Abstract
Cell death is regulated by a myriad of intracellular molecular pathways, with many involving protein phosphorylation and dephosphorylation. In this review, we will focus on Ser/Thr phosphatases-mediated regulation in cell apoptosis as well as on their potential roles in cell necrosis. The emerging functional importance of Ser/Thr protein phosphatases in cell death regulation adds new dimension to the signaling mechanisms of cellular function, physiology, and diseases.
Protein phosphorylation/dephosphorylation plays a central role in regulating protein functions and thus virtually every aspect of cellular physiology. The dynamic and reversible process of protein phosphorylation is executed by protein kinases and protein phosphatases. Kinases add a phosphate group to proteins, and phosphatases remove it. In eukaryotes, protein phosphorylation typically occurs on three amino acid residues: serine, threonine, and tyrosine. There are 518 putative protein kinases forming the kinome encoded by human genome (80). Of them, 85 are catalytically active protein tyrosine kinases matched with 81 counteracting protein tyrosine phosphatases (2). For the 428 Ser/Thr protein kinases annotated in human genome, only a handful of Ser/Thr phosphatase (STP) catalytic subunits are identified. However, it is believed that, comparable with the complexity of the kinome, the function of STPs is mostly determined by a large number of regulatory subunits (35). Tyrosine phosphorylation is distinct from serine and threonine phosphorylation in many aspects and has been reviewed extensively in other articles and thus will not be part of the discussion here (50, 51, 96, 112). The general information on the function, structure, and regulatory mechanisms of STPs also has been summarized recently in detail (107, 123). Readers are suggested to find relevant information from those excellent reviews. The focus of this article is on the regulatory functions of STPs in cell death.
Cell death plays a critical role in development, maintenance of tissue homeostasis, and disease initiation and progression. To date, three major types of cell death have been characterized: apoptosis, necrosis, and autophagy, although the latter is still being debated. Apoptosis, used to be referred to as programmed cell death, is the most extensively studied mode of cell death. Apoptosis is an energy-dependent process associated with characteristic morphological changes including cell blebbing, shrinkage, chromatin condensation, and DNA fragmentation. Numerous proteins have been identified in this process, and many of them are regulated by phosphorylation. Correspondingly, many protein phosphatases have been tightly linked with regulation of apoptosis (79). Necrosis generally is not energy dependent and is not associated with characteristic apoptotic morphology. Necrotic cells lose the integrity of plasma membrane at very early stage accompanied with dilated organelles. Necrotic cell death has long been considered as nonprogrammed. But in the past several years, the notion that necrosis is also under the control of a highly regulated molecular network has emerged, and several key players have been identified, including proteins subjected to protein phosphorylation (82, 120). Autophagy is generally viewed as an adaptive response to stress that promotes cell survival under most circumstances. However, uncontrolled autophagy has been linked to cell death and contributes to disease development (69). The major focus of this review will be the involvement of STPs in cell death regulation and how STPs regulate the survival/death signaling and the executioners of apoptosis (FIGURE 1). The role of STPs in necrosis regulation has not been established and will be presented as our perspectives. Finally, we will outline the major challenges and some future directions in STP studies in the context of cell death regulation. Due to space limitations, many reports are not discussed if they are already cited in other review articles.
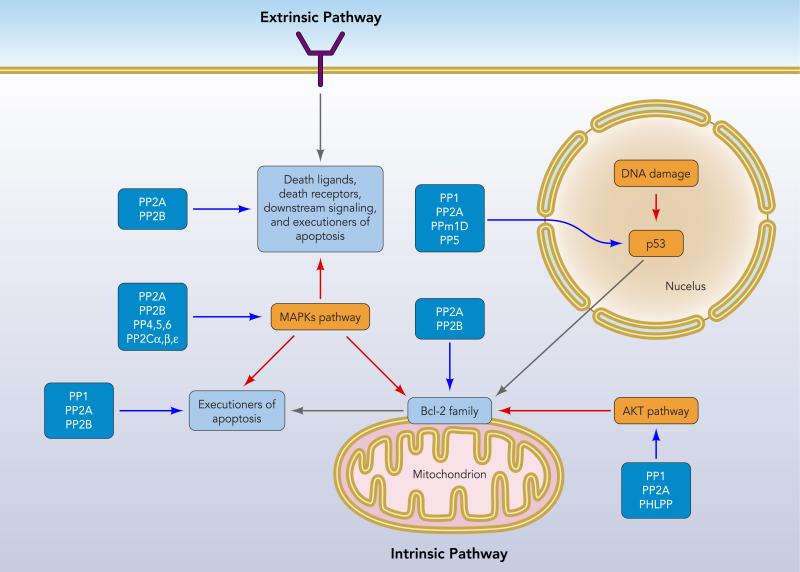
FIGURE 1. Protein Ser/Thr phosphatases implicated in both intrinsic and extrinsic apoptotic pathways.
The protein phosphatases are listed in dark blue boxes. Blue arrows indicate direct targets of protein phosphatases, and red arrows indicate indirect targets via MAP kinases and AKT and p53 pathways.
STPs Superfamily and Cell Death Regulation
Based on structural features, STP superfamily can be classified into three categories: phosphoprotein phosphatases (PPPs), metal-dependent protein phosphatases (PPM), and aspartate-based phosphatases (also called FCP/SCP, for TFIIF-associating component of RNA polymerase II CTD phosphatase or small CTD phosphatase) (107). The PPP family comprises several subfamilies, including PP1, PP2A, PP2B, PP4, PP5, PP6, and PP7. There are only a handful of catalytic core subunits in this family. However, a large number of regulatory subunits in combination with the catalytic cores constitute the functional diversity and specificity of the PPP holoenzymes. Unlike PPPs, PPM family members are monomeric in general. At least 18 members of PPM family are encoded by mammalian genome (77). In addition to a conserved catalytic domain, many PPM enzymes have evolved special domains with substantial structural and sequence variance (77). The functional roles of PPPs in cell death regulation have been well documented. To date, most studies focused on PP2A and PP2B, and only a few studies directly implicate PP1, PP4, PP5, PP6, and PP7 members in cell death.
PP2A in Cell Death Regulation
PP2A is the most abundant STP and accounts for the majority of STP activities measured in cells (20). PP2A is a heterotrimeric complex consisted of catalytic (C), scaffolding (A), and regulatory subunit (B). In mammals, catalytic and scaffolding subunits are produced by two genes (α and β, respectively), whereas a handful of genes have been suggested to encode the regulatory subunits that determine the substrate specificity and intracellular targeting.
PP2A has demonstrated both pro-apoptotic and pro-survival characteristics (41). For example, downregulation of PP2A scaffold subunit Aα by siRNA decreased PP2A activity and triggered apoptotic cell death (110). But truncated form of the Epstein-Barr virus protein EBNA-LP inhibited PP2A and protected cells from apoptosis (39). In addition, it has been shown that chemical inhibitors of PP2A suppressed intrinsic apoptosis (18), whereas protein inhibitor of PP2A suppressed death receptor-mediated apoptosis (48). Okadaic acid, a selective PPPs inhibitor and a well known tumor promoter (111), induced apoptosis in mammalian cells (10).
It remains elusive whether the discrepancy of PP2A's pro-survival and pro-apoptotic functions was due to different experimental settings including but not limited to, for example, different concentrations or nonspecific targeting of okadaic acid (29, 34). Nevertheless, one possibility is that PP2A regulates a diverse array of substrates that either promotes or inhibits cell death. Thus direct inhibition of PP2A catalytic activity might interfere with various physiological processes, resulting in cell survival or death under different conditions. To further understand the regulatory mechanism of PP2A on cell death, recent research has focused on specific PP2A complexes. Tsao et al. reported that activation of a PP2A/B56α complex was required for ischemia-induced apoptotic and necrotic death in kidney epithelial proximal tubule cells. Overexpression of PP2A B56α but not B55 enhanced ischemia-induced cell death (119). In a different report, human adenovirus E4orf4 protein was found to reduce B55-specific PP2A activity, leading to cell cycle arrest and death (71). B56ε (PPP2R5E), another B56 family member of PP2A regulatory subunit, was shown to have both anti- and pro-apoptotic functions by suppressing p53-independent apoptosis while triggering p53-dependent apoptosis (59). These findings suggest that specific PP2A complexes exert different regulatory functions on cell death.
PP2B/Calcineurin in Cell Death Regulation
PP2B/calcineurin has been linked with cell death regulation directly, but whether it is pro- or anti-cell death remains controversial. Some studies showed that calcineurin mediated cell death induced by calcium, excitotoxicity, and hormones in lymphocyte, cardiomyocyte, and neural cells (6, 58, 81, 102, 108, 118, 124, 125, 127). In addition, calcineurin Aα overexpressing transgenic mice showed increased apoptosis following ischemia/reperfusion injury in heart (22). However, other results suggested a protective function of calcineurin in cell death regulation. For example, PP2B protected T cells from glucocorticoid-induced apoptosis (130). Chemical inhibitors of PP2B canceled the inhibitory effect of ET-1 on apoptosis induced by oxidative stress (61). More importantly, calcineurin Aβ ablation predisposed cardiomyocytes to ischemia-induced apoptosis in heart (16), and constitutively active PP2B protected against serum starvation and hydrogen peroxide-induced apoptosis in neonatal cardiomyocytes (13). Therefore, although studies unequivocally pointed out the implication of PP2B in cell death regulation, its exact functions may depend on the nature of the stimuli and specific complex involved.
