible factor 1α, p62, and LC3 were assessed by immunoblot. (B, Middle) Untreated (−CQ) or chloroquine (60 µM) treated (+CQ) cells cultured under normoxia (N) or hypoxia (H) were assessed by immunoblot for the expression of LC3. (B, Lower) GFP–LC3 expressing normoxic or hypoxic MCF-7 cells were analyzed by fluorescence microscopy for the autophagosome formation (dots). (Scale bar, 10 μm.) (C) MCF-7 cells were cultured under normoxia, hypoxia, or starvation [Earle's balanced salt solution (EBSS)]. Cytotoxicity assays were performed using NK cells isolated from a healthy donor (D12). The percentage of tumor cell lysis is reported as an average (±SEM) of three experiments performed. Statistically significant differences are indicated by asterisks (*P < 0.05; **P < 0.005; ***P < 0.0005). (D, Upper Left) Autophagy-competent and -defective MCF-7 cells were generated by culturing cells expressing BECN1 under the control of a tetracycline-responsive promoter (BECN1) in the absence (−) or presence (+) of doxycycline (100 ng/mL), respectively. Beclin1 expression was assessed by immunoblot. (D, Right) The formation of autophagosomes (dot-like structures) in autophagy-competent (−Dox) and autophagy-defective (+Dox) hypoxic cells was assessed using immunofluorescence microscopy with an Alexa-Fluor-488–coupled LC3 antibody. Nuclei were stained with DAPI. (Scale bar, 10 µm.) (D, Lower Left) Cytotoxicity assays were performed on autophagy competent (BECN1+) or defective (BECN1−) MCF-7 cells cultured under normoxic (N) or hypoxic (H) conditions with NK cells isolated from a healthy donor (D13). The percentage of tumor cell lysis is reported as an average (±SEM) of three experiments, and statistically significant differences are indicated by asterisks (*P < 0.05).
This impairment correlated with the induction of the autophagic flux as indicated by the degradation of p62/Sequestosome 1 (SQSTM1), the accumulation of microtubule-associated protein light chain-3 II (LC3-II) in chloroquine (CQ)-treated cells and the formation of autophagosomes in hypoxic cells (Fig. 1B and Fig. S1A). Time-lapse video microscopy of GFP–LC3-expressing normoxic or hypoxic MCF-7 cells (Fig. S1B) cocultured with PKH-26–stained NK cells confirmed that normoxic tumor cells were efficiently lysed by NK cells (Movie S1) compared with hypoxic tumor cells, which displayed several autophagosomes (Movie S2). In addition, time-lapse video microscopy performed under the same conditions using propidium iodide provided compelling evidence that normoxic tumor cells underwent NK-mediated cell death (Movie S3) compared with hypoxic tumor cells (Movie S4). Similar results were obtained using the T47D breast cancer cell line (Fig. S1C), which confirms that the impairment of breast tumor cell susceptibility to NK-mediated lysis during hypoxia is not restricted to the MCF-7 cell line. In addition, we demonstrated that, independently of hypoxia, autophagy induction by other stimuli (e.g., starvation) also impairs NK-mediated lysis (Fig. 1C). To determine the extent to which the induction of autophagy is implicated in the impairment of NK-cell–mediated lysis, we used the MCF-7 cell line expressing Beclin1 (MCF-7BECN1) under the control of a tetracycline-responsive promoter (tet-off). Using this cell line, autophagy-competent (MCF-7BECN1+) or autophagy-defective (MCF-7BECN1−) MCF-7 cells can be generated by culturing cells in the absence (−) or presence (+) of doxycycline, respectively (Fig. 1D, Upper Left and Right). Cytotoxic assay (Fig. 1D, Lower Left) clearly demonstrated that NK cells kill autophagy-deficient (BECN1−) MCF-7 cells more efficiently than autophagy-competent (BECN1+) MCF-7 cells under hypoxic conditions. Similar results were obtained in ATG5-deficient MCF-7 cells (Fig. S1D). These data indicate that autophagy itself is a key determinant in the impairment of NK-mediated lysis of MCF-7 tumor cells.
Impaired NK-Mediated Lysis of Hypoxic Tumor Cells Is Not Related to a Defect in Tumor Cell Recognition by NK Cells.
