Significance
Drugs against disease-causing microbes are among the major achievements of modern medicine, but many microbes show a tenacious ability to develop resistance, so they are no longer killed by available drugs. We show here for an important class of these drugs, represented by the common drug metronidazole, that broad modifications of the basic drug structure can improve drug activities against several clinically important microbes and unexpectedly overcome different forms of resistance. Several of these new drugs cure infections in animal models and are safe in initial toxicity evaluations. These findings provide reasons to develop this class of drugs as human medicines in the ongoing fight against disease-causing microbes.
Keywords: infectious diseases, antibiotics, medicinal chemistry
Abstract
Metronidazole and other 5-nitroimidazoles (5-NI) are among the most effective antimicrobials available against many important anaerobic pathogens, but evolving resistance is threatening their long-term clinical utility. The common 5-NIs were developed decades ago, yet little 5-NI drug development has since taken place, leaving the true potential of this important drug class unexplored. Here we report on a unique approach to the modular synthesis of diversified 5-NIs for broad exploration of their antimicrobial potential. Many of the more than 650 synthesized compounds, carrying structurally diverse functional groups, have vastly improved activity against a range of microbes, including the pathogenic protozoa Giardia lamblia and Trichomonas vaginalis, and the bacterial pathogens Helicobacter pylori, Clostridium difficile, and Bacteroides fragilis. Furthermore, they can overcome different forms of drug resistance, and are active and nontoxic in animal infection models. These findings provide impetus to the development of structurally diverse, next-generation 5-NI drugs as agents in the antimicrobial armamentarium, thus ensuring their future viability as primary therapeutic agents against many clinically important infections.
Antibiotics are among the greatest advances in medicine, yet their utility is constantly threatened by the development of resistance due to the high genetic adaptability of many target microbes. Most common antibiotics belong to a small number of functional and structural classes that target a limited set of microbial processes, including cell wall synthesis, protein translation, DNA replication, RNA transcription, and unique metabolic pathways. Despite these seemingly limited targeting opportunities, improved compounds have been developed within specific antibiotics classes over several drug generations with expanded potency and microbial range, as best illustrated by next-generation β-lactam antibiotics (1).
Of particular importance among antibiotics are 5-nitro drugs, characterized by a nitro functional group in the 5-position of a five-membered heterocycle (imidazole, thiazole, or furan). The prototype and most commonly used drug of this class is the 5-nitroimidazole (5-NI) compound, metronidazole (Mz). Listed as an essential medicine by the World Health Organization, it is one of the most versatile antibiotics in clinical practice, targeting a wide range of anaerobic microbes from protozoa, including Giardia lamblia, Trichomononas vaginalis, and Entamoeba histolytica, to bacteria, such as Helicobacter pylori, Clostridium difficile, and Bacteroides fragilis (2).
Mz and other 5-nitro antimicrobials are prodrugs that must be activated by reduction in the target microbe to generate toxic, short-lived radical intermediates. The radicals form adducts with different microbial molecules, including DNA, proteins, and lipids, although the exact molecular targets and specific functional consequences are incompletely understood. The microbial specificity of 5-nitro drugs stems largely from the requirement for low redox potential electron transfers that do not occur in mammalian cells (3), although other, poorly defined aspects may also be important (4).
Antimicrobial therapy with Mz is usually effective, with reported success rates of 70–99%, depending on the specific infectious agent and patient population (5). However, Mz treatment failure and resistance occur for all target pathogens. For example, >50% of H. pylori cases are resistant to Mz in some developing countries (6). As many as 10–20% of patients with giardiasis show clinical resistance to Mz (7), while 2–4% of clinical T. vaginalis isolates display varying degrees of Mz resistance (8). In some cases, Mz resistance can be overcome by treatment with other 5-NI drugs, but many resistant microbial strains exhibit cross-resistance between the major currently available 5-NI drugs (9). Multiple mechanisms have been implicated in 5-NI drug resistance, including a diminished capacity to reduce and activate 5-nitro prodrugs (10, 11) and detoxification of nitro drug radicals (12).
The common 5-NI drugs have different simple side chains at the 1-position of the imidazole, e.g., Mz possesses a hydroxyethyl group (Fig. 1A) and tinidazole has an ethylsulfonylethyl group. These modifications mostly affect the pharmacokinetic properties of the drugs but have only limited influence on drug potency or ability to overcome resistance (9). However, other structural modifications of 5-NI compounds can improve antimicrobial activity and resistance profiles (4, 13) or confer new antimicrobial activities, as shown for the kinetoplastid Trypanosoma cruzi (14). Despite these promising observations, the full antimicrobial potential of 5-NI drugs is not known, partly because commercial drug development largely ceased after approval of the first-generation 5-NI drugs beginning in the 1960s. Concerns about long-term safety may have contributed to this situation, but extensive clinical studies have shown that these compounds are safe and have no relevant long-term toxicity in humans. For example, a follow-up of 771 women treated with Mz 10 or more years previously revealed no excess cancer risk (15).
