Abstract
The antioxidant activity and free radical scavenging capacity of the essential oil and three different extracts of wildly grown Mentha longifolia (M. longifolia) were studied. The essential oil from M. longifolia aerial parts was isolated by hydrodistillation technique using Clevenger-type apparatus. The extracts were prepared with three solvents of different polarity (n-hexane, dichloromethane, and methanol) using Soxhlet extractor. Maximum extract yield was obtained with methanol (12.6 g/100 g) while the minimum with dichloromethane (3.50 g/100 g). The essential oil content was found to be 1.07 g/100 g. A total of 19 constituents were identified in the M. longifolia oil using GC/MS. The main components detected were piperitenone oxide, piperitenone, germacrene D, borneol, and β-caryophyllene. The total phenolics (TP) and total flavonoids (TF) contents of the methanol extract of M. longifolia were found to be significantly higher than dichloromethane and hexane extracts. The dichloromethane and methanol extracts exhibited excellent antioxidant activity as assessed by 2,2′-diphenyl-1-picrylhydrazyl (DPPH) free radical scavenging ability, bleaching β-carotene, and inhibition of linoleic acid peroxidation assays. The essential oil and hexane extract showed comparatively weaker antioxidant and free radical scavenging activities. The results of the study have validated the medicinal and antioxidant potential of M. longifolia essential oil and extracts.
1. Introduction
Free radicals are considered to initiate oxidation that leads to aging and causes diseases in human beings [1, 2]. Moreover, activated oxygen incorporates reactive oxygen species (ROS) which consists of free radicals (1O2, O2 ∙−, ∙OH, ONOO−) and nonfree radicals (H2O2, NO, and R–OOH) [3]. ROS are liberated by virtue of stress, and thus, an imbalance is developed in the body that damages cells in it and causes health problems [2, 4]. Moreover, oxidation in processed foods, enriched with fats and oils, during storage leads to spoilage and quality deterioration [5].
The use of synthetic antioxidants such as butylated hydroxyanisole (BHA), and butylated hydroxytoluene (BHT) and tertiary butylhydroquinone (TBHQ) have been restricted because of their carcinogenicity and other toxic properties [3, 6]. Thus, the interest in natural antioxidants has increased considerably. Natural antioxidants can be phenolic compounds (tocopherols, flavonoids, and phenolic acids) and carotenoids (lutein, lycopene, and carotene). Growing evidence has shown an inverse correlation between the intake of dietary antioxidants and the risk of chronic diseases such as coronary heart disease, cancer, and several other aging-related health concerns [1, 7].
Natural antioxidant compounds exhibit their antioxidant activity by various mechanisms including chain breaking by donation of hydrogen atoms or electrons that convert free radicals into more stable species and decomposing lipid peroxides into stable final products [1]. Different in vitro assays simply provide an idea of the protective efficacy of the test model. Thus it is necessary to use at least two methods depending on the expected antioxidant potential and/or on the origin of the substance. Most commonly used methods for the determination of antioxidant activity of plant essential oils and extracts are 2,2-di(4-tert-octaphenyl)-1-picrylhydrazyl (DPPH∙) radical scavenging assay, inhibition of linoleic acid peroxidation, and bleaching of β-carotene in linoleic acid system assays. DPPH radical scavenging assay is the most popular and frequently used for the determination of antioxidant activity of essential oils and plant extracts [1, 7, 8]. Bleachability of β-carotene in linoleic acid system is another simple, reproducible, and time efficient method for rapid evaluation of antioxidant properties [1, 7, 8]. Measurement of inhibition of linoleic acid peroxidation is also an effective method for the assessment of antioxidant activity of the plant samples.
Mentha longifolia (wild mint) belongs to genus Mentha (family Lamiaceae) and grows widely throughout the temperate regions of the world [8]. The different herbal and food products from Mentha species have been in use since ancient times for the treatment of heart burns, indigestion, colic, flatulence, coughs and flu, nausea, irritable bowel syndrome, gall-bladder and bile ducts, herpes, and certain skin infections including acne and pigmentation [7–9]. Research work on plants from different regions resulted in the innovation of biologically active substances [8, 10]. Therefore the study was conducted to investigate the chemical composition and antioxidant and antimicrobial activities of essential oil and three different extracts from M. longifolia native to dry region of Pakistan.