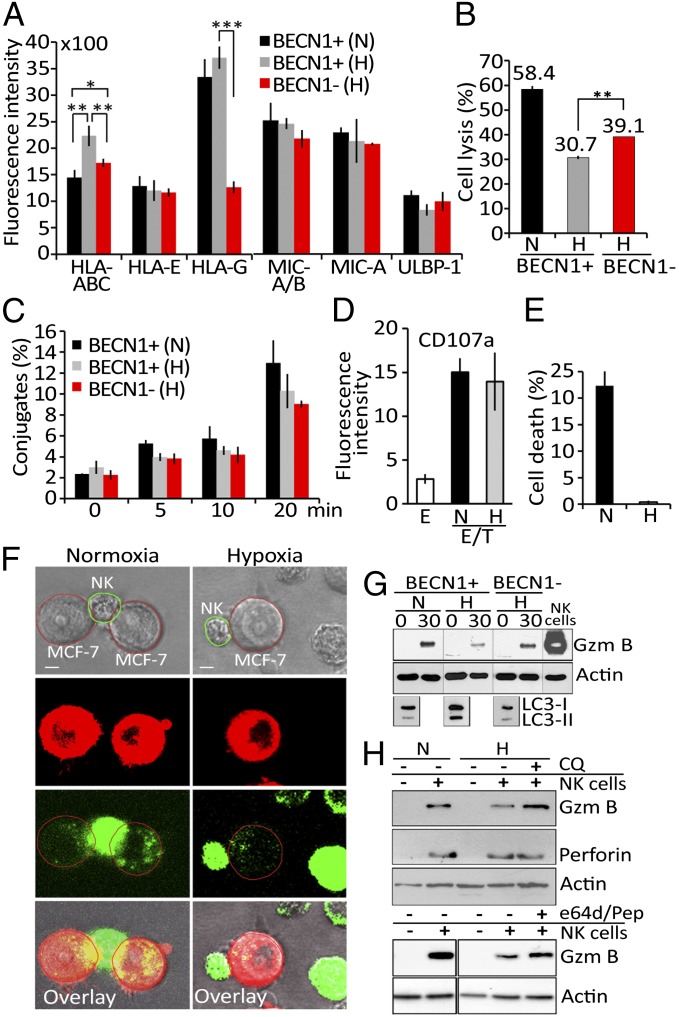
We investigated whether the resistance of hypoxic tumor cells to NK-mediated lysis is related to an increase in NK-inhibitory MHC class I molecules or a decrease in NK-activating NKG2D ligands on the cell surface. Among all analyzed molecules (Fig. 2A), only HLA-A, B, and C were found to be significantly up-regulated on the surface of autophagy competent hypoxic MCF-7 cells [BECN1+ (H)] and down-regulated on autophagy-defective cells cultured under the same conditions [BECN1− (H)]. We next investigated whether the resistance of hypoxic MCF-7 cells to NK-mediated lysis was related to an increase in HLA class I molecules. Our data demonstrated that even when HLA class I molecules are blocked, the lysis of hypoxic (BECN1−) MCF-7 cells is significantly improved compared with hypoxic (BECN1+) MCF-7 cells (Fig. 2B). We therefore suggest that independently of HLA class I expression level, the autophagic status of target cells plays a key role in the resistance to NK-mediated lysis in our model. Similarly, the data presented in Fig. 2C demonstrated a time-dependent increase in the percentage of conjugates between NK and tumor cells, but no significant difference in conjugate formation was observed between autophagy-competent (BECN1+) and -defective (BECN1−) cells cultured under normoxic or hypoxic conditions. Representative images from time-lapse experiments support the conclusion that NK cells maintain their ability to interact with hypoxic cells in our model (Fig. S2). We also addressed whether the degranulation activity of NK cells was affected by hypoxic tumor cells. Fig. 2D showed a basal level of CD107a on the surface of NK cells cultured alone (E), but a significantly higher level was detected when NK cells were cocultured with normoxic or hypoxic tumor cells (E/T). As no difference in the level of CD107a was observed when NK cells were cocultured with normoxic and hypoxic tumor cells, the resistance of hypoxic tumor cells to NK-mediated lysis does not appear to be related to a defect in NK activity. Our results further suggest that resistance is dependent on an intrinsic mechanism that makes tumor cells less sensitive to the cytotoxic granules released by NK cells. This hypothesis was supported by data (Fig. 2E) demonstrating that under conditions where normoxic and hypoxic tumor cells were treated with the pore-forming protein streptolysin-O, GzmB only killed normoxic cells.
Fig. 2.
Hypoxia-induced autophagy impairs tumor cell susceptibility to NK-mediated lysis without affecting NK cell function. (A) Autophagy-competent (BECN1+) and -defective (BECN1−) MCF-7 cells cultured under normoxia (N) or hypoxia (H) were assessed by flow cytometry for the expression of MHC class I molecules and NK cell-activating NKG2D ligands . Fluorescence intensity is reported as an average (±SEM) of three experiments. Statistically significant differences are indicated by asterisks (*P < 0.05; **P < 0.005; ***P < 0.0005). (B) BECN1+ or BECN1− MCF-7 cells were pretreated with mouse IgM anti-pan HLA class I A6-136 mAb and incubated under normoxic (N) or hypoxic (H) conditions before presentation to NK cells at a 5/1 E/T ratio. The percentage of target cell lysis is reported. Statistically significant differences are indicated by asterisks (**P < 0.005). (C) BECN1+ or BECN1− MCF-7 cells cultured under normoxia (N) or hypoxia (H) were incubated with NK cells. The percentage of conjugate formation at indicated time was determined by flow cytometry. Results are reported as an average (±SEM) of three independent experiments. No statistically significant differences were observed. (D) NK cells as effectors (E) were cultured alone or with normoxic (N) or hypoxic (H) MCF-7 cells at a 5/1 E/T ratio. The level of CD107a (a degranulation marker) on the surface of the NK cells was assessed by flow cytometry. Fluorescence intensity is reported as an average (±SEM) of five experiments performed with NK cells from different donors. (E) Normoxic (N) or hypoxic (H) MCF-7 cells were loaded with exogenous, activated granzyme B (0.8 μg/mL) using the pore-forming protein streptolysin-O. The percentage of cell death was determined by flow cytometry. Results are reported as an average (±SEM) of three experiments. (F) PKH-26–stained normoxic or hypoxic MCF-7 cells (red) were cocultured with YT–Indy–NK cells expressing GFP–GzmB (green) at 5/1 E/T ratio. The content of NK-derived GFP–GzmB in target cells was monitored after 30 min of coculture by a Zeiss laser-scanning confocal microscope (LSM-510 Meta) with a 60× oil immersion objective. (Scale bar, 10 μm.) (G) Autophagy-competent (BECN1+) or -defective (BECN1−) MCF-7 cells were incubated under normoxia (N) or hypoxia (H) and cocultured with NK cells at 5/1 E/T ratio for 0 and 30 min. Following separation, tumor cells were lysed and subjected to immunoblot for the intracellular GzmB content. NK cell lysate was used as a control for GzmB detection. The expression of LC3 was reported as a marker for autophagy. (H) Normoxic (N) or hypoxic (H) MCF-7 cells were cultured alone (−) or with NK cells (+) at 5/1 ratio for 30 min in the presence (+) or absence (−) of chloroquine (CQ) or e64d/pepstatin. Tumor cells separated from NK were subjected to immunoblot analysis to evaluate the intracellular GzmB and perforin content.
