Abstract
To assess gap junctional intercellular communication we have developed a tracer-based methodology which is both highly sensitive and potentially adaptable for in vivo measurements. We found that injection of serotonin revealed significantly more intercellular communication than that injection of the most permeant synthetic tracer currently in use, neurobiotin. Furthermore, mechanical tracer loading steps can be replaced by transfection with human serotonin transporter and the inclusion of serotonin in the medium. Tracer and transporter are detected using immunocytochemical techniques and the presence of cells that are tracer-positive but transporter-negative indicates junctional communication. Tracer loading in vivo using transgenesis, electroporation or viral transduction to direct expression of transporter should be more easily accomplished than with mechanical loading methods.
Keywords: gap junction, connexin, dye transfer, serotonin
Introduction
Intercellular communication through gap junctions plays important roles in coordinating physiological responses of cells to stimuli, maintaining cell and tissue homeostasis. Its importance is underscored by the variety of human diseases resulting from mutations in connexins, the family of 20–21 proteins that comprise gap junctional intercellular channels in vertebrates (Goodenough and Paul, 2009). While measurements of junctional communication in tissue culture cells are routine, studies of intercellular communication in vivo are severely constrained by the complexity of its assessment. Two broad approaches have been widely utilized to monitor communication. The first, and most widely used, employs optical methods to detect the cell-to-cell movement of molecules small enough to permeate intercellular channels, and the second employs electrophysiological techniques to directly or indirectly measure electrical conductance between cells.
All optical methods require the introduction of a tracer molecule into the cytoplasm of a donor cell and a mechanism to follow its movement into neighboring cells. The introduction of tracer has been accomplished by microelectrode injection (Kanno and Loewenstein, 1964), by scrape-loading (El-Fouly et al., 1987), by electroporation (Raptis et al., 1994) and by ballistic (gene gun) delivery (Bittman et al., 2004). Each of these methods has different strengths but all are invasive and involve some degree of damage to cells and tissues. Non-invasive loading can be accomplished with membrane-permeant dyes that undergo hydrolysis and become trapped in the cytosol. Fluorescence recovery after photobleaching (FRAP) can then be employed to assess junctional coupling (Wade et al., 1986). However, none of these methods are easily adapted to measurements in vivo.
In addition, tracer-based methods vary greatly in terms of sensitivity, in part because tracers which are optimal from one standpoint may be suboptimal from others. Lucifer yellow, for example, is a widely used tracer because it is highly fluorescent, membrane-impermeant and aldehyde-fixable. However, it is relatively large (443 Da) and therefore permeates gap junctions comprised of certain connexins poorly (Beltramello et al., 2003) or not at all (Teubner et al., 2000). Ultimately, the sensitivity will depend on how well the tracer can permeate gap junctions, the rate of tracer loss from cells by leakage, secretion or degradation and the signal-to-noise of the detection system. There are no tracer systems that are both sensitive and readily adaptable for in vivo applications.
We have developed a tracer-based methodology which is both highly sensitive and potentially adaptable for in vivo measurements. To increase sensitivity, we have employed a biological tracer, serotonin (187 Da), which is smaller than the most permeant synthetic tracer currently in use, neurobiotin (287Da). To bypass mechanical loading of tracer, cells are transfected with plasmid DNAs encoding the human serotonin transporter (Ramamoorthy et al., 1993). Tracer and transporter are detected using immunocytochemical techniques and the presence of cells that are tracer-positive but transporter-negative indicates junctional communication. Here, we show that serotonin is significantly more sensitive than neurobiotin for reporting junctional transfer in cultured cells. Furthermore, both tracers could be readily adapted for in vivo studies using conventional transgenesis, in vivo electroporation or adeno-associated viral transduction to facilitate cell-specific expression of transporter.
Materials and Methods
HeLa cell lines
Characterization of the parental and connexin-expressing HeLa cell lines has been previously presented (Magnotti et al., 2011). Cells were grown in high glucose Dulbecco’s Modified Eagle Media (DMEM; Invitrogen, Carlsbad, CA) with sodium pyruvate containing 10% FBS (HyClone, Logan, UT) and 1% penicillin/streptomycin (Invitrogen) unless otherwise indicated.
Inhibitors
100X stock solutions of carbenoxlone (40mM), meclofenamic acid (20mM) and flufenamic acid (50mM) (Sigma-Aldrich, St. Louis, MO) were made in water or DMSO (flufenamic acid). Inhibitors were added to culture medium and injection medium immediately before use and applied to the cells one hour before dye injection.