PP2C in Cell Death Regulation
PPM (represented by PP2C here) subfamily members have been linked with cell death directly. PP2Cα and PP2Cβ can be activated by oleic acid, an unsaturated fatty acid, and siRNA knockdown of these phosphatases protected cells from fatty acid-induced apoptosis (105). PP2Cε and PP2Cδ/ILKAP regulated TNF-α, hydrogen peroxide, and integrin-induced cell death (114). PP2Cm is encoded by PPm1κ gene and resident in mitochondria matrix. PP2Cm was essential for cell survival through regulation of mitochondrial permeability transition pore (75). The role of other PP2C members in cell death has remained elusive.
Other STPs in Cell Death Regulation
It has been shown that inhibition of PP1 with either chemical inhibitor or overexpression of PP1 inhibitor-1 gene prevented apoptosis of tumor cells and cardiomyocytes, respectively (18, 90). PP4c overexpression enhanced and downregulation of PP4c suppressed apoptosis in HEK293T cell and T cell (87, 88). PP5 demonstrates a broad diversity of substrates and regulates multiple signaling pathways with some of them involved in stress response (42, 52). It has been shown that overexpression of PP5 inhibited rapamycin-induced apoptosis in p53 mutant Rh30 cells (57). There is no clear evidence linking PP7/PPEF (protein phosphatases with EF-hand domains) with cell death yet (5). Members of the FCP/SCP family are capable of dephosphorylating the carboxyl terminal domain of RNA polymerase II (62). No other substrate of FCP/SCP has been revealed (107). To date, no link between FCP/SCP and cell death has been reported.
Signaling Mechanism in STPs Mediated Cell Death Regulation
Many intracellular signaling pathways are involved in cell death/survival regulation. Protein phosphorylation/dephosphorylation plays a critical role in the transduction of signals to ultimately decide the fate of cells. In the current context, we will focus on three pathways with extensive STP implication: AKT, MAPK, and DNA damage response pathways, with details summarized in Table 1.
Table 1.
List of protein Ser/Thr phosphatases (STPs) involved in cell death regulation and their reported targets
| STPs | Target | Phosphorylation Residue | Reference |
|---|---|---|---|
| PP1 | AKT | Thr450 | 126 |
| Caspase-9 | Thr125 | 25 | |
| Cofilin | 92 | ||
| PP1cγ1 | JNK1 | 117 | |
| p53 | 117 | ||
| PP2A | Caspase-9 | Thr125 | 1 |
| Cofilin | Ser3 | 92 | |
| Bcl-2 | Ser70 | 24 | |
| Bax | 40 | ||
| Bim | 128 | ||
| AKT | Ser473/Thr308 | 128 | |
| AKT | Ser473 | 74 | |
| p53 | Ser46 | 84 | |
| ERK | 53 | ||
| p38 MAPK | Thr-180/Tyr-182 | 3 | |
| Caspase-3 | Ser150 | 3 | |
| p38 MAPK | Thr-180/Tyr-182 | 12 | |
| ASK-1 | Ser976 | 85 | |
| PP2A/α4 | MEK3 | Thr193 | 95 |
| p53 | Ser18 | 65 | |
| Jun | Ser63 | 65 | |
| PP2A/PR55 | c-SRC | Ser12 | 27 |
| PP2A/B56γ | CREB | Ser-108/111/114 and Ser-121 | 106 |
| PP2A/B56γ | ATF1 | Ser-47/50/51 | 106 |
| PP2A/PR65β | DAPK | Ser308 | 43 |
| PP2B/Calcineurin | Caspase-3 | 100 | |
| Bad | 124 | ||
| ARC | Thr149 | 115 | |
| ASK-1 | Ser967 | 73 | |
| PP4 | NFκB | Thr43 | 21 |
| JNK | 21 | ||
| PP5 | ASK-1 | Thr845 | 67 |
| ASK-1 | 131 | ||
| p53BP1 | Ser25/Ser1778 | 63 | |
| PP6 | TAK1 | 14 | |
| PP2Cα | p38 MAPK | 113 | |
| JNK | 113 | ||
| PP2Cβ | p38 MAPK | 46 | |
| JNK | 46 | ||
| TAK1 | 47 | ||
| PP2Cε | ASK1 | Thr845 | 101 |
| TAK1 | 70 | ||
| PP2CA/B56β | AKT | Thr308 | 93 |
| PHLPP | AKT | Ser473 | 38 |
| PKCβII | Ser660/Thr641 | 37 | |
| PHLPP isoforms | AKT isoforms | Ser473/Thr308 | 15 |
| PPm1D/Wip | p53 | Ser15 | 78 |
AKT is a protein serine/threonine kinase and plays important roles in the transduction of signals of growth factors and other extracellular stimuli to regulate cell growth, survival, and death (109). AKT is phosphorylated at multiple sites including serine 473 and threonine 308 to be fully activated. AKT activation promotes cell survival. Several STPs have been reported to negatively regulate AKT pathway. It has been shown that PP1 dephosphorylates AKT and regulates cell survival (126). AKT dephosphorylation by PP2A contributed to 4-hydroxynonenal-induced apoptosis (74). In addition, mammalian PP2A/B56β regulated AKT phosphorylation at threonine 308 site (93). PPM subfamily members PHLPP dephosphorylated AKT serine 473 in AKT and promotes apoptosis (37, 38), whereas further characterization showed that PHLPP isoforms targeted specific AKT isoforms (15).
Mitogen-activated protein kinase (MAPK) cascades consist of four major branches: p38, JNK, ERK, and ERK5 pathways (99). JNK and p38 MAPK pathways are mostly involved in stress signal transduction with implication in cell death regulation. The activation of MAPKs is mediated through three layers of a phosphorylation relay mechanism. Meanwhile, the activity of some components can be inhibited through phosphorylation. Therefore, protein dephosphorylation by phosphatases can regulate MAPK pathways both positively and negatively. Numerous STPs have been shown to regulate p38 and JNK pathways to modulate cell death. PP1cγ1 overexpression promoted VSMC survival by interfering with JNK1-mediated apoptosis (117). Dephosphorylation of p38 MAPK by PP2A was reported to be essential to trigger apoptosis in neutrophils (3) and coordinately regulated T-cell survival (12). PP2A and a “B” subunit PR55γ dephosphorylated c-SRC to negatively regulate JNK MAPK pathway and cell survival (27). PP2A dephosphorylated ASK1 at Ser976, which facilitated JNK activation in response to TNF (85). In addition, PP2A, through regulatory subunit α4, regulated MEK3/p38 pathway-mediated cell death induced by cytokine (95) and transcription-dependent apoptosis through Jun (65). PP2B/calcineurin affected cardiomyocyte cell death through modulation of ASK1 activity (73). Other PPPs implicated in MAPK-mediated apoptosis include PP4 for JNK (21), PP5 for ASK1 (67, 131), and PP6 for transforming growth factor beta-activated kinase 1 (TAK1) kinase as part of tumor necrosis factor (TNF), interleukin 1, and Toll-like receptor signaling pathways (14). Finally, PPM subfamily members, including PP2Cα, PP2Cβ, and PP2Cε, were reported to regulate p38 and JNK MAPK pathways (46, 47, 70, 101, 113), although not all of them were directly implicated in apoptosis.
DNA damage occurs when cells are insulted by genotoxins such as ultraviolet radiation (UV), reactive oxygen species, ionizing radiation, and some chemotherapy drugs. Protein phosphatases involved in DNA damage response have been reviewed recently (33, 94), and details will not be discussed here. One of the key proteins determining the survival or death outcome of DNA damage response is p53. p53 and its partners are regulated by intricate phosphorylation events. It has been shown that PP1cγ1 overexpression enhanced cell survival by interfering with p53 phosphorylation (117). In addition, PP2A regulated transcription-dependent apoptosis through regulation of p53 (65) and dephosphorylated p53 at Ser46 site to regulate DNA-damage response (84). Furthermore, PPm1D/Wip dephosphorylated p53 at Ser15 in DNA-damage response (78), and PP5 regulated DNA-damage response through p53 binding protein 1 (63). Therefore, dephosphorylation of p53 at different sites by STPs provides a major contribution to p53 function and cell death during DNA-damage response.
