Abstract
Three-dimensional conformal radiation therapy (3DCRT) has emerged as a preferred treatment for gynecologic malignancies. Yet its superiority to conventional radiotherapy (2-dimensional radiotherapy (2DRT)) for gynecologic malignancies has not been well established. Data from the 2005 to 2010 National Health Insurance Research Database (NHIRD) provided by the National Research Institutes in Taiwan were analyzed to address this issue. Patients were initially diagnosed as having cervical cancer according to the International Classification of Disease, Ninth Revision, Clinical Modification (ICD-9-CM) code 180, and this clinical diagnosis was confirmed histopathologically or cytologically. Kaplan-Meier method and Cox proportional hazards regression were used to analyze the reported data. Between January 2005 and December 2010, there were 776 patients with newly diagnosed cervical cancer without metastasis, local recurrence, or surgical treatment before RT and 132 and 644 patients, respectively, who received 2DRT and 3DCRT. After adjustment for age, diabetes mellitus, hypertension, coronary heart disease, hyperlipidemia, side effects, urbanization level, geographic region, and enrollee category in the 5-year follow-up period, the HR was 1.82 (95% CI, 1.16–2.85, P = 0.009). The 5-year survival rate in the 2DRT and 3DCRT groups was 73.0% and 82.3%, P = 0.007, respectively. Cervical cancer patients treated with 3DCRT had better overall survival.
1. Introduction
Cervical cancer is the second most frequent cancer among women worldwide and the most frequent cancer among women in Africa, Asia, and South America [1]. Concurrent chemotherapy with external beam radiotherapy (EBRT) shows benefit for patients with bulky and locally advanced cervical cancer [2–5]. Though dose is related to local control and overall survival, the risk of tissue toxicity (acute or late) currently limits the total radiation dose that can safely be delivered [6, 7]. Risk factors for morbidity include the volume of irradiated normal tissue, total tumor dose, EBRT dose, fraction size, and age [8–10]. These factors can lead to unplanned treatment breaks and long overall treatment times that may negatively influence the outcome. Therefore, dose escalation, decreasing toxicity to normal tissues, and the use of novel systemic agents have tremendous potentials to improve the outcome.
Conventional radiotherapy (2-dimensional radiotherapy (2DRT)) uses bony landmarks to define the target volume for pelvic radiotherapy. Treatment is delivered either with anterior and posterior opposed fields or with a four-field box technique, which reduces the volume of small bowel in the treated volume [11, 12]. However, studies assessing the adequacy of the standard fields for target volume coverage have reported an underdosing of the designated lymph node regions in 30–40% of patients [13–15]. In the late 1990s, the technique of three-dimensional conformal radiation therapy (3DCRT) emerged as a preferred treatment for gynecologic malignancies, since it gave better and more precise target coverage (20% reduction in the risk of a geographical miss) and significantly reduced the volume of radiation-exposed bladder and bowel [16, 17]. These results are consistent with the findings of reduced dose to normal structures such as the gastrointestinal tract (32% and 19% grade 2 toxicity of 3DCRT and 2DRT, resp., for prostate cancer; P = 0.02) [18].
The proven benefits of 3DCRT over the 2DRT technique have led to studies to determine whether 3DCRT is superior to 2DRT for the clinical treatment of gynecologic malignancies. To prove the superiority of 3DCRT, a large-scale, nationwide, controlled cohort study was conducted in Taiwan to investigate the relative benefits of 3DCRT and 2DRT in patients with gynecologic malignancies.
2. Materials and Methods
2.1. Ethics Statement
The study protocol was approved by the Buddhist Dalin Tzu Chi General Hospital Institutional Review Boards. Informed consent was not needed and waived because only deidentified retrospective data released to the public for research was collected and analyzed.
2.2. Database
The study analyzed data from the 2005 to 2010 National Health Insurance Research Database (NHIRD) provided by the National Research Institutes in Taiwan. The National Health Insurance program was implemented in Taiwan in 1995. The database contains comprehensive information on insured subjects, including dates of clinical visits, diagnostic codes, details of prescriptions, and expenditure amounts. The NHIRD contains the medical benefit claims for 97% of the population and a registry of board-certified physicians and contracted medical facilities.
