Abstract
We sought to characterize perfusion patterns of progressive multifocal leukoencephalopathy lesions by arterial spin labelling perfusion magnetic resonance imaging and to analyse their association with immune reconstitution inflammatory syndrome, and survival. A total of 22 patients with progressive multifocal leukoencephalopathy underwent a clinical evaluation and magnetic resonance imaging of the brain within 190 days of symptom onset. The presence of immune reconstitution inflammatory syndrome was determined based on clinical and laboratory criteria. Perfusion within progressive multifocal leukoencephalopathy lesions was determined by arterial spin labelling magnetic resonance imaging. We observed intense hyperperfusion within and at the edge of progressive multifocal leukoencephalopathy lesions in a subset of subjects. This hyperperfusion was quantified by measuring the fraction of lesion volume showing perfusion in excess of twice normal appearing grey matter. Hyperperfused lesion fraction was significantly greater in progressive multifocal leukoencephalopathy progressors than in survivors (12.8% versus 3.4% P = 0.02) corresponding to a relative risk of progression for individuals with a hyperperfused lesion fraction ≥ 4.0% of 9.1 (95% confidence interval of 1.4–59.5). The presence of hyperperfusion was inversely related to the occurrence of immune reconstitution inflammatory syndrome at the time of scan (P = 0.03). Indeed, within 3 months after symptom onset, hyperperfusion had a positive predictive value of 88% for absence of immune reconstitution inflammatory syndrome. Arterial spin labelling magnetic resonance imaging recognized regions of elevated perfusion within lesions of progressive multifocal leukoencephalopathy. These regions might represent virologically active areas operating in the absence of an effective adaptive immune response and correspond with a worse prognosis.
Keywords: progressive multifocal leukoencephalopathy, magnetic resonance imaging, perfusion imaging, neuroinflammation, neuroimmunology
Introduction
Progressive multifocal leukoencephalopathy (PML) is a devastating demyelinating disease of the CNS caused by JC virus. This polyomavirus infects ∼50% of the adult population without causing any disease (Berger et al., 2013). However, in the setting of immunosuppression, JC virus may reactivate, causing a lytic infection of oligodendrocytes and astrocytes, leading to the development of PML (Gheuens et al., 2013). PML was first described in a patient with chronic lymphocytic leukaemia (Astrom et al., 1958), and was initially reported mainly in patients with haematological malignancies. Since the 1980s the incidence of PML increased dramatically concurrent with the AIDS epidemic. More recently, the occurrence of PML in patients with auto-immune diseases treated with monoclonal antibodies has led to a renewal of interest for this disease (Kleinschmidt-DeMasters and Tyler, 2005; Langer-Gould et al., 2005).
In individuals with untreated HIV infection, PML survival is only 10% but increases to 43–75% with initiation of combined antiretroviral therapy (Berger et al., 1998; Falco et al., 2008; Gasnault et al., 2011). However, recovery of the immune system after combined antiretroviral therapy or by withdrawal of an immunosuppressive agent can be complicated by a clinical worsening known as immune reconstitution inflammatory syndrome (IRIS). The mechanism of PML-IRIS is incompletely understood; however, it is characterized histologically by inflammation and by lymphocytic infiltration of the brain (Vendrely et al., 2005). Of great concern is the importance of a prompt response to IRIS as the neurological damage incurred by the inflammatory response often is irreversible (Johnson and Nath, 2011). An incomplete understanding of the pathogeneisis of IRIS in addition to an absence of reliable indicators of PML severity and of IRIS complicates clinical management. Therefore, in the setting of HIV, early initiation of combined antiretroviral therapy before the patient experiences immunosuppression has been proposed as a strategy in reducing the risk of developing IRIS and its associated morbidity (Martin-Blondel et al., 2011). Advanced neuroimaging has the potential to improve disease characterization. A dark lesion core surrounded by elevated signal on diffusion weighted MRI has been reported in acute PML, but it has not been associated with progression of disease or IRIS (Mader et al., 2003; Bergui et al., 2004; Cosottini et al., 2008; Yousry et al., 2012). Magnetic resonance spectroscopy results suggest that the ratio of certain metabolites is associated with IRIS in PML. In particular, the ratio of myo-inositol to creatine was found to be higher in PML survivors than in progressors and elevated ratios of these and other metabolites were associated with IRIS (Katz-Brull et al., 2004; Gheuens et al., 2012). As inflammation is often associated with elevated metabolism and perfusion, we hypothesized that perfusion MRI might provide useful measures of PML and immune response with potentially higher spatial resolution and sensitivity than magnetic resonance spectroscopy.
Arterial spin-labelling (ASL) is a newer magnetic resonance perfusion method that has recently become available on clinical imaging platforms (Williams et al., 1992). ASL uses magnetic fields to alter the magnetization of water in the inflowing arterial blood and then measures the effect of the inflow of altered magnetization on the tissue signal. Because the ‘tracer’ used is the endogenous blood water, ASL does not require any injections or other contrast agent. The use of freely diffusible water as a tracer has other benefits including simpler absolute quantification of cerebral blood flow and better detection of high flow regions than in methods with an intravascular tracer such as perfusion with CT or MRI contrast agents (Wang et al., 2012).
ASL MRI has been applied to research and diagnosis in numerous cerebral pathologies (Alsop et al., 2000; Du et al., 2006; Duhameau et al., 2010; Ozsunar et al., 2010; Scheef et al., 2010; Detre et al., 2012). Decreased caudate perfusion was associated with increasing HIV-associated neurocognitive impairment (Ances et al., 2006). ASL has also shown areas of increased perfusion in subjects with untreated relapsing-remitting multiple sclerosis. This finding was attributed to the presence of higher metabolic and cell activity (Rashid et al., 2004).
We evaluated ASL as a tool to characterize brain perfusion in PML and PML-IRIS and gain insight into PML pathogenesis.
Materials and methods
Standard protocol approvals, registration and patient consent
This observational study was registered at ClinicalTrials.gov (NCT01132053). Patients gave written consent to participate according to the institution’s guidelines and were enrolled and followed in an outpatient neurology clinic between 2008 and 2012.
Study subjects and design
PML diagnosis was established according to consensus criteria (Berger et al., 2013). Of a total of 22 consecutively enrolled patients, four (18.2%) patients had histology-confirmed PML, 14 (63.6%) patients had laboratory-confirmed PML with positive JC virus DNA PCR in CSF, and four (18.2%) patients had possible PML based on clinical and radiological findings, and ruling out other opportunistic infections and brain tumours (Table 1). All patients studied had their initial MRI within 190 days from PML symptoms onset (22 MRIs). Patients with PML were divided retrospectively into progressors or survivors depending on survival shorter or longer than 1 year from onset of neurological symptoms (Marzocchetti et al., 2009). There were 11 patients each in both the survivor and the progressor groups. In the PML survivors, seven (63.6%) patients were HIV-positive, whereas three (27.3%) patients in the PML progressors were HIV-positive. The median CD4 count in HIV-positive individuals in the PML survivors group was 232 compared with 148 in the PML progressors group.
Table 1.
