Sir, it has been reported that microcysts from macular oedema occur in 4.7% of patients with multiple sclerosis (Gelfand et al., 2012). It is now evident that these microcysts do not represent oedema, nor is their occurrence a specific feature of demyelinating disease, because they have been identified in patients with Leber’s hereditary optic neuropathy, Kjer’s dominant optic atrophy, and optic glioma (Abegg et al., 2012; Barboni et al., 2013). However, their precise aetiology has remained a mystery.
As pointed out by Abegg et al. (2012), the classical ocular pathology literature provides a clue to the formation of microcysts. Van Buren (1963) described retrograde trans-synaptic degeneration of the inner nuclear layer in histological sections of the retina after lesions of the optic nerve or chiasm, causing ‘cystic degeneration’ that resembles the ‘microcysts’ now being imaged with optical coherence tomography. Gills and Wadsworth (1967) found a 15–60% reduction in cell population in the inner nuclear layer in nine patients with longstanding blindness from compressive or traumatic lesions of the optic nerve. In at least three cases, microcycsts were present in the inner nuclear layer (Fig. 1). In two patients with more recent blindness, cell loss was not observed. Presumably, sufficient time had not elapsed for trans-synaptic degeneration to occur.
Figure 1.
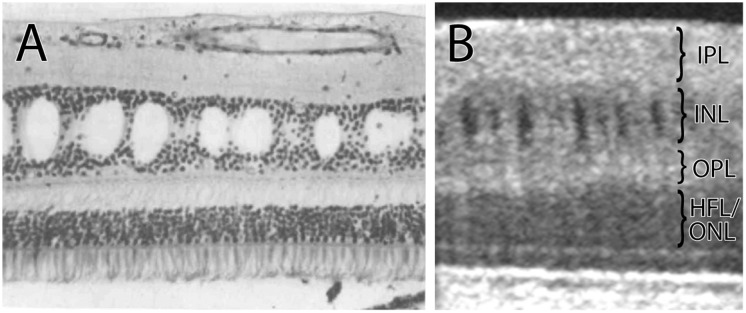
(A) Histological section of the retina showing cysts in the inner nuclear layer following retrograde trans-synaptic degeneration from a tumour of the optic nerve. Reproduced from Gills and Wadsworth (1967) with permission from James P. Gills. (B) Image obtained by optical coherence tomography from a patient with optic atrophy, showing cysts in the inner nuclear layer. IPL = inner plexiform layer; INL = inner nuclear layer; OPL = outer plexiform layer; HFL = Henle fibre layer; ONL = outer nuclear layer.
Gills and Wadsworth (1967) also reported cell loss in the inner nuclear layer in patients with optic atrophy from multiple sclerosis. They concluded that ‘The loss of cells in the inner nuclear layer in eyes of patients with advanced multiple sclerosis is compatible with trans-synaptic degeneration of the inner nuclear layer’. Gelfand et al. (2012) have succeeded admirably at imaging trans-synaptic degeneration in living patients by using optical coherence tomography. Not surprisingly, they found that patients with microcysts were older, with longer disease duration and worse disability. Such individuals are likely to have more severe trans-synaptic degeneration. The microcysts should not be treated with steroids or angiogenesis blockers, because they do not signify macular oedema.
It has been controversial whether retrograde trans-synaptic degeneration occurs in the visual system. The dispute has been settled by evidence that the retinal nerve fibre layer becomes atrophic after damage to the visual cortex (Jindahra et al., 2009). Shifting forward one synapse, there is no reason why the inner nuclear layer should not become atrophic after damage to ganglion cells. A recent observation by Sigler et al. (2013) provides the most direct evidence that microcysts of the inner nuclear layer are due to retrograde trans-synaptic degeneration. In eight patients the nerve fibre and ganglion cell layers were damaged by surgical removal of an epi-retinal membrane. A month postoperatively no microcysts were present in the inner nuclear layer. However, microcysts appeared at a mean of 3.3 months following surgery, and continued to increase in size for up to 18 months. This observation suggests that with the passage of time, progressive trans-synaptic degeneration in the inner nuclear layer leads to the formation of microcysts.
Barboni et al. (2013) denied that microcysts are due to trans-synaptic degeneration, because they develop in only a minority of patients, whereas trans-synaptic degeneration occurs in all patients given sufficient time. As an alternative mechanism, they proposed a combined effect from optic atrophy and vitreous traction. According to their idea, loss of the nerve fibre and ganglion cell layers allows the vitreous to tug directly on the inner nuclear layer, resulting in schisis.
