Significance
Histone-modifying enzymes play an important role in regulating chromatin-associated processes such as transcription. In addition to modifying histones, these enzymes control gene expression through modifying nonhistone proteins, including transcription factors. In this study, we show that SET and MYND domain containing 2 (SMYD2), a histone H3K4 and H3K36 methyltransferase, directly methylates estrogen receptor alpha (ERα) protein at lysine 266 and represses ERα transactivation activity. Upon estrogen activation, this repressive mark is relieved by the histone H3K4 demethylase lysine-specific demethylase 1, followed by p300/cAMP response element-binding protein–binding protein (CBP)-mediated protein acetylation. Our study suggests that the cross-talk of distinct posttranslational modifications in the hinge region of ERα plays an important role in fine tuning the functions of ERα at chromatin in hormone response.
Keywords: ERα hinge region, lysine methylation, LSD1
Abstract
Estrogen receptor alpha (ERα) is a ligand-activated transcription factor. Upon estrogen stimulation, ERα recruits a number of coregulators, including both coactivators and corepressors, to the estrogen response elements, modulating gene activation or repression. Most coregulator complexes contain histone-modifying enzymes to control ERα target gene expression in an epigenetic manner. In addition to histones, these epigenetic modifiers can modify nonhistone proteins including ERα, thereby constituting another layer of transcriptional regulation. Here we show that SET and MYND domain containing 2 (SMYD2), a histone H3K4 and H3K36 methyltransferase, directly methylates ERα protein at lysine 266 (K266) both in vitro and in cells. In breast cancer MCF7 cells, SMYD2 attenuates the chromatin recruitment of ERα to prevent ERα target gene activation under an estrogen-depleted condition. Importantly, the SMYD2-mediated repression of ERα target gene expression is mediated by the methylation of ERα at K266 in the nucleus, but not the methylation of histone H3K4. Upon estrogen stimulation, ERα–K266 methylation is diminished, thereby enabling p300/cAMP response element-binding protein–binding protein to acetylate ERα at K266, which is known to promote ERα transactivation activity. Our study identifies a previously undescribed inhibitory methylation event on ERα. Our data suggest that the dynamic cross-talk between SMYD2-mediated ERα protein methylation and p300/cAMP response element-binding protein–binding protein-dependent ERα acetylation plays an important role in fine-tuning the functions of ERα at chromatin and the estrogen-induced gene expression profiles.
Estrogen receptors (ERs) are a subfamily of nuclear receptors that control cellular responses to estrogens (1). There are two different forms of ER, usually referred to as ERα and ERβ, and ERα is the dominant form expressed in breast and ovary tissues. The regulation of hormone-responsive gene expression by ERα as well as other nuclear receptors is a complex process involving a variety of cellular responses. One essential step is the recruitment of transcriptional coregulators—namely, nuclear receptor coactivators (NCOAs; also known as steroid receptor coactivators; e.g., SRC1, 2, and 3) or nuclear receptor corepressors (NCORs)—in a hormone-dependent manner (2). Most coactivator complexes comprise histone lysine (K) acetyltransferases such as p300/cAMP response element-binding protein–binding protein (CBP) (3), which can put on acetylation marks on histones. Histone acetylation helps open up chromatin around the estrogen response element (ERE) regions to facilitate the loading of RNA polymerase II transcriptional machinery. In the absence of its hormone ligands, ERα interacts with corepressor complexes, which normally consist of histone deacetylases (HDACs), to remove acetylation on histones, leading to gene repression (4).
In addition to modifying histones, these nuclear receptor coregulators can modify nonhistone proteins, including ERα. For instance, p300/CBP acetylates ERα at several K residues in the hinge region (5, 6). Interestingly, acetylation of ERα on different K residues is associated with distinct functions: acetylation of ERα on K266/288 promotes ERα transactivation activity, whereas acetylation of ERα on K302/303 inhibits ERα target gene expression (5, 6). Besides acetylation, ERα undergoes many other posttranslational modifications—including phosphorylation, sumoylation, and ubiquitylation—that regulate ERα protein stability, subcellular localization, and hormone sensitivity (7). Some modifications of ERα are associated with distinct biological and clinical outcomes, suggesting that these modifications have great potential as markers for prognosis or prediction of endocrine therapy response. For example, phosphorylation of ERα on serine (S) 305 is associated with tamoxifen resistance (8), whereas patients whose tumors express ERα with phosphorylation on S118 and/or S167 often have a better clinical outcome to tamoxifen therapy (9, 10).
Compared with what is known about the phosphorylation and acetylation of ERα, much less is known about the protein methylation of ERα. Until recently, only one ERα protein K methylation event, which is catalyzed by SET domain containing 7/9 (SET7/9) to control ERα protein stability, has been reported (11). In addition, arginine 260 of ERα is methylated by the protein arginine methyltransferase 1, which regulates the nongenomic function of ERα in the cytoplasm (12). In fact, ERα recruits a number of enzymes involved in the regulation of histone methylation dynamics. Coactivator-associated arginine methyltransferase 1 is a well-known ERα coactivator that methylates histone H3R17 to promote chromatin recruitment of p300/CBP to EREs (13–16). Recent studies have demonstrated that ERα also recruits a number of K methyltransferases (KMTs) and K demethylases (KDMs), including the MLL/KMT2 family of proteins, G9a/KMT1C, SMYD3, KDM4B, and lysine-specific demethylase 1 (LSD1)/KMT1A (reviewed in ref. 17 and references therein). Most of these enzymes function as ERα coactivators by depositing active methylation marks or removing repressive methylation marks from histones. However, whether these enzymes also directly modify ERα protein to control ERα activities remains unknown.
In the present study, we screened nearly 30 KMTs and identified an inhibitory methylation event on ERα, which is catalyzed by SET and MYND domain containing 2 (SMYD2, a.k.a. KMT3C), a H3K4 and H3K36 methyltransferase (18, 19). SMYD2 specifically methylates ERα at K266 in the hinge region both in vitro and in cultured cells. ERα–K266 monomethylation (ERα–K266me1) prevents ERα from activating target genes by inhibiting the chromatin recruitment of ERα. Our results demonstrate that the transcriptional repression by ERα–K266me1 is, at least in part, through inhibition of K266/268 acetylation, a known mark of active ERα (5). Furthermore, we found that knockdown of LSD1 leads to increased ERα–K266 methylation and decreased K266/268 acetylation, suggesting that ERα–K266 methylation is dynamically regulated by SMYD2 and LSD1, which are known to also control the methylation of histone H3K4 and p53K370 (20, 21). Our study suggests that the cross-talk of distinct modifications in the hinge region of ERα plays an important role in fine tuning the functions of ERα at chromatin in hormone response.
Results
SMYD2 Methylates ERα in Vitro.