Study subjects characteristics
| PML survivors | PML progressors | |
|---|---|---|
| Total number of patients | 11 | 11 |
| Number of patients with IRIS | 8 | 1 |
| Number of days between PML onset and initial MRI range (median) | 50–190 (107) | 23–180 (91) |
| Age, range (median) | 20–75 (44) | 40–69 (63) |
| Gender (M/F) | 10/1 | 7/4 |
| Ethnicity (Caucasian/African American/other) | 8/2/1 | 10/1/0 |
| Total number of HIV-positive patients | 7 | 3 |
| Total number of HIV-negative patients | 4 | 8 |
| Malignancy, haematologica | 1 | 4 |
| Haematologic disease, otherb | 1 | 3 |
| Autoimmune diseasec | 2 | 1 |
| HIV-positive CD4+ at time of first MRI, counts/µl, range (median) | 33–833 (232); n = 6 | 24–251 (148); n = 3 |
| HIV-negative CD4+ at time of first MRI, counts/ µl, range (median) | 112–669 (544); n = 4 | 61–1072 (324); n = 7 |
| Karnofsky score at time of first MRI, range (median) | 30–90 (70); n = 11 | 30–90 (35); n = 10 |
| Modified Rankin scale score at time of initial MRI, range (median) | 1–5 (3); n = 11 | 1–4 (4); n = 10 |
| PML diagnosed by: | ||
| Biopsy | 2 | 2 |
| CSF (PCR) | 6 | 8 |
| Clinical criteria + MRI | 3 | 1 |
| Diagnosis of hypertension | 2 | 4 |
aChronic lymphocytic leukemia (2), non-Hodgkin lymphoma, NK cell leukemia, cutaneous B cell lymphoma.
bIdiopathic lymphocytopenia, lymphomatosis granulomatosis, Waldenstrom macroglobulinemia (2).
cMultiple sclerosis treated with natalizumab, lupus erythematosus, dermatomyositis.
To determine the evolution of perfusion in PML lesions over time, 12/22 patients had multiple MRIs (two to four). An additional five PML survivors had their first MRI between 223–597 days from PML symptoms onset. In total, 54 MRIs of 27 patients with PML were included in a separate analysis regarding the evolution of perfusion.
The presence of IRIS was determined on the basis of laboratory evaluation, including: (i) evidence of immune reconstitution demonstrated by an increase of CD4+ T cell counts and a decrease in HIV plasma RNA after starting on combined antiretroviral therapy in HIV-positive patients or after discontinuation of immunosuppressive medications in HIV-negative individuals; (ii) sudden worsening of neurological signs and symptoms occurring in the context of improved immunological and virological response; and (iii) absence of other pathogens, inflammatory processes, or tumours. These criteria were further supported by the presence of swelling, mass effect, or contrast enhancement on MRI in some cases.
Patients had a full neurological examination by two neurologists (S.G., I.J.K.). CD4+ T cell counts, comorbidities, and Karnofsky score, and modified Rankin Scale were recorded. We performed brain imaging at the time of enrollment and after 3, 6 and 12 months when possible.
Brain magnetic resonance imaging
All brain MRIs were performed on a single 3-T scanner (GE HDxt, General Electric Healthcare) and included anatomical imaging, ASL perfusion, and diffusion imaging. All imaging was performed with a vendor supplied eight channel receive head coil and body coil transmission. T1-weighted imaging was performed with a Modified Driven-Equilibrium Fourier Transform (MDEFT) magnetization prepared 3D sequence that provides excellent grey–white matter contrast (Deichmann et al., 2004). This sequence used multiple presaturation pulses followed by a delay and then an adiabatic inversion pulse at 500 ms before image acquisition. A 64-slice encode centric ordered spoiled gradient echo with 15° flip angle was used for acquisition. A 24-cm field of view, 3-mm slice thickness, and a 256 × 256 matrix were selected. Fluid-attenuated inversion recovery (FLAIR) was performed with the 2D product sequence, with 3-mm slices, 24-cm field of view, and a 256 × 256 matrix. Diffusion imaging was performed with a field of view of 24 cm, a 128 × 128 matrix, 5 mm slices, b = 1000 s/mm2, echo time/repetition time 129 ms/10 s, six directions and one b = 0 image. After all other images were acquired, patients received 0.1 mM/kg of intravenous MRI contrast agent (Magnevist® Bayer Healthcare). A post-contrast sequence identical to the 3D precontrast T1 was run 5 min after administration and as the last sequence of the examination.
ASL was performed before contrast injection using a prototype application similar to a later released vendor product application (3DASL, GE Healthcare). ASL was achieved using pseudocontinuous labelling for 1.5 s (Dai et al., 2008), with a post-labelling delay of 1.5 s (Alsop and Detre, 1996). Optimally timed inversion pulses were used to suppress background signal to minimize noise and artefacts from motion or other instabilities (Ye et al., 2000; Maleki et al., 2012). Images were acquired with a 3D stack of interleaved spirals fast spin echo sequence. Slice encodes were centrically ordered and each of the eight spiral interleaves were acquired in separate repetitions with a repetition time of 6 s. Estimated in-plane resolution was 3.5 mm and 44 slices of 4 mm thickness were selected. A reference proton density weighted image was also acquired to enable quantification. Perfusion was automatically quantified on the scanner using the equations and assumptions previously described (Pfefferbaum et al., 2010; Dai et al., 2012).
Image analysis
Imaging data were transferred to off-line workstations for postprocessing. Images were transformed into a standardized space with custom software using manually selected landmarks on the 3D T1 images and affine transformation. Regions containing all lesions on the FLAIR images were defined by a semi-automatic region growing algorithm within MRIcron (Chris Rorden, http://www.mricro.com). In all cases the PML lesions on FLAIR were larger than and contained the entirety of lesions defined by any other sequence, so the FLAIR lesion was used to define the total lesion volume.
Segmentation of the images was performed within MIPAV (http://mipav.cit.nih.gov) using both the FLAIR and 3D T1 images. Custom software within MATLAB (Mathworks) was used to calculate the mean blood flow in normal-appearing grey matter outside any FLAIR lesion, as well as the fraction of the voxels within the lesion volume with blood flow greater than twice normal grey matter blood flow. Grey matter was selected as a reference because reliable measurement of the very low perfusion in normal white matter with ASL can be challenging (van Gelderen et al., 2008; van Osch et al., 2009). This hyperperfused lesion fraction (HLF) was motivated by the observation of high signal within the PML lesions for a number of the patients, and represents the percentage of the lesion volume harbouring hyperperfusion.
The presence of contrast enhancement and of abnormally high intensity on calculated isotropic diffusion weighted images and low intensity on apparent diffusion coefficient maps was determined by visual examination.
Cellular immune response against JC virus
The T cell response against JC virus in the blood was measured by intracellular cytokine staining or ELISpot as previously described (Gheuens et al., 2011).
Statistical analysis
Because of the small sample size and the non-normal distribution of the data, we used the non-parametric Wilcoxon Rank Sum test to compare the number of days from PML onset to MRI, CD4+ T cell count, Karnofsky score and modified Rankin scale, and age between PML survivors and PML progressors. Fisher’s exact test was used to compare the proportions of ethnicity, gender, HIV status, and presence of hypertension and hyperlipidaemia between the two groups. We analysed the HLF in PML lesions on the initial MRI from 22 subjects also using the Wilcoxon Rank Sum test. To find the optimal cut-off point for the HLF threshold to discriminate between PML survivors and progressors, we divided the distribution of the HLF into multiple cut-off points and at each point we dichotomized the data and computed the sensitivity and specificity. After calculating the area under the operating characteristic curve for each point, the optimal cut-off for HLF was identified at 4.0%. Using this value, the relative risk, sensitivity, specificity, positive predictive value, negative predictive value and also their respective 95% confidence intervals (CI) were calculated.
