Significance
Breast milk provides all nutrients required for the growth and development of the newborn child. In addition to energy source, milk contains other biomolecules, such as oligosaccharides, which contribute to the intestinal colonization through microbiota and development of mucosal immunity. This study shows that specific milk oligosaccharides stimulate intestinal immune responses through binding and activation of dendritic cells. We demonstrate that the milk oligosaccharide sialyl(α2,3)lactose is directly recognized by dendritic cells, resulting in a proinflammatory stimulation. This finding provides evidence that oligosaccharides from dietary sources indeed modulate mucosal immunity.
Keywords: carbohydrate, glycan, prebiotics, mouse innate immunity
Abstract
Breast milk oligosaccharides shape the intestinal environment by affecting mucosal immunity and bacterial colonization. To clarify the role of milk oligosaccharide sialyl(α2,3)lactose (3SL) in intestinal physiology and disease, we investigated colitis development in Il10−/− mice exposed to normal or 3SL-deficient milk during lactation. Onset and progression of intestinal inflammation were delayed in Il10−/− mice deficient for the α2,3 sialyltransferase 4 (ST3GAL4) responsible for 3SL biosynthesis. The proinflammatory role of 3SL was confirmed by showing that oral supplementation of newborn Il10−/−;St3gal4−/− mice with 3SL increased colitis severity. Conversely, fostering of newborn Il10−/− mice to lactating St3gal4−/− mothers reduced colitis severity. 3SL directly stimulated mesenteric lymph node CD11c+ dendritic cells and induced production of cytokines required for expansion of TH1 and TH17 T cells. The stimulatory effect of 3SL was attenuated in Tlr4-deficient CD11c+ cells, demonstrating that 3SL induces inflammation through Toll-like receptor 4 (TLR4) signaling. Thus, 3SL directly modulates mucosal immunity, which increases susceptibility to colitis.
Inflammatory bowel disease (IBD) affects up to 0.8% of the Western population and this number is constantly growing worldwide (1). The etiology of IBD is not fully understood, although a number of genetic and environmental factors leading to aberrant mucosal immune responses have been identified (2). Nutrition and especially breastfeeding affects the risk for IBD (3). Breastfeeding for at least 3 mo, accordingly, contributes to a lower incidence for IBD (4); however, these data still are controversial. Oligosaccharides are major constituents of breast milk fulfilling various functions, such as promoting growth of beneficial bacteria, acting as soluble receptors preventing attachment of pathogens in the gastrointestinal tract, and reducing adhesion of leukocytes (4, 5). The exact functions of individual oligosaccharides remain however largely unknown.
The limited structural diversity of mouse milk oligosaccharides, including only sialyl(α2,3)lactose (3SL) and sialyl(α2,6)lactose (6SL), enables assessing the specific functional contribution of these oligosaccharides to intestinal homeostasis (6). A recent study provided unique evidence that milk-derived 3SL, but not 6SL, increased susceptibility to dextran sulfate sodium (DSS)-induced acute colitis (6). These findings indicate that individual milk oligosaccharides may not only mediate protective effects, but may promote inflammation as well.
Mucosal innate immunity has a pivotal role in regulating inflammatory responses (2). Dendritic cells (DCs) and macrophages sense luminal antigens and provide signals for induction of tolerance to ingested antigens and commensal bacteria or for initiation of inflammatory immune responses, facilitating activation of adaptive immunity (7, 8). Intestinal DCs consist of two functionally distinct subsets based on expression of CD103 and chemokine (C-X3-C motif) receptor 1 (CX3CR1) (9). CD103+CX3CR1− cells are the main population of migratory intestinal DCs influencing regulatory T cells in a TGF-β and retinoic-acid–dependent manner (10, 11). In contrast, CD103−CX3CR1+ DCs are resident cells sampling luminal antigens, thereby initiating local immune responses (12).
Mucosal immunity is a key to the maintenance of gut homeostasis. Several classes of pattern recognition receptors mediate innate immune responses of intestinal epithelial cells and lamina propria-resident leukocytes. Toll-like receptors (TLR) and Nod-like receptors facilitate the recognition of bacterial fragments such as peptidoglycan (TLR2), lipopolysaccharides (TLR4), flaggelin (TLR5), unmethylated CpG DNA sequences (TLR9), and muramyl di- and tripeptides (Nod1 and Nod2) (13). In particular, glycans are recognized by different families of receptors, such as C-type lectins, galectins, and siglecs, which are expressed both by antigen-presenting cells and intestinal epithelial cells (14). Although, beneficial effects of milk oligosaccharides are well known, the mechanisms mediating the “sensing” of these oligosaccharides remain to be determined.
The present study addresses the proinflammatory effect of milk oligosaccharide 3SL in contributing to spontaneous colitis in Il10−/− mice. The 3SL supplementation of adult mice demonstrated the ability of milk oligosaccharides to directly affect mucosal immunity.
Results
α2,3-Sialic Acid Contributes to the Early Onset and Development of Colitis in Il10−/− Mice.
