Significance
The developing nervous systems of birds and mammals exhibit rhythmic waves of electrical activity, but their importance in early circuit formation events, such as initial axon pathfinding, has been unclear. By using flashes of light to activate exogeneously expressed light-sensitive ion channels in intact chick embryos, we show that the two major motoneuron pathfinding decisions (dorsal-ventral and muscle-specific) were differentially sensitive to the precise frequency of waves, with accurate pathfinding requiring the normal control frequency. Thus, many drugs that are known to alter wave frequency in this circuit have the potential to disrupt normal motor circuit formation.
Keywords: axonal guidance, spinal cord development, motoneuron development, spontaneous neural activity
Abstract
Rhythmic waves of spontaneous electrical activity are widespread in the developing nervous systems of birds and mammals, and although many aspects of neural development are activity-dependent, it has been unclear if rhythmic waves are required for in vivo motor circuit development, including the proper targeting of motoneurons to muscles. We show here that electroporated channelrhodopsin-2 can be activated in ovo with light flashes to drive waves at precise intervals of approximately twice the control frequency in intact chicken embryos. Optical monitoring of associated axial movements ensured that the altered frequency was maintained. In embryos thus stimulated, motor axons correctly executed the binary dorsal-ventral pathfinding decision but failed to make the subsequent pool-specific decision to target to appropriate muscles. This observation, together with the previous demonstration that slowing the frequency by half perturbed dorsal-ventral but not pool-specific pathfinding, shows that modest changes in frequency differentially disrupt these two major pathfinding decisions. Thus, many drugs known to alter early rhythmic activity have the potential to impair normal motor circuit development, and given the conservation between mouse and avian spinal cords, our observations are likely relevant to mammals, where such studies would be difficult to carry out.
Propagating waves of spontaneous, rhythmic electrical activity are widespread in the developing nervous systems of birds and mammals (1) and in the visual system contribute to the refinement of central connections (2). Spontaneous waves also occur in the developing spinal cords of birds and mammals and the networks that generate this activity exhibit many similarities. However, their role in motor circuit development, including initial axon pathfinding, has only recently begun to be explored. The extent to which the frequency of waves/bursting episodes affects the development of in vivo motor circuits can be studied by experimentally varying frequency. However, because of homeostatic mechanisms that restore frequency toward normal (3–5), it is essential to ensure that the altered frequency is maintained throughout the desired time window. The chicken embryo presents two unique advantages for studying the effect of altering the frequency of activity. First, it enables in vivo activation of channelrhodopsin 2 (ChR2) to drive waves of bursting activity at precise frequencies. Second, because each wave causes an S-shaped movement of the embryo’s trunk, the interval between waves can be precisely characterized during chronic stimulation (6).
Embryonic chick spinal cords generate waves of spontaneous bursting activity while motoneurons are still pathfinding to their targets (4), and these are driven by acetylcholine, by GABA acting on GABAA receptors, and by glycine, all three neurotransmitters being excitatory at these developmental stages (4). To determine the significance of bursting activity for motoneuron pathfinding, its frequency was previously decreased in ovo by picrotoxin, a GABAA receptor antagonist. Motoneurons were observed to make dorsal-ventral (D-V) pathfinding errors at the base of the limb and to express lower levels of ephrin (Eph)A4, EphB1, and polysiliac acid (PSA) (6, 7), critical guidance molecules for D-V pathfinding (8–10). We subsequently showed that altering the frequency of bursting episodes, and not GABAA signaling, caused these D-V pathfinding errors (6).
In contrast, when bursting frequency was increased by sarcosine, a glycine uptake inhibitor, D-V pathfinding was unaffected but motor axons made apparent pool-specific pathfinding errors (11). To conclusively show that increased bursting frequency rather than enhanced glycine signaling caused the pool-specific pathfinding errors, we electroporated embryonic cords with ChR2 to drive bursting activity at twice the control frequency, similar to that achieved with sarcosine. We were able to chronically drive activity at this higher-than-normal frequency in intact embryos, resulting in pool-specific but not D-V pathfinding errors. The pool-specific nature of these errors was shown by characterizing the expression of Er81, an E26 (ETS) transcription factor expressed in specific motoneuron pools (12), and by motoneuron pool-specific bursting patterns (11). Thus, the normal in vivo bursting frequency rather than glycine signaling is necessary for motoneurons to target to their appropriate muscles. Furthermore, rather than simply requiring a threshold level of activity, motoneurons in vivo were highly sensitive to the precise bursting frequency with reductions below (6) or increases above (present study) the control frequency, resulting in D-V or pool-specific pathfinding errors, respectively.
Results
Electroporated ChR2 Can Drive Neural Activity at Twice the Normal Frequency During in Vivo Motor Axon Outgrowth.
To test if increasing the frequency of activity alone results in improper motoneuron pool-specific pathfinding, we increased bursting frequency by light activation of ChR2. Although acute in ovo light activation of ChR2 could drive activity at twice the normal frequency (6), it was necessary to show that the increased frequency could be maintained during both the D-V and pool-specific pathfinding decisions [stages (St) 23–26].
