Significance
Oral drug delivery is the most convenient administration route for patients. Docetaxel, a potent anticancer drug, elicits severe side effects following intravenous administration. Furthermore, oral docetaxel absorption is prevented by biochemical barriers in the intestine. An oral formulation of docetaxel nanocapsules (NCs) embedded in microparticles was developed and elicited higher plasma docetaxel concentrations than intravenous administration of the commercial product. These unexpected results were explained by the penetration of the docetaxel NCs within the enterocytes, circumventing the barriers, where their coating was reinforced prior reaching, intact, the circulation via the lymphatic system. The oral formulation significantly improves docetaxel anticancer efficacy. This delivery concept has potential for clinical translation, allowing docetaxel chemotherapy to be switched from intravenous to oral delivery.
Keywords: lymphatic system, nanoparticles, nanocarrier, taxanes
Abstract
An original oral formulation of docetaxel nanocapsules (NCs) embedded in microparticles elicited in rats a higher bioavailability compared with the i.v. administration of the commercial docetaxel solution, Taxotere. In the present study, various animal studies were designed to elucidate the absorption process of docetaxel from such a delivery system. Again, the docetaxel NC formulation elicited a marked enhanced absorption compared with oral Taxotere in minipigs, resulting in relative bioavailability and Cmax values 10- and 8.4-fold higher, respectively, confirming the previous rat study results. It was revealed that orally absorbed NCs altered the elimination and distribution of docetaxel, as shown in the organ biodistribution rat study, due to their reinforced coating, while transiting through the enterocytes by surface adsorption of apoproteins and phospholipids. These findings were demonstrated by the cryogenic-temperature transmission electron microscopy results and confirmed by the use of a chylomicron flow blocker, cycloheximide, that prevented the oral absorption of docetaxel from the NC formulation in an independent pharmacokinetic study. The lipoproteinated NCs reduced the docetaxel release in plasma and its distribution among the organs. The improved anticancer activity compared with i.v. Taxotere, observed in the metastatic lung cancer model in Severe Combined Immune Deficiency-beige (SCID-bg) mice, should be attributed to the extravasation effect, leading to the lipoproteinated NC accumulation in lung tumors, where they exert a significant therapeutic action. To the best of our knowledge, no study has reported that the absorption of NCs was mediated by a lymphatic process and reinforced during their transit.
Docetaxel is widely used for lung cancer treatment, and its i.v. administration is often associated with acute hypersensitive reactions owing to the presence of polysorbate 80 in the formulation that is needed for drug solubilization (1, 2). Docetaxel also exhibits low and variable oral bioavailability due to the active efflux by the Permeability-Glycoprotein (P-gp) pump and to CYP3A4 gut metabolism (3). Thus, intensive efforts are being invested in the design of novel oral formulations of docetaxel, either combined with P-gp or CYP3A4 inhibitors (4, 5) or without such inhibitors (6). Most of these studies emphasize the absence of ethanol and polysorbate and the opportunity to investigate more schedule-intensive treatment regimens as major advantages (7). The clinical rationale behind such a trend is that shorter schedules result in more frequent exposure of docetaxel to tumor cells, whereas lower maximum plasma levels are reached, thus reducing the incidence of severe side effects (8). It should be emphasized that all of the formulations improved the oral bioavailability of docetaxel compared with Taxotere but still remain well below the absolute bioavailability elicited by the same dose of docetaxel injected i.v.
We developed a unique polysorbate-free oral delivery system of docetaxel nanocapsules (NCs) embedded in gastro-resistant microparticles (MPs). Recently, we reported that the oral delivery of docetaxel NCs to rats elicited a higher bioavailability compared with the i.v. or oral administration of Taxotere (9). These unexpected results could only be explained if the pharmacokinetics of docetaxel had been modified (9). In this study, the drug absorption of the selected poly(dl-lactide-coglycolide) (PLGA) formulation was also investigated following oral administration to minipigs to verify if animal size might affect the oral bioavailability improvement observed in rats. Then, the in vivo efficacy of oral docetaxel NCs was investigated in mice using a metastatic lung cancer model as an additional milestone toward clinical trials. In addition, docetaxel organ biodistribution of the oral microparticulate formulation was investigated and compared with an oral solution of Taxotere, which had been combined with blank NCs embedded in MPs or i.v.-administered solutions of either docetaxel NCs or Taxotere alone. In addition, Lipiodol NCs were prepared and monitored by cryogenic-temperature transmission electron microscopy (cryo-TEM) following oral absorption of these NCs embedded in the MPs and respective controls in rats. Finally, to validate the results of the cryo-TEM, a third pharmacokinetic study of oral absorption of docetaxel-loaded nanoparticles (NPs) embedded in MPs was carried out in the absence and presence of cycloheximide, a protein synthesis inhibitor known to inhibit the secretion of chylomicrons from the enterocyte (10).
Results
Docetaxel Content in NCs and MPs.
The drug content of the two different batches of MPs embedding docetaxel NCs was 4% and 6% (wt/wt), with a nanoencapsulation efficiency >95%. The mean particle diameter of the batches of NCs with high and low docetaxel content was around 300 nm. The NPs exhibited a negative zeta potential value of −53 mV. SEM pictures of the MPs embedding docetaxel NCs are depicted in Fig. S1. Most of the MPs, with a diameter between 2 and 10 µm, are deflated due to the internal void volume collapse on drying of the SEM specimen (Fig. S1 A and B). The MPs kept their spherical structure, and no NCs were observed following incubation at pH 1.2 for 1 h (Fig. S1 C and D). The NCs were released only when immersed for 1 h at pH 7.4, because both polymers forming the microcapsule matrix dissolved above pH 5.5, as expected (Fig. S1 E and F). These results are confirmed by the in vitro kinetic results (Fig. S2).
