Abstract
Schizophrenia is a severe mental disorder that has a strong genetic basis. Converging evidence suggests that schizophrenia is a progressive neurodevelopmental disorder, with earlier onset cases resulting in more profound brain abnormalities. Siblings of patients with schizophrenia provide an invaluable resource for differentiating between trait and state markers, thus highlighting possible endophenotypes for ongoing research. However, findings from sibling studies have not been systematically put together in a coherent story across the broader age span. We review here the cortical grey matter abnormalities in siblings of patients with schizophrenia from childhood to adulthood, by reviewing sibling studies from both childhood-onset schizophrenia, and the more common adult-onset schizophrenia. When reviewed together, studies suggest that siblings of patients with schizophrenia display significant brain abnormalities that highlight both similarities and differences between the adult and childhood populations, with shared developmental risk patterns, and segregating trajectories. Based on current research it appears that the cortical grey matter abnormalities in siblings are likely to be an age-dependent endophenotype, which normalize by the typical age of onset of schizophrenia unless there has been more genetic or symptom burdening. With increased genetic burdening (e.g. discordant twins of patients) the grey matter abnormalities in (twin) siblings are progressive in adulthood. This synthesis of the literature clarifies the importance of brain plasticity in the pathophysiology of the illness, indicating that probands may lack protective factors critical for healthy development.
Keywords: schizophrenia, imaging, grey matter, endophenotype
Introduction
Schizophrenia is a debilitating mental illness that is characterized by widespread brain abnormalities. Neuroimaging studies have demonstrated that both cortical and deeper brain structures, including the white matter, in patients with schizophrenia differ significantly from healthy control subjects, and that those abnormalities change over the lifespan, with the deficits becoming more pronounced with longer illness duration (Wright et al., 1999; Pantelis et al., 2005; DeLisi, 2008; Ellison-Wright et al., 2008; Gogtay, 2008; Gogtay and Rapoport, 2008; Hulshoff Pol and Kahn, 2008; Fornito et al., 2009; Olabi et al., 2011). The most consistent anatomical abnormalities are reduction in cortical grey matter volume, particularly in prefrontal and temporal cortices, reduced volumes of medial temporal lobe structures, and increased lateral ventricular volume (Wright et al., 2000; Shenton et al., 2001, 2010). In the patients with typical adult-onset schizophrenia, the extent of annualized progressive brain tissue loss (−0.5%) is twice that of healthy control subjects (−0.2%) (Hulshoff Pol and Kahn, 2008) and these deficits are more pronounced in the severe phenotype of childhood-onset cases (Rapoport et al., 2005; Gogtay and Rapoport, 2008; Gogtay et al., 2008).
The molecular mechanism of anatomical brain abnormalities in schizophrenia remains unclear, and an important question is whether these abnormalities are related to the illness state itself, or a more tied to genetic risk, as an endophenotype. Characterizing endophenotypes, a new focus in psychiatry and other medical disciplines (Gottesman and Gould, 2003), is a compelling venture because they may represent less complex disease antecedents that are riper for study than the illness syndrome itself. By definition, they must be associated with illness in the population, be heritable, and be primarily state independent (not more common in the disease than in high-risk relatives) (Gould and Gottesman, 2006). Brain structures present as a good endophenotype, since many are highly heritable (Peper et al., 2007), and influenced by genetic variants that are beginning to emerge based on large-scale studies (Stein et al., 2012). In the realm of complex neuropsychiatric disorders such as schizophrenia, endophenotypes may simplify and prioritize biological precursors for study that are highly genetic, and not directly tied to the illness itself. Non-psychotic siblings and discordant twins provide ideal populations to study this question, given that siblings and fraternal twins share on average 50% of their segregating genes with the proband, while identical twins share virtually 100% of their genes, but not the clinical phenotype (psychosis). Some criticisms have been raised about endophenotypic studies, particularly that an endophenotype may be able to satisfy all of its criteria while still being unrelated to the disease pathway (Walters and Owen, 2007), and that there may be a bi-directional relationship between the illness state and the putative endophenotype (Kendler and Neale, 2010). These potential flaws of endophenotypes must be kept in mind any time this type of research is undertaken.
Sibling studies in schizophrenia, both in adult and childhood patient populations, have attempted to address this question. A recent meta-analysis of family studies in schizophrenia showed that first-degree relatives had lower hippocampal, total grey matter, and ventricular volumes, compared with healthy volunteers (Boos et al., 2007). Although these findings in schizophrenia siblings show that—at least in part—the structural brain changes in schizophrenia are familial, the extent to which these changes change over the course of development, and whether the young and adult siblings overlap in their findings or diverge is important to know. This requires a longitudinal setup to study developmental brain changes in family members of patients. Findings from these studies may tell us more about the resilience and risks that accompany developmental brain changes.
The goal of this review is to assess and summarize the structural brain imaging findings of developmental changes in siblings and twin pairs discordant for schizophrenia. It will incorporate studies on siblings of patients with childhood-onset schizophrenia, in which the siblings tend to be children and adolescents and hence provide a window into early brain development, and the more common adult-onset schizophrenia, in which the siblings are adults and thus of older average age. We seek to synthesize these results from a comprehensive neurodevelopmental perspective, of sibling brain development across a wide age range from early childhood to mid-adult age, which encompasses a large window around the typical age of onset for schizophrenia. Not only does this body of literature demonstrate the strong genetic basis of schizophrenia, but it may also lead to the identification of meaningful biological trait markers and endophenotypes for further hypothesis generation.
Siblings of patients with childhood-onset schizophrenia
Childhood-onset schizophrenia is a rare and severe form of the disorder defined by the onset of psychosis before the age of 13, which is neurobiologically continuous with the adult-onset illness (Rapoport and Gogtay, 2011). Childhood-onset schizophrenia shows profound and progressive cortical grey matter loss that spreads in a dynamic wave in a parieto-frontal and parieto-temporal direction during adolescence, finally localizing to prefrontal and temporal cortices as children become young adults (Gogtay et al., 2008; Greenstein et al., 2008). Independent research that highlights the positive association between cortical thickness and cognitive and overall functioning supplements these findings (Shaw et al., 2006; Greenstein et al., 2008), and strengthens the supposition that grey matter deficits in childhood-onset schizophrenia contribute to the illness and functional impairment. As part of ongoing investigations at the NIMH, full siblings of patients with childhood-onset schizophrenia are prospectively followed and re-scanned every 2 years.
The initial cross-sectional, volumetric analyses of structural abnormalities in healthy siblings of patients with childhood-onset schizophrenia showed that, compared with healthy control subjects, childhood-onset schizophrenia siblings had smaller total cerebellar volume and total, frontal and parietal grey matter volumes, particularly in younger ages which also suggested that sibling deficits may mirror the grey matter loss pattern that has been documented in childhood-onset schizophrenia probands (Gogtay et al., 2003). This evidence provided a starting point; given that there is similarity between the probands and siblings, prospective inspection might show evidence that the neurodevelopmental trajectory itself could also be a trait marker.