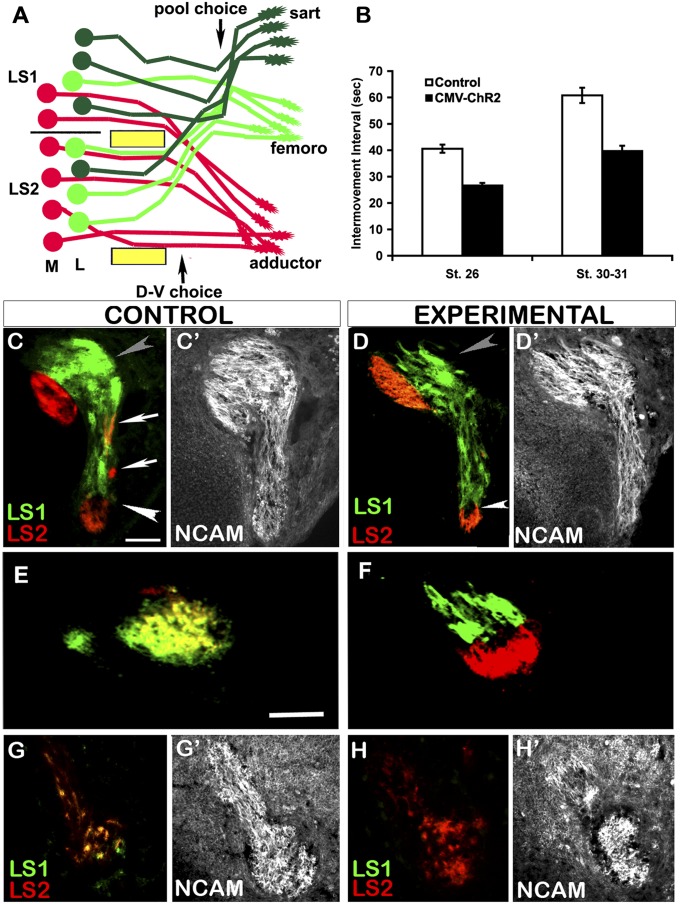
As motor axons extend into the limb, they are guided by a series of attractive and repulsive molecular guidance cues (13). Just before the convergence of lumbosacral (LS) spinal nerves 1–3 in the crural plexus at the base of the limb, axons of motoneurons located laterally in the lateral motor column (LMCL) defasciculate from those of medially located motoneurons (LMCM) and project dorsally into dorsal mesenchyme; the LMCM axons project ventrally to the adductor muscles via the obturator trunk (Fig. 1A). Just distal to this D-V choice, axons belonging to different pools resort into pool-specific fascicles, which project to regions of mesenchyme from which specific muscles will arise, making the pool-specific pathfinding choice (14–16). Spontaneous bursting activity has been recorded from limb-innervating motor fascicles from St 23 (4) but, because it might occur earlier, light treatment was begun at St 20. In St 23–25 isolated cord-limb preparations, each wave of activity (spontaneous or generated by electrical or light stimulation) activates axial muscles and generates an S-shaped body movement that can be monitored in vitro and in intact embryos (6, 7, 17). Importantly, as a reporter of spontaneous waves occurring in intact developing embryos, visualization of these in ovo movements enabled us to precisely characterize the pattern and frequency of bursting episodes, which we express here as intermovement intervals (Fig. 1B).
Fig. 1.
Light activation of ChR2 increases bursting frequency and results in segmental misprojections and altered axonal fasciculation. (A) Schematic showing the D-V and subsequent pool-specific pathfinding decisions made by sartorius (dark green), femorotibialis (light green), and adductor (red) motoneurons at base of limb. (B) Bar graph showing intervals between episodes of axial movements in controls and embryos chronically activated by light between St 20–26 (n = 4) or St 20–St 30/31 (n = 4) (mean ± SEM). (C–H) Transverse limb sections showing axons orthogradely labeled from LS1 with Di-Asp (green) and LS2 with Di-I (red) in control and stimulated embryos. (C and D) At D-V, choice point gray and white arrowheads indicate dorsal and ventral trunks, respectively. White arrows in C show intermingling of LS1 and LS2 axons in control. (E and F) Axons from LS1 and LS2 in control intermingle extensively in the ventral obturator nerve trunk but remain as separate fascicles in the experimental embryo. (G) More distally, both LS1 and LS2 axons contribute to the femorotibialis nerves and became extensively intermingled as shown by colocalization of red and green dye (yellow) in controls. (H) After treatment in some embryos, as in this case, only LS2, contributed. (C′, D′, E′, F′, G′, and H′) Same sections as shown in C, D, E, F, G, and H immunostained with NCAM antibody to reveal the entire nerve pattern. In C–H, dorsal is up, anterior is right. (Scale bars, 50 μm.)
In embryonic chick cords, an episode of activity occurs when the network of recurrent excitatory connections reaches a threshold for generating a propagating wave of activity. Following each episode is a period of network depression when it is difficult to elicit another episode (18), recovery from depression largely determining the length of the interepisode interval (19). As the embryo matures the intervals between episodes increase (4, 18). This circuit thus produces highly rhythmic episodes of activity throughout development.
By monitoring axial in ovo movements, we found that St 26 embryos had highly rhythmic activity, with an episode occurring every 40.6 ± 1.5 s (mean ± SEM) (Fig. 1B). At St 30–31, episodes occurred every 60.8 ± 2.9 s (mean ± SEM). Embryos were activated by light from St 20 until either St 26 or St 31. Although network properties, such as the time to recover from depression (19), prevented us from driving activity at precisely twice the normal frequency, we were able to elicit episodes close to this, specifically every 26.6 ± 0.9 s (mean ± SEM) for St 26 embryos and 39.7 ± 2.0 s (mean ± SEM) for St 30–31 embryos, with little variation in interepisode intervals at either stage (Fig. 1B). Previous control experiments showed that expression of ChR2 had no effect on the frequency of spontaneous activity in the absence of light stimulation (6, 17). Additionally, because ChR2 was electroporated at the cervical level, lumbar motoneurons were activated by descending waves of activity, previously shown to not differ detectably from those generated spontaneously (17). This process avoided potential complications from chronic light stimulation of the motoneurons under study.
Increasing Bursting Frequency Results in Segmental Pathfinding Errors and Prevents the Rearrangement of Axons into Pool-Specific Fascicles.
When bursting frequency was increased by the glycine uptake inhibitor, sarcosine, lumbar motoneurons did not make D-V pathfinding errors, but did misproject along the A-P axis, making apparent pool-specific pathfinding errors (11). To characterize segmental motoneuron projections, individual spinal nerves LS1, LS2, or LS3 were injected in pair-wise combinations with Di-I or Di-Asp, and the labeled axons traced to their termination sites in the limb bud.
Injection of LS1 with Di-Asp and LS2 with Di-I (Fig. 1 C and D) revealed that at the D-V choice point, both spinal nerves contributed to the dorsal and ventral nerve trunks in control (n = 4) as well as stimulated (n = 6) embryos, but in the stimulated embryos axons originating in different LS segments were less intermingled in both spinal nerves and dorsal nerve trunks compared with controls (Fig. 1 C and D). Similarly, LS1 and LS2 axons became extensively intermingled more distally in the ventral obturator nerve trunk in controls, but remained as distinct spinal nerve-specific fascicles after treatment (Fig. 1 E and F). This finding suggests that increasing bursting frequency interferes with the ability of motor axons to reorganize in response to pool-specific guidance cues, the first step in sorting into pool-specific fascicles (15, 20). In this process, axons from different spinal nerves but belonging to the same motoneuron pool come together to form pool-specific fascicles within the major dorsal and ventral nerve trunks, as shown in Fig. 1A.