Dye Injection
Three days prior to the dye transfer assay, cells were plated on 35mm glass bottom Fluorodishes (World Precision Instruments, Sarasota, FL) and the medium was supplemented with 400 ng/µl unlabeled avidin (Sigma) to suppress background from endogenous biotinylated proteins. Cells were grown to confluence and transferred to a HEPES buffered solution containing 150 mM NaCl, 5 mM KCl, 2 mM CaCl2, 1 mM MgCl2, and 10 mM D-glucose, pH 7.4 immediately prior to injection. Neurobiotin (Vector Laboratories, Burlingame, CA) and serotonin or glycine (Sigma) were dissolved at 10% and 1mM respectively, along with 3% dextran-Cascade Blue, (MW=10,000; Invitrogen) in a HEPES buffered solution of 140 mM KCl, 2 mM MgCl2, 6 mM EGTA, and 5 mM CsCl. 100 MΩ pipettes were loaded with tracer solution and iontophoretic injection was carried out by applying alternating currents of −1 nA and +1 nA at 200 ms each for a total of 1 minute. Ten injections were performed per dish and the total time of the experiment never exceeded 15 minutes. A minimum of 3 dishes were used for each cell type for a total of 30 injections. Following injection, cells were fixed in 4% paraformaldehyde for 15 minutes and stored in PBS at 4°C. Data were presented as mean±SD and the significance of the difference between 5-HT and NB dye spreads was analyzed using the Student’s t-Test.
Plasmids and HeLa cell transfection
pcDNA3-hSERT plasmid was purchased from Addgene (plasmid 15483). The insert was released by EcoRV/XhoI digestion and subcloned into the AfeI and XhoI sites of pIRES2-Cerulean (Magnotti et al., 2011). For transfection, cells were cultured on 10mm coverslips in 24-well plates as described above except that 24 hrs before transfection, medium containing serum that had been dialyzed to remove 5-HT was introduced. HeLa cells were transfected with pIRES2-hSERT-Cerulean using Lipofectamine (Invitrogen, Carlsbad, CA) according to the manufacturer’s directions. 24 hours after transfection, HeLa cells were washed in PBS and incubated with 400 nM serotonin in DMEM for 20 minutes in incubator, washed in PBS 3 times for 5 minutes each at room temperature, fixed with 4% paraformaldehyde for 15 minutes at room temperature and stored in PBS at 4°C. For the experiment in which transfected cells were diluted with untransfected ones, cells were trypsinized 24 hours after transfection and counted, mixed with untransfected cells at a 1:10 ratio and seeded at 3×105 cells/well in a 24-well plate. The following day, incubation with 5-HT transfer as described above was performed.
Immunostaining
For NB injections, cells were blocked with 1% fish skin gelatin in PBS containing 0.2% Tween-20 for 30 minutes, and incubated in block solution typically containing NeutrAvidin-tetramethylrhodamine (1:1000; Invitrogen) and rabbit anti-serotonin antibody (1:1000; Sigma S5545) for 1 hour at room temperature. Cells were then washed in PBS 3 times for 10 minutes each, incubated with Alexa 488-labeled donkey anti-rabbit (1:500; Invitrogen) for 1hr and washed again. For transfections, cells were blocked with 10% donkey serum in PBS containing 0.2% Tween-20, and incubated for 1 hour at room temperature with mouse anti-serotonin transporter (1:2000; Millipore, MAB5618) and rabbit anti-serotonin (1:1000). After washing, cells were incubated for most experiments with Rhodamine-labeled donkey anti-mouse (1:1000; Millipore) and Alexa488- labeled donkey anti-rabbit (1:500, Invitrogen). In some experiments, fluorophores were altered and/or exchanged, to insure that there were no fluorophore-dependent effects on sensitivity. Cells were photographed using a Nikon TE2000 inverted microscope with epifluorescence optics and SPOT RT camera (Diagnostic Instr. CA).
Results
To qualitatively compare the sensitivity of glycine, serotonin (5-HT) and neurobiotin (NB) as indicators of junctional communication, we performed microinjections using a parental HeLa cell strain that consistently showed little or no neurobiotin transfer and non-clonal lines expressing Cx32 or Cx43 derived by lentiviral transduction (Magnotti et al., 2011). After injection, cells were aldehyde-fixed and neurobiotin was detected with fluorescently-labeled avidin while glycine and 5-HT were detected with commercially available antibodies. Control HeLa cells display an avidin-dependent signal due to biotin-containing compounds in mitochondria (Hollinshead et al., 1997) which was eliminated by adding unlabeled avidin to the culture medium 72hrs before injection (Magnotti et al., 2011).
