Abstract
Sir2, a member of the sirtuin family of protein acylases, deacetylates lysine residues within many proteins and is associated with lifespan extension in a variety of model organisms. Recent studies have questioned the positive effects of Sir2 on lifespan in Drosophila. Several studies have shown that increased expression of the Drosophila Sir2 homolog (dSir2) extends life span while other studies have reported no effect on life span or suggested that increased dSir2 expression was cytotoxic. To attempt to reconcile the differences in these observed effects of dSir2 on Drosophila life span, we hypothesized that a critical level of dSir2 may be necessary to mediate life span extension. Using approaches that allow us to titrate dSir2 expression, we describe here a strong dose-dependent effect of dSir2 on life span. Using the two transgenic dSir2 lines that were reported not to extend life span, we are able to show significant life span extension when dSir2 expression is induced between 2 and 5-fold. However, higher levels decrease life span and can induce cellular toxicity, manifested by increased expression of the JNK-signaling molecule Puc phosphatase and induction of dnaJ-H. Our results help to resolve the apparently conflicting reports by demonstrating that the effects of increased dSir2 expression on life span in Drosophila are dependent upon dSir2 dosage.
Keywords: aging, life span, Sir2, sirtuin, SIRT1, dnaJ-H, dose response, Drosophila melanogaster, deacetylase, HDAC
INTRODUCTION
Silent Information Regulator 2 (Sir2) is an NAD+ -dependent deacetylase that is highly conserved from bacteria to mammalian systems. While yeast Sir2 was originally reported to deacetylate histones, the mammalian Sir2 homologs (SIRT1-7) have since been shown to deacetylate a wide variety of other proteins including p53, Foxo, PGC-1α, Ku70 and NF- κB and play key roles in age-related diseases [1]. Increased expression of sirtuins has been found to increase life span in yeast [2], C. elegans [3,4], Drosophila melanogaster [5,6,7] and male mice [8]. Many of the identified targets of sirtuins have also been shown to have significant effects on longevity [1].
Increasing the expression of dSir2 increases fly life span when expression is induced from the endogenous locus by either the ubiquitous Tubulin-Gal4 driver or the neuron-specific ELAV-Gal4 driver [5]. Importantly, life span is also increased when increased expression of dSir2 is temporally restricted to adulthood and spatially to either neurons [5,6] or abdominal fat body [7] by use of the Gene Switch system. The Gene Switch system has the advantage of allowing selective expression in adults via the presence of the inducing agent, RU-486, in the fly food. Therefore, genetically identical siblings from the same cohort as the experimental flies that are not induced with RU-486 can serve as controls, thereby eliminating any potential concern regarding genetic background effects on life span. In these studies [5,6,7], increased dSir2 expression was mediated from the endogenous dSir2 locus through use of a P-element mediated insertion of the UAS sequence upstream of dSir2 to generate an “EP” line [9], allowing induced expression of dSir2 from its endogenous locus when the GAL4 protein is present. While the most commonly used EP line is dSir2EP2300, other similarly constructed fly lines have also been shown to extend life span when dSir2 expression is increased using some of the same Gal4 drivers [5].
A recent study reported no life span extension when dSir2 was over expressed by using the constitutive, ubiquitously expressing Tubulin-Gal4 driver, either with the dSir2EP2300 line, or with either of two newly constructed lines containing an inducible UAS-dSir2 transgene in addition to the endogenous copy of dSir2 [10]. The authors attributed the lack of lifespan extension to the use of more appropriate genetic background controls [5]. However, increased expression of dSir2 in the dSir2EP2300 line did show extended life span when driven by the inducible neuron-specific ELAV-Gene Switch driver [5,6] and more recently by the inducible abdominal fat-body specific S1-106-Gene Switch driver [7], where the controls are genetically identical flies from the same cohort, ruling out genetic background as the cause for life span extension.
Another recent study found that ubiquitous or neuronal over-expression of dSir2 from a UAS-dSir2 transgene was developmentally lethal [12]. Furthermore, expression of this UAS-dSir2 gene under control of the strong eye-specific GMR-Gal4 driver caused developmental apoptosis of cells of the eye, while simultaneously inducing JNK-signaling. The genetic locus of dSir2 partially overlaps with that of a gene that is transcribed in the opposite direction, the dnaJ-homologue (dnaJ-H) gene. While the specific function of DNAJ-H has not been shown, it has homology to the Heat Shock Protein 40 family of proteins, which are known to be involved in protein folding, translation, and degradation [11]. Interestingly, expression of dSir2 from the native dSir2 locus in the eye using the same robust GMR-Gal4 driver with the dSir2EP2300 line resulted in a greater than 12-fold increase in dSir2 mRNA and a 2-fold induction of dnaJ-H mRNA expression [12]. From these results, the authors concluded that increased dSir2 expression is lethal and that dSir2 normally regulates cell survival and death in Drosophila. They hypothesized that the life span extension seen in the dSir2EP2300 line was likely a result of the simultaneous over-expression of both dSir2 and dnaJ-H. In contrast, subsequent reports demonstrated that 2.5-fold, 3-fold, and 5-fold increased expression of dSir2 from the native dSir2 locus results in life span extension without an increase in dnaJ-H mRNA expression [6,7].