2. Materials and Methods
2.1. Collection and Pretreatment of Plant Material
Aerial parts of wild mint (M. longifolia L.) were collected during May-June from South Punjab, Pakistan. The specimens were further identified and authenticated by a taxonomist, Dr. Qasim Ali (Assistant Professor), Department of Botany, Government College University Faisalabad. Collected specimens were dried at 35°C in a hot air oven (IM-30 Irmec, Germany) and grinded to 80 mesh and stored in polyethylene bags at −4°C.
2.2. Chemicals and Reagents
Linoleic acid, 2,2′-diphenyl-1-picrylhydrazyl, gallic acid, Folin-Ciocalteu reagent, ascorbic acid, trichloroacetic acid, sodium nitrite, aluminum chloride, ammonium thiocyanate, ferrous chloride, ferric chloride, potassium ferricyanide, butylated hydroxytoluene (99.0%), and homologous series of C9–C24 n-alkanes and various reference chemicals used to identify the constituents were obtained from Sigma Chemical Co. (St. Louis, MO, USA). All other chemicals (analytical grade), that is, anhydrous sodium carbonate ferrous chloride, ammonium thiocyanate, chloroform, and methanol, used in this study were purchased from Merck (Darmstadt, Germany), unless stated otherwise. All culture media and standard antibiotic discs were purchased from Oxoid Ltd. (Hampshire, UK).
2.3. Isolation of Essential Oil
The oven-dried and ground fennel seeds (80 mesh) were subjected to hydrodistillation for 4 h, using a Clevenger-type apparatus. The obtained essential oil was dried over anhydrous sodium sulfate, filtered, and stored at −4°C until analyzed.
2.4. Preparation of Extracts
Ground (80 mesh) M. longifolia sample (100 g) was subjected to extraction for 4 h using Soxhlet unit. The plant materials were extracted in sequence with three solvents of different polarity, that is, n-hexane, dichloromethane and methanol. The extracts were concentrated under vacuum at 45°C, using a vacuum rotary evaporator (N–N Series, Eyela, Rikakikai Co. Ltd., Tokyo, Japan), and stored at −4°C until used for further analyses.
2.5. Analysis of the Essential Oil
2.5.1. Gas Chromatography/Mass Spectrometry Analysis
The M. longifolia essential oil composition was determined on Agilent-Technologies (Little Falls, CA, USA) 6890N Network gas chromatographic (GC) system, equipped with an Agilent-Technologies 5975 inert XL Mass selective detector and Agilent-Technologies 7683B series autoinjector. Compounds were separated on HP-5 MS capillary column (30 m × 0.25 mm, film thickness 0.25 μm; Little Falls, CA, USA). A sample of 1.0 μL was injected in the split mode with split ratio 1 : 100. Helium was used as a carrier gas at a flow rate of 1.5 mL/min. For GC/MS detection, an electron ionization system, with ionization energy of 70 eV, was used. The column oven temperature was programmed from 80°C to 220°C at the rate of 4°C/min; initial and final temperatures were held for 3 and 10 min, respectively. Mass scanning range was 50–550 m/z while the injector and MS transfer line temperatures were set at 220 and 290°C, respectively. All quantifications were done by a built-in data-handling program of the equipment used (Perkin-Elmer, Norwalk, CT, USA). The composition was reported as a relative percentage of the total peak area.
2.5.2. Compounds Identification
The components of the M. longifolia essential oil were identified by comparison of their retention indices relative to (C9–C24) n-alkanes either with those of published data or with authentic compounds [11, 12]. Compounds were further identified and authenticated using their complete mass fragmentation data compared to the NIST02.L and WILEY7n.L mass spectral libraries and published mass spectra and, wherever possible, by coinjection with authentic standards (α-pinene, β-pinene, limonene, cis-β-ocimene, δ-terpinene, 1,8-cineol, linalool, borneol, α-terpineol, thymol, piperitenone, piperitenone oxide, β-caryophyllene, germacrene D, calamenene, cis-jasmone, and caryophyllene oxide) [1, 13, 14].