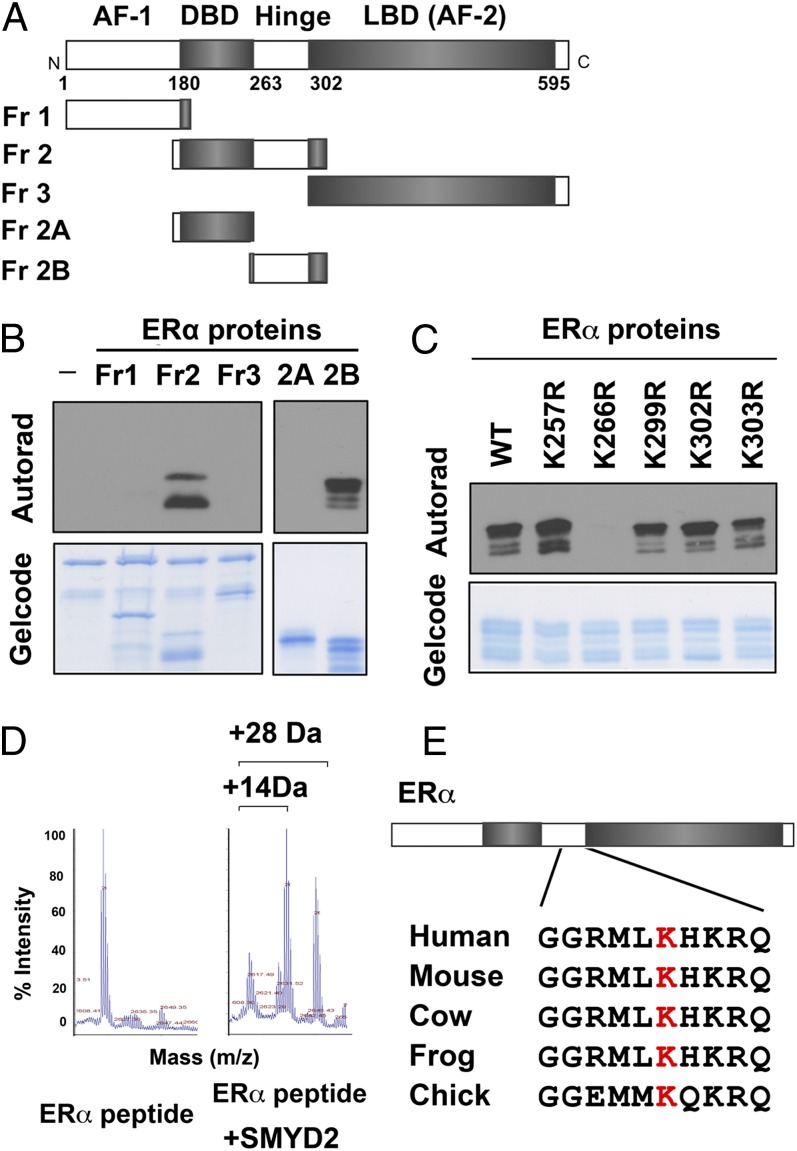
To determine whether ERα protein can be methylated, we cloned and purified the SET domains of ∼30 known and potential KMTs (22) and performed in vitro methylation assays with these enzymes and recombinant ERα protein. We found that in addition to the previously reported SET7/9 (11), SMYD2 exhibited strong methylation activity on ERα protein (SI Appendix, Fig. S1). To identify the residues on ERα that are methylated by SMYD2, we first divided ERα protein into three fragments: fragment 1 contained an N-terminal regulatory domain known as activation function domain 1; fragment 2 contained a central DNA-binding domain and a flexible hinge region; and fragment 3 contained a C-terminal ligand-binding domain that is also known as activation function domain 2 (Fig. 1A). In vitro methylation assays using these ERα fragments revealed that SMYD2 methylated only the hinge region within fragment 2 (Fig. 1B). Using a series of point mutant proteins of ERα for methylation assays, we found that SMYD2 specifically methylated K266, but not any other K residues in the hinge region (Fig. 1C). Furthermore, we carried out mass spectrometric (MS) analysis of the ERα peptide (amino acids 258–276) that was methylated by SMYD2, and we determined that, compared with the unmethylated peptide, the SMYD2 methylated peptide had mass increases of 14 and 28 Da, indicating that SMYD2 can carry out both monomethylation and dimethylation on ERα (Fig. 1D). The sequences of K266 and its neighboring residues are conserved among ERα proteins in multiple species (Fig. 1E), but distinct from ERβ and other nuclear receptors (5), suggesting that SMYD2-dependent methylation on this residue may have a conserved functional role specific to ERα.
Fig. 1.
SMYD2 methylates ERα in vitro. (A) Schematic representation of ERα protein domains. (B and C) ERα is methylated at K266 by SMYD2. Autoradiograms of SMYD2-dependent in vitro methylation of recombinant ERα fragments (B) and point mutant proteins (C) are shown. GelCode Blue staining shows equal amount of ERα and SMYD2 proteins used in the assays. (D) ERα is monomethylated and dimethylated by SMYD2. MS analysis of ERα peptide (amino acids 258–276) with or without SMYD2 incubation is shown. (E) ERα–K266 is evolutionally conserved. Alignment of amino acid sequences surrounding K266 in human ERα and ERα proteins from several other species is shown.
ERα Is Methylated by SMYD2 at K266 in Cells.
To determine whether SMYD2 methylates ERα at K266 in cells, we developed a polyclonal antibody that specifically recognizes ERα–K266me1. The antibody did not recognize the unmethylated and dimethylated ERα–K266 peptide or monomethylated and dimethylated H3K4, a histone substrate of SMYD2 (19), on a dot blot (SI Appendix, Fig. S2A), suggesting that this antibody was specific to ERα–K266me1. The specificity of this antibody was further verified by probing the recombinant ERα hinge-region protein methylated by SMYD2 in vitro (SI Appendix, Fig. S2B). The antibody recognized ERα protein incubated with SMYD2 and the methyl donor S-adenosyl methionine (SAM), but not the ERα protein without SAM or SMYD2. Importantly, we did not detect any signals on the ERα–K266R mutant that could not be methylated by SMYD2 (SI Appendix, Fig. S2B).