2.3. Study Population
Patients were initially identified as having cervical cancer according to the International Classification of Disease, Ninth Revision, Clinical Modification (ICD-9-CM) code 180, and then, the clinical diagnosis was validated using the cross-linked data from the registry for catastrophic illness (n = 33, 205). Next, we identified 8134 patients with cervical cancer newly diagnosed between January 2005 to December 2010. From this patient cohort, we excluded patients with distant metastases at the time of diagnosis (n = 481), prior surgery (n = 6161), no treatment plan (n = 703), and errors in coded data (n = 13). Finally, we identified and divided 776 cervical cancer patients receiving radiotherapy into two study groups: one receiving 2DRT (n = 132) and the other 3DCRT (n = 644). The flow diagram in Figure 1 shows the allocation of patients to the two study groups.
Figure 1.
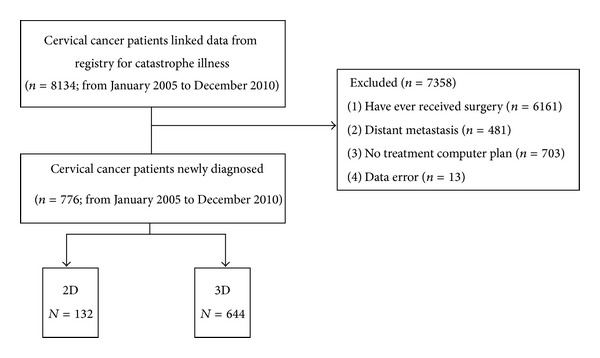
A flowchart of this population-based study showing the selection and group allocation of the cohort used for analysis.
2.4. Measurements
The key dependent variable of interest was the 5-year survival rate. Survival was measured from the time of cervical cancer diagnosis to the time of death. The independent variables were age, comorbidities, side effects, geographic region, urbanization level, and socioeconomic status. Comorbidities included hypertension (ICD-9-CM codes 401–405), diabetes (ICD-9-CM code 250), coronary heart disease (ICD-9-CM codes 410–414), and hyperlipidemia (ICD-9-CM codes 2720–2724). Diarrhea or radiation proctitis, gastroenteritis, and colitis due to radiation (ICD-9-CM code 5581), radiation cystitis, or overactive bladder (ICD-9-CM codes 59582, 59589, and 59651), dermatitis due to other radiation (ICD-9-CM code 69282), other myelopathy (ICD-9-CM code 3368), late effect of radiation (ICD-9-CM code 9092), and other unspecific radiation side effects (ICD-9-CM code 990) were defined as side effects. There were five geographic regions (northern, central, southern, eastern, and other) and four urbanization levels (urban, suburban, rural, and other). This study also used enrollee category (EC) in the NHIRD as a proxy measure of socioeconomic status. All patients were divided into 4 subgroups: EC 1 (civil servants, full-time or regular paid personnel with a government affiliation), EC 2 (employees of privately owned institutions), EC 3 (self-employed individuals, other employees, and members of farmers' or fishermens' associations), and EC 4 (veterans, low-income families, and substitute service draftees).
2.5. Statistical Analysis
The statistical software packages SAS (version 9.2; SAS Institute, Inc., Cary, NC, USA) and SPSS (version 17; SPSS Inc., Chicago, IL, USA) were used for data analysis. Pearson's chi-square tests were used to explore the differences between categorical variables in the different plan groups. The 5-year survival rate was estimated using the Kaplan-Meier method and compared by the logrank test. A Cox proportional hazard regression model was used to calculate the relative risk of cervical cancer patients between different plan modalities after adjusting for age, diabetes mellitus, hypertension, coronary heart disease, hyperlipidemia, side effects, residence urbanization level, and socioeconomic status. A P < 0.05 was defined as statistically significant.
3. Results
The characteristics and comorbidities of the patients included in the study are shown in Table 1. After adjustment for age, diabetes mellitus, hypertension, coronary heart disease, hyperlipidemia, side effects, urbanization level, geographic region, and EC in the 5-year follow-up period, HR was 1.82 (95% CI, 1.16–2.85, P = 0.009) (Table 2). Five-year survival rate for 2DRT and 3DCRT was significantly different (73.0% and 82.3%, resp., P = 0.007; Figure 2). Additionally, age older than 55 years, hypertension, diabetes, coronary heart disease, and hyperlipidemia were used to stratify the cervical cancer patients into a low-risk group (n = 221) and high-risk group (n = 555). The 5-year cumulative survival rate for the two-risk groups was 86.5% (3DCRT) and 78.0% (2DRT; P = 0.05; Figure 3). The incidence trend of side effect after 2DRT and 3DCRT was showed in Figure 4.
Table 1.