The conflicting results of these studies, demonstrating in one case that increased expression of dSir2 is lethal [12], in a second that there is no effect on life span [10], and in third, fourth and fifth cases, that there is significant life span extension [5,6,7], have been a barrier to understanding the role of dSir2 in Drosophila aging. In the present study, we present data showing that when dose-dependent effects of dSir2 are taken into account, these many discrepancies can be resolved. Through a detailed series of studies in which we examine the effects of expression of dSir2 at low, moderate and high levels on life span, we demonstrate that modest levels of dSir2 are indeed effective at extending life span, and that high levels of expression likely induce a cellular stress response that is reflected by induction of dnaJ-H. Thus, our studies show that moderately increased levels of dSir2 can extend life span in Drosophila melanogaster.
RESULTS
dSir2 expression from UAS-dSir2 transgenes can increase or decrease life span
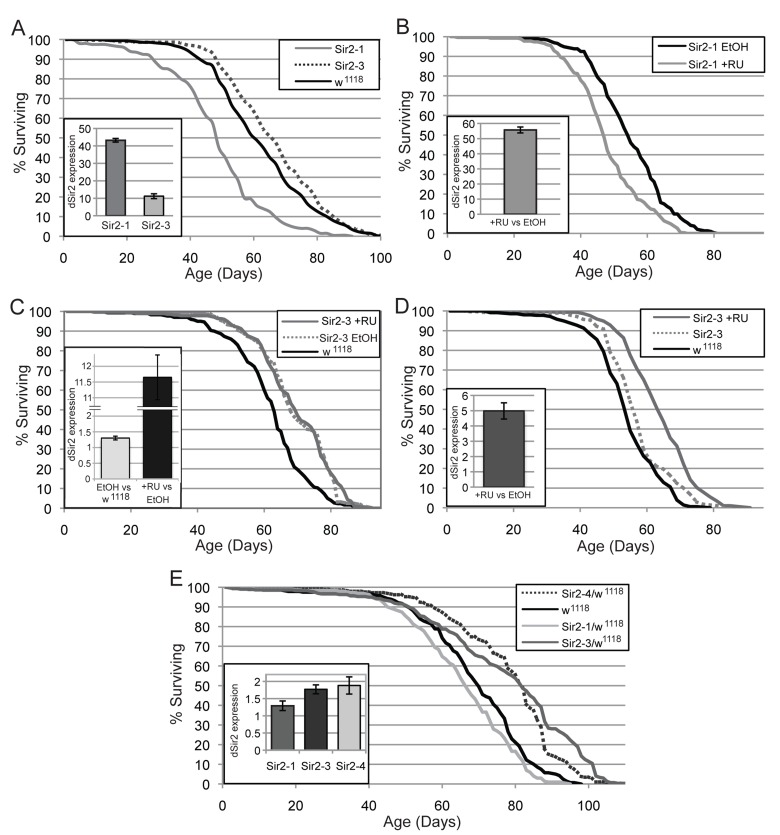
To examine the effect of increasing dSir2 expression on longevity, we examined the life span of flies carrying either of two newly generated UAS-dSir2 transgenes (Sir2-1 and Sir2-3) driven by the ubiquitous Tubulin-Gal4 driver. The life span of these flies was compared to Tubulin-Gal4 flies crossed to wild type flies from the same genetic background as the UAS-dSir2 transgenes. Under these conditions, Tubulin-Gal4>Sir2-3 flies showed life span extension, while Tubulin-Gal4>Sir2-1 flies had a shortened life span (Fig. 1A). The discrepancy of finding of a decrease in life span with the Sir2-1 transgene and an increase with the Sir2-3 transgene led us to measure the levels of dSir2 expression in these flies. Due to difficulties in establishing a functional dSIR2 western protocol, we instead utilized RT-qPCR to measure mRNA levels in this study. We found that flies containing the Sir2-1 transgene over expressed dSir2 at very high levels (~ 45-fold increase), compared to the Sir2-3 transgene (~ 11-fold increase) (Fig. 1A, inset).
Figure 1. Effects of increased dSir2 expression on life span vary under different conditions.
(A) When two different UAS-dSir2 expressing lines are driven by the Tubulin-Gal4 driver, one line extends life span (Sir2-3), while the other is detrimental (Sir2-1), compared to the control (Tubulin-Gal4>w1118). (A, inset) Sir2-1 displays very highly increased dSir2 expression, while Sir2-3 increases dSir2 expression more moderately. (B) When the Sir2-1 transgene is induced to express dSir2 by the Tubulin-Gene Switch driver, life span is decreased. (B, inset) dSir2 is expressed at greater than 50-fold Tubulin-Gene Switch>Sir2-1 flies. (C) In the Tubulin-Gene Switch>Sir2-3 flies, dSir2 is mildly elevated and life span extended when comparing the ethanol-only condition (no RU-486) to the genetic background control. (C, inset) The induction of higher levels of dSir2 from Sir2-3 using RU-486 does not further increase life span. (D) When the Sir2-3 transgene is expressed using the neuron-specific ELAV Gene Switch driver, life span is extended when RU-486 is used. (D, inset) dSir2 expression is 5-fold increased in ELAV-Gene Switch>Sir2-3 flies. (E, inset) Some UAS-dSir2 lines show elevated dSir2 levels in a heterozygous state without the presence of a driver. (E) Fly lines that show an increase in dSir2 levels also extend life span in the absence of a driver. Error bars represent SD of 3 biological replicates. Life span statistics can be found in table S1, and qPCR p-values in table S2.
