Abstract
Among the myriad of intra-cellular signaling networks that govern the cardiac development and pathogenesis, mitogen-activated protein kinases (MAPKs) are prominent players that have been the focus of extensive investigations in the past decades. The four best characterized MAPK subfamilies, ERK1/2, JNK, p38, and ERK5, are the targets of pharmacological and genetic manipulations to uncover their roles in cardiac development, function, and diseases. However, information reported in the literature from these efforts has not yet resulted in a clear view about the roles of specific MAPK pathways in heart. Rather, controversies from contradictive results have led to a perception that MAPKs are ambiguous characters in heart with both protective and detrimental effects. The primary object of this review is to provide a comprehensive overview of the current progress, in an effort to highlight the areas where consensus is established verses the ones where controversy remains. MAPKs in cardiac development, cardiac hypertrophy, ischemia/reperfusion injury, and pathological remodeling are the main focuses of this review as these represent the most critical issues for evaluating MAPKs as viable targets of therapeutic development. The studies presented in this review will help to reveal the major challenges in the field and the limitations of current approaches and point to a critical need in future studies to gain better understanding of the fundamental mechanisms of MAPK function and regulation in the heart.
I. INTRODUCTION
Cellular responses to various stimuli are mediated via complex but coordinated signaling pathways. In the heart, a cast of molecules participate in a choreographed drama of signal transduction events during cardiac development, physiological adaptation, and pathological manifestation. Mitogen-activated protein kinases (MAPKs) are a well-studied family of proteins that play an integral role in these signaling events. Like any good drama, MAPK members consist of both angels and demons that can protect or injure the heart. In this review, we focus on our current understanding of the roles these different MAPK members play in cardiac development, function, and diseases and discuss efforts to harness their activities to treat heart failure.
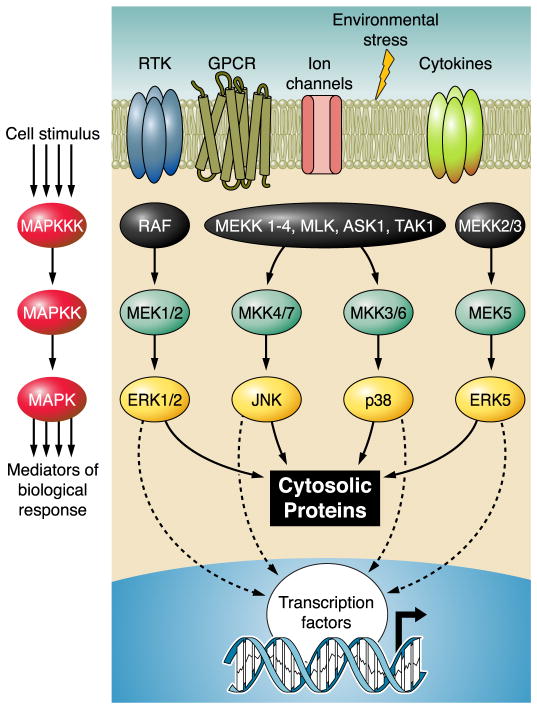
Highly conserved from yeast to human (429), MAPKs are involved in a diverse repertoire of biological events including proliferation, differentiation, metabolism, motility, survival, and apoptosis. These biological events are the culmination of signal transduction and regulation by primarily four MAPK subfamilies including extracellular signal-regulated kinases (ERK1/2), c-Jun NH2-terminal kinases (JNK1, -2 and -3), p38 kinase (α, β, γ, δ), and big MAPK (BMK or ERK5) (185, 318, 329). Activation of MAPKs requires dual phosphorylation of a Thr-X-Tyr motif (where X is either a Gly, Pro, or Glu) in the regulatory loop (62, 330). The typical event leading to this phosphorylation is a well-conserved three-tiered kinase cascade in which a MAPK kinase kinase (MAPKKK, MAP3K, MEKK, or MKKK) activates a MAPK kinase (MAPKK, MAP2K, MEK, or MKK) which in turn activates the MAPK through serial phosphorylation (Fig. 1). This canonical activation cascade allows for signal amplification, modulation, and specificity in response to different stimuli (120). As with many signaling pathways, complex regulatory mechanisms are utilized to direct the functional outcome mediated by MAPKs. The prototypic ERK1/2 pathway is found to be mainly responsive to stimulation by growth factors (333), while JNK and p38 are collectively called stress-activated MAPKs (SAPKs) due to their induction by physical, chemical and physiological stressors [such as ultraviolet (UV) light, oxidant stress, osmotic shock, infection, and cytokines] (221). In addition, the ERK5/BMK pathway is implicated in both growth and stress signaling (155). The specificity and efficiency of MAPK signaling pathways are often dictated by specific docking and binding partners (180, 332, 336). These include positive and negative modulators and scaffolding proteins which help to bring upstream and downstream signaling components together (95, 285, 