Demographic characteristics and comorbidities of cervical cancer patients in the 2D and 3D groups.
| 2D | 3D | P value | |||
|---|---|---|---|---|---|
| (n = 132) | (n = 644) | ||||
| n | % | n | % | ||
| Age (in years) | 0.67 | ||||
| 0–44 | 16 | 12.1 | 83 | 12.9 | |
| 45–54 | 32 | 24.2 | 183 | 28.4 | |
| 55–64 | 36 | 27.3 | 152 | 23.6 | |
| 65–74 | 33 | 25.0 | 139 | 21.6 | |
| 75+ | 15 | 11.4 | 87 | 13.5 | |
| Diabetes mellitus | 0.01 | ||||
| Yes | 41 | 31.1 | 138 | 21.4 | |
| No | 91 | 68.9 | 506 | 78.6 | |
| Hypertension | 0.75 | ||||
| Yes | 54 | 40.9 | 273 | 42.4 | |
| No | 78 | 59.1 | 371 | 57.6 | |
| Coronary heart disease | 0.13 | ||||
| Yes | 35 | 26.5 | 133 | 20.7 | |
| No | 97 | 73.5 | 511 | 79.3 | |
| Hyperlipidemia | 0.13 | ||||
| Yes | 41 | 31.1 | 160 | 24.8 | |
| No | 91 | 68.9 | 484 | 75.2 | |
| Side effects | 0.04 | ||||
| Yes | 41 | 31.1 | 148 | 23.0 | |
| No | 91 | 68.9 | 496 | 77.0 | |
| Urbanization level | 0.18 | ||||
| Urban | 27 | 20.5 | 189 | 29.3 | |
| Suburban | 65 | 49.2 | 270 | 41.9 | |
| Rural | 39 | 29.5 | 177 | 27.5 | |
| Other | 1 | 0.8 | 8 | 1.2 | |
| Geographic region | 0.60 | ||||
| Northern | 50 | 37.9 | 289 | 44.9 | |
| Central | 49 | 37.1 | 197 | 30.6 | |
| Southern | 3 | 2.3 | 14 | 2.2 | |
| Eastern | 29 | 22.0 | 139 | 21.6 | |
| Other | 1 | 0.8 | 5 | 0.8 | |
| EC | 0.06 | ||||
| EC 1, 2 | 32 | 24.2 | 111 | 17.2 | |
| EC 3 | 50 | 37.9 | 221 | 34.3 | |
| EC 4 | 20 | 15.2 | 96 | 14.9 | |
| Other | 30 | 22.7 | 216 | 33.5 | |
EC indicates enrollee category.
Table 2.
Crude and adjusted hazard ratio for the two groups in the 5-year follow-up period.
| Event % | Unadjusted HR | P value | Adjusted HR | P value | |
|---|---|---|---|---|---|
| (95% CI) | (95% CI) | ||||
| Cervical cancer patients with 3D treatment (n = 644) | 67 (10.4) | 1 | 0.008 | 1 | 0.009 |
| Cervical cancer patients with 2D treatment (n = 132) | 29 (22.0) | 1.80 (1.16–2.79) | 1.82 (1.16–2.85) |
Adjusted for age, diabetes mellitus, hypertension, coronary heart disease, hyperlipidemia, side effects, urbanization level, geographic region, and enrollee category.
Figure 2.
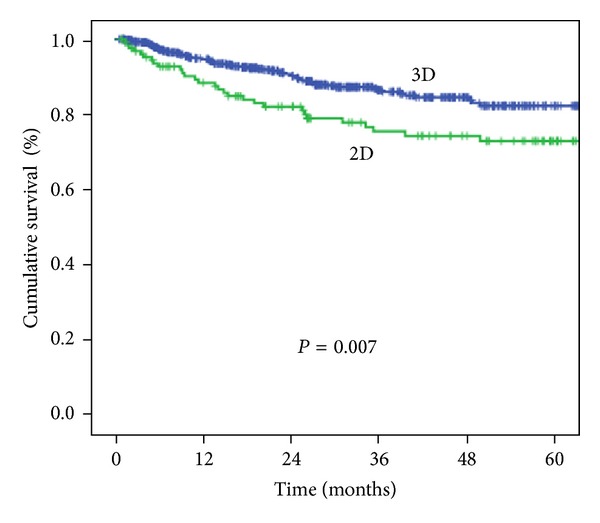
Cumulative survival of the 2DRT and 3DCRT groups from 2005 to 2010.