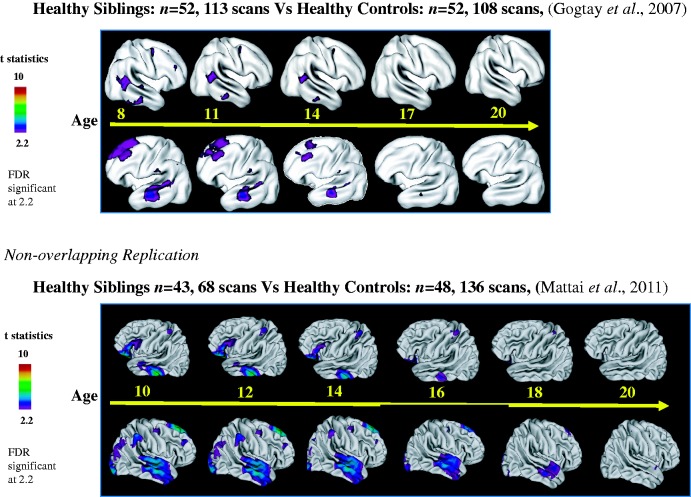
These observations have since been extended in large prospectively acquired childhood-onset schizophrenia sibling samples. In the first longitudinal analyses from ages 8 to 28, healthy childhood-onset schizophrenia siblings showed an interesting pattern of cortical grey matter thickness development (Gogtay et al., 2007). Non-psychotic siblings (n = 52, 113 scans) showed grey matter deficits in parietal, prefrontal and temporal regions compared with control subjects (n = 52, 108 scans) in early ages, but the deficits normalized by late adolescence (age 20), the typical age of onset of schizophrenia (Fig. 1). This intriguing finding was subsequently replicated in an independent, non-overlapping sample of non-psychotic childhood-onset schizophrenia siblings (n = 43, 68 scans) and healthy control subjects (n = 86, 136 scans) from ages 5 to 26 years, where the early prefrontal and temporal deficits again showed normalization by the age of 20 (Fig. 1) (Mattai et al., 2011b). A 3D dynamic sequence of deficit normalization with age from a combined sample (childhood-onset schizophrenia: n = 92, 193 scans, siblings: n = 91, 185 scans, ages 8–24 years) is available online at https://www.dropbox.com/s/bhwksqoolv3y7q8/Sibling.mov.zip.
Figure 1.
Grey matter loss in healthy childhood-onset schizophrenia siblings.
There are several implications that stem from this replicated result. The first is that the pattern of cortical grey matter deficit progression in healthy siblings appears to parallel that of childhood-onset schizophrenia probands except they may even begin earlier in life. The second is that there is a significant divergence from the childhood-onset schizophrenia trajectory in siblings in which their early deficits normalize, signifying protective factors in healthy siblings not present in cases with childhood-onset schizophrenia. Together these provide the basis for classifying early (prefrontal and temporal) grey matter deficits as a potential endophenotype in schizophrenia, in other words, a hypothesized antecedent and biological underpinning of this disorder that is highly genetic, and may not by itself cause disease symptoms. These results also suggest that studies on prefrontal and temporal neurocircuitry development, particularly in healthy siblings, could provide important clues in pathophysiological (or protective) mechanisms in schizophrenia.
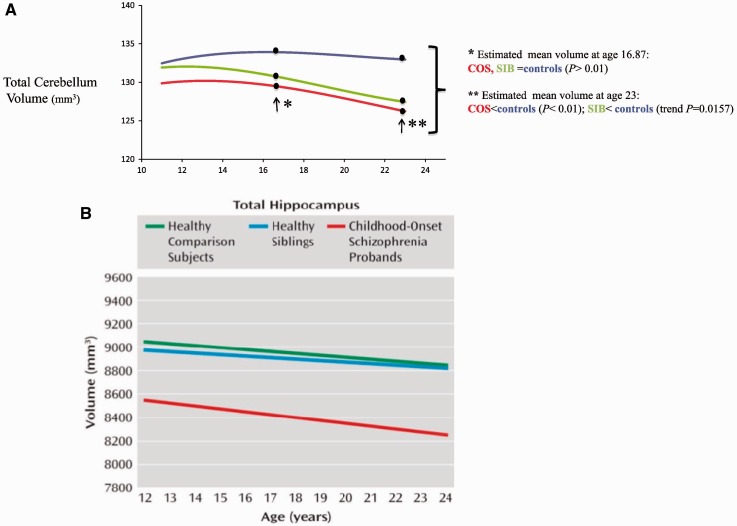
These analyses were recently extended to the hippocampus, due to the fact that hippocampal volume loss is one of the most consistently reported findings in schizophrenia (Nelson et al., 1998). In a recent study analysing a large longitudinal sample of patients with childhood-onset schizophrenia (n = 89, 198 scans), non-psychotic siblings (n = 78, 172 scans), and matched controls (n = 79, 198 scans), patients with childhood-onset schizophrenia showed 6–7% loss in hippocampal volume, which appeared to be a ‘fixed’ deficit across ages 10 to 29 years, whereas the trajectories of hippocampal volume were not different between patients and control subjects. Childhood-onset schizophrenia siblings, however, did not differ from control subjects in either hippocampal volume or its trajectory over time (Mattai et al., 2011a). This suggested that, unlike prefrontal and temporal cortical grey matter deficits, hippocampal volume loss in childhood-onset schizophrenia is a state-marker or disease-related, perhaps secondary to some already postulated environmental associations such as early life stress (Cannon et al., 2002a). These results also suggest that the grey matter deficits in childhood-onset schizophrenia and schizophrenia generally are not only age dependent, but also regionally dependent endophenotypes, further buoyed by a recent meta-analysis of brain abnormalities in adult-onset schizophrenia that found no progressive hippocampal changes (Olabi et al., 2011).
In neuroimaging, longitudinal profiles can be more informative than cross-sectional time points (Giedd and Rapoport, 2010). The age dependent amelioration of cortical grey matter deficits in siblings offers information that would not be captured using cross-sectional data. This was also highlighted in our recent study of cerebellar development in childhood-onset schizophrenia (Greenstein et al., 2011). In a longitudinal sample of probands (n = 94, 208 scans), siblings (n = 80, 165 scans), and unrelated control subjects (n = 110, 269 scans), the non-psychotic siblings did not differ significantly from control subjects in terms of total cerebellar volumes, but did so in the developmental trajectories of the total and right cerebellum, and the left inferior posterior left superior posterior and superior vermis (Greenstein et al., 2011). The developmental trajectory of the siblings’ cerebellar measures was not as severely abnormal as their ill brothers and sisters, but significantly more so than the control subjects. Furthermore, cross-sectional observations of early versus later age points would yield inconsistent and hence misrepresentative results. Interestingly, unlike the cortex, normalization of deficits did not occur for cerebellum in healthy siblings, again indicating a lack of synchrony, and subregional specificity of endophenotypes (Fig. 2) (Greenstein et al., 2011). Alternatively, cerebellar development might be an endophenotype relegated to ‘later’ ages, whereas cortical grey matter deficits are endophenotypes relegated to ‘early’ ages and hippocampus volume loss in childhood-onset schizophrenia is a disease effect in entirety.
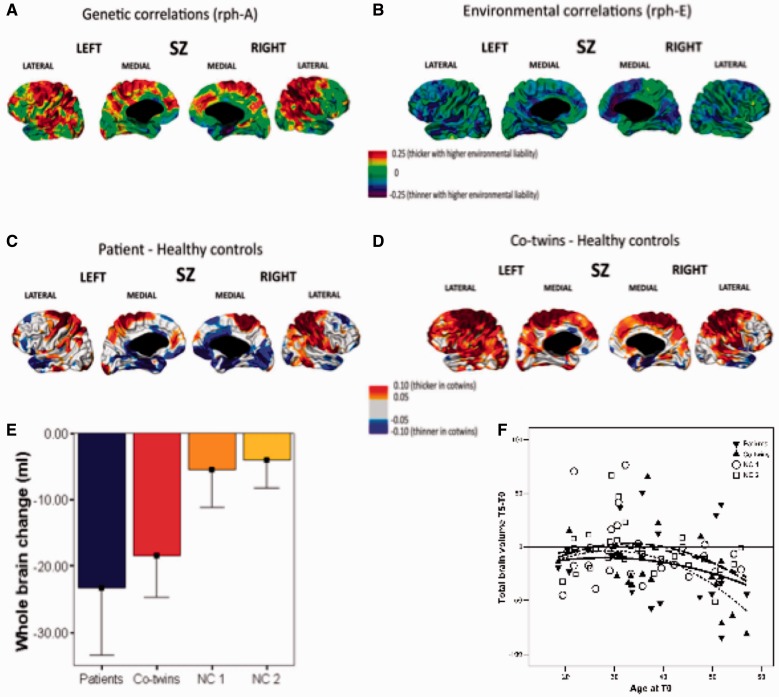
Figure 3.