In Vitro Hypoxic Tumor Cells Degrade NK Cell-Derived Granzyme B in Lysosomes via Autophagy.
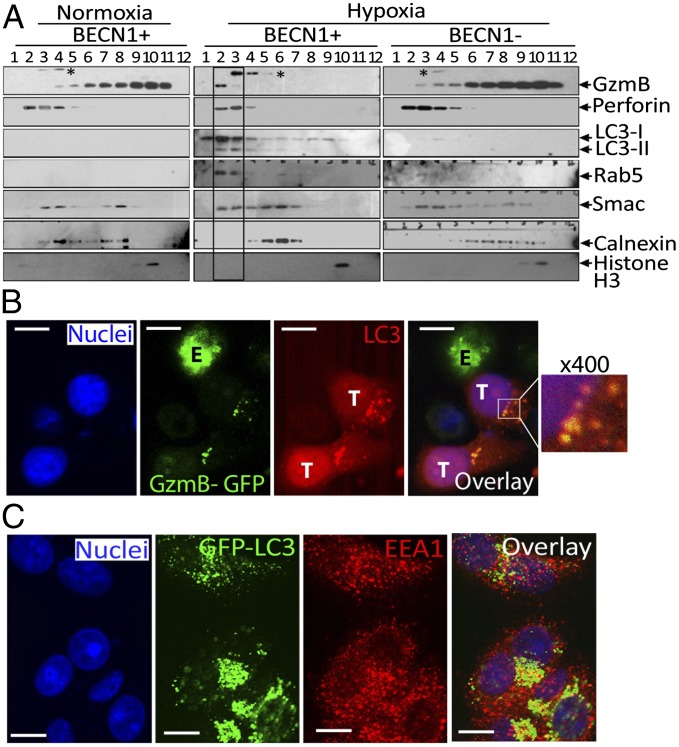
To investigate the mechanism underlying the observed reduction of GzmB-mediated killing of H cells, we analyzed the intracellular GzmB content of target cells. PKH-26–stained normoxic or hypoxic MCF-7 cells were cocultured with YT–Indy–NK cells expressing GFP–GzmB. Significantly lower levels of GFP–GzmB were detected in hypoxic cells than in normoxic (N) cells (Fig. 2F). We next evaluated whether targeting autophagy by knocking down BECN1 restores GzmB level in hypoxic cells. Our results demonstrated that the level of GzmB is restored in BECN1-defective cells (Fig. 2G). As the NK-mediated lysis of hypoxic cells was also restored by targeting ATG5, it remains to be determined whether the level of GzmB was affected in ATG5-defective cells. Nevertheless, these results suggest that following its delivery to hypoxic cells, GzmB is loaded into autophagosomes and subsequently degraded in lysosomes. This was further supported by our data (Fig. 2H) showing that the level of GzmB was restored by autophagy and lysosomal hydrolases inhibitors chloroquine and e64d/Pepstatin, respectively. In contrast, the level of perforin in target cells was not affected by hypoxia or autophagy inhibition (Fig. 2H). The subcellular distribution of NK-derived GzmB was further analyzed by fractionation of autophagy-competent (BECN1+) or -defective (BECN1−) MCF-7 cells cultured under normoxic or hypoxic conditions. Fig. 3A showed a dramatic difference in the distribution pattern of GzmB between normoxic and hypoxic (BECN1+) cells. GzmB is mostly present in fractions 4 to 11 in normoxic cells; however, it is exclusively detected in fraction 2 and to a lesser extent in fraction 3 in hypoxic cells. Interestingly, the GzmB-containing fractions 2 and 3 are positive for LC3 (autophagosomes) and Rab5 (endosomes), suggesting that these fractions may correspond to amphisomes (structures generated from the fusion of autophagosomes and late endosomes). Taken together, these results suggest that endosomes containing GzmB and perforin fuse with autophagosomes upon activation of autophagy in hypoxic cells, leading to the specific degradation of GzmB. The selectivity of GzmB degradation by autophagy was further supported by our data demonstrating that inhibition of the autophagy cargo protein p62 restores GzmB level in hypoxic targets (Fig. S3). Importantly, targeting autophagy in hypoxic cells dramatically changes the subcellular distribution of GzmB to a profile similar to that observed in normoxic cells. The presence of NK-derived GzmB in autophagosomes of hypoxic cells was further confirmed by immunofluorescence data showing colocalization of GzmB–GFP with autophagosomes (LC3-stained structures) (Fig. 3B); those autophagosomes also contained the endosomal marker EEA1 (Fig. 3C).