2.6. Antioxidant Activity
2.6.1. Determination of Total Phenolics (TP) and Total Flavonoids (TF) Contents
Amounts of total phenolics (TP) and total flavonoids (TF) in the M. longifolia extracts were determined using Folin-Ciocalteu reagent method and aluminum chloride colorimetric assay, respectively, as reported previously [15].
2.6.2. DPPH Radical Scavenging Assay
2,2′-Diphenyl-1-picrylhydrazyl (DPPH) free radical assay was carried out to measure the free radical scavenging activity as reported previously [9]. Briefly, M. longifolia essential oil, extracts, piperitenone compound, and BHT concentrations in methanol (1–100 μg/mL) were mixed with 2 mL of 90 μM methanol solution of DPPH. After 30 min incubation period at room temperature, the absorbance was read at 517 nm. The scavenging (%) was calculated by the following formula:
| (1) |
where A blank is the absorbance of the DPPH solution and A sample is the absorbance of the extract solution. Extract concentration providing 50% scavenging (IC50) was calculated from the graph plotted between scavenging percentage and extract concentration.
2.6.3. Antioxidant Activity Determination in Linoleic Acid System
The antioxidant activity of M. longifolia essential oil and extracts weas determined in terms of measurement of % inhibition of peroxidation in linoleic acid system following the reported method with some modification [16]. Essential oil and extracts (5 mg) were added to a solution mixture of linoleic acid (0.13 mL), 99.8% ethanol (10 mL), and 10 mL of 0.2 M sodium phosphate buffer (pH 7). Total mixture was diluted to 25 mL with distilled water. The solution was incubated at 40°C for 175 h. The extent of oxidation was measured by peroxide value using the colorimetric method as reported previously [15].
2.6.4. Bleaching of β-Carotene in Linoleic Acid System
Antioxidant activity of M. longifolia essential oil and extracts was also assessed by bleaching of β-carotene/linoleic acid emulsion system as reported previously [1]. Briefly, a stock solution of β-carotene-linoleic acid mixture was prepared by dissolving 0.1 mg β-carotene, 20 mg linoleic acid, and 100 mg Tween 40 in 1.0 mL of chloroform (HPLC grade). The chloroform was removed under vacuum in rotary evaporator at 50°C. Then, 50 mL of distilled water saturated with oxygen (30 min, 100 mL min−1) was added with vigorous shaking. A 5.0 mL of this reaction mixture was dispensed to test tubes with 200 μL of the essential oil or trans-anethole solution, prepared at 4.0 g L−1 concentrations, and the absorbance was immediately (t = 0) measured at 490 nm against a blank, consisting of an emulsion without β-carotene. Then emulsion was incubated for 50 h at room temperature, and the absorbance was recorded at different time intervals. The same procedure was repeated with BHT and blank. Antioxidant capacities of the fennel essential oils were compared with BHT and blank.
2.7. Statistical Analysis
All the experiments were conducted in triplicate unless stated otherwise, and data are presented as mean ± standard deviation (SD). Statistical analysis of the data was performed by Analysis of Variance (ANOVA) using STATISTICA 5.5 (Stat Soft Inc, Tulsa, OK, USA) software, and probability value P ≤ 0.05 was considered to denote a statistically significant difference.
3. Results and Discussion
3.1. Percentage Yield of Essential Oil and Different Extracts
Yield (g/100 g of dry plant material) of Mentha longifolia essential oil and n-hexane, dichloromethane, and methanol extracts is given in Table 1. Maximum yield was obtained with methanol (12.60 g/100 g). The minimum yield was obtained with dichloromethane (3.50 g/100 g). The essential oil yield from the aerial parts of M. longifolia was found to be 1.07 g/100 g. Nonpolar extract yield (n-hexane) was found to be 7.30 g/100 g. Tukey's range test revealed the significant (P < 0.05) difference among the extract yield with solvents of different polarities. Differences in yield of extracts from different solvents might be attributed to the availability of extractable component of different polarities.
Table 1.
Yield of M. longifolia essential oil and hexane, dichloromethane, and methanol extracts.
| Samples | Yield (g/100 g)* |
|---|---|
| Essential oil | 1.07 ± 0.10a |
| n-Hexane extract | 7.30 ± 0.32c |
| Dichloromethane extract | 3.50 ± 0.21b |
| Methanol extract | 12.60 ± 0.70d |
*Values are mean ± SD of three samples of M. longifolia analyzed individually in triplicate.