Minipig Results.
The mean and individual docetaxel plasma levels produced by the NC-embedded microparticulate formulation were markedly higher than the respective levels yielded by Taxotere following oral administration (Figs. S3 and S4, respectively). The oral delivery in minipigs of docetaxel NC-loaded MPs elicited a significantly enhanced relative bioavailability compared with oral Taxotere, resulting in a calculated area under the plasma concentration-time curve (AUC) and Cmax values 10- and 8.4-fold higher, respectively (Table 1), confirming the rat pharmacokinetic results (9).
Table 1.
Comparing pharmacokinetic parameter values of docetaxel solution and formulation following oral administration in minipigs
| Oral formulation | Cmax (ng/mL) | AUC (h⋅ng/mL) | CL (L/h/kg) |
| Docetaxel solution* | 97.6 ± 221† | 797.7 ± 1,141† | 4 ± 2.8† |
| Docetaxel formulation‡ | 817.9 ± 558† | 7,923.1 ± 5,644† | 0.4 ± 0.5† |
Average pharmacokinetic parameter values (mean ± SD) following oral administration of 1.25 mg/kg of Taxotere in fasted minipigs, n = 6.
Cmax, AUC, and CL of 1 vs. 2: P < 0.03 significantly.
Average pharmacokinetic parameter values (mean ± SD) following oral administration of docetaxel-loaded NCs embedded in microparticles in fasted minipigs, n = 6.
Efficacy Study.
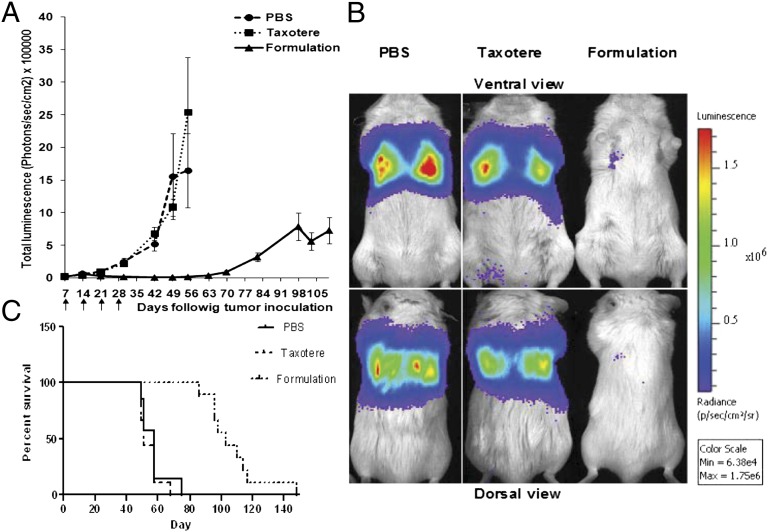
Tumor luminescence intensity demonstrated that in PBS- and Taxotere-treated animals, a rapidly progressing disease had already developed by 21 d after tumor inoculation, whereas the docetaxel NCs microparticulate formulation effectively inhibited tumor growth for up to 70 d following tumor cell injection. Absolute luminescence values measured in Taxotere- and PBS-treated animals were around 2 × 106 at 56 d, as opposed to 1.8 × 104 in NC-treated animals (Fig. 1 A and B). Notably, animal survival was prolonged by docetaxel NC treatment [formulation containing 4% (wt/wt) docetaxel] with 100% survival as opposed to 0% survival at 75 d in PBS- and Taxotere-treated animals. The first animal in the docetaxel NC formulation group died at day 90, and none survived past day 129 (Fig. 1C). Docetaxel NC oral delivery was well tolerated, inducing a slight but insignificant and reversible inhibition in weight gain during the initial treatment period (Fig. S5).
Fig. 1.
(A) Detection and evaluation of A549-luc-C8 metastatic lung cancer growth in SCID-bg mice using a CCCD camera. Mice were treated once weekly for 4 wk (treatment days are indicated by arrows). Results are presented as the average total luminescence in radiance units (photons/s/cm2) ± SEM, n = 9. No significant difference (P > 0.05) of tumor growth at days 28, 42, 49, and 56 was observed between PBS and Taxotere groups, whereas an extremely significant difference (P < 0.0001) was noted between PBS and Taxotere groups compared with the docetaxel formulation. (B) Representative images of the total tumor luminescence in tumor bearing mice at day 42 after tumor cells inoculation and 35 d following treatments. (C) Survival rate analysis comparing treatment arms.
Organ Biodistribution Results.