To eliminate the possibility that increased expression of dSir2 during development in the presence of the constitutively expressed Tubulin-Gal4 driver might contribute to the deleterious effect we see on adult life span in the Sir2-1 line, we utilized the RU-486 inducible Gene Switch system in which control and experimental flies are genetically identical offspring from the same cohort and increased expression can be induced in, and thus restricted only to adult life. We examined the life span of flies with increased expression of dSir2 from the Sir2-1 transgene driven by the conditional Tubulin Gene Switch driver (Tubulin-Gene Switch). When expression of the Sir2-1 construct was induced using RU-486 in the food of adult flies, the life span of these flies was shortened, as seen for the Tubulin-Gal4>Sir2-1 life span (Fig. 1B). We measured the level of dSir2 mRNA in these flies, and found an approximately 50-fold induction of dSir2, consistent with the high level of induction seen with the constitutive Tubulin-Gal4 driver (Fig. 1B, inset).
Additionally, we crossed another UAS-dSir2 line, Sir2-4, to the Tubulin Gene Switch driver and observed an increase of ~15-fold in dSir2 levels for RU-486-induced flies compared to EtOH controls, without any observable change in median life span (Fig. S1).
Given that the level of dSir2 induction using both the Sir2-1 and Sir2-4 transgenes in these studies was high, we hypothesized that these levels of dSir2 might be detrimental to life span. In order to better test the effects of increased dSir2 expression at more moderate levels, we performed life span and mRNA expression experiments using the Sir2-3 transgene, which induced dSir2 expression more moderately than Sir2-1. We also included an additional genetic background control in this experiment, where we crossed w1118 flies (injection stock for the transgene lines Sir2-1, Sir2-3, Sir2-4) to the Tubulin-Gene Switch driver, and measured life span in these flies with and without RU-486 induction. Life spans of control w1118 flies carrying the Tubulin-Gene Switch driver was the same with or without RU-486, demonstrating that RU-486 treatment does not directly affect life span in this w1118 strain.
When the Sir2-3 transgene was expressed ubiquitously using the Tubulin-Gene Switch driver in the presence of RU-486, we observed no life span extension compared with genetically identical uninduced (ethanol-fed) flies. However, inclusion of the additional w1118 genetic background control in this study allowed for a comparison of uninduced control flies (Tubulin-Gene Switch> Sir2-3) with the w1118 controls. Surprisingly, we found that in comparison to the Tubulin-Gene Switch>w1118 controls, both the uninduced and the induced Tubulin-Gene Switch>Sir2-3 flies exhibited an extended life span (Fig. 1C). This result was unexpected, given that the absence of RU-486 in the food should have meant that there was no induction of dSir2. In order to clarify this result, we also examined the levels of dSir2 mRNA in all of these flies, and found that the diluent, ethanol-fed control Tubulin-Gene Switch>Sir2-3 flies had a mild but significant elevation in dSir2 levels (~1.5-fold induction) when compared with the Tubulin-Gene Switch>w1118 controls. The Tubulin-Gene Switch>Sir2-3 flies that were induced with RU-486 in the food showed an ~ 11-fold increase in dSir2 levels (Fig. 1C, inset). This finding suggests that in these conditions, a modest ~1.5-fold and significantly higher ~11-fold elevation of dSir2 can extend life span.
Finally, we crossed the Sir2-3 transgenic flies to the neuron-specific ELAV-Gene Switch driver and evaluated life span extension and dSir2 mRNA levels. Again, we included the ELAV-Gene Switch>w1118 genetic background control to allow for comparison with the uninduced ELAV-Gene Switch>Sir2-3 ethanol controls. In this experiment, the uninduced ELAV-Gene Switch>Sir2-3 flies did not have significantly elevated dSir2 levels compared to the ELAV-Gene Switch>w1118 genetic control. Consistent with this finding, we saw no life span extension for the uninduced ELAV-Gene Switch>Sir2-3. However, the RU-486 induced ELAV-Gene Switch>Sir2-3 flies had a 5-fold increase in dSir2 mRNA levels and extended life span as compared to the uninduced Elav-Gene Switch>Sir2-3 control flies and the Elav-Gene Switch>w1118 control flies (Fig. 1D).
Moderate increases in dSir2 expression from UAS-dSir2 transgenes extend life span
The ability of the Sir2-3 line to induce dSir2 expression in the uninduced condition of the Tubulin-Gene Switch life span led us to examine whether the additional copy of dSir2 from the UAS-dSir2 transgene may under certain circumstances express dSir2 in the absence of Gal4. To directly test whether the presence of one copy of the UAS-dSir2 transgene could lead to increased expression of dSir2 without the presence of a Gal4 driver, we crossed three different homozygous UAS-dSir2 transgenic lines (Sir2-1, Sir2-3, and Sir2-4) to their genetic background control (w1118) and measureddSir2 mRNA levels in the F1 heterozygotes (Fig. 1E, inset). One copy of the Sir2-1 insertion (Sir2-1 / +) did not result in significantly elevated dSir2 levels, but both Sir2-3 / + and Sir2-4 / + exhibited significantly increased dSir2 mRNA levels (~ 2-fold increase over w1118dSir2 levels). This result suggests that the life span extension observed in the control condition of the Tubulin-Gene Switch>Sir2-3 and Sir2-4 life spans may be due to uninduced expression of the dSir2 construct under some conditions.