Different letters in superscript represent significant (P < 0.05) difference within solvents.
3.2. Essential Oil Composition
The retention indices, percentage composition, and identification methods for the essential oil of M. longifolia are given in Table 2. Nineteen compounds, 96.79% of the total oil, were identified from the oil (Figure 1). The most abundant constituents (>5%) in the essential oil of M. longifolia were found to be piperitenone oxide (28.3%), piperitenone (24.9%), germacrene D (8.16%), borneol (5.96%), and β-caryophyllene (5.94%). Analyzed essential oil mainly consisted of oxygenated monoterpenes (67.24%) followed by sesquiterpene hydrocarbons (17.19%), monoterpene hydrocarbons (7.31%), and oxygenated sesquiterpenes (5.05%).
Table 2.
Chemical composition of M. longifolia essential oil.
| Componentsb | RIc | Molecular mass | % Compositiona | Mode of identificationd | Quality (%)e |
|---|---|---|---|---|---|
| Monoterpene hydrocarbons | (7.31) | ||||
| α-Pinene | 939 | 136 | 0.76 ± 0.06 | RT, RI, MS | 97 |
| β-Pinene | 979 | 136 | 2.14 ± 0.19 | RT, RI, MS | 96 |
| Limonene | 1029 | 136 | 1.80 ± 0.19 | RT, RI, MS | 94 |
| cis-β-Ocimene | 1037 | 136 | 1.98 ± 0.11 | RT, RI, MS | 97 |
| δ-Terpinene | 1089 | 136 | 0.63 ± 0.04 | RI, MS | 96 |
| Oxygenated monoterpenes | (67.24) | ||||
| 1,8-Cineol | 1031 | 154 | 2.00 ± 0.17 | RT, RI, MS | 98 |
| Linalool | 1097 | 154 | 0.98 ± 0.10 | RT, RI, MS | 98 |
| Borneol | 1169 | 154 | 5.96 ± 0.44 | RT, RI, MS | 96 |
| α-Terpineol | 1189 | 154 | 1.17 ± 0.09 | RT, RI, MS | 98 |
| Thymol | 1290 | 150 | 2.85 ± 0.20 | RT, RI, MS | 99 |
| Piperitenone | 1343 | 150 | 24.9 ± 1.34 | RT, RI, MS | 97 |
| Thymol acetate | 1352 | 192 | 1.08 ± 0.08 | RI, MS | 94 |
| Piperitenone oxide | 1370 | 166 | 28.3 ± 1.6 | RT, RI, MS | 96 |
| Sesquiterpene hydrocarbons | (17.19) | ||||
| α-Gurjunene | 1410 | 204 | 1.11 ± 0.18 | RI, MS | 96 |
| β-Caryophyllene | 1421 | 204 | 5.94 ± 0.32 | RT, RI, MS | 99 |
| Germacrene D | 1485 | 204 | 8.16 ± 1.01 | RT, RI, MS | 99 |
| Calamenene | 1540 | 202 | 1.98 ± 0.17d | RT, RI, MS | 98 |
| Oxygenated sesquiterpenes | (5.05) | ||||
| cis-Jasmone | 1393 | 164 | 1.13 ± 0.11a | RT, RI, MS | 96 |
| Caryophyllene oxide | 1583 | 220 | 3.92 ± 0b | RT, RI, MS | 97 |
|
| |||||
| Total | 96.79 | ||||
aValues are mean ± standard deviation of three samples of M. longifolia essential oil, analyzed individually in triplicate.
bCompounds are listed in order of elution from a HP-5MS column; cretention indices relative to C9–C24 n-alkanes on the HP-5MS column; dmode of identifications; RT: identification based on retention time; RI: identification based on retention index; MS: identification based on comparison of MS data compared with those from the NIST02.L and WILEY7n.L mass spectral libraries; ematching percentage with the NIST02.L and WILEY7n.L mass spectral libraries.
Figure 1.