STPs Mediated Regulation on Apoptosis Executioner Machinery
Both intrinsic and extrinsic pathways have been extensively characterized for apoptosis. In extrinsic pathway, ligand binding to death receptor activates caspase-8 or -10, which leads to cleavage and activation of either effector caspase-3 or Bid, an initiator of intrinsic pathway. In intrinsic apoptosis pathway, the BH3-only proteins of the Bcl-2 family, including Bid, Bim, and Bad, are activated by stimuli such as UV, DNA damage, oxidative stress, and other apoptotic injury. Activation of BH3-only proteins triggers oligomerization of pro-apoptotic Bcl-2 family members such as Bax and Bak. The resulting pores formed by these oligomers in outer membrane of mitochondria causes leakage of protein factors (such as cytochrome c) from the mitochondrial intermembrane space into cytosol. An apoptosome consisting of cytochrome c, Apaf-1, and caspase-9 will be formed to activate caspase-9. Active caspase-9 then cleaves and activates caspase-3 to initiate apoptosis. Pro-survival Bcl-2 family members such as Bcl-2 and Bcl-xl suppress the apoptotic process by sequestering proapoptotic Bcl-2 family members on mitochondrial or SR and block the leakage of mitochondrial outer membrane. These components comprise the execution machinery of apoptosis.
It has been well recognized that the execution machinery of apoptosis is tightly regulated by protein phosphorylation. Apoptotic caspase-2, -3, -8, and -9 are targets of tyrosine and serine/threonine kinases (64, 66). Bcl-2 family members, such as Bcl-2, Bad, Bax, and Bcl-xl, are all phosphoproteins as well (64). Phosphorylation of these components is either pro-apoptotic or pro-survival, thus providing a regulatory mechanism on cell death by integrating both environmental and intracellular signals. Accordingly, protein phosphatases may contribute to apoptosis regulation by dephosphorylating these executioners.
STPs can regulate cell death through modifying the molecular components of the apoptosis executioners as summarized in Table 1. First of all, STPs can regulate caspases through a variety of approaches. PP1α dephosphorylated Thr125 site of caspase-9 and activated caspase-9 to mediate IL-2 deprivation-induced apoptosis (25). In another experimental setting, ERK-mediated phosphorylation of caspase-9 Thr125 site was shown to be sensitive to okadaic acid, indicating the involvement of PP2A (1). Dephosphorylation of caspase-3 at Ser150 site by PP2A increased caspase-3 activity, which was essential to trigger apoptosis in neutrophils (3). In addition, it has been shown that PP2B interacted with procaspase-3 and promoted caspase-3 maturation (100). On the other hand, protein phosphatases can be cleaved and regulated by caspases (66). For example, PP1 protein inhibitor-3 was a target of caspase-3, and its degradation contributed to apoptosis (56). Regulatory subunit Aα of PP2A was another substrate of caspase-3 (103). During neuroexcitotoxicity, PP2B was cleaved and activated by calpain to mediate cell death (125).
Bcl-2 family members are also targeted by STPs. Activation of PP2A by either C2-ceramide treatment or PP2A catalytic subunit overexpression inhibited Bcl-2 Ser70 phosphorylation, which enhanced p53/Bcl-2 interaction and apoptotic cell death (24). PP2A also dephosphorylated Bax and regulated Bax translocation to mitochondria in verotoxin-induced apoptosis (40). Furthermore, instead of dephosphorylation, PP2A contributed to amyloid-β-peptide-induced cerebrovascular endothelial cell death by regulating Bim gene expression (128). Finally, BAD dephosphorylation by PP2B has been reported to contribute to Ca2+-induced apoptosis (58, 124). In addition to caspase and Bcl-2 family members, Thr149 of apoptosis repressor with caspase recruitment domain (ARC) was dephosphorylated by PP2B to regulate apoptosis in heart (115). Moreover, dephosphorylation of cofilin by PP1 and PP2A at Ser3 site mediated the tumor-suppressing properties of ALDH1L1 in promoting apoptosis (92). Therefore, STPs can target different components of the executioners and regulators to modulate cell apoptosis under a variety of pathophysiological conditions.
Potential STPs Mediated Regulation on Necrosis
Necrosis has been considered a form of accidental and uncontrollable cell death for decades, yet accumulating evidence in the past several years has revealed that, like apoptosis, necrosis is also a highly regulated process involving specific molecular networks (121). The notion of “programmed necrosis” was raised by Chan et al. in 2003 (17), and, since then, both protein and non-protein players such as reactive oxygen species and calcium have been implicated in this process (32, 86). More than 40 protein components of the molecular machinery for one form of death receptor-induced necrosis (necropotosis) (23) have been proposed, and many of them are represented by specific protein families (121).
The involvement of specific molecular pathways in necrosis regulation raises the possibility that protein phosphorylation might also play a role in the necrotic cell death. In fact, two key regulator of necrosis so far identified are protein kinases: receptor-interacting protein 1 and 3 (RIP1 and RIP3; also known as RIPK1 and RIPK3) (31, 36, 120). RIP3 likely undergoes autophosphorylation at Ser199 and regulates RIP1 phosphorylation at Ser161 (19, 49). It remains unclear whether RIP1 and RIP3 can phosphorylate other proteins that may be important players of necropotosis. Nevertheless, the known phosphorylation events are critical for RIP1 and RIP3 to form necrosome to initiate necrosis. Therefore, it is intriguing to find out whether any STP(s) regulates these phosphorylation events. Clearly, the dephosphorylation of RIP1 and RIP3 and potentially other RIP substrates should play critical roles in the necropotosis regulation.
Recent studies elucidated an intriguing cross talk between apoptosis and necropotosis, suggesting that apoptosis could suppress necrosis under physiological conditions (60, 91, 129). Caspase-8 appeared to serve as the mediator of this cross talk. Caspase-8 cleaved both RIPK1 and RIPK3, inhibiting caspase-independent cell death (30, 72). It has been reported that p38 MAPK phosphorylated caspase-8 at Ser364 (4). Whether protein phosphatase plays a role in this cross talk remains unknown.
In addition to death receptor-regulated necrosis, ischemia/reperfusion or reactive oxygen species also induces necrosis (32, 121, 122). Although the targets remain elusive, at least two STPs have been linked with ischemia-/reperfusion-induced necrosis. Overexpression of PP1 inhibitor-1 gene protected cardiomyocyte from ischemia-/reperfusion-induced necrotic cell death (90). It was also reported that knockdown of PP2A scaffold subunit decreased PP2A activity and triggered necrotic and apoptotic cell death (110). On the other hand, activation of PP2A/B56α was shown to be required for ischemia-induced apoptotic and necrotic death in kidney epithelial proximal tubule cells (119).
During severe ischemia or postischemia/reperfusion injury, ATP depletion due to the loss of mitochondrial inner membrane potential is likely an initiating factor of necrotic cell death (122). The mitochondrial permeability transition pore (MPTP) modulates the integrity of mitochondrial inner membrane, and thus plays a central role in necrosis (7, 8, 122, 132). The functional consequence of MPTP opening as a nonspecific pore on mitochondrial inner membrane is clearly described (45). Under severe and prolonged stress, MPTP opening will cause leakage of mitochondrial inner membrane to molecules of <1.5 kDa, including protons, leading to diminished electrochemical gradient across the inner membrane and abolished ATP production and necrosis.
Although MPTP is known as a key regulator of cell death, its molecular components remain to be fully identified. Several proteins, including adenine nucleotide translocase (ANT), voltage-dependent anion channel (VDAC), phosphate carrier (PiC), and cyclophilin D, have been suggested to be the pore-forming components or regulator of MPTP opening, although the roles of some of them are still controversial (45). ANT, VDAC, and cyclophilin D are all phosphoproteins (97, 132). Several protein kinases, including hexokinase, PKCε, GSK3β, and ERK, transduce signals to MPTP with or without known targets (98). To date, only one STP, PP2Cm, has been linked with MPTP opening regulation (75). However, the mechanistic link between PP2Cm-mediated MPTP regulation and its known target of branched chain keto acid dehydrogenase (BCKD) (76) remains to be determined.
Clearly, limited information is available about the role of STPs in the regulation of necrotic cell death. Nevertheless, protein phosphorylation has been unequivocally linked with necrosis process, and it would not be surprising to find more involvement of STPs in necrosis from future studies.
Challenges and Perspectives
Cell death plays a critical role in physiology and pathology. Many diseases are associated with dys-regulation of cell death. Preventing cell loss during ischemia/reperfusion, infection, and neurodegeneration or promoting cell death in cancer has been the primary goal for many therapeutic interventions. STPs regulate cell death and may serve as potential targets for drug development. For example, large-scale screening has identified numerous protein phosphatases with tumor-suppressor-like activity (79). Therefore, our understanding of the function and mechanism of STPs in cell death regulation has important biological and clinical significance. Although much progress has been made in the last decade, major challenges remain in the field.