Structural brain changes in twins discordant for schizophrenia. Cortical thickness and genetic (A) and environmental (B) liabilities for schizophrenia (SZ) are shown based on structural equation modelling, and cortical thickness decreases (in blue) and increases (in red) in patients (C) and their discordant co-twins (D) as compared to control twins are shown based on planned comparisons (adapted from Hulshoff Pol et al., 2012). Brain volume change over time is expressed as ml change from 0 in mean (SD) (E) and age (F) plots in patients with schizophrenia, their discordant co-twins and control twins over a 5-year interval (based on data from Brans et al., 2008a).
Figure 2.
Volume trajectories for (A) cerebellum (Greenstein et al., 2011) and (B) hippocampus (Mattai et al., 2011a) among childhood-onset schizophrenia siblings and healthy controls. COS = childhood-onset schizophrenia; SIB = sibling.
An implicit goal of finding endophenotypes is to locate markers or antecedents of a disease that are more upstream than the overt clinical phenotype. In terms of neurological and psychiatric diseases, this can happen by moving from structural abnormalities to the genetic variants that may cause them. One such variant is a polymorphism for an enzyme responsible for dopamine catabolism, a neurotransmitter theorized to be dysregulated in patients with schizophrenia (Howes and Kapur, 2009) and may in part mediate the deficits in cortical thickness common to this disorder. The polymorphism is a substitution of the amino acid valine for methionine, and results in an enzyme that catabolizes dopamine at 40% greater rates, leaving far lower levels available in the CNS (Raznahan et al., 2011). In one of the largest childhood-onset schizophrenia studies to date (Raznahan et al., 2011), interaction between COMT genotype, structural imaging data, and age showed that among patients with childhood-onset schizophrenia and their non-psychotic siblings, homozygous Val/Val status was associated with a significantly steeper slope of cortical grey matter loss in overlapping areas (primarily the dorsolateral prefrontal cortex, along with bilateral cingulate) between childhood-onset schizophrenia probands and their healthy siblings. Given also that this was not the case for unrelated control subjects, where in fact higher doses were related to attenuated cortical thickness losses in the bilateral prefrontal cortex and temporal and superior parietal regions, these findings strongly point towards genetic polymorphism as a compelling variable, along with specific subregion and age, that influences the structural endophenotype. This effect has also been reported in young adults (aged 16–25) at high genetic risk of psychosis (McIntosh et al., 2007), and those with a 22q11.2 deletion (Gothelf et al., 2005). Together, this growing body of work suggests that brain abnormalities related to psychosis may develop in part because of a disruption in the pathway that links dopaminergic function to cortical maturation (Raznahan et al., 2011).
To summarize the results of studies of healthy siblings of patients with childhood-onset schizophrenia, the potential endophenotypes that merit further scrutiny to date, are temporal and frontal cortical thickness deficits in early age, decreased cerebellar volume in later age, and the influence of individual genetic polymorphisms. The reflection that is built from the sum of these findings is also that the healthy (non-psychotic) siblings of patients with childhood-onset schizophrenia appear to have plastic nervous systems that can correct developmental abnormalities and normalize during adolescence. The specific regions identified must be pursued individually, but so too should the notion that brain plasticity in general, and the neurological basis of that ability, may be the key to treatment that can delay and even prevent the onset of psychosis for those at even the highest risk of this severely impairing disorder.
Siblings of patients with adult-onset schizophrenia
Adult-onset schizophrenia has an onset that typically occurs during late adolescence and young adulthood, is far more common (with a lifetime risk of 1% of the general population) than childhood-onset schizophrenia, and its body of research is understandably much larger (Ayuso-Mateos et al., 2006). Of note, adult-onset schizophrenia, or schizophrenia such as it is normally defined, is here contrasted with childhood-onset schizophrenia, but also includes individuals and their family members with a disease onset before the age of 18 years. In adult-onset schizophrenia, similar attempts have been made in the adult population to differentiate state and trait markers in sibling studies, including twin studies, and these have provided important insights. Twin studies are the most informative as they offer a comparison between monozygotic twins with near identical genetic profiles, with dizygotic twins that can be considered effectively as full siblings with a 50% share of their segregating genes on average. Twin populations discordant and concordant for schizophrenia are rare and difficult to study, and practically impossible to obtain in childhood-onset schizophrenia. Concordance rate for schizophrenia in monozygotic twins, despite sharing all genes, is not 100%, but closer to 50% with an estimated heritability of 81% (Sullivan et al., 2003) and possibly lower, (Lichtenstein et al., 2009) suggesting a complex interplay between genetics and other vulnerability factors; making the study of endophenotypes even more relevant (Baare et al., 2001).
The adult-onset schizophrenia sibling brain picture has been developed over time, and one of the earliest findings was from an analysis of thalamic volume in relatives of patients with schizophrenia. This research followed studies that indicated thalamic volume in patients with adult-onset schizophrenia (n = 16) was reduced compared with the general population, and measured mean total thalamic volume corrected for total brain volume (Staal et al., 1998). With this metric, it was found that patient thalamic volume was the smallest, but also that sibling volume (n = 16) was significantly greater than probands, but lower than healthy control subjects (n = 32). Although small and cross-sectional, this study strengthened the idea that thalamic volume is a possible endophenotype and related to the genetic liability to develop schizophrenia.
Many adult-onset schizophrenia sibling studies followed that examined other brain regions in terms of structure and function among siblings of patients with adult-onset schizophrenia, which are summarized in Table 1.
Table 1.
Brain abnormalities in siblings of adults with schizophrenia
| Study* | Participants** | Positive findings | Negative findings*** |
|---|---|---|---|
| (Noga et al., 1996) |
|
|
|
| (Seidman et al., 1997) |
|
Reduced hippocampal, right amygdala, and right putamen volumes | Total cerebral hemisphere, adjusted cerebral grey matter and white matter |
| (Staal et al., 1998) |
|
Decreased thalamic volume | Total brain volume |
| (Cannon et al., 1998) |
|
Cortical grey matter reductions | White matter volume reductions |
| (Seidman et al., 1999) |
|
|
Total brain volume, grey matter volume, lateral ventricles, cerebellum |
| (Sharma et al., 1999) |
|
Total brain volume, prefrontal, premotor, sensorimotor, occipitoparietal volume | |
| (Staal et al., 2000) |
|
Third ventricle enlargement | Intracranial, cerebellum, amygdala, hippocampus, parahippocampal gyrus, and CSF volume |
| (Baare et al., 2001) |
|
|
|
| (Narr et al., 2002) |
|
Lower left hippocampal volume in MZ not DZ co-twins, supporting genetic influences | |
| (Cannon et al., 2002b) |
|
Polar PFC grey matter volume | DLPFC grey matter volume |
| (Van Erp et al., 2002) |
|
Lower hippocampal volume | |
| (Tepest et al., 2003) |
|
Lower hippocampus volume, inward deformity of the head of the hippocampus | |
| (Hulshoff Pol et al., 2004) |
|
Lower white matter volume | Grey matter volume |
| (van Haren et al., 2004) |
|
Lower whole brain volumes | Third ventricle, and lateral ventricles |
| (van Erp et al., 2004) |
|
Lower hippocampus volumes | |
| (Rijsdijk et al., 2005) |
|
|
Lateral ventricle volume |
| (Hulshoff Pol et al., 2006) |
|
|
|
| (Ettinger et al., 2007) |
|
Reduced thalamic volume | |
| (Brans et al., 2008a) |
|
Progressive total brain frontal lobe and temporal lobe tissue loss due to genes common to schizophrenia risk | |
| (Brans et al., 2008b) |
|
Progressive change over time in whole brain and cerebral grey matter volumes | |
| (Honea et al., 2008) |
|
Grey matter deficits in medial frontal, superior temporal, and insular cortices | |
| (Goldman et al., 2009) |
|
Brain wide cortical thickness | |
| (Harms et al., 2010) |
|
Reduced inferior frontal grey matter volume | Middle frontal grey matter volume |
| (Rasetti et al., 2011) |
|
Abnormal DL PFC coupling with hippocampus | |
| (Hulshoff Pol et al., 2012) |
|
|
|
| (Boos et al., 2012) |
|
Total brain, grey matter, white matter, lateral and third ventricle, cerebellum volumes, cortical thickness and grey matter density | |
| (Ettinger et al., 2012) |
|
Lower superior frontal cortex volume | Inferior, medial and orbitofrontal cortices volumes |
| (van Haren et al., 2012) |
|
|
Cerebral grey matter |
*Studies between 1997 and 2005 are included in the meta-analysis on structural brain changes in siblings of patients with schizophrenia finding significantly smaller hippocampus and grey matter volume as well as significantly larger third ventricle volume in non-psychotic first-degree relatives of patients with schizophrenia (Boos et al., 2007).