Structure of major compounds detected from M. longifolia essential oil. (a) α-Pinene; (b) β-pinene; (c) limonene; (d) cis-β-ocimene; (e) δ-terpinene; (f) 1,8-cineole; (g) linalool; (h) borneol; (i) α-terpineol; (j) thymol; (k) piperitenone; (l) thymol acetate; (m) piperitenone oxide; (n) α-gurjunene; (o) β-caryophyllene; (p) germacrene D; (q) calamenene; (r) cis-jasmone; (s) caryophyllene oxide.
The variation in the essential oil composition of M. longifolia is reported in the literature from different part of the world [8, 17, 18]. Our results reported the essential oil composition of M. longifolia essential oil from South Punjab, Pakistan, where the weather conditions are very hot and dry. Variations in the chemical compositions of essential oil across countries might be attributed to the varied agroclimatic (climatical, seasonal, and geographical) conditions of the regions, isolation regimes, and adaptive metabolism of plants.
3.3. Antioxidant Activity
3.3.1. Total Phenolics (TP) and Total Flavonoids (TF) Contents
Amount of total phenolics and total flavonoids is given in Figure 2. The highest TP was found in methanol extract (71.43 mg/g acid of dry plant material, measured as gallic equivalent) and the lowest in hexane extract (1.7 mg/g acid of dry plant material, measured as gallic equivalent). Similarly, the amount of TF in methanol, dichloromethane, and hexane extracts was found to be 12.35, 4.7, and 1.1 mg/g acid of dry plant material, measured as catechin equivalent. The effect of different solvent systems on the amount of TP and TF was significant (P < 0.05). Methanol has been proven as effective solvent to extract phenolic compounds [6].
Figure 2.
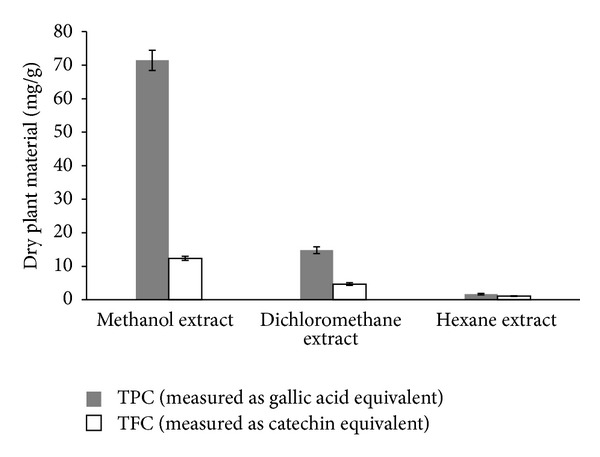
Total phenolics (TP) and total flavonoids (TF) contents of n-hexane, dichloromethane, and methanol extracts of M. longifolia.
3.3.2. DPPH Radical Scavenging Assay
The ability of M. longifolia essential oil and different extract to donate proton to DPPH free radical and change its color from violet to yellow is accessed in this assay. Concentration of extracts and essential oil scavenging 50% of DPPH radical is shown in Table 3. IC50 value ranged from 6.70 to 33.3 μg/mL. Greater IC50 value (maximum radical scavenging activity) was observed with methanol extract of M. longifolia, and lesser IC50 value was recorded with n-hexane extract. IC50 values of M. longifolia essential oil and dichloromethane extracts were found to be comparable with IC50 value of the piperitenone, a major compound of M. longifolia essential oil. IC50 value of methanol extract is significantly (P < 0.05) better than hexane and dichloromethane extracts and essential oil and comparable with synthetic antioxidant, BHT.
Table 3.
Antioxidant activity of M. longifolia essential oil and n-hexane, dichloromethane, and methanol extracts.
| Samples | Antioxidant activity* | |
|---|---|---|
| DPPH, IC50, (µg mL−1) |
Inhibition of linoleic acid peroxidation (%) |
|
| Essential oil | 21.8 ± 1.2c | 37.3 ± 1.3c |
| n-Hexane extract | 33.3 ± 1.7d | 9.9 ± 0.7a |
| DCM extract | 21.2 ± 1.7c | 89.3 ± 2.9d |
| Methanol extract | 6.70 ± 0.3a | 91.6 ± 2.3d |
| Piperitenone | 22.7 ± 1.5c | 31.3 ± 2.1b |
| BHT | 9.90 ± 0.2b | 90.9 ± 2.7d |
*Values are mean ± standard deviation of three samples of each Thymus species, analyzed individually in triplicate. Mean followed by different superscript letters in the same column represents significant difference (P < 0.05).