First, potent and specific inhibitors of STP activity remain elusive. As discussed above, direct targeting of PP2A has yielded a different outcome of cell death regulation. Some of those discrepancies may come from the utilization of chemical inhibitors that have poor specific PP2A catalytic activity (34). Similarly, cyclosporine A and FK-506 affect proteins other than PP2B/calcineurin. Indeed, another important target of cyclosporine A is cyclophilin D, a potent regulator of MPTP. Ablation of cyclophilin D protected cells from necrosis (9, 89, 104). Therefore, interpretation of results obtained from studies using cyclosporine A should be taken with caution. Given the importance and complexity of STP-mediated cell death regulation, developing more specific and potent small molecule inhibitors and agonists should be recognized with more urgent needs. In addition to small chemicals targeted to enzymatic activities, new chemicals can be developed to interfere with the STP complex formation (83). Such molecules are of special interest because they may be utilized to target a specific STP complex to avoid the side-effects of catalytic subunit inhibitors. In recent years, alternative approaches to manipulate STP activities have also been evaluated. For example, peptides have been designed to target phosphatases (44, 116). Like small chemicals, these peptides can be designed to target either catalytic subunits or complex formation, either inhibiting or activating STPs. Moreover, siRNA as a new type of molecular medicine has been exploited (26, 28, 68). Since siRNA targets individual genes specifically, the specific manipulation of targeted STPs can be potentially achieved to regulate cell death as an alternative approach to intervene in disease initiation and progression.
Second, the importance of local regulation of protein phosphorylation in mitochondria has been increasingly recognized in the context of cell death regulation. Many phosphoproteins have been identified in mitochondria (11, 54). Numerous kinases have been found either in mitochondria matrix or in the cytoplasmic surface of mitochondria (55). Many of them, such as AKT and p38/JNK MAPK, are involved in cell death/survival regulation, as discussed above. The substrates of these kinases include apoptosis executioner such as Bcl-2 family proteins, channels (including MPTP), metabolism enzymes, respiration complex, and antioxidant proteins, among many others (55). In contrast, only two examples of STPs have been well characterized in mitochondria: PP2Cm/PPM1K (75) and pyruvate dehydrogenase phosphatase isoforms. It is clear that more STPs in or on mitochondria can be anticipated. The identification of their substrates and the mechanisms of dephosphorylation regulation should further advance our current understanding of cell death and the development of new therapeutic approaches.
In summary, with more understanding of the biology of cell death and the development of novel therapeutic approaches, we anticipate that the function of STPs in cell death regulation will become clearer, and the targeting of these phosphatases can provide powerful tools to either enhance or inhibit undesired cell death in difference diseases.
Acknowledgments
The authors acknowledge the support of Shanghai Jiaotong University K. C. Wong Medical Fellowship Fund. We thank Erik Anderson for assistance in proofreading.
This work is supported in part by National Heart, Lung, and Blood Institute Grants HL-108186, HL-103205, HL-098954. H. Sun is supported by AHA post doc fellowship (Great Western Affiliate) and AHA Science Development Grant (National Research Center).
Footnotes
Author contributions: H.S. and Y.W. conception and design of research; H.S. and Y.W. analyzed data; H.S. and Y.W. prepared figures; H.S. and Y.W. drafted the manuscript; H.S. and Y.W. edited and revised the manuscript; H.S. and Y.W. approved the final version of the manuscript.
No conflicts of interest, financial or otherwise, are declared by the author(s).
References
- 1.Allan LA, Morrice N, Brady S, Magee G, Pathak S, Clarke PR. Inhibition of caspase-9 through phosphorylation at Thr 125 by ERK MAPK. Nat Cell Biol. 2003;5:647–654. doi: 10.1038/ncb1005. [DOI] [PubMed] [Google Scholar]
- 2.Alonso A, Sasin J, Bottini N, Friedberg I, Friedberg I, Osterman A, Godzik A, Hunter T, Dixon J, Mustelin T. Protein tyrosine phosphatases in the human genome. Cell. 2004;117:699–711. doi: 10.1016/j.cell.2004.05.018. [DOI] [PubMed] [Google Scholar]
- 3.Alvarado-Kristensson M, Andersson T. Protein phosphatase 2A regulates apoptosis in neutrophils by dephosphorylating both p38 MAPK and its substrate caspase 3. J Biol Chem. 2005;280:6238–6244. doi: 10.1074/jbc.M409718200. [DOI] [PubMed] [Google Scholar]
- 4.Alvarado-Kristensson M, Melander F, Leandersson K, Rönnstrand L, Wernstedt C, Andersson T. p38-MAPK signals survival by phosphorylation of caspase-8 and caspase-3 in human neutrophils. J Exp Med. 2004;199:449–458. doi: 10.1084/jem.20031771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Andreeva A, Kutuzov M. PPEF/PP7 protein Ser/Thr phosphatases. Cell Mol Life Sci. 2009;66:3103–3110. doi: 10.1007/s00018-009-0110-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Ankarcrona M, Dypbukt JM, Orrenius S, Nicotera P. Calcineurin and mitochondrial function in glutamate-induced neuronal cell death. FEBS Lett. 1996;394:321–324. doi: 10.1016/0014-5793(96)00959-3. [DOI] [PubMed] [Google Scholar]
- 7.Baines C. The mitochondrial permeability transition pore and the cardiac necrotic program. Pediatric Cardiol. 2011:1–5. doi: 10.1007/s00246-010-9880-9. [DOI] [PubMed] [Google Scholar]
- 8.Baines C. Role of the mitochondrion in programmed necrosis. Front Physiol. 2010;1:156. doi: 10.3389/fphys.2010.00156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Baines CP, Kaiser RA, Purcell NH, Blair NS, Osinska H, Hambleton MA, Brunskill EW, Sayen MR, Gottlieb RA, Dorn GW, Robbins J, Molkentin JD. Loss of cyclophilin D reveals a critical role for mitochondrial permeability transition in cell death. Nature. 2005;434:658–662. doi: 10.1038/nature03434. [DOI] [PubMed] [Google Scholar]
- 10.Boe R, Gjertsen BT, Vintermyr OK, Houge G, Lanotte M, Doskeland SO. The protein phosphatase inhibitor okadaic acid induces morphological changes typical of apoptosis in mammalian cells. Exp Cell Res. 1991;195:237–246. doi: 10.1016/0014-4827(91)90523-w. [DOI] [PubMed] [Google Scholar]
- 11.Boja ES, Phillips D, French SA, Harris RA, Balaban RS. Quantitative mitochondrial phosphoproteomics using iTRAQ on an LTQ-Orbitrap with high energy collision dissociation. J Proteome Res. 2009;8:4665–4675. doi: 10.1021/pr900387b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Boudreau RTM, Conrad DM, Hoskin DW. Apoptosis induced by protein phosphatase 2A (PP2A) inhibition in T leukemia cells is negatively regulated by PP2A-associated p38 mitogen-activated protein kinase. Cell Signal. 2007;19:139–151. doi: 10.1016/j.cellsig.2006.05.030. [DOI] [PubMed] [Google Scholar]
- 13.Bousette N, Chugh S, Fong V, Isserlin R, Kim KH, Volchuk A, Backx PH, Liu P, Kislinger T, MacLennan DH, Emili A, Gramolini AO. Constitutively active calcineurin induces cardiac endoplasmic reticulum stress and protects against apoptosis that is mediated by α-crystallin-B. Proc Natl Acad Sci USA. 2010;107:18481–18486. doi: 10.1073/pnas.1013555107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Broglie P, Matsumoto K, Akira S, Brautigan DL, Ninomiya-Tsuji J. Transforming growth factor beta-activated kinase 1 (TAK1) kinase adaptor, TAK1-binding protein 2, plays dual roles in TAK1 signaling by recruiting both an activator and an inhibitor of TAK1 kinase in tumor necrosis factor signaling pathway. J Biol Chem. 2010;285:2333–2339. doi: 10.1074/jbc.M109.090522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Brognard J, Sierecki E, Gao T, Newton AC. PHLPP and a second isoform, PHLPP2, differentially attenuate the amplitude of Akt signaling by regulating distinct Akt isoforms. Mol Cell. 2007;25:917–931. doi: 10.1016/j.molcel.2007.02.017. [DOI] [PubMed] [Google Scholar]