By following labeled axons distally, we detected segmental projection errors in some of the embryos in which bursting frequency was increased. Although both LS1 and LS2 always contributed to the sartorius nerve in controls, in three of six light-activated embryos LS2 axons did not contribute. Similarly, LS1 failed to project to the femorotibialis nerve in three of six light-activated embryos (Fig. 1 G and H and Table S1). To better confirm the existence of pool-specific pathfinding errors, we further characterized motoneuron projections via retrograde labeling from muscles.
ChR2-Stimulated Embryos Exhibit Pool-Specific Misprojections Based on Somal Location and Er81 Expression.
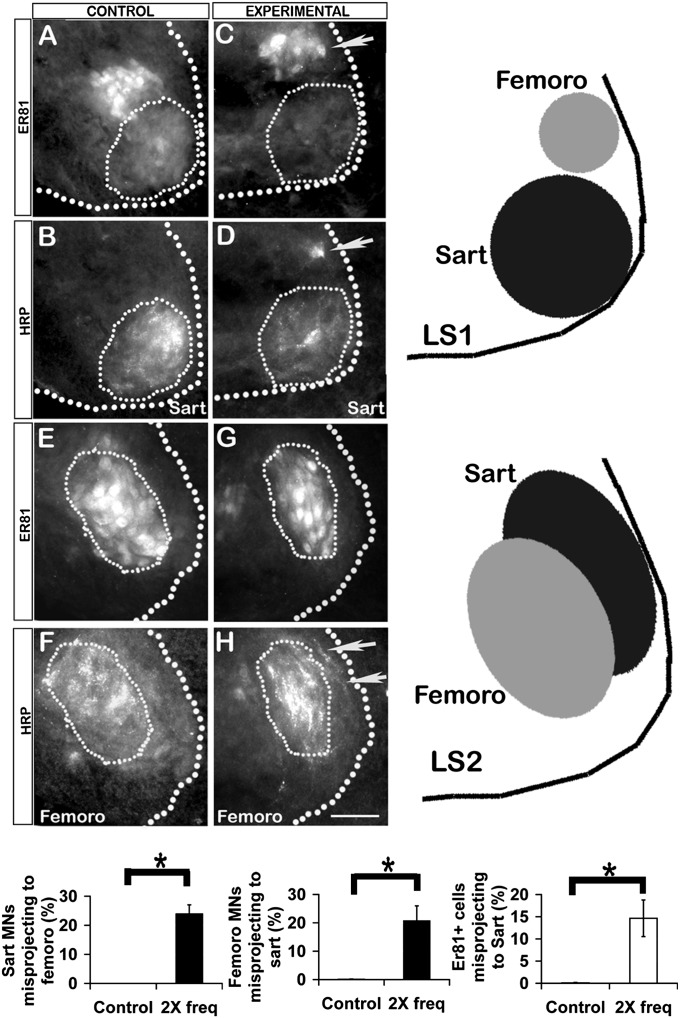
Motoneuron pools occupy stereotyped positions within the transverse plane of the spinal cord (16) and some pools can be distinguished by molecular markers. For example, the femorotibialis pool expresses the transcription factor Er81, whereas the sartorius pool does not (12) (Fig. S1), and in LS1 the femorotibialis pool is located dorsal to the sartorius pool, whereas in LS2 it is located more laterally (Fig. 2 A, B, E, F, and schematic at right). In St 29 controls all motoneurons labeled from the sartorius muscle were within the sartorius pool boundary and did not express Er81 (Fig. 2 A and B). In light-stimulated embryos, some HRP labeled motoneurons were within the femorotibialis pool (Fig. 2D, arrow) and expressed the femorotibialis pool marker Er81 (Fig. 2C, arrow). Similarly in controls, all motoneurons labeled from the femorotibialis muscle were within that pool’s boundary (Fig. 2F) and expressed Er81 (Fig. 2E). In light-activated embryos, some motoneurons labeled from the femorotibialis were located more laterally in the position of the sartorius pool (Fig. 2H, arrows); these did not express Er81 and thus, by this criterion, were sartorius and not femorotibialis motoneurons.
Fig. 2.
The location of HRP+ and Er81+ motoneuron somas after retrograde labeling from St 30–31 sartorius or femorotibialis muscles in controls and light-activated (experimental) embryos. Motoneurons retrogradely labeled with HRP from the sartorius in control (B) and experimental embryo (D). Thin dotted line denotes the normal sartorius motoneuron pool boundary at LS1. (A and C) Same sections showing Er81 expression. Arrows (C and D) show misplaced motoneuron expressing Er81. (F and H) Motoneurons labeled from femorotibialis in control and experimental embryo. Thin dotted line denotes normal femorotibialis pool boundary at LS2. Arrows in H show misplaced motoneurons. (E and G) Same sections showing that these misplaced motoneurons do not express Er81. (Scale bar, 30 µm.) In A–H, the thick dotted line shows lateral edge of gray matter; dorsal is up, lateral is right. (Right) Schematic shows sartorius and femorotibialis pool locations at LS1 and LS2. (Bottom) Left and Center bar graphs show proportion of sartorius or femorotibilais motoneurons, respectively, that wrongly projected to the other muscle in controls and embryos activated at twice the normal frequency (n = 3, *P < 0.05 for both). Right bar graph shows proportion of motoneurons retrogradely labeled from sartorius that were Er81-positive (n = 3, *P < 0.05).
When these data were quantified and combined (Fig. 2, bar graphs), 24.1% of the back-labeled sartorius motoneurons had misprojected to the femorotibialis muscle based on somal location (Fig. 2, Left graph, n = 4), and 22.7% of the femorotibialis motoneurons had misprojected to the sartorius (Fig. 2, Center graph, n = 3). The proportion of motoneurons projecting to the sartorius that were Er81-positive was 17 ± 9% (Fig. 2, Right bar graph). In three control embryos no misprojecting motoneurons were detected. Following treatment, motoneurons labeled by injecting the sartorius or femorotibialis muscles were within the LMCL as in controls and not the LMCM, indicating that increasing the frequency of activity did not perturb D-V pathfinding.