Fig. 3.
In vitro tumor cells degrade NK cell-derived granzyme B in lysosomes via autophagy. (A) NK cells were cocultured with autophagy-competent (BECN1+) or -defective (BECN1−) normoxic or hypoxic MCF-7 cells. Cell lysates of separated tumor cells were subjected to subcellular fractionation. Fractions 1 to 12 were characterized by Western blot using the indicated antibodies. * indicates an unspecific band. (B) Chloroquine-treated hypoxic MCF-7 cells (T) were cocultured with GzmB–GFP–expressing NK cells (E). Target cells were stained with an Alexa-Fluor-568–coupled rabbit anti-LC3 antibody to visualize autophagosomes (red) and with DAPI to stain nuclei (blue). Colocalization of GzmB with LC3 in autophagosomes was visualized by confocal microscopy using a 100× oil immersion objective. An enlarged image (400×) of the overlay (box) is shown. (Scale bar, 10 µm.) (C) Chloroquine treated GFP–LC3 –expressing MCF-7 cell were cultured under hypoxia stained with anti-EEA1 Alexa-Fluor-568–coupled anti-rabbit IgG antibody (red) and DAPI for nuclei. Cells were analyzed by confocal microscopy. The overlay image shows colocalization of LC3 and EEA1 (yellow dots). (Scale bar, 10 µm.)
Targeting Autophagy Increased NK-Mediated Tumor Regression in Vivo.
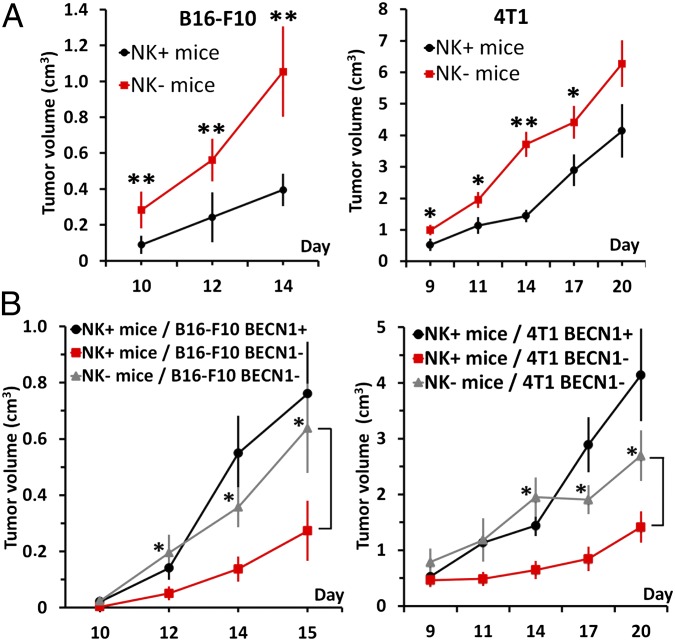
To assess whether inhibition of autophagy improves NK-mediated tumor regression in vivo, we used two aggressive syngeneic murine models: B16–F10 melanoma tumors and 4T1 breast carcinoma tumors. B16–F10 tumors have been previously reported as highly hypoxic, with a selective activation of autophagy in hypoxic zones (16), and 4T1 tumors have been reported as a suitable experimental animal model for human mammary cancer (26). We first evaluated whether host NK cells affect B16–F10 and 4T1 tumor growth in C57BL/6 and BALB/c mice, respectively. To address this issue, control (NK+) and NK-depleted (NK−) mice, which were generated by repeated injection of normal rabbit IgG and antiasialo GM1, respectively, were engrafted with B16–F10 or 4T1 cells. The efficiency and selectivity of antiasialo GM1 in depleting murine NK cells in C57BL/6 and BALB/c mice are shown in Fig. S4A. The data presented in Fig. 4A demonstrated a significant increase in B16–F10 and 4T1 tumor volume in NK− mice compared with NK+ mice, indicating that NK cells play a role in B16–F10 and 4T1 tumor regression in vivo. To determine the impact of autophagy on NK-mediated lysis in vivo, we analyzed the growth of autophagy-defective (BECN1−) B16–F10 and 4T1 tumor cells in both NK+ and NK− mice. B16–F10BECN1− and 4T1BECN1− cells were generated using BECN1 shRNA lentiviral particles. B16–F10 and 4T1 cells infected with scrambled shRNA-expressing vectors (B16–F10BECN1+ and 4T1BECN1+) were used as autophagy-competent control cells. Stable clones of B16–F10BECN1− and 4T1BECN1− cells were selected, and their in vitro growth was determined (Fig. S4B). The in vivo experimental design is shown in Fig. S4C. Fig. 4B demonstrated that in NK+ mice, the volume of B16–F10BECN1− and 4T1BECN1− tumors (red curves) was significantly reduced compared with that of BECN1+ tumors (black curves). This reduction is most likely due to the ability of NK cells to eliminate autophagy-defective cells more efficiently than autophagy-competent cells. Consistent with this hypothesis, in NK-depleted mice (NK−), the regression of BECN1− tumors was no longer observed (gray vs. red curves). Taken together, these results suggest that blocking autophagy in tumors facilitates and improves their elimination by NK cells in vivo.