318). On the other hand, selective interaction between the MKK’s docking sites (D sites) and their cognate MAPKs helps to segregate different branches of MAPKs into specific signaling pathways (27–29, 143, 163, 336). Once activated, MAPKs can phosphorylate serine or threonine residues in a specific Pro-X-Thr/Ser-Pro motif on their target proteins (377). The duration and level of MAPK signaling are subject to negative-feedback regulation by Try, Ser/Thr, or dual-specificity phosphatases (261, 311). The resulting balance between kinase activation and inactivation by these phosphatases adds yet another layer of regulation by which MAPK signaling is tightly controlled to achieve the desired outcome. While there is a large degree of specificity in different MAPK cascades, there is also significant overlap observed among them. Both upstream activators and downstream targets can be shared between different subfamilies, allowing for potential cross-talk and feedback (329, 411). Likewise, some phosphatases activated by one pathway (e.g., protein phosphatase 2A stimulation by p38) can act as a negative regulator of another pathway (e.g., ERK), demonstrating the close connection between different signaling events of MAPK family members (186). Furthermore, in addition to the classic kinase phosphorylation cascades just discussed, several noncanonical mechanisms have also been identified for MAPK activation, adding to the molecular complexity of MAPK signal transduction (348). In short, MAPKs form complex signaling networks that can be induced by a large array of external stimuli and can achieve highly specific cellular effects through multitudes of regulatory mechanisms.
FIG. 1.
Canonical mitogen-activated protein kinase (MAPK) signaling. MAPK are prototypically activated by canonical three-tiered sequential phosphorylation events. The most well-known MAPKKK and MAPKK are listed for each MAPK; however, this is only a small representation of all identified upstream kinases. Furthermore, multiple steps may exist between the cell stimulus and activation of the MAPKKK and between activation of the MAPK and the biological response.
II. MITOGEN-ACTIVATED PROTEIN KINASE FAMILY MEMBERS
There are four classic MAPK subfamilies. Each of these family members has been studied extensively in a multitude of cellular settings and has been reviewed in great detail by others (31, 221, 318, 332, 333, 348). For this reason, only a brief introduction to each subfamily will be given here. Furthermore, other atypical MAPKs, including ERK3/4, NLK, and ERK7, are much less studied and are not discussed in this review (81).
A. ERK1/2
First discovered in the early 1980s for its ability to phosphorylate microtubule-associated protein-2 (MAP-2) in 3T3-L1 adipocytes in response to insulin stimulation (18), extracellular signal-regulated kinases (ERKs) are now one of the most widely studied signaling pathways in cellular biology. ERK1 and ERK2 are 83% identical, share most of the same signaling activities, and, as a result, are usually referred to simply as ERK1/2. However, these two proteins are not completely functionally redundant as demonstrated by gene knockout experiments. ERK1 null mice have, in general, a normal phenotype (139, 312), but ERK2 null mice are embryonic lethal between E6.5 and E8.5 (139, 151, 350, 454). ERK1/2 is ubiquitously expressed and has many diverse cellular and physiological functions. At the cellular level, ERK1/2 regulates cell cycle progression, proliferation, cytokinesis, transcription, differentiation, senescence, cell death, migration, GAP junction formation, actin and microtubule networks, and cell adhesion (333). ERK1/2’s role in cellular biology translates it into a prominent player in physiological settings, influencing the immune system and heart development and contributing to the response of many hormones, growth factors, and insulin. Furthermore, because of its role in so many biological processes, ERK1/2 has likewise been shown to play a significant part in various pathologies including cancer, diabetes, and cardiovascular disease. This extensive and diverse functional ability is the result of ERK1/2’s ability to phosphorylate over 100 possible substrates (456).