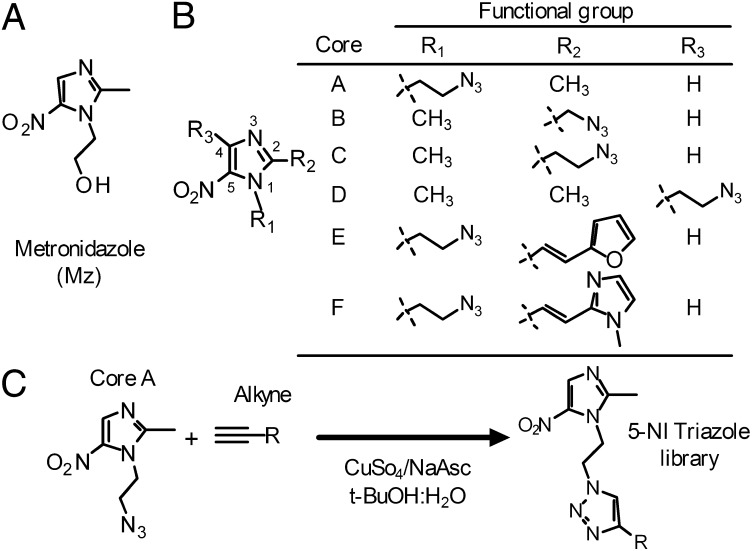
Fig. 1.
Synthesis of comprehensive new 5-NI library. (A) Structure of Mz. (B) Six different 5-NI cores (A−F) were synthesized with a “clickable” azide (N3) functional group. (C) Scheme of click chemistry-facilitated synthesis of 5-NI triazole library.
The goal of the present studies was to generate a large library of structurally diverse 5-NI compounds for comprehensive and unbiased evaluation of the therapeutic potential of this important class of antimicrobials. Using a unique modular approach to 5-NI synthesis, we show here that many of the >650 synthesized 5-NI compounds have vastly improved activity against a range of microbial pathogens, as they display marked improvements in broad-spectrum activity, can overcome different forms of 5-NI drug resistance, and are active and nontoxic in animal infection models. These findings substantially broaden the potential structural space suitable for further development of alternative nitro antimicrobials for ultimate clinical use, and strongly suggest that the systematic development of next-generation 5-NI drugs can lead to superior agents in the armamentarium of antimicrobials against a wide range of clinically important infections.
Results
Click Chemistry for Broad Structural Diversification of 5-NI Compounds.
Mz has an imidazole core substituted with 5-nitro and 1-hydroxyethyl groups (Fig. 1A). The nitro group is critical for antimicrobial activity, whereas the 3-position cannot be functionalized without fundamentally altering the core heterocycle, leaving the 1-, 2-, and 4-positions for potential modifications to improve activity. Prior studies on side chain modifications had not revealed any clear structural themes that could be exploited to generate compounds with superior activity (4, 13). As an alternative, we reasoned that generating broad unbiased structural diversity might be more powerful than incremental optimization for developing improved antimicrobials. Therefore, we designed a synthetic approach for exponentially leveraging the diversity of two reaction partners to generate a broad range of 5-NI derivatives. The chemistry exploits the copper(I)-catalyzed azide alkyne cycloaddition (“click reaction”), in which an azide and an alkyne are united to yield a 1,4-substituted 1,2,3-triazole (16). This reaction is particularly suitable for generating libraries for antimicrobial drug development, as yields are practically quantitative under simple, mild, and safe reaction conditions, leaving few offensive by-products (17). The reaction is also regiospecific and readily scalable (17), allowing rapid production of substantial quantities for preclinical efficacy and safety testing.
We first synthesized six azido derivatives of 5-NI, in which one of the three available positions in the imidazole ring was functionalized with azidoalkyl groups (Fig. 1B). The distance between the azido group and the imidazole core was minimized to limit structural restrictions on the products of the subsequent cycloaddition, although direct linkage of the azido group to the imidazole core proved impossible, presumably because it compromised the stability of the heterocycle. We also generated 1-azidoalkyl imidazole compounds with conjugated furan- or imidazole-based side chain in the 2-position (cores E and F, Fig. 1B), as our previous studies had suggested that these modifications can yield potent antimicrobials (4).
We next assembled a library of 63 structurally diverse alkynes as reaction partners for the azido cores (SI Appendix, Table S1). The alkynes, which were obtained commercially or synthesized de novo, were selected to achieve maximal structural diversity with little consideration of conventional pharmacological criteria such as hydrophobicity, electrochemical properties, or previously successful side chains. We then performed all possible copper(I)-catalyzed cycloadditions of the six azido-imidazole cores and 63 alkynes to synthesize 378 5-NIs (Fig. 1C and SI Appendix, Table S1). Initial studies showed similar antimicrobial activities in crude reaction mixtures compared with purified compounds, indicating that the reaction mixtures could be rapidly screened for activity without the time-consuming isolation of the products.
Broad Antimicrobial Activities of Diverse 5-NI Compounds.