Results
Study subject characterization
Of the 22 patients, 11 (50%) were PML survivors and 11 (50%) PML progressors (Table 1). PML progressor patients tended to be older than PML survivors (P = 0.07). There was no significant difference between the groups in gender (P = 0.31) and ethnicity (P = 0.59). HIV-positive patients accounted for 63.6% of PML survivors and 27.3% of PML progressors (P = 0.20). The CD4+T cell count at the first MRI was not significantly different between the HIV-positive PML survivor and HIV-positive PML progressor groups (P = 0.38) or the HIV-negative PML survivor and HIV-negative PML progressor groups (P = 0.65). IRIS was more common in the PML survivor group (72.7%) than the PML progressors (9.1%) (P = 0.008). Five patients with PML-IRIS received steroids before the first MRI (three within 1 month of the MRI with one of those patients receiving steroids at the time of the MRI).
The Karnofsky score and modified Rankin scale at the time of the first MRI tended to be better in PML survivors than PML progressors (P = 0.05 and P = 0.07, respectively). The difference in the median number of days between PML onset and MRI between the two groups did not reach significance (P = 0.19). The prevalence of hypertension between the PML survivor (18.2%) and PML progressor (36.4%) groups was not statistically significant (P = 0.64).
We compared the CD4+T cell count in the IRIS and non-IRIS groups. In all patients, the median CD4+ T cell count was 446.4/µl in the IRIS group (seven patients) and 321.5/µl in the non-IRIS group (13 patients) (P = 0.30). In those who were HIV-positive, the median CD4+ T cell count was 385.6/µl in the IRIS group (five patients) and 114/µl in the non-IRIS group (four patients) (P = 0.11).
Hyperperfusion is frequent in progressive multifocal leukoencephalopathy lesions
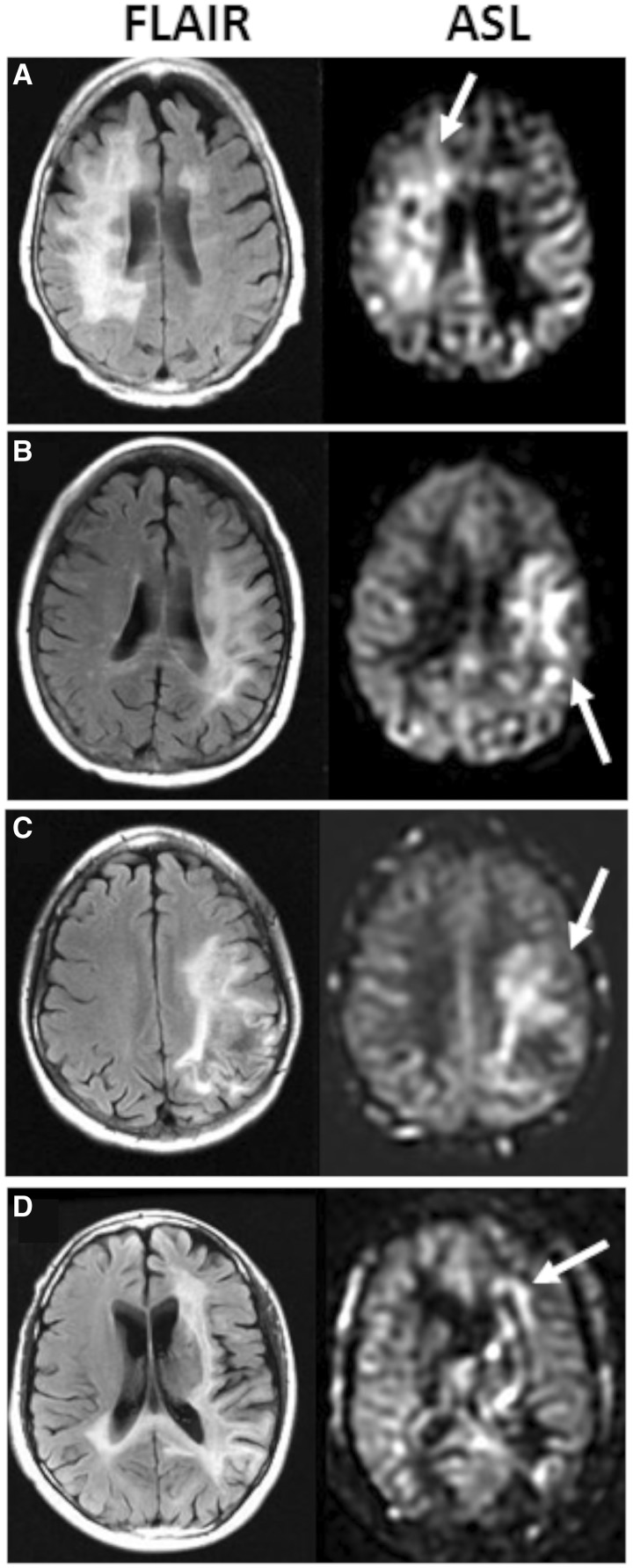
We observed visibly elevated perfusion within PML lesions of 10 patients. This perfusion exceeded not only the perfusion of nearby white matter but also that of more highly perfused grey matter. Examples of hyperperfusion and corresponding FLAIR lesions for six subjects are shown in Fig. 1. Of the 10 patients with visibly high perfusion in PML lesions on ASL, seven (70%) demonstrated perfusion primarily along the edge of the lesion.
Figure 1.
PML lesions contain areas of elevated perfusion. Four representative cases of PML lesions on FLAIR with corresponding ASL perfusion sequences are shown. Areas of increased perfusion are marked by an arrow. (A) Right frontal lesion in a PML progressor. HLF of the PML lesion was 10.9%. (B) Left fronto-parietal lesion in a PML progressor with an HLF of 26.0%. (C) Left fronto-parietal lesion in a PML survivor with an HLF of 34.6% (D) Left fronto-parietal and splenium of corpus callosum lesion in a PML progressor with an HLF of 11.3%. Perfusion was more commonly elevated along the edge rather than the core of the PML lesions (B, C and D).