As discussed previously, ERK1/2 is activated via a canonical three-tiered kinase cascade by both extracellular and intracellular stimuli (Fig. 2A). Growth factors, serum, and phorbol esters strongly activate the pathway, but it can also be activated by G protein-coupled receptors, cytokines, microtubule disorganization, and other stimuli (140, 270, 332). Prototypically, growth factor (such as fibroblast growth factor, FGF) binding to their respective receptor tyrosine kinase (RTK) activates Ras which recruits and activates Raf (MAP3K) at the plasma membrane. Once activated, Raf phosphorylates and activates MEK1/2 (MAP2K). MEK1/2 in turn activates ERK1/2 by phosphorylation of the Thr and Tyr residues in the conserved Thr-Glu-Tyr motif within its regulatory loop. Activated ERK1/2 can phosphorylate downstream proteins in the cytoplasm or nucleus, including many transcription factors.
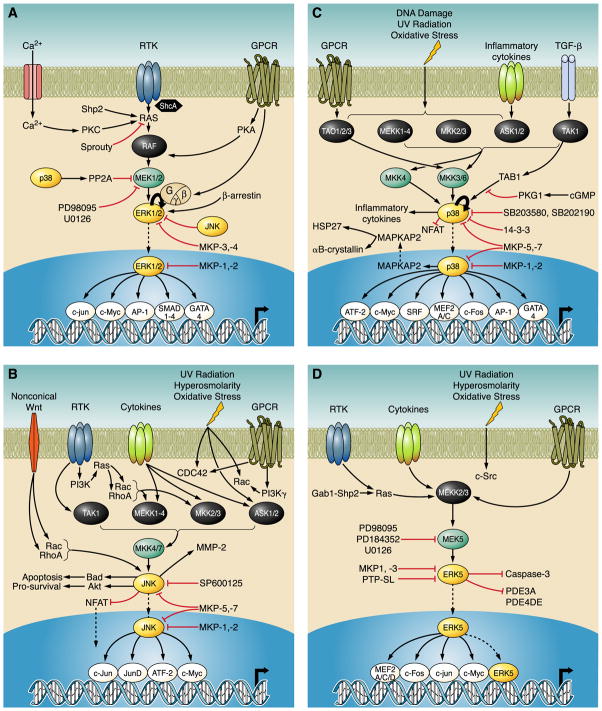
FIG. 2.
Representative MAPK signaling in the heart. MAPK signaling events that play a role in cardiac signaling. Not all connections necessarily represent a direct interaction but rather may represent the end product of multiple steps. These are only a general representation of a sample of signaling events in the heart and do not represent all known MAPK signaling. A: ERK signaling. B: JNK signaling. C: p38 signaling. D: ERK5 signaling.
As mentioned in section I, MAPK signaling is subject to many mechanisms of modulation that determine the specificity and magnitude of the signaling outcome. Interactions with scaffold proteins are one of these mechanisms. ERK has a number of known scaffold proteins including kinase suppressor of Ras (KSR), MEK partner 1 (MP1), MAPK organizer 1 (MORG1), and β-arrestin (95). Structural studies also reveal specific docking site motifs that help direct the specificity of ERK1/2 signaling, including the ERK docking (ED) motif, the docking site for ERK and FXFG (DEF) motif, and the common docking (CD) motif (332). Protein phosphatases are a third mechanism that contributes to MAPK regulation. ERK signaling has been shown to be regulated by various phosphatases including dual-specificity MAPK phosphatases (MKP1,-2, -3, and -4), protein serine/threonine phosphatases (PP2A, PPM1α), and protein tyrosine phosphatases (SHP-2 PTP, hematopoietic PTP, STEP, PTP-ε) (186, 311). The final way that MAPK activity is regulated is by positive and negative feedback regulation from other components of the MAPK signaling network. This includes negative regulation of ERK by other MAPKs such as JNK and p38 (186).