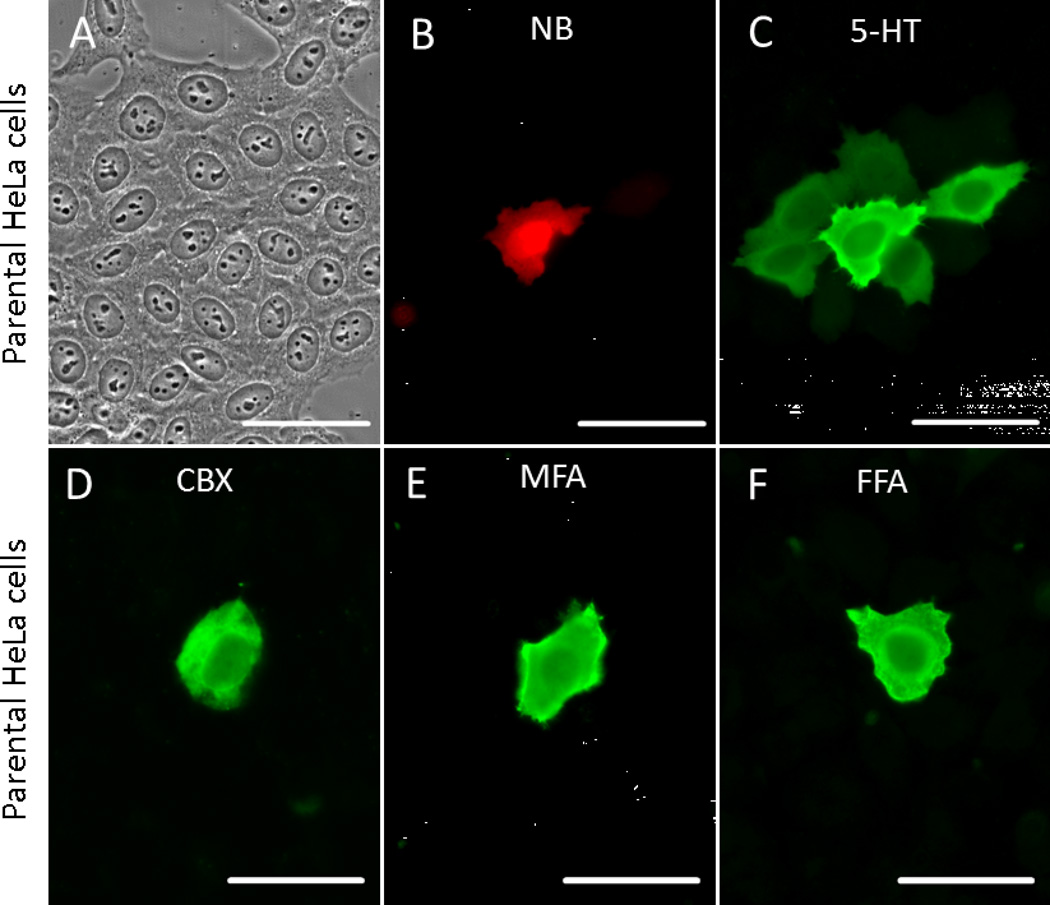
When NB and 5-HT were co-injected into parental HeLa cells, NB was rarely detected beyond the injected cell (Fig. 1B). However, spread of 5-HT into the first order neighboring cells was routinely observed (Fig. 1C) and signal in second order cells was occasionally observed (data not shown). On average, NB was detected in 1.21±0.31 per injection (N=40),. while serotonin was detected in 7.88±2.15 (N=40; P<0.001). Our HeLa strain was specifically selected for lack of communication by assessing NB transfer and thus 5-HT transfer was unexpected. This could reflect a very low level of endogenous connexin expression, insufficient to allow NB transfer. On the other hand, movement of 5-HT could occur by a non-junctional mechanism such as release and re-uptake by neighboring cells and/or the persistence of cytoplasmic bridges after cell division. However, addition of 1mM 5-HT to the medium did not result in detectable uptake (data not shown) and a role for cytoplasmic bridges is ruled out by the lack of movement of co-injected NB. Furthermore, the inclusion of gap junction blockers carbenoxolone (Goldberg et al., 1996), meclofenamic acid or flufenamic acid (Harks et al., 2001) in the culture medium and post-injection washes (Fig. 1D–F) restricted 5-HT to the injected cell, consistent with the hypothesis that junctional communication was responsible for 5-HT diffusion. Thus, injected 5-HT is clearly a more sensitive indicator of junctional communication in parental HeLa cells than injected NB.