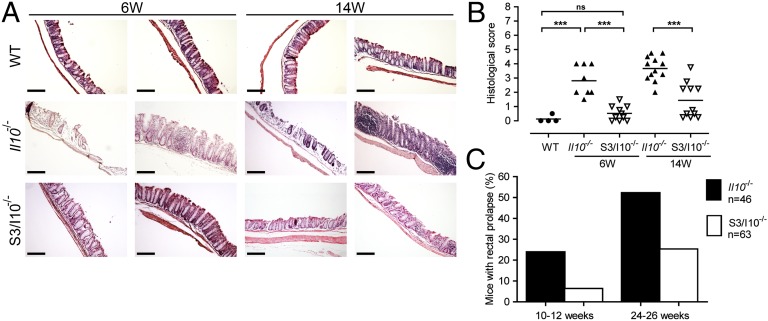
The α2,3-sialyltransferase 4 (ST3GAL4) synthesizes sialyl(α2,3)lactose (3SL) in lactating mammary gland. Previously, we have shown that milk oligosaccharide 3SL exacerbates acute colitis in mice (6). To address the contribution of 3SL in a model of spontaneous chronic intestinal inflammation, we generated and studied double St3gal4−/−;Il10−/− mice (S3/I10−/−). The absence of anti-inflammatory cytokine IL-10 leads to increased immune response against commensal microbiota and thereby to chronic colitis (15). S3/I10−/− mice were compared with St3gal4+/+;Il10−/− (further referred to as Il10−/−) mice and colitis development was monitored at 6 and 14 wk of age. Histological analysis of Il10−/− mice revealed severe colitis in the distal colon at the age of 6 wk accompanied by massive leukocyte infiltration, loss of crypts, and epithelial damage (Fig. 1A). In contrast, S3/I10−/−mice showed no histological alterations at the same age. By 14 wk, S3/I10−/− mice showed delayed onset of intestinal inflammation as histologically assessed (Fig. 1B). The incidence of prolapsed rectum, a sign of overt intestinal inflammation (16), was monitored for up to 46 wk. Prolapsed rectum was threefold more frequent at the age of 10–12 wk and twice more frequent by the age of 24–46 wk in Il10−/− mice than in S3/I10−/− mice (Fig. 1C).
Fig. 1.
St3gal4 deficiency attenuates spontaneous intestinal inflammation in Il10−/− mice. (A) Microscopic analysis of colon sections from 6- and 14-wk-old Il10−/− and S3/I10−/− mice, stained with H&E. Representative images from three independent experiments. (Scale bar, 200 μm.) (B) Histological score based on evaluation of morphological changes of epithelium and immune cell infiltration. Six- and 14-wk-old Il10−/− and S3/I10−/− mice (n = 8–12) were analyzed. (C) Frequency of rectal prolapse in Il10−/− and S3/I10−/− mice. Il10−/− mice (n = 46) and S3/I10−/− mice (n = 63) were monitored over a period of 26 wk. The graph represents percentage of mice with rectal prolapse from the total number mice at the age of 10–12 wk and 24–26 wk, respectively. WT, wild-type mice; S3/I10−/−, St3g4−/−; Il10−/− mice; 6W, 6-wk-old mice; 14W, 14-wk-old mice.
St3gal4 Deficiency Decreases Leukocyte Infiltration.
To assess the effect of St3gal4 deficiency on intestinal inflammation in Il10−/− mice, we analyzed leukocytes both in blood circulation and in colonic lamina propria from Il10−/− and S3/I10−/− mice. Peripheral blood leukocytes isolated from 6-wk-old mice showed twofold increase in CD11b+ cells in Il10−/− mice, whereas no changes were visible in S3/I10−/− mice compared with wild-type (WT) mice (Fig. S1A). In addition, twofold increases in proinflammatory Ly-6Chi monocytes and Ly-6G+ cells were observed in Il10−/− mice, indicating an inflammatory response that was unseen in the circulation of S3/I10−/− mice (Fig. S1A).
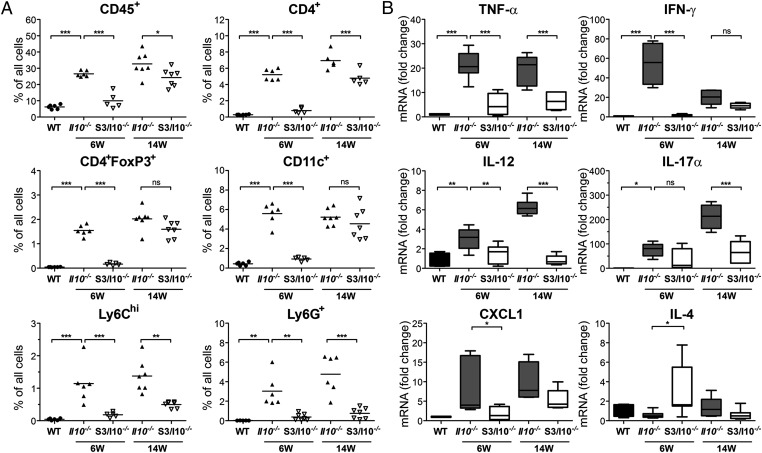
Analysis of lamina propria leukocytes (LPLs) revealed no changes in leukocyte numbers (CD45+ cells) in S3/I10−/− mice compared with WT controls at 6 wk of age (Fig. 2A and Fig. S2). A threefold increase in CD45+ cell infiltration was however observed in Il10−/− mice. Increase in CD3+ T cells (composed of CD4+, CD8+, and CD4+FoxP3+ cells), Ly-6Chi inflammatory monocytes, Ly-6G+ granulocytes, and CD11c+ cells was observed in Il10−/− mice (Fig. 2A and Fig. S1B). Interestingly, immune cell subpopulations remained in S3/I10−/− mice at similar levels as found in healthy WT mice. Thus, intestinal inflammation in Il10−/− mice was accompanied by a 10-fold increase in CD3+CD4+ T cells (5% in Il10−/− mice vs. 0.5% in WT mice) that could be further identified as an increase in TH1 and TH17 cells, based on mRNA expression of IFN-γ, TNF-α, and IL-17α, respectively (Fig. 2B and Table S1). Although increased numbers of regulatory T cells (Treg, CD4+FoxP3+) were observed in Il10−/− mice, their inability to produce IL-10 had no effect on inflammation (Fig. 2A). In addition, infiltration of CD11c+ cells together with proinflammatory Ly-6Chi monocytes were a sign of pronounced colonic inflammation, as previously observed in patients with IBD (17). Differences in leukocyte infiltration between Il10−/− (33%) and S3/I10−/− mice (22%) persisted at 14 wk of age (Fig. 2A and Fig. S1B).