Lesions of progressive multifocal leukoencephalopathy progressors have larger areas of hyperperfusion than those of survivors
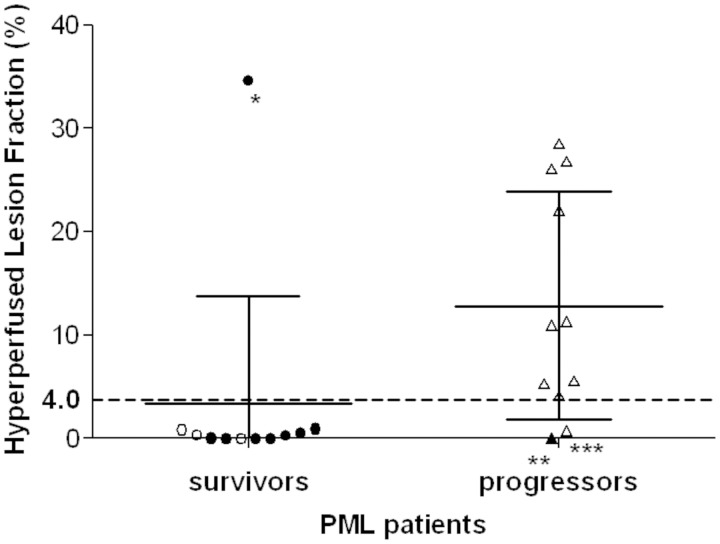
To quantify the high perfusion within PML lesions, we calculated the hyperperfusion lesion fraction. This quantity reflects the fraction—or percentage—of the lesion volume defined on FLAIR with perfusion greater than twice normal-appearing grey matter. Contrary to our hypothesis, the mean HLF was lower, 3.43%, in PML survivors compared with PML progressors, 12.83% (P = 0.02). Indeed only one PML survivor demonstrated HLF >4.0% (Fig. 2). Of note, all PML lesions with HLF ≥4.0% displayed hyperintense signal on ASL sequences that could readily be identified visually. Conversely, PML lesions with HLF <4.0% were not visualized.
Figure 2.
Perfusion is increased in brain lesions of PML progressors. Each circle (PML survivors) or triangle (PML progressors) represents the HLF in PML lesions of a given patient at the first MRI. A filled symbol represents patients with active IRIS. The dotted line indicates the 4.0 cut-off separating survivors and progressors. The bars represent the standard deviations and the means.
*the only PML survivor with an HLF >4.0. This 37-year-old HIV-infected male had a CD4+ T cell count of 47/µl at the time of the first MRI and had been on combined anti-retroviral therapy for ∼3 months. The CD4+ T cell count increased to 161/µl when he had his second MRI months later.
**the only PML progressor with IRIS.
***the only PML progressor with contrast enhancement.
The relative risk of PML progression with HLF ≥4.0% was 9.1 (95% CI of 1.4–59.5). Using 4.0% as the cut-off point to indicate the PML progressor status, the sensitivity of this test was 81.8% (95% CI: 0.59–1.00) whereas the specificity was 90.9 % (95% CI: 0.74–1.00). The positive predictive value of the test in determining PML progressor status at this cut-off point (likelihood that a patient with HLF ≥4% is a PML progressor), was 90% (95% CI: 0.71–1.00) and the negative predictive value of the test (likelihood that a patient with HLF <4% is a PML survivor), was 83% (95% CI: 0.62–1.00).
Lesions of PML-IRIS patients had smaller areas of hyperperfusion than those without IRIS
The mean HLF was 4.05% in PML lesions of patients with IRIS at the time of the MRI (n = nine patients), compared with 10.95% (P = 0.03) in those without IRIS (n = 13 patients). The only PML progressor with IRIS (Fig. 2) was also one of only two progressors with an HLF <4.0%. In our data, which includes results up to 190 days (∼6 months) post-disease onset, the positive predictive value (likelihood that a patient with an HLF ≥4.0% does not have IRIS) was 90% (95% CI: 0.56–0.99), whereas the negative predictive value (likelihood that a patient with an HLF <4.0% has IRIS) was 67% (95% CI, 0.35–0.90). The sensitivity and specificity of this test is 69% (95% CI: 0.39–0.91) and 90% (95% CI: 0.52–0.99), respectively. However, if only patients with imaging obtained in a more acute setting of 94 days (∼3 months) were analysed, the positive predictive value was 88% (95% CI: 0.47–0.99), the negative predictive value was 80% (95% CI: 0.28–0.99), sensitivity was 88% (95% CI: 0.47–0.99), and specificity of 80% (95% CI: 0.28 to 0.99). Of 22 patients, nine (40.9%) had IRIS including 8/11 PML survivors and 1/11 PML progressors. This difference was statistically significant (P = 0.008).
Hyperperfusion was not associated with the presence of contrast enhancement
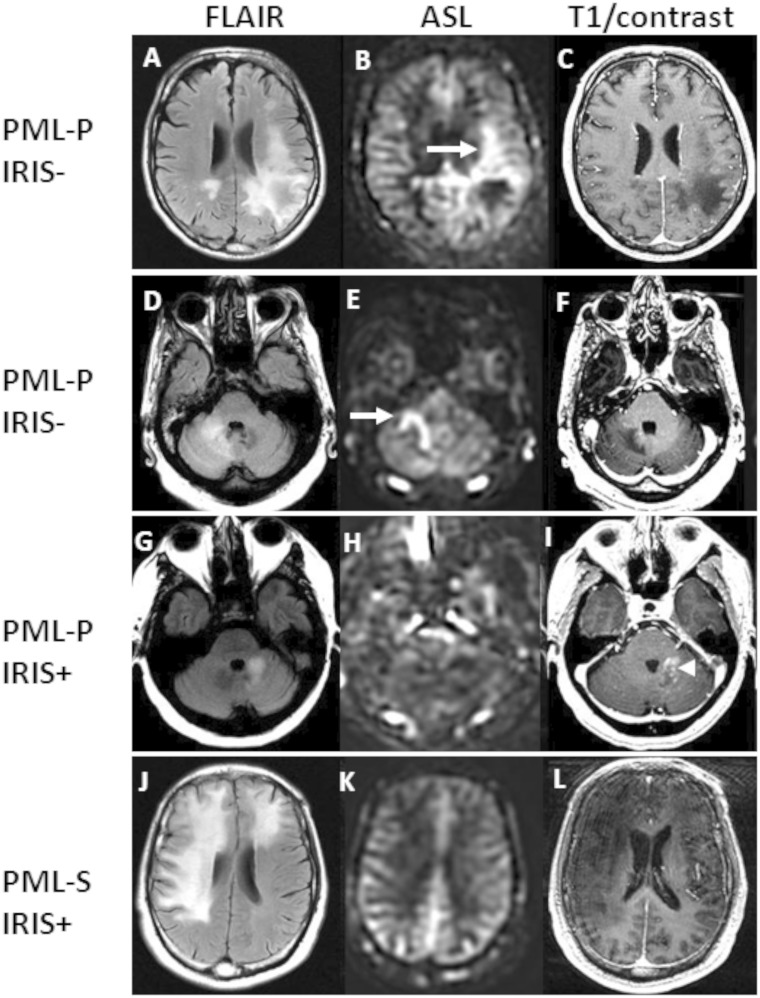
Among the 21 patients who received intravenous contrast, the mean HLF was 0.19% in contrast-enhancing lesions (n = 4) and 10.23% in non-enhancing lesions (n = 17) (P = 0.06). The only PML progressors (Fig. 2) with contrast-enhancing lesions did not have an HLF >4.0%. Hence HLF reflects elevated perfusion without substantial damage to the blood–brain barrier. Representative examples of discrepancies between hyperperfusion and enhancement in PML lesions are shown in Fig. 3.
Figure 3.