- 16.Bueno OF, Lips DJ, Kaiser RA, Wilkins BJ, Dai YS, Glascock BJ, Klevitsky R, Hewett TE, Kimball TR, Aronow BJ, Doevendans PA, Molkentin JD. Calcineurin Abeta gene targeting predisposes the myocardium to acute ischemia-induced apoptosis and dysfunction. Circ Res. 2004;94:91–99. doi: 10.1161/01.RES.0000107197.99679.77. [DOI] [PubMed] [Google Scholar]
- 17.Chan FKM, Shisler J, Bixby JG, Felices M, Zheng L, Appel M, Orenstein J, Moss B, Lenardo MJ. A role for tumor necrosis factor receptor-2 and receptor-interacting protein in programmed necrosis and antiviral responses. J Biol Chem. 2003;278:51613–51621. doi: 10.1074/jbc.M305633200. [DOI] [PubMed] [Google Scholar]
- 18.Chatfield K, Eastman A. Inhibitors of protein phosphatases 1 and 2A differentially prevent intrinsic and extrinsic apoptosis pathways. Biochem Biophys Res Commun. 2004;323:1313–1320. doi: 10.1016/j.bbrc.2004.09.003. [DOI] [PubMed] [Google Scholar]
- 19.Cho Y, Challa S, Moquin D, Genga R, Ray TD, Guildford M, Chan FKM. Phosphorylation-driven assembly of the RIP1-RIP3 complex regulates programmed necrosis and virus-induced inflammation. Cell. 2009;137:1112–1123. doi: 10.1016/j.cell.2009.05.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Cohen PTW. Novel protein serine/threonine phosphatases: variety is the spice of life. Trends Biochem Sci. 1997;22:245–251. doi: 10.1016/s0968-0004(97)01060-8. [DOI] [PubMed] [Google Scholar]
- 21.Cohen PTW, Philp A, Vázquez-Martin C. Protein phosphatase 4–from obscurity to vital functions. FEBS Lett. 2005;579:3278–3286. doi: 10.1016/j.febslet.2005.04.070. [DOI] [PubMed] [Google Scholar]
- 22.De Windt LJ, Lim HW, Taigen T, Wencker D, Condorelli G, Dorn GW, II, Kitsis RN, Molkentin JD. Calcineurin-mediated hypertrophy protects cardiomyocytes from apoptosis in vitro and in vivo: an apoptosis-independent model of dilated heart failure. Circ Res. 2000;86:255–263. doi: 10.1161/01.res.86.3.255. [DOI] [PubMed] [Google Scholar]
- 23.Degterev A, Huang Z, Boyce M, Li Y, Jagtap P, Mizushima N, Cuny GD, Mitchison TJ, Moskowitz MA, Yuan J. Chemical inhibitor of nonapoptotic cell death with therapeutic potential for ischemic brain injury. Nat Chem Biol. 2005;1:112–119. doi: 10.1038/nchembio711. [DOI] [PubMed] [Google Scholar]
- 24.Deng X, Gao F, May WS. Protein phosphatase 2A inactivates Bcl2's antiapoptotic function by dephosphorylation and up-regulation of Bcl2-p53 binding. Blood. 2009;113:422–428. doi: 10.1182/blood-2008-06-165134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Dessauge F, Cayla X, Albar JP, Fleischer A, Ghadiri A, Duhamel M, Rebollo A. Identification of PP1α as a caspase-9 regulator in IL-2 deprivation-induced apoptosis. J Immunol. 2006;177:2441–2451. doi: 10.4049/jimmunol.177.4.2441. [DOI] [PubMed] [Google Scholar]
- 26.Dykxhoorn DM, Palliser D, Lieberman J. The silent treatment: siRNAs as small molecule drugs. Gene Ther. 2006;13:541–552. doi: 10.1038/sj.gt.3302703. [DOI] [PubMed] [Google Scholar]
- 27.Eichhorn PJA, Creyghton MP, Wilhelmsen K, van Dam H, Bernards R. A RNA interference screen identifies the protein phosphatase 2A subunit PR55γ as a stress-sensitive inhibitor of c-SRC. PLoS Genet. 2007;3:e218. doi: 10.1371/journal.pgen.0030218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Elmen J, Lindow M, Schutz S, Lawrence M, Petri A, Obad S, Lindholm M, Hedtjarn M, Hansen HF, Berger U, Gullans S, Kearney P, Sarnow P, Straarup EM, Kauppinen S. LNA-mediated microRNA silencing in non-human primates. Nature. 2008;452:896–899. doi: 10.1038/nature06783. [DOI] [PubMed] [Google Scholar]
- 29.Espiña B, Louzao M, Cagide E, Alfonso A, Vieytes MR, Yasumoto T, Botana LM. The methyl ester of okadaic acid is more potent than okadaic acid in disrupting the actin cytoskeleton and metabolism of primary cultured hepatocytes. Br J Pharmacol. 2010;159:337–344. doi: 10.1111/j.1476-5381.2009.00512.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Feng S, Yang Y, Mei Y, Ma L, Zhu De Hoti N, Castanares M, Wu M. Cleavage of RIP3 inactivates its caspase-independent apoptosis pathway by removal of kinase domain. Cell Signal. 2007;19:2056–2067. doi: 10.1016/j.cellsig.2007.05.016. [DOI] [PubMed] [Google Scholar]
- 31.Festjens N, Vanden Berghe T, Cornelis S, Vandenabeele P. RIP1, a kinase on the crossroads of a cell's decision to live or die. Cell Death Differ. 2007;14:400–410. doi: 10.1038/sj.cdd.4402085. [DOI] [PubMed] [Google Scholar]
- 32.Festjens N, Vanden Berghe T, Vandenabeele P. Necrosis, a well-orchestrated form of cell demise: signalling cascades, important mediators and concomitant immune response. Biochim Biophys Acta. 2006;1757:1371–1387. doi: 10.1016/j.bbabio.2006.06.014. [DOI] [PubMed] [Google Scholar]
- 33.Freeman AK, Monteiro AN. Phosphatases in the cellular response to DNA damage. Cell Commun Signal. 2010;8:27. doi: 10.1186/1478-811X-8-27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Fujiki H, Suganuma M. Unique features of the okadaic acid activity class of tumor promoters. J Cancer Res Clin Oncol. 1999;125:150–155. doi: 10.1007/s004320050257. [DOI] [PubMed] [Google Scholar]
- 35.Gallego M, Virshup DM. Protein serine/threonine phosphatases: life, death, and sleeping. Curr Opin Cell Biol. 2005;17:197–202. doi: 10.1016/j.ceb.2005.01.002. [DOI] [PubMed] [Google Scholar]
- 36.Galluzzi L, Kepp O, Kroemer G. RIP kinases initiate programmed necrosis. J Mol Cell Biol. 2009;1:8–10. doi: 10.1093/jmcb/mjp007. [DOI] [PubMed] [Google Scholar]
- 37.Gao T, Brognard J, Newton AC. The phosphatase PHLPP controls the cellular levels of protein kinase C. J Biol Chem. 2008;283:6300–6311. doi: 10.1074/jbc.M707319200. [DOI] [PubMed] [Google Scholar]
- 38.Gao T, Furnari F, Newton AC. PHLPP: a phosphatase that directly dephosphorylates Akt, promotes apoptosis, and suppresses tumor growth. Mol Cell. 2005;18:13–24. doi: 10.1016/j.molcel.2005.03.008. [DOI] [PubMed] [Google Scholar]
- 39.Garibal J, Hollville E, Bell AI, Kelly GL, Renouf B, Kawaguchi Y, Rickinson AB, Wiels J. Truncated form of the Epstein-Barr virus protein EBNA-LP protects against caspase-dependent apoptosis by inhibiting protein phosphatase 2A. J Virol. 2007;81:7598–7607. doi: 10.1128/JVI.02435-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Garibal J, Hollville É , Renouf B, Tétaud C, Wiels J. Caspase-8-mediated cleavage of Bid and protein phosphatase 2A-mediated activation of Bax are necessary for Verotoxin-1-induced apoptosis in Burkitt's lymphoma cells. Cell Signal. 2010;22:467–475. doi: 10.1016/j.cellsig.2009.10.018. [DOI] [PubMed] [Google Scholar]
- 41.Gausdal G, Krakstad C, Herfindal L, Døskeland SO. Serine/threonine protein phosphatases in apoptosis. In: Srivastava R, editor. Apoptosis, Cell Signaling, and Human Diseases. Humana; New York: 2007. pp. 151–166. [Google Scholar]
- 42.Golden T, Swingle M, Honkanen R. The role of serine/threonine protein phosphatase type 5 (PP5) in the regulation of stress-induced signaling networks and cancer. Cancer Metastasis Rev. 2008;27:169–178. doi: 10.1007/s10555-008-9125-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Guenebeaud C, Goldschneider D, Castets M, Guix C, Chazot G, Delloye-Bourgeois C, Eisenberg-Lerner A, Shohat G, Zhang M, Laudet V, Kimchi A, Bernet A, Mehlen P. The dependence receptor UNC5H2/B triggers apoptosis via PP2A-mediated dephosphorylation of DAP kinase. Molecular Cell. 2010;40:863–876. doi: 10.1016/j.molcel.2010.11.021. [DOI] [PubMed] [Google Scholar]