Figure 3.
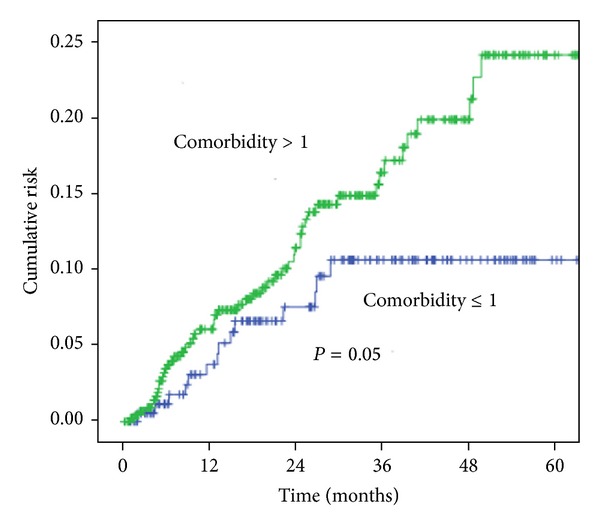
The survival of cervical cancer patients with >1 risk factor compared with that in patients ≤1 risk factor.
Figure 4.
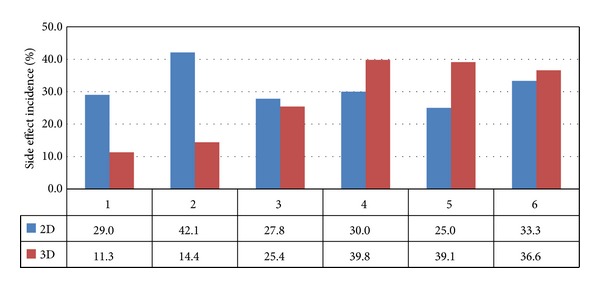
Annual incidence trend of side effect postradiotherapy. *X axis means interval year of postradiotherapy; Y axis means side effect incidence (%).
4. Discussion
Optimal nodal coverage is critical in the treatment of cervical cancer, and RT has been shown to sterilize nodes. Moreover, pelvic failure is associated with decreased survival [19, 20]. 2DRT consists of a single beam from one to four directions. Beam setups are usually quite simple; plans frequently consist of opposed lateral fields or four-field “boxes” and use bony landmarks to confirm. However, use of bony landmarks is associated with a degree of uncertainty. The inadequacy of the standard fields for target volume coverage and underdosing in lymph node regions in around 30–40% of patients has been reported [13–15]. Kim [21] noted that margins are inadequate in 39–50% of cases. Russell et al. [22] also noted that the rate of missed therapeutic margins and the rate of incomplete coverage of the uterine fundus are as high as 24% and 62.5%, respectively. Even the use of 4-field radiation to the pelvis was potentially dangerous without a CT scan to confirm. The incidence of inadequate margin ranged from 39% to 50% and was independent of the stage of the disease, and the most common site of inadequate margin was the rectum [21]. Therefore, defining the lymph node structures to be included in the treatment plan and radiotherapy target volume is an important issue for cervical cancer patients.
To confirm the area of lymph node targeting in 2DRT, direct visualization with lymphangiography (LAG) has been suggested [13]. Pendlebury et al. [23] using lymphangiography reported that a margin of 2.5 cm lateral to the pelvic sidewall would be required to cover the pelvic lymph nodes in 90% of patients. In their analysis, the superior border of the anterior-posterior (AP)/PA portals in 14% of patients has to be altered from the L5/S1 junction to L4/L5 to cover the common iliac nodes. Since computed tomography (CT) scans have become available in many radiotherapy departments, several attempts to improve treatment planning have been made by taking into account the anatomy of individual patients. Because sectional CT enables the visualization and delineation of the cervix, uterus, vagina, iliac vessels, and organs at risk such as bladder, rectum, and intestine, 3DCRT has become a preferred treatment for gynecologic malignancies. It gives better, more precise target coverage while reducing the risk of a geographical miss by 20% [16]. Although 4-field radiation technique spares the small bowel anteriorly and a portion of the rectum posteriorly, it is potentially dangerous to use the 4-field pelvic technique without knowledge of the precise tumor volume. Therefore, Kim [21] strongly recommended CT treatment planning. With more targeted treatment, better results can be predicted. These characteristics resulted in improved overall survival in our study. The survival rate was better in our 3DCRT group than in our 2DRT group (82.3% and 73.0%, P = 0.05) (Figure 1).