Discrepancies between perfusion in PML lesions, contrast enhancement and IRIS. FLAIR, ASL sequences and post-gadolinium T1-weighted images of representative cases are shown. (A) Left parietal lesion of a PML progressor (PML-P) without IRIS shows increased perfusion (B, arrow) and no enhancement (C). The HLF was 26.7%; (D) right cerebellar lesion from a PML progressor without IRIS has increased perfusion (E, arrow) and no enhancement (F). The HLF of the PML lesion was 22.0%; (G) left cerebellar lesion from a PML progressor with IRIS has normal perfusion (H) and does enhance after contrast administration (I, arrowhead). The HLF of the PML lesion was 0%; (J) bilateral frontal lesions of a PML survivor (PML-S) with IRIS have normal perfusion (K) and no enhancement (L). The HLF of the PML lesion was 0.02%. Elevated perfusion along the edge of the PML lesions can be seen in B and E.
Hyperperfusion of progressive multifocal leukoencephalopathy lesions tended to be associated with low immune response
The presence of a JC virus-specific T cell response was determined by ELISpot or intracellular cytokine staining in 21 patients. In those with detectable immune response (n = 17), the mean HLF was 5.32% compared with 13.45% (P = 0.21) in those with undetectable immune response (n = 4). Of nine HIV-positive patients with laboratory data available within 2 months from the time of MRI, the mean HLF in those with a CD4+ T cell count >200/µl was 1.03% (n = 4) compared with 14.06% in those with a CD4+ T cell count <200/µl (n = 5) (P = 0.10).
Longitudinal studies of perfusion in progressive multifocal leukoencephalopathy
Of 22 patients, 12 had two to four MRIs, including 11 PML survivors and one PML progressor. Moreover, five additional PML survivors had one to four MRI scans 223–597 days from symptom onset, for a combined total of 54 MRIs (42 PML survivors, 12 PML progressors). There were no images obtained beyond 190 days after PML onset that demonstrated areas of elevated perfusion. In the sole PML survivor demonstrating hyperperfusion at Day 94 of the initial study (Fig. 2), the perfusion had normalized at Day 165 and remained normal at Day 452. Interestingly, this patient showed an increase in CD4+ T cell count subsequent to antiviral therapy between the first two scans (from 47/µl to 161/µl). In the one PML progressor who had two studies, the perfusion remained elevated on Day 59 and also on Day 164 after symptom onset.
Lesion volume or location was not associated with progressive multifocal leukoencephalopathy progression
To determine whether PML progressors had larger lesions than PML survivors, total FLAIR lesion volume was tabulated. There was no significant difference between the two groups as the mean lesion volume of the PML survivors (n = 11) was 162.3 cm3 compared with 109.2 cm3 in PML progressors (n = 11) (P = 0.40). In addition, FLAIR sequences were reviewed for the presence of infratentorial lesions. There was no significant difference between the two groups as two PML survivor patients and five PML progressor patients had infratentorial lesions (P = 0.36).
Hyperintensity on diffusion weighted imaging was not associated with hyperperfusion
Numerous lesions demonstrated hyperintensity on diffusion weighted imaging, but no lesions showed measurable decrease in apparent diffusion coefficient. The bright signal on diffusion weighted imaging thus reflected elevated T2 with normal diffusion coefficient. Bright diffusion weighted imaging was not significantly associated with hyperperfusion or progression (P = 0.76 and P = 0.61, respectively).
Discussion
Contrary to our initial hypothesis, areas of hyperperfusion within PML lesions were associated with disease progression and were inversely related to the presence of IRIS. We observed a trend between hyperperfusion and low immune responses. These findings suggest that hyperperfusion reflects viral activity rather than immune response or inflammation. Consistent with this conclusion, our results also demonstrate that areas of increased perfusion in PML lesions predict a relative risk of disease progression of 9.1.
Our results suggest that ASL may be useful in the clinical management of patients with PML. Clinicians managing patients with PML encounter a diagnostic dilemma when the patient’s clinical status deteriorates shortly after withdrawal of the offending immunosuppressants in HIV-negative patients, or after initiation of combined antiretroviral therapy in HIV-positive individuals, concomitant to immune reconstitution. Standard MRI with injection of contrast material cannot reliably differentiate progression of PML from PML-IRIS (Johnson and Nath, 2011). Recently, we demonstrated that in the presence of contrast enhancement, and elevated Lip1/Cr ratio in PML lesions measured by proton magnetic resonance spectroscopy yielded a 79% probability of IRIS (Gheuens et al., 2012). As this study suggests, ASL—which does not require injection of contrast— has the potential to serve as a non-invasive and simpler alternative to differentiate clinical worsening due to the classic evolution of PML from that due to PML-IRIS. Indeed, HLF of 4% or greater within PML lesions are clearly visualized as hyperintense on ASL sequences, whereas values below this threshold are not.
The intensity of the hyperperfusion in affected white matter and the use of the relatively new ASL perfusion technique naturally raises questions of systematic effects in the measurement. ASL can show elevated signal in larger arteries when flow is very slow (Chalela et al., 2000), but the high flow nearby and the spatial distribution of the hyperperfusion argue against this explanation. Perfusion can appear artificially high if long labelling durations are used and T1 of the lesions is much higher than normal (Tanaka et al., 2011). Though we cannot exclude the possibility of slight overestimation of perfusion from these causes, the modest labelling duration of 1.5 s and the intermediate T1 observed at the edge of the PML lesions do not support a major contribution to the observed hyperperfusion.
The relationship found between increased perfusion and absence of IRIS and enhancement is a surprising finding. This raises the possibility that the source of the increased perfusion is induced by (i) the virus; (ii) the innate immune system; or (iii) an interplay between the two. One candidate mechanism for the hyperperfusion is nitric oxide, a potent vasodilator capable of greatly increasing blood flow (Abrams, 1984). Various investigations have demonstrated that neurotropic viruses, including influenza, bornavirus, herpes simplex-1, rabies, and coxsackievirus can induce the immune system to produce nitric oxide synthase (NOS) (Hooper et al., 1995; Akaike and Maeda, 2000). The human paramyxovirus respiratory syncytial virus is also capable of directly upregulating inducible nitric oxide synthase (iNOS) in human type two alveolar epithelial cells independent of pro-inflammatory cytokines (Tsutsumi et al., 1999). Additionally, reactive astrocytes and neurons have the capability of activating nitric oxide synthase (Faraci and Breese, 1993; Sofroniew, 2005). At low levels of nitric oxide, the host Th1-immune response (and CD8+ T cell activation) is enhanced; however, at high levels, nitric oxide will suppress Th1 cell differentiation and proliferation and can also induce apoptosis of T cells (Tarrant et al., 1999; Aiello et al., 2000; van der Veen, 2001; Niedbala et al., 2006). The Th1 immune response is classically considered to be the primary immune response against viruses and also the driving force behind IRIS (Sun and Singh, 2009; Johnson and Nath, 2011). We are not aware of any studies of JC virus and nitric oxide production. This complex interplay between nitric oxide and the immune system might explain our finding of increased perfusion in the absence of IRIS. Furthermore, the location of increased perfusion along the periphery of the lesions supports the role of the innate immune system in response to advancing JC virus infection. Indeed, the periphery of PML lesions contain the greatest viral activity and active demyelination (Horger et al., 2012). If nitric oxide production involvement in the progression of PML is confirmed, then nitric oxide synthase inhibition may become a potential therapeutic target (Salerno et al., 2002; Charriaut-Marlangue et al., 2013).
