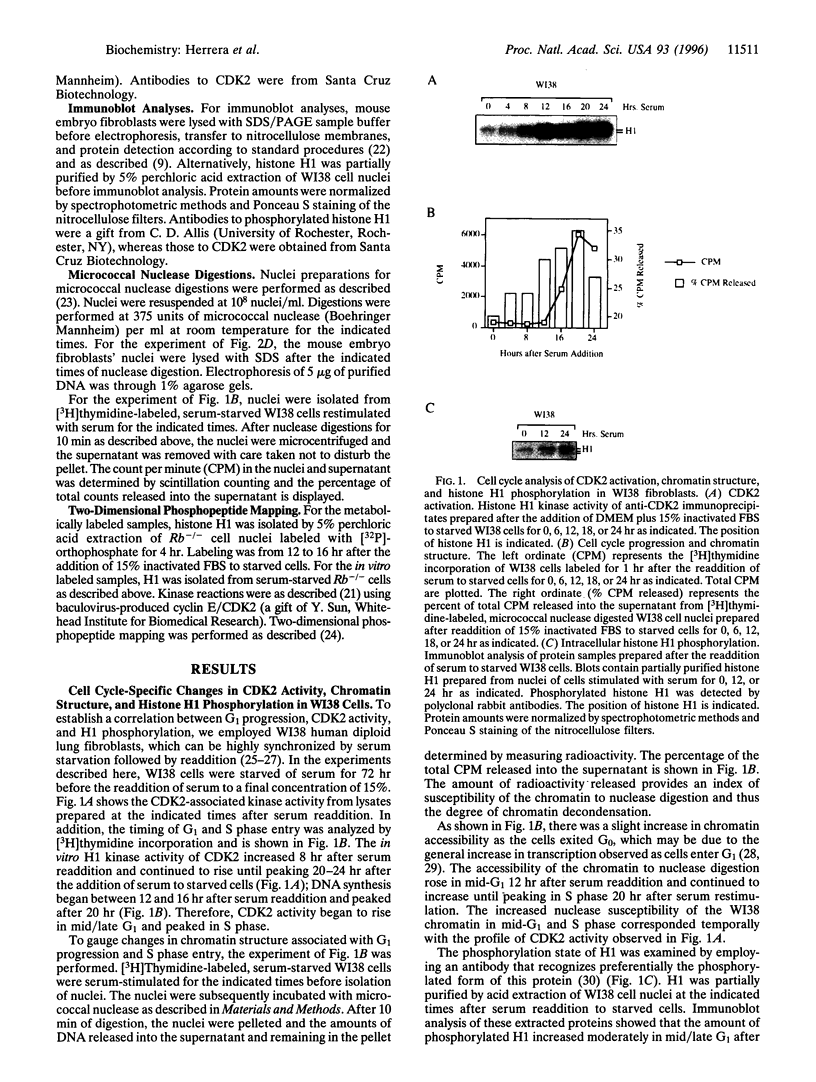
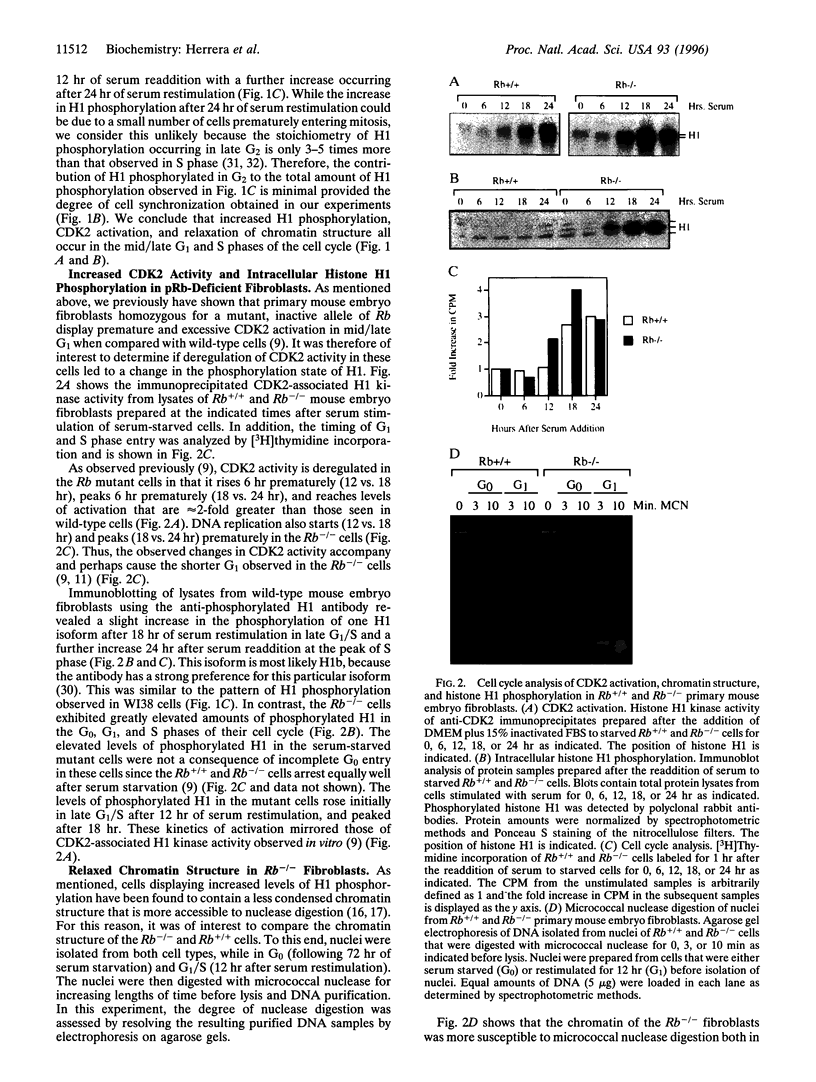
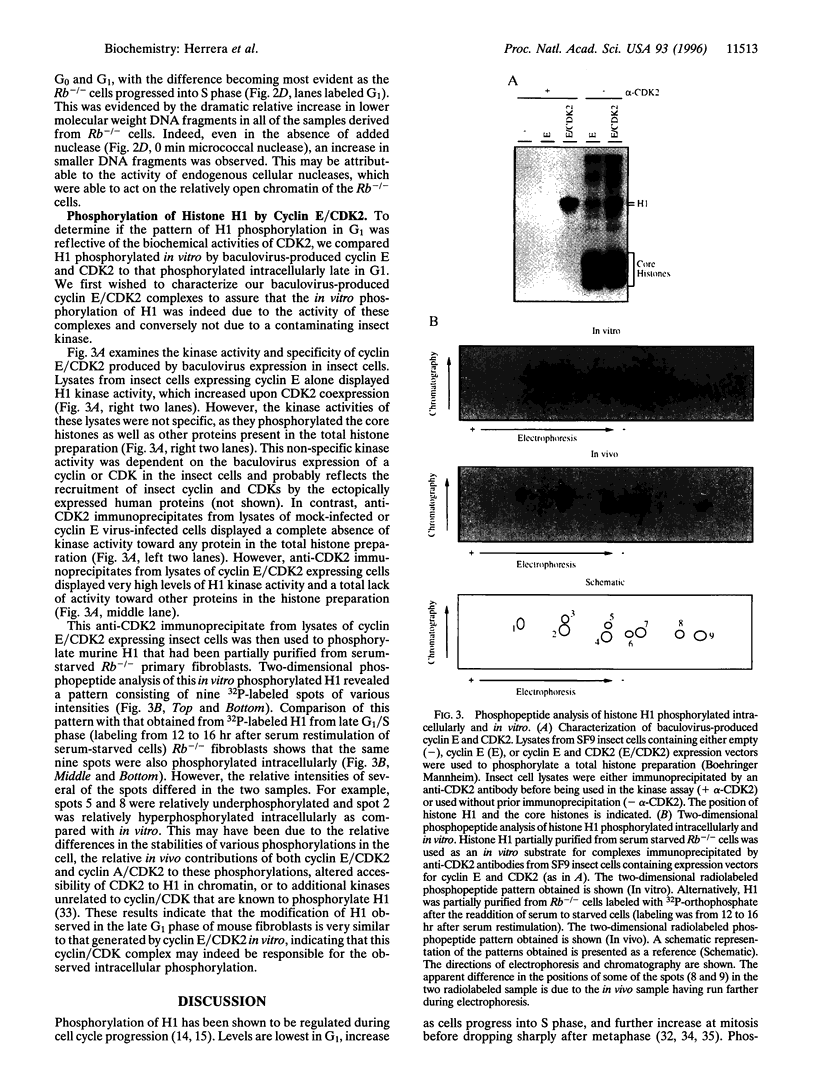
Abstract
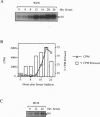
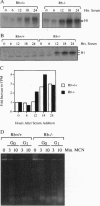
Fibroblasts derived from embryos homozygous for a disruption of the retinoblastoma gene (Rb) exhibit a shorter G1 than their wild-type counterparts, apparently due to highly elevated levels of cyclin E protein and deregulated cyclin-dependent kinase 2 (CDK2) activity. Here we demonstrate that the Rb-/- fibroblasts display higher levels of phosphorylated H1 throughout G1 with the maximum being 10-fold higher than that of the Rb+/+ fibroblasts. This profile of intracellular H1 phosphorylation corresponds with deregulated CDK2 activity observed in in vitro assays, suggesting that CDK2 may be directly responsible for the in vivo phosphorylation of H1. H1 phosphorylation has been proposed to lead to a relaxation of chromatin structure due to a decreased affinity of this protein for chromatin after phosphorylation. In accord with this, chromatin from the Rb-/- cells is more susceptible to micrococcal nuclease digestion than that from Rb+/+ fibroblasts. Increased H1 phosphorylation and relaxed chromatin structure have also been observed in cells expressing several oncogenes, suggesting a common mechanism in oncogene and tumor suppressor gene function.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Ajiro K., Borun T. W., Cohen L. H. Phosphorylation states of different histone 1 subtypes and their relationship to chromatin functions during the HeLa S-3 cell cycle. Biochemistry. 1981 Mar 17;20(6):1445–1454. doi: 10.1021/bi00509a007. [DOI] [PubMed] [Google Scholar]
- Ajiro K., Borun T. W., Shulman S. D., McFadden G. M., Cohen L. H. Comparison of the structures of human histone 1A and 1B and their intramolecular phosphorylation sites during the HeLa S-3 cell cycle. Biochemistry. 1981 Mar 17;20(6):1454–1464. doi: 10.1021/bi00509a008. [DOI] [PubMed] [Google Scholar]
- Bradbury E. M., Inglis R. J., Matthews H. R. Control of cell division by very lysine rich histone (F1) phosphorylation. Nature. 1974 Feb 1;247(5439):257–261. doi: 10.1038/247257a0. [DOI] [PubMed] [Google Scholar]