**Several studies have overlapping cohorts.
***Significant changes in volumes may be present in these areas in patients with schizophrenia in these same studies. For meta-analyses on structural brain changes in schizophrenia see Wright et al. (2000), Olabi et al. (2011) and Haijma et al. (2012).
AOS = adult-onset schizophrenia; DZ = dizygotic; MZ = monozygotic; DL = dorsolateral; PFC = prefrontal cortex.
Thus, there are a considerable number of studies in adult-onset schizophrenia siblings, although samples between studies are overlapping. The majority of studies suggest familial influences on at least part of the structural brain abnormalities in schizophrenia. This is confirmed in a meta-analysis on structural brain changes in non-psychotic siblings (including co-twins) of patients with schizophrenia finding significant smaller hippocampus and grey matter volume as well as significant larger third ventricle volume in studies published until 2005 (Boos et al., 2007). In the more than 10 studies published since, studies in twins largely confirm volume changes in schizophrenia to have a familial component. Importantly, these twin studies add that the brain volume abnormalities in schizophrenia are at least in part due to genes implicated in the disease. However, a few, considerably large studies in non-psychotic singleton adult-onset schizophrenia siblings do not show any brain abnormalities (Honea et al., 2008; Boos et al., 2012).
There could be several reasons for these inconsistencies. We cannot exclude that studies differed in their exclusion criteria for siblings regarding (schizophrenia spectrum) problems on axis I or axis II and this may explain some of these findings. However, more likely and importantly, they seem to reflect a difference in genetic loading between siblings and twins (Sullivan et al., 2003). The estimated 9% increased risk for the disease in healthy singleton siblings from patients with schizophrenia is for the most part not reflected in brain abnormalities, whereas higher risks such as the estimated 50% increased risk in monozygotic co-twins from patients with schizophrenia can be reflected in the brain abnormalities. Alternatively, we cannot exclude that this lack of detectable differences at lower levels of relatedness, however, may not be a signal of a cut-off between genetic risk and progressive brain abnormalities, but rather that there appears to be a lower incidence of schizotypal personality disorder among siblings than twins. Studies in relatives and twins generally support familial aggregation with schizophrenia, particularly for the affective and interpersonal components (Kremen et al., 1998; Lichtenstein et al., 2009; Picchioni et al., 2010) and that in some instances older samples with decreasing chances of disease-transition, that measured with less sensitive techniques, such as voxel-based morphology (Honea et al., 2008) were employed. Indeed, studies in monozygotic and dizygotic twins concordant and discordant for adult-onset schizophrenia revealed not only that some of the brain abnormalities in schizophrenia are familial, but also that part of the brain abnormalities in schizophrenia are due to genes implicated in the disease (Baare et al., 2001; Cannon et al., 2002b; van Haren et al., 2004; Hulshoff Pol et al., 2006, 2012). Interestingly, some of the changes in brain structure, particularly loss of white matter volume and thinner cortex of parahippocampal gyri and right orbitofrontal cortex, were found not only to be heritable in adult-onset schizophrenia but also in bipolar disorder, whereas other brain changes were specific for the genetic risk for adult-onset schizophrenia but not bipolar disorder, suggesting both overlapping and segregating effects on brain structures of genetic risks for adult-onset schizophrenia and bipolar disorder (Hulshoff Pol et al., 2012).
Few longitudinal studies on adult-onset schizophrenia sibling samples have been carried out. We are not aware of any studies outside of the few cohorts followed by researchers at the University Medical Centre in Utrecht, The Netherlands. The Utrecht studies involved singleton siblings and monozygotic and dizygotic twins being re-scanned at a 5-year follow-up period. Healthy siblings of adult-onset schizophrenia did not show any evidence for progressive grey matter loss over time. However, when adult monozygotic and dizygotic twin pairs discordant for schizophrenia were studied longitudinally, evidence for progressive brain tissue loss in family members of adult-onset schizophrenia was found (Brans et al., 2008b). The progressive volume loss of total brain and particularly of frontal and temporal lobes was at least for 50% due to genes implicated in schizophrenia (Brans et al., 2008b). The genes implicated in these progressive brain tissue changes in schizophrenia may differ from those implicated in brain maturation per se, since in healthy childhood and adulthood separate gene pools are implicated in brain structure change and brain plasticity (Brans et al., 2010; van Soelen et al., 2012). Thus, increased genetic risk for schizophrenia is not only associated with brain tissue loss but also with progressive brain tissue loss in adulthood.
Synthesis and discussion
Studies from unaffected siblings of patients with both childhood and adult-onset schizophrenia demonstrate an interesting and divergent pattern of brain development compared to unrelated healthy controls. Based on the continuity that has been established between these two forms of the disorder (Giedd et al., 1999), the opportunity exists to bridge and combine the findings from both sibling groups for a more comprehensive picture. Together, these data indicate that the sibling brain often lies in between that of the patients with schizophrenia and healthy controls, with significant, but not as severe regional volume reductions. These findings strengthen the familial and genetic basis of schizophrenia, in which siblings provide an opportunity to highlight brain development as a structural and hence functional endophenotype. Even more important than the similarities, the differences between the childhood and adult-onset cases may indicate the temporal nature of the endophenotypes in question.
In the childhood-onset schizophrenia siblings, prefrontal and temporal grey matter deficits were detected at early ages. In co-twins of patients with adult-onset schizophrenia prefrontal and temporal grey matter deficits are found that are progressive in adulthood. This suggests that the decrease in prefrontal and temporal grey matter is familial (and associated with increased genetic risk) in schizophrenia irrespective of age. There have been documented cross-sectional and longitudinal brain changes in relatives at high risk for schizophrenia, which includes siblings as well as other close relatives (Boos et al., 2007; McIntosh et al., 2011), but singleton adult-onset schizophrenia siblings brain changes are predominantly absent and do not progress over time, although this finding is based on only a small number of longitudinal follow-up sibling samples.