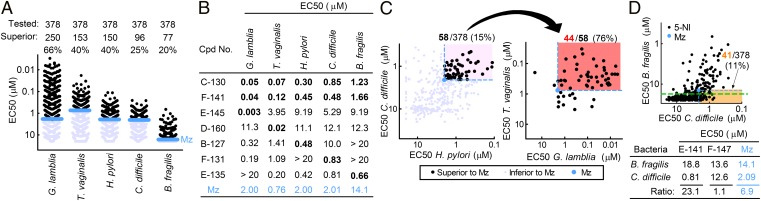
To test the antimicrobial activity of the 5-NI library, we used a range of clinically important protozoa and bacteria that are commonly treated with Mz. The intestinal parasite, G. lamblia, is a major worldwide protozoal cause of diarrheal disease, T. vaginalis is a common protozoal cause of sexually transmitted disease of the genitourinay tract, and H. pylori and C. difficile are major bacterial causes of infectious disease in stomach and colon, respectively. Using quantitative growth and survival assays, we found that 66% of the 378 tested compounds had superior activity relative to Mz against G. lamblia and 40% against T. vaginalis, with up to 50- to 500-fold lower EC50 relative to Mz (Fig. 2A and SI Appendix, Tables S2 and S3). Marked activity improvements were also observed for the bacterial pathogens: 40% of the compounds were superior to Mz against H. pylori and 25% against C. difficile (Fig. 2A and SI Appendix, Table S4).
Fig. 2.
Expanded antimicrobial activity range of structurally diverse 5-NI compounds. (A) The activities of 378 compounds were tested against the indicated protozoa and bacteria in 24- to 48-h growth assays using ATP levels or OD600 as readouts. Each data point or number represents the mean EC50 for one compound, with Mz shown in blue for comparison. (B) Examples of compounds with enhanced broad-spectrum or pathogen-selective activity (key values in bold). (C) Relationships between activities of individual compounds against the four target pathogens. Compounds that exceeded the activity of Mz (blue point) for both bacteria (black circles in light pink-shaded region, Left) were examined for their activities against the two protozoa (Right). The region containing compounds with superior activity against all four pathogens is shaded in salmon color. (D) The relationship between compound activities against two colonic bacteria (Upper). The orange-shaded region highlights compounds more active than Mz against the opportunistic pathogen, C. difficile, but less active than Mz against the commensal, B. fragilis. Assay sensitivity is depicted by the dashed green line. The table (Lower) lists examples of compounds that showed greater or lesser selectivity than Mz against C. difficile compared with B. fragilis.
Some compounds displayed highly selective improvement in activity against one of the target microbes, whereas others exhibited greater activity against more than one microbe (Fig. 2B and SI Appendix, Tables S2−S4). Analysis of the relationships between different antimicrobial activities revealed modest but significant positive correlations between the protozoa (r = 0.38, P < 0.001) and bacteria (r = 0.53, P < 0.001) (Fig. 2C and SI Appendix, Fig. S1). Moreover, superior antibacterial activity correlated with improved antiprotozoal activity, and conversely, superior antiprotozoal activity correlated with better antibacterial activity (Fig. 2C and SI Appendix, Fig. S1). For example, 44 of 58 (76%) of the compounds that were more active than Mz against both bacterial pathogens were also more active against both protozoa. Together, 12% (n = 44) of the 378 tested compounds were superior to Mz against all four pathogens (Fig. 2C). This percentage of broadly active compounds significantly exceeded (by >fourfold) the predicted 2.6% of compounds if improvements in activity against each individual microbe were entirely independent from improvement against the other microbes, as calculated by multiplying the individual percentages of superior compounds for each of the four target pathogens (i.e., 66%, 40%, 40%, and 25%; Fig. 2A).
Given the broad-spectrum activity of several compounds, we questioned whether the nitro compounds were also active against intestinal commensals, which might impact their clinical utility. None of the 378 nitro compounds exhibited activity against the commensal Escherichia coli (strain K12) up to a maximum concentration of 100 µM, suggesting that the newly synthesized 5-NI drugs, like Mz, do not have nonspecific toxicity in facultative microbes naturally resistant to this drug class. B. fragilis is an important commensal that normally resides in the intestinal lumen but can cause peritoneal infections when translocated due to gut perforations. A substantial fraction (20%) of compounds had superior activity against B. fragilis compared with Mz (Fig. 2 A and B and SI Appendix, Table S4). Importantly, comparison of activities against B. fragilis and C. difficile, both of which colonize the colon, revealed a range of ratios (Fig. 2D), indicating that some nitro drugs exist with improved relative selectivity against the opportunistic pathogen C. difficile compared with the commensal B. fragilis.
Collectively, these results indicate that many of the synthesized 5-NIs have a marked, and for some highly selective, increase in activity against each of the individual microbes. Furthermore, a significantly greater than expected number of compounds were superior to Mz against multiple different microbes, making these compounds strong candidates for broad-spectrum antibiotics. Importantly, the vast majority of the compounds (362/378, >95%) had no cytotoxicity in human HeLa cells and many showed therapeutic indices equal or superior to Mz (SI Appendix, Table S5).
Structurally Diverse 5-NI Compounds Can Overcome Different Forms of Mz Resistance.
Natural variability in antimicrobial susceptibility is an important consideration in drug development. To address this issue, we tested additional isolates of three of the target pathogens and related the data to the initial screens. Activities showed generally good correlations (with r values of 0.896, 0.695, and 0.388 for Giardia, Trichomonas, and Helicobacter, respectively; P < 0.01 for all three; SI Appendix, Fig. S2 and Tables S2−S4), but differences were apparent between the pathogens. For Giardia, 63% of the compounds exhibited superior activity over Mz against both clinical isolates, whereas this percentage was lower for T. vaginalis (21%) and H. pylori (26%), underlining the importance of testing a range of isolates to avoid pursuit of compounds with limited, isolate-specific activity. Nonetheless, these data demonstrate that the 5-NI library had multiple compounds with superior activities against different clinical isolates of the target pathogens.