We examined other factors that could affect cerebral perfusion and influence the outcome of the study. van Laar et al. (2008) studied various conditions (including diabetes, hypertension, and tobacco use) that could affect cerebral blood flow as assessed by ASL and found that only hypertension was associated with increased regional cerebral blood flow. In our study, hypertension was not associated with increased HLF. Decreased resting cerebral blood flow has previously been demonstrated in individuals with HIV (Ances et al., 2009). However, although the PML survivor group in our study had a larger proportion of HIV-positive individuals than the PML progressor group this difference was not statistically significant. Additionally, the dramatic differences in perfusion as seen in our study cannot be explained by the reduction in blood flow of HIV-positive individuals alone.
Our study does have several limitations. First, in our patient population, the PML survivor group overlapped with those with PML-IRIS. It might appear intuitive that patients with immune reconstitution—and thus the ability to mount a response to the JC virus—would be more likely to survive PML. However, the use of the presence of PML-IRIS as a prognostic indicator for survival remains an area of controversy (Harrison et al., 2011). Nonetheless, the increased survival seen in those without an increase in perfusion might be related to their ability to mount an immune response as evidenced by IRIS.
Second, the functional status recorded at initial MRI was worse in the PML progressor group than in the PML survivor group (median Karnofsky scores of 35 and 70, respectively). The differences in initial disability scores in the PML, survivor and PML progressor populations could contribute to the differences in survivorship. Because of the small sample size (which is largely a function of the incidence of PML), we were unable to perform a multivariable analysis to control for potential confounders such as functional status, as well as age, gender, CD4+ count, PML survivor/progressor, or presence or absence of IRIS.
Third, of the nine patients with IRIS in our study, five had received corticosteroids before the MRI. Steroid use in brain tumours can cause a decrease in cerebral blood flow (Leenders et al., 1985). However, these changes were minimal compared with the large difference in perfusion observed in our study.
This study is the first to show that ASL can demonstrate high perfusion patterns in PML lesions. Areas of elevated perfusion might indicate virologically active PML, which portends a poorer prognosis compared with a virologically inactive PML devoid of elevated perfusion. Additionally, and likely related to the lack of an appropriate immune response and hence an undeterred virologic progression, ASL has the potential to be a useful tool in distinguishing the natural evolution of PML from PML-IRIS as the cause of clinical worsening. In the study previously mentioned, we used proton magnetic resonance spectroscopy (1H-MRS) to explore the metabolic profile within PML lesions with and withour IRIS (Gheuens et al., 2012). We plan to validate these data in another cohort of patients with PML, combining ASL and 1H-MRS with the purpose of evaluating MRS metabolic parameters in areas of increased perfusion. A benefit of ASL over other techniques is that results are readily visualized on MRI images, it is not invasive, does not require intravenous injection of contrast, and is also a relatively short MRI sequence. The information obtained by ASL MRI technique can provide valuable insight into the course of PML and will guide physicians in the management of their patients.
Funding
This work was supported in part by National Institutes of Health [grant number T32 AI052074] to M.N.K.; [grants R01 NS 074995, NS047029 and K24 NS 060950] to I.J.K., X.W. and D.C.A; [grant T32-AI07387-21] to S.G. S.G. was a fellow of the Clinical Investigator Training Program: Beth Israel Deaconess Medical Center–Harvard/MIT Health Sciences and Technology, in collaboration with Pfizer Inc. and Merck & Co. D.C.A. is an inventor on patents related to the arterial spin labeling techniques used in this study and receives licensing royalties from GE Healthcare through his current and former academic institutions.
Glossary
Abbreviations
- ASL
arterial spin-labelling; HLF = hyperperfused lesion fraction; IRIS = immune reconstitution inflammatory syndrome; PML = progressive multifocal leukoencephalopathy
References
- Abrams J. Nitrate delivery systems in perspective. A decade of progress. Am J Med. 1984;76:38–46. doi: 10.1016/0002-9343(84)91041-6. [DOI] [PubMed] [Google Scholar]
- Aiello S, Noris M, Piccinini G, Tomasoni S, Casiraghi F, Bonazzola S, et al. Thymic dendritic cells express inducible nitric oxide synthase and generate nitric oxide in response to self- and alloantigens. J Immunol. 2000;164:4649–58. doi: 10.4049/jimmunol.164.9.4649. [DOI] [PubMed] [Google Scholar]
- Akaike T, Maeda H. Nitric oxide and virus infection. Immunology. 2000;101:300–8. doi: 10.1046/j.1365-2567.2000.00142.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alsop DC, Detre JA. Reduced transit-time sensitivity in noninvasive magnetic resonance imaging of human cerebral blood flow. J Cereb Blood Flow Metab. 1996;16:1236–49. doi: 10.1097/00004647-199611000-00019. [DOI] [PubMed] [Google Scholar]
- Alsop DC, Detre JA, Grossman M. Assessment of cerebral blood flow in Alzheimer's disease by spin-labeled magnetic resonance imaging. Ann Neurol. 2000;47:93–100. [PubMed] [Google Scholar]
- Ances BM, Roc AC, Wang J, Korczykowski M, Okawa J, Stern J, et al. Caudate blood flow and volume are reduced in HIV+ neurocognitively impaired patients. Neurology. 2006;66:862–6. doi: 10.1212/01.wnl.0000203524.57993.e2. [DOI] [PubMed] [Google Scholar]
- Ances BM, Sisti D, Vaida F, Liang CL, Leontiev O, Perthen JE, et al. Resting cerebral blood flow: a potential biomarker of the effects of HIV in the brain. Neurology. 2009;73:702–8. doi: 10.1212/WNL.0b013e3181b59a97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Astrom KE, Mancall EL, Richardson EP., Jr Progressive multifocal leuko-encephalopathy; a hitherto unrecognized complication of chronic lymphatic leukaemia and Hodgkin's disease. Brain. 1958;81:93–111. doi: 10.1093/brain/81.1.93. [DOI] [PubMed] [Google Scholar]
- Berger JR, Pall L, Lanska D, Whiteman M. Progressive multifocal leukoencephalopathy in patients with HIV infection. J Neurovirol. 1998;4:59–68. doi: 10.3109/13550289809113482. [DOI] [PubMed] [Google Scholar]