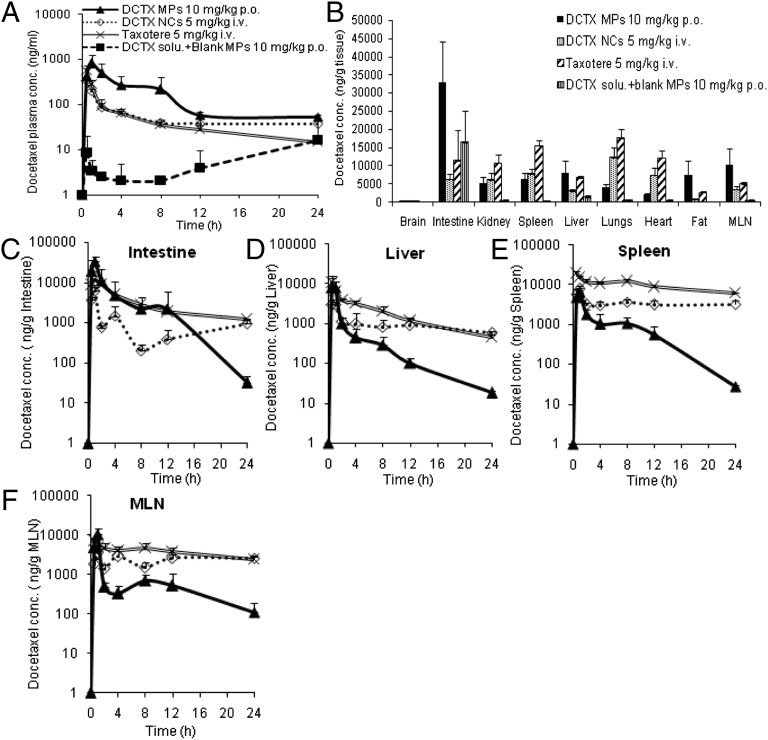
The average plasma docetaxel concentration profiles elicited by the docetaxel NCs embedded in MPs [6% (wt/wt), drug content] and Taxotere combined with blank NCs embedded in MPs administered orally, compared with Taxotere and docetaxel NCs injected i.v., are depicted in Fig. 2A. It can be observed that no docetaxel was absorbed from the oral Taxotere solution, whereas the microparticulate formulation again elicited the highest absorption profile, even compared with the Taxotere solution and docetaxel NCs injected i.v. with a normalized increase in AUC of 177% and 141%, respectively (Table 2). Most importantly, the docetaxel plasma profile and relative plateau occurring over 8 h is not reflected in the organ distribution profile (Fig. 2 and Fig. S6). Docetaxel was widely and rapidly distributed into most tissues following oral administration. The order in docetaxel AUC from the highest to the lowest for the oral formulation was as follows: intestine > spleen > mesenteric lymph node > lungs > liver > kidney > heart > fat > brain. It should be emphasized that the levels of docetaxel elicited by the NC-embedded MPs over time in the various organs were lower than for Taxotere or docetaxel NCs injected i.v. (Fig. 2 C–F and Fig. S6) with the exception of the intestine, mesenteric lymph node, and fat (Fig. 2B).
Fig. 2.
(A) Mean plasma docetaxel level–time profiles following i.v. administration of docetaxel NCs at a dose of 5 mg/kg, oral administration of docetaxel solution with blank MPs, or docetaxel formulation at a dose of 10 mg/kg, n = 4. (B) Organ distribution of docetaxel levels after oral administration of docetaxel formulation at a dose of 10 mg/kg at 1 h, n = 4. (C–F) Mean concentration–time profiles of docetaxel in tissues following i.v. administration of Taxotere or docetaxel NCs at a dose of 5 mg/kg, and oral administration of docetaxel solution with blank MPs, or docetaxel formulation at a dose of 10 mg/kg in fasted rats, n = 4.
Table 2.
Comparing pharmacokinetic parameters values of docetaxel following i.v. and oral administration in rats
| Formulation | Route | Cmax (ng/mL) | AUC (h⋅ng/mL) | CL (L/h/kg) |
| Taxotere | i.v. | — | 1,822 ± 350 | 2.8 ± 0.5 |
| Docetaxel NCs | i.v. | — | 2,289 ± 468 | 2.3 ± 0.5 |
| Docetaxel NCs | Oral | 132 | 270.9 | 36.9 |
| Docetaxel solution + blank MPs | Oral | 16.2 ± 30.6 | — | — |
| Docetaxel formulation | Oral | 1,022 ± 237 | 6,448 ± 2,439 | 1.7 ± 0.6 |
Cmax of row 5 vs. 4: P = 0.0002 extremely significant. AUC of 5 vs.1 and 2: P < 0.01 very significant. CL of 5 vs.1 and 2: P > 0.05 ns. i.v. administration of Taxotere and docetaxel NCs (mean ± SD) at a dose of 5 mg/kg, oral administration of docetaxel NCs, docetaxel solution with blank MPs, and docetaxel formulation (mean ± SD) at a dose of 10 mg/kg in fasted rats, n = 4.
Cryo-TEM Imaging.
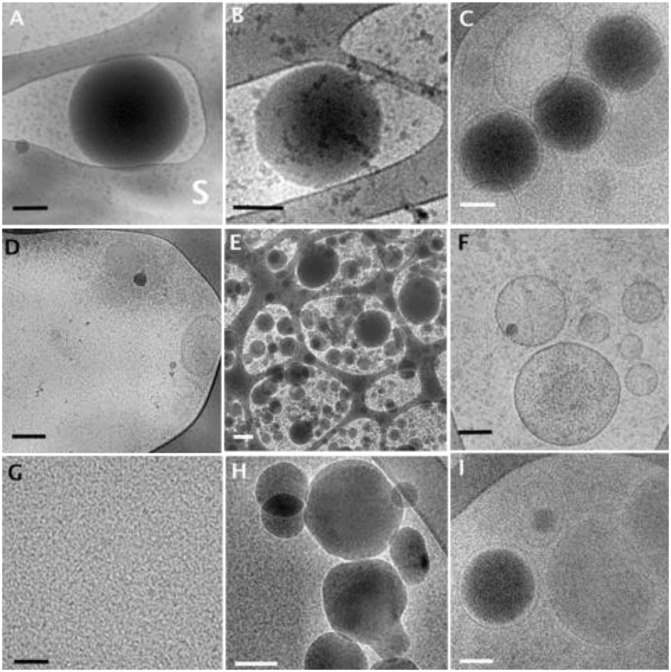
Lipiodol, an oil contrast agent, which can be detected by cryo-TEM because of its high electron density, was used to follow the path of the NCs either administered i.v. or orally following their embedding in the MPs. The interior structures of Lipiodol NCs (Fig. 3 A and B), serving as controls in water and plasma, respectively, were clearly distinguishable. Spherical structures with dark internal cores, attributed to Lipiodol, surrounded by a lighter rim due to the polymeric envelope/shell layer were noted. Particle size of the NCs was about 300 nm in diameter, confirming photon correlation spectroscopy (PCS) results. Fig. 3C shows three Lipiodol NCs at a time interval of 6 h following i.v. injection of the Lipiodol NCs to the rats. Only a few intact NCs could be seen in plasma compared with the plasma control micrograph of Lipiodol NCs, because of the high in vivo dilution (Fig. 3B). It can be observed that the structure of the NCs in plasma is preserved and is similar to the control. In contrast to i.v. administration, numerous intact NCs can be seen in the mesenteric lymph node extract at 0.5 h following oral administration of the Lipiodol NCs embedded in MPs, whereas at 1 h, their concentration decreased markedly (Fig. 3 E and F, respectively). More interestingly, the same electron-dense nanostructures can be detected in plasma at 0.5 and 1 h (Fig. 3 H and I) compared with their respective time 0 controls (Fig. 3 D and G). The micrograph of the NC in Fig. 3I clearly shows the presence of a second envelope around the NC resembling a vesicle (natural liposomes or semisynthetic chylomicron).