Fig. 1. 5-HT is a more sensitive indicator of GJIC than NB or Glycine at low levels of coupling.
When NB (red) and 5-HT (green) were co-injected into the parental HeLa line, both were detected only in the injected cell (A–C). Spread of 5-HT into the first order neighboring cells was routinely observed (C). NB was rarely detected beyond the injected cell while serotonin was detected in many cells. The presence of gap junction blockers carbenoxolone (D), meclofenamic acid (E) or flufenamic acid (F) eliminate intercellular diffusion of 5-HT, supporting the notion that GJIC was responsible for 5-HT diffusion. Scale bars: 50 µm.
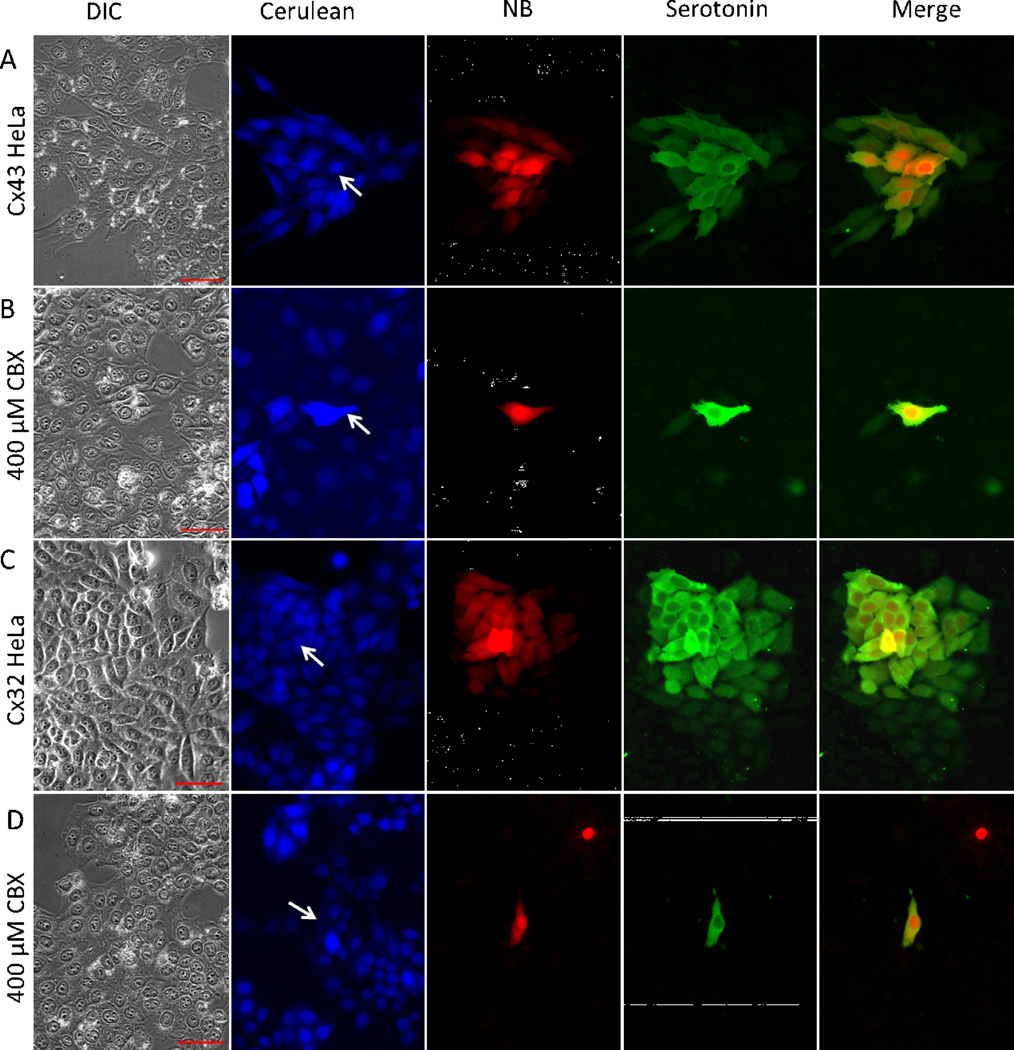
To evaluate the permeation of NB and 5-HT through gap junctions comprised of specific connexins, we produced HeLa cells expressing exogenous human Cx43 (alpha 1; GJA1) or rat Cx32 (beta 1; GJB1) using lentiviral transduction (Magnotti et al., 2011). Infected cells were marked by the fluorescent protein Cerulean (Fig.2). These cultures were not clonal and contained a low percentage of uninfected cells which provided internal negative controls. Transfer of NB and 5-HT was much more extensive in cells expressing exogenous connexins (Cx43 - Fig.2A; Cx32 - Fig. 2C) than in the parental cells. However, similar to parental cells, 5-HT transfer was more extensive than that of NB. 5-HT was detected in 40.74±14.65 (N=19) cells per injection while NB was seen in 27.95±7.25 (N=19; P<0.001). 5-HT was routinely detected in 3rd order cells while NB was routinely detected only in 2nd order cells. NB transfer was completely inhibited by the gap junction blockers carbenoxlone (Fig. 2B, 2D), meclofenamic acid (data not shown) and flufenamic acid (data not shown). 5-HT transfer was almost completely inhibited by carbenoxolone (Fig. 2B, 2D) while the other blockers produced significant but not complete inhibition (data not shown), consistent with the notion that 5-HT permeates gap junctions more efficiently than NB.