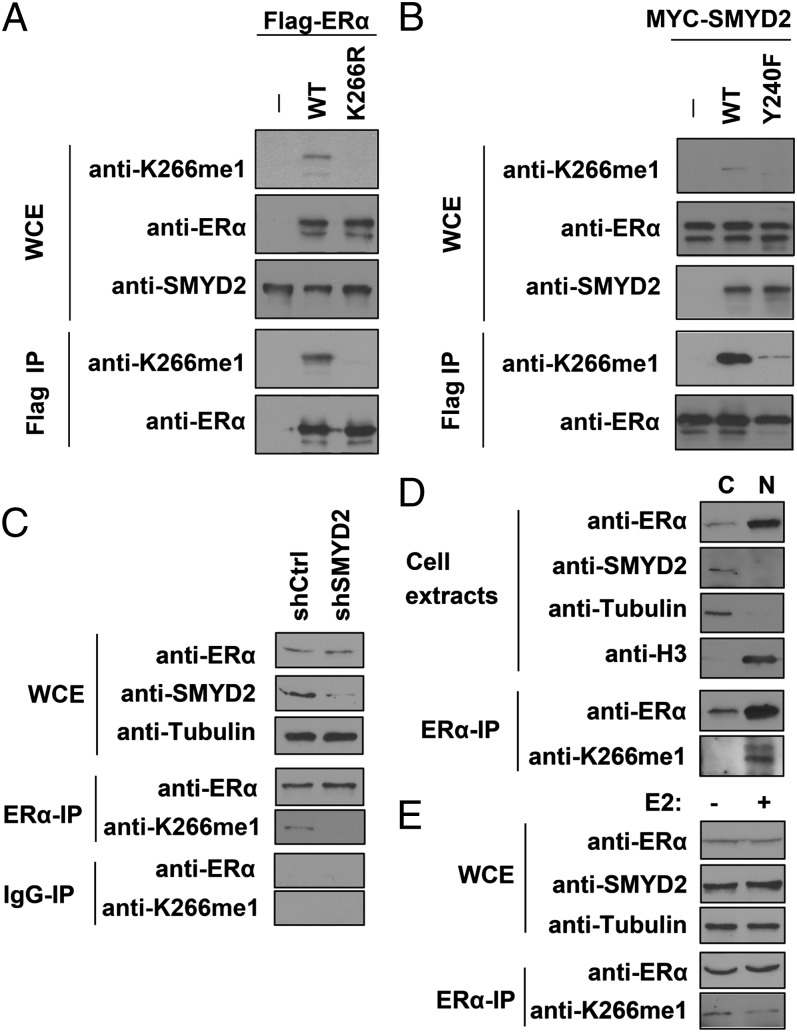
Next, we used the ERα–K266me1-specific antibody to determine whether SMYD2 methylates ERα at K266 in cells. We cotransfected HEK 293T cells with Myc-SMYD2 and Flag-tagged wild-type (WT) ERα or the K266R mutant, and we probed whole-cell extracts (WCEs) and the immunoprecipitated (IP) Flag-ERα proteins with the anti-ERα–K266me1 antibody. We detected a clear signal in both the WCEs and Flag-IP samples in the cells coexpressing SMYD2 and WT ERα (Fig. 2A). We did not detect any signals in the cells coexpressing SMYD2 and the ERα–K266R mutant, indicating that this signal was methylation specific. Furthermore, this methylation is dependent on the methyltransferase activity of SMYD2, because the methylation-specific signal was greatly diminished in cells coexpressing ERα and a catalytic mutant SMYD2–Y240F (18), compared to the cells coexpressing ERα and the WT SMYD2 (Fig. 2B).
Fig. 2.
ERα is methylated by SMYD2 in cells. (A) SMYD2 methylates ectopic ERα at K266 in cells. Western blot analysis of ERα–K266me1 and total ERα levels in WCE and Flag IP of 293T cells cotransfected with Flag-WT ERα or K266R mutants and Myc-SMYD2 are shown. (B) The enzymatic activity of SMYD2 is required for ERα–K266 methylation in cells. Western blot analysis of ERα–K266me1 and total ERα levels in WCE and Flag IP of 293T cells cotransfected with Flag-ERα and MYC-WT SMYD2 or the Y240F catalytic mutant are shown. (C) SMYD2 is required for endogenous ERα–K266 methylation in MCF7 cells. Western blot analysis of ERα–K266me1 and total ERα levels in WCE and ERα IP in control (shCtrl) and SMYD2-knockdown (shSMYD2) MCF7 cells are shown. IgG IP is shown as a negative control. Tubulin is shown as a loading control. (D) K266-methylated ERα mainly resides in the nucleus. Western blot analysis of ERα, SMYD2, and ERα–K266me1 levels in the cytoplasmic and nuclear fractions of MCF7 cells is shown. Tubulin and histone H3 are shown as markers of the cytoplasm and nucleus, respectively. (E) ERα–K266 methylation levels decrease upon E2 treatment. Western blot analysis of ERα–K266me1 and total ERα levels in WCE and ERα IP of MCF7 cells with (+) or without (−) E2 treatment are shown.
Next, we sought to determine whether the anti-ERα–K266me1 antibody was able to detect K266 methylation on endogenous ERα protein. We used an anti-ERα antibody to IP endogenous ERα protein from MCF7 cells and probed the IP samples with the anti-ERα–K266me1 antibody. We detected a clear signal in the ERα IP, but not control IgG IP, samples (Fig. 2C). To further determine whether the signal we observed was ERα–K266 methylation-specific, we knocked down SMYD2 using shRNA and probed endogenous ERα immunoprecipitated from the SMYD2-knockdown cells. The ERα–K266me1 signal was greatly diminished in SMYD2-knockdown cells, comparing to the control MCF7 cells treated with nontargeting shRNA, whereas the levels of total ERα proteins remained unchanged (Fig. 2C). These results suggested that methylation of endogenous ERα at K266 is dependent on SMYD2. To detect endogenous ERα–K266 methylation using an independent approach, we performed liquid chromatography-tandem MS (LC-MS/MS) analysis of endogenous ERα proteins purified from MCF7 cells. LS-MS/MS identified several unique modifications of ERα, including acetylation of K32 and K171, monomethylation of K171, and dimethylation of K171, K180, R277, and K401 (SI Appendix, Table S1 and Fig. S3), in addition to acetylation of K299 that has been reported (6). However, because the hinge region around K266 is highly enriched with positively charged residues, both Arg-C and Asp-N protease digestions did not yield visible peptides that contained K266 in our MS analysis.
SMYD2-Methylated ERα Proteins Reside in the Nucleus and the ERα–K266 Methylation Levels Decrease upon E2 Treatment.