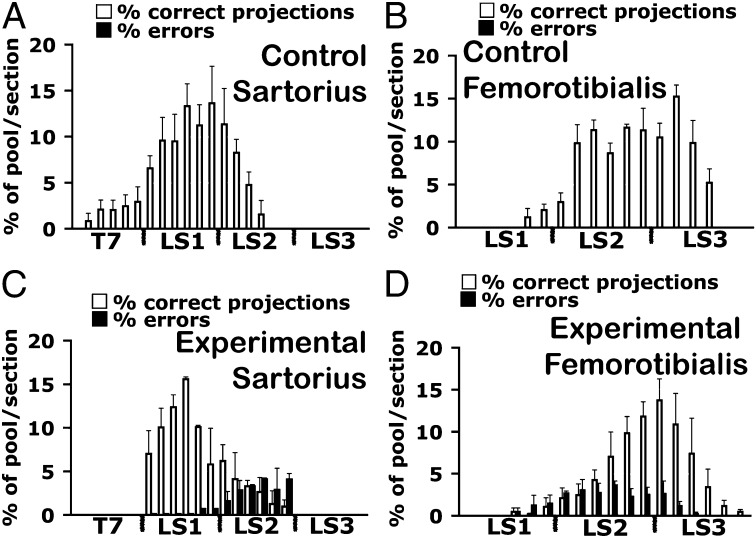
The segmental distribution of motoneurons that had made pool-specific errors is shown in more detail in Fig. 3, where the percent of sartorius or femorotibialis motoneurons that had projected either appropriately (white bars) or inappropriately (black bars) is plotted for every third section from T7 through LS3. The sartorius muscle is innervated by motoneurons in LS1 and the anterior part of LS2, with a minor contribution from T7 (Fig. 3A). The femorotibialis is innervated by LS2 and LS3 with a small contribution from LS1 (Fig. 3B). After light treatment, femorotibialis motoneurons from posterior L1 and throughout LS2 misprojected to the sartorius muscle (black bars in Fig. 3C). In addition, the T7 contribution to the sartorius was lost (Fig. 3C). Motoneurons within the sartorius pool in LS1 and LS2 also misprojected to the femorotibialis (black bars in Fig. 3D). Taken together, these data provide evidence of ∼20% of motoneurons making pool-specific misprojections when bursting frequency was increased. Although we focused here on two motoneuron pools, our previous study (11) indicated that many if not all motoneuron pools, including those in the sciatic portion of the cord, make A-P/pool-specific pathfinding errors.
Fig. 3.
Segmental distribution of motoneurons properly (white bars) or improperly (black bars) projecting to the sartorius and the femorotibialis muscles in control and light-activated embryos. Histograms of the rostrocaudal locations of motoneuron somas in cross-sections of the spinal cord after retrograde labeling with HRP from the control sartorius (A) or femorotibialis (B) muscles as a percentage of the total HRP labeled cells. In controls, all motoneurons were located in the appropriate pool position within the spinal cord (n = 3 in A; n = 4 in B). (C and D) In treated embryos a proportion of motoneurons (black bars) in LS1 and LS2 were found in inappropriate pool locations for the sartorius (n = 3) and the femorotibialis (n = 4).
Pool-Specific Bursting Patterns Confirm the Occurrence of Pool-Specific Pathfinding Errors.
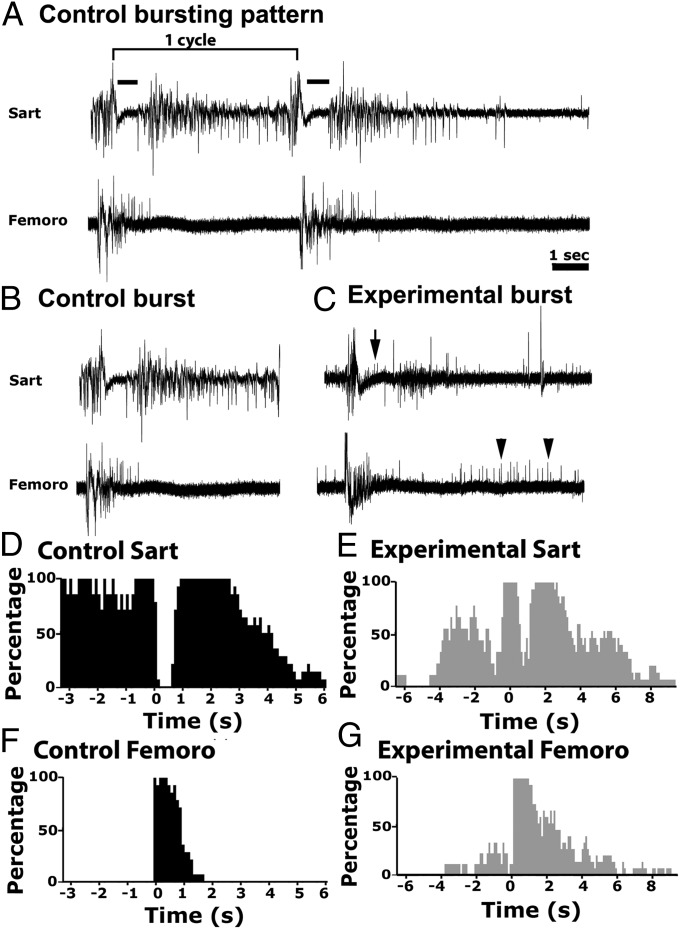
Individual chick motoneuron pools exhibit highly stereotyped episodes of activity recorded in isolated spinal cord preparations or in ovo (21). Because these bursting patterns are autonomous to each pool and are not altered when motoneurons are experimentally caused to innervate a foreign muscle (22), they can be used to determine pool identity. The sartorius, a flexor, and the femorotibialis, an extensor, are normally activated out of phase and each has a distinct bursting pattern. Using suction electrode recordings from these muscles, we compared bursting patterns in control and ChR2-stimulated embryos between St 31–33 (Fig. 4 A–C) when each episode consists of between three and four bursts or cycles. Two cycles of typical bursts from a control sartorius (Upper) and femorotibialis (Lower) muscle are shown in Fig. 4A. At the onset of each cycle the two muscles are activated simultaneously, after which the sartorius muscle undergoes an inhibitory period (Fig. 4, black bar), and the femorotibialis muscle continues to fire. Subsequently the femorotibialis burst terminates, and after its inhibitory period the sartorius muscle resumes bursting, often continuing until the onset of the next cycle (Fig. 4B). In contrast, in stimulated embryos, the sartorius muscle exhibited unit activity (Fig. 4B, black arrow) during its normal inhibitory period (Fig. 4C, Upper), and the femorotibialis muscle exhibited unit activity when it is usually silent (Fig. 4C, Lower, black arrowheads).