In the study by Souhami et al. [24] using conventional concurrent chemoradiation therapy to treat cervical cancer followed by high dose brachytherapy, the rate of response was high but severe late gastrointestinal complications developed in 28% of 50 patients. In the phase 3 study by Rose et al. [2], 38% of patients treated with 2DRT concurrent with cisplatin experienced grade 3 and 4 toxicities and 35% of patients treated with 2DRT + cisplatin for bulky stage IB cervical cancers experienced grade 3 or grade 4 adverse effects [25]. In the recent update of the Radiation Therapy Oncology Group (RTOG) 90-01 trial, 12% of patients treated with extended-field radiotherapy for common iliac or para-aortic lymph node involvement had late grade 3 and 4 toxicities [26]. In the RTOG 79-02 report, the risk of grade 4 and 5 toxicities doubled with extended-field treatment compared with involved-field treatment [27]. The addition of concurrent chemotherapy to extended-field radiotherapy magnifies the acute toxicity. The grade 3 and 4 acute bowel toxicity was 49% with conventional delivery of extended-field radiotherapy via opposed AP/PA fields to the para-aortic lymph nodes [28]. Furthermore, as the focus of extended treatment can encompass a large volume of bone marrow [29], potential hematologic depression could lead to untoward treatment interruptions, reducing the number and intensity of chemotherapy cycles [30].
Studies have shown that 3DCRT improves patient tolerance to curative treatment and allows for dose escalation [31]. Gerstner and colleagues reported that 3DCRT (compared with 2DRT) significantly reduces the volume of radiation exposure in the bladder (up to 34%) and bowel (up to 254 cm3) of cervical cancer patients [16]. Additionally, 3DCRT (compared with 2DRT) also decreases the dose to the small bowel up to 33% in postoperative node-positive cervical cancer patients [17]. Similarly, Hanks et al. [32] showed that conformal RT (compared with standard techniques of external beam therapy) decreased RTOG-EORTC grade 2 acute morbidity in prostate cancer patients. In other studies, the use of 3D planning for the entire course of treatment, rather than just the last part of the treatment, reduced the incidence of gastrointestinal complications [33, 34]. These results were consistent with dose reduction to normal structures in prostate cancer patients treated by 3DCRT (19% and 32% grade 2 gastrointestinal toxicity for 3DCRT and 2DRT, resp., P = 0.02) [18]. In the current study, side effects of 3DCRT were significantly lower than those of 2DRT (31% versus 23%, P = 0.04). This could be one reason why survival rate was better in the 3DCRT treatment group.
This analysis has several limitations. First, the diagnosis of cervical cancer, and any other comorbid conditions, was completely dependent on ICD codes. Nonetheless, the Bureau of National Health Insurance in Taiwan randomly reviews records and interviews patients to verify the accuracy of the diagnosis [35]. Second, cancer stages were not considered because this information was not available from the database. Instead of cancer-specific survival rate, overall survival rate was used, because the former could not be determined from this registry data. Third, several studies have reported that diabetes mellitus could be a factor influencing survival [36, 37], and the rate of diabetes mellitus in the current study was 31% in the 2DRT group and 21% in the 3DRCT group (P = 0.01). However, after adjustment for diabetes, the hazard ratio for the two groups was still significantly different (HR = 1.82, P = 0.009), suggesting that the influence of diabetes on overall survival could be less important.
In summary, 3DCRT (compared with 2DRT) leads to a better overall survival rate in cervical cancer patients. Cervical cancer patients with more comorbidities have poorer survival rate. Strategies to reduce the risk of comorbidity should be assessed.
Authors' Contribution
Chen-Hsi Hsieh and Shiang-Jiun Tsai contributed equally to this work.
Acknowledgments
This study utilizes data from the National Health Insurance Research Database provided by the Bureau of National Health Insurance, Department of Health and managed by the National Health Research Institutes (registry no. 99029). The interpretation and conclusions contained herein are not those of the Bureau of National Health Insurance, Department of Health, or National Health Research Institutes.