Fig. 4.
Targeting autophagy in vivo improves tumor elimination by NK cells. (A) Control (NK+) and NK-depleted (NK−) C57BL/6 (Left) or BALB/c (Right) mice were engrafted with B16–F10 murine melanoma cells and 4T1 mammary carcinoma cells, respectively. Tumor growth in NK+ and NK− C57BL/6 (n = 7) and BALB/c (n = 10) mice was monitored using caliper measurements on the indicated days. Statistically significant differences in tumor volume are indicated by asterisks (*P < 0.05; **P < 0.005). (B) Autophagy-competent (BECN1+) or -defective (BECN1−) B16–F10 or 4T1 cells were injected s.c. (Left) or in the mammary fat pad (Right), respectively, in control (NK+) and NK-depleted (NK−) C57BL/6 (n = 7) and BALB/c (n = 10) mice. Tumor growth was monitored using caliper measurements on the indicated days. Statistically significant differences are indicated by asterisks (*P < 0.05).
Discussion
In this report, we identify autophagy as a major player in the resistance of breast cancer cells to NK-mediated lysis. Our in vivo data validate this concept and highlight the inhibition of autophagy as a potential therapeutic approach to improve NK-based cancer immunotherapy. In addition, this report elucidates the mechanism by which autophagy impairs the susceptibility of tumor cells to NK-mediated killing. This study demonstrates that the activation of autophagy under hypoxic conditions causes the degradation of NK-derived GzmB in vitro, which compromises the ability of NK cells to eliminate tumor cells. As the impairment of NK-mediated lysis of tumor cells was also observed under starvation conditions, it remains important to determine whether the degradation of GzmB occurs independently of the nature of stimuli involved in the activation of autophagy in target tumor cells.
NK-based immunotherapy is a promising therapy for solid and hematologic cancers, and it can potentially be combined with chemotherapy, radiation, or monoclonal antibody therapy (27). One of the major obstacles for defining successful NK-based therapeutic protocols is the ability of tumor cells to activate several mechanisms that lead to tumor escape from NK-mediated lysis. Extensive efforts have been made in recent years to identify these mechanisms (28). Inefficient killing of tumor cells by NK cells may be due, in part, to the down-regulation of NKG2D-activating ligands on the surface of tumor cells. Consistent with this hypothesis, hypoxia has been reported to increase the shedding of MIC from the surface of prostate cancer cells (23). In MCF-7 cells, however, we did not detect any decrease in the expression of MIC on the surface of H cells, which suggests that in our model, resistance to NK-mediated killing is not related to modulation in the expression of this NKG2D ligand. Interestingly, our data demonstrate a significant up-regulation of the expression of NK cell-inhibiting MHC class I molecules on the surface of hypoxic cells. Although the causal mechanisms underlying this increase remain unknown, our result indicated that resistance to NK-mediated lysis under hypoxia occurs independently of MHC class I expression levels. In addition, it is unlikely that resistance is related to a defect in NK/target cell interaction, as no difference in the formation of conjugates between NK cells and normoxic or hypoxic cells was observed. Based on these data, we exclude the possibility of autophagy-induced destabilization of the immunological synapse in hypoxic tumor cells, as recently described (29). Our results provide evidence that there is no difference in the degranulation level of NK cells when cocultured with normoxic or hypoxic cells. This eliminates the possibility of a defect in the cytotoxic potential of NK cells toward hypoxic tumor cells as a mechanism of resistance. Having demonstrated that NK cells fulfill their cytotoxic functions toward both hypoxic (H) and normoxic (N) cells, and that exogenous GzmB was unable to kill hypoxic tumor cells, we propose that autophagy operates as an intrinsic resistance mechanism in tumor cells.
The delivery of the lytic effector proteins perforin and granzymes to target cells occurs by at least three mechanisms. GzmB can be taken up by endosomes within the target cell by fluid-phase endocytosis or by mannose 6–phosphate receptor (M6PR)-mediated endocytosis (30). The third mechanism involves heat shock protein 70 (HSP70) bound to GzmB at the cell surface (31). We did not detect any differences in the cell surface expression of HSP70 or M6PR of normoxic and hypoxic cells (Fig. S5). These results rule out a defect in the transfer of the lytic effector proteins, perforin and granzymes, to hypoxic target cells. It is still debated whether granzymes enter via perforin pores formed at the plasma membrane or whether perforin and granzymes are endocytosed with subsequent release of granzymes from endosomes into the cytoplasm (32). Recent studies indicated that perforin activates clathrin- and dynamin-dependent endocytosis, which removes perforin and granzymes from the plasma membrane to enlarged early endosomes called “gigantosomes.” Subsequently, perforin forms pores in the gigantosome membrane, allowing for the gradual release of GzmB (33). Once released inside target cells, GzmB initiates apoptotic cell death. It has been reported that early endosomes can fuse with autophagic vacuoles to form amphisomes. This seems to be a prerequisite for the maturation of autophagic vacuoles, their subsequent fusion with lysosomes, and the formation of autophagolysosome (34). Based on these findings, we propose that during the intracellular trafficking, GzmB is subjected to degradation in autophagosomes under hypoxic conditions in vitro. Several data reported in this study support such a mechanism: i) the level of NK-derived GzmB detected in hypoxic cells is significantly lower than that in normoxic cells; ii) inhibition of autophagy by targeting BECN1 restores the level of GzmB and subsequently restores NK-mediated lysis of hypoxic cells; and iii) NK-derived GzmB is detected in LC3- and Rab5-positive cellular compartments, suggesting its presence within amphisomes in hypoxic cells. Together, our data identify a potential mechanism by which autophagy impairs the susceptibility of tumor cells to NK-mediated lysis in vitro. A schematic representation of this mechanism is provided in Fig. S6.