- Bradbury E. M. Reversible histone modifications and the chromosome cell cycle. Bioessays. 1992 Jan;14(1):9–16. doi: 10.1002/bies.950140103. [DOI] [PubMed] [Google Scholar]
- Chadee D. N., Taylor W. R., Hurta R. A., Allis C. D., Wright J. A., Davie J. R. Increased phosphorylation of histone H1 in mouse fibroblasts transformed with oncogenes or constitutively active mitogen-activated protein kinase kinase. J Biol Chem. 1995 Aug 25;270(34):20098–20105. doi: 10.1074/jbc.270.34.20098. [DOI] [PubMed] [Google Scholar]
- Chen F., Weinberg R. A. Biochemical evidence for the autophosphorylation and transphosphorylation of transforming growth factor beta receptor kinases. Proc Natl Acad Sci U S A. 1995 Feb 28;92(5):1565–1569. doi: 10.1073/pnas.92.5.1565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coppock D. L., Kopman C., Scandalis S., Gilleran S. Preferential gene expression in quiescent human lung fibroblasts. Cell Growth Differ. 1993 Jun;4(6):483–493. [PubMed] [Google Scholar]
- DeGregori J., Leone G., Ohtani K., Miron A., Nevins J. R. E2F-1 accumulation bypasses a G1 arrest resulting from the inhibition of G1 cyclin-dependent kinase activity. Genes Dev. 1995 Dec 1;9(23):2873–2887. doi: 10.1101/gad.9.23.2873. [DOI] [PubMed] [Google Scholar]
- Ewen M. E., Sluss H. K., Sherr C. J., Matsushime H., Kato J., Livingston D. M. Functional interactions of the retinoblastoma protein with mammalian D-type cyclins. Cell. 1993 May 7;73(3):487–497. doi: 10.1016/0092-8674(93)90136-e. [DOI] [PubMed] [Google Scholar]
- Geng Y., Eaton E. N., Picón M., Roberts J. M., Lundberg A. S., Gifford A., Sardet C., Weinberg R. A. Regulation of cyclin E transcription by E2Fs and retinoblastoma protein. Oncogene. 1996 Mar 21;12(6):1173–1180. [PubMed] [Google Scholar]
- Gope M. L., Chun M., Gope R. Comparative study of the expression of Rb and p53 genes in human colorectal cancers, colon carcinoma cell lines and synchronized human fibroblasts. Mol Cell Biochem. 1991 Sep 18;107(1):55–63. doi: 10.1007/BF02424576. [DOI] [PubMed] [Google Scholar]
- Gurley L. R., Walters R. A., Tobey R. A. Sequential phsophorylation of histone subfractions in the Chinese hamster cell cycle. J Biol Chem. 1975 May 25;250(10):3936–3944. [PubMed] [Google Scholar]