The longitudinal findings from the childhood-onset sample allows for a more nuanced perspective of the adult results given that the grey matter deficits in childhood-onset schizophrenia siblings normalize by young adulthood. Together, the early grey matter deficits in childhood-onset schizophrenia siblings that normalize by late adolescence combined with the largely negative grey matter findings in adult-onset schizophrenia singleton siblings—but not in adult-onset schizophrenia co-twins—suggest that grey matter deficits are an age-dependent endophenotype that are subregionally specific and highlight the neurodevelopmental nature of schizophrenia, and the plasticity of the healthy (singleton) sibling brain. Such plastic processes may reflect genetic processes implicated in subregionally specific changes in the normal brain at different ages (Brans et al., 2010; van Soelen et al., 2012). For example, large-scale healthy participant studies indicate that later developing brain regions, such as the prefrontal cortex and temporal lobes, show increasingly prominent genetic effects with brain maturation (Lenroot et al., 2009), highlighting how age (through longitudinal work) might provide the ideal filter to detecting endophenotypes. Interestingly, age-dependent genetic influences are not uncommon in medicine, including examples in Alzheimer’s disease (Shi and Gibson, 2007) and Huntington’s disease (Reiner et al., 2011), and add further emphasis to the notion that brain changes take place over specific and critical periods throughout life.
This allows for a model to be developed for the sibling brain that stretches in two dimensions. The first dimension is age, and the synthesis of the different populations by age indicate that cortical grey matter deficits are more prevalent earlier in life, normalize over adolescence, and are all but absent during young adulthood provided the siblings do not have schizophrenia spectrum abnormalities or higher genetic loading as in the case of monozygotic discordant twins. The second dimension is degree of genetic loading and the degree of symptom/disease burden. Structural abnormalities become more prominent and remain evident in later ages in monozygotic twins compared with non-twins, and also among childhood-onset schizophrenia and adult-onset schizophrenia siblings that are diagnosed with schizophrenia spectrum disorders. Additionally, studies that include other relatives beyond siblings (Olabi et al., 2011) can also add focus to the larger genetic and environmental causes of schizophrenia. Together these dimensions of analysis demonstrate strongly that structural deficits in schizophrenia are genetically influenced, and represent a dimensional construct as opposed to an ‘all or nothing’ model of psychosis. The unification of the childhood and adult literature also adds weight to a gene dosage effect at play in schizophrenia, especially given the work done that supports childhood-onset schizophrenia as representing a more genetic form of the disorder (Eckstrand et al., 2008).
In addition to addressing the endophenotype question, sibling studies provide the basis for future hypotheses and specific directions forward. Prefrontal and temporal deficits appear to be consistent abnormalities in schizophrenia, appearing as early age endophenotypes, at least in siblings of patients with childhood-onset schizophrenia. It is important to explore factors that cause these deficits in healthy siblings, what protects siblings from schizophrenia symptoms in both children and adults, and what is underlying the (temporary) normalization of these deficits by late adolescence. Volumetric studies may not be sensitive enough to reflect the subtler involvement differences seen in adult siblings. Other imaging techniques, such as diffusion tensor imaging, magnetic transfer ratio, functional MRI, or magnetoencephalography may provide more acute insight into developing neurocircuitries, particularly to see if these observed patterns of grey matter loss reflect connectivity network changes that have been documented in patients with schizophrenia (Ellison-Wright and Bullmore, 2009; van den Heuvel et al., 2010; Pettersson-Yeo et al., 2011; Fornito et al., 2012; Hulshoff Pol and Bullmore, 2013), and their siblings (Whitfield-Gabrieli et al., 2009; Collin et al., 2011; Repovs et al., 2011), as has been validated recently in other conditions (Seeley et al., 2009).
Indeed, impaired cerebellar functional connectivity (Collin et al., 2011) as well as hyperactivity and hyperconnectivity of the default network (Whitfield-Gabrieli et al., 2009) have been found in siblings of patients with adult-onset schizophrenia. Longitudinal studies in healthy siblings may show ‘normalization’ of the abnormal brain activity in siblings as they get older, or a continued deficit pattern at a milder degree compared to the probands. Thus studying functional neurodevelopment in siblings, particularly from early ages through the typical age of onset of schizophrenia, may provide unique insights into which neurocircuitries are vulnerable to genetic variants and how they normalize over time. Together, a complete functional understanding of neurodevelopment can synergize with the structural one, and indicate the crucial link, or lack thereof, between the physical changes to the brain, and its illness related activity.
The benefit that comes from comparing childhood and adult-onset forms of schizophrenia should not only be employed in terms of brain changes, but in any instance that can improve the understanding and treatment of this disorder. The human body changes throughout the lifespan, and analysis that incorporates that progression is best suited to spot time and age dependent effects. Whether this involves neural dysfunction, growth, or normalization, critical information arises from longitudinal study of individuals at the highest risk for developing schizophrenia, to better analyse its complex causes.
Funding
The present research was funded by the Intramural Research Program (IRP) at the National Institute of Mental Health in Bethesda, MD.
References
- Ayuso-Mateos JL, Gutierrez-Recacha P, Haro JM, Chisholm D. Estimating the prevalence of schizophrenia in Spain using a disease model. Schizophr Res. 2006;86:194–201. doi: 10.1016/j.schres.2006.06.003. [DOI] [PubMed] [Google Scholar]
- Baare WF, van Oel CJ, Hulshoff Pol HE, Schnack HG, Durston S, Sitskoorn MM, et al. Volumes of brain structures in twins discordant for schizophrenia. Arch Gen Psychiatry. 2001;58:33–40. doi: 10.1001/archpsyc.58.1.33. [DOI] [PubMed] [Google Scholar]
- Boos HB, Aleman A, Cahn W, Hulshoff Pol H, Kahn RS. Brain volumes in relatives of patients with schizophrenia: a meta-analysis. Arch Gen Psychiatry. 2007;64:297–304. doi: 10.1001/archpsyc.64.3.297. [DOI] [PubMed] [Google Scholar]
- Boos HB, Cahn W, van Haren NE, Derks EM, Brouwer RM, Schnack HG, et al. Focal and global brain measurements in siblings of patients with schizophrenia. Schizophr Bull. 2012;38:814–825. doi: 10.1093/schbul/sbq147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brans RG, Kahn RS, Schnack HG, van Baal GC, Posthuma D, van Haren NE, et al. Brain plasticity and intellectual ability are influenced by shared genes. J Neurosci. 2010;30:5519–24. doi: 10.1523/JNEUROSCI.5841-09.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brans RG, van Haren NE, van Baal GC, Schnack HG, Kahn RS, Hulshoff Pol HE. Heritability of changes in brain volume over time in twin pairs discordant for schizophrenia. Arch Gen Psychiatry. 2008a;65:1259–68. doi: 10.1001/archpsyc.65.11.1259. [DOI] [PubMed] [Google Scholar]