Fig. 2. 5-HT to permeates intracellular channels composed of Cx43 or Cx32 more efficiently than NB.
Expression of a fluorescent marker (Cerulean) and either human Cx43 or rat Cx32 in HeLa cells was induced using Lentiviral infection. Robust transfer of injected NB and 5-HT to adjacent Cerulean-positive cells (A, C) was observed but 5-HT was detected in significantly more cells than NB. NB transfer was completely inhibited by the gap junction blocker carbenoxlone while transfer of 5-HT was largely inhibited (B, D). Arrows point to the injected cell. Scale bars: 50 µm.
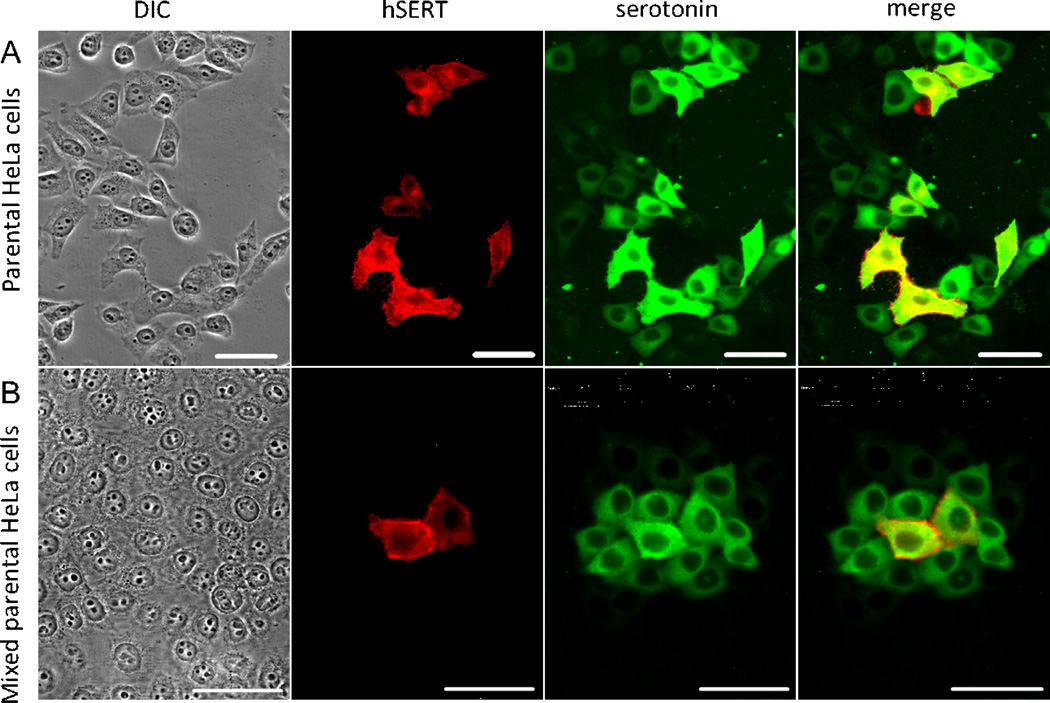
To explore the utility of serotonin as a non-invasive tracer, we replaced 5-HT microinjection with expression of the human serotonin transporter (hSERT). Transiently transfected cells were incubated with 400 nM serotonin for 20 minutes, aldehyde fixed and co-immunostained for 5-HT and hSERT. All cells positive for hSERT (red) were also positive for 5-HT (green, Fig. 3A), indicating that the transporter was functional. In addition, serotonin was detected in a transporter-negative adjacent cell population (Fig 3A-serotonin). The transporterPage negative adjacent cells likely received 5-HT via junctional transfer from their transporter-positive neighbor.
