Abstract
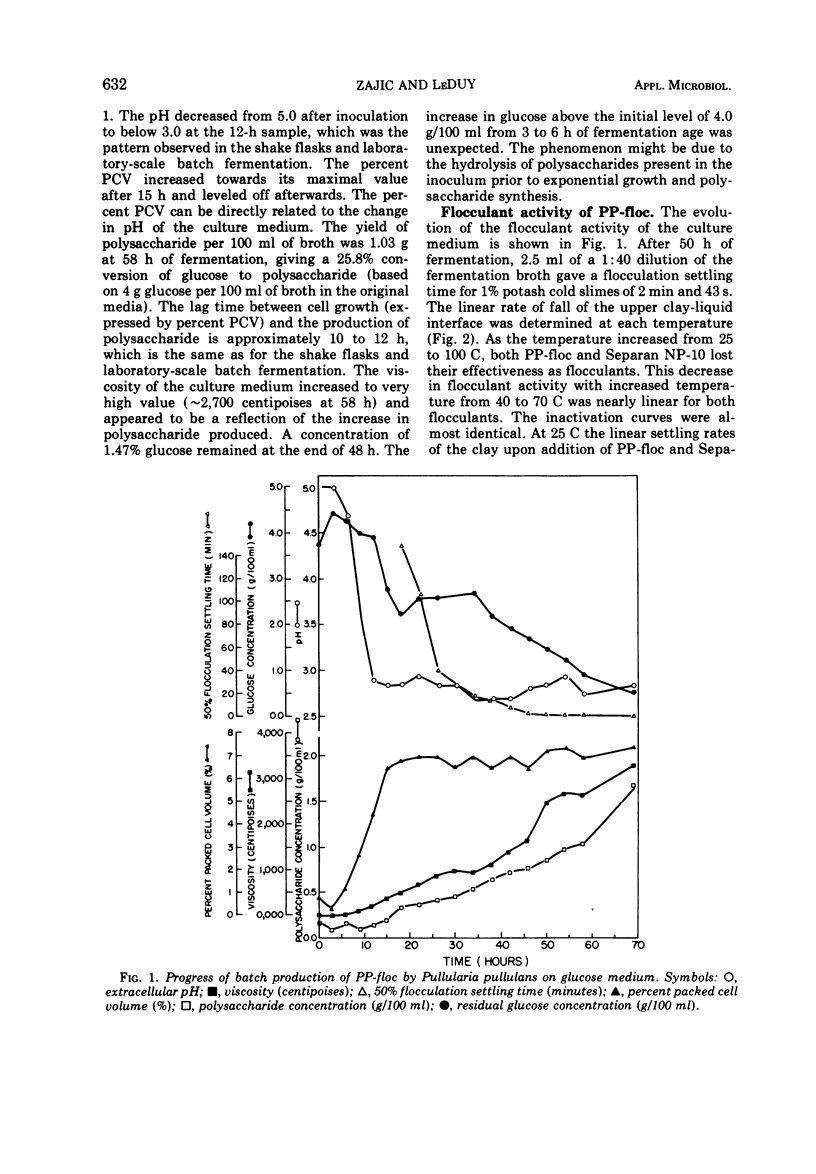
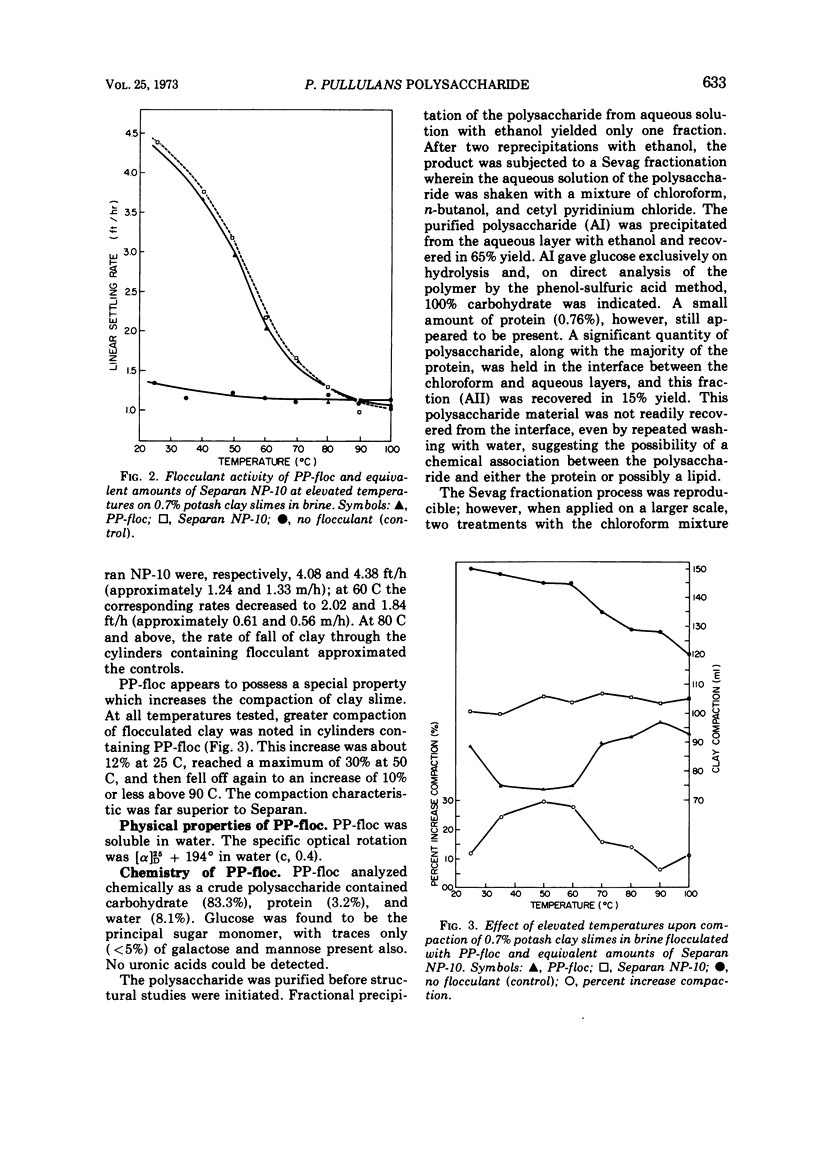
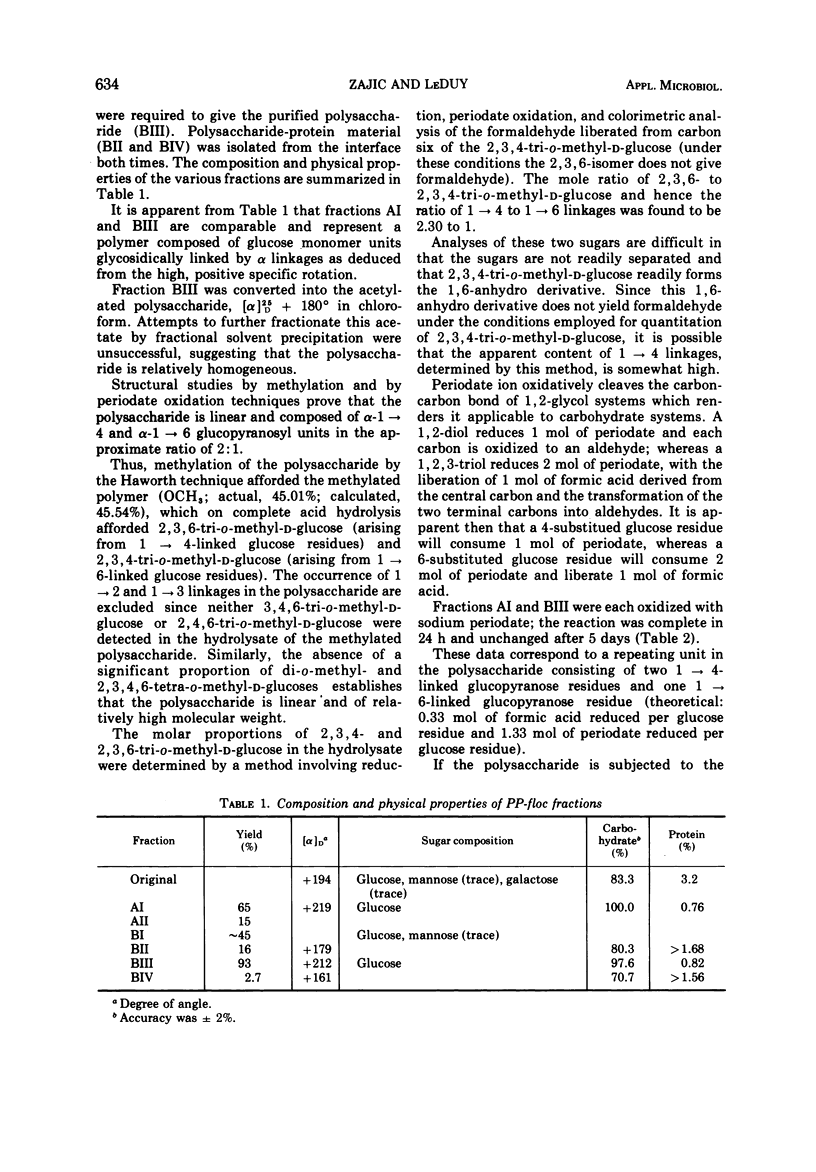
An extracellular polysaccharide, PP-floc, was synthesized from glucose by Pullularia pullulans (or Aureobasidium pullulans) in a pilot plant batch fermentor containing 175 liters of culture medium. At 58 h of fermentation, the concentration of PP-floc was 1.03 g/100 ml, giving a 25.8% conversion of initial glucose to polysaccharide. The flocculant activity of the culture medium increased during the fermentation process and reached its maximum at 50 h of culture age. Less PP-floc (0.33 lb/ton of slimes [approximately 149.7 g/0.907 t]) was required to give the same flocculant activity as a synthetic polymer of acrylamide, Separan NP-10 (0.5 lb/ton of slimes [approximately 226.8 g/0.907 t]), at all temperatures from 25 to 100 C. The degree of inactivation of PP-floc and Separan NP-10 at elevated temperatures was almost identical, and they were completely inactivated at