To evaluate the impact of autophagy on the regulation of NK-mediated immune responses to tumors in vivo, we used C57BL/6 and BALB/c mice transplanted with syngeneic murine B16–F10 melanoma and 4T1 breast adenocarcinoma tumor cells. The use of B16–F10 transplantation in mice to evaluate the role of autophagy in vivo provides several advantages, as previously reported (16). Our results clearly demonstrate that NK cells control B16–F10 and 4T1 tumor development in vivo, as the depletion of NK cells dramatically increases tumor growth. After establishing the role of NK cells in the control of both B16–F10 and 4T1 tumor growth, we further demonstrated that the growth of genetically engineered autophagy-defective B16–F10 and 4T1 cells was substantially inhibited in immunocompetent mice. Our data, demonstrating that an inhibition of tumor growth was no longer observed when NK cells were depleted, highlight the key role of autophagy in the impairment of NK-mediated tumor cell killing in vivo.
The TME plays a critical role in the control of immune protection by a number of overlapping mechanisms, which ultimately lead to tumor evasion from immunosurveillance. Tumors have evolved to use hypoxic stress to their own advantage by activating key biochemical and cellular pathways that are important for progression, survival, and metastasis. In this regard, our study underlines the inhibition of autophagy as a cutting-edge approach for the formulation of more effective NK-based cancer immunotherapies. Although there are several ongoing clinical trials using NK cells for cancer treatment, this study highlights the importance of integrating autophagy inhibitors as an innovative strategy in NK-based cancer immunotherapy.
Materials and Methods
Cytotoxicity Assay.
Target cells were labeled with 10 μM of CellTrace carboxyfluorescein diacetate succinimidyl ester (CFSE) (Invitrogen), coincubated with effector cells at 1/1 and 5/1 effector to target cell (E/T) ratios for 4 h at 37 °C and analyzed by flow cytometry (FACSCanto). TO-PRO-3 (Invitrogen) was used to assess cell death. HLA-class I was blocked using a mouse IgM anti-pan HLA class I A6-136 mAb (35).
Granzyme B Transfer.
YT–INDY–NK cells (1.25 × 106) were coincubated with target cells (E/T ratio: 5/1) at 37 °C for 0 and 30 min, washed twice with a solution of 1% (vol/vol) FCS and 2 mM EDTA in PBS, and submitted to high-speed vortexing to disrupt E/T conjugates. Target cells were separated from NK cells using a CD56 MicroBeads kit (Miltenyi Biotec). The purity of the fraction containing the target MCF-7 cells was assessed by flow cytometry (FACSCanto). The presence of granzyme B in target cells was evaluated by immunoblot. The transfer of granzyme B to target cells was also assessed using a Laser scanning microscopy (LSM)-510-Meta (Carl Zeiss) after coincubation with GFP–granzyme B-expressing YT–INDY–NK cells.
Subcellular Fractionation.
Normoxic or hypoxic MCF-7 cells were treated with e64d and pepstatin before coincubation with YT–INDY–NK cells for 30 min at 37 °C. Following separation from NK cells, subcellular fractionation of the target cells was performed according the protocol established by Fulvio Reggiori (Department of Cell Biology, University Medical Centre Utrecht, Utrecht, The Netherlands) to isolate autophagosomes. Details of the experimental procedure are described in the SI Materials and Methods.
In Vivo Experiments.
Ten-week-old C57BL/6 or 7-wk-old BALB/c female mice were injected s.c. with anti-asialo GM1 antibody (3.8 mg per mouse) (Cedarlane CL8955) at indicated days to generate NK-depleted mice (NK−). The experimental design is presented in Fig. 4B, Upper. Control (NK+) mice were injected with normal rabbit IgG (3.8 mg per mouse) (Dako). Three days after the first NK cell depletion, mice were injected with tumor cells. C57BL/6 mice were injected s.c. with control (B16–F10BECN1+) or autophagy-defective (B16–F10BECN1−) B16–F10 cells (2.3 × 105 cells per mouse). BALB/c mice were injected orthotopically in the mammary fat pad with control (4T1BECN1+) or autophagy-defective (4T1BECN1−) 4T1 cells (5 × 104 cells per mouse). Tumor growth was monitored using caliper measurements on the indicated days, and tumor volume was calculated as follows: Volume (cm3) = (width × length × height) × 0.5236. C57BL/6 and BALB/c mice were killed on days 15 and 20, respectively. Animal experiments have been approved by the national and institutional (CRP-Sante) review boards responsible for animal studies in Luxembourg.