Fig. 3.
Cryo-TEM images of Lipiodol NCs. (A and B) Control Lipiodol NCs (A) in water; S denotes the perforated carbon support film. (B) In rat plasma. (C) Rat plasma at 6 h following i.v. administration of Lipiodol NCs into the jugular vein. (D–F) Extract of rat abdominal mesenteric lymph nodes at different time intervals following oral administration by gavage of embedded Lipiodol NCs in MP formulation: at 0 h (D); with nonspecific structures, at 0.5 h (E); a low-magnified image illustrating how many Lipiodol droplets are found, at 1 h (F). (G–I) Rat plasma samples at different time intervals following oral administration by gavage of embedded Lipiodol NCs in MP formulation at 0 (G), 0.5 (H), and 1 h (I). (Scale bars: A–D and F–I, 100 nm; E, 200 nm.)
Intestinal Lymphatic Transport Investigation Following Chylomicron Flow Blockade.
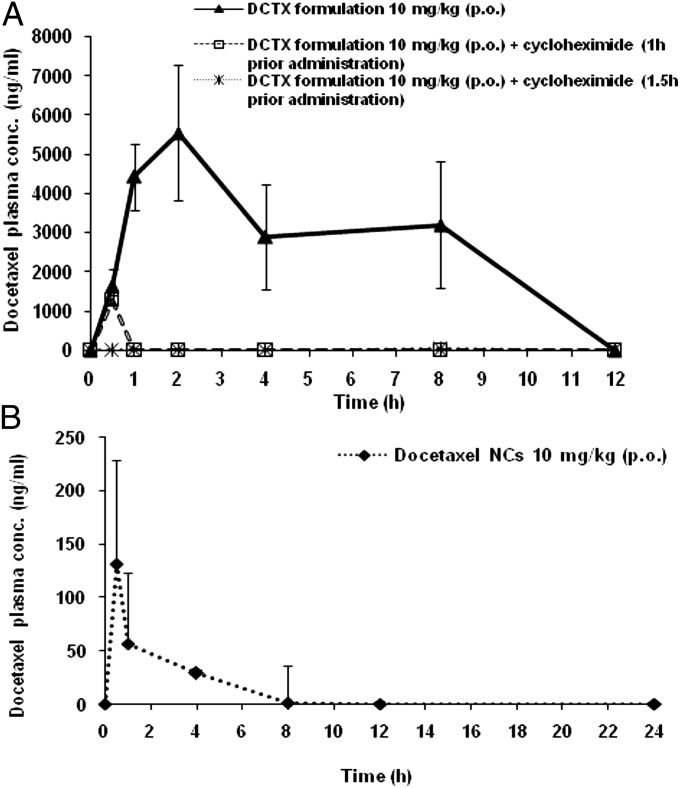
Fig. 4A exhibits data obtained in the preliminary pilot study, where the formulation was administered orally 1 h after injecting cycloheximide to rats. During the first 30 min, there was still an absorption of docetaxel, reaching 1,200 ng/mL in plasma after which there was a total blockade. However, in the docetaxel-treated group, when administered with cycloheximide 1.5 h in advance to allow it to fully exert its effects, no absorption of docetaxel was observed (Fig. 4A). In the docetaxel-treated group without cycloheximide, the absorption of docetaxel was markedly enhanced and confirmed previous results (Fig. 4A).
Fig. 4.
(A) Plasma docetaxel concentration–time profiles (±SD) obtained following oral administration of 10 mg/kg docetaxel MPs formulation to nontreated rats, n = 4, to cycloheximide-treated rats 1 h before administration, n = 2, and 1.5 h before administration, n = 4. (B) Plasma docetaxel concentration–time profiles following oral administration of docetaxel-NCs at a dose of 10 mg/kg to fasted rats, n = 5. The docetaxel NCs were prepared exactly as the NC of docetaxel formulation.