Under an unstimulated condition, ERα resides in the cytoplasm; upon binding to its hormone ligands, such as 17β-estradiol (E2), ERα dimerize and translocates into the nucleus (1). Next, we asked where ERα–K266 methylation occurs and what is the dynamics of the methylation event during E2 response. To address this question, we biochemically separated the MCF7 cells into cytoplasmic and nuclear fractions and immunoprecipitated the endogenous ERα protein to determine ERα–K266 methylation levels. We found that under a regular growth condition that contains low levels of E2, ERα mainly resided in the nucleus, whereas SMYD2 was predominantly a cytoplasmic protein. Surprisingly, in contrast to the cytoplasmic localization of SMYD2, K266-methylated ERα was observed only in the nucleus (Fig. 2D). To assess the dynamics of ERα–K266 methylation in response to E2 treatment, we cultured MCF7 cells in E2-depleted medium for 3 d, treated the cells with 10 nM E2 for 1 h, and determined the methylation levels of the ERα proteins. Although the levels of both SMYD2 and total ERα proteins remained largely the same before and after E2 treatment, the ERα–K266 methylation levels decreased drastically upon E2 treatment (Fig. 2E), suggesting that ERα–K266 methylation may play an inhibitory role in regulating ERα activities.
SMYD2-Mediated ERα–K266 Methylation Negatively Regulates ERα Target Gene Activation During E2 Response.
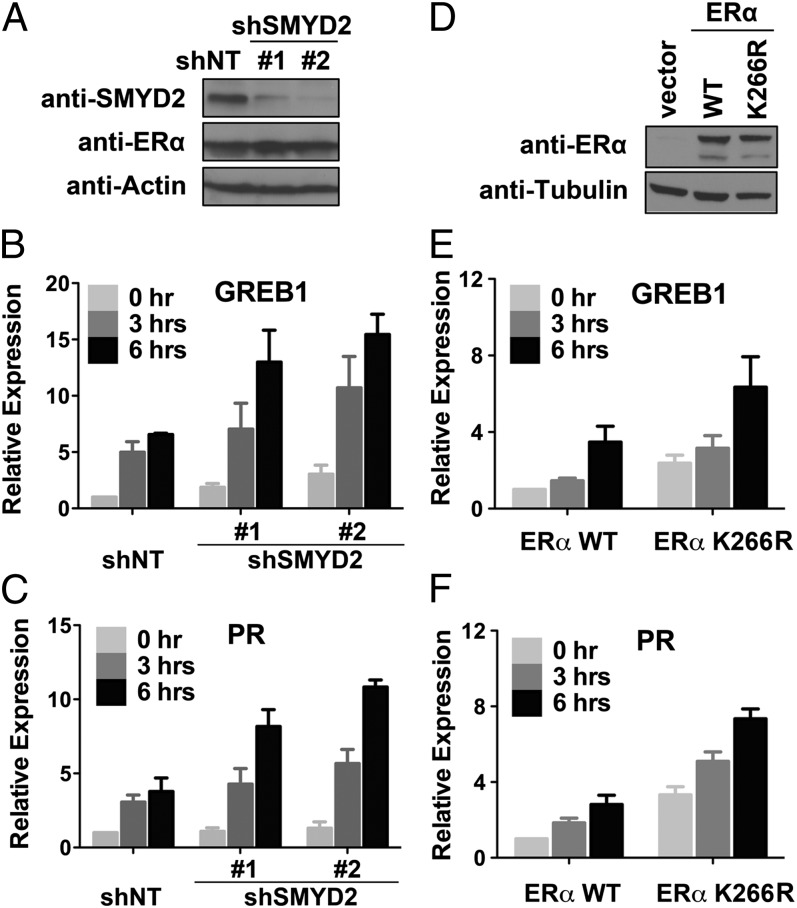
ERα activates a large number of target genes in response to E2 treatment. We asked whether SMYD2-mediated ERα–K266 methylation plays a role in regulating ERα-dependent gene activation, and we sought to address this question by assessing the expression of endogenous ERα target gene in MCF7 cells upon SMYD2 depletion. We used two shRNAs to independently knockdown endogenous SMYD2 in MCF7 cells, in which the mRNA and proteins levels of ERα were not affected (Fig. 3A and SI Appendix, Fig. S4 A and B). We treated the cells with 10 nM E2 for 3 or 6 h and then determined the expression of several ERα target genes in both the control and SMYD2-knockdown MCF7 cells. E2 treatment activated the expression of several ERα target genes in MCF7 cells, including growth regulation by estrogen in breast cancer 1 (GREB1), progesterone receptor (PR), trefoil factor 1 (TFF1, a.k.a. pS2), and IGFBP4. Importantly, compared with the control cells, SMYD2-depleted cells exhibited a higher expression of the ERα target genes (Fig. 3 B and C and SI Appendix, Fig. S4C), suggesting that endogenous SMYD2 negatively controls the expression of ERα target genes during E2 response.
Fig. 3.
Depletion of SMYD2 enhances the expression of ERα target genes. (A) Western blot analysis of ERα and SMYD2 protein levels in control and SMYD2-knockdown MCF7 cells. (B and C) Knockdown of SMYD2 enhances the expression of ERα target genes in MCF7 cells. Quantitative reverse transcription PCR (qPCR) analysis of gene expression in cells as in A at 3 and 6 h after E2 treatment. (D) Western blot analysis of ERα proteins in MDA-MB 231 cells stably expressing the WT ERα or ERα–K266R mutant. (E and F) ERα–K266R mutant exhibits increased transactivation activity compared with the WT ERα. qPCR analysis of gene expression in cells as in D at 3 and 6 h after E2 treatment is shown. Error bars represent SEM of three experiments.
Next, we sought to determine whether the suppression of ERα target gene activation by SMYD2 is dependent on methylation of ERα at K266. We generated stable cells expressing either WT ERα or the K266R mutant in the ERα-negative MDA-MB 231 breast cancer cells (Fig. 3D), and we determined their transactivation activities by assessing the expression of endogenous ERα target genes. The ectopic ERα proteins enabled the MDA-MB 231 cells to respond to E2 treatment (Fig. 3 E and F). Notably, ERα–K266R mutant possesses a higher transactivation activity than the WT ERα in these cells, not only under E2 treatment, but also under unstimulated condition without E2 (Fig. 3 E and F). Importantly, the cells expressing ERα–K266R mutant exhibited a higher proliferation rate than the cells expressing WT ERα under the grow condition without E2 (SI Appendix, Fig. S5). These results strongly suggest that SMYD2-mediated ERα–K266 methylation plays an inhibitory role in regulating ERα target gene expression.
SMYD2 Attenuates ERα Chromatin Recruitment.