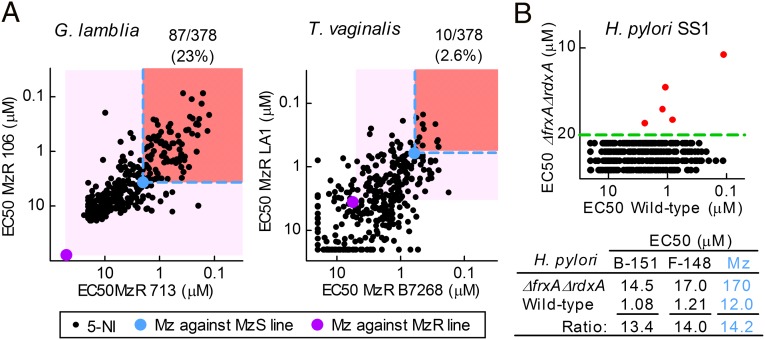
Beyond natural variability in antimicrobial susceptibility, resistance to Mz can develop during antimicrobial therapy, and Mz resistance can be readily induced in bacteria and protozoa in the laboratory (9). Importantly, currently approved 5-NI drugs exhibit cross-resistance to Mz and show similar clinical failure rates (9). To determine whether the 5-NI compounds can overcome acquired Mz resistance, we used two independently derived syngeneic Mz-resistant (MzR) lines of G. lamblia and two clinical MzR isolates of T. vaginalis. Remarkably, all of the 378 tested compounds exhibited activity against the two MzR Giardia lines that was superior (i.e., lower EC50) to Mz (Fig. 3A, light pink shading; SI Appendix, Table S2). Moreover, 23% of the compounds (n = 87) were also more active against both MzR lines than Mz was against the corresponding parental Mz-sensitive (MzS) lines (Fig. 3A, salmon-colored shading). Thus, these 87 compounds effectively overcame acquired Mz resistance in Giardia, as they could kill MzR lines at least as effectively as Mz could kill MzS lines.
Fig. 3.
New 5-NI compounds overcome Mz resistance. (A) Compounds were tested against MzR strains of G. lamblia (Left) and T. vaginalis (Right), with those more active than Mz (purple) highlighted by light pink boxes. Of these, compounds more active against both MzR lines than Mz against the respective MzS lines (light blue) are further highlighted by salmon-colored boxes. (B) Activities against wild-type and double mutant (ΔfrxA ΔrdxA) strains of H. pylori. Five compounds (red dots) had measurable activity against the mutant; all others were below the assay sensitivity (dashed green line, Upper). Examples of active compounds are listed in the table (Lower).
For T. vaginalis, 176 of 377 tested compounds (47%) were superior to Mz in two unrelated MzR isolates (Fig. 3A, light pink shading; SI Appendix, Table S3). Of these, 10 compounds (2.6% of all compounds) exhibited activity against both MzR lines that exceeded Mz activity against the MzS lines (Fig. 3A, salmon-colored shading). Thus, the range of activities, if not the frequency of compounds with superior activity, against T. vaginalis was similar to G. lamblia, supporting the conclusion that our comprehensive 5-NI drug library contains compounds with diverse activities against a wide range of microbes and forms of drug resistance.
As a further test of the ability to overcome nitro drug resistance, we used a double mutant of H. pylori for two reductases, NADPH flavin oxidoreductase (FrxA) and oxygen-insensitive NADPH nitroreductase (RdxA), that activate nitro drugs and are involved in clinical drug resistance (18). Screening of the library revealed that 98.6% of compounds lost activity against the double mutant (Fig. 3B and SI Appendix, Table S4), which demonstrates that reduction by one or both of these reductases is critical for compound activity and further underlines the high molecular specificity of the 5-NI compounds. Interestingly, we also found five compounds that remained active in the 10- to 20-µM range in the double mutant. One of the compounds was also toxic in HeLa cells, but the other four had no apparent cytotoxicity in these cells (and no activity against E. coli up to 100 µM), suggesting that they retain microbial specificity and raising the possibility that alternative reductive pathways may exist in H. pylori to activate these nitro compounds.
Distinct and Predictive Bioactivity Landscapes of 5-NI Compounds.
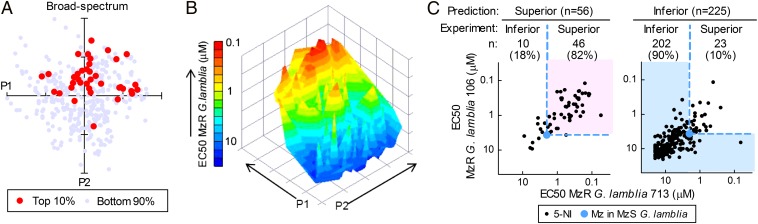
To begin to understand the relationship between compound structures and antimicrobial activities, we used principal component analysis to visualize the 5-NI compounds in a structural space and then highlighted the most active compounds within that space. The top 10% compounds with broad-spectrum activity against all four target pathogens were located in different regions within the space (Fig. 4A). By comparison, localization of the most active compounds against each of the individual pathogens revealed distinct patterns (“activity fingerprints”) for the four pathogens (SI Appendix, Fig. S3), which overlapped only partly with the pattern of broad-spectrum compounds. These data suggest that the structural requirements for activity improvement heavily depend on the particular microbes, but also show that broad-spectrum activity can be achieved in multiple structural domains.