- Brans RG, van Haren NE, van Baal GC, Staal WG, Schnack HG, Kahn RS, et al. Longitudinal MRI study in schizophrenia patients and their healthy siblings. Br J Psychiatry. 2008b;193:422–3. doi: 10.1192/bjp.bp.107.041467. [DOI] [PubMed] [Google Scholar]
- Cannon M, Jones PB, Murray RM. Obstetric complications and schizophrenia: historical and meta-analytic review. Am J Psychiatry. 2002a;159:1080–92. doi: 10.1176/appi.ajp.159.7.1080. [DOI] [PubMed] [Google Scholar]
- Cannon TD, Thompson PM, van Erp TG, Toga AW, Poutanen VP, Huttunen M, et al. Cortex mapping reveals regionally specific patterns of genetic and disease-specific gray-matter deficits in twins discordant for schizophrenia. Proc Natl Acad Sci USA. 2002b;99:3228–33. doi: 10.1073/pnas.052023499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cannon TD, van Erp TG, Huttunen M, Lonnqvist J, Salonen O, Valanne L, et al. Regional gray matter, white matter, and cerebrospinal fluid distributions in schizophrenic patients, their siblings, and controls. Arch Gen Psychiatry. 1998;55:1084–91. doi: 10.1001/archpsyc.55.12.1084. [DOI] [PubMed] [Google Scholar]
- Collin G, Hulshoff Pol HE, Haijma SV, Cahn W, Kahn RS, van den Heuvel MP. Impaired cerebellar functional connectivity in schizophrenia patients and their healthy siblings. Front Psychiatry. 2011;2:73. doi: 10.3389/fpsyt.2011.00073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DeLisi LE. The concept of progressive brain change in schizophrenia: implications for understanding schizophrenia. Schizophr Bull. 2008;34:312–21. doi: 10.1093/schbul/sbm164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eckstrand K, Addington AM, Stromberg T, Merriman B, Miller R, Gochman P, et al. Sex chromosome anomalies in childhood onset schizophrenia: an update. Mol Psychiatry. 2008;13:910–1. doi: 10.1038/mp.2008.67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ellison-Wright I, Bullmore E. Meta-analysis of diffusion tensor imaging studies in schizophrenia. Schizophr Res. 2009;108:3–10. doi: 10.1016/j.schres.2008.11.021. [DOI] [PubMed] [Google Scholar]
- Ellison-Wright I, Glahn DC, Laird AR, Thelen SM, Bullmore E. The anatomy of first-episode and chronic schizophrenia: an anatomical likelihood estimation meta-analysis. Am J Psychiatry. 2008;165:1015–23. doi: 10.1176/appi.ajp.2008.07101562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ettinger U, Picchioni M, Landau S, Matsumoto K, van Haren NE, Marshall N, et al. Magnetic resonance imaging of the thalamus and adhesio interthalamica in twins with schizophrenia. Arch Gen Psychiatry. 2007;64:401–9. doi: 10.1001/archpsyc.64.4.401. [DOI] [PubMed] [Google Scholar]
- Ettinger U, Schmechtig A, Toulopoulou T, Borg C, Orrells C, Owens S, et al. Prefrontal and striatal volumes in monozygotic twins concordant and discordant for schizophrenia. Schizophr Bull. 2012;38:192–203. doi: 10.1093/schbul/sbq060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fornito A, Yucel M, Patti J, Wood SJ, Pantelis C. Mapping grey matter reductions in schizophrenia: an anatomical likelihood estimation analysis of voxel-based morphometry studies. Schizophr Res. 2009;108:104–13. doi: 10.1016/j.schres.2008.12.011. [DOI] [PubMed] [Google Scholar]
- Fornito A, Zalesky A, Pantelis C, Bullmore ET. Schizophrenia, neuroimaging and connectomics. Neuroimage. 2012;62:2296–314. doi: 10.1016/j.neuroimage.2011.12.090. [DOI] [PubMed] [Google Scholar]
- Giedd JN, Jeffries NO, Blumenthal J, Castellanos FX, Vaituzis AC, Fernandez T, et al. Childhood-onset schizophrenia: progressive brain changes during adolescence. Biol Psychiatry. 1999;46:892–8. doi: 10.1016/s0006-3223(99)00072-4. [DOI] [PubMed] [Google Scholar]
- Giedd JN, Rapoport JL. Structural MRI of pediatric brain development: what have we learned and where are we going? Neuron. 2010;67:728–34. doi: 10.1016/j.neuron.2010.08.040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gogtay N. Cortical brain development in schizophrenia: insights from neuroimaging studies in childhood-onset schizophrenia. Schizophr Bull. 2008;34:30–6. doi: 10.1093/schbul/sbm103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gogtay N, Greenstein D, Lenane M, Clasen L, Sharp W, Gochman P, et al. Cortical brain development in nonpsychotic siblings of patients with childhood-onset schizophrenia. Arch Gen Psychiatry. 2007;64:772–80. doi: 10.1001/archpsyc.64.7.772. [DOI] [PubMed] [Google Scholar]
- Gogtay N, Lu A, Leow AD, Klunder AD, Lee AD, Chavez A, et al. Three-dimensional brain growth abnormalities in childhood-onset schizophrenia visualized by using tensor-based morphometry. Proc Natl Acad Sci USA. 2008;105:15979–84. doi: 10.1073/pnas.0806485105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gogtay N, Rapoport JL. Childhood-onset schizophrenia: insights from neuroimaging studies. J Am Acad Child Adolesc Psychiatry. 2008;47:1120–4. doi: 10.1097/CHI.0b013e31817eed7a. [DOI] [PubMed] [Google Scholar]
- Gogtay N, Sporn A, Clasen LS, Greenstein D, Giedd JN, Lenane M, et al. Structural brain MRI abnormalities in healthy siblings of patients with childhood-onset schizophrenia. Am J Psychiatry. 2003;160:569–71. doi: 10.1176/appi.ajp.160.3.569. [DOI] [PubMed] [Google Scholar]
- Goldman AL, Pezawas L, Mattay VS, Fischl B, Verchinski BA, Chen Q, et al. Widespread reductions of cortical thickness in schizophrenia and spectrum disorders and evidence of heritability. Arch Gen Psychiatry. 2009;66:467–77. doi: 10.1001/archgenpsychiatry.2009.24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gothelf D, Eliez S, Thompson T, Hinard C, Penniman L, Feinstein C, et al. COMT genotype predicts longitudinal cognitive decline and psychosis in 22q11.2 deletion syndrome. Nat Neurosci. 2005;8:1500–2. doi: 10.1038/nn1572. [DOI] [PubMed] [Google Scholar]
- Gottesman II, Gould TD. The endophenotype concept in psychiatry: etymology and strategic intentions. Am J Psychiatry. 2003;160:636–45. doi: 10.1176/appi.ajp.160.4.636. [DOI] [PubMed] [Google Scholar]
- Gould TD, Gottesman II. Psychiatric endophenotypes and the development of valid animal models. Genes Brain Behav. 2006;5:113–9. doi: 10.1111/j.1601-183X.2005.00186.x. [DOI] [PubMed] [Google Scholar]