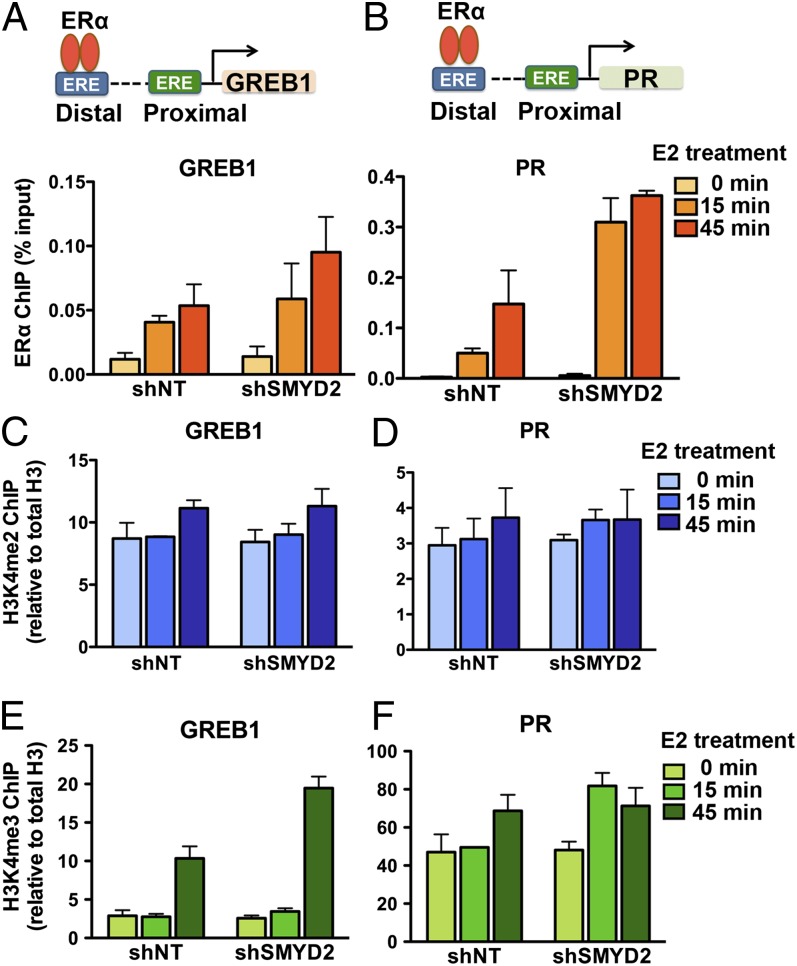
What is the molecular mechanism by which SMYD2-mediated ERα–K266 methylation inhibits ERα target gene activation? Because K266-methylated ERα resides mainly in the nucleus, whereas SMYD2 is predominantly a cytoplasmic protein, one possibility is that SMYD2 controls the translocation of ERα from the cytoplasm to the nucleus in response to E2. However, fractionation experiments in both control and SMYD2-knockdown MCF7 cells revealed that SMYD2 depletion did not affect the overall distribution of ERα proteins in the cytoplasm and nucleus (SI Appendix, Fig. S6). We then investigated another possibility, which is that SMYD2-mediated ERα methylation in the nucleus regulates ERα chromatin recruitment. To test this hypothesis, we performed ERα ChIP experiments in both control and SMYD2-knockdown MCF7 cells. We treated the cells with 10 nM E2 for 15 or 45 min and determined the occupancy of ERα on its strong binding ERE sites on target genes, including the distal EREs/enhancers of GREB1 and PR and the proximal ERE/promoter of TFF1 (Fig. 4 A and B and SI Appendix, Fig. S7A). ERα occupancy on these EREs gradually increased during the time course of E2 treatment. Importantly, compared with the nontargeting shRNA-infected control cells, the SMYD2-knockdown cells exhibited a higher ERα occupancy (Fig. 4 A and B and SI Appendix, Fig. S7A), suggesting that SMYD2 prevents ERα chromatin recruitment.
Fig. 4.
SMYD2 attenuates ERα chromatin recruitment. (A and B) Depletion of SMYD2 enhances ERα chromatin recruitment. qPCR analysis of ERα ChIP in control and SMYD2-knockdown MCF7 cells 15 and 45 min after E2 treatment is shown. ERα occupancies on the ERE sites of target genes are shown in Upper. (C and D) Depletion of SMYD2 does not affect H3K4me2 levels on the enhancers of ERα target genes. qPCR analysis of H3K4me2 ChIP in control and SMYD2-knockdown MCF7 cells as in A is shown. (E and F) Depletion of SMYD2 does not decrease H3K4me3 levels on the promoters of ERα target genes. qPCR analysis of H3K4me3 ChIP in cells as in A is shown. All error bars indicate SEM of two or three independent experiments.
The SMYD family protein SMYD3 has been shown to methylate H3K4 at the promoters of ERα target genes (23). Because SMYD2 can methylate both histone H3K4 and ERα–K266, we next sought to determine whether the increase of ERα occupancy in SMYD2-knockdown cells is due to the loss of ERα–K266 methylation or the loss of H3K4 methylation. First, we performed ChIP experiments using anti-ERα–K266me1 antibody to determine the levels of methylated ERα on EREs. However, we did not detect any signals on the enhancers of GREB1 and PR genes or the promoter of TFF1 gene before or after E2 treatment (SI Appendix, Fig. S7B), suggesting that K266 methylation prevents ERα from binding to chromatin. Next, we performed ChIP experiments using H3K4me2 and H3K4me3 antibodies to determine whether SMYD2 depletion leads to decreased H3K4 methylation levels at the enhancers and promoters of the ERα target genes. However, we found no apparent decrease in H3K4me2 levels at the enhancers of the ERα target genes in SMYD2-knockdown cells compared with the control MCF7 cells (Fig. 4 C and D and SI Appendix, Fig. S7A). Instead, the H3K4me3 levels at the promoters of the ERα target genes were even slightly higher in SMYD2-knockdown cells (Fig. 4 E and F and SI Appendix, Fig. S7A), suggesting that SMYD2 is not responsible for maintaining the histone H3K4 methylation levels at ERα target genes. Together, these results demonstrated that SMYD2 attenuates ERα chromatin recruitment through the methylation of ERα, but not the methylation of histone H3K4.
SMYD2-Mediated Methylation Inhibits ERα K266/268 Acetylation.
The “histone code” hypothesis proposed that modifications on histone can induce or repel interactions with “reader” proteins and can cross-talk with other modifications (24). We speculated that the methylation on nonhistone proteins may function via similar mechanisms. First, we determined whether ERα–K266 methylation is recognized by any reader proteins. We screened >300 reader domains using a chromatin-associated domain array but did not identify any specific readers of ERα–K266me1 (SI Appendix, Fig. S8).