- Berger JR, Aksamit AJ, Clifford DB, Davis L, Koralnik IJ, Sejvar JJ, et al. PML diagnostic criteria: consensus statement from the AAN Neuroinfectious Disease Section. Neurology. 2013;80:1430–8. doi: 10.1212/WNL.0b013e31828c2fa1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bergui M, Bradac GB, Oguz KK, Boghi A, Geda C, Gatti G, et al. Progressive multifocal leukoencephalopathy: diffusion-weighted imaging and pathological correlations. Neuroradiology. 2004;46:22–5. doi: 10.1007/s00234-003-1115-9. [DOI] [PubMed] [Google Scholar]
- Chalela JA, Alsop DC, Gonzalez-Atavales JB, Maldjian JA, Kasner SE, Detre JA. Magnetic resonance perfusion imaging in acute ischemic stroke using continuous arterial spin labeling. Stroke. 2000;31:680–7. doi: 10.1161/01.str.31.3.680. [DOI] [PubMed] [Google Scholar]
- Charriaut-Marlangue C, Bonnin P, Pham H, Loron G, Leger PL, Gressens P, et al. Nitric oxide signaling in the brain: a new target for inhaled nitric oxide? Ann Neurol. 2013 doi: 10.1002/ana.23842. [Epub ahead of print] [DOI] [PubMed] [Google Scholar]
- Cosottini M, Tavarelli C, Del Bono L, Doria G, Giannelli M, De Cori S, et al. Diffusion-weighted imaging in patients with progressive multifocal leukoencephalopathy. Eur Radiol. 2008;18:1024–30. doi: 10.1007/s00330-007-0845-1. [DOI] [PubMed] [Google Scholar]
- Dai W, Garcia D, de Bazelaire C, Alsop DC. Continuous flow-driven inversion for arterial spin labeling using pulsed radio frequency and gradient fields. Magn Reson Med. 2008;60:1488–97. doi: 10.1002/mrm.21790. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dai W, Robson PM, Shankaranarayanan A, Alsop DC. Reduced resolution transit delay prescan for quantitative continuous arterial spin labeling perfusion imaging. Magn Reson Med. 2012;67(5):1252–65. doi: 10.1002/mrm.23103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deichmann R, Schwarzbauer C, Turner R. Optimisation of the 3D MDEFT sequence for anatomical brain imaging: technical implications at 1.5 and 3 T. Neuroimage. 2004;21:757–67. doi: 10.1016/j.neuroimage.2003.09.062. [DOI] [PubMed] [Google Scholar]
- Detre JA, Rao H, Wang DJ, Chen YF, Wang Z. Applications of arterial spin labeled MRI in the brain. J Magn Reson Imaging. 2012;35:1026–37. doi: 10.1002/jmri.23581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Du AT, Jahng GH, Hayasaka S, Kramer JH, Rosen HJ, Gorno-Tempini ML, et al. Hypoperfusion in frontotemporal dementia and Alzheimer disease by arterial spin labeling MRI. Neurology. 2006;67:1215–20. doi: 10.1212/01.wnl.0000238163.71349.78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duhameau B, Ferre JC, Jannin P, Gauvrit JY, Verin M, Millet B, et al. Chronic and treatment-resistant depression: a study using arterial spin labeling perfusion MRI at 3Tesla. Psychiatry Res. 2010;182:111–6. doi: 10.1016/j.pscychresns.2010.01.009. [DOI] [PubMed] [Google Scholar]
- Falco V, Olmo M, del Saz SV, Guelar A, Santos JR, Gutierrez M, et al. Influence of HAART on the clinical course of HIV-1-infected patients with progressive multifocal leukoencephalopathy: results of an observational multicenter study. J Acquir Immune Defic Syndr. 2008;49:26–31. doi: 10.1097/QAI.0b013e31817bec64. [DOI] [PubMed] [Google Scholar]
- Faraci FM, Breese KR. Nitric oxide mediates vasodilatation in response to activation of N-methyl-D-aspartate receptors in brain. Circ Res. 1993;72:476–80. doi: 10.1161/01.res.72.2.476. [DOI] [PubMed] [Google Scholar]
- Gasnault J, Costagliola D, Hendel-Chavez H, Dulioust A, Pakianather S, Mazet AA, et al. Improved survival of HIV-1-infected patients with progressive multifocal leukoencephalopathy receiving early 5-drug combination antiretroviral therapy. PLoS One. 2011;6:e20967. doi: 10.1371/journal.pone.0020967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gheuens S, Wuthrich C, Koralnik IJ. Progressive multifocal leukoencephalopathy: why gray and white matter. Annu Rev Pathol. 2013;8:189–215. doi: 10.1146/annurev-pathol-020712-164018. [DOI] [PubMed] [Google Scholar]
- Gheuens S, Ngo L, Wang X, Alsop DC, Lenkinski RE, Koralnik IJ. Metabolic profile of PML lesions in patients with and without IRIS: an observational study. Neurology. 2012;79:1041–8. doi: 10.1212/WNL.0b013e318268465b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gheuens S, Bord E, Kesari S, Simpson DM, Gandhi RT, Clifford DB, et al. Role of CD4+ and CD8+ T-cell responses against JC virus in the outcome of patients with progressive multifocal leukoencephalopathy (PML) and PML with immune reconstitution inflammatory syndrome. J Virol. 2011;85:7256–63. doi: 10.1128/JVI.02506-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harrison DM, Newsome SD, Skolasky RL, McArthur JC, Nath A. Immune reconstitution is not a prognostic factor in progressive multifocal leukoencephalopathy. J Neuroimmunol. 2011;238:81–6. doi: 10.1016/j.jneuroim.2011.07.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hooper DC, Ohnishi ST, Kean R, Numagami Y, Dietzschold B, Koprowski H. Local nitric oxide production in viral and autoimmune diseases of the central nervous system. Proc Natl Acad Sci USA. 1995;92:5312–6. doi: 10.1073/pnas.92.12.5312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Horger M, Beschorner R, Beck R, Nagele T, Schulze M, Ernemann U, et al. Common and uncommon imaging findings in progressive multifocal leukoencephalopathy (PML) with differential diagnostic considerations. Clin Neurol Neurosurg. 2012;114:1123–30. doi: 10.1016/j.clineuro.2012.06.018. [DOI] [PubMed] [Google Scholar]
- Johnson T, Nath A. Immune reconstitution inflammatory syndrome and the central nervous system. Curr Opin Neurol. 2011;24:284–90. doi: 10.1097/WCO.0b013e328346be57. [DOI] [PubMed] [Google Scholar]
- Katz-Brull R, Lenkinski RE, Du Pasquier RA, Koralnik IJ. Elevation of myoinositol is associated with disease containment in progressive multifocal leukoencephalopathy. Neurology. 2004;63:897–900. doi: 10.1212/01.wnl.0000137420.58346.9f. [DOI] [PubMed] [Google Scholar]
- Kleinschmidt-DeMasters BK, Tyler KL. Progressive multifocal leukoencephalopathy complicating treatment with natalizumab and interferon beta-1a for multiple sclerosis. N Engl J Med. 2005;353:369–74. doi: 10.1056/NEJMoa051782. [DOI] [PubMed] [Google Scholar]
- Langer-Gould A, Atlas SW, Green AJ, Bollen AW, Pelletier D. Progressive multifocal leukoencephalopathy in a patient treated with natalizumab. N Engl J Med. 2005;353:375–81. doi: 10.1056/NEJMoa051847. [DOI] [PubMed] [Google Scholar]