- Greenstein D, Lenroot R, Clausen L, Chavez A, Vaituzis AC, Tran L, et al. Cerebellar development in childhood onset schizophrenia and non-psychotic siblings. Psychiatry Res. 2011;193:131–7. doi: 10.1016/j.pscychresns.2011.02.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Greenstein DK, Wolfe S, Gochman P, Rapoport JL, Gogtay N. Remission status and cortical thickness in childhood-onset schizophrenia. J Am Acad Child Adolesc Psychiatry. 2008;47:1133–40. doi: 10.1097/CHI.0b013e3181825b0c. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haijma SV, Van Haren N, Cahn W, Koolschijn PC, Hulshoff Pol HE, Kahn RS. Brain volumes in schizophrenia: a meta-analysis in over 18 000 subjects. Schizophr Bull. 2012 doi: 10.1093/schbul/sbs118. Oct 5 (Epub) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harms MP, Wang L, Campanella C, Aldridge K, Moffitt AJ, Kuelper J, et al. Structural abnormalities in gyri of the prefrontal cortex in individuals with schizophrenia and their unaffected siblings. Br J Psychiatry. 2010;196:150–7. doi: 10.1192/bjp.bp.109.067314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Honea RA, Meyer-Lindenberg A, Hobbs KB, Pezawas L, Mattay VS, Egan MF, et al. Is gray matter volume an intermediate phenotype for schizophrenia? A voxel-based morphometry study of patients with schizophrenia and their healthy siblings. Biol Psychiatry. 2008;63:465–74. doi: 10.1016/j.biopsych.2007.05.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Howes OD, Kapur S. The dopamine hypothesis of schizophrenia: version III—the final common pathway. Schizophr Bull. 2009;35:549–62. doi: 10.1093/schbul/sbp006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hulshoff Pol H, Bullmore E. Neural networks in psychiatry. Eur Neuropsychopharmacol. 2013;23:1–6. doi: 10.1016/j.euroneuro.2012.12.004. [DOI] [PubMed] [Google Scholar]
- Hulshoff Pol HE, Brans RG, van Haren NE, Schnack HG, Langen M, Baare WF, et al. Gray and white matter volume abnormalities in monozygotic and same-gender dizygotic twins discordant for schizophrenia. Biol Psychiatry. 2004;55:126–30. doi: 10.1016/s0006-3223(03)00728-5. [DOI] [PubMed] [Google Scholar]
- Hulshoff Pol HE, Kahn RS. What happens after the first episode? A review of progressive brain changes in chronically ill patients with schizophrenia. Schizophr Bull. 2008;34:354–66. doi: 10.1093/schbul/sbm168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hulshoff Pol HE, Schnack HG, Mandl RC, Brans RG, van Haren NE, Baare WF, et al. Gray and white matter density changes in monozygotic and same-sex dizygotic twins discordant for schizophrenia using voxel-based morphometry. Neuroimage. 2006;31:482–8. doi: 10.1016/j.neuroimage.2005.12.056. [DOI] [PubMed] [Google Scholar]
- Hulshoff Pol HE, van Baal GC, Schnack HG, Brans RG, van der Schot AC, Brouwer RM, et al. Overlapping and segregating structural brain abnormalities in twins with schizophrenia or bipolar disorder. Arch Gen Psychiatry. 2012;69:349–59. doi: 10.1001/archgenpsychiatry.2011.1615. [DOI] [PubMed] [Google Scholar]
- Kendler KS, Neale MC. Endophenotype: a conceptual analysis. Mol Psychiatry. 2010;15:789–97. doi: 10.1038/mp.2010.8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kremen WS, Faraone SV, Toomey R, Seidman LJ, Tsuang MT. Sex differences in self-reported schizotypal traits in relatives of schizophrenic probands. Schizophr Res. 1998;34:27–37. doi: 10.1016/s0920-9964(98)00081-4. [DOI] [PubMed] [Google Scholar]
- Lenroot RK, Schmitt JE, Ordaz SJ, Wallace GL, Neale MC, et al. Differences in genetic and environmental influences on the human cerebral cortex associated with development during childhood and adolescence. Hum Brain Map. 2009;30:163–74. doi: 10.1002/hbm.20494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lichtenstein P, Yip BH, Bjork C, Pawitan Y, Cannon TD, Sullivan PF, et al. Common genetic determinants of schizophrenia and bipolar disorder in Swedish families: a population-based study. Lancet. 2009;373:234–9. doi: 10.1016/S0140-6736(09)60072-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mattai A, Hosanagar A, Weisinger B, Greenstein D, Stidd R, Clasen L, et al. Hippocampal volume development in healthy siblings of childhood-onset schizophrenia patients. Am J Psychiatry. 2011a;168:427–35. doi: 10.1176/appi.ajp.2010.10050681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mattai AA, Weisinger B, Greenstein D, Stidd R, Clasen L, Miller R, et al. Normalization of cortical gray matter deficits in nonpsychotic siblings of patients with childhood-onset schizophrenia. J Am Acad Child Adolesc Psychiatry. 2011b;50:697–704. doi: 10.1016/j.jaac.2011.03.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McIntosh AM, Baig BJ, Hall J, Job D, Whalley HC, Lymer GK, et al. Relationship of catechol-O-methyltransferase variants to brain structure and function in a population at high risk of psychosis. Biol Psychiatry. 2007;61:1127–34. doi: 10.1016/j.biopsych.2006.05.020. [DOI] [PubMed] [Google Scholar]
- McIntosh AM, Owens DC, Moorhead WJ, Whalley HC, Stanfield AC, Hall J, et al. Longitudinal volume reductions in people at high genetic risk of schizophrenia as they develop psychosis. Biol Psychiatry. 2011;69:953–8. doi: 10.1016/j.biopsych.2010.11.003. [DOI] [PubMed] [Google Scholar]
- Narr KL, van Erp TG, Cannon TD, Woods RP, Thompson PM, Jang S, et al. A twin study of genetic contributions to hippocampal morphology in schizophrenia. Neurobiol Dis. 2002;11:83–95. doi: 10.1006/nbdi.2002.0548. [DOI] [PubMed] [Google Scholar]
- Nelson MD, Saykin AJ, Flashman LA, Riordan HJ. Hippocampal volume reduction in schizophrenia as assessed by magnetic resonance imaging: a meta-analytic study. Arch Gen Psychiatry. 1998;55:433–40. doi: 10.1001/archpsyc.55.5.433. [DOI] [PubMed] [Google Scholar]
- Noga JT, Bartley AJ, Jones DW, Torrey EF, Weinberger DR. Cortical gyral anatomy and gross brain dimensions in monozygotic twins discordant for schizophrenia. Schizophr Res. 1996;22:27–40. doi: 10.1016/0920-9964(96)00046-1. [DOI] [PubMed] [Google Scholar]
- Olabi B, Ellison-Wright I, McIntosh AM, Wood SJ, Bullmore E, Lawrie SM. Are there progressive brain changes in schizophrenia? A meta-analysis of structural magnetic resonance imaging studies. Biol Psychiatry. 2011;70:88–96. doi: 10.1016/j.biopsych.2011.01.032. [DOI] [PubMed] [Google Scholar]
- Pantelis C, Yucel M, Wood SJ, Velakoulis D, Sun D, Berger G, et al. Structural brain imaging evidence for multiple pathological processes at different stages of brain development in schizophrenia. Schizophr Bull. 2005;31:672–96. doi: 10.1093/schbul/sbi034. [DOI] [PubMed] [Google Scholar]
- Peper JS, Brouwer RM, Boomsma DI, Kahn RS, Hulshoff Pol HE. Genetic influences on human brain structure: a review of brain imaging studies in twins. Hum Brain Mapp. 2007;28:464–73. doi: 10.1002/hbm.20398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pettersson-Yeo W, Allen P, Benetti S, McGuire P, Mechelli A. Dysconnectivity in schizophrenia: where are we now? Neurosci Biobehav Rev. 2011;35:1110–24. doi: 10.1016/j.neubiorev.2010.11.004. [DOI] [PubMed] [Google Scholar]
- Picchioni MM, Walshe M, Toulopoulou T, McDonald C, Taylor M, Waters-Metenier S, et al. Genetic modelling of childhood social development and personality in twins and siblings with schizophrenia. Psychol Med. 2010;40:1305–16. doi: 10.1017/S0033291709991425. [DOI] [PubMed] [Google Scholar]
- Rapoport JL, Addington A, Frangou S. The neurodevelopmental model of schizophrenia: what can very early onset cases tell us? Curr Psychiatry Rep. 2005;7:81–2. doi: 10.1007/s11920-005-0001-z. [DOI] [PubMed] [Google Scholar]
- Rapoport JL, Gogtay N. Childhood onset schizophrenia: support for a progressive neurodevelopmental disorder. Int J Dev Neurosci. 2011;29:251–8. doi: 10.1016/j.ijdevneu.2010.10.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rasetti R, Sambataro F, Chen Q, Callicott JH, Mattay VS, Weinberger DR. Altered cortical network dynamics: a potential intermediate phenotype for schizophrenia and association with ZNF804A. Arch Gen Psychiatry. 2011;68:1207–17. doi: 10.1001/archgenpsychiatry.2011.103. [DOI] [PubMed] [Google Scholar]