Discussion
The oral bioavailability of P-gp drug substrates, especially taxanes that exhibit very poor absorption, can be enhanced by encapsulation in polymeric NP, probably as a result of active NP uptake by enterocytes mediated by a transcytosis or endocytosis process (11). Different modulation strategies for the coating polymer have been applied: grafting of P-gp inhibitory moieties to the coating polymer (12) or surface functionalization of the NPs by folic acid (13). Although these strategies improved the oral bioavailability of docetaxel compared with the orally administered solution, none compared with the absolute bioavailability elicited by the i.v. administration of Taxotere at an identical dose. In contrast, our approach where docetaxel is dissolved in the oil core of PLGA NCs, which are embedded in MPs consisting of a blend of polymers (Eudragit L and hydroxypropylmethylcellulose (HPMC)), not only significantly improved oral bioavailability of docetaxel without affecting the physiological activity of the P-gp and CYP3A4, but also elicited much higher Cmax and AUC values than the respective values yielded by Taxotere injected i.v. at an equivalent dose (9). The results of the physicochemical characterization indicate that the experimental conditions for the preparation of the NC-loaded MP formulations were well controlled, as good reproducibility, in terms of particle size distribution, zeta potential, and drug content, was achieved. The difference in morphologies of the spherical micromatrices as a function of pH (Fig. S1) is due to the aqueous solubility properties of the polymers. Although HPMC is soluble in an aqueous solution irrespective of pH, Eudragit L dissolves only at a pH >5.5. The release of the NCs noted in Fig. S1 E and F at pH 7.4 should be attributed to the massive erosion of the MP matrices due to complete dissolution of HPMC and Eudragit L. The curves (Fig. S2) show the total amount of docetaxel released from the MPs (free and incorporated in NCs) following filtration of the sink solution samples using 0.8-µm filters, as well as the free released docetaxel fraction only following filtration using 300,000 molecular weight cutoff membranes. In fact, after 15 min, almost 75% of the docetaxel was released from the MPs, whereas less than 30% was released from the NCs.
Batches with different docetaxel contents in the NCs embedded in the MPs were prepared to evaluate the drug content effect on docetaxel oral absorption. The batch with 4% docetaxel in NCs was tested and reported in extensive previous rat studies (9). For the purpose of confirming the enhanced docetaxel oral absorption in large animals, in this study, the same batch was tested in minipigs, in addition to the efficacy evaluation in the metastatic SCID-bg mice model. Finally, in anticipation of future human studies where high drug-loaded formulations will be needed to reduce the orally administered total dose of formulation and limit of docetaxel detection, in the various organs, the biodistribution study was carried out with the 6% (wt/wt) drug content instead of 4% (wt/wt), keeping all of the other ingredient concentrations constant in the formulation.
It was noted from the individual minipig results that despite the marked deviation of one animal from each group, which in fact significantly increased the deviation ranges for Cmax and AUC of the NC-loaded MP formulation and docetaxel solution, the pharmacokinetic parameter values are significantly different (Table 1 and Figs. S3 and S4) and markedly in favor of the docetaxel NC formulation (Cmax and AUC of NCs vs. solution, P < 0.03). Consequently, the clearance (CL) values were decreased significantly (CL of docetaxel NCs vs. docetaxel solution, P < 0.03) as previously reported (9). It can also be deduced that the formulation is in fact gastro-resistant, because Cmax is delayed compared with the rat study, in which the formulation was diluted into an aqueous dispersion, whereas in the minipigs, the formulation was packed in a conventional solid-capsule dosage form (Fig. S4).
The very low absorption of docetaxel from the docetaxel solution with blank MPs (Fig. 2A) indicates that the blank MPs do not inhibit the P-gp pump in rats, confirming previous findings (9). Following i.v. administration, docetaxel NCs behavior is similar to Taxotere, suggesting that most of the docetaxel is rapidly released from the NCs in the plasma, where infinite dilution prevail. Furthermore, significant rapid elimination of the i.v. bolus in comparison with p.o. was observed in Fig. 2A, indicating a fast clearance of the drug via the liver. More importantly, the behavior of the orally administered docetaxel NCs embedded in MPs differs markedly from the behavior elicited by the docetaxel NCs injected i.v., because a controlled released profile is observed in the plasma over 8 h (Fig. 2A), suggesting that the NCs have apparently been modified by some natural physiological process while transiting via the enterocytes. This deduction is also confirmed by the organ biodistribution study results. With the exception of the mesenteric lymph node and fat tissues, the docetaxel levels elicited by the orally administered NC-embedded MPs were much lower than the levels of docetaxel yielded by Taxotere and docetaxel NCs injected i.v. (Fig. 2 B–F). Probably, the coating of the orally administered NCs had been reinforced significantly, reducing leakage and release of docetaxel from the oil in the plasma, subsequently altering the distribution within the organs. It should be stressed that the data for the orally administered Taxotere solution with the empty MPs are not shown in Fig. 2 C–F because of the low plasma concentrations yielded by such a combination. Furthermore, the docetaxel concentration in the brain was very low, irrespective of the formulation tested (Fig. 2B). There is no evidence that docetaxel NCs accumulate more in reticuloendothelial system (RES) tissues (liver, lungs, and spleen) compared with other tissues (Fig. 2 D and E). However, the marked concentrations of docetaxel noted in the intestine, mesenteric lymph node, and fat tissues (Fig. 2 B, C, and F), combined with the cryo-TEM findings (Fig. 3), indicate the involvement of the lymphatic route in NC oral absorption. These observations suggest that the NCs released from the MPs penetrated the enterocytes and moved into the circulation via the lymphatic system. The entrapment of the docetaxel NCs in the polymeric MP matrix is essential because the aqueous dispersion of the same docetaxel NCs administered orally elicited very low docetaxel plasma levels (Fig. 4B). These findings are not surprising because most of the entrapped docetaxel in the NCs is expected to be released in the gut lumen as the result of the occurrence of gastrointestinal sink conditions. The released docetaxel is subjected to the P-gp pump efflux yielding a bioavailability of 7.4% and 4.2% with respect to Taxotere and the oral MP formulation, respectively, as calculated from the respective values presented in Table 2.