- 44.Guergnon J, Dessauge F, Dominguez V, Viallet J, Bonnefoy S, Yuste VJ, Mercereau-Puijalon O, Cayla X, Rebollo A, Susin SA, Bost PE, Garcia A. Use of penetrating peptides interacting with PP1/PP2A proteins as a general approach for a drug phosphatase technology. Mol Pharmacol. 2006;69:1115–1124. doi: 10.1124/mol.105.019364. [DOI] [PubMed] [Google Scholar]
- 45.Halestrap AP. What is the mitochondrial permeability transition pore? J Mol Cell Cardiol. 2009;46:821–831. doi: 10.1016/j.yjmcc.2009.02.021. [DOI] [PubMed] [Google Scholar]
- 46.Hanada M, Kobayashi T, Ohnishi M, Ikeda S, Wang H, Katsura K, Yanagawa Y, Hiraga A, Kanamaru R, Tamura S. Selective suppression of stress-activated protein kinase pathway by protein phosphatase 2C in mammalian cells. FEBS Lett. 1998;437:172–176. doi: 10.1016/s0014-5793(98)01229-0. [DOI] [PubMed] [Google Scholar]
- 47.Hanada M, Ninomiya-Tsuji J, Komaki Ki Ohnishi M, Katsura K, Kanamaru R, Matsumoto K, Tamura S. Regulation of the TAK1 signaling pathway by protein phosphatase 2C. J Biol Chem. 2001;276:5753–5759. doi: 10.1074/jbc.M007773200. [DOI] [PubMed] [Google Scholar]
- 48.Harmala-Brasken AS, Mikhailov A, Soderstrom TS, Meinander A, Holmstrom T, Damuni Z, Eriksson JE. Type-2A protein phosphatase activity is required to maintain death receptor responsiveness. Oncogene. 2003;22:7677–7686. doi: 10.1038/sj.onc.1207077. [DOI] [PubMed] [Google Scholar]
- 49.He S, Wang L, Miao L, Wang T, Du F, Zhao L, Wang X. Receptor interacting protein kinase-3 determines cellular necrotic response to TNF-alpha. Cell. 2009;137:1100–1111. doi: 10.1016/j.cell.2009.05.021. [DOI] [PubMed] [Google Scholar]
- 50.Hendriks WJAJ, Elson A, Harroch S, Stoker AW. Protein tyrosine phosphatases: functional inferences from mouse models and human diseases. FEBS J. 2008;275:816–830. doi: 10.1111/j.1742-4658.2008.06249.x. [DOI] [PubMed] [Google Scholar]
- 51.Hertog JD, Östman A, Böhmer FD. Protein tyrosine phosphatases: regulatory mechanisms. FEBS J. 2008;275:831–847. doi: 10.1111/j.1742-4658.2008.06247.x. [DOI] [PubMed] [Google Scholar]
- 52.Hinds TD, Jr, Sánchez ER. Protein phosphatase 5. Intl J Biochem Cell Biol. 2008;40:2358–2362. doi: 10.1016/j.biocel.2007.08.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Ho Y, Samarasinghe R, Knoch ME, Lewis M, Aizenman E, DeFranco DB. Selective inhibition of mitogen-activated protein kinase phosphatases by zinc accounts for extracellular signal-regulated kinase 1/2-dependent oxidative neuronal cell death. Mol Pharmacol. 2008;74:1141–1151. doi: 10.1124/mol.108.049064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Hopper RK, Carroll S, Aponte AM, Johnson DT, French S, Shen RF, Witzmann FA, Harris RA, Balaban RS. Mitochondrial matrix phosphoproteome: effect of extra mitochondrial calcium. Biochemistry. 2006;45:2524–2536. doi: 10.1021/bi052475e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Horbinski C, Chu CT. Kinase signaling cascades in the mitochondrion: a matter of life or death. Free Radic Biol Med. 2005;38:2–11. doi: 10.1016/j.freeradbiomed.2004.09.030. [DOI] [PubMed] [Google Scholar]
- 56.Huang HS, Lee EYC. Protein phosphatase-1 inhibitor-3 is an in vivo target of caspase-3 and participates in the apoptotic response. J Biol Chem. 2008;283:18135–18146. doi: 10.1074/jbc.M709735200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Huang S, Shu L, Easton J, Harwood FC, Germain GS, Ichijo H, Houghton PJ. Inhibition of mammalian target of rapamycin activates apoptosis signal-regulating kinase 1 signaling by suppressing protein phosphatase 5 activity. J Biol Chem. 2004;279:36490–36496. doi: 10.1074/jbc.M401208200. [DOI] [PubMed] [Google Scholar]
- 58.Jayaraman T, Marks AR. Calcineurin is downstream of the inositol 1,4,5-trisphosphate receptor in the apoptotic and cell growth pathways. J Biol Chem. 2000;275:6417–6420. doi: 10.1074/jbc.275.9.6417. [DOI] [PubMed] [Google Scholar]
- 59.Jin Z, Wallace L, Harper SQ, Yang J. PP2A:B56ε, a substrate of caspase-3, regulates p53-dependent and p53-independent apoptosis during development. J Biol Chem. 2010;285:34493–34502. doi: 10.1074/jbc.M110.169581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Kaiser WJ, Upton JW, Long AB, Livingston-Rosanoff D, Daley-Bauer LP, Hakem R, Caspary T, Mocarski ES. RIP3 mediates the embryonic lethality of caspase-8-deficient mice. Nature. 2011;471:368–372. doi: 10.1038/nature09857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Kakita T, Hasegawa K, Iwai-Kanai E, Adachi S, Morimoto T, Wada H, Kawamura T, Yanazume T, Sasayama S. Calcineurin pathway is required for endothelin-1-mediated protection against oxidant stress-induced apoptosis in cardiac myocytes. Circ Res. 2001;88:1239–1246. doi: 10.1161/hh1201.091794. [DOI] [PubMed] [Google Scholar]
- 62.Kamenski T, Heilmeier S, Meinhart A, Cramer P. Structure and mechanism of RNA polymerase ii ctd phosphatases. Molecular Cell. 2004;15:399–407. doi: 10.1016/j.molcel.2004.06.035. [DOI] [PubMed] [Google Scholar]
- 63.Kang Y, Lee JH, Hoan NN, Sohn HM, Chang IY, You HJ. Protein phosphatase 5 regulates the function of 53bp1 after neocarzinostatin-induced dna damage. J Biol Chem. 2009;284:9845–9853. doi: 10.1074/jbc.M809272200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Kitazumi I, Tsukahara M. Regulation of DNA fragmentation: the role of caspases and phosphorylation. FEBS J. 2011;278:427–441. doi: 10.1111/j.1742-4658.2010.07975.x. [DOI] [PubMed] [Google Scholar]
- 65.Kong M, Fox CJ, Mu J, Solt L, Xu A, Cinalli RM, Birnbaum MJ, Lindsten T, Thompson CB. The PP2A-associated protein alpha4 is an essential inhibitor of apoptosis. Science. 2004;306:695–698. doi: 10.1126/science.1100537. [DOI] [PubMed] [Google Scholar]
- 66.Kurokawa M, Kornbluth S. Caspases and kinases in a death grip. Cell. 2009;138:838–854. doi: 10.1016/j.cell.2009.08.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Kutuzov MA, Andreeva AV, Voyno-Yasenetskaya TA. Regulation of apoptosis signal-regulating kinase 1 (ASK1) by polyamine levels via protein phosphatase 5. J Biol Chem. 2005;280:25388–25395. doi: 10.1074/jbc.M413202200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Lanford RE, Hildebrandt-Eriksen ES, Petri A, Persson R, Lindow M, Munk ME, Kauppinen S, Ørum H. Therapeutic silencing of microRNA-122 in primates with chronic hepatitis C virus infection. Science. 2010;327:198–201. doi: 10.1126/science.1178178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Levine B, Kroemer G. Autophagy in the pathogenesis of disease. Cell. 2008;132:27–42. doi: 10.1016/j.cell.2007.12.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Li MG, Katsura K, Nomiyama H, Komaki Ki Ninomiya-Tsuji J, Matsumoto K, Kobayashi T, Tamura S. Regulation of the interleukin-1-induced signaling pathways by a novel member of the protein phosphatase 2c family (PP2Cε). J Biol Chem. 2003;278:12013–12021. doi: 10.1074/jbc.M211474200. [DOI] [PubMed] [Google Scholar]
- 71.Li S, Brignole C, Marcellus R, Thirlwell S, Binda O, McQuoid MJ, Ashby D, Chan H, Zhang Z, Miron MJ, Pallas DC, Branton PE. The adenovirus E4orf4 protein induces G2/M arrest and cell death by blocking protein phosphatase 2A activity regulated by the B55 subunit. J Virol. 2009;83:8340–8352. doi: 10.1128/JVI.00711-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Lin Y, Devin A, Rodriguez Y, Liu Zg. Cleavage of the death domain kinase RIP by Caspase-8 prompts TNF-induced apoptosis. Genes Development. 1999;13:2514–2526. doi: 10.1101/gad.13.19.2514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Liu Q, Wilkins BJ, Lee YJ, Ichijo H, Molkentin JD. Direct interaction and reciprocal regulation between ASK1 and calcineurin-NFAT control cardiomyocyte death and growth. Mol Cell Biol. 2006;26:3785–3797. doi: 10.1128/MCB.26.10.3785-3797.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Liu W, Akhand AA, Takeda K, Kawamoto Y, Itoigawa M, Kato M, Suzuki H, Ishikawa N, Nakashima I. Protein phosphatase 2A-linked and -unlinked caspase-dependent pathways for downregulation of Akt kinase triggered by 4-hydroxynonenal. Cell Death Differ. 2003;10:772–781. doi: 10.1038/sj.cdd.4401238. [DOI] [PubMed] [Google Scholar]