- Raznahan A, Greenstein D, Lee Y, Long R, Clasen L, Gochman P, et al. Catechol-o-methyl transferase (COMT) val158met polymorphism and adolescent cortical development in patients with childhood-onset schizophrenia, their non-psychotic siblings, and healthy controls. Neuroimage. 2011;57:1517–23. doi: 10.1016/j.neuroimage.2011.05.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reiner A, Dragatsis I, Dietrich P. Genetics and neuropathology of Huntington's disease. Int Rev Neurobiol. 2011;98:325–72. doi: 10.1016/B978-0-12-381328-2.00014-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Repovs G, Csernansky JG, Barch DM. Brain network connectivity in individuals with schizophrenia and their siblings. Biol Psychiatry. 2011;69:967–73. doi: 10.1016/j.biopsych.2010.11.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rijsdijk FV, van Haren NE, Picchioni MM, McDonald C, Toulopoulou T, Hulshoff Pol HE, et al. Brain MRI abnormalities in schizophrenia: same genes or same environment? Psychol Med. 2005;35:1399–409. doi: 10.1017/S0033291705005167. [DOI] [PubMed] [Google Scholar]
- Seeley WW, Crawford RK, Zhou J, Miller BL, Greicius MD. Neurodegenerative diseases target large-scale human brain networks. Neuron. 2009;62:42–52. doi: 10.1016/j.neuron.2009.03.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seidman LJ, Faraone SV, Goldstein JM, Goodman JM, Kremen WS, Matsuda G, et al. Reduced subcortical brain volumes in nonpsychotic siblings of schizophrenic patients: a pilot magnetic resonance imaging study. Am J Med Genet. 1997;74:507–14. doi: 10.1002/(sici)1096-8628(19970919)74:5<507::aid-ajmg11>3.0.co;2-g. [DOI] [PubMed] [Google Scholar]
- Seidman LJ, Faraone SV, Goldstein JM, Goodman JM, Kremen WS, Toomey R, et al. Thalamic and amygdala-hippocampal volume reductions in first-degree relatives of patients with schizophrenia: an MRI-based morphometric analysis. Biol Psychiatry. 1999;46:941–54. doi: 10.1016/s0006-3223(99)00075-x. [DOI] [PubMed] [Google Scholar]
- Sharma T, Lancaster E, Sigmundsson T, Lewis S, Takei N, Gurling H, et al. Lack of normal pattern of cerebral asymmetry in familial schizophrenic patients and their relatives—The Maudsley Family Study. Schizophr Res. 1999;40:111–20. doi: 10.1016/s0920-9964(99)00143-7. [DOI] [PubMed] [Google Scholar]
- Shaw P, Greenstein D, Lerch J, Clasen L, Lenroot R, Gogtay N, et al. Intellectual ability and cortical development in children and adolescents. Nature. 2006;440:676–9. doi: 10.1038/nature04513. [DOI] [PubMed] [Google Scholar]
- Shenton ME, Dickey CC, Frumin M, McCarley RW. A review of MRI findings in schizophrenia. Schizophr Res. 2001;49:1–52. doi: 10.1016/s0920-9964(01)00163-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shenton ME, Whitford TJ, Kubicki M. Structural neuroimaging in schizophrenia: from methods to insights to treatments. Dialogues Clin Neurosci. 2010;12:317–32. doi: 10.31887/DCNS.2010.12.3/mshenton. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shi Q, Gibson GE. Oxidative stress and transcriptional regulation in Alzheimer disease. Alzheimer Dis Assoc Disord. 2007;21:276–91. doi: 10.1097/WAD.0b013e31815721c3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Staal WG, Hulshoff Pol HE, Schnack H, van der Schot AC, Kahn RS. Partial volume decrease of the thalamus in relatives of patients with schizophrenia. Am J Psychiatry. 1998;155:1784–6. doi: 10.1176/ajp.155.12.1784. [DOI] [PubMed] [Google Scholar]
- Staal WG, Hulshoff Pol HE, Schnack HG, Hoogendoorn ML, Jellema K, Kahn RS. Structural brain abnormalities in patients with schizophrenia and their healthy siblings. Am J Psychiatry. 2000;157:416–21. doi: 10.1176/appi.ajp.157.3.416. [DOI] [PubMed] [Google Scholar]
- Stein JL, Medland SE, Vasquez AA, Hibar DP, Senstad RE, Winkler AM, et al. Identification of common variants associated with human hippocampal and intracranial volumes. Nat Genet. 2012;44:552–61. doi: 10.1038/ng.2250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sullivan PF, Kendler KS, Neale MC. Schizophrenia as a complex trait: evidence from a meta-analysis of twin studies. Arch Gen Psychiatry. 2003;60:1187–92. doi: 10.1001/archpsyc.60.12.1187. [DOI] [PubMed] [Google Scholar]
- Tepest R, Wang L, Miller MI, Falkai P, Csernansky JG. Hippocampal deformities in the unaffected siblings of schizophrenia subjects. Biol Psychiatry. 2003;54:1234–40. doi: 10.1016/s0006-3223(03)00702-9. [DOI] [PubMed] [Google Scholar]
- van den Heuvel MP, Mandl RC, Stam CJ, Kahn RS, Hulshoff Pol HE. Aberrant frontal and temporal complex network structure in schizophrenia: a graph theoretical analysis. J Neurosci. 2010;30:15915–26. doi: 10.1523/JNEUROSCI.2874-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Erp TG, Saleh PA, Huttunen M, Lonnqvist J, Kaprio J, Salonen O, et al. Hippocampal volumes in schizophrenic twins. Arch Gen Psychiatry. 2004;61:346–53. doi: 10.1001/archpsyc.61.4.346. [DOI] [PubMed] [Google Scholar]
- Van Erp TG, Saleh PA, Rosso IM, Huttunen M, Lonnqvist J, et al. Contributions of genetic risk and fetal hypoxia to hippocampal volume in patients with schizophrenia or schizoaffective disorder, their unaffected siblings, and healthy unrelated volunteers. Am J Psychiatry. 2002;159:1514–20. doi: 10.1176/appi.ajp.159.9.1514. [DOI] [PubMed] [Google Scholar]
- van Haren NE, Picchioni MM, McDonald C, Marshall N, Davis N, et al. A controlled study of brain structure in monozygotic twins concordant and discordant for schizophrenia. Biol Psychiatry. 2004;56:454–61. doi: 10.1016/j.biopsych.2004.06.033. [DOI] [PubMed] [Google Scholar]
- van Haren NE, Rijsdijk F, Schnack HG, Picchioni MM, Toulopoulou T, Weisbrod M, et al. The genetic and environmental determinants of the association between brain abnormalities and schizophrenia: the schizophrenia twins and relatives consortium. Biol Psychiatry. 2012;71:915–21. doi: 10.1016/j.biopsych.2012.01.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Soelen IL, Brouwer RM, van Baal GC, Schnack HG, Peper JS, Collins DL, et al. Genetic influences on thinning of the cerebral cortex during development. Neuroimage. 2012;59:3871–80. doi: 10.1016/j.neuroimage.2011.11.044. [DOI] [PubMed] [Google Scholar]
- Walters JT, Owen MJ. Endophenotypes in psychiatric genetics. Mol Psychiatry. 2007;12:886–90. doi: 10.1038/sj.mp.4002068. [DOI] [PubMed] [Google Scholar]
- Whitfield-Gabrieli S, Thermenos HW, Milanovic S, Tsuang MT, Faraone SV, McCarley RW, et al. Hyperactivity and hyperconnectivity of the default network in schizophrenia and in first-degree relatives of persons with schizophrenia. Proc Natl Acad Sci USA. 2009;106:1279–84. doi: 10.1073/pnas.0809141106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wright IC, Rabe-Hesketh S, Woodruff PW, David AS, Murray RM, Bullmore ET. Meta-analysis of regional brain volumes in schizophrenia. Am J Psychiatry. 2000;157:16–25. doi: 10.1176/ajp.157.1.16. [DOI] [PubMed] [Google Scholar]
- Wright IC, Sharma T, Ellison ZR, McGuire PK, Friston KJ, Brammer MJ, et al. Supra-regional brain systems and the neuropathology of schizophrenia. Cereb Cortex. 1999;9:366–78. doi: 10.1093/cercor/9.4.366. [DOI] [PubMed] [Google Scholar]