NT: not tested.
3.3.3. Antioxidant Activity Determination in Terms of Inhibition of Linoleic Acid Peroxidation
The antioxidants activity has also been assessed as ability to prevent the oxidation of linoleic acid. Therefore, inhibition of linoleic acid oxidation was also used to assess the antioxidant activity of M. longifolia extracts and essential oil. All extracts and essential oil exhibited appreciable inhibition of linoleic acid peroxidation (Table 3) ranging from 9.9 to 91.6%. Methanol extract showed maximum antioxidant activity (91.6%) followed by dichloromethane extract (89.3%) which is comparable with the activity of BHT standard (90.6%). M. longifolia essential oil and hexane extract showed weaker antioxidant activity. Polar extract exhibited significantly (P ≤ 0.05) higher antioxidant activity than nonpolar extracts which might be due to the higher concentration of TP and TF contents [15].
3.3.4. Antioxidant Activity Determination in Terms of Bleaching of β-Carotene in Linoleic Acid System
Bleaching β-carotene with linoleic acid system as antioxidant activity of the M. longifolia essential oil and extracts is presented in Figure 3. The greater is the effectiveness of an antioxidant, the slower will be the colour depletion. In Figure 3 smaller decline in absorbance of β-carotene indicates a lower rate of oxidation of linoleic acid and higher antioxidant activity in the presence of M. longifolia methanol and dichloromethane extracts and BHT. Hexane extract and essential oil showed poor antioxidant activity.
Figure 3.
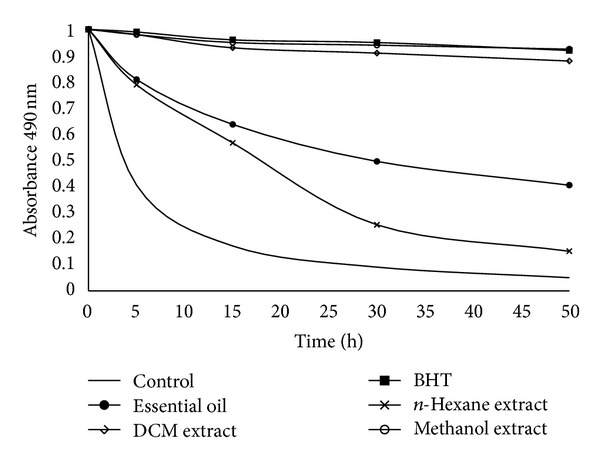
Antioxidant activity of M. longifolia essential oil and n-hexane, dichloromethane, and methanol extracts in terms of bleaching of β-carotene-linoleic acid emulsion.
4. Conclusion
Methanol extracts of Mentha longifolia exhibited excellent antioxidant activity and free radical scavenging capacity followed by dichloromethane extract, essential oil, and hexane extract. High TP and TF contents and antioxidant potential of M. longifolia extracts lead to its possible use as a food preservative. Moreover, they may be used in pharmaceutical and natural therapies for treatment of oxidative stress.
Acknowledgment
Grants supports by the Higher Education Commission (HEC), Islamabad, Pakistan, under the National Research Program for University (NRPU) scheme are highly acknowledged.