In flies from the same cohort as those used for qPCR, we tested whether dSir2 expression from the UAS-dSir2 transgenes in the absence of a Gal4 driver was sufficient to extend life span (Fig. 1E). A single copy of Sir2-1 that did not show elevated dSir2 mRNA levels did not extend lifespan, whereas one copy of either Sir2-3 or Sir2-4 did increase lifespan, compared to the genetically matched w1118 controls. These results suggest that moderately increased dSir2 expression, here from un-driven UAS-dSir2 constructs, can extend lifespan in flies.
dSir2-mediated life span extension is dose-dependent
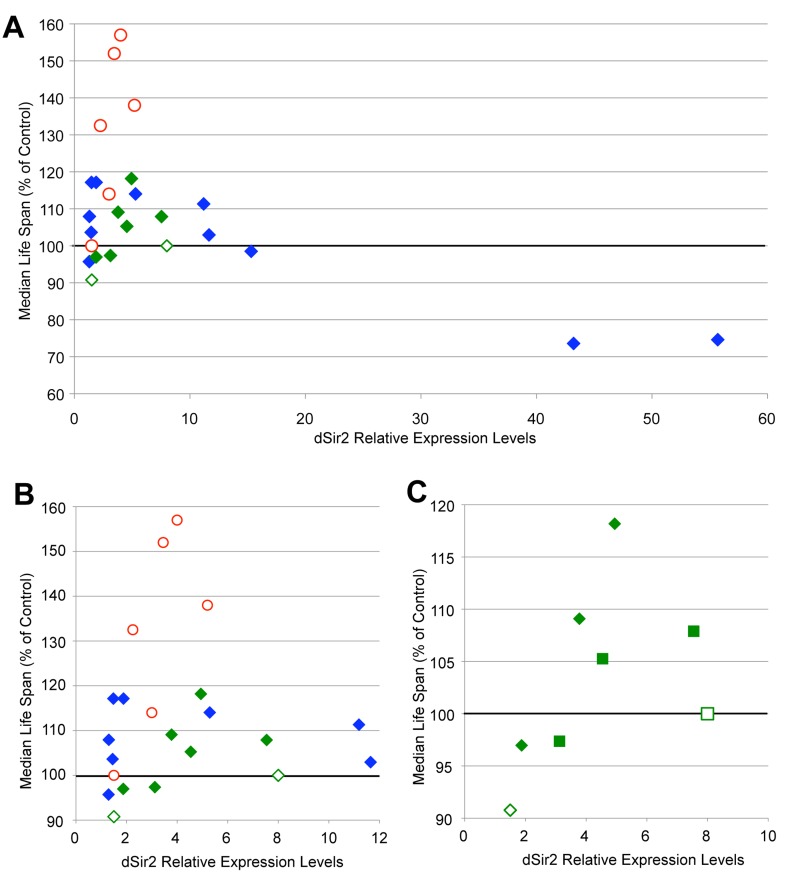
The extension of life span seen when dSir2 expression is moderately increased led to the hypothesis that there may be a dose-dependent element to the effect of dSir2 expression on life span. Previous studies showing that increased dSir2 expression extends life span used fly lines that expressed dSir2 from its endogenous locus at relatively modest levels (~3-5 fold increase) [5,6]. We noted that the flies with increased dSir2 expression from the Sir2-3 and Sir2-4 transgenes express dSir2 to a similar extent, from 2-5 fold increased, when life span is extended, while some of the conditions that we had tested had much higher levels of dSir2, and rarely extended life span. We noted that the recent study demonstrating no effect of increasing dSir2 on life span reported that their induced dSir2 levels were either 1.5- fold or 8-fold increased [10]. In order to examine whether increased dSir2 expression might have a dose-dependent effect on life span, we plotted dSir2 expression levels and life span extension from both our studies and those of published life spans (Fig. 2A and B).
Figure 2. Increased dSir2 expression extends life span dose-dependently.
(A) Median life span change (shown as % of control) is plotted as a function of dSir2 expression levels for life span experiments described in this study and in previous reports. (B) A finer view of x-axis dSir2 levels below 12-fold is shown. For all figures, previously published life spans are shown as open data points [5,6,7,10]. For figures A and B, life spans using dSir2EP2300 are shown as red circles and UAS-dSir2 transgene life spans are shown as diamonds. Life spans using the UAS-Sir2-myc lines from [10] are shown in green, while life spans conducted using UAS-Sir2-1, Sir2-3, or Sir2-4 lines in this study are shown in blue. (C) dSir2 expression levels and median life span extension from [10] (diamonds = Myc2, squares = Myc9, open data points indicate data from [10]) and from our studies using the Tubulin-Gene Switch driver and three levels of RU-486 (100 μM, 200 μM, and 500 μM) (green diamond = Tubulin-Gene Switch>Myc2, green square = Tubulin-Gene Switch>Myc9). In life span experiments published in [5], additional EP lines that over-express dSir2 from the endogenous locus also extended life span, but are not included in this graph because of the lack of data on their level of dSir2 expression. Life span statistics can be found in table S1, and qPCR p-values in table S2.
As can be seen in Fig. 2, increased dSir2 expression is associated with life span extension when mRNA levels are elevated approximately 2 to 5-fold compared to controls. In addition, very high dSir2 expression (greater than 30-fold increase) is associated with shortened life span, suggesting that such high levels of dSir2 may have a toxic effect. Interestingly, increased expression of dSir2 in the dSir2EP2300 line extends life span more reliably and to a greater extent than induction at similar levels from the UAS-dSir2 transgenes. This suggests that the presence of endogenous regulatory elements may be beneficial. Given that all of the dSir2 expression levels for flies studied in [10] were outside the predicted optimal range that we report here, we hypothesized that we should be able to extend life span with these transgenic flies if dSir2 expression within our predicted optimal window could be achieved.