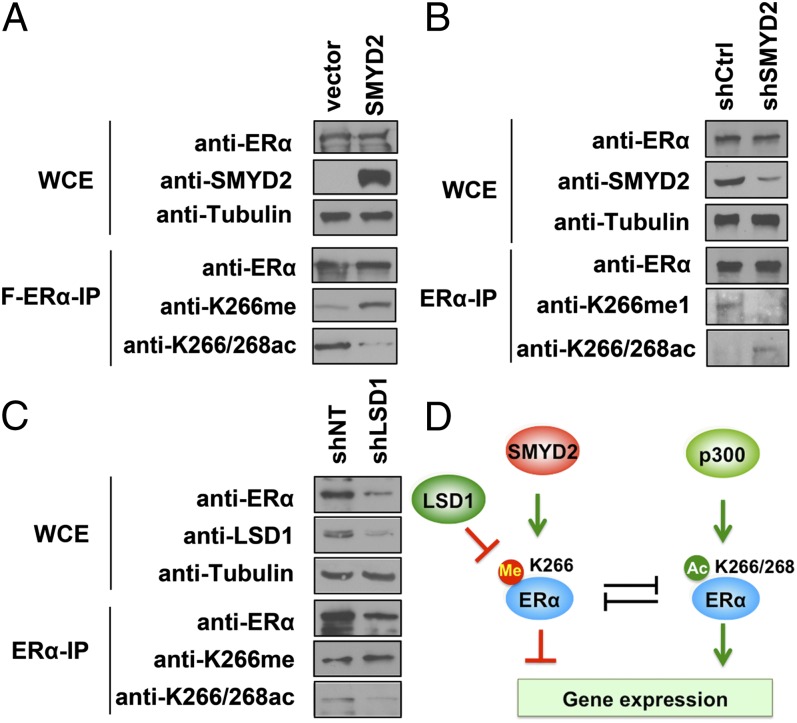
Next, we determined whether K266 methylation could cross-talk with other modifications of ERα protein. ERα–K266 and the neighboring K268 can be acetylated by p300/CBP, which enhances ERα transactivation activity (5). Because SMYD2 methylates ERα at the same residue (K266) and has the opposite function of p300/CBP in regulating ERα transactivation activity, we hypothesized that SMYD2-mediated ERα methylation antagonizes p300/CBP-dependent K266/268 acetylation. To test this hypothesis, we cotransfected HEK 293 cells with Flag-ERα and Myc-SMYD2 and assessed ERα-K266me1 and K266/268 acetylation levels by Western blot analysis. We found that overexpression of SMYD2 resulted in increased K266 methylation and decreased K266/268 acetylation of ERα protein (Fig. 5A), demonstrating the negative correlation between these two modifications. We then sought to determine whether the cross-talk between methylation and acetylation on K266 also occurs on the endogenous ERα protein. We knocked down SMYD2 in MCF7 cells and determined ERα–K266me1 and –K266/268 acetylation levels in the immunoprecipitated endogenous ERα protein. The results revealed that K266 methylation on the endogenous ERα protein was diminished in the SMYD2-knockdown cells. Notably, the decreased K266 methylation was accompanied by increased K266/268 acetylation (Fig. 5B), demonstrating the methylation/acetylation cross-talk on the endogenous ERα protein.
Fig. 5.
SMYD2-mediated methylation inhibits ERα–K266/268 acetylation. (A) Overexpression of SMYD2 increases ERα–K266 methylation and decreases K266/268 acetylation. Western blot analysis of ERα–K266me1 and ERα–K266/268ac and total ERα levels in WCE and Flag-ERα IP of 293T cells transfected with Flag-ERα with or without SMYD2 are shown. (B) Depletion of SMYD2 decreases K266 methylation and increases K266/268 acetylation on endogenous ERα protein. Western blot analysis of ERα–K266me1 and ERα–K266/268ac and total ERα levels in WCE and ERα IP in control and SMYD2-knockdown MCF7 cells are shown. (C) Depletion of LSD1 increases K266 methylation and decreases K266/268 acetylation on endogenous ERα protein. Western blot analysis of ERα–K266me1 and ERα–K266/268ac and total ERα levels in WCE and ERα IP in control and LSD1-knockdown MCF7 cells are shown. (D) Model of ERα–K266 methylation dynamically regulated by SMYD2 and LSD1 and its cross-talk with p300/CBP-dependent ERα–K266/268 acetylation in regulating ERα target gene expression.
LSD1 is the first identified histone demethylase (25). In addition to demethylating histone H3K4, LSD1 has recently been found to remove the methyl groups from p53 and heat shock protein 90 (HSP90); both are methylated by SMYD2 (21, 26). Thus, LSD1 may antagonize SMYD2 in the regulation of methylation dynamics on a variety of protein substrates. Interestingly, LSD1 is known to act as a coactivator of ERα and androgen receptor (27, 28), a function opposite from that of SMYD2. Therefore, we hypothesized that LSD1 could remove the repressive K266-methylation mark on ERα. To test this hypothesis, first we cotransfected 293T cells with LSD1 and Flag-ERα that was premethylated by SMYD2, and we found that overexpression of LSD1 reduced SMYD2-mediated ERα–K266 methylation (SI Appendix, Fig. S9). Next, we knocked down LSD1 in MCF7 cells and determined K266 methylation and K266/268 acetylation levels on endogenous ERα protein. We fond that LSD1 knockdown led to decreased ERα protein levels as well as ERα–K266/268 acetylation levels; in contrast, the levels of ERα–K266 methylation were slightly increased upon LSD1 depletion (Fig. 5C), suggesting that LSD1 negatively regulates ERα–K266 methylation in cells. Together, these results suggest a model in which SMYD2 and LSD1 control the dynamics of ERα–K266 methylation and its cross-talk with K266/268 acetylation, thereby modulating ERα functions in breast cancer cells (Fig. 5D).
Discussion
The regulation of estrogen-induced gene expression is a complex process mediated by ERs. Upon estrogen stimulation, ERα recruits numerous coregulators, including a number of histone-modifying enzymes, which can modify histones to facilitate chromatin relaxation, thereby allowing the binding of ERα and the transcriptional machinery to EREs (17). Increasing evidence suggests that these enzymes can also modify nonhistone proteins such as ERα and other nuclear receptors, thus constituting another layer of regulation of the hormone-responsive gene expression. The first methylation event on ERα was reported a few years ago—that ERα protein is methylated by SET7/9 at K302, the methylation of which stabilizes ERα proteins by inhibiting ERα ubiquitylation and therefore promotes ERα transactivation activity (11). Our study identifies SMYD2-mediated K266 methylation as a previously undescribed inhibitory methylation event on ERa.