B. JNK
In the early 1990s, 10 years after the discovery of ERK, JNK was discovered as a second subfamily of MAPKs for its ability to phosphorylate microtubule-associated protein 2 in rat liver following cycloheximide injection. It was further detailed for its ability to phosphorylate the transcription factor c-jun at two sites following UV radiation (159, 219, 220). JNK1, JNK2, and JNK3 are encoded by three separate genes, and alternative splicing can produce 10 different protein sequences that share >80% homology (31). JNK1 and JNK2 are ubiquitously expressed, while JNK3 is predominantly found in the brain, heart, and testis (93). While there is some redundancy in the functions of the three isoforms, gene knockout studies have shown specific roles for different JNK isoforms in vivo (41, 139). Like ERK, JNK plays a role in a number of different biological processes including cell proliferation, differentiation, apoptosis, cell survival, actin reorganization, cell mobility, metabolism, and cytokine production (43, 93, 332). This translates into JNK’s physiological role in insulin signaling, the immune response and inflammation, and its pathological role in neurological disorders, arthritis, obesity, diabetes, atherosclerosis, cardiac disease, liver disease, and cancer (41).
Activation of the JNK pathway occurs in response to a number of different stimuli. As a stress-activated protein kinase, JNK responds most robustly to inflammatory cytokines and cellular stresses such as heat shock, hyperosmolarity, ischemia-reperfusion, UV radiation, oxidant stress, DNA damage, and ER stress (41, 332). However, they are also activated to a lesser extent by growth factors, G protein-coupled receptors, and noncanonical Wnt pathway signaling (140, 196, 317). Once stimulated, JNK is activated by the previously described three-tiered kinase cascade (Fig. 2B). After the cell is stimulated, signaling occurs which eventually leads to the activation of the first tier. The MAP3Ks that can activate JNKs are MEKK1, MEKK2, and MEKK3, as well as mixed lineage kinase 2 and 3 (MLK2 and MLK3) and others (332). These kinases then activate the MAP2Ks involved in the JNK cascade, MKK4 and MKK7. MKK4/7 then activates JNK by phosphorylation on a conserved Thr-Pro-Tyr motif. It has been shown that MKK4 has a preference for Tyr phosphorylation while MKK7 has a preference for Thr in the TPY motif, allowing these two kinases to work synergistically in JNK activation (227). Activated JNK has a large number of downstream substrates, including nuclear and cytoplasmic proteins. Similar to the other MAPKs, JNK has the ability to shuttle between the cytoplasm and the nucleus to exert its effects depending on the specific cellular stimuli. The diversity of JNK signaling can be conferred by signaling via more than 25 nuclear substrates and more than 25 nonnuclear substrates for any specific stimulus (43).
JNKs, like all MAPKs, utilize the same mechanisms to impart specificity and degree of magnitude to its signaling. Interaction with scaffold proteins such as JNK-interacting proteins (JIP1, JIP2), JNK/stress-activated protein kinase-associated protein 1 (JSAP1/JIP3), JNK-associated leucine-zipper protein (JLP), and plenty of SH3 (POSH) help direct the specificity of this pathway (95). The specificity of JNK’s interaction with these scaffold proteins and its up and downstream partners is also mediated, in part, through specific docking sites, including D motifs, MAPK-docking sites, and others (332). Like all protein kinases, JNK activity is also counterregulated by phosphatases including dual specific phosphatases MKP1, -2, -5, and -7 (311).
C. p38
Around the same time that JNK was discovered, another subfamily of SAPKs from the MAPK family was also identified. p38 was originally isolated as a tyrosine phosphorylated protein found in LPS-stimulated macrophages (147, 148). At the same time, it was also reported as a molecule that binds pyridinyl imidazoles which inhibit the production of proinflammatory cytokines (229). Since then, four different p38 isoforms have been identified, including the prototypic p38α (often referred to as simply p38), p38β (184), p38γ (237), and p38δ (228). p38 and p38β are ubiquitously expressed, while p38γ is expressed primarily in skeletal muscle and p38δ is found in lung, kidney, testis, pancreas, and small intestine (309). The four isoforms share structural similarities (>60% homology within the group and even higher in their kinase domains) and substrate similarities as well. However, it is unclear in vivo if activity towards a given substrate can vary between isoforms and if each isoform also has its own set of specific substrates. This is demonstrated by gene knockout experiments in which deletion of the p38α gene leads to embryonic lethality due to placental and erythroid differentiation defects (286, 397), but mice carrying deletion of any of the other three isoforms are phenotypically normal (139). Like other MAPK subfamilies, p38 kinases also play numerous biological roles. Most prominently, p38 signaling is involved in the immune response, promoting expression of proinflammatory cytokines [interleukin (IL)-1β, tumor necrosis factor (TNF)-α, and IL-6], cell adhesion molecules (VCAM-1), and other inflammatory related molecules and regulating the proliferation, differentiation, and function of immune cells (221, 342). p38 also plays a role in many other biological functions, namely, apoptosis, cell survival, cell cycle regulation, differentiation, senescence, and cell growth and migration (406, 459). Physiologically, this translates into a role for p38 in chronic inflammatory diseases (rheumatoid arthritis, Crohn’s disease, psoriasis, and chronic asthma), tumorgenesis, cardiovascular disease, and Alzheimer’s disease (83).