Fig. 4.
Electromyograms of bursting patterns from sartorius and femorotibialis muscles in control and light treated embryos. (A) Traces of two consecutive bursts within a bursting episode recorded simultaneously from the sartorius (Upper) and the femorotibialis (Lower) muscles of a stage 33.5 control embryo. The bracket above the first burst indicates one cycle of activity. The sartorius inhibitory period is shown by the black bars. (Scale bar, 1 s.) (B and C) A single burst from a control (B) and treated (C) embryo. Arrow and arrowheads indicate motoneurons firing at inappropriate times in the experimental sartorius and femorotibialis, respectively. (D–G) Histograms of traces from two St 34 embryos, a control (D and F, n = 7) and a light-activated embryo (E and G, n = 6) showing the probability of a muscle firing during the cycle preceding and subsequent to the 0 time point of a given cycle. Following treatment, motoneurons fire at inappropriate times during a cycle, consistent with motoneurons innervating wrong muscles (see text for further explanation). All six light-treated embryos examined exhibited motoneurons firing at inappropriate times whereas none of the five controls did.
Based on previous studies (11, 22), these observations suggest that some femorotibialis motoneurons have projected to the sartorius muscle and fire during its normal inhibitory period. We similarly interpret the units firing after the normal femorotibialis burst would have terminated, as sartorius motoneurons that have inappropriately projected to the femorotibialis muscle. Histograms in which multiple bursts are pooled, and which indicate the proportion of times each muscle is active during any 100-ms bin preceding or subsequent to the beginning of a given cycle, demonstrate the distinct and out-of-phase bursting patterns of these two muscles in control embryos (Fig. 4 D and F). In contrast, after treatment the sartorius exhibited activity during the usually silent inhibitory period (Fig. 4E), and the femorotibialis exhibited activity both preceding and following its normal burst period (Fig. 4G). Thus, we conclude that this aspect of the sartorius and femorotibialis motoneuron pool identity was not altered by increasing bursting frequency, supporting our observations that used Er81 expression to define pool identity.
Discussion
The present study shows that ChR2-mediated increases in in vivo motoneuron bursting frequency produced segmental pathfinding errors similar to sarcosine treatment (11) and confirmed that these were pool-specific in nature, indicating the importance of maintaining the precise control bursting frequency rather than normal glycinergic signaling for proper motoneuron pool-specific pathfinding. The distinct type of pathfinding errors (D-V versus pool-specific) produced by moderate decreases versus increases in bursting frequency demonstrates that this circuit is exquisitely sensitive to bursting frequency and that normal pathfinding does not simply require a threshold level of activity. These observations strongly suggest that different downstream signaling pathways must be activated in the two cases.
In various systems both the frequency and pattern of Ca2+ transients, as well as the source of Ca2+, are known to activate different patterns of gene transcription and intracellular signaling pathways (23–26). The frequency of activity has also been shown to modulate some aspects of spinal cord circuit formation, including transmitter phenotype (see ref. 27 for review). We have found that as chick motoneurons are making their D-V and pool-specific pathfinding choices, spontaneous bursts generate Ca2+ transients primarily via Ca2+ entry through T- and L-type Ca2+ channels with little contribution from internal stores. Importantly, the drugs used previously to increase or decrease burst frequency and thus to cause either pool-specific or D-V pathfinding errors, only altered the frequency of the Ca2+ transients and not their duration or amplitude (28). For proper D-V pathfinding, EphA4 on dorsally projecting motor axons and EphB1 on ventrally projecting ones are required for growth cones to respond to the corresponding inhibitory ligands, thus preventing axon extension into inappropriate D-V limb regions (8, 9). PSA on neural cell-adhesion molecule (NCAM) is also required to reduce axon-axon fasciculation and allow both dorsally and ventrally projecting axons to respond to their respective D-V guidance cues (10, 29). It is interesting that EphA4, EphB1, and PSA were all down-regulated on distal axons by slowing bursting with picrotoxin and rescued by driving activity at normal levels with ChR2 (6, 11), suggesting some coordinate form of regulation. Although the transcription of neural genes can be regulated by activity (30, 31), the down-regulation of EphA4 on distal axons following reduced activity was not accompanied by a reduction in EphA4 message or protein in the soma (7). Furthermore, although the phenotypic differentiation of LMCL and LMCM motoneurons, including their expression of EphRs, is regulated by the differential expression of the Islet-1 and Lim-1 genes (8, 32), the downstream pathways linking these transcription factors to the regulation of EphRs is unknown. Changes in activity did alter the expression of these LIM transcription factors in motoneurons (6, 7), but only after the D-V pathfinding errors had been made.