To test this hypothesis we used the Gene Switch system to increase expression of dSir2 from the myc-tagged UAS-dSir2 transgenic fly lines used in [10]. The Tubulin-Gene Switch driver permitted us to express dSir2 in the same tissues as in the original study, and to limit the expression to only adult life, avoiding any potential deleterious effects of increased dSir2 during development. At the same time, this system allowed us to vary the concentration of RU-486 in the food, thereby titrating dSir2 expression levels in these flies. We used three concentrations of RU-486 (100, 200, and 500 μM) to induce dSir2 expression, and measured the dSir2 transcript levels for each of these conditions. We then plotted the median life span increase for both our own new life spans and those previously published with these constructs as a function of the measured dSir2 levels (Fig. 2C). Life span was extended to the greatest extent when dSir2 was expressed at moderate levels. These results demonstrate a dose-dependent response for increased dSir2 expression on fly life span, even for transgenic constructs previously reported not to extend life span.
High levels of dSir2 expressed from transgenes induce cellular toxicity and dnaJ-H expression
The UAS insertion in the dSir2EP2300 over-expression line is located within the 5' UTR of dSir2, oriented in a direction so as to specifically drive expression of dSir2 and not the adjacent gene dnaJ-H [9]. Although one study concluded that increased dSir2 transgene expression is lethal and that dnaJ-H suppresses this phenotype [12], in a subsequent study using the same dSir2EP2300 line driven by ELAV-Gene Switch at two different doses of RU-486, a dose-dependent increase in life span was observed coincident with increased dSir2 but not dnaJ-H levels [6]. Similarly, a life span extending increase in dSir2 expression from dSir2EP2300 in fat body using the S1-106 Gene Switch driver showed no increase in dnaJ-H expression [7]. These conflicting studies led us to test whether the expression of dnaJ-H in the eye might be unrelated to expression of dSir2 by the dSir2EP2300 EP element.
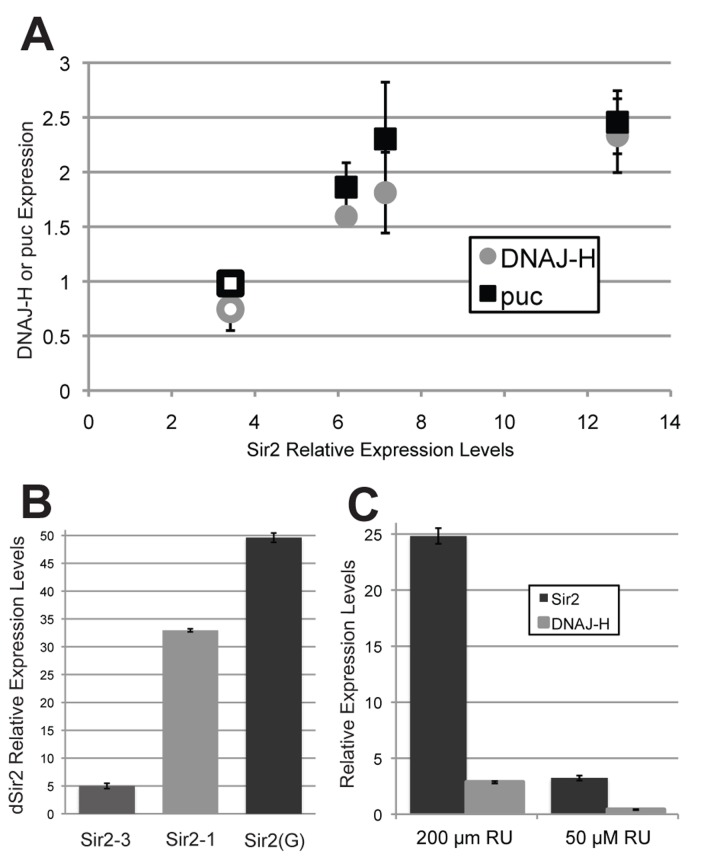
We measured dnaJ-H and dSir2 transcript levels in the heads of ELAV-Gene Switch>dSir2EP2300 flies and observed that induction of dSir2EP2300 from the native dSir2 locus as in [5] leads to a 3.5-fold increase in dSir2 and no increase in dnaJ-H levels (Fig. S2). We also measured dSir2 and dnaJ-H transcripts in three of our own UAS-dSir2 transgene containing fly lines (Sir2-1, Sir2-3, and Sir2-4), driven by the ELAV-Gal4 driver (Fig. 3A). Expression of dnaJ-H is increased in some of the UAS-dSir2 transgenic induced expression conditions but only when the level of dSir2 induction is greater than 6-fold (Fig. 3A, diamonds).
Figure 3. High levels of dSir2 induce both puc phosphatase and dnaJ-H expression, but lower levels of dSir2 expression do not.
(A) Expression of dSir2 at high levels using the constitutive neuronal driver Elav-Gal4 leads to dnaJ-H induction when expression is high, even when expression is not from the native dSir2 site (filled diamonds = UAS-dSir2 transgenes, open diamond = dSir2EP2300). Levels of dSir2 expression that induce dnaJ-H also induce expression of puc phosphatase, a JNK-signaling target gene (filled squares = UAS-dSir2 transgenes, open square = dSir2EP2300). (B) On 500 μM RU-486, the neuronal inducible ELAV-Gene Switch driver and dSir2(G) transgene from [12], induces dSir2 at levels that are extremely high, even when compared to a highly expressing line from our own studies (Sir2-1). (C) When the Sir2 (G) transgene is driven by the ubiquitous inducible Tubulin-Gene Switch driver at two different concentrations of RU-486, it can be seen that a high level of dSir2 expression (with 200 μM RU-486) induces dnaJ-H expression, while a lower level of expression (with 50 μM RU-486) does not. Error bars represent SD of 3 biological replicates. p-values can be found in table S3.