SMYD2 was initially identified as a histone methyltransferase that can deposit methyl groups on histones H3K4 and H3K36, two epigenetic marks of active transcription; therefore, SMYD2 was thought to be a transcription coactivator (18, 19). However, recent studies and the findings of the present study suggest that SMYD2 can also methylate diverse nonhistone protein substrates. In contrast to its activity on histones, SMYD2 normally plays an inhibitory role in regulating nonhistone proteins. For instance, SMYD2 directly methylates p53 and pRb and inhibits their transactivation and tumor suppression functions (20, 29, 30). In the present study, we found that SMYD2 was a negative regulator of ERα, and the inhibition of ERα target gene activation by SMYD2 was through the methylation of ERα protein at K266, but not histone H3K4 methylation. These results suggest that the enzymatic activity of SMYD2 toward histones and nonhistone proteins is precisely controlled for transcription activation or repression. However, how the substrate specificity is controlled remains unknown. HSP90 was shown to interact with SMYD2, and this interaction enhances SMYD2 histone methyltransferase activity toward histone H3K4, but not H3K36 (19). HSP90 is known to function as a chaperone of ERα and many other nuclear receptors (31). Interestingly, a recent study revealed that SMYD2 can also methylate HSP90 in the cytoplasm (26). Additional studies are needed to determine whether HSP90 regulates the enzymatic activity of SMYD2 toward ERα protein and histone H3K4 during hormone response.
Our study suggests that the SMYD2-dependent methylation of ERα is reversible and that, similar to methylation of histone H3K4 and p53, the removal of ERα–K266 methylation is likely mediated by LSD1. LSD1 was initially identified as an H3K4 demethylase and was thus thought to be a transcription corepressor (25). However, several studies have demonstrated that LSD1 functions as a transcription coactivator of ERα and androgen receptor (27, 28). It has been proposed that, when associated with nuclear receptors, LSD1 switches its enzymatic activity from H3K4 to H3K9 (27, 32); however, direct biochemical evidence is lacking. Our finding of demethylation of the repressive methylation mark on ERα protein provides an alternative mechanism by which LSD1 activates ERα target gene expression in its demethylation activity-dependent manner.
The hinge region of ERα is a flexible fragment that links the DNA-binding domain to the ligand-binding AF2 domain. The hinge region is heavily posttranslationally modified, indicating a regulatory role in fine-tuning ERα activities (7). ERα–K266 and –K268, as well as –K299, –K302, and –K303, are subject to acetylation and sumoylation (5, 33). In addition, ERα K302 and K303 are ubiquitinated, and K302 is methylated (11, 34). Both acetylation and sumoylation on K266/268 are believed to enhance ERα transactivation activity. In contrast, our study demonstrates that K266 methylation by SMYD2 plays an inhibitory role. Interestingly, we found that knockdown of LSD1, which is known to abolish ERα target gene activation (28), decreased ERα–K266/268 acetylation, whereas knockdown of SMYD2 increased ERα–K266/268 acetylation and the expression of ERα target genes. These results are consistent with a previous observation that ERα–K266/268 acetylation promotes ERα transactivation activity (5). Together, our findings point to a model in which SMYD2 represses ERα target gene activation, at least partly through the inhibition of ERα–K266/268 acetylation. Interestingly, SMYD2 has been shown to interact with the Sin3 homolog A (SIN3A)/HDAC complex (18). Additional studies are needed to determine whether SMYD2 associates with Sin3A/HDAC to maintain ERα protein at a K266-hypoacetylated and -hypermethylated status during the quiescent stage and whether LSD1 cooperates with p300/CBP to facilitate ERα protein acetylation and target gene activation upon estrogen stimulation.
Materials and Methods
In vitro methylation assays were carried out using recombinant proteins and 3H-labelled S-adanosylmathionine at 30 °C for 4 h. Cell culture, RNA interference, ChIP, cell fractionation, co-IP, reverse-transcription PCR, and real-time PCR experiments were performed as described with slight modifications (35). Materials and detailed methods are available in SI Appendix.
Supplementary Material
Acknowledgments
We thank M. T. Bedford, W. L. Kraus, C. M. Chiang, J. Sage, M. Lee, and L. Ma for reagents; D. Weber (University of California Davis Proteomics Facility) for MS data collection; and Joseph Munch for editing the manuscript. This work was supported by American Cancer Society Grant RSG-13-290-01-TBE (to X.S.) Cancer Prevention Research Institute of Texas Grant RP110471 (to X.S.), Welch Grant G1719 (to X.S.), National Institutes of Health/MD Anderson (MDA) Cancer Center Support Grant CA016672 (to X.S.), MDA Institutional Research Grant (to H.W.), the Leukemia Research Foundation (Z.Y.), and Aplastic Anemia & Myelodysplastic Syndromes International Foundation (Z.Y.). X.Z. is a recipient of an MDA Center for Cancer Epigenetics Postdoc Scholar Award, and X.S. is a recipient of a Kimmel Scholar Award.
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1307959110/-/DCSupplemental.
References
- 1.Tsai MJ, O’Malley BW. Molecular mechanisms of action of steroid/thyroid receptor superfamily members. Annu Rev Biochem. 1994;63:451–486. doi: 10.1146/annurev.bi.63.070194.002315. [DOI] [PubMed] [Google Scholar]
- 2.Xu L, Glass CK, Rosenfeld MG. Coactivator and corepressor complexes in nuclear receptor function. Curr Opin Genet Dev. 1999;9(2):140–147. doi: 10.1016/S0959-437X(99)80021-5. [DOI] [PubMed] [Google Scholar]
- 3.Xu J, Wu RC, O’Malley BW. Normal and cancer-related functions of the p160 steroid receptor co-activator (SRC) family. Nat Rev Cancer. 2009;9(9):615–630. doi: 10.1038/nrc2695. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Perissi V, Jepsen K, Glass CK, Rosenfeld MG. Deconstructing repression: Evolving models of co-repressor action. Nat Rev Genet. 2010;11(2):109–123. doi: 10.1038/nrg2736. [DOI] [PubMed] [Google Scholar]
- 5.Kim MY, Woo EM, Chong YT, Homenko DR, Kraus WL. Acetylation of estrogen receptor alpha by p300 at lysines 266 and 268 enhances the deoxyribonucleic acid binding and transactivation activities of the receptor. Mol Endocrinol. 2006;20(7):1479–1493. doi: 10.1210/me.2005-0531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Wang C, et al. Direct acetylation of the estrogen receptor alpha hinge region by p300 regulates transactivation and hormone sensitivity. J Biol Chem. 2001;276(21):18375–18383. doi: 10.1074/jbc.M100800200. [DOI] [PubMed] [Google Scholar]