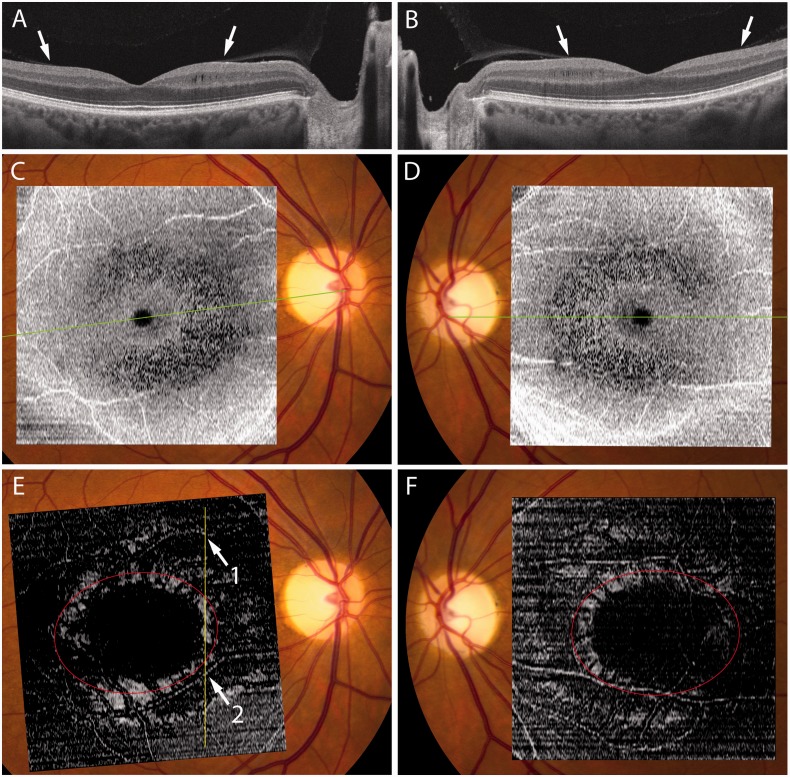
Here we present imaging data that support their proposal that traction on the retina is necessary for the occurrence of microcysts. However, we suggest that trans-synaptic degeneration is also important. A 49-year-old female with dominant optic atrophy had a 5-year history of blurred vision and dychromatopsia. The visual acuity was 20/30 in each eye and there were bilateral central scotomas. Fundus examination was normal except for marked temporal pallor of both optic discs. An angiogram showed no fluorescein leakage, but optical coherence tomography revealed extensive microcysts in the inner nuclear layer of both eyes (Fig. 2A and B). An en face reconstruction showed that the microcysts were distributed in a C-shaped ring that was incomplete temporally (Fig. 2C and D). Abegg et al. (2012) illustrated a similar distribution of microcysts, although they did not comment upon it. Gelfand (personal communication) has also confirmed that in their cohort, microcysts were more extensive on the nasal compared with the temporal side of the fovea.
Figure 2.
(A and B) Optical coherence tomography scans through the optic nerve and fovea in each eye of a 49-year-old female with dominant optic atrophy showing vitreous insertion (arrows). Nasally, but not temporally, vitreous traction has thickened the inner nuclear layer and produced microcysts. Note severe attenuation of the ganglion cell and nerve fibre layer. (C and D) Ring of microcysts between 3–8° visualized in en face reconstructions of 512 × 128 macular cubes achieved using Cirrus HD-OCT advanced visualization software (version 6.0) with a 68 µm RPE-based contour passing through the inner nuclear layer. The green lines correspond to the orientation of the scans shown above. (E and F) 20 µm internal limiting membrane based slab showing a ring of hyper-reflectivity where the retinal surface is perpendicular to incident light, presumably from vitreous traction. Red oval marks the insertion of the posterior hyaloid membrane, determined from inspection of serial cross-sections. In E, the vertical yellow line corresponds to the orientation of Fig. 3, with two prominent blood vessels denoted as 1 and 2.
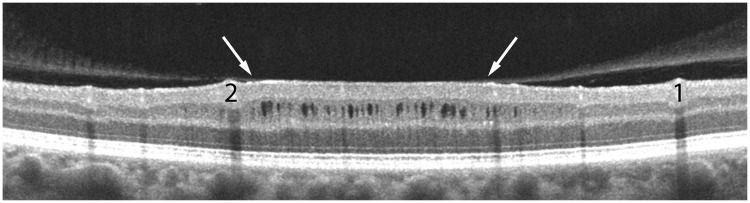
In our patient, the microcysts were concentrated at an eccentricity between 3–8° in the macula. The posterior hyaloid membrane inserted into the ring of microcysts at an eccentricity of ∼6°. Optical coherence tomography revealed a zone of bright signal in an en face reconstruction at the level of the vitreo-retinal interface, corresponding to the ring of microcysts (Fig. 2E and F). In cross-section the retina was tented upwards by the posterior hyaloid membrane, causing flattening of the retinal surface, relative thickening of the inner nuclear layer, and the development of microcysts (Fig. 3). In temporal retina, where microcysts were absent, the retina was not elevated by the posterior hyaloid membrane (Fig. 2A and B).