In order to explore the mechanism by which dnaJ-H is elevated in response to transgenically expressed dSir2, we measured the level of expression of the JNK signaling gene, puc phosphatase (puc), in these conditions and found that puc follows the same pattern as dnaJ-H, exhibiting increased expression when dSir2 reaches high levels (Fig. 3A, squares). Thus, expression of dSir2 at high levels may induce dnaJ-H as part of a cellular stress response, not because it is located adjacent to dSir2, as was suggested in a previous report [12]. Importantly, moderate levels of increased dSir2, such as those typically seen under conditions associated with life span extension, do not induce dnaJ-H expression.
These results led us to consider that the lethality previously observed in [12] might have resulted from toxically high levels of dSir2. Since crossing the UAS-dSir2 line from that study (referred to here as Sir2(G)) [12] to constitutive GAL4 drivers results in developmental and cellular lethality, we used the ELAV-Gene Switch driver to induce expression from the Sir2(G) transgene in neurons for 10 days during adult life, and measured dSir2 levels in fly heads. We compared dSir2 expression from the ELAV-Gene Switch>Sir2(G) flies with ELAV-Gene Switch>Sir2-1 and ELAV-Gene Switch>Sir2-3 flies under the same conditions, and found that Sir2-3, which has moderate levels of increased expression, and Sir2-1, which shows very high dSir2 levels, both express dSir2 at much lower levels than the Sir2(G) line, which showed a 50-fold increase in mRNA levels under these conditions (Fig. 3B). We conclude that the lethality of the Sir2(G) transgene resulted from extremely high dSir2 levels.
Since the Sir2(G) transgene expresses dSir2 at very high levels, and dnaJ-H is elevated in our highly expressing dSir2 transgenes, we would predict that under conditions of high dSir2 induction, the Sir2(G) transgene should also induce dnaJ-H. Additionally, we expect that induction of dSir2 expression from Sir2(G) at lower levels should not induce dnaJ-H. To test this, we crossed the lethal Sir2(G) line to the ubiquitous Tubulin-Gene Switch driver and induced dSir2 at different levels by using two different doses of RU-486 (Fig. 3C). At a low concentration of RU-486 (50 μM), dSir2 is increased 3-fold and dnaJ-H levels are not elevated, while at a higher concentration of RU-486 (200 μM), dSir2 is induced 25-fold over control levels and dnaJ-H expression is increased almost 3-fold. Therefore, the Sir2(G) transgene induces dnaJ-H induction when dSir2 levels are high, but not when dSir2 is slightly induced.
Finally, we tested whether dnaJ-H induction was specific to elevated dSir2 by expressing the foreign GFP mRNA using the strong, ubiquitous, constitutive daughterless-Gal4 driver and measuring dnaJ-H levels (Fig. S3). Interestingly, expressing GFP with this driver led to a 2.5-fold increase in dnaJ-H. The induction of a strong dnaJ-H expression response when proteins other than dSir2 are expressed indicates that the induction of dnaJ-H when dSir2 is highly over-expressed is likely part of the cellular stress response itself, and not due to a specific function of dSir2.
DISCUSSION
Here we show that in Drosophila, increased expression of dSir2 extends life span in a dose-dependent manner, thereby resolving apparent controversies in the field about the role of sirtuins in fly aging. By measuring life span while directly titrating the increase in dSir2 expression through use of a series of new and available UAS-dSir2 transgenes, and by determining the level of dSir2 expression under conditions for previously published life span studies, we show that when dSir2 expression is increased to moderate levels (approximately 2-5 fold increased over normal), life span is consistently extended. Expression below this range (less than 2-fold increase), or slightly above it (between 5-10 fold increase) inconsistently extends life span, while higher levels of expression are detrimental to life span and can induce JNK signaling and dnaJ-H expression.
Consistent with our findings that the dose of dSir2 is important in longevity determination, previous work has shown that a reduction in dSir2 levels shortens life span [13]. Our demonstration that the dose of dSir2 is critical to life span extension suggests an explanation for the contrary findings reporting an inability to detect life span extension when using dSir2EP2300 or two new UAS-dSir2 transgenes [10]. In that report [10], dSir2 expression from the Sir2-myc2 and dSir2EP2300 transgenes were both reported to be below the critical level we determined is necessary for life span extension (less than 1.5-fold increase), while the Sir2-myc9 transgene was expressing at a level of expression that is likely too high (~8-fold) to consistently extend life span. To test the hypothesis that the optimal range for dSir2 expression was not achieved in this study [10], we used these same two lines (Sir2-myc2 and Sir2-myc9) in conjunction with the conditional Gene Switch system to increase expression of dSir2 between 2 and 5-fold. In this report, we demonstrate that when dSir2 expression is increased to within an optimal range, both of these UAS-Sir2 transgenes extend life span compared with their genetically identical sibling cohorts in which dSir2 is not increased (Fig. 2C).
We note that induction of dSir2 from its native locus using the dSir2EP2300 insertion allele extends life span more reliably and to a greater extent than increased expression from the dSir2 transgenes at other genomic locations. This may be due to the lower level of dSir2 induction seen with dSir2EP2300, but it could also be due to the presence of favorable endogenous regulatory elements that are maintained when dSir2 is expressed from its native genomic location.