- 75.Lu G, Ren S, Korge P, Choi J, Dong Y, Weiss J, Koehler C, Chen JN, Wang Y. A novel mitochondrial matrix serine/threonine protein phosphatase regulates the mitochondria permeability transition pore and is essential for cellular survival and development. Genes Dev. 2007;21:784–796. doi: 10.1101/gad.1499107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Lu G, Sun H, She P, Youn JY, Warburton S, Ping P, Vondriska TM, Cai H, Lynch CJ, Wang Y. Protein phosphatase 2Cm is a critical regulator of branched-chain amino acid catabolism in mice and cultured cells. J Clin Invest. 2009;119:1678–1687. doi: 10.1172/JCI38151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Lu G, Wang Y. Functional diversity of mammalian type 2C protein phosphatase isoforms: new tales from an old family. Clin Exp Pharmacol Physiol. 2008;35:107–112. doi: 10.1111/j.1440-1681.2007.04843.x. [DOI] [PubMed] [Google Scholar]
- 78.Lu X, Nannenga B, Donehower LA. PPM1D dephosphorylates Chk1 and p53 and abrogates cell cycle checkpoints. Genes Dev. 2005;19:1162–1174. doi: 10.1101/gad.1291305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.MacKeigan JP, Murphy LO, Blenis J. Sensitized RNAi screen of human kinases and phosphatases identifies new regulators of apoptosis and chemoresistance. Nat Cell Biol. 2005;7:591–600. doi: 10.1038/ncb1258. [DOI] [PubMed] [Google Scholar]
- 80.Manning G, Whyte DB, Martinez R, Hunter T, Sudarsanam S. The protein kinase complement of the human genome. Science. 2002;298:1912–1934. doi: 10.1126/science.1075762. [DOI] [PubMed] [Google Scholar]
- 81.Mano A, Tatsumi T, Shiraishi J, Keira N, Nomura T, Takeda M, Nishikawa S, Yamanaka S, Matoba S, Kobara M, Tanaka H, Shirayama T, Takamatsu T, Nozawa Y, Matsubara H. Aldosterone directly induces myocyte apoptosis through calcineurin-dependent pathways. Circulation. 2004;110:317–323. doi: 10.1161/01.CIR.0000135599.33787.CA. [DOI] [PubMed] [Google Scholar]
- 82.McCall K. Genetic control of necrosis: another type of programmed cell death. Curr Opin Cell Biol. 2010;22:882–888. doi: 10.1016/j.ceb.2010.09.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.McConnell JL, Wadzinski BE. Targeting protein serine/threonine phosphatases for drug development. Mol Pharmacol. 2009;75:1249–1261. doi: 10.1124/mol.108.053140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Mi J, Bolesta E, Brautigan DL, Larner JM. PP2A regulates ionizing radiation-induced apoptosis through Ser46 phosphorylation of p53. Mol Cancer Therap. 2009;8:135–140. doi: 10.1158/1535-7163.MCT-08-0457. [DOI] [PubMed] [Google Scholar]
- 85.Min W, Lin Y, Tang S, Yu L, Zhang H, Wan T, Luhn T, Fu H, Chen H. AIP1 recruits phosphatase PP2A to ASK1 in tumor necrosis factor-induced ASK1-JNK activation. Circ Res. 2008;102:840–848. doi: 10.1161/CIRCRESAHA.107.168153. [DOI] [PubMed] [Google Scholar]
- 86.Moquin D, Chan FKM. The molecular regulation of programmed necrotic cell injury. Trends Biochem Sci. 2010;35:434–441. doi: 10.1016/j.tibs.2010.03.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Mourtada-Maarabouni M, Williams GT. Protein phosphatase 4 regulates apoptosis in leukemic and primary human T-cells. Leuk Res. 2009;33:1539–1551. doi: 10.1016/j.leukres.2009.05.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Mourtada-Maarabouni M, Williams GT. Protein phosphatase 4 regulates apoptosis, proliferation and mutation rate of human cells. Biochim Biophys Acta. 2008;1783:1490–1502. doi: 10.1016/j.bbamcr.2008.03.005. [DOI] [PubMed] [Google Scholar]
- 89.Nakagawa T, Shimizu S, Watanabe T, Yamaguchi O, Otsu K, Yamagata H, Inohara H, Kubo T, Tsujimoto Y. Cyclophilin D-dependent mitochondrial permeability transition regulates some necrotic but not apoptotic cell death. Nature. 2005;434:652–658. doi: 10.1038/nature03317. [DOI] [PubMed] [Google Scholar]
- 90.Nicolaou P, Rodriguez P, Ren X, Zhou X, Qian J, Sadayappan S, Mitton B, Pathak A, Robbins J, Hajjar RJ, Jones K, Kranias EG. Inducible expression of active protein phosphatase-1 inhibitor-1 enhances basal cardiac function and protects against ischemia/reperfusion injury. Circ Res. 2009;104:1012–1020. doi: 10.1161/CIRCRESAHA.108.189811. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Oberst A, Dillon CP, Weinlich R, McCormick LL, Fitzgerald P, Pop C, Hakem R, Salvesen GS, Green DR. Catalytic activity of the caspase-8-FLIPL complex inhibits RIPK3-dependent necrosis. Nature. 2011;471:363–367. doi: 10.1038/nature09852. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Oleinik NV, Krupenko NI, Krupenko SA. ALDH1L1 inhibits cell motility via dephosphorylation of cofilin by PP1 and PP2A. Oncogene. 2010;29:6233–6244. doi: 10.1038/onc.2010.356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Padmanabhan S, Mukhopadhyay A, Narasimhan SD, Tesz G, Czech MP, Tissenbaum HA. A PP2A regulatory subunit regulates C. elegans insulin/IGF-1 signaling by modulating AKT-1 phosphorylation. Cell. 2009;136:939–951. doi: 10.1016/j.cell.2009.01.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Peng A, Maller JL. Serine/threonine phosphatases in the DNA damage response and cancer. Oncogene. 2010;29:5977–5988. doi: 10.1038/onc.2010.371. [DOI] [PubMed] [Google Scholar]
- 95.Prickett TD, Brautigan DL. Cytokine activation of p38 mitogen-activated protein kinase and apoptosis is opposed by alpha-4 targeting of protein phosphatase 2a for site-specific dephosphorylation of MEK3. Mol Cell Biol. 2007;27:4217–4227. doi: 10.1128/MCB.00067-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Pulido R, Van Huijsduijnen RH. Protein tyrosine phosphatases: dual-specificity phosphatases in health and disease. FEBS J. 2008;275:848–866. doi: 10.1111/j.1742-4658.2008.06250.x. [DOI] [PubMed] [Google Scholar]
- 97.Rasola A, Sciacovelli M, Chiara F, Pantic B, Brusilow WS, Bernardi P. Activation of mitochondrial ERK protects cancer cells from death through inhibition of the permeability transition. Proc Natl Acad Sci USA. 2010;107:726–731. doi: 10.1073/pnas.0912742107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Rasola A, Sciacovelli M, Pantic B, Bernardi P. Signal transduction to the permeability transition pore. FEBS Lett. 2010;584:1989–1996. doi: 10.1016/j.febslet.2010.02.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Rose BA, Force T, Wang Y. Mitogen-activated protein kinase signaling in the heart: angels versus demons in a heart-breaking tale. Physiol Rev. 2010;90:1507–1546. doi: 10.1152/physrev.00054.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Saeki M, Irie Y, Ni L, Itsuki Y, Terao Y, Kawabata S, Kamisaki Y. Calcineurin potentiates the activation of procaspase-3 by accelerating its proteolytic maturation. J Biol Chem. 2007;282:11786–11794. doi: 10.1074/jbc.M609347200. [DOI] [PubMed] [Google Scholar]
- 101.Saito Ji Toriumi S, Awano K, Ichijo H, Sasaki K, Kobayashi T, Tamura S. Regulation of apoptosis signal-regulating kinase 1 by protein phosphatase 2Cε. Biochem J. 2007;405:591–596. doi: 10.1042/BJ20070231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Saito S, Hiroi Y, Zou Y, Aikawa R, Toko H, Shibasaki F, Yazaki Y, Nagai R, Komuro I. β-Adrenergic pathway induces apoptosis through calcineurin activation in cardiac myocytes. J Biol Chem. 2000;275:34528–34533. doi: 10.1074/jbc.M002844200. [DOI] [PubMed] [Google Scholar]