Fig. 2.
Decreased leukocyte infiltration and inflammation in colons of St3gal4−/−/Il10−/− mice. (A) Flow cytometry analysis of lamina propria leukocytes (LPLs) isolated from a distal part of the colon of 6- and 14-wk-old Il10−/− and S3/I10−/− mice (n = 5–7) or 6- to 8-wk-old WT controls. Data are presented as percentage of CD45+, CD4+, CD4+FoxP3+ (Treg), CD11c+, Ly6Chi, and Ly6G+ cells from all isolated cells. (B) Expression levels of cytokines in colons of 6- and 14-wk-old Il10−/−, S3/I10−/− mice (n = 6–8) and WT control mice (n = 5–6) were determined by real-time PCR and normalized to GAPDH.
The difference in colonic inflammation was further corroborated by analysis of cytokine expression in the colon tissue samples. Increased levels of IFN-γ, TNF-α, and IL-17α transcripts were detected in Il10−/− but not in S3/I10−/− mice, indicating that St3gal4 products contribute to TH1/TH17-driven intestinal inflammation (Fig. 2B and Fig. S1C). In contrast, increased IL-4 expression was observed in S3/I10−/− mice, indicating Th2-mediated control of intestinal inflammation. The enhanced infiltration of CD11c+ cells correlated with the large increase of IL-12 expression in Il10−/− mice, which is mainly produced by activated DCs (Fig. 2B). These results indicate that oligosaccharide products of ST3GAL4 aggravate the development of spontaneous colitis in the absence of IL-10.
Milk Oligosaccharide 3SL Aggravates Intestinal Inflammation.
Milk oligosaccharides act as prebiotics that may affect maturation of intestinal mucosal immunity (18). We tested whether the absence of 3SL during lactation affects development of spontaneous colitis in Il10−/− mice. Il10−/− newborn mice were cross-fostered to WT or ST3gal4−/− (3SL-deficient milk) mothers and mice were analyzed at the age of 6 wk. In comparison with mice fed on 3SL-containing milk, a twofold decrease of infiltrating CD45+ cells in the lamina propria was observed in Il10−/− mice fed on 3SL-deficient milk (Fig. S3A). All leukocyte subpopulations previously detected during intestinal inflammation were decreased in Il10−/− mice fed on 3SL-deficient milk. Specifically, CD3+ T cells and their CD4+, CD8+, and CD4+FoxP3+ subsets were decreased by fourfold, CD11b+ myeloid cells, Ly-6Chi and Ly-6G+, and CD11c+ cells by threefold. To confirm changes in the inflammation status of Il10−/− mice upon feeding with 3SL-deficient milk, we analyzed cytokine gene expression in colon specimen by real time-PCR (Fig. S3B). Accordingly, TNF-α, IL-17α, IL-1β, and IL-12 transcripts were decreased in Il10−/− mice fed with 3SL-deficient milk compared with Il10−/− mice fed with 3SL-containing milk. Of note, we did not detect any difference in IFN-γ gene expression.
We tested whether milk-derived 3SL alters microflora and thereby affects spontaneous inflammation in Il10−/− mice. The comparison of microflora of 3-wk-old mice showed reduction in Enterobacteriaceae, clostridial cluster IV, and Lactobacillaceae in S3/I10−/− mice compared with Il10−/− mice (Fig. S4A). To assess whether specific microbiota can be transferred between Il10−/− and S3/I10−/− mice and consequently alter the onset of inflammation, we performed a cohousing experiment. Il10−/− and S3/I10−/− young mice were housed conventionally or cohoused together for 4 wk after weaning. Analysis of cohoused Il10−/− and S3/I10−/− mice revealed no major changes in bacterial composition between both groups of mice (Fig. S4A). Similarly, cohousing did not alter onset of inflammation as determined by analysis of infiltrating leukocytes (Fig. S4B). The analysis of mice from cross-fostering experiments did not reveal any major changes in bacterial composition among the groups (Fig. S3C). Taken together, the exposure to 3SL during lactation increased susceptibility to colitis, indicative of a long-lasting effect on mucosal immunity.
3SL Supplementation Facilitates Development of Chronic Colitis.