In a previous study, an increase in dnaJ-H was observed when dSir2EP2300 was used to increase expression of dSir2 in the eye using a GMR-Gal4 driver [12], indicating that Gal4 induction of dSir2EP2300 may increase expression of both dnaJ-H and dSir2 due to their overlapping genetic locus and that increased co-expression may account for the observed life span extension. However, it has subsequently been shown that dnaJ-H is not induced when dSir2EP2300 is used to increase expression of dSir2 in life span-extending conditions in adult neurons [6] or in adult fat body cells [7]. Furthermore, we found that dnaJ-H is induced when dSir2 or GFP is highly expressed from transgenic lines that are not located near the native dSir2 / dnaJ-H genomic locus. The dSir2 expression conditions that exhibited an increase in dnaJ-H levels also showed elevated transcripts of puc phosphatase, a target of JNK signaling. Taken together, these results show that increased expression of dSir2 can induce JNK signaling/puc phosphatase as previously reported, but only when dSir2 is expressed at high levels.
Our observation that high levels of dSir2 over expression is toxic fits with previously published results showing that expression of dSir2 from a transgene induced lethality and activated JNK signaling [12]. In this previous publication, it was reported that any increased expression of dSir2 from a transgene distant from the native dSir2 site is lethal [12]. However, the system used in that study consistently induces dSir2 expression at very high levels, indicating that lethality occurred because dSir2 was induced too strongly.
The finding that high levels of dSir2 expression can lead to cytotoxicity is perhaps not surprising given the many known interacting partners of dSir2, including proteins central to metabolism (FOXO), mitochondrial biogenesis (PGC-1ɑ), and genomic defense (p53, Ku70) [1]. Additionally, it has been shown that high level expression of the yeast Sir2p from a high-copy plasmid is toxic [14]. It is important to note that lethality in a previous study [12] was shown and measured using the eye-specific GMR-Gal4 driver, which has previously been shown to induce mild developmental defects and apoptosis in the Drosophila eye, even without induction of expression from another gene [15]. This suggests that the GMR-Gal4 system that was used to demonstrate lethality may have already been partially sensitized to apoptotic phenotypes.
We conclude that increasing dSir2 expression in Drosophila can extend life span, but caution that experiments testing the overexpression of dSir2 should ensure that dSir2 levels are increased to a sufficient extent to induce a positive effect on life span, but not high enough to induce cytotoxicity. The conflicting reports in the literature over whether increased dSir2 expression extends life span are resolved when the dosage of dSir2 is taken into consideration.
METHODS
Drosophila Stocks and Maintenance
All flies were maintained at 25°C in a temperature-controlled incubator at 50% humidity with a 12-hour light/dark cycle. The Tubulin-Gal4 (5138), ELAV-Gal4 (Bloomington 458), and Daughterless-Gal4 (Bloomington 5460) lines were from Bloomington Stock Center. ELAV Gene Switch was a gift from H. Keshishian (Yale University, New Haven, CT). dSir2EP2300 from the Bloomington stock center was backcrossed 9 times into the w1118 control line before use in qPCR studies. The lethal UAS-dSir2(G) line was a kind gift from KT Min (Indiana University, Bloomington, IN). The Tubulin Gene Switch and Myc-tagged UAS-dSir2 lines (Myc2 and Myc9) were kind gifts from S. Pletcher (University of Michigan, Ann Arbor, MI).
Construction of UAS-dSir2 lines
To generate the UAS-dSir2 lines (Sir2-1, Sir2-4 and Sir2-4), full length dSir2 was cloned from an adult Drosophila cDNA library into pDONR221 (Invitrogen) and then the pTW vector (Drosophila Gateway Vector Collection, Carnegie Institute) using the Gateway system. Primers used were dSir2-GW-F: GGGGACAAGTTTGTACAAAAAAGC AGGC TGCACCATGATGGAAAATTACGAGGAA, and dSir2-GW-R: GGGGACCACTTTGTACAAGAA AGCTGGGTCCACTGCTGCTAACTGTCCTGG, amplifying a 2.4 kb fragment. Embryo injection of pTW-dSir2 into the w1118 background was performed by Bestgene, Inc. (Chino Hills, CA). Flies were selected for germline transformation, and kept as homozygous stocks. Different lines were established from three separate integration events.
Life span Assays
All life span experiments were performed on food containing 150 g/L sucrose, 150 g/L autolysed yeast, 20 g/L cornmeal, and 20 g/L agar, all w/v. Life span experiments using UAS-Sir2 Myc2 or UAS-Sir2 Myc9 lines were performed on food containing 150 g/L dextrose, 150 g/L autolysed yeast, 20 g/L cornmeal, and 20 g/L agar, all w/v. Life span experiments using Gene Switch drivers were conducted on food containing these ingredients plus either RU-486 (Cayman Chemical, Ann Arbor, MI) in ethanol at the stated concentration, or the diluent alone (20 ml/L ethanol) for the control. Flies were collected under light CO2 anesthesia, randomly divided into treatment groups, and housed at a density of 25 males and 25 females per vial. Flies were passed onto fresh food every other day (Experiments labeled “EOD” in Table S1) or every day (labeled “ED” in Table S1), and the number and sex of dead flies recorded.