References
- 1.Pisani P, Parkin DM, Bray F, Ferlay J. Estimates of the worldwide mortality from 25 cancers in 1990. International Journal of Cancer Journal International Du Cancer. 1999;83(1):18–29. doi: 10.1002/(sici)1097-0215(19990924)83:1<18::aid-ijc5>3.0.co;2-m. [DOI] [PubMed] [Google Scholar]
- 2.Rose PG, Bundy BN, Watkins EB, et al. Concurrent cisplatin-based radiotherapy and chemotherapy for locally advanced cervical cancer. New England Journal of Medicine. 1999;340(15):1144–1153. doi: 10.1056/NEJM199904153401502. [DOI] [PubMed] [Google Scholar]
- 3.Peters WA, III, Liu PY, Barrett RJ, II, et al. Concurrent chemotherapy and pelvic radiation therapy compared with pelvic radiation therapy alone as adjuvant therapy after radical surgery in high-risk early-stage cancer of the cervix. Journal of Clinical Oncology. 2000;18(8):1606–1613. doi: 10.1200/JCO.2000.18.8.1606. [DOI] [PubMed] [Google Scholar]
- 4.Forrest JL, Ackerman I, Barbera L, et al. Patient outcome study of concurrent chemoradiation, external beam radiotherapy, and high-dose rate brachytherapy in locally advanced carcinoma of the cervix. International Journal of Gynecological Cancer. 2010;20(6):1074–1078. doi: 10.1111/IGC.0b013e3181e6f321. [DOI] [PubMed] [Google Scholar]
- 5.Huguet F, Cojocariu O-M, Levy P, et al. Preoperative concurrent radiation therapy and chemotherapy for bulky stageIB2, IIA, and IIB carcinoma of the uterine cervix with proximal parametrial invasion. International Journal of Radiation Oncology Biology Physics. 2008;72(5):1508–1515. doi: 10.1016/j.ijrobp.2008.03.054. [DOI] [PubMed] [Google Scholar]
- 6.Snijders-Keilholz A, Von Acht M, De Vroome H, Hermans J, Trimbos JB, Leer JWH. Pelvic failure rate and radiation toxicity in relation to total dose of radiation alone for the treatment of cancer of the uterine cervix. Clinical Oncology. 1993;5(1):6–10. doi: 10.1016/s0936-6555(05)80684-3. [DOI] [PubMed] [Google Scholar]
- 7.Davidson MTM, Yuen J, D’Souza DP, Batchelar DL. Image-guided cervix high-dose-rate brachytherapy treatment planning: does custom computed tomography planning for each insertion provide better conformal avoidance of organs at risk? Brachytherapy. 2008;7(1):37–42. doi: 10.1016/j.brachy.2007.12.003. [DOI] [PubMed] [Google Scholar]
- 8.Ferrigno R, dos Santos Novaes PER, Pellizzon ACA, et al. High-dose-rate brachytherapy in the treatment of uterine cervix cancer. Analysis of dose effectiveness and late complications. International Journal of Radiation Oncology Biology Physics. 2001;50(5):1123–1135. doi: 10.1016/s0360-3016(01)01533-4. [DOI] [PubMed] [Google Scholar]
- 9.Roeske JC, Mundt AJ, Halpern H, et al. Late rectal sequelae following definitive radiation therapy for carcinoma of the uterine cervix: a dosimetric analysis. International Journal of Radiation Oncology Biology Physics. 1997;37(2):351–358. doi: 10.1016/s0360-3016(96)00490-7. [DOI] [PubMed] [Google Scholar]
- 10.Dale E, Hellebust TP, Skjønsberg A, Høgberg T, Olsen DR. Modeling normal tissue complication probability from repetitive computed tomography scans during fractionated high-dose-rate brachytherapy and external beam radiotherapy of the uterine cervix. International Journal of Radiation Oncology Biology Physics. 2000;47(4):963–971. doi: 10.1016/s0360-3016(00)00510-1. [DOI] [PubMed] [Google Scholar]
- 11.Gallagher MJ, Brereton HD, Rostock RA, et al. A prospective study of treatment techniques to minimize the volume of pelvic small bowel with reduction of acute and late effects associated with pelvic irradiation. International Journal of Radiation Oncology Biology Physics. 1986;12(9):1565–1573. doi: 10.1016/0360-3016(86)90279-8. [DOI] [PubMed] [Google Scholar]