Fig. 4.
Predictive bioactivity landscape of 5-NI compounds. A structural space was generated by principal component analysis using activity data of the 378 compounds in the 5-NI library against the four target microbes. (A) The individual compounds were plotted in the resulting space with the top 10% most potent broad-spectrum compounds shown as red circles. (B) A structural space was constructed from the activity data against MzR G. lamblia. Activities of all compounds were plotted in the space (z axis), and a contour graph was generated using the indicated color scale. (C) A service vector machine model was constructed from the activity data of the 378 compounds against MzR Giardia (training set) and applied prospectively to a new set of 281 independently synthesized 5-NI compounds (test set). Correctly predicted compounds, as defined by coincidence of model prediction and assay-determined activity against the two MzR Giardia lines, are highlighted by background coloring (light pink and light blue) for predicted superior (Left) and inferior (Right) compounds. Each data point represents one compound. The light blue dots show Mz activity against the MzS parental lines for comparison.
Further evaluation of the structure−activity relationships by principal component analysis for one of the pathogens, MzR G. lamblia, revealed a nonrandom distribution of bioactivity in the chemical space occupied by the compounds, with distinct activity peaks and valleys (Fig. 4B). Based on this observation, we applied machine learning tools to construct a mathematical model for bioactivity prediction. Although decision tree analysis yielded excellent (84%) cross-validation in the set of 378 training compounds, even better predictability (87%) was achieved with a service vector machine approach. To test the predictive power of the model, we synthesized another set of 281 test compounds by click chemistry using the same six 5-NI cores A−F (Fig. 1B) but 47 different alkynes (SI Appendix, Table S6). These alkynes were structurally distinct from those used in the initial library, but occupied an overlapping chemical space (SI Appendix, Fig. S4). The model was used to divide the test compounds into two groups with predicted superior or inferior activity in MzR cells relative to Mz in the parental MzS Giardia cells. Some 82% of predicted superior compounds exhibited superior activity in antimicrobial testing, while 90% of predicted inferior compounds had inferior activity in the tests (Fig. 4C and SI Appendix, Table S7). Together, bioactivity of 86% (248 of 281) of the test compounds against MzR G. lamblia was correctly predicted, indicating the excellent utility of the predictive model. The findings experimentally validate prior contentions that structural analysis by chemical descriptors can be exploited to define chemical spaces with promising structure−activity relationships (19, 20). As for the training compounds, the vast majority of test compounds (260/279, 93%) had no measurable cytotoxicity in human HeLa cells (SI Appendix, Table S8).
Structural Determinants of Superior 5-NI Activity.
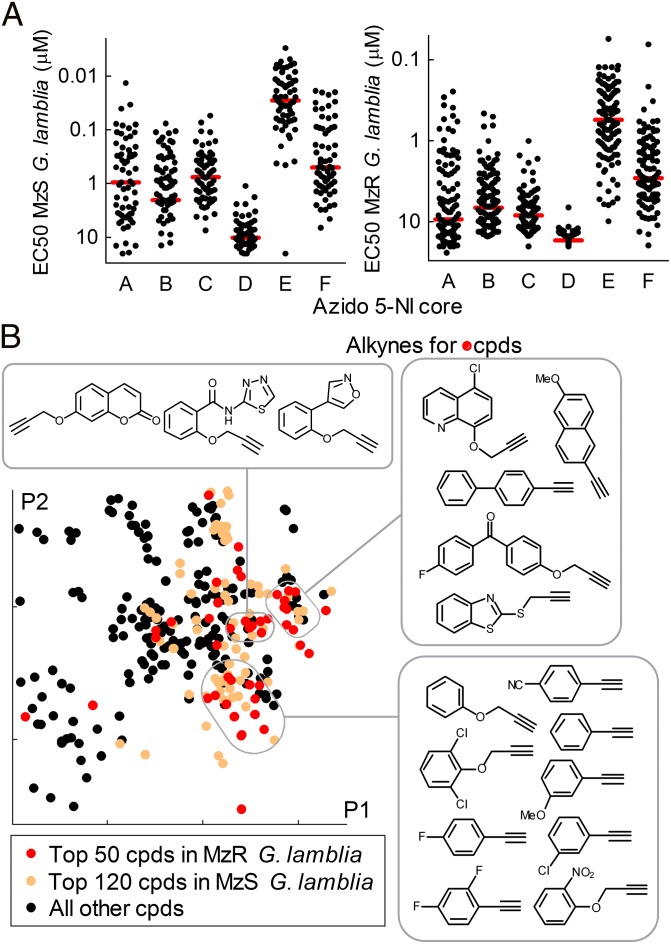
To define the structural determinants of superior antimicrobial activity of the 5-NI triazole compounds, we first examined the importance of the azido-imidazole core. Compounds based on cores A−C had similar average activities against MzS and MzR Giardia (Fig. 5A). Core D compounds had low or no activity against either, whereas the E series compounds had, on average, significantly higher activities than those of the other cores against both MzS and MzR Giardia (Fig. 5A). To explore the contribution of the alkyne partners to triazole bioactivity, we focused on the antigiardial activity of A−C series compounds to minimize core bias. Principal component analysis revealed a nonrandom, patchy distribution of the superior triazoles, occupying several distinct areas in the chemical space of the entire group of core A−C compounds (Fig. 5B). The corresponding alkynes in the active regions showed marked structural similarities within each of these regions, but little between different regions. These results indicate that several structurally unrelated groups of compounds can yield superior antigiardials.