Supplementary Material
Acknowledgments
We thank Prof. Rolf Bjerkvig (University of Bergen) for helpful discussions and critical reading of the manuscript; Dr. Beth Levine (University of Texas Southwest Medical Center) for providing MCF-7BECN1 tet-off cells; Prof. Alessandro Moretta (Department of Experimental Medicine, University of Genova) for providing anti-pan HLA class I A6-136 mAb; and Dr. Fulvio Reggiori (University Medical Centre Utrecht). We also thank Dr. Etienne Moussay, Dr. Jérome Paggetti, Hakim Boulenouar, Loredana Jacobs, Virginie Baus, and Nicolaas H. C. Brons for technical assistance. This work was supported by grants from Public Research Center for Health, Luxembourg (REC-LHCE-2009-0201) and Fondation Cancer, Luxembourg (FC/2012/02) (to B.J.); grants from the Ligue Contre le Cancer (Comité de Val de Marne), Institut National du Cancer (10128), and Association de Recherche sur le Cancer (R01354) (to S.C.); and by the Canadian Institutes of Health Research (to R.C.B. and I.S.G.). J.B. is a recipient of an Aide à la Formation-Recherche (AFR) grant (2009-1201) from the Fonds National de la Recherche, Luxembourg. S.C. and B.J. were coprincipal investigators of this work.
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission. N.M. is a guest editor invited by the Editorial Board.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1304790110/-/DCSupplemental.
References
- 1.Zitvogel L, Tesniere A, Kroemer G. Cancer despite immunosurveillance: immunoselection and immunosubversion. Nat Rev Immunol. 2006;6(10):715–727. doi: 10.1038/nri1936. [DOI] [PubMed] [Google Scholar]
- 2.Whiteside TL. The tumor microenvironment and its role in promoting tumor growth. Oncogene. 2008;27(45):5904–5912. doi: 10.1038/onc.2008.271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Swartz MA, et al. Tumor microenvironment complexity: Emerging roles in cancer therapy. Cancer Res. 2012;72(10):2473–2480. doi: 10.1158/0008-5472.CAN-12-0122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Smyth MJ, Hayakawa Y, Takeda K, Yagita H. New aspects of natural-killer-cell surveillance and therapy of cancer. Nat Rev Cancer. 2002;2(11):850–861. doi: 10.1038/nrc928. [DOI] [PubMed] [Google Scholar]
- 5.Bryceson YT, March ME, Ljunggren HG, Long EO. Synergy among receptors on resting NK cells for the activation of natural cytotoxicity and cytokine secretion. Blood. 2006;107(1):159–166. doi: 10.1182/blood-2005-04-1351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Smyth MJ, et al. Differential tumor surveillance by natural killer (NK) and NKT cells. J Exp Med. 2000;191(4):661–668. doi: 10.1084/jem.191.4.661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Lv L, et al. The accumulation and prognosis value of tumor infiltrating IL-17 producing cells in esophageal squamous cell carcinoma. PLoS ONE. 2011;6(3):e18219. doi: 10.1371/journal.pone.0018219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.van Herpen CM, et al. Intratumoral recombinant human interleukin-12 administration in head and neck squamous cell carcinoma patients modifies locoregional lymph node architecture and induces natural killer cell infiltration in the primary tumor. Clin Cancer Res. 2005;11(5):1899–1909. doi: 10.1158/1078-0432.CCR-04-1524. [DOI] [PubMed] [Google Scholar]
- 9.Ishigami S, et al. Prognostic value of intratumoral natural killer cells in gastric carcinoma. Cancer. 2000;88(3):577–583. [PubMed] [Google Scholar]
- 10.Ménard C, et al. Natural killer cell IFN-gamma levels predict long-term survival with imatinib mesylate therapy in gastrointestinal stromal tumor-bearing patients. Cancer Res. 2009;69(8):3563–3569. doi: 10.1158/0008-5472.CAN-08-3807. [DOI] [PubMed] [Google Scholar]
- 11.Stanietsky N, Mandelboim O. Paired NK cell receptors controlling NK cytotoxicity. FEBS Lett. 2010;584(24):4895–4900. doi: 10.1016/j.febslet.2010.08.047. [DOI] [PubMed] [Google Scholar]
- 12.Stern-Ginossar N, Mandelboim O. An integrated view of the regulation of NKG2D ligands. Immunology. 2009;128(1):1–6. doi: 10.1111/j.1365-2567.2009.03147.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kärre K, Ljunggren HG, Piontek G, Kiessling R. Selective rejection of H-2-deficient lymphoma variants suggests alternative immune defence strategy. 1986. J Immunol. 2005;174(11):6566–6569. [PubMed] [Google Scholar]