The docetaxel NCs that reached and penetrated the enterocytes as a result of the MP coating are probably subjected to a physiological process resembling the process of chylomicron formation (14). Chylomicrons are triglyceride-rich particles ranging in size from 75 to 600 nm formed during the peak of lipid absorption and stabilized by active adsorption of surface constituents such as apoproteins and phospholipids (15). A similar process could be envisioned in the present study. The NC, when internalized in the enterocyte, is recognized as a triglyceride-rich particle. Then, the NC is subsequently assembled with the primordial lipoprotein particle that consists of a protein moiety, neutral lipids, and a phospholipid monolayer (NC-LP). This process, called core expansion, is the last event of the biosynthesis of chylomicron in agreement with the hypothesis of the sequential assembly of chylomicrons (16). Therefore, the lipoproteinated NCs are exocytosed from the enterocyte into the lamina propria like chylomicrons with access to the systemic circulation, through preferential uptake into the mesenteric lymphatic system (17). This hypothesis is supported by the cryo-TEM findings and organ biodistribution results. Indeed, in the present study, the surfaces of the NCs are modified in the enterocytes and are covered by apoproteins and phospholipids, as depicted in Fig. 3 H and I, where a second natural coating can be observed (Fig. 3I), typical of phospholipid layers (18). However, TEM evidence remains qualitative and needs to be validated by alternative quantitative independent techniques. For such a purpose, it was decided to apply the technique published by Dahan and Hoffman (16) who made use of cycloheximide (3 mg/kg), a protein synthesis inhibitor known to inhibit the secretion of chylomicrons from the enterocyte (10). They showed that even though the chylomicron flow blockade induced by cycloheximide is irreversible, this did not affect other absorption pathways. In our case, we started with a preliminary pilot pharmacokinetic study on two rats and observed, as depicted in Fig. 4A, that during the first 30 min, there was continual absorption of docetaxel until 1,200 ng/mL in plasma, followed by a total blockade. It was therefore decided to inject four additional rats with cycloheximide (3 mg/kg) and wait 1.5 h before orally administering NCs embedded in the MPs, to allow the cycloheximide to fully exert its effects. As noted from the curve in Fig. 4A, although the cycloheximide nontreated animal group elicited up to 5,500 ng/mL peak concentration of docetaxel in plasma and the absorption profile resembled previous profiles already reported, no absorption of docetaxel was observed in the treated group (Fig. 4A). It can be deduced that the use of a chemical inhibitor of chylomicron flow completely blocked the docetaxel absorption of the NC formulation. These findings confirm the cryo-TEM results and demonstrate that the oral absorption of the docetaxel NCs is mediated by the lymphatic transport system.
The formation and secretion of chylomicrons (or NC-LPs) are rapid as already reported (19), and noted in this study, because numerous Lipiodol NCs were detected at 0.5 h but disappeared after 1 h (Fig. 3 E and F). Similar findings were observed with the mesenteric lymph node from the second animal of each group, indicating that the results were formulation related. Apparently, these NCs, once internalized in the enterocytes and transformed into lipoproteinated drug nanocarriers, behave like chylomicrons. The NCs are transported and drain via the lymphatic pathway to the blood circulation through a physiological absorption process similar to the process of digested lipids and remain constrained to the plasma compartment until the adsorbed apoproteins and phospholipids surface coating are distributed to the HDL fraction of plasma (20). The chylomicron remnants are then eliminated by the liver. It was reported that the half-life of chylomicrons in the plasma ranged from 63 to 346 min in humans (21). Thus, we can anticipate similar behavior from the NCs-LP. Once the second lipoprotein coating is removed and distributed to the plasma HDL fraction, the sustained docetaxel retained within the NC oil core is rapidly released and eliminated, similar to docetaxel NCs injected i.v.. This phenomenon can explain the plasma profiles of the docetaxel elicited by the various NC-loaded MPs. In all these animal experiments, a relative plateau of docetaxel levels is noted up to 8 h after oral administration, with a sudden docetaxel concentration decrease afterward until final disappearance within the 12- to 24-h time interval. It should be emphasized that there is a discrepancy with some data published previously. We stated in our previous publication (9) that most of the docetaxel is not within the NCs, but rather free in the plasma protein fraction, considering that any docetaxel in the plasma protein fraction is free. This hypothesis was in fact a misinterpretation of the results, based on a wrong assumption. Indeed, most of the docetaxel was in the plasma protein fraction, not free, but rather entrapped in the NCs-LP (associated chylomicrons) that could not be separated like the free NCs, the fraction of which was negligible (9). In addition, in the same publication (9), we labeled the PLGA with a Near Infra-Red (NIR) fluorescent probe and quantitatively monitored the fluorescence with time in various organs including the liver. It was seen that the liver fluorescence increased moderately with time, reaching its maximum at 8 h after oral administration, as can be expected from chylomicron remnant elimination. All previous and current results support the hypothesis and confirm the results presented here. Therefore, if docetaxel is entrapped in the NCs-LP over time and constrained to the plasma compartment, without being eliminated by the organs of the RES, the issue of efficacy should be addressed. It can be noted that the unique oral NC formulation significantly improves docetaxel anticancer efficacy compared with Taxotere, both in tumor inhibition effect and the survival curve (Fig. 1). The hypothesis that the presence of protective modified NCs of docetaxel circulating in the bloodstream, while preventing massive drug release and being targeted passively via the enhanced permeability and retention (EPR) effect (22) or extravasated within the lung tumors of the mice, appears a plausible explanation for the markedly improved efficacy, in agreement with the overall results of the organ biodistribution study. It is more and more recognized that active targeting mediated by nanocarriers is complex and should be explored on a case-by-case basis, as reported by Farokhzad and Langer (23), and recently highlighted by Kwon et al. (24). Indeed, NPs tend to passively extravasate through the leaky vasculature, characteristic of solid tumors and inflamed tissue, and preferentially accumulate in the tumors (24). In this present study, the docetaxel-loaded NCs-LP probably accumulated in the lung tumors and exerted a marked therapeutic action. To the best of our knowledge, no study has reported any orally administered nanoparticulate formulation that possibly elicited a passive systemic targeting in solid tumors, because intact surface-modified NCs permeated the enterocytes, behaved as a nanotransporter without markedly affecting the physiologic activity of P-gp, and reached the circulation via the intestinal lymphatic system, bypassing all of the biochemical barriers. The overall results may advocate the feasibility of reducing the dose and intensifying the tumor-tissue targeting ability of docetaxel following oral administration.