Fig. 5.
Structure−activity relationships of 5-NI building blocks. The entire library of 659 5-NI compounds (composed of the 378 training and 281 test compounds) was examined for structure−activity relationships of the two building blocks, azido-5-NI and alkyne, used for the click chemistry-facilitated synthesis. (A) The influence of the azido-5-NI cores (A−F, Fig. 1B) on activity against Mz-sensitive (MzS, Left) and Mz-resistant (MzR, Right) Giardia, with average activities shown as red lines. (B) Data of all compounds (cpds) generated from cores A−C (to minimize core bias in the alkyne evaluation) in a structural space derived by principal component analysis. The top most active compounds against MzS and MzR Giardia are highlighted in light brown and red, respectively. Several regions with clustering of the most active compounds are boxed, and the corresponding alkynes used for compound synthesis are depicted.
In Vivo Efficacy of 5-NI Triazoles in Animal Infection Models.
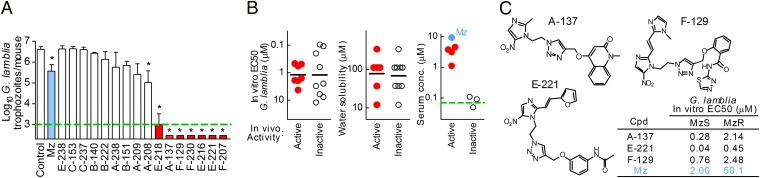
Because many 5-NI compounds exhibited promising antimicrobial activities in vitro, we next examined their in vivo efficacy in a murine model of giardiasis. Out of 16 structurally representative compounds, 8 (50%) displayed significant activity against giardiasis, and 7 (45%) were more efficacious at clearing Giardia than Mz at a standard dose of 10 mg/kg (Fig. 6A, red). Acute toxicity was not observed over the 3-d treatment course. In vivo efficacy could not be explained by greater in vitro antigiardial activity or altered aqueous solubility (Fig. 6B). However, active compounds had higher serum levels than inactive compounds by 2 h after oral administration (Fig. 6B), suggesting that systemic bioavailability was important for antigiardial efficacy. Examination of the structure−activity relationships revealed five chemical attributes, different from those in the Lipinski rules commonly used to predict bioavailability of new compounds (21), that could effectively differentiate in vivo active and inactive compounds in the chemical space of all library compounds (SI Appendix, Figs. S5 and S6). Examples of active compounds show that promising activities against giardiasis can be found among very different chemical structures (Fig. 6C). Initial evaluation of the toxicity profile of several of these compounds by micronucleus assay with mammalian CHO cells using current Organization for Economic Cooperation and Development guidelines revealed no acute genotoxicity (SI Appendix, Fig. S7). Furthermore, oral administration of five high doses (100 mg/kg; equivalent to ∼10 × effective dose) to adult mice over 3 d revealed no toxicity, as assessed by liver enzyme panels and histological and hematological evaluation.
Fig. 6.
In vivo efficacy of 5-NI compounds against giardiasis. (A) Adult C57BL/6 mice were orally infected with G. lamblia and given five 10 mg/kg oral doses of the indicated compounds over 3 d at 12-h intervals, and trophozoite numbers in the small intestine were determined. The dashed green line represents the assay sensitivity. Data are mean + SEM (n ≥ 5); *P < 0.05 vs. vehicle-treated controls. Compounds significantly more efficacious than Mz (light blue) are highlighted in red. (B) The relationships of in vivo bioactivity, in vitro activity (Left), aqueous solubility (Center), and measured serum drug concentrations (Right, green dashed line represents the assay sensitivity; Mz is shown in blue for comparison). (C) Examples for in vivo active compounds, along with their in vitro activities (Table in Lower Right) against MzS and MzR Giardia.
Discussion
Our results indicate that next-generation 5-NI drug candidates can be synthesized and rapidly tested in vitro and in vivo by combining the ease of modular click chemistry with cell culture assays, predictive machine learning tools, and animal models. Thus, it is readily feasible to advance from initial synthesis of hundreds of compounds to focused animal testing in mere weeks, thereby significantly shortening the initial development cycle in drug design (17). This acceleration could promote a timely and cost-effective drug development response to the emergence of antimicrobial resistance that threatens public health. Adequate preclinical safety testing will be needed before commencing human trials, but even this step can be significantly shortened and made more cost-effective by rapid production of kilogram quantities of new drug candidates by click chemistry or other chemistries suitable for combinatorial synthesis and ready side chain preservation.