Alternatively, activity might affect local protein translation in growth cones, which is necessary for some pathfinding decisions (33), or affect the insertion or removal of proteins required for D-V pathfinding from growth cone membranes (34). Increasing burst frequency with ChR2 selectively affected pool-specific pathfinding. Although Hox genes and their cofactor FoxP1 have been shown to regulate pool-specific identity (35, 36), the guidance molecules and receptors for pool-specific pathfinding are unknown. The ability of motoneurons displaced by early embryonic surgery (20), or of axons that have made pathfinding errors in the plexus following alterations of activity, to home in on their target muscles by taking novel pathways within the limb (11), suggests that they are responding to target-derived diffusible cues. Evidence supports FGF as a diffusible chemoattractant for medial motor column epaxial motoneurons (37) but similar chemoattractants for limb motoneurons have not yet been identified. We showed here that increasing the frequency of activity did not alter motoneuron pool identity as judged by either pool-specific bursting patterns (21, 22) or the expression of Er81 (12). We previously showed that axons that had made pathfinding errors at the plexus region were rapidly able to respond to presumed target-derived cues and reach their muscles by novel trajectories when activity was allowed to recover to normal levels (11). Thus, increased activity may simply prevent growth cones from responding to target-derived cues by interfering with some intervening signaling event within the growth cone (for examples, see ref. 34). In culture, electrically activating Xenopus growth cones altered, within minutes, their ability to respond to diffusible guidance cues (38). Periodic activity of the appropriate frequency is required for Drosophila motoneurons to retract from inappropriate muscles in response to a semaphorin-mediated signal (39). A recent in vivo study also showed that zebrafish primary motoneurons required spontaneous Ca2+ transients to pathfind accurately (40). However, both the characteristics of the spontaneous activity and the downstream cellular/molecular mechanisms differ from those in chick cord, suggesting possible species and cell-type diversity and the need to study these events in multiple organisms.
Our results suggest that when growth cones are unable to detect or respond to target-derived guidance cues, they project down nonspecific, permissive pathways and make inappropriate but functionally effective synapses with foreign muscles (11, 20, and present study). This finding might explain why axons from individual spinal nerves failed to defasciculate and regroup into target-specific fascicles when the frequency of activity was increased with ChR2 (11 and present study), because responses to such cues have been suggested to underlie these axonal rearrangements (41). Alternatively, molecules possibly involved with selective fasciculation and segregation of subsets of motoneurons, such as semaphorins (42), may have been affected. Altering the amount of spontaneous activity in developing spinal cord circuits can trigger homeostatic changes that restore activity toward normal (3–5), and can also alter the proportion of neurons expressing excitatory versus inhibitory transmitters (27). We did not observe such changes when decreasing (7) or increasing (6 and present study) the frequency of spontaneous bursting episodes. The modest amounts by which we altered frequency, although detected by the motoneurons and resulting in axonal pathfinding errors, may have been insufficient to trigger these compensatory changes.
Given the robust and divergent alterations in motoneuron pathfinding in response to either increasing or decreasing burst frequency, and our ability to precisely control burst frequency in vivo, this system should be an excellent one for discovering the intracellular signaling pathways underlying these divergent activity dependent responses, and for further elucidating the role of rhythmic spontaneous activity in neural circuit formation. A major advantage of this approach over others, such as altering activity in an inexact way via exogenous expression of K+ or Na+ channels, is the precise control over the pattern of bursts, allowing determination of the extent to which frequency versus pattern and rhythmicity are important. Finally, the fact that the two major pathfinding decisions made by motoneurons, as well as their expression of homeodomain transcription factors were affected by modest alterations in spontaneous burst frequency, suggests that many drugs—including prescribed medications, which affect cholinergic, GABAergic, or glycinergic transmission—could all have deleterious effects on circuit formation in developing embryos, including human.
Materials and Methods
Light Activation of Bursts by ChR2 and Quantification of in Ovo Movements.
Methods for light activation of ChR2 at the cervical level to drive propagating bursts at precise intervals was described previously (6). Briefly, between St 20 and either St 26 or St 30–31, 1-s light flashes were used to trigger waves of activity at approximately twice the control frequency. In ovo movements were quantified as previously described (6). In embryos used to characterize motoneuron-specific bursting patterns, treatment was continued until St 31–33.
Characterization of Motoneuron Bursting Patterns via Electromyogram Recordings.
Isolated spinal cords were superfused with oxygenated Tyrode’s solution at 30 °C. Electromyograms were recorded simultaneously from sartorius and femorotibialis muscles with suction electrodes (for additional details, see ref. 21 and SI Text).
Orthograde Labeling of Motoneurons with Di-Asp and Di-I.
Following decapitation and ventral laminectomy of embryos, spinal nerves at lumbosacral segments LS1, LS2, and LS3 were exposed and injected with Di-Asp or Di-I (5 mg/mL; Molecular Probes), and kept at 34 °C in oxygenated tyrode for 6 h, then maintained in 1% (vol/vol) formaldehyde at room temperature for 2 wk. Following postfixation in 3.7% (vol/vol) formaldehyde, serial frozen transverse sections of St 27 limb were prepared at 16 µm, as previously described (6).
Retrograde Labeling of Motoneurons with HRP.
Following a ventral laminectomy, sartorius and femorotibialis muscles were exposed and injected with 10% (wt/vol) HRP (Invitrogen) in isolated spinal cord-hindlimb preparations and incubated for 6 h at 32 °C in oxygenated Tyrode’s solution. The cords were then fixed in 3.7% (vol/vol) formaldehyde in PBS for 30 min and processed as described in ref. 6.
Immunohistochemistry.
Transverse lumbar cord sections were incubated with antibodies against HRP (rabbit anti-HRP 1:400; Jackson Immunoresearch) and Er81 (mouse anti-Er81 72.5B10 1:50; Developmental Studies Hybridoma Bank) for 2 h at room temperature followed by 1-h incubation with appropriate secondary antibodies, and mounted with ProLong Antifade reagent (Invitrogen). Di-I and Di-ASP labeled axonal projections were analyzed in 16-µm transverse limb sections before immunohistochemistry to avoid dye diffusion, then incubated with antibodies against NCAM, 5E, 10 µg/mL (7), and appropriate secondary antibodies, as described above.
Image Acquisition.
Images were captured on an upright Nikon Microphot-FX with a digital camera (Retiga Exi, QImaging) using the QCapture software, as detailed previously (6).
Quantification of Misplaced Motoneurons and Pool-Specific Pathfinding Errors.
Serial 16-μm cross-sections from the spinal cords of control and light-activated St 30–31 embryos, whose sartorius or femorotibialis muscles had been injected with HRP, were stained with anti-HRP antibodies. For both muscles, the number of HRP+ cell bodies present in a spinal cord location inappropriate for the muscle to which they had projected was expressed as a percentage of all HRP+ cells labeled from that muscle. The percentages were then averaged and depicted in bar graphs or in histograms showing the percent of correctly and incorrectly projecting HRP+ motoneurons occurring in every third section along the A-P axis of the cord from T7 through LS3.