- 12.Perez CA, Grigsby PW, Chao KSC, Mutch DG, Lockett MA. Tumor size, irradiation dose, and long-term outcome of carcinoma of uterine cervix. International Journal of Radiation Oncology Biology Physics. 1998;41(2):307–317. doi: 10.1016/s0360-3016(98)00067-4. [DOI] [PubMed] [Google Scholar]
- 13.Bonin SR, Lanciano RM, Corn BW, Hogan WM, Hartz WH, Hanks GE. Bony landmarks are not an adequate substitute for lymphangiography in defining pelvic lymph node location for the treatment of cervical cancer with radiotherapy. International Journal of Radiation Oncology Biology Physics. 1996;34(1):167–172. doi: 10.1016/0360-3016(95)02055-1. [DOI] [PubMed] [Google Scholar]
- 14.Zunino S, Rosato O, Lucino S, Jauregui E, Rossi L, Venencia D. Anatomic study of the pelvis in carcinoma of the uterine cervix as related to the box technique. International Journal of Radiation Oncology Biology Physics. 1999;44(1):53–59. doi: 10.1016/s0360-3016(98)00538-0. [DOI] [PubMed] [Google Scholar]
- 15.McAlpine J, Schlaerth JB, Lim P, Chen D, Eisenkop SM, Spirtos NM. Radiation fields in gynecologic oncology: correlation of soft tissue (surgical) to radiologic landmarks. Gynecologic Oncology. 2004;92(1):25–30. doi: 10.1016/j.ygyno.2003.09.008. [DOI] [PubMed] [Google Scholar]
- 16.Gerstner N, Wachter S, Knocke TH, Fellner C, Wambersie A, Pötter R. The benefit of Beam’s eye view based 3D treatment planning for cervical cancer. Radiotherapy and Oncology. 1999;51(1):71–78. doi: 10.1016/s0167-8140(99)00038-9. [DOI] [PubMed] [Google Scholar]
- 17.Olofsen-van Acht MJJ, Quint S, Seven M, et al. Three-dimensional treatment planning for postoperative radiotherapy in patients with node-positive cervical cancer. Comparison between a conventional and a conformal technique. Strahlentherapie und Onkologie. 1999;175(9):462–469. doi: 10.1007/s000660050037. [DOI] [PubMed] [Google Scholar]
- 18.Koper PCM, Stroom JC, van Putten WLJ, et al. Acute morbidity reduction using 3DCRT for prostate carcinoma: a randomized study. International Journal of Radiation Oncology Biology Physics. 1999;43(4):727–734. doi: 10.1016/s0360-3016(98)00406-4. [DOI] [PubMed] [Google Scholar]
- 19.Stock RG. Node-positive cervical cancer: impact of pelvic irradiation and patterns of failure. International Journal of Radiation Oncology Biology Physics. 1995;31(1):31–36. doi: 10.1016/0360-3016(94)00391-W. [DOI] [PubMed] [Google Scholar]
- 20.Suprasert P, Charoenkwan K, Khunamornpong S. Pelvic node removal and disease-free survival in cervical cancer patients treated with radical hysterectomy and pelvic lymphadenectomy. International Journal of Gynecology and Obstetrics. 2012;116(1):43–46. doi: 10.1016/j.ijgo.2011.08.001. [DOI] [PubMed] [Google Scholar]
- 21.Kim RY. Conventional four-field pelvic radiotherapy technique without CT treatment planning in cancer of the cervix: potential geographic miss. Radiotherapy and Oncology. 1994;30(2):140–145. doi: 10.1016/0167-8140(94)90043-4. [DOI] [PubMed] [Google Scholar]
- 22.Russell AH, Walter JP, Anderson MW, Zukowski CL. Sagittal magnetic resonance imaging in the design of lateral radiation treatment portals for patients with locally advanced squamous cancer of the cervix. International Journal of Radiation Oncology Biology Physics. 1992;23(2):449–455. doi: 10.1016/0360-3016(92)90767-c. [DOI] [PubMed] [Google Scholar]
- 23.Pendlebury SC, Cahill S, Crandon AJ, Bull CA. Role of bipedal lymphangiogram in radiation treatment planning for cervix cancer. International Journal of Radiation Oncology Biology Physics. 1993;27(4):959–962. doi: 10.1016/0360-3016(93)90474-a. [DOI] [PubMed] [Google Scholar]
- 24.Souhami L, Seymour R, Roman TN, et al. Weekly cisplatin plus external beam radiotherapy and high dose rate brachytherapy in patients with locally advanced carcinoma of the cervix. International Journal of Radiation Oncology Biology Physics. 1993;27(4):871–878. doi: 10.1016/0360-3016(93)90462-5. [DOI] [PubMed] [Google Scholar]