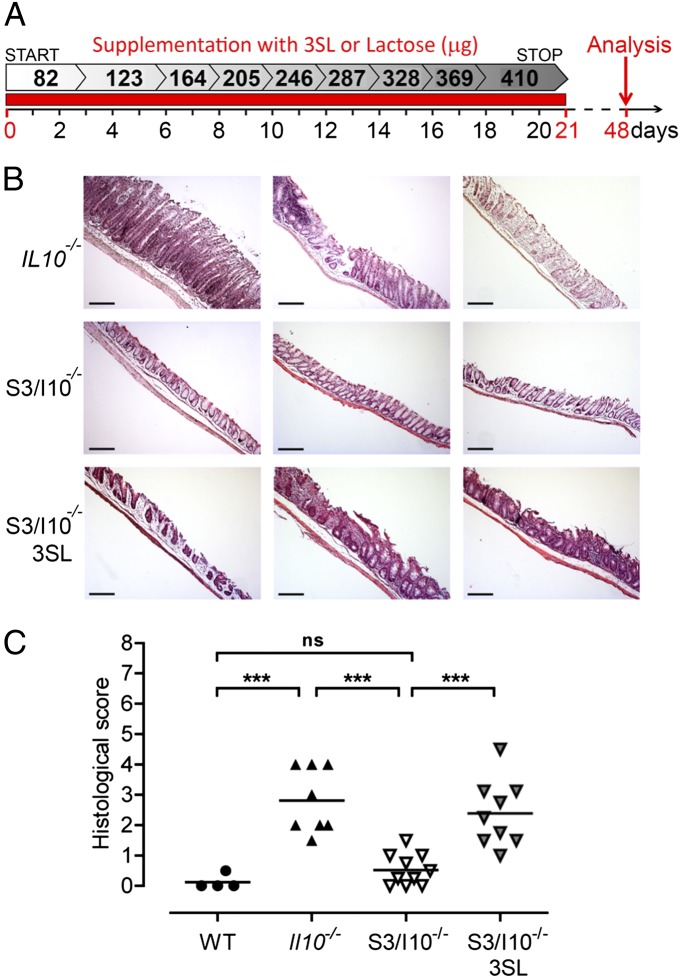
To demonstrate that exposure to 3SL during lactation sustainably affects mucosal immunity, we supplemented S3/I10−/− mice orally with 3SL, lactose, or water during the first 3 wk of life and monitored inflammatory parameters at the age of 6 wk (Fig. 3A). Histological analysis in 3SL-supplemented S3/I10−/− mice revealed colitis scores comparable to those observed in Il10−/− mice of the same age (Fig. 3 B and C). Increased infiltration of CD45+ cells was detected in colon tissues of 3SL-supplemented S3/I10−/− mice compared with lactose or water-treated controls (Fig. S5A). Accordingly, elevated numbers of CD11c+ and Ly-6Chi and CD4+ cells correlated with aggravated intestinal inflammation observed in Il10−/− mice (Fig. S5A). Increased transcript levels of TH1-associated cytokines, IFN-γ, TNF-α, and IL-12, were detected in 3SL-supplemented S3/I10−/− mice compared with controls (Fig. S5B). In addition, IL-17α was also increased in 3SL-supplemented mice. Increased expression levels of CXCL1 correlated with Ly-6G+ cell infiltration in the colon of 3SL-supplemented mice (Fig. S5B). Of note, IL-4 expression levels were higher in S3/I10−/− control mice (Fig. S5B).
Fig. 3.
3SL supplementation aggravates colonic inflammation. S3/I10−/− mice were fed daily from birth until weaning (21 d) with 25 mM 3SL or lactose; control mice were fed with water. Mice were analyzed at the age of 6 wk (day 48). (A) Schematic representation of 3SL and lactose supplementation. (B) Representative microscopic images (n = 9) of colon sections from 6-wk-old Il10−/− and S3/I10−/− mice either with or without 3SL supplementation stained with hematoxylin and eosin. (Scale bar, 200 μm.) (C) Histological score based on evaluation of morphological changes of epithelium and immune cell infiltration from WT (n = 4), Il10−/−, and S3/I10−/− mice (n = 8–9). WT, wild-type mice; S3/I10−/−, St3g4−/−; Il10−/− mice; 3SL, sialyl(α2,3)lactose.
Dendritic Cells Are Major Mediators of Intestinal Inflammation.
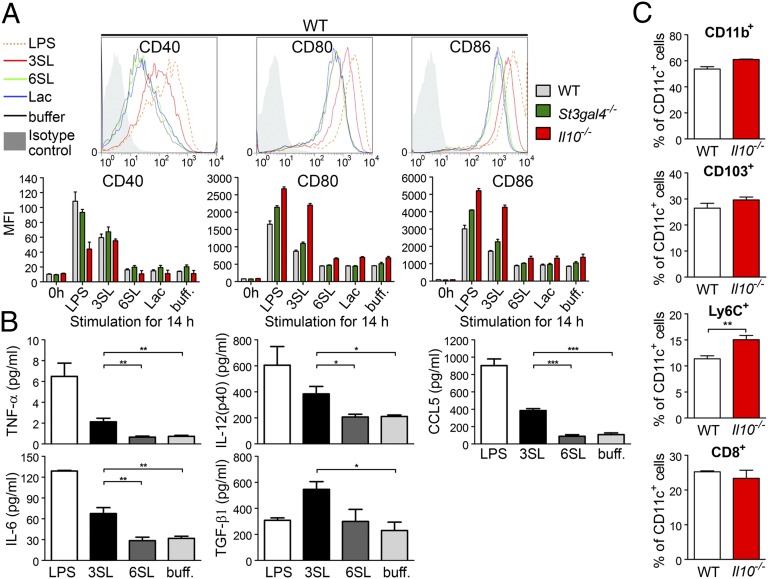
Intestinal DCs sample luminal antigens and trigger either proinflammatory or tolerogenic responses (12). Our observations of increased levels of CD11c+ cells infiltrating colons of St3gal4+/+ mice but not St3gal4−/− mice (Fig. 2A) suggested an involvement of DCs in 3SL-mediated colitis. To test whether 3SL can activate DCs directly, we stimulated mesenteric lymph-node–derived DCs with increasing 3SL concentration (Fig. S6A). Stimulation of DCs with 3SL for 14 h increased CD40, CD80, and CD86 expression to levels observed upon LPS stimulation (Fig. 4A and Fig. S6B). Strikingly, the structurally similar oligosaccharide 6SL failed to stimulate DCs. DCs isolated from St3gal4−/− mice responded to 3SL stimulation to the same extent as DCs from WT mice, even though these cells were not previously exposed to 3SL in vivo. DCs isolated from Il10−/− mice showed elevated expression of CD80 and CD86 markers, indicating that these cells were already primed in vivo by ongoing intestinal inflammation. Increased levels of IL-6, IL-12, TNF-α, and the inflammatory chemokine (C-C motif) ligand 5 (CCL5) were detected in 3SL-stimulated DCs but not 6SL-stimulated cells (Fig. 4B). Finally, 3SL stimulation resulted in increased TGF-β1 production, which is linked to induction of TH17 differentiation in the inflammatory environment (19).