To verify that motoneurons that misprojected to the sartorius were femorotibialis motoneurons, 16-μm cross-sections of St 30–31 chick spinal cords were costained with antibodies against Er81, a marker of femorotibialis but not sartorius neurons (12), and HRP. The number of motoneurons projecting to the sartorius muscle that were Er81-positive was expressed as the percentage of all HRP+ cells in the LMCL. Statistical significance was determined by one-way ANOVA. A P value of less than 0.05 was considered as significant.
Supplementary Material
Acknowledgments
We thank Yuka Maeno-Hikichi for advice throughout the experiments; Yuka Maeno-Hikichi, David Katz, and Jerry Silver for helpful comments on the manuscript; and William Frank for help in constructing the light-activation apparatus. The Er81 antibodies were obtained from the Developmental Studies Hybridoma Bank under the auspices of the National Institute of Child Health and Human Development and maintained by the University of Iowa Department of Biology. This work was supported by National Institutes of Health Grants NS19640 and NS074199.
Footnotes
The authors declare no conflict of interest.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1316457110/-/DCSupplemental.
References
- 1.Blankenship AG, Feller MB. Mechanisms underlying spontaneous patterned activity in developing neural circuits. Nat Rev Neurosci. 2010;11(1):18–29. doi: 10.1038/nrn2759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Huberman AD, Feller MB, Chapman B. Mechanisms underlying development of visual maps and receptive fields. Annu Rev Neurosci. 2008;31:479–509. doi: 10.1146/annurev.neuro.31.060407.125533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Chub N, O’Donovan MJ. Blockade and recovery of spontaneous rhythmic activity after application of neurotransmitter antagonists to spinal networks of the chick embryo. J Neurosci. 1998;18(1):294–306. doi: 10.1523/JNEUROSCI.18-01-00294.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Milner LD, Landmesser LT. Cholinergic and GABAergic inputs drive patterned spontaneous motoneuron activity before target contact. J Neurosci. 1999;19(8):3007–3022. doi: 10.1523/JNEUROSCI.19-08-03007.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Wilhelm JC, Rich MM, Wenner P. Compensatory changes in cellular excitability, not synaptic scaling, contribute to homeostatic recovery of embryonic network activity. Proc Natl Acad Sci USA. 2009;106(16):6760–6765. doi: 10.1073/pnas.0813058106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kastanenka KV, Landmesser LT. In vivo activation of channelrhodopsin-2 reveals that normal patterns of spontaneous activity are required for motoneuron guidance and maintenance of guidance molecules. J Neurosci. 2010;30(31):10575–10585. doi: 10.1523/JNEUROSCI.2773-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Hanson MG, Landmesser LT. Normal patterns of spontaneous activity are required for correct motor axon guidance and the expression of specific guidance molecules. Neuron. 2004;43(5):687–701. doi: 10.1016/j.neuron.2004.08.018. [DOI] [PubMed] [Google Scholar]
- 8.Kania A, Jessell TM. Topographic motor projections in the limb imposed by LIM homeodomain protein regulation of ephrin-A:EphA interactions. Neuron. 2003;38(4):581–596. doi: 10.1016/s0896-6273(03)00292-7. [DOI] [PubMed] [Google Scholar]
- 9.Luria V, Krawchuk D, Jessell TM, Laufer E, Kania A. Specification of motor axon trajectory by ephrin-B:EphB signaling: Symmetrical control of axonal patterning in the developing limb. Neuron. 2008;60(6):1039–1053. doi: 10.1016/j.neuron.2008.11.011. [DOI] [PubMed] [Google Scholar]
- 10.Tang J, Landmesser L, Rutishauser U. Polysialic acid influences specific pathfinding by avian motoneurons. Neuron. 1992;8(6):1031–1044. doi: 10.1016/0896-6273(92)90125-w. [DOI] [PubMed] [Google Scholar]
- 11.Hanson MG, Landmesser LT. Increasing the frequency of spontaneous rhythmic activity disrupts pool-specific axon fasciculation and pathfinding of embryonic spinal motoneurons. J Neurosci. 2006;26(49):12769–12780. doi: 10.1523/JNEUROSCI.4170-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Lin JH, et al. Functionally related motor neuron pool and muscle sensory afferent subtypes defined by coordinate ETS gene expression. Cell. 1998;95(3):393–407. doi: 10.1016/s0092-8674(00)81770-5. [DOI] [PubMed] [Google Scholar]
- 13.Bonanomi D, Pfaff SL. Motor axon pathfinding. Cold Spring Harb Perspect Biol. 2010;2(3):a001735. doi: 10.1101/cshperspect.a001735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Landmesser L. The development of motor projection patterns in the chick hind limb. J Physiol. 1978;284:391–414. doi: 10.1113/jphysiol.1978.sp012546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Lance-Jones C, Landmesser LT. Pathway selection by chick lumbosacral motoneurons during normal development. Proc R Soc Lond B Biol Sci. 1981;214(1194):1–18. doi: 10.1098/rspb.1981.0079. [DOI] [PubMed] [Google Scholar]
- 16.Landmesser L. The distribution of motoneurones supplying chick hind limb muscles. J Physiol. 1978;284:371–389. doi: 10.1113/jphysiol.1978.sp012545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Li X, et al. Fast noninvasive activation and inhibition of neural and network activity by vertebrate rhodopsin and green algae channelrhodopsin. Proc Natl Acad Sci USA. 2005;102(49):17816–17821. doi: 10.1073/pnas.0509030102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Chub N, O’Donovan MJ. Post-episode depression of GABAergic transmission in spinal neurons of the chick embryo. J Neurophysiol. 2001;85(5):2166–2176. doi: 10.1152/jn.2001.85.5.2166. [DOI] [PubMed] [Google Scholar]