about the same temperature (80 C). PP-floc also gave better compaction of slimes than Separan NP-10 at all temperatures tested. PP-floc was soluble in water and its specific optical rotation was [α]D25 + 194° in water (c, 0.4). PP-floc contained 83.3% carbohydrate, 3.2% protein, and 8.1% water. Glucose was found to be the principal sugar monomer with traces (>5%) of galactose and mannose present. Structural studies on the fractions of purified polysaccharide by methylation and by periodate oxidation techniques prove that PP-floc is linear and composed of α-(1 → 4) and α-(1 → 6) glucopyranosyl units in the approximate ratio of 2:1. The action of pullulanase on crude PP-floc suggested the ordered arrangement of two consecutive α-(1 → 4)-linked glucopyranosyl units flanked by α-(1 → 6)-linked glucopyranose residues.
Full text
PDF




Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- BENDER H., LEHMANN J., WALLENFELS K. [Pullulan, an extracellular glucan from Pullularia pullulans]. Biochim Biophys Acta. 1959 Dec;36:309–316. doi: 10.1016/0006-3002(59)90172-6. [DOI] [PubMed] [Google Scholar]
- BERNIER B. The production of polysaccharides by fungi active in the decomposition of wood and forest litter. Can J Microbiol. 1958 Jun;4(3):195–204. doi: 10.1139/m58-020. [DOI] [PubMed] [Google Scholar]
- Catley B. J. Role of pH and nitrogen limitation in the elaboration of the extracellular polysaccharide pullulan by Pullularia pullulans. Appl Microbiol. 1971 Oct;22(4):650–654. doi: 10.1128/am.22.4.650-654.1971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Catley B. J. Utilization of carbon sources by Pullularia pullulans for the elaboration of extracellular polysaccharides. Appl Microbiol. 1971 Oct;22(4):641–649. doi: 10.1128/am.22.4.641-649.1971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morris D. L. Quantitative Determination of Carbohydrates With Dreywood's Anthrone Reagent. Science. 1948 Mar 5;107(2775):254–255. doi: 10.1126/science.107.2775.254. [DOI] [PubMed] [Google Scholar]