- Halmer L., Gruss C. Effects of cell cycle dependent histone H1 phosphorylation on chromatin structure and chromatin replication. Nucleic Acids Res. 1996 Apr 15;24(8):1420–1427. doi: 10.1093/nar/24.8.1420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herrera R. E., Sah V. P., Williams B. O., Mäkelä T. P., Weinberg R. A., Jacks T. Altered cell cycle kinetics, gene expression, and G1 restriction point regulation in Rb-deficient fibroblasts. Mol Cell Biol. 1996 May;16(5):2402–2407. doi: 10.1128/mcb.16.5.2402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hinds P. W., Mittnacht S., Dulic V., Arnold A., Reed S. I., Weinberg R. A. Regulation of retinoblastoma protein functions by ectopic expression of human cyclins. Cell. 1992 Sep 18;70(6):993–1006. doi: 10.1016/0092-8674(92)90249-c. [DOI] [PubMed] [Google Scholar]
- Jacks T., Fazeli A., Schmitt E. M., Bronson R. T., Goodell M. A., Weinberg R. A. Effects of an Rb mutation in the mouse. Nature. 1992 Sep 24;359(6393):295–300. doi: 10.1038/359295a0. [DOI] [PubMed] [Google Scholar]
- Kaplan L. J., Bauer R., Morrison E., Langan T. A., Fasman G. D. The structure of chromatin reconstituted with phosphorylated H1. Circular dichroism and thermal denaturation studies. J Biol Chem. 1984 Jul 25;259(14):8777–8785. [PubMed] [Google Scholar]
- Kato J., Matsushime H., Hiebert S. W., Ewen M. E., Sherr C. J. Direct binding of cyclin D to the retinoblastoma gene product (pRb) and pRb phosphorylation by the cyclin D-dependent kinase CDK4. Genes Dev. 1993 Mar;7(3):331–342. doi: 10.1101/gad.7.3.331. [DOI] [PubMed] [Google Scholar]
- Koff A., Giordano A., Desai D., Yamashita K., Harper J. W., Elledge S., Nishimoto T., Morgan D. O., Franza B. R., Roberts J. M. Formation and activation of a cyclin E-cdk2 complex during the G1 phase of the human cell cycle. Science. 1992 Sep 18;257(5077):1689–1694. doi: 10.1126/science.1388288. [DOI] [PubMed] [Google Scholar]
- Koh J., Enders G. H., Dynlacht B. D., Harlow E. Tumour-derived p16 alleles encoding proteins defective in cell-cycle inhibition. Nature. 1995 Jun 8;375(6531):506–510. doi: 10.1038/375506a0. [DOI] [PubMed] [Google Scholar]