- 25.Keys HM, Bundy BN, Stehman FB, et al. Cisplatin, radiation, and adjuvant hysterectomy compared with radiation and adjuvant hysterectomy for bulky stage IB cervical carcinoma. New England Journal of Medicine. 1999;340(15):1154–1161. doi: 10.1056/NEJM199904153401503. [DOI] [PubMed] [Google Scholar]
- 26.Eifel PJ, Winter K, Morris M, et al. Pelvic irradiation with concurrent chemotherapy versus pelvic and para-aortic irradiation for high-risk cervical cancer: an update of radiation therapy oncology group trial (RTOG) 90-01. Journal of Clinical Oncology. 2004;22(5):872–880. doi: 10.1200/JCO.2004.07.197. [DOI] [PubMed] [Google Scholar]
- 27.Rotman M, Pajak TF, Choi K, et al. Prophylactic extended-field irradiation of para-aortic lymph nodes in stages IIB and bulky IB and IIA cervical carcinomas: ten-year treatment results of RTOG 79-20. The Journal of the American Medical Association. 1995;274(5):387–393. [PubMed] [Google Scholar]
- 28.Grigsby PW, Heydon K, Mutch DG, Kim RY, Eifel P. Long-term follow-up of RTOG 92-10: cervical cancer with positive para-aortic lymph nodes. International Journal of Radiation Oncology Biology Physics. 2001;51(4):982–987. doi: 10.1016/s0360-3016(01)01723-0. [DOI] [PubMed] [Google Scholar]
- 29.Ellis RE. The distribution of active bone marrow in the adult. Physics in Medicine and Biology. 1961;5(3, article 302):255–258. doi: 10.1088/0031-9155/5/3/302. [DOI] [PubMed] [Google Scholar]
- 30.Sood BM, Gorla GR, Garg M, et al. Extended-field radiotherapy and high-dose-rate brachytherapy in carcinoma of the uterine cervix: clinical experience with and without concomitant chemotherapy. Cancer. 2003;97(7):1781–1788. doi: 10.1002/cncr.11248. [DOI] [PubMed] [Google Scholar]
- 31.Hanks GE, Lee WR, Hanlon AL, et al. Conformal technique dose escalation for prostate cancer: biochemical evidence of improved cancer control with higher doses in patients with pretreatment prostate-specific antigen ≤10 ng/ml. International Journal of Radiation Oncology Biology Physics. 1996;35(5):861–868. doi: 10.1016/0360-3016(96)00207-6. [DOI] [PubMed] [Google Scholar]
- 32.Hanks GE, Schultheiss TE, Hunt MA, Epstein B. Factors influencing incidence of acute grade 2 morbidity in conformal and standard radiation treatment of prostate cancer. International Journal of Radiation Oncology Biology Physics. 1995;31(1):25–29. doi: 10.1016/0360-3016(94)00366-S. [DOI] [PubMed] [Google Scholar]
- 33.Michalski JM, Winter K, Purdy JA, et al. Toxicity after three-dimensional radiotherapy for prostate cancer with RTOG 9406 dose level IV. International Journal of Radiation Oncology Biology Physics. 2004;58(3):735–742. doi: 10.1016/S0360-3016(03)01578-5. [DOI] [PubMed] [Google Scholar]
- 34.Michalski JM, Winter K, Purdy JA, et al. Preliminary evaluation of low-grade toxicity with conformal radiation therapy for prostate cancer on RTOG 9406 dose levels I and II. International Journal of Radiation Oncology Biology Physics. 2003;56(1):192–198. doi: 10.1016/s0360-3016(03)00072-5. [DOI] [PubMed] [Google Scholar]
- 35.Tseng C-H. Mortality and causes of death in a national sample of diabetic patients in Taiwan. Diabetes Care. 2004;27(7):1605–1609. doi: 10.2337/diacare.27.7.1605. [DOI] [PubMed] [Google Scholar]
- 36.Hopkins MP, Morley GW. Stage 1B squamous cell cancer of the cervix: clinicopathologic features related to survival. American Journal of Obstetrics and Gynecology. 1991;164(6, part 1):1520–1529. doi: 10.1016/0002-9378(91)91431-u. [DOI] [PubMed] [Google Scholar]
- 37.Kapp DS, Fischer D, Gutierrez E. Pretreatment prognostic factors in carcinoma of the uterine cervix: a multivariable analysis of the effect of age, stage, histology and blood counts on survival. International Journal of Radiation Oncology Biology Physics. 1983;9(4):445–455. doi: 10.1016/0360-3016(83)90060-3. [DOI] [PubMed] [Google Scholar]