Fig. 4.
3SL directly stimulates dendritic cells (DCs). DCs were isolated from mesenteric lymph nodes of 6-wk-old WT, St3gal4−/−, and Il10−/− mice and purified with CD11c MicroBeads. (A) CD11c+ cells were stimulated for 14 h with 625 μM of 3SL, 6SL, or lactose (Lac). Stimulations with LPS (500 ng/mL) or PBS (buff.) were used as controls. Cell surface expression of CD40, CD80, and CD86 was analyzed by flow cytometry. (B) Measurement of secreted cytokines in the medium of stimulated CD11c+ cells. (C) Increase in Ly-6C+/CD11c+ cells in Il10−/− mice during inflammation. Data are presented as percentage of CD11b+, CD103+, Ly-6C+, or CD8α+ cells from CD11c+ cells (n = 6). MFI, mean fluorescence intensity; 3SL, sialyl(α2,3)lactose; 6SL, sialyl(α2,6)lactose.
3SL Stimulates Ly-6Chi/CD11c+ Dendritic Cells and Contributes to Inflammation.
Severe intestinal inflammation was observed in mice reconstituted with CD11b+/Ly-6Chi/CX3CR1+ DCs upon DSS challenge, thus implicating these cells in colitis development (9). To define the subpopulations of DCs responding to 3SL, we analyzed mesenteric lymph node (MLN)-derived CD11c+ cells using the markers CD8α, CD11b, CD103, and Ly-6C. Increased levels of CD11c+Ly-6Chi cells were detected in Il10−/− mice compared with WT mice (Fig. 4C). This finding was in line with previous studies showing the association of Ly-6Chi–derived DCs with progression of intestinal inflammation (20). Next, we tested DCs isolated from Ccr2−/− mice that have reduced levels of circulating Ly-6Chi cells (21). CD11c+ DC subpopulations isolated from MLNs showed a twofold decrease in Ly-6Chi cells and a 1.5-fold increase in CD103+ cells in Ccr2−/− mice compared with WT mice (Fig. S7 A and B). Stimulation of DCs isolated from MLNs of Ccr2−/− mice with 3SL resulted in reduced expression of costimulatory molecules on Ly-6C+/CD11c+ cells, indicating that these cells respond to 3SL (Fig. S7 C and D). By contrast, CD103+ DCs expressed activation markers independently from the stimulus. These results show that CD11c+Ly-6Chi cells are the main subpopulation of DCs involved in 3SL sensing and driving a proinflammatory response.
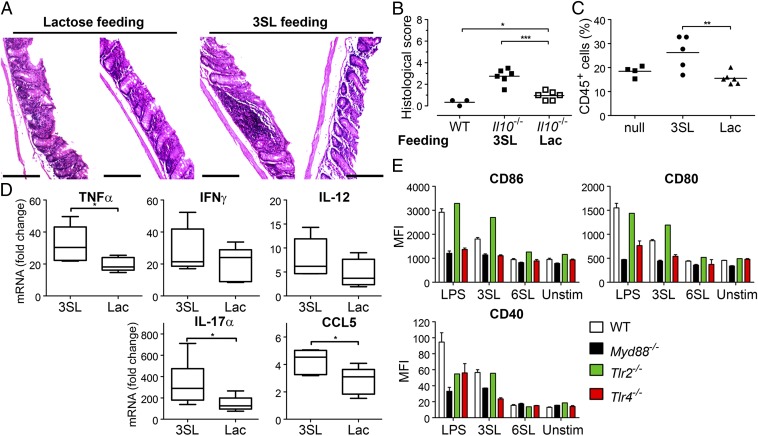
To test whether 3SL exposure induces intestinal inflammation in adult animals, we supplemented 6-wk-old Il10−/− mice orally with 3SL for 4 d. We observed increased inflammation upon 3SL supplementation compared with lactose-fed mice as assessed by histological scoring (Fig. 5 A and B). Infiltration of CD45+ leukocytes was increased in the lamina propria of 3SL-treated mice compared with lactose-treated or control mice (Fig. 5C). Enhanced colon inflammation was further confirmed by analysis of the expression levels of intestinal cytokines as shown by increased levels of TNF-α, IL-17α, and CCL5 in 3SL-supplemented mice (Fig. 5D).
Fig. 5.
3SL feeding induces colon inflammation and 3SL is sensed by TLR4. Six-week-old Il10−/− mice were treated with 3SL, lactose (Lac), or control (null) for 4 d and analyzed at day 5 (n = 6 per group). (A) Microscopic analysis of colon sections. (Scale bar, 200 μm.) (B) Histological score based on evaluation of morphological changes of epithelium and immune cell infiltration. (C) Lamina propria CD45+ cells are presented as percentage of all isolated cells. (D) Expression levels of cytokines in colons of mice fed with 3SL and lactose (Lac) for 4 d were determined by real-time PCR and normalized to GAPDH (n = 5). (E) CD11c+ cells from MLN of WT, Myd88−/−, Tlr2−/−, and Tlr4−/− mice were stimulated with 625 μM of 3SL or 6SL for 14 h. Stimulation with LPS (500 ng/mL) or PBS (buffered) was used as controls. Cell surface expression of CD80, CD86, and CD40 on CD11c+/MHCII+ cells was analyzed by flow cytometry and quantified in two independent experiments (n = 4). MFI, mean fluorescence intensity; 3SL, sialyl(α2,3)lactose; 6SL, sialyl(α2,6)lactose; Unstim, nonstimulated cells.