- 103.Santoro MF, Annand RR, Robertson MM, Peng YW, Brady MJ, Mankovich JA, Hackett MC, Ghayur T, Walter G, Wong WW, Giegel DA. Regulation of protein phosphatase 2A activity by caspase-3 during apoptosis. J Biol Chem. 1998;273:13119–13128. doi: 10.1074/jbc.273.21.13119. [DOI] [PubMed] [Google Scholar]
- 104.Schinzel AC, Takeuchi O, Huang Z, Fisher JK, Zhou Z, Rubens J, Hetz C, Danial NN, Moskowitz MA, Korsmeyer SJ. Cyclophilin D is a component of mitochondrial permeability transition and mediates neuronal cell death after focal cerebral ischemia. Proc Natl Acad Sci USA. 2005;102:12005–12010. doi: 10.1073/pnas.0505294102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Schwarz S, Hufnagel B, Dworak M, Klumpp S, Krieglstein J. Protein phosphatase type 2Cα and 2Cβ are involved in fatty acid-induced apoptosis of neuronal and endothelial cells. Apoptosis. 2006;11:1111–1119. doi: 10.1007/s10495-006-6982-1. [DOI] [PubMed] [Google Scholar]
- 106.Shanware NP, Zhan L, Hutchinson JA, Kim SH, Williams LM, Tibbetts RS. Conserved and distinct modes of CREB/ATF transcription factor regulation by PP2A/B56γ and genotoxic stress. PLos One. 2010;5:e12173. doi: 10.1371/journal.pone.0012173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Shi Y. Serine/threonine phosphatases: mechanism through structure. Cell. 2009;139:468–484. doi: 10.1016/j.cell.2009.10.006. [DOI] [PubMed] [Google Scholar]
- 108.Shibasaki F, McKeon F. Calcineurin functions in Ca2+-activated cell death in mammalian cells. J Cell Biol. 1995;131:735–743. doi: 10.1083/jcb.131.3.735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Song G, Ouyang G, Bao S. The activation of Akt/PKB signaling pathway and cell survival. J Cell Mol Med. 2005;9:59–71. doi: 10.1111/j.1582-4934.2005.tb00337.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Strack S, Cribbs JT, Gomez L. Critical role for protein phosphatase 2A heterotrimers in mammalian cell survival. J Biol Chem. 2004;279:47732–47739. doi: 10.1074/jbc.M408015200. [DOI] [PubMed] [Google Scholar]
- 111.Suganuma M, Fujiki H, Suguri H, Yoshizawa S, Hirota M, Nakayasu M, Ojika M, Wakamatsu K, Yamada K, Sugimura T. Okadaic acid: an additional non-phorbol-12-tetradecanoate-13-acetate-type tumor promoter. Proc Natl Acad Sci USA. 1988;85:1768–1771. doi: 10.1073/pnas.85.6.1768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Tabernero L, Aricescu AR, Jones EY, Szedlacsek SE. Protein tyrosine phosphatases: structure-function relationships. FEBS J. 2008;275:867–882. doi: 10.1111/j.1742-4658.2008.06251.x. [DOI] [PubMed] [Google Scholar]
- 113.Takekawa M, Maeda T, Saito H. Protein phosphatase 2Calpha inhibits the human stress-responsive p38 and JNK MAPK pathways. EMBO J. 1998;17:4744–4752. doi: 10.1093/emboj/17.16.4744. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Tamura S, Toriumi S, Saito Ji Awano K, Kudo Ta, Kobayashi T. PP2C family members play key roles in regulation of cell survival and apoptosis. Cancer Sci. 2006;97:563–567. doi: 10.1111/j.1349-7006.2006.00219.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Tan WQ, Wang JX, Lin ZQ, Li YR, Lin Y, Li PF. Novel cardiac apoptotic pathway: the dephosphorylation of apoptosis repressor with caspase recruitment domain by calcineurin. Circulation. 2008;118:2268–2276. doi: 10.1161/CIRCULATIONAHA.107.750869. [DOI] [PubMed] [Google Scholar]
- 116.Tappan E, Chamberlin AR. Activation of protein phosphatase 1 by a small molecule designed to bind to the enzyme's regulatory site. Chem Biol. 2008;15:167–174. doi: 10.1016/j.chembiol.2008.01.005. [DOI] [PubMed] [Google Scholar]
- 117.Tchivilev I, Madamanchi NR, Vendrov AE, Niu XL, Runge MS. Identification of a protective role for protein phosphatase 1cγ1 against oxidative stress-induced vascular smooth muscle cell apoptosis. J Biol Chem. 2008;283:22193–22205. doi: 10.1074/jbc.M803452200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Tombal B, Weeraratna AT, Denmeade SR, Isaacs JT. Thapsigargin induces a calmodulin/calcineurin-dependent apoptotic cascade responsible for the death of prostatic cancer cells. Prostate. 2000;43:303–317. doi: 10.1002/1097-0045(20000601)43:4<303::aid-pros10>3.0.co;2-v. [DOI] [PubMed] [Google Scholar]
- 119.Tsao CC, Nica AF, Kurinna SM, Jiffar T, Mumby M, Ruvolo PP. Mitochondrial protein phosphatase 2A regulates cell death induced by simulated ischemia in kidney NRK-52E cells. Cell Cycle. 2007;6:2377–2385. doi: 10.4161/cc.6.19.4737. [DOI] [PubMed] [Google Scholar]
- 120.Vandenabeele P, Declercq W, Van Herreweghe F, Vanden Berghe T. The role of the kinases RIP1 and RIP3 in TNF-induced necrosis 101126/scisignal3115re4. Sci Signal. 2010;3:re4. doi: 10.1126/scisignal.3115re4. [DOI] [PubMed] [Google Scholar]
- 121.Vandenabeele P, Galluzzi L, Vanden Berghe T, Kroemer G. Molecular mechanisms of necroptosis: an ordered cellular explosion. Nat Rev Mol Cell Biol. 2010;11:700–714. doi: 10.1038/nrm2970. [DOI] [PubMed] [Google Scholar]
- 122.Vanlangenakker N, Vanden Berghe T, Krysko DV, Festjens N, Vandenabeele P. Molecular mechanisms and pathophysiology of necrotic cell death. Curr Mol Med. 2008;8:207–220. doi: 10.2174/156652408784221306. [DOI] [PubMed] [Google Scholar]
- 123.Virshup DM, Shenolikar S. From promiscuity to precision: protein phosphatases get a makeover. Mol Cell. 2009;33:537–545. doi: 10.1016/j.molcel.2009.02.015. [DOI] [PubMed] [Google Scholar]
- 124.Wang HG, Pathan N, Ethell IM, Krajewski S, Yamaguchi Y, Shibasaki F, McKeon F, Bobo T, Franke TF, Reed JC. Ca2+-induced apoptosis through calcineurin dephosphorylation of BAD. Science. 1999;284:339–343. doi: 10.1126/science.284.5412.339. [DOI] [PubMed] [Google Scholar]
- 125.Wu HY, Tomizawa K, Oda Y, Wei FY, Lu YF, Matsushita M, Li ST, Moriwaki A, Matsui H. Critical role of calpain-mediated cleavage of calcineurin in excitotoxic neurodegeneration. J Biol Chem. 2004;279:4929–4940. doi: 10.1074/jbc.M309767200. [DOI] [PubMed] [Google Scholar]
- 126.Xiao L, Gong LL, Yuan D, Deng M, Zeng XM, Chen LL, Zhang L, Yan Q, Liu JP, Hu XH, Sun SM, Liu J, Ma HL, Zheng CB, Fu H, Chen PC, Zhao JQ, Xie SS, Zou LJ, Xiao YM, Liu WB, Zhang J, Liu Y, Li DWC. Protein phosphatase-1 regulates Akt1 signal transduction pathway to control gene expression, cell survival and differentiation. Cell Death Differ. 2010;17:1448–1462. doi: 10.1038/cdd.2010.16. [DOI] [PubMed] [Google Scholar]
- 127.Xifró X, García-Martínez JM, Del Toro D, Alberch J, Pérez-Navarro E. Calcineurin is involved in the early activation of NMDA-mediated cell death in mutant huntingtin knock-in striatal cells. J Neurochem. 2008;105:1596–1612. doi: 10.1111/j.1471-4159.2008.05252.x. [DOI] [PubMed] [Google Scholar]
- 128.Yin KJ, Hsu CY, Hu XY, Chen H, Chen SW, Xu J, Lee JM. Protein Phosphatase 2A regulates bim expression via the Akt/FKHRL1 signaling pathway in amyloid-beta peptide-induced cerebrovascular endothelial cell death. J Neurosci. 2006;26:2290–2299. doi: 10.1523/JNEUROSCI.5103-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Zhang H, Zhou X, McQuade T, Li J, Chan FKM, Zhang J. Functional complementation between FADD and RIP1 in embryos and lymphocytes. Nature. 2011;471:373–376. doi: 10.1038/nature09878. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Zhao Y, Tozawa Y, Iseki R, Mukai M, Iwata M. Calcineurin activation protects T cells from glucocorticoid-induced apoptosis. J Immunol. 1995;154:6346–6354. [PubMed] [Google Scholar]
- 131.Zhou G, Golden T, Aragon IV, Honkanen RE. Ser/Thr protein phosphatase 5 inactivates hypoxia-induced activation of an apoptosis signal-regulating kinase 1/MKK-4/JNK signaling cascade. J Biol Chem. 2004;279:46595–46605. doi: 10.1074/jbc.M408320200. [DOI] [PubMed] [Google Scholar]
- 132.Zorov DB, Juhaszova M, Yaniv Y, Nuss HB, Wang S, Sollott SJ. Regulation and pharmacology of the mitochondrial permeability transition pore. Cardiovasc Res. 2009;83:213–225. doi: 10.1093/cvr/cvp151. [DOI] [PMC free article] [PubMed] [Google Scholar]