Fig. 3. Intracellular 5-HT induced by transfection of a serotonin transporter can function as an indicator of GJIC.
Cells transfected with hSERT were briefly incubated with 400nM 5-HT, washed, fixed and co-immunostained for 5-HT and hSERT (A). All cells positive for hSERT (red) were also positive for 5-HT (green) indicating that the transporter was functional. In addition, 5-HT was detected in a substantial number of hSERT-negative cells, potentially indicating GJIC. However, to address the possibility this results from undetectably low levels of hSERT we reseeded the transfected cells with untransfected ones at 1:10 ratio then performed the 5-HT uptake assay (B). Under these conditions, 5-HT-positive, hSERT-negative cells were generally found in distinct, isolated groups with one or two hSERT-positive cells at their center. Thus, transfected hSERT can increase intracellular 5-HT which can then function as a gap junction-permeant tracer. Scale bars: 50 µm.
It remained possible that some of the 5-HT-positive neighboring cells expressed transporter at a low level not detectable with our antibody. To address this possibility, we reseeded the transfected cells with untransfected ones at 1:10 ratio then incubated with 400 nM serotonin as before. Under these conditions, the majority of cells were untransfected and the presence of 5-HT in hSERT-negative cells surrounding an hSERT-positive one would be most easily explained by gap junctional transfer. Under these conditions, 5-HT-positive, hSERT-negative cells were generally found in distinct, isolated groups with one or two hSERT-positive cells at their center (Fig.3B). Thus, transfected hSERT appears to be a reliable way to increase intracellular 5-HT which can then function as a gap junction-permeant tracer.
Discussion
Here we show that 5-HT offers two important advantages over NB as an indicator of gap junctional communication. First, it is significantly more sensitive, most likely because it is smaller and can diffuse more readily through intercellular channels. Second, it can be introduced to specific cells using genetic methods rather than requiring invasive mechanical methods that are difficult to implement in vivo.
While we have not yet used 5-HT as an in vivo tracer, a serendipitous proof of principle involving glycine is provided by the retina. A unique characteristic of certain cone bipolar cells is that they accumulate glycine despite the absence of a functional glycine transporter (Cohen and Sterling, 1986; Vaney et al., 1998). Since transporter-positive amacrine cells often form gap junctions with ON cone bipolars, it was suggested that glycine could diffuse through the junctions, increasing cone bipolar glycine levels. In support of this idea, it was found that cone bipolar accumulation of glycine was eliminated by treatment with carbenoxolone (Vaney and Weiler, 2000) and by genetic ablation of the connexin expressed by the glycinergic AII amacrine (Deans et al., 2002).
The utility of glycine as a tracer may be limited by its presence in most if not all cells, reducing the signal-to-noise ratio. In contrast, 5-HT in mammals is found primarily in enterochromaffin cells of the gastrointestinal tract and in blood platelets. It is also found in a relatively small subset of central neurons found mainly in brainstem raphe nuclei, although projections to other regions are extensive (Dahlstrom and Fuxe, 1964). Thus, 5-HT could be useful for assessments of junctional communication throughout the body, even in many parts of the CNS. For example, there are no serotoninergic neurons in the retina and only a very small number of cells that express a transporter (Vaney, 1986; Wassle and Chun, 1988). In addition, the affinity of hSERT for its substrate is very high; ~300 times higher than the GLTY1B glycine transporter (Smith et al., 1992) and in the retina, 5-HT loading of cells with endogenous transporter can be accomplished quickly in vivo by intraocular injection (Sandell and Masland, 1986).
In vivo uses of this technique will be limited by the specific physiological properties of the cells in question. For example, it has been reported that junctional coupling in developing rat somatosensory cortex is inhibited by 5-HT (Rorig and Sutor, 1996). However, that study used 5-HT at ~100X higher concentrations than ours. Furthermore, 5-HT concentration in our assay can be reduced 10-fold and similar sensitivity is obtained if the incubation period is extended to 1 hr (data not shown). Thus, it remains possible that lower concentrations of tracer might be used to explore coupling in the developing cortex. 5-HT has also been reported to affect coupling in cultured neonatal, although not adult, rat atrial myocytes (Derangeon et al., 2010). However, in this case the effect was a modest increase in coupling not likely to significantly affect interpretation of the assay. The utility of the assay may also be affected by 5-HT in serum where it is present at 0.5 to 17 µM (Veenstra-VanderWeele et al., 2012) which would prevent temporal control over the introduction of tracer. However, in a situation where transporter expression is restricted to a particular cell type and the goal is to ascertain which other cell types might be coupled to them, the presence of serotonin in the extracellular milieu might simplify the assay because the introduction of exogenous tracer is not necessary. In organs/tissues where vascular permeability is low (i.e. CNS), presence of serotonin in serum would not be relevant.