TLR4 Senses 3SL.
Induction of DC maturation is mediated by TLR-activated signals in a MyD88-dependent manner and yields production of inflammatory cytokines through activation of the NF-κB pathway (22). To test whether TLR-dependent signaling is involved in DC sensing of 3SL, we stimulated DCs isolated from Myd88−/− mice with 3SL and 6SL. CD80 and CD86 expression was almost completely absent, whereas CD40 expression was reduced but not abrogated in 3SL-stimulated cells (Fig. 5E). Next, we repeated the experiment on DCs isolated from Tlr2−/− and Tlr4−/− mice. Whereas the loss of TLR2 did not affect DC activation, absence of TLR4 strongly decreased activation following 3SL stimulation (Fig. 5E). The minimal yet detectable increase in CD40 expression both in Myd88−/− and Tlr4−/− DCs suggested additional TLR-independent 3SL-sensing pathways. These findings demonstrated that TLR4 on DCs can directly sense milk oligosaccharide 3SL and thereby stimulate the intestinal immune system.
Discussion
The present study demonstrates the regulatory activity of milk oligosaccharide 3SL on intestinal mucosal immunity. The application of mice deficient for selected glycosyltransferases allows addressing the function of specific milk oligosaccharides in intestinal inflammation. We have previously found that 3SL affects bacterial colonization in a mouse model of acute colitis (6) and the current study shows that 3SL equally affects the course and severity of spontaneous colitis in Il10−/− mice, which more closely resembles the pathophysiology of IBD. Although differences in intestinal bacterial colonization accounted for the effect of 3SL on DSS-mediated acute colitis (6), intestinal bacteria did not mediate the proinflammatory effect of milk 3SL in the Il10−/− spontaneous colitis model, as shown through cohousing of Il10−/− and S3/I10−/− mice. We cannot, however, completely dismiss the contribution of microbiota to colitis development, and further analysis in context of 3SL will be required. Although demonstrating the direct stimulatory effect of 3SL on DCs, our findings raise several questions regarding the contribution of milk oligosaccharides to intestinal homeostasis.
The first question relates to the cells involved in milk oligosaccharide sensing. The interface of the intestinal immune system encompasses epithelial cells and intestinal-resident leukocytes, both expressing several paternal recognition and lectin-type receptors detecting antigens present in the intestinal lumen (14). Only a few studies have demonstrated a direct role of milk oligosaccharides on mucosal immunity. Treatment of newborn rats with a specific fraction of human milk oligosaccharides comprising disialyllacto-N-tetraose prevented necrotizing enterocolitis (23). I.p. injection of lacto-N-neotetraose in mice induced accumulation of immune-suppressive Gr1+ cells associated with higher production of IL-10 (24). In the present study, we showed that milk trisaccharide 3SL directly stimulates MLN-derived DCs, which resulted in production of cytokines driving TH1 and TH17-dependent inflammation. Through their ability to orchestrate protective immunity, DCs have a key role in shaping intestinal immune response (12). We show that MLN-derived CD11c+ DCs sense 3SL and mediate proinflammatory properties such as increased secretion of inflammatory cytokines (e.g., IL-6, IL-12, and TNF-α). Ccr2−/− mice have generally reduced levels of Ly6Chi cells and are also less susceptible to DSS-induced colitis (21). We detected low levels of CD11c+/Ly6C+ cells in MLNs of Ccr2−/− mice and these cells were also less responsive to stimulation with 3SL. Importantly, Ly-6Chi monocytes invading colon during colitis differentiate into inflammatory DCs, producing high levels of IL-12, IL-23, and TNF-α (20). Our observations indicate that monocyte-derived DCs in the colon are mainly responsible for 3SL sensing, but further work is necessary to characterize the sensing cell population in the lamina propria.
What is the mechanism of glycan sensing in the intestine? The direct contact of the mucosal immune system with glycans from dietary sources or derived from the host cell surfaces may exercise immunomodulatory functions (14). The mechanism involved in glycan sensing by DCs indicated involvement of siglecs and a C-type lectin DC-SIGN (14, 25). DC-SIGN directly binds to α-glucan and mannose-containing glycans of mycobacterial capsules, thereby contributing to immune suppression (25). Campylobacter jejuni-derived oligosaccharides containing α2,3-sialic acid induced Th2 responses in Siglec-7–expressing DCs, whereas α2,8-sialic acid induced TH1 responses (26). We demonstrated that milk trisaccharide 3SL directly stimulates DCs through interaction with TLR4 and MyD88-dependent signaling pathway. Interestingly, 6SL trisaccharide (α2,6-linked lactose) did not stimulate DCs, indicating a specific sensing of 3SL (α2,3-linked sialic acid) in a TLR4-dependent manner.