- Laitinen J., Sistonen L., Alitalo K., Hölttä E. Cell transformation by c-Ha-rasVal12 oncogene is accompanied by a decrease in histone H1 zero and an increase in nucleosomal repeat length. J Cell Biochem. 1995 Jan;57(1):1–11. doi: 10.1002/jcb.240570102. [DOI] [PubMed] [Google Scholar]
- Laitinen J., Sistonen L., Alitalo K., Hölttä E. c-Ha-rasVal 12 oncogene-transformed NIH-3T3 fibroblasts display more decondensed nucleosomal organization than normal fibroblasts. J Cell Biol. 1990 Jul;111(1):9–17. doi: 10.1083/jcb.111.1.9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Langan T. A., Gautier J., Lohka M., Hollingsworth R., Moreno S., Nurse P., Maller J., Sclafani R. A. Mammalian growth-associated H1 histone kinase: a homolog of cdc2+/CDC28 protein kinases controlling mitotic entry in yeast and frog cells. Mol Cell Biol. 1989 Sep;9(9):3860–3868. doi: 10.1128/mcb.9.9.3860. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lu M. J., Dadd C. A., Mizzen C. A., Perry C. A., McLachlan D. R., Annunziato A. T., Allis C. D. Generation and characterization of novel antibodies highly selective for phosphorylated linker histone H1 in Tetrahymena and HeLa cells. Chromosoma. 1994 Apr;103(2):111–121. doi: 10.1007/BF00352320. [DOI] [PubMed] [Google Scholar]
- Lu M. J., Mpoke S. S., Dadd C. A., Allis C. D. Phosphorylated and dephosphorylated linker histone H1 reside in distinct chromatin domains in Tetrahymena macronuclei. Mol Biol Cell. 1995 Aug;6(8):1077–1087. doi: 10.1091/mbc.6.8.1077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lukas J., Bartkova J., Rohde M., Strauss M., Bartek J. Cyclin D1 is dispensable for G1 control in retinoblastoma gene-deficient cells independently of cdk4 activity. Mol Cell Biol. 1995 May;15(5):2600–2611. doi: 10.1128/mcb.15.5.2600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lukas J., Parry D., Aagaard L., Mann D. J., Bartkova J., Strauss M., Peters G., Bartek J. Retinoblastoma-protein-dependent cell-cycle inhibition by the tumour suppressor p16. Nature. 1995 Jun 8;375(6531):503–506. doi: 10.1038/375503a0. [DOI] [PubMed] [Google Scholar]