- 7.Le Romancer M, et al. Cracking the estrogen receptor’s posttranslational code in breast tumors. Endocr Rev. 2011;32(5):597–622. doi: 10.1210/er.2010-0016. [DOI] [PubMed] [Google Scholar]
- 8.Michalides R, et al. Tamoxifen resistance by a conformational arrest of the estrogen receptor alpha after PKA activation in breast cancer. Cancer Cell. 2004;5(6):597–605. doi: 10.1016/j.ccr.2004.05.016. [DOI] [PubMed] [Google Scholar]
- 9.Yamashita H, et al. Low phosphorylation of estrogen receptor alpha (ERalpha) serine 118 and high phosphorylation of ERalpha serine 167 improve survival in ER-positive breast cancer. Endocr Relat Cancer. 2008;15(3):755–763. doi: 10.1677/ERC-08-0078. [DOI] [PubMed] [Google Scholar]
- 10.Jiang J, et al. Phosphorylation of estrogen receptor-alpha at Ser167 is indicative of longer disease-free and overall survival in breast cancer patients. Clin Cancer Res. 2007;13(19):5769–5776. doi: 10.1158/1078-0432.CCR-07-0822. [DOI] [PubMed] [Google Scholar]
- 11.Subramanian K, et al. Regulation of estrogen receptor alpha by the SET7 lysine methyltransferase. Mol Cell. 2008;30(3):336–347. doi: 10.1016/j.molcel.2008.03.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Le Romancer M, et al. Regulation of estrogen rapid signaling through arginine methylation by PRMT1. Mol Cell. 2008;31(2):212–221. doi: 10.1016/j.molcel.2008.05.025. [DOI] [PubMed] [Google Scholar]
- 13.Wang H, et al. Methylation of histone H4 at arginine 3 facilitating transcriptional activation by nuclear hormone receptor. Science. 2001;293(5531):853–857. doi: 10.1126/science.1060781. [DOI] [PubMed] [Google Scholar]
- 14.Strahl BD, et al. Methylation of histone H4 at arginine 3 occurs in vivo and is mediated by the nuclear receptor coactivator PRMT1. Curr Biol. 2001;11(12):996–1000. doi: 10.1016/s0960-9822(01)00294-9. [DOI] [PubMed] [Google Scholar]
- 15.Koh SS, Chen D, Lee YH, Stallcup MR. Synergistic enhancement of nuclear receptor function by p160 coactivators and two coactivators with protein methyltransferase activities. J Biol Chem. 2001;276(2):1089–1098. doi: 10.1074/jbc.M004228200. [DOI] [PubMed] [Google Scholar]
- 16.Yang Y, et al. TDRD3 is an effector molecule for arginine-methylated histone marks. Mol Cell. 2010;40(6):1016–1023. doi: 10.1016/j.molcel.2010.11.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Wu SC, Zhang Y. Minireview: Role of protein methylation and demethylation in nuclear hormone signaling. Mol Endocrinol. 2009;23(9):1323–1334. doi: 10.1210/me.2009-0131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Brown MA, Sims RJ, 3rd, Gottlieb PD, Tucker PW. Identification and characterization of Smyd2: A split SET/MYND domain-containing histone H3 lysine 36-specific methyltransferase that interacts with the Sin3 histone deacetylase complex. Mol Cancer. 2006;5:26. doi: 10.1186/1476-4598-5-26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Abu-Farha M, et al. The tale of two domains: Proteomics and genomics analysis of SMYD2, a new histone methyltransferase. Mol Cell Proteomics. 2008;7(3):560–572. doi: 10.1074/mcp.M700271-MCP200. [DOI] [PubMed] [Google Scholar]
- 20.Huang J, et al. Repression of p53 activity by Smyd2-mediated methylation. Nature. 2006;444(7119):629–632. doi: 10.1038/nature05287. [DOI] [PubMed] [Google Scholar]
- 21.Huang J, et al. p53 is regulated by the lysine demethylase LSD1. Nature. 2007;449(7158):105–108. doi: 10.1038/nature06092. [DOI] [PubMed] [Google Scholar]
- 22.Qian C, Zhou MM. SET domain protein lysine methyltransferases: Structure, specificity and catalysis. Cell Mol Life Sci. 2006;63(23):2755–2763. doi: 10.1007/s00018-006-6274-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kim H, et al. Requirement of histone methyltransferase SMYD3 for estrogen receptor-mediated transcription. J Biol Chem. 2009;284(30):19867–19877. doi: 10.1074/jbc.M109.021485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Strahl BD, Allis CD. The language of covalent histone modifications. Nature. 2000;403(6765):41–45. doi: 10.1038/47412. [DOI] [PubMed] [Google Scholar]
- 25.Shi Y, et al. Histone demethylation mediated by the nuclear amine oxidase homolog LSD1. Cell. 2004;119(7):941–953. doi: 10.1016/j.cell.2004.12.012. [DOI] [PubMed] [Google Scholar]
- 26.Abu-Farha M, et al. Proteomic analyses of the SMYD family interactomes identify HSP90 as a novel target for SMYD2. J Mol Cell Biol. 2011;3(5):301–308. doi: 10.1093/jmcb/mjr025. [DOI] [PubMed] [Google Scholar]
- 27.Metzger E, et al. LSD1 demethylates repressive histone marks to promote androgen-receptor-dependent transcription. Nature. 2005;437(7057):436–439. doi: 10.1038/nature04020. [DOI] [PubMed] [Google Scholar]
- 28.Garcia-Bassets I, et al. Histone methylation-dependent mechanisms impose ligand dependency for gene activation by nuclear receptors. Cell. 2007;128(3):505–518. doi: 10.1016/j.cell.2006.12.038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Saddic LA, et al. Methylation of the retinoblastoma tumor suppressor by SMYD2. J Biol Chem. 2010;285(48):37733–37740. doi: 10.1074/jbc.M110.137612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Cho HS, et al. RB1 methylation by SMYD2 enhances cell cycle progression through an increase of RB1 phosphorylation. Neoplasia. 2012;14(6):476–486. doi: 10.1593/neo.12656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Pratt WB, Welsh MJ. Chaperone functions of the heat shock proteins associated with steroid receptors. Semin Cell Biol. 1994;5(2):83–93. doi: 10.1006/scel.1994.1012. [DOI] [PubMed] [Google Scholar]
- 32.Metzger E, et al. Phosphorylation of histone H3T6 by PKCbeta(I) controls demethylation at histone H3K4. Nature. 2010;464(7289):792–796. doi: 10.1038/nature08839. [DOI] [PubMed] [Google Scholar]
- 33.Sentis S, Le Romancer M, Bianchin C, Rostan MC, Corbo L. Sumoylation of the estrogen receptor alpha hinge region regulates its transcriptional activity. Mol Endocrinol. 2005;19(11):2671–2684. doi: 10.1210/me.2005-0042. [DOI] [PubMed] [Google Scholar]
- 34.Berry NB, Fan M, Nephew KP. Estrogen receptor-alpha hinge-region lysines 302 and 303 regulate receptor degradation by the proteasome. Mol Endocrinol. 2008;22(7):1535–1551. doi: 10.1210/me.2007-0449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Wen H, et al. Recognition of histone H3K4 trimethylation by the plant homeodomain of PHF2 modulates histone demethylation. J Biol Chem. 2010;285(13):9322–9326. doi: 10.1074/jbc.C109.097667. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.