A major question raised by our work relates to why the naturally occurring milk oligosaccharide 3SL should promote inflammation. Sialic acid is often found on pathogenic bacteria, such as C. jejuni, Haemophilus influenzae, Neisseria gonorrheae, Neisseria meningitidis, and Pasteurella multocida (27). The incorporation of host-derived α2,3-linked sialic acid into H. influenzae is a major virulence factor for experimental otitis (28). The presence of sialic acid on these bacteria is thought to mimic sialylated human glycans and thereby ease evasion from the host immune system. Interestingly, on most pathogenic bacteria, sialic acid occurs α2,3-linked to galactose (29), thus in the same conformation as found in 3SL. Accordingly, elevated local levels of α2,3-linked sialylated structures could act as a signal to prime innate immune cells in the intestinal mucosa. It is tentative to speculate that milk 3SL provides such a priming signal in early infancy during lactation. The stimulatory action of 3SL on DCs through TLR4 is in agreement with recent findings on the recognition of sialylated bacterial glycans. For example, α2,3-sialylated lipooligosaccharides on C. jejuni are sensed by DCs through the TLR4 pathway (30). Therefore, we propose that specific milk oligosaccharides such as 3SL provide structural cues to educate the early innate immune system and prepare the infant for a possible encounter with mimicking pathogenic bacteria. Whether 3SL first promotes colonization of certain pathogenic bacteria thereby causing colitis or 3SL directly affects the immune system remains to be identified. Future work will show if other milk oligosaccharides share the proinflammatory properties of 3SL or rather induce tolerogenic responses, which are equally important to enable the colonization of the intestine by commensal bacteria.
Materials and Methods
SI Materials and Methods provides additional detail.
Mice.
Sialyltransferase St3gal4−/− mice (C57BL/6) (31) were provided by T. Hennet (University of Zürich, Zürich). WT C57BL/J6 mice were purchased from The Jackson Laboratory. Il10−/− mice in C57BL/J6 background (15) were provided by C. Wagner (University of Zürich, Zürich). St3gal4−/−; Il10−/− (S3/I10−/−) mice were generated by breeding St3gal4−/− and Il10−/− mice. Mothers of all pups used in this study were either S3/I10−/− or Il10−/− genotype. All animal experiments were performed according to Swiss animal welfare laws and were approved by the Veterinary Office of the Canton Zürich.
Mice Supplementation with Oligosaccharides.
Chemically synthesized 3SL and 6SL were provided by Norbert Spengler (Nestlé Research Center, Lausanne, Switzerland). Endotoxin levels of all oligosaccharides (3SL, 6SL, and lactose) were measured with limulus assay (Associates of Cape Cod, Inc.). In 3SL and 6SL at concentrations of 63 mg/mL and in lactose at 1 mg/mL, there was no endotoxin levels detectable (limit of detection 0.05 endotoxin unit/mL). S3/I10−/− mice were fed daily with 25 mM 3SL from birth until the age of 3 wk. Amount of supplemented 3SL varied from 5 μL directly after birth to 25 μL at the end of supplementation (Fig. 3). In parallel, mice were supplemented with 25 mM lactose or water for control. Adult Il10−/− mice (6 wk old) were supplemented with 3 mg of 3SL and lactose daily per gavage for 4 d. Mice were killed 24 h after the last treatment and lamina propria cells were isolated and analyzed.
Supplementary Material
Acknowledgments
We thank Norbert Sprenger (Nestlé Research Center) for generously providing 3SL and 6SL. This work was supported by the Zürich Center for Integrative Human Physiology, the Swiss National Foundation, and Hartmann Müller-Stiftung (L.B.).
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1306322110/-/DCSupplemental.
References
- 1.Molodecky NA, et al. Increasing incidence and prevalence of the inflammatory bowel diseases with time, based on systematic review. Gastroenterology. 2012;142(1):46–54, e42, quiz e30. doi: 10.1053/j.gastro.2011.10.001. [DOI] [PubMed] [Google Scholar]
- 2.Khor B, Gardet A, Xavier RJ. Genetics and pathogenesis of inflammatory bowel disease. Nature. 2011;474(7351):307–317. doi: 10.1038/nature10209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Chapman-Kiddell CA, Davies PS, Gillen L, Radford-Smith GL. Role of diet in the development of inflammatory bowel disease. Inflamm Bowel Dis. 2010;16(1):137–151. doi: 10.1002/ibd.20968. [DOI] [PubMed] [Google Scholar]
- 4.Gearry RB, Richardson AK, Frampton CM, Dodgshun AJ, Barclay ML. Population-based cases control study of inflammatory bowel disease risk factors. J Gastroenterol Hepatol. 2010;25(2):325–333. doi: 10.1111/j.1440-1746.2009.06140.x. [DOI] [PubMed] [Google Scholar]
- 5.Bode L. Human milk oligosaccharides: Prebiotics and beyond. Nutr Rev. 2009;67(Suppl 2):S183–S191. doi: 10.1111/j.1753-4887.2009.00239.x. [DOI] [PubMed] [Google Scholar]
- 6.Fuhrer A, et al. Milk sialyllactose influences colitis in mice through selective intestinal bacterial colonization. J Exp Med. 2010;207(13):2843–2854. doi: 10.1084/jem.20101098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Hadis U, et al. Intestinal tolerance requires gut homing and expansion of FoxP3+ regulatory T cells in the lamina propria. Immunity. 2011;34(2):237–246. doi: 10.1016/j.immuni.2011.01.016. [DOI] [PubMed] [Google Scholar]
- 8.Niess JH. Role of mucosal dendritic cells in inflammatory bowel disease. World J Gastroenterol. 2008;14(33):5138–5148. doi: 10.3748/wjg.14.5138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Varol C, et al. Intestinal lamina propria dendritic cell subsets have different origin and functions. Immunity. 2009;31(3):502–512. doi: 10.1016/j.immuni.2009.06.025. [DOI] [PubMed] [Google Scholar]