Figure 3.
Optical tomography coherence scan of the right eye, corresponding to the yellow line in Fig. 2E with blood vessels marked 1 and 2, showing tenting of the inner nuclear layer from vitreous traction (arrows). The thickness of the inner nuclear layer is increased by 40% in the zone where it is elevated by traction, giving rise to ∼30 microcysts.
Why does vitreous traction combined with trans-synaptic degeneration produce microcysts? In normal subjects, the inner nuclear layer is thickest and most densely populated in a ring from 3–8°. Hence, following trans-synaptic degeneration, this zone of the inner nuclear layer suffers the most pronounced reduction in tissue volume. We propose that collapse of the inner nuclear layer is prevented by inwards and lateral traction exerted by the intact or partially detached posterior hyaloid membrane. This membrane inserts into the internal limiting membrane of the retina, which is formed by the footplates of Müller cells. These cells serve as a scaffold for the layers of the retina. Traction exerted by the vitreous body counteracts the loss of volume caused by trans-synaptic cellular degeneration. The volume formerly occupied by cells that have degenerated must be replaced by something. Hence, fluid-filled microcysts begin to form in the inner nuclear layer. Of course, the lost bipolar cells were once present diffusely within the inner nuclear layer, but intercellular adhesion between the surviving neurons promotes the development of cystic clefts. In patients with relatively mild volume loss, weak vitreous traction, or strong intercellular adhesion, microcysts do not occur. They also do not occur in patients with optic neuropathies that spare the macula.
Several questions remain. Why are no cysts present between the fovea and 3°, or at eccentricities beyond 8°? The inner nuclear layer is thinner in these regions, so volume loss is less. In addition, retinal elevation is maximal where the posterior hyaloid membrane inserts, and dissipates with increasing distance from the site of vitreous-retinal contact. Why is the ring of cysts incomplete temporally? Although the vitreous inserts into the retina temporally, it exerts less traction along the temporal raphe, judging from the lack of tissue elevation (Fig. 2A and B). Why do cysts occur from epi-retinal membrane peeling, even after the vitreous has been removed (Sigler et al., 2013)? This question is difficult to answer, but residual epi-retinal membrane and scarring may be responsible.
Barboni et al. (2013) are correct that microcysts in the inner nuclear layer of the macula revealed by optical coherence tomography are simply a consequence of long standing optic atrophy. Therefore, this imaging technology may not advance our understanding of the biology of multiple sclerosis as much as hoped.
Acknowledgements
Technical support was provided by Valerie L. Wu.
Funding
This work was supported by grants EY10217 (J.C.H.), EY017269 (B.J.L.), and EY02162 (Beckman Vision Center) from the National Eye Institute.
References
- Abegg M, Zinkernagel M, Wolf S. Microcystic macular degeneration from optic neuropathy. Brain. 2012;135:e225. doi: 10.1093/brain/aws215. [DOI] [PubMed] [Google Scholar]
- Barboni P, Carelli V, Savini G, Carbonelli M, La Morgia C, Sadun AA. Microcystic macular degeneration from optic neuropathy: not inflammatory, not trans-synaptic degeneration. Brain. 2013 doi: 10.1093/brain/awt014. Advance Access published on February 8, 2013. [DOI] [PubMed] [Google Scholar]
- Gelfand JM, Nolan R, Schwartz DM, Graves J, Green AJ. Microcystic macular oedema in multiple sclerosis is associated with disease severity. Brain. 2012;135:1786–93. doi: 10.1093/brain/aws098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gills JP, Wadsworth JA. Retrograde transsynaptic degeneration of the inner nuclear layer of the retina. Invest Ophthalmol Vis Sci. 1967;6:437–48. [Google Scholar]
- Jindahra P, Petrie A, Plant GT. Retrograde trans-synaptic retinal ganglion cell loss identified by optical coherence tomography. Brain. 2009;132:628–34. doi: 10.1093/brain/awp001. [DOI] [PubMed] [Google Scholar]
- Sigler EJ, Randolph JC, Charles S. Delayed onset inner nuclear layer cystic changes following internal limiting membrane removal for epimacular membrane. Graefes Arch Clin Exp Ophthalmol. 2013 doi: 10.1007/s00417-012-2253-8. Advance Access published on January 10, 2013. [DOI] [PubMed] [Google Scholar]
- Van Buren JM. Trans-synaptic retrograde degeneration in the visual system of primates. J Neurol Neurosurg Psychiatry. 1963;26:402–9. doi: 10.1136/jnnp.26.5.402. [DOI] [PMC free article] [PubMed] [Google Scholar]