- 19.Tabak J, Rinzel J, O’Donovan MJ. The role of activity-dependent network depression in the expression and self-regulation of spontaneous activity in the developing spinal cord. J Neurosci. 2001;21(22):8966–8978. doi: 10.1523/JNEUROSCI.21-22-08966.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Lance-Jones C, Landmesser LT. Pathway selection by embryonic chick motoneurons in an experimentally altered environment. Proc R Soc Lond B Biol Sci. 1981;214(1194):19–52. doi: 10.1098/rspb.1981.0080. [DOI] [PubMed] [Google Scholar]
- 21.Landmesser LT, O’Donovan MJ. Activation patterns of embryonic chick hind limb muscles recorded in ovo and in an isolated spinal cord preparation. J Physiol. 1984;347:189–204. doi: 10.1113/jphysiol.1984.sp015061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Landmesser LT, O’Donovan MJ. The activation patterns of embryonic chick motoneurones projecting to inappropriate muscles. J Physiol. 1984;347:205–224. doi: 10.1113/jphysiol.1984.sp015062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Dolmetsch RE, Lewis RS, Goodnow CC, Healy JI. Differential activation of transcription factors induced by Ca2+ response amplitude and duration. Nature. 1997;386(6627):855–858. doi: 10.1038/386855a0. [DOI] [PubMed] [Google Scholar]
- 24.Dolmetsch RE, Xu K, Lewis RS. Calcium oscillations increase the efficiency and specificity of gene expression. Nature. 1998;392(6679):933–936. doi: 10.1038/31960. [DOI] [PubMed] [Google Scholar]
- 25.Wu GY, Deisseroth K, Tsien RW. Spaced stimuli stabilize MAPK pathway activation and its effects on dendritic morphology. Nat Neurosci. 2001;4(2):151–158. doi: 10.1038/83976. [DOI] [PubMed] [Google Scholar]
- 26.Bengtson CP, Freitag HE, Weislogel JM, Bading H. Nuclear calcium sensors reveal that repetition of trains of synaptic stimuli boosts nuclear calcium signaling in CA1 pyramidal neurons. Biophys J. 2010;99(12):4066–4077. doi: 10.1016/j.bpj.2010.10.044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Borodinsky LN, Belgacem YH, Swapna I. Electrical activity as a developmental regulator in the formation of spinal cord circuits. Curr Opin Neurobiol. 2012;22(4):624–630. doi: 10.1016/j.conb.2012.02.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Wang S, Polo-Parada L, Landmesser LT. Characterization of rhythmic Ca2+ transients in early embryonic chick motoneurons: Ca2+ sources and effects of altered activation of transmitter receptors. J Neurosci. 2009;29(48):15232–15244. doi: 10.1523/JNEUROSCI.3809-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Tang J, Rutishauser U, Landmesser L. Polysialic acid regulates growth cone behavior during sorting of motor axons in the plexus region. Neuron. 1994;13(2):405–414. doi: 10.1016/0896-6273(94)90356-5. [DOI] [PubMed] [Google Scholar]
- 30.Flavell SW, Greenberg ME. Signaling mechanisms linking neuronal activity to gene expression and plasticity of the nervous system. Annu Rev Neurosci. 2008;31:563–590. doi: 10.1146/annurev.neuro.31.060407.125631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Kim T-K, et al. Widespread transcription at neuronal activity-regulated enhancers. Nature. 2010;465(7295):182–187. doi: 10.1038/nature09033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Kania A, Johnson RL, Jessell TM. Coordinate roles for LIM homeobox genes in directing the dorsoventral trajectory of motor axons in the vertebrate limb. Cell. 2000;102(2):161–173. doi: 10.1016/s0092-8674(00)00022-2. [DOI] [PubMed] [Google Scholar]
- 33.Lin AC, Holt CE. Function and regulation of local axonal translation. Curr Opin Neurobiol. 2008;18(1):60–68. doi: 10.1016/j.conb.2008.05.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.O’Donnell M, Chance RK, Bashaw GJ. Axon growth and guidance: Receptor regulation and signal transduction. Annu Rev Neurosci. 2009;32:383–412. doi: 10.1146/annurev.neuro.051508.135614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Dasen JS, De Camilli A, Wang B, Tucker PW, Jessell TM. Hox repertoires for motor neuron diversity and connectivity gated by a single accessory factor, FoxP1. Cell. 2008;134(2):304–316. doi: 10.1016/j.cell.2008.06.019. [DOI] [PubMed] [Google Scholar]
- 36.Dasen JS, Tice BC, Brenner-Morton S, Jessell TM. A Hox regulatory network establishes motor neuron pool identity and target-muscle connectivity. Cell. 2005;123(3):477–491. doi: 10.1016/j.cell.2005.09.009. [DOI] [PubMed] [Google Scholar]
- 37.Shirasaki R, Lewcock JW, Lettieri K, Pfaff SL. FGF as a target-derived chemoattractant for developing motor axons genetically programmed by the LIM code. Neuron. 2006;50(6):841–853. doi: 10.1016/j.neuron.2006.04.030. [DOI] [PubMed] [Google Scholar]
- 38.Ming G, Henley J, Tessier-Lavigne M, Song H, Poo M. Electrical activity modulates growth cone guidance by diffusible factors. Neuron. 2001;29(2):441–452. doi: 10.1016/s0896-6273(01)00217-3. [DOI] [PubMed] [Google Scholar]
- 39.Carrillo RA, Olsen DP, Yoon KS, Keshishian H. Presynaptic activity and CaMKII modulate retrograde semaphorin signaling and synaptic refinement. Neuron. 2010;68(1):32–44. doi: 10.1016/j.neuron.2010.09.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Plazas PV, Nicol X, Spitzer NC. Activity-dependent competition regulates motor neuron axon pathfinding via PlexinA3. Proc Natl Acad Sci USA. 2013;110(4):1524–1529. doi: 10.1073/pnas.1213048110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Landmesser LT. The acquisition of motoneuron subtype identity and motor circuit formation. Int J Dev Neurosci. 2001;19(2):175–182. doi: 10.1016/s0736-5748(00)00090-3. [DOI] [PubMed] [Google Scholar]
- 42.Cohen S, et al. A semaphorin code defines subpopulations of spinal motor neurons during mouse development. Eur J Neurosci. 2005;21(7):1767–1776. doi: 10.1111/j.1460-9568.2005.04021.x. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.