- Leenders KL, Beaney RP, Brooks DJ, Lammertsma AA, Heather JD, McKenzie CG. Dexamethasone treatment of brain tumor patients: effects on regional cerebral blood flow, blood volume, and oxygen utilization. Neurology. 1985;35:1610–6. doi: 10.1212/wnl.35.11.1610. [DOI] [PubMed] [Google Scholar]
- Mader I, Herrlinger U, Klose U, Schmidt F, Kuker W. Progressive multifocal leukoencephalopathy: analysis of lesion development with diffusion-weighted MRI. Neuroradiology. 2003;45:717–21. doi: 10.1007/s00234-003-0966-4. [DOI] [PubMed] [Google Scholar]
- Maleki N, Dai W, Alsop DC. Optimization of background suppression for arterial spin labeling perfusion imaging. Magma. 2012;25:127–33. doi: 10.1007/s10334-011-0286-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martin-Blondel G, Delobel P, Blancher A, Massip P, Marchou B, Liblau RS, et al. Pathogenesis of the immune reconstitution inflammatory syndrome affecting the central nervous system in patients infected with HIV. Brain. 2011;134(Pt 4):928–46. doi: 10.1093/brain/awq365. [DOI] [PubMed] [Google Scholar]
- Marzocchetti A, Tompkins T, Clifford DB, Gandhi RT, Kesari S, Berger JR, et al. Determinants of survival in progressive multifocal leukoencephalopathy. Neurology. 2009;73:1551–8. doi: 10.1212/WNL.0b013e3181c0d4a1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Niedbala W, Cai B, Liew FY. Role of nitric oxide in the regulation of T cell functions. Ann Rheum Dis. 2006;65(Suppl 3):iii37–40. doi: 10.1136/ard.2006.058446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ozsunar Y, Mullins ME, Kwong K, Hochberg FH, Ament C, Schaefer PW, et al. Glioma recurrence versus radiation necrosis? A pilot comparison of arterial spin-labeled, dynamic susceptibility contrast enhanced MRI, and FDG-PET imaging. Acad Radiol. 2010;17:282–90. doi: 10.1016/j.acra.2009.10.024. [DOI] [PubMed] [Google Scholar]
- Pfefferbaum A, Chanraud S, Pitel AL, Shankaranarayanan A, Alsop DC, Rohlfing T, et al. Volumetric cerebral perfusion imaging in healthy adults: regional distribution, laterality, and repeatability of pulsed continuous arterial spin labeling (PCASL) Psychiatry Res. 2010;182:266–73. doi: 10.1016/j.pscychresns.2010.02.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rashid W, Parkes LM, Ingle GT, Chard DT, Toosy AT, Altmann DR, et al. Abnormalities of cerebral perfusion in multiple sclerosis. J Neurol Neurosurg Psychiatry. 2004;75:1288–93. doi: 10.1136/jnnp.2003.026021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salerno L, Sorrenti V, Di Giacomo C, Romeo G, Siracusa MA. Progress in the development of selective nitric oxide synthase (NOS) inhibitors. Curr Pharm Des. 2002;8:177–200. doi: 10.2174/1381612023396375. [DOI] [PubMed] [Google Scholar]
- Scheef L, Manka C, Daamen M, Kuhn KU, Maier W, Schild HH, et al. Resting-state perfusion in nonmedicated schizophrenic patients: a continuous arterial spin-labeling 3.0-T MR study. Radiology. 2010;256:253–60. doi: 10.1148/radiol.10091224. [DOI] [PubMed] [Google Scholar]
- Sofroniew MV. Reactive astrocytes in neural repair and protection. Neuroscientist. 2005;11:400–7. doi: 10.1177/1073858405278321. [DOI] [PubMed] [Google Scholar]
- Sun HY, Singh N. Immune reconstitution inflammatory syndrome in non-HIV immunocompromised patients. Curr Opin Infect Dis. 2009;22:394–402. doi: 10.1097/QCO.0b013e32832d7aff. [DOI] [PubMed] [Google Scholar]
- Tanaka Y, Nagaoka T, Nair G, Ohno K, Duong TQ. Arterial spin labeling and dynamic susceptibility contrast CBF MRI in postischemic hyperperfusion, hypercapnia, and after mannitol injection. J Cereb Blood Flow Metab. 2011;31:1403–11. doi: 10.1038/jcbfm.2010.228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tarrant TK, Silver PB, Wahlsten JL, Rizzo LV, Chan CC, Wiggert B, et al. Interleukin 12 protects from a T helper type 1-mediated autoimmune disease, experimental autoimmune uveitis, through a mechanism involving interferon gamma, nitric oxide, and apoptosis. J Exp Med. 1999;189:219–30. doi: 10.1084/jem.189.2.219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsutsumi H, Takeuchi R, Ohsaki M, Seki K, Chiba S. Respiratory syncytial virus infection of human respiratory epithelial cells enhances inducible nitric oxide synthase gene expression. J Leukoc Biol. 1999;66:99–104. [PubMed] [Google Scholar]
- van der Veen RC. Nitric oxide and T helper cell immunity. Int Immunopharmacol. 2001;1:1491–500. doi: 10.1016/s1567-5769(01)00093-5. [DOI] [PubMed] [Google Scholar]
- van Gelderen P, de Zwart JA, Duyn JH. Pittfalls of MRI measurement of white matter perfusion based on arterial spin labeling. Magn Reson Med. 2008;59:788–95. doi: 10.1002/mrm.21515. [DOI] [PubMed] [Google Scholar]
- van Laar PJ, van der Graaf Y, Mali WP, van der Grond J, Hendrikse J. Effect of cerebrovascular risk factors on regional cerebral blood flow. Radiology. 2008;246:198–204. doi: 10.1148/radiol.2453061932. [DOI] [PubMed] [Google Scholar]
- van Osch MJ, Teeuwisse WM, van Walderveen MA, Hendrikse J, Kies DA, van Buchem MA. Can arterial spin labeling detect white matter perfusion signal? Magn Reson Med. 2009;62:165–73. doi: 10.1002/mrm.22002. [DOI] [PubMed] [Google Scholar]
- Vendrely A, Bienvenu B, Gasnault J, Thiebault JB, Salmon D, Gray F. Fulminant inflammatory leukoencephalopathy associated with HAART-induced immune restoration in AIDS-related progressive multifocal leukoencephalopathy. Acta Neuropathol. 2005;109:449–55. doi: 10.1007/s00401-005-0983-y. [DOI] [PubMed] [Google Scholar]
- Wang DJ, Alger JR, Qiao JX, Hao Q, Hou S, Fiaz R, et al. The value of arterial spin-labeled perfusion imaging in acute ischemic stroke: comparison with dynamic susceptibility contrast-enhanced MRI. Stroke. 2012;43:1018–24. doi: 10.1161/STROKEAHA.111.631929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williams DS, Detre JA, Leigh JS, Koretsky AP. Magnetic resonance imaging of perfusion using spin inversion of arterial water. Proc Natl Acad Sci USA. 1992;89:212–6. doi: 10.1073/pnas.89.1.212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ye FQ, Frank JA, Weinberger DR, McLaughlin AC. Noise reduction in 3D perfusion imaging by attenuating the static signal in arterial spin tagging (ASSIST) Magn Reson Med. 2000;44:92–100. doi: 10.1002/1522-2594(200007)44:1<92::aid-mrm14>3.0.co;2-m. [DOI] [PubMed] [Google Scholar]
- Yousry TA, Pelletier D, Cadavid D, Gass A, Richert ND, Radue EW, et al. Magnetic resonance imaging pattern in natalizumab-associated progressive multifocal leukoencephalopathy. Ann Neurol. 2012;72:779–87. doi: 10.1002/ana.23676. [DOI] [PubMed] [Google Scholar]