References
- 1.Hussain AI, Anwar F, Sherazi STH, Przybylski R. Chemical composition, antioxidant and antimicrobial activities of basil (Ocimum basilicum) essential oils depends on seasonal variations. Food Chemistry. 2008;108(3):986–995. doi: 10.1016/j.foodchem.2007.12.010. [DOI] [PubMed] [Google Scholar]
- 2.Shahidi F, Wanasundara PK. Phenolic antioxidants. Critical Reviews in Food Science abd Nutrition. 1992;32(1):67–103. doi: 10.1080/10408399209527581. [DOI] [PubMed] [Google Scholar]
- 3.Sultana B, Anwar F, Przybylski R. Antioxidant activity of phenolic components present in barks of Azadirachta indica, Terminalia arjuna, Acacia nilotica, and Eugenia jambolana Lam. trees. Food Chemistry. 2007;104(3):1106–1114. [Google Scholar]
- 4.Huang D, Boxin OU, Prior RL. The chemistry behind antioxidant capacity assays. Journal of Agricultural and Food Chemistry. 2005;53(6):1841–1856. doi: 10.1021/jf030723c. [DOI] [PubMed] [Google Scholar]
- 5.Chatha SAS, Hussain AI, Bajwa J, Sagir M. Antioxidant activity of different solvent extracts of rice bran at accelerated storage of sunflower oil. Journal of Food Lipids. 2006;13(4):424–433. [Google Scholar]
- 6.Siddhuraju P, Becker K. Antioxidant properties of various solvent extracts of total phenolic constituents from three different agroclimatic origins of drumstick tree (Moringa oleifera Lam.) leaves. Journal of Agricultural and Food Chemistry. 2003;51(8):2144–2155. doi: 10.1021/jf020444+. [DOI] [PubMed] [Google Scholar]
- 7.Hussain AI, Anwar F, Chatha SAS, Jabbar A, Mahboob S, Nigam PS. Rosmarinus officinalis essential oil: antiproliferative, antioxidant and antibacterial activities. Brazilian Journal of Microbiology. 2010;41(4):1070–1078. doi: 10.1590/S1517-838220100004000027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Hussain AI, Anwar F, Nigam PS, Ashraf M, Gilani AH. Seasonal variation in content, chemical composition and antimicrobial and cytotoxic activities of essential oils from four Mentha species. Journal of the Science of Food and Agriculture. 2010;90(11):1827–1836. doi: 10.1002/jsfa.4021. [DOI] [PubMed] [Google Scholar]
- 9.Hussain AI, Anwar F, Rasheed S, Nigam PS, Janneh O, Sarker SD. Composition, antioxidant and chemotherapeutic properties of the essential oils from two Origanum species growing in Pakistan. Revista Brasileira de Farmacognosia. 2011;21(6):943–952. [Google Scholar]
- 10.Anwar F, Hussain AI, Sherazi STH, Bhanger MI. Changes in composition and antioxidant and antimicrobial activities of essential oil of fennel (Foeniculum vulgare Mill.) fruit at different stages of maturity. Journal of Herbs, Spices and Medicinal Plants. 2009;15(2):187–202. [Google Scholar]
- 11.Massada Y. Analysis of Essential Oils by Gas Chromatography and Mass Spectrometry. New York, NY, USA: John Wiley & Sons; 1976. [Google Scholar]
- 12.Adam RP. Identification of Essential Oils Components by Gas Chromatography/Quadrupole Mass Spectroscopy. Carol Stream, Ill, USA: Allured Publishing; 2001. [Google Scholar]
- 13.Anwar F, Ali M, Hussain AI, Shahid M. Antioxidant and antimicrobial activities of essential oil and extracts of fennel (Foeniculum vulgare Mill.) seeds from Pakistan. Flavour and Fragrance Journal. 2009;24(4):170–176. [Google Scholar]
- 14.Vagionas K, Graikou K, Ngassapa O, Runyoro D, Chinou I. Composition and antimicrobial activity of the essential oils of three Satureja species growing in Tanzania. Food Chemistry. 2007;103(2):319–324. [Google Scholar]
- 15.Hussain AI, Chatha SAS, Noor S, et al. Effect of extraction techniques and solvent systems for the extraction of antioxidant components from peanut (Arachis hypogaea L.) Hulls. Food Analytical Methods. 2012;5(4):890–896. [Google Scholar]
- 16.Iqbal S, Bhanger MI, Anwar F. Antioxidant properties and components of some commercially available varieties of rice bran in Pakistan. Food Chemistry. 2005;93(2):265–272. [Google Scholar]
- 17.Viljoen AM, Petkar S, van Vuuren SF, Figueiredo AC, Pedro LG, Barroso JG. The chemo-geographical variation in essential oil composition and the antimicrobial properties of “wild mint”—Mentha longifolia subsp. polyadena (Lamiaceae) in Southern Africa. Journal of Essential Oil Research. 2006;18:60–65. [Google Scholar]
- 18.Gulluce M, Sahin F, Sokmen M, Ozer H, Daferera D, Sokmen A. Antimicrobial and antioxidant properties of the essential oils and methanol extract from Mentha longifolia L. ssp. longifolia . Food Chemistry. 2007;103(4):1449–1456. [Google Scholar]