- 10.Schulz O, et al. Intestinal CD103+, but not CX3CR1+, antigen sampling cells migrate in lymph and serve classical dendritic cell functions. J Exp Med. 2009;206(13):3101–3114. doi: 10.1084/jem.20091925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Coombes JL, et al. A functionally specialized population of mucosal CD103+ DCs induces Foxp3+ regulatory T cells via a TGF-beta and retinoic acid-dependent mechanism. J Exp Med. 2007;204(8):1757–1764. doi: 10.1084/jem.20070590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Coombes JL, Powrie F. Dendritic cells in intestinal immune regulation. Nat Rev Immunol. 2008;8(6):435–446. doi: 10.1038/nri2335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Abreu MT, Fukata M, Arditi M. TLR signaling in the gut in health and disease. J Immunol. 2005;174(8):4453–4460. doi: 10.4049/jimmunol.174.8.4453. [DOI] [PubMed] [Google Scholar]
- 14.de Kivit S, Kraneveld AD, Garssen J, Willemsen LE. Glycan recognition at the interface of the intestinal immune system: Target for immune modulation via dietary components. Eur J Pharmacol. 2011;668(Suppl 1):S124–S132. doi: 10.1016/j.ejphar.2011.05.086. [DOI] [PubMed] [Google Scholar]
- 15.Kühn R, Löhler J, Rennick D, Rajewsky K, Müller W. Interleukin-10-deficient mice develop chronic enterocolitis. Cell. 1993;75(2):263–274. doi: 10.1016/0092-8674(93)80068-p. [DOI] [PubMed] [Google Scholar]
- 16.Nagle D. Rectal prolapse and fecal incontinence. Prim Care. 1999;26(1):101–111. doi: 10.1016/s0095-4543(05)70104-4. [DOI] [PubMed] [Google Scholar]
- 17.Flanagan K, et al. Intestinal epithelial cell up-regulation of LY6 molecules during colitis results in enhanced chemokine secretion. J Immunol. 2008;180(6):3874–3881. doi: 10.4049/jimmunol.180.6.3874. [DOI] [PubMed] [Google Scholar]
- 18.Gibson GR, Probert HM, Loo JV, Rastall RA, Roberfroid MB. Dietary modulation of the human colonic microbiota: Updating the concept of prebiotics. Nutr Res Rev. 2004;17(2):259–275. doi: 10.1079/NRR200479. [DOI] [PubMed] [Google Scholar]
- 19.Veldhoen M, Hocking RJ, Atkins CJ, Locksley RM, Stockinger B. TGFbeta in the context of an inflammatory cytokine milieu supports de novo differentiation of IL-17-producing T cells. Immunity. 2006;24(2):179–189. doi: 10.1016/j.immuni.2006.01.001. [DOI] [PubMed] [Google Scholar]
- 20.Rivollier A, He J, Kole A, Valatas V, Kelsall BL. Inflammation switches the differentiation program of Ly6Chi monocytes from antiinflammatory macrophages to inflammatory dendritic cells in the colon. J Exp Med. 2012;209(1):139–155. doi: 10.1084/jem.20101387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Platt AM, Bain CC, Bordon Y, Sester DP, Mowat AM. An independent subset of TLR expressing CCR2-dependent macrophages promotes colonic inflammation. J Immunol. 2010;184(12):6843–6854. doi: 10.4049/jimmunol.0903987. [DOI] [PubMed] [Google Scholar]
- 22.Hou B, Reizis B, DeFranco AL. Toll-like receptors activate innate and adaptive immunity by using dendritic cell-intrinsic and -extrinsic mechanisms. Immunity. 2008;29(2):272–282. doi: 10.1016/j.immuni.2008.05.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Jantscher-Krenn E, et al. The human milk oligosaccharide disialyllacto-N-tetraose prevents necrotising enterocolitis in neonatal rats. Gut. 2012;61(10):1417–1425. doi: 10.1136/gutjnl-2011-301404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Terrazas LI, Walsh KL, Piskorska D, McGuire E, Harn DA., Jr The schistosome oligosaccharide lacto-N-neotetraose expands Gr1(+) cells that secrete anti-inflammatory cytokines and inhibit proliferation of naive CD4(+) cells: A potential mechanism for immune polarization in helminth infections. J Immunol. 2001;167(9):5294–5303. doi: 10.4049/jimmunol.167.9.5294. [DOI] [PubMed] [Google Scholar]
- 25.Geijtenbeek TB, et al. Mycobacteria target DC-SIGN to suppress dendritic cell function. J Exp Med. 2003;197(1):7–17. doi: 10.1084/jem.20021229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Bax M, et al. Campylobacter jejuni lipooligosaccharides modulate dendritic cell-mediated T cell polarization in a sialic acid linkage-dependent manner. Infect Immun. 2011;79(7):2681–2689. doi: 10.1128/IAI.00009-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Severi E, Hood DW, Thomas GH. Sialic acid utilization by bacterial pathogens. Microbiology. 2007;153(Pt 9):2817–2822. doi: 10.1099/mic.0.2007/009480-0. [DOI] [PubMed] [Google Scholar]
- 28.Bouchet V, et al. Host-derived sialic acid is incorporated into Haemophilus influenzae lipopolysaccharide and is a major virulence factor in experimental otitis media. Proc Natl Acad Sci USA. 2003;100(15):8898–8903. doi: 10.1073/pnas.1432026100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Audry M, et al. Current trends in the structure-activity relationships of sialyltransferases. Glycobiology. 2011;21(6):716–726. doi: 10.1093/glycob/cwq189. [DOI] [PubMed] [Google Scholar]
- 30.Kuijf ML, et al. TLR4-mediated sensing of Campylobacter jejuni by dendritic cells is determined by sialylation. J Immunol. 2010;185(1):748–755. doi: 10.4049/jimmunol.0903014. [DOI] [PubMed] [Google Scholar]
- 31.Ellies LG, et al. Sialyltransferase ST3Gal-IV operates as a dominant modifier of hemostasis by concealing asialoglycoprotein receptor ligands. Proc Natl Acad Sci USA. 2002;99(15):10042–10047. doi: 10.1073/pnas.142005099. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.