- 14.Campoli M, Ferrone S. Tumor escape mechanisms: Potential role of soluble HLA antigens and NK cells activating ligands. Tissue Antigens. 2008;72(4):321–334. doi: 10.1111/j.1399-0039.2008.01106.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Lee CT, Mace T, Repasky EA. Hypoxia-driven immunosuppression: A new reason to use thermal therapy in the treatment of cancer? Int J Hyperthermia. 2010;26(3):232–246. doi: 10.3109/02656731003601745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Noman MZ, et al. Blocking hypoxia-induced autophagy in tumors restores cytotoxic T-cell activity and promotes regression. Cancer Res. 2011;71(18):5976–5986. doi: 10.1158/0008-5472.CAN-11-1094. [DOI] [PubMed] [Google Scholar]
- 17.Bosco MC, Varesio L. Dendritic cell reprogramming by the hypoxic environment. Immunobiology. 2012;217(12):1241–1249. doi: 10.1016/j.imbio.2012.07.023. [DOI] [PubMed] [Google Scholar]
- 18.Youssef MM, Symonds P, Ellis IO, Murray JC. EMAP-II-dependent lymphocyte killing is associated with hypoxia in colorectal cancer. Br J Cancer. 2006;95(6):735–743. doi: 10.1038/sj.bjc.6603299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Chouaib S, et al. Hypoxia promotes tumor growth in linking angiogenesis to immune escape. Front Immunol. 2012;3:21. doi: 10.3389/fimmu.2012.00021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Grimshaw MJ, Naylor S, Balkwill FR. Endothelin-2 is a hypoxia-induced autocrine survival factor for breast tumor cells. Mol Cancer Ther. 2002;1(14):1273–1281. [PubMed] [Google Scholar]
- 21.Sceneay J, et al. Primary tumor hypoxia recruits CD11b+/Ly6Cmed/Ly6G+ immune suppressor cells and compromises NK cell cytotoxicity in the premetastatic niche. Cancer Res. 2012;72(16):3906–3911. doi: 10.1158/0008-5472.CAN-11-3873. [DOI] [PubMed] [Google Scholar]
- 22.Flavell RA, Sanjabi S, Wrzesinski SH, Licona-Limón P. The polarization of immune cells in the tumour environment by TGFbeta. Nat Rev Immunol. 2010;10(8):554–567. doi: 10.1038/nri2808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Siemens DR, et al. Hypoxia increases tumor cell shedding of MHC class I chain-related molecule: Role of nitric oxide. Cancer Res. 2008;68(12):4746–4753. doi: 10.1158/0008-5472.CAN-08-0054. [DOI] [PubMed] [Google Scholar]
- 24.Noman MZ, Janji B, Berchem G, Mami-Chouaib F, Chouaib S. Hypoxia-induced autophagy: A new player in cancer immunotherapy? Autophagy. 2012;8(4):704–706. doi: 10.4161/auto.19572. [DOI] [PubMed] [Google Scholar]
- 25.Michaud M, et al. Autophagy-dependent anticancer immune responses induced by chemotherapeutic agents in mice. Science. 2011;334(6062):1573–1577. doi: 10.1126/science.1208347. [DOI] [PubMed] [Google Scholar]
- 26.Heppner GH, Miller FR, Shekhar PM. Nontransgenic models of breast cancer. Breast Cancer Res. 2000;2(5):331–334. doi: 10.1186/bcr77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Hanahan D, Weinberg RA. Hallmarks of cancer: The next generation. Cell. 2011;144(5):646–674. doi: 10.1016/j.cell.2011.02.013. [DOI] [PubMed] [Google Scholar]
- 28.Ljunggren HG, Malmberg KJ. Prospects for the use of NK cells in immunotherapy of human cancer. Nat Rev Immunol. 2007;7(5):329–339. doi: 10.1038/nri2073. [DOI] [PubMed] [Google Scholar]
- 29. Wildenberg ME, et al. (2012) Autophagy attenuates the adaptive immune response by destabilizing the immunologic synapse. Gastroenterology 142(7):1493–1503. [DOI] [PubMed]
- 30.Wowk ME, Trapani JA. Cytotoxic activity of the lymphocyte toxin granzme B. Microbes Infect. 2004;6(8):752–758. doi: 10.1016/j.micinf.2004.03.008. [DOI] [PubMed] [Google Scholar]
- 31.Gross C, Koelch W, DeMaio A, Arispe N, Multhoff G. Cell surface-bound heat shock protein 70 (Hsp70) mediates perforin-independent apoptosis by specific binding and uptake of granzyme B. J Biol Chem. 2003;278(42):41173–41181. doi: 10.1074/jbc.M302644200. [DOI] [PubMed] [Google Scholar]
- 32.Lopez JA, Brennan AJ, Whisstock JC, Voskoboinik I, Trapani JA. Protecting a serial killer: Pathways for perforin trafficking and self-defence ensure sequential target cell death. Trends Immunol. 2012;33(8):406–412. doi: 10.1016/j.it.2012.04.001. [DOI] [PubMed] [Google Scholar]
- 33.Thiery J, et al. Perforin pores in the endosomal membrane trigger the release of endocytosed granzyme B into the cytosol of target cells. Nat Immunol. 2011;12(8):770–777. doi: 10.1038/ni.2050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Longatti A, Tooze SA. Recycling endosomes contribute to autophagosome formation. Autophagy. 2012;8(11):1682–1683. doi: 10.4161/auto.21486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Ciccone E, et al. General role of HLA class I molecules in the protection of target cells from lysis by natural killer cells: evidence that the free heavy chains of class I molecules are not sufficient to mediate the protective effect. Int Immunol. 1995;7(3):393–400. doi: 10.1093/intimm/7.3.393. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.