In conclusion, the significant improvement in docetaxel oral absorption should be attributed to the unique nanoplatform. The lymphatic route was shown to be involved in NC absorption, therefore circumventing the first-pass effects and protecting the drug from CYP3A4 degradation either in the gut or systemic circulation, resulting in higher drug blood levels than those achieved with the i.v.-injected free drug, which was markedly metabolized by CYP3A4 in the circulation. Overall, the findings are encouraging and represent further progress in the steps toward clinical studies in humans, at least in the treatment of solid tumors widely irrigated by the leaky vasculature. To the best of our knowledge, no study has reported that NCs have been reported to be subjected to such a lymphatic absorption process and reinforced during their transit.
Materials and Methods
A summary of the techniques is presented here. For full details for materials and methods, please see SI Materials and Methods.
Preparation of Docetaxel-Loaded NCs for Embedding in the MPs.
Docetaxel NCs were prepared as follows (9): 1,500 mg of glyceryl tributyrate, 300 mg of oleoyl polyoxylglycerides, 300 mg of PLGA 4,000 Da, and an increasing amount of docetaxel starting from 180 mg were dissolved in 100 mL acetone. Then, with constant stirring, 70 mL of bidistilled water was slowly added to the organic phase.
Microencapsulation of the NCs Using the Spray-Drying Method.
To prepare microencapsulated NC formulations, aqueous solutions containing Eudragit L and HPMC solution, the exact composition of which is described in SI Materials and Methods, were combined and added to the NC dispersed mixture. The final suspension was diluted to 500 mL with bidistilled water. The PLGA suspension was spray-dried with a Buchi mini spray-dryer B-290. The powder consisting of NCs embedded in spherical MPs was collected in the cyclone separator and weighed.
Two separate batches containing 4% and 6% (wt/wt) docetaxel were used in these studies. The determination of docetaxel content in NCs, the drug content in the MPs, the measurement of the zeta potential, particle size distribution, SEM studies, and in vitro drug release profile evaluation under sink conditions are depicted in SI Materials and Methods.
Animal Experiments.
All animal experimentation was carried out in accordance with the rules and guidelines concerning the care and use of laboratory animals and was approved by the Authority for Biological and Biomedical Models at the Hebrew University of Jerusalem.
The minipig absorption experiment was designed as a cross-over study involving six animals. The in vivo efficacy studies of docetaxel NCs metastatic lung tumors in SCID-bg mice was established following injection to the tail of A549-luc-C8.
The organ biodistribution evaluation was carried out in healthy Sprague–Dawley male rats, as well as the detection of Lipiodol NCs in plasma by cryo-TEM following oral and i.v. administration. All animal studies are described in detail in SI Materials and Methods.
Intestinal Lymphatic Transport Investigation Following Chylomicron Flow Blockade.
In this study, Sprague–Dawley male rats weighing 300–325 g were used. They were fasted overnight and had free access to water. Eight animals were divided randomly into two groups of four as follows. Group I (A, B, C, and D) was administered orally by gavage with the docetaxel MP formulation (BI) at a dose of 10 mg/kg. Group II (E, F, G, and H) was treated i.p. with cycloheximide (3 mg/kg) dissolved in saline [3 mg/mL (wt/vol)]. Ninety minutes after injection, the animals were given an oral gavage of the docetaxel MP formulation (BI).
Cycloheximide is a protein synthesis inhibitor known to inhibit the secretion of chylomicrons from the enterocyte, apparently through antimicrotubular effects, thus inhibiting lymphatic transport (16). A preliminary pilot study using two rats was carried out where the formulation was administered orally 1 h after injecting cycloheximide.
Blood samples (∼0.5 mL) were collected from the tail in heparin-containing tubes at 0, 0.5, 1, 2, 4, 8, and 12 h and immediately centrifuged at 2370 × g for 5 min, after which the plasma was transferred to clean tubes. All samples were immediately frozen at −80 °C until analyzed by LC/MS-MS.
Supplementary Material
Acknowledgments
This research was supported in part by the Israel Science Foundation (Grant 576/08).
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1313839110/-/DCSupplemental.