- Matsushime H., Quelle D. E., Shurtleff S. A., Shibuya M., Sherr C. J., Kato J. Y. D-type cyclin-dependent kinase activity in mammalian cells. Mol Cell Biol. 1994 Mar;14(3):2066–2076. doi: 10.1128/mcb.14.3.2066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Medema R. H., Herrera R. E., Lam F., Weinberg R. A. Growth suppression by p16ink4 requires functional retinoblastoma protein. Proc Natl Acad Sci U S A. 1995 Jul 3;92(14):6289–6293. doi: 10.1073/pnas.92.14.6289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mäkelä T. P., Tassan J. P., Nigg E. A., Frutiger S., Hughes G. J., Weinberg R. A. A cyclin associated with the CDK-activating kinase MO15. Nature. 1994 Sep 15;371(6494):254–257. doi: 10.1038/371254a0. [DOI] [PubMed] [Google Scholar]
- Norbury C., Nurse P. Animal cell cycles and their control. Annu Rev Biochem. 1992;61:441–470. doi: 10.1146/annurev.bi.61.070192.002301. [DOI] [PubMed] [Google Scholar]
- Reik A., Schütz G., Stewart A. F. Glucocorticoids are required for establishment and maintenance of an alteration in chromatin structure: induction leads to a reversible disruption of nucleosomes over an enhancer. EMBO J. 1991 Sep;10(9):2569–2576. doi: 10.1002/j.1460-2075.1991.tb07797.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roth S. Y., Allis C. D. Chromatin condensation: does histone H1 dephosphorylation play a role? Trends Biochem Sci. 1992 Mar;17(3):93–98. doi: 10.1016/0968-0004(92)90243-3. [DOI] [PubMed] [Google Scholar]
- Taylor W. R., Chadee D. N., Allis C. D., Wright J. A., Davie J. R. Fibroblasts transformed by combinations of ras, myc and mutant p53 exhibit increased phosphorylation of histone H1 that is independent of metastatic potential. FEBS Lett. 1995 Dec 11;377(1):51–53. doi: 10.1016/0014-5793(95)01314-8. [DOI] [PubMed] [Google Scholar]
- Tsai L. H., Lees E., Faha B., Harlow E., Riabowol K. The cdk2 kinase is required for the G1-to-S transition in mammalian cells. Oncogene. 1993 Jun;8(6):1593–1602. [PubMed] [Google Scholar]
- Tupper J. T., Kaufman L., Bodine P. V. Related effects of calcium and serum on the G1 phase of the human W138 fibroblast. J Cell Physiol. 1980 Jul;104(1):97–103. doi: 10.1002/jcp.1041040113. [DOI] [PubMed] [Google Scholar]
- Weinberg R. A. The retinoblastoma protein and cell cycle control. Cell. 1995 May 5;81(3):323–330. doi: 10.1016/0092-8674(95)90385-2. [DOI] [PubMed] [Google Scholar]