In summary, 5-HT offers significant advantages over NB for assessment of junctional communication. Like NB, it is aldehyde fixable and is readily detected using fluorescently-labeled secondary reagents. However, it is superior to NB because it permeates intercellular channels of at least two different connexins more efficiently, providing a higher sensitivity and because cell loading can be accomplished in a non-invasive fashion, in theory facilitating in vivo analyses of communication that are otherwise not feasible.
Serotonin has significant advantages over neurobiotin as an indicator of gap junctional intercellular communication.
Serotonin is more sensitive than neurobiotin in microinjection studies.
Serotonin loading can be accomplished in a non-invasive manner by transfection with cDNA encoding a transporter
Acknowledgments
This work was supported by NIH RO1 GM37751 and EY014127 to DLP.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Beltramello M, Bicego M, Piazza V, Ciubotaru CD, Mammano F, D'Andrea P. Permeability and gating properties of human connexins 26 and 30 expressed in HeLa cells. Biochem. Biophys. Res Commun. 2003;305:1024–1033. doi: 10.1016/s0006-291x(03)00868-4. [DOI] [PubMed] [Google Scholar]
- Bittman KS, Panzer JA, Balice-Gordon RJ. Patterns of cell-cell coupling in embryonic spinal cord studied via ballistic delivery of gap-junction-permeable dyes. J. Comp Neurol. 2004;477:273–285. doi: 10.1002/cne.20253. [DOI] [PubMed] [Google Scholar]
- Cohen E, Sterling P. Accumulation of (3H)glycine by cone bipolar neurons in the cat retina. Journal of Comparative Neurology. 1986;250:1–7. doi: 10.1002/cne.902500102. [DOI] [PubMed] [Google Scholar]
- Dahlstrom A, Fuxe K. Localization of monoamines in the lower brain stem. Experientia. 1964;20:398–399. doi: 10.1007/BF02147990. [DOI] [PubMed] [Google Scholar]
- Deans MR, Volgyi B, Goodenough DA, Bloomfield SA, Paul DL. Connexin36 is essential for transmission of rod-mediated visual signals in the Mammalian retina. Neuron. 2002;36:703–712. doi: 10.1016/s0896-6273(02)01046-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Derangeon M, Bozon V, Defamie N, Peineau N, Bourmeyster N, Sarrouilhe D, Argibay JA, Herve JC. 5-HT4 and 5-HT2 receptors antagonistically influence gap junctional coupling between rat auricular myocytes. Journal of molecular and cellular cardiology. 2010;48:220–229. doi: 10.1016/j.yjmcc.2009.07.005. [DOI] [PubMed] [Google Scholar]
- El-Fouly MH, Trosko JE, Chang CC. Scrape-loading and dye transfer. A rapid and simple technique to study gap junctional intercellular communication. Experimental Cell Research. 1987;168:422–430. doi: 10.1016/0014-4827(87)90014-0. [DOI] [PubMed] [Google Scholar]
- Goldberg GS, Moreno AP, Bechberger JF, Hearn SS, Shivers RR, Macphee DJ, Zhang YC, Naus CCG. Evidence that disruption of connexon particle arrangements in gap junction plaques is associated with inhibition of gap junctional communication by a glycyrrhetinic acid derivative. Experimental Cell Research. 1996;222:48–53. doi: 10.1006/excr.1996.0006. [DOI] [PubMed] [Google Scholar]
- Goodenough DA, Paul DL. Gap junctions. Cold Spring Harbor Perspect. Biol. 2009;1:a002576. doi: 10.1101/cshperspect.a002576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harks EG, de Roos AD, Peters PH, de Haan LH, Brouwer A, Ypey DL, van Zoelen EJ, Theuvenet AP. Fenamates: a novel class of reversible gap junction blockers. J. Pharmacol. Exp. Ther. 2001;298:1033–1041. [PubMed] [Google Scholar]
- Hollinshead M, Sanderson J, Vaux DJ. Anti-biotin antibodies offer superior organelle-specific labeling of mitochondria over avidin or streptavidin. Journal of Histochemistry & Cytochemistry. 1997;45:1053–1057. doi: 10.1177/002215549704500803. [DOI] [PubMed] [Google Scholar]