The observed increases in antimicrobial activity were striking (>100-fold) for several of the 5-NI compounds, although the underlying reasons remain to be established. Despite the widespread use of Mz and other 5-NI drugs, their mechanisms of antimicrobial killing remain incompletely understood. Reduction of the prodrugs is required for activity, so higher affinity or faster reaction kinetics of more potent compounds with the relevant reductases may be important. The stability or molecular targeting efficiency of activated nitro drug radicals may be more favorable for the new compounds (22), although chemical reduction with dithionite followed by immediate antimicrobial testing led to complete activity loss, suggesting that the toxic products formed by reduction are not very stable under these conditions. Alternatively, the spectrum of nitro drug adduction targets may be different for the most potent compounds (4, 23). In any case, the observation that many compounds showed improved broad-spectrum activity would suggest that the underlying mechanisms are likely to entail broadly relevant drug properties, such as improved membrane permeability, that are not limited to particular molecular targets or microbial features.
A key observation of our studies is that many new compounds could effectively overcome nitro drug resistance in different microbes. Mz resistance is functionally heterogeneous, with several causative mechanisms for each of the target microbes and between different microbes (7, 24). For example, resistance in Giardia involves down-regulation of nitro drug-activating systems, including different reductases and redox proteins and metabolites (11, 25), whereas Trichomonas also produces resistance proteins that directly detoxify nitro drugs (12) and Bacteroides has transporters that can remove nitro drugs from the cell (26). Despite this mechanistic diversity, for each of the resistant microbes, we were able to identify nitro compounds that could combat resistance, and some compounds were broadly effective against different resistant microbes. Several basic explanations, alone or in combination, may account for this finding. New nitro compounds may be activated by alternative pathways that are not involved in Mz resistance, or they may bypass Mz detoxification or efflux pathways. Alternatively, improved nitro compounds may be activated by the same pathways that are suppressed in Mz resistance, but the residual activity of these pathways, which may not be completely shut down due to significant fitness costs (9), is sufficient to mediate adequate activation of the most potent nitro compounds.
Our data suggest that incremental structural modifications, as frequently used for lead compound optimization (27), may not be an optimal strategy for 5-NI drugs, as compounds with different and unpredictable structural features had desirable antimicrobial properties in vitro and in vivo. Although the precise mechanistic reasons for this counterintuitive observation remain to be determined, 5-NIs can be activated by different microbial pathways and probably target multiple microbial molecules (11, 12, 23) whose relative importance may depend on the compound and microbe. Because the specific target molecules of 5-NI drugs are not fully understood (23), a strategy focused on achieving effective whole-cell antimicrobial activity in the relevant systems may be the best choice for making rapid progress in developing improved antimicrobials in the clinically indispensable 5-NI class.
Materials and Methods
Azido-alkyl-5NI heterocycles and 60 alkynes were synthesized de novo, and 50 additional alkynes were purchased. Click reactions were performed with azides and alkynes in t-BuOH:H2O with sodium ascorbate and CuSO4. In vitro activities (EC50) were determined with single-step ATP assay or OD600 after 24–48 h growth in culture. Structure–activity relationships were analyzed with chemical descriptors (E-Dragon 1.0) and machine learning software (WEKA). In vivo efficacy was assessed in C57BL/6 mice infected with G. lamblia GS/M. See SI Appendix for details.
Supplementary Material
Acknowledgments
We thank Elaine Hanson, Lucia Hall, and Christina Yoon for technical assistance. This work was supported by National Institutes of Health Grants AI075527, DK035108, and DK080506.
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1302664110/-/DCSupplemental.
References
- 1.Drawz SM, Bonomo RA. Three decades of beta-lactamase inhibitors. Clin Microbiol Rev. 2010;23(1):160–201. doi: 10.1128/CMR.00037-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Brook I. Antimicrobial treatment of anaerobic infections. Expert Opin Pharmacother. 2011;12(11):1691–1707. doi: 10.1517/14656566.2011.576672. [DOI] [PubMed] [Google Scholar]
- 3.Edwards DI. Nitroimidazole drugs—Action and resistance mechanisms. I. Mechanisms of action. J Antimicrob Chemother. 1993;31(1):9–20. doi: 10.1093/jac/31.1.9. [DOI] [PubMed] [Google Scholar]
- 4.Valdez CA, et al. Synthesis and electrochemistry of 2-ethenyl and 2-ethanyl derivatives of 5-nitroimidazole and antimicrobial activity against Giardia lamblia. J Med Chem. 2009;52(13):4038–4053. doi: 10.1021/jm900356n. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Löfmark S, Edlund C, Nord CE. Metronidazole is still the drug of choice for treatment of anaerobic infections. Clin Infect Dis. 2010;50(Suppl 1):S16–S23. doi: 10.1086/647939. [DOI] [PubMed] [Google Scholar]