RNA Isolation and qPCR
Flies for qPCR analysis were collected in a 24-hour window and grown under the same conditions as flies in life span experiments. They were passed onto new food every other day (or every day for life spans passed every day), and on day 10, were snap-frozen in liquid nitrogen and stored at −80°C. For mRNA isolation, 30 whole male flies (for ubiquitious driver experiments) or male fly heads (for ELAV-Gal4 and ELAV-Gene Switch experiments) were used to isolate mRNA using the Dynabeads mRNA DIRECT kit (Life Technologies). mRNA was reverse transcribed using the iScript cDNA Synthesis Kit (Bio-Rad). 50 ng of cDNA was used as qPCR template. qPCR was performed on a ABI 7500 FAST Real-Time PCR machine, using SYBR Green PCR Master Mix (Life Technologies). Each qPCR run was performed using three biological replicates in triplicate. Primer sequences used for amplification can be found in Table S4. GAPDH was used as a normalizing gene.
Statistical Analysis
All life spans were analyzed in a Log Rank test using the Survival package in R. Student's t-test was used to analyze the statistical significance of qPCR data.
SUPPLEMENTARY FIGURES AND TABLES
Acknowledgments
We would like to thank Suzanne Hosier and Chengyi Chang for technical assistance, and Will Lightfoot for fly food preparation. We also thank S. Pletcher, H. Keshishian, KT Min, and the Bloomington Drosophila Stock Center for fly strains. This work was supported by NIA grants AG16667, AG24353 and AG25277 to S.L.H. S.L.H. is an Ellison Medical Research Foundation Senior Investigator and recipient of a Glenn Award for Research in Biological Mechanisms of Aging.
RW, JW, and SLH designed research; RW, SF, RM, LB, and MH performed research; RW, SLH, and JW wrote the paper.
The authors declare no conflicts of interest.
REFERENCES
- Haigis MC, Guarente LP. Mammalian sirtuins--emerging roles in physiology, aging, and calorie restriction. Genes Dev. 2006;20:2913–2921. doi: 10.1101/gad.1467506. [DOI] [PubMed] [Google Scholar]
- Kaeberlein M, McVey M, Guarente L. The SIR2/3/4 complex and SIR2 alone promote longevity in Saccharomyces cerevisiae by two different mechanisms. Genes Dev. 1999;13:2570–2580. doi: 10.1101/gad.13.19.2570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tissenbaum HA, Guarente L. Increased dosage of a sir-2 gene extends lifespan in Caenorhabditis elegans. Nature. 2001;410:227–230. doi: 10.1038/35065638. [DOI] [PubMed] [Google Scholar]
- Viswanathan M, Guarente L. Regulation of Caenorhabditis elegans lifespan by sir-2.1 transgenes. Nature. 2011;477:E1–2. doi: 10.1038/nature10440. [DOI] [PubMed] [Google Scholar]
- Rogina B, Helfand SL. Sir2 mediates longevity in the fly through a pathway related to calorie restriction. Proc Natl Acad Sci USA. 2004;101:15998–16003. doi: 10.1073/pnas.0404184101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bauer JH, Morris SN, Chang C, Flatt T, Wood JG, Helfand SL. dSir2 and Dmp53 interact to mediate aspects of CR-dependent lifespan extension in D. melanogaster. Aging (Albany NY) 2009;1:38–48. doi: 10.18632/aging.100001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Banerjee KK, Ayyub C, Ali SZ, Mandot V, Prasad NG, Kolthur-Seetharam dSir2 in the adult fat body, but not in muscles, regulates life span in a diet-dependent manner. Cell Rep. 2012;2:1485–1491. doi: 10.1016/j.celrep.2012.11.013. [DOI] [PubMed] [Google Scholar]
- Kanfi Y, Naiman S, Amir G, Peshti V, Zinman G, Nahum L, Bar-Joseph Z, Cohen HY. The sirtuin SIRT6 regulates lifespan in male mice. Nature. 2012;483:218–21. doi: 10.1038/nature10815. [DOI] [PubMed] [Google Scholar]
- Rorth P. A modular misexpression screen in Drosophila detecting tissue-specific phenotypes. Proc. Natl. Acad. Sci. USA. 1996;93:12418–12422. doi: 10.1073/pnas.93.22.12418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burnett C, et al. Absence of effects of Sir2 overexpression on lifespan in C. elegans and Drosophila. Nature. 477:482–485. doi: 10.1038/nature10296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chan HY, Warrick JM, Gray-Board GL, Paulson HL, Bonini NM. Mechanisms of chaperone suppression of polyglutamine disease: selectivity, synergy and modulation of protein solubility in Drosophila. Hum Mol Genet. 2000;9:2811–2820. doi: 10.1093/hmg/9.19.2811. [DOI] [PubMed] [Google Scholar]
- Griswold AJ, Chang KT, Runko AP, Knight MA, Min KT. Sir2 mediates apoptosis through JNK-dependent pathways in Drosophila. Proc Natl Acad Sci USA. 2008;105:8673–8678. doi: 10.1073/pnas.0803837105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Astrom SU, Cline TW, Rine J. The Drosophila melanogaster sir2(+) Gene Is Nonessential and Has Only Minor Effects on Position-Effect Variegation. Genetics. 2003;163:931–937. doi: 10.1093/genetics/163.3.931. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holmes SG, Rose AB, Steuerle K, Saez E, Sayegh S, Lee YM, Broach JR. Hyperactivation of the silencing proteins, Sir2p and Sir3p, causes chromosome loss. Genetics. 1997;145:605–14. doi: 10.1093/genetics/145.3.605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kramer JM, Staveley BE. GAL4 causes developmental defects and apoptosis when expressed in the developing eye of Drosophila melanogaster. Genet Mol Res. 2003;2:43–47. [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.