- Kanno Y, Loewenstein WR. Intercellular Diffusion. Science. 1964;143:959–960. doi: 10.1126/science.143.3609.959. [DOI] [PubMed] [Google Scholar]
- Magnotti LM, Goodenough DA, Paul DL. Functional heterotypic interactions between astrocyte and oligodendrocyte connexins. Glia. 2011;59:26–34. doi: 10.1002/glia.21073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ramamoorthy S, Bauman AL, Moore KR, Han H, Yang-Feng T, Chang AS, Ganapathy V, Blakely RD. Antidepressant- and cocaine-sensitive human serotonin transporter: molecular cloning, expression, and chromosomal localization. Proc Natl Acad Sci U S A. 1993;90:2542–2546. doi: 10.1073/pnas.90.6.2542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raptis LH, Brownell HL, Firth KL, Mackenzie LW. A novel technique for the study of intercellular, junctional communication: electroporation of adherent cells on a partly conductive slide. DNA and Cell Biology. 1994;13:963–975. doi: 10.1089/dna.1994.13.963. [DOI] [PubMed] [Google Scholar]
- Rorig B, Sutor B. Serotonin regulates gap junction coupling in the developing rat somatosensory cortex. European Journal of Neuroscience. 1996;8:1685–1695. doi: 10.1111/j.1460-9568.1996.tb01312.x. [DOI] [PubMed] [Google Scholar]
- Sandell JH, Masland RH. A system of indoleamine-accumulating neurons in the rabbit retina. Journal of Neuroscience. 1986;6:3331–3347. doi: 10.1523/JNEUROSCI.06-11-03331.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith KE, Borden LA, Hartig PR, Branchek T, Weinshank RL. Cloning and expression of a glycine transporter reveal colocalization with NMDA receptors. Neuron. 1992;8:927–935. doi: 10.1016/0896-6273(92)90207-t. [DOI] [PubMed] [Google Scholar]
- Teubner B, Degen J, Sohl G, Guldenagel M, Bukauskas FF, Trexler EB, Verselis VK, De Zeeuw CI, Lee CG, Kozak CA, Petrasch-Parwez E, Dermietzel R, Willecke K. Functional Expression of the Murine Connexin 36 Gene Coding for a Neuron-Specific Gap Junctional Protein. Journal of Membrane Biology. 2000;176:249–262. doi: 10.1007/s00232001094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vaney DI. Morphological identification of serotonin-accumulating neurons in the living retina. Science (Wash.) 1986;233:444–446. doi: 10.1126/science.3726538. [DOI] [PubMed] [Google Scholar]
- Vaney DI, Nelson JC, Pow DV. Neurotransmitter Coupling through Gap Junctions in the Retina. Journal of Neuroscience. 1998;18:10594–10602. doi: 10.1523/JNEUROSCI.18-24-10594.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vaney DI, Weiler R. Gap junctions in the eye: evidence for heteromeric, heterotypic and mixed-homotypic interactions. Brain Res. Brain Res. Rev. 2000;32:115–120. doi: 10.1016/s0165-0173(99)00070-3. [DOI] [PubMed] [Google Scholar]
- Veenstra-VanderWeele J, Muller CL, Iwamoto H, Sauer JE, Owens WA, Shah CR, Cohen J, Mannangatti P, Jessen T, Thompson BJ, Ye R, Kerr TM, Carneiro AM, Crawley JN, Sanders-Bush E, McMahon DG, Ramamoorthy S, Daws LC, Sutcliffe JS, Blakely RD. Autism gene variant causes hyperserotonemia, serotonin receptor hypersensitivity, social impairment and repetitive behavior. Proc Natl Acad Sci U S A. 2012;109:5469–5474. doi: 10.1073/pnas.1112345109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wade MH, Trosko JE, Schindler M. A fluorescence photobleaching assay of gap junction-mediated communication between human cells. Science. 1986;232:525–528. doi: 10.1126/science.3961495. [DOI] [PubMed] [Google Scholar]
- Wassle H, Chun MH. Dopaminergic and indoleamine-accumulating amacrine cells express GABA-like immunoreactivity in the cat retina. Journal of Neuroscience. 1988;8:3383–3394. doi: 10.1523/JNEUROSCI.08-09-03383.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]