- 6.Secka O, et al. Antimicrobial susceptibility and resistance patterns among Helicobacter pylori strains from The Gambia, West Africa. Antimicrob Agents Chemother. 2013;57(3):1231–1237. doi: 10.1128/AAC.00517-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Upcroft P, Upcroft JA. Drug targets and mechanisms of resistance in the anaerobic protozoa. Clin Microbiol Rev. 2001;14(1):150–164. doi: 10.1128/CMR.14.1.150-164.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Krashin JW, et al. Trichomonas vaginalis prevalence, incidence, risk factors and antibiotic-resistance in an adolescent population. Sex Transm Dis. 2010;37(7):440–444. doi: 10.1097/OLQ.0b013e3181cfcd8c. [DOI] [PubMed] [Google Scholar]
- 9.Tejman-Yarden N, et al. Impaired parasite attachment as fitness cost of metronidazole resistance in Giardia lamblia. Antimicrob Agents Chemother. 2011;55(10):4643–4651. doi: 10.1128/AAC.00384-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Dan M, Wang AL, Wang CC. Inhibition of pyruvate-ferredoxin oxidoreductase gene expression in Giardia lamblia by a virus-mediated hammerhead ribozyme. Mol Microbiol. 2000;36(2):447–456. doi: 10.1046/j.1365-2958.2000.01863.x. [DOI] [PubMed] [Google Scholar]
- 11.Leitsch D, et al. Pyruvate:ferredoxin oxidoreductase and thioredoxin reductase are involved in 5-nitroimidazole activation while flavin metabolism is linked to 5-nitroimidazole resistance in Giardia lamblia. J Antimicrob Chemother. 2011;66(8):1756–1765. doi: 10.1093/jac/dkr192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Pal D, et al. Giardia, Entamoeba, and Trichomonas enzymes activate metronidazole (nitroreductases) and inactivate metronidazole (nitroimidazole reductases) Antimicrob Agents Chemother. 2009;53(2):458–464. doi: 10.1128/AAC.00909-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Upcroft JA, et al. 5-Nitroimidazole drugs effective against metronidazole-resistant Trichomonas vaginalis and Giardia duodenalis. Antimicrob Agents Chemother. 2006;50(1):344–347. doi: 10.1128/AAC.50.1.344-347.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Bahia MT, et al. (2012) Fexinidazole: A potential new drug candidate for chagas disease. PLoS Negl Trop Dis 6(11):e1870. [DOI] [PMC free article] [PubMed]
- 15.Roe FJ. Safety of nitroimidazoles. Scand J Infect Dis Suppl. 1985;46:72–81. [PubMed] [Google Scholar]
- 16.Kolb HC, Finn MG, Sharpless KB. Click chemistry: Diverse chemical function from a few good reactions. Angew Chem Int Ed Engl. 2001;40(11):2004–2021. doi: 10.1002/1521-3773(20010601)40:11<2004::AID-ANIE2004>3.0.CO;2-5. [DOI] [PubMed] [Google Scholar]
- 17.Kolb HC, Sharpless KB. The growing impact of click chemistry on drug discovery. Drug Discov Today. 2003;8(24):1128–1137. doi: 10.1016/s1359-6446(03)02933-7. [DOI] [PubMed] [Google Scholar]
- 18.Jeong JY, et al. Roles of FrxA and RdxA nitroreductases of Helicobacter pylori in susceptibility and resistance to metronidazole. J Bacteriol. 2001;183(17):5155–5162. doi: 10.1128/JB.183.17.5155-5162.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Gamo FJ, et al. Thousands of chemical starting points for antimalarial lead identification. Nature. 2010;465(7296):305–310. doi: 10.1038/nature09107. [DOI] [PubMed] [Google Scholar]
- 20.Reymond J-L, van Deursen R, Blum LC, Ruddigkeit L. Chemical space as a source for new drugs. Med. Chem. Comm. 2010;1(1):30–38. [Google Scholar]
- 21.Ghose AK, Viswanadhan VN, Wendoloski JJ. A knowledge-based approach in designing combinatorial or medicinal chemistry libraries for drug discovery. 1. A qualitative and quantitative characterization of known drug databases. J Comb Chem. 1999;1(1):55–68. doi: 10.1021/cc9800071. [DOI] [PubMed] [Google Scholar]
- 22.Aravena CM, et al. Potent 5-nitrofuran derivatives inhibitors of Trypanosoma cruzi growth: Electrochemical, spectroscopic and biological studies. Spectrochim Acta A Mol Biomol Spectrosc. 2011;79(2):312–319. doi: 10.1016/j.saa.2011.02.007. [DOI] [PubMed] [Google Scholar]
- 23.Leitsch D, Kolarich D, Wilson IB, Altmann F, Duchêne M. Nitroimidazole action in Entamoeba histolytica: A central role for thioredoxin reductase. PLoS Biol. 2007;5(8):e211. doi: 10.1371/journal.pbio.0050211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Land KM, Johnson PJ. Molecular basis of metronidazole resistance in pathogenic bacteria and protozoa. Drug Resist Updat. 1999;2(5):289–294. doi: 10.1054/drup.1999.0104. [DOI] [PubMed] [Google Scholar]
- 25.Nillius D, Müller J, Müller N. Nitroreductase (GlNR1) increases susceptibility of Giardia lamblia and Escherichia coli to nitro drugs. J Antimicrob Chemother. 2011;66(5):1029–1035. doi: 10.1093/jac/dkr029. [DOI] [PubMed] [Google Scholar]
- 26.Pumbwe L, Glass D, Wexler HM. Efflux pump overexpression in multiple-antibiotic-resistant mutants of Bacteroides fragilis. Antimicrob Agents Chemother. 2006;50(9):3150–3153. doi: 10.1128/AAC.00141-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Nagle A, et al. Imidazolopiperazines: Lead optimization of the second-generation antimalarial agents. J Med Chem. 2012;55(9):4244–4273. doi: 10.1021/jm300041e. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.