References
- 1.Aapro M. The scientific rationale for developing taxoids. Anticancer Drugs. 1996;7(Suppl 2):33–36. [PubMed] [Google Scholar]
- 2.Engels FK, Verweij J. Docetaxel administration schedule: From fever to tears? A review of randomised studies. Eur J Cancer. 2005;41(8):1117–1126. doi: 10.1016/j.ejca.2005.02.016. [DOI] [PubMed] [Google Scholar]
- 3.van Waterschoot RA, et al. Absence of both cytochrome P450 3A and P-glycoprotein dramatically increases docetaxel oral bioavailability and risk of intestinal toxicity. Cancer Res. 2009;69(23):8996–9002. doi: 10.1158/0008-5472.CAN-09-2915. [DOI] [PubMed] [Google Scholar]
- 4.Oostendorp RL, et al. Coadministration of ritonavir strongly enhances the apparent oral bioavailability of docetaxel in patients with solid tumors. Clin Cancer Res. 2009;15(12):4228–4233. doi: 10.1158/1078-0432.CCR-08-2944. [DOI] [PubMed] [Google Scholar]
- 5.Yan YD, et al. Effect of dose and dosage interval on the oral bioavailability of docetaxel in combination with a curcumin self-emulsifying drug delivery system (SEDDS) Eur J Drug Metab Pharmacokinet. 2012;37(3):217–224. doi: 10.1007/s13318-011-0078-1. [DOI] [PubMed] [Google Scholar]
- 6.Saremi S, Atyabi F, Akhlaghi SP, Ostad SN, Dinarvand R. Thiolated chitosan nanoparticles for enhancing oral absorption of docetaxel: Preparation, in vitro and ex vivo evaluation. Int J Nanomedicine. 2011;6:119–128. doi: 10.2147/IJN.S15500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Koolen SLW, Oostendorp RL, Beijnen JH, Schellens JHM, Huitema ADW. Population pharmacokinetics of intravenously and orally administered docetaxel with or without co-administration of ritonavir in patients with advanced cancer. Br J Clin Pharmacol. 2010;69(5):465–474. doi: 10.1111/j.1365-2125.2010.03621.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Di Maio M, et al. Individual patient data meta-analysis of docetaxel administered once every 3 weeks compared with once every week second-line treatment of advanced non-small-cell lung cancer. J Clin Oncol. 2007;25(11):1377–1382. doi: 10.1200/JCO.2006.09.8251. [DOI] [PubMed] [Google Scholar]
- 9.Nassar T, et al. High plasma levels and effective lymphatic uptake of docetaxel in an orally available nanotransporter formulation. Cancer Res. 2011;71(8):3018–3028. doi: 10.1158/0008-5472.CAN-10-3118. [DOI] [PubMed] [Google Scholar]
- 10.Sabesin SM, Isselbacher KJ. Protein synthesis inhibition: Mechanism for the production of impaired fat absorption. Science. 1965;147(3662):1149–1151. doi: 10.1126/science.147.3662.1149. [DOI] [PubMed] [Google Scholar]
- 11. Herrero EP, Alonso MJ, Csaba N (2012) Polymer-based oral peptide nanomedicines. Ther Deliv 3(5):657–668. [DOI] [PubMed]
- 12.Feng S-S, Mei L, Anitha P, Gan CW, Zhou W. Poly(lactide)-vitamin E derivative/montmorillonite nanoparticle formulations for the oral delivery of Docetaxel. Biomaterials. 2009;30(19):3297–3306. doi: 10.1016/j.biomaterials.2009.02.045. [DOI] [PubMed] [Google Scholar]
- 13.Roger E, et al. Folic acid functionalized nanoparticles for enhanced oral drug delivery. Mol Pharm. 2012;9:2103–2110. doi: 10.1021/mp2005388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Bisgaier CL, Glickman RM. Intestinal synthesis, secretion, and transport of lipoproteins. Annu Rev Physiol. 1983;45:625–636. doi: 10.1146/annurev.ph.45.030183.003205. [DOI] [PubMed] [Google Scholar]
- 15.Zilversmit DB. The composition and structure of lymph chylomicrons in dog, rat, and man. J Clin Invest. 1965;44(10):1610–1622. doi: 10.1172/JCI105267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Dahan A, Hoffman A. Evaluation of a chylomicron flow blocking approach to investigate the intestinal lymphatic transport of lipophilic drugs. Eur J Pharm Sci. 2005;24(4):381–388. doi: 10.1016/j.ejps.2004.12.006. [DOI] [PubMed] [Google Scholar]
- 17.Porter CJH, Trevaskis NL, Charman WN. Lipids and lipid-based formulations: Optimizing the oral delivery of lipophilic drugs. Nat Rev Drug Discov. 2007;6(3):231–248. doi: 10.1038/nrd2197. [DOI] [PubMed] [Google Scholar]
- 18.Konikoff FM, Danino D, Weihs D, Rubin M, Talmon Y. Microstructural evolution of lipid aggregates in nucleating model and human biles visualized by cryogenic transmission electron microscopy. Hepatology. 2000;31(2):261–268. doi: 10.1002/hep.510310202. [DOI] [PubMed] [Google Scholar]
- 19.Jersild RA., Jr A time sequence study of fat absorption in the rat jejunum. Am J Anat. 1966;118(1):135–162. doi: 10.1002/aja.1001180108. [DOI] [PubMed] [Google Scholar]
- 20.Tall AR, Green PHR, Glickman RM, Riley JW. Metabolic fate of chylomicron phospholipids and apoproteins in the rat. J Clin Invest. 1979;64(4):977–989. doi: 10.1172/JCI109564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Berr F. Characterization of chylomicron remnant clearance by retinyl palmitate label in normal humans. J Lipid Res. 1992;33(6):915–930. [PubMed] [Google Scholar]
- 22.Maeda H, Bharate GY, Daruwalla J. Polymeric drugs for efficient tumor-targeted drug delivery based on EPR-effect. Eur J Pharm Biopharm. 2009;71(3):409–419. doi: 10.1016/j.ejpb.2008.11.010. [DOI] [PubMed] [Google Scholar]
- 23.Farokhzad OC, Langer R. Impact of nanotechnology on drug delivery. ACS Nano. 2009;3(1):16–20. doi: 10.1021/nn900002m. [DOI] [PubMed] [Google Scholar]
- 24.Kwon IK, Lee SC, Han B, Park K. Analysis on the current status of targeted drug delivery to tumors. J Control Release. 2012;164(2):108–114. doi: 10.1016/j.jconrel.2012.07.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.