As a stress-activated kinase, p38 responds to most of the same stimuli as JNK as well as others that are specific to p38. p38 can be activated by such stimuli as UV radiation, heat, osmotic shock, pathogens, inflammatory cytokines, growth factors, and others. Making this pathway complicated, p38 can respond to over 60 different extracellular stimuli in a cell-specific manner, making it challenging to elucidate its exact functional role in vivo (309). Regardless of the exact stimuli, the canonical pathway of p38 activation is the same as for ERK and JNK (Fig. 2C). A number of upstream kinases are implicated in the phosphorylation cascades leading to the activation of p38, including MEKK1–4, TAK1, and ASK1 at the MAP3K level and MKK3, -6, and, possibly, -4 at the MAP2K level. These MAP2Ks activate p38 by phosphorylation of its conserved Thr-Gly-Tyr motif. Of interesting note, p38 can be activated in noncanonical ways as well. One way is TAB-1-mediated autophosphorylation (138, 399), and another is T-cell receptor-induced activation of p38 through ZAP70 (353). Once activated, p38 can function in the cytoplasm or translocate to the nucleus. Substrates for p38 include transcription factors, other nuclear proteins, and cytoplasmic proteins (309).
The magnitude of the signal and the specificity of the p38 pathway are determined by similar mechanisms as both ERK and JNK. While scaffold proteins have been shown to be important in p38 signaling, there have only been three such proteins identified so far: osmosensing scaffold for MEKK1 (OSM), JIP2, and JLP (95). p38 also utilizes specific domains, such as CD motifs, ED motifs, and D motifs to facilitate its interaction with other proteins (332). Finally, protein phosphatases are yet another form of p38 regulation, including dual specific MKPs (MKP1, -2, -5, -7) and protein Ser/Thr phosphatases (PP2C) (186, 311).
D. ERK5
ERK5 is the final classic MAPK subfamily and the least studied among the four. Discovered in the mid 1990s by two groups simultaneously, many questions remain to be answered, although progress is rapidly being made on many fronts. The first group identified ERK5 using a yeast two-hybrid screen with the upstream activator MEK5 as the bait (25), while the second group used a degenerate PCR strategy to clone novel MAPKs (230). The most distinguishing feature of this MAPK is its size, 816 amino acids, making it more than twice the size of the other MAPK family members (thus the alternative name big MAPK or BMK). This increased size is due to a large 396-amino acid COOH-terminal extension. While only one ERK5 gene has been identified, it undergoes alternative splicing to produce four different protein species: ERK5a, ERK5b, ERK5c, and ERK-T. ERK5a is the most prominently expressed, and the other three appear to function as negative regulators of ERK5a (268, 448). This kinase is ubiquitously expressed, and gene knockout studies show global deletion of ERK5 is embryonic lethal due to what was initially thought to be cardiac defects (335). However, cardiomyocyte specific inactivation of ERK5 results in normal development, indicating that the lethality from the global knockout is due to defects in vascular formation (154, 155). Diverse biological roles of ERK5 are also identified, including cell survival, differentiation, proliferation, and growth. ERK5 is reported to play a physiological role in neuronal survival, endothelial cell response to sheer stress, prostate and breast cancer, cardiac hypertrophy, and atherosclerosis (155, 304, 424).
ERK5 is activated in response to both growth and stress stimuli. This includes a wide variety of growth factors [epidermal growth factor, nerve growth factor, vascular endothelial growth factor (VEGF), FGF-2], serum, phorbol ester, hyperosmosis, oxidative stress, laminar flow sheer stress, and UV radiation (155). Whatever the activating stimuli, ERK5 follows the same canonical three-tiered pathway as the other MAPKs (Fig. 2D). Because of the relative paucity of investigation for this pathway, there are fewer known upstream kinases. The most well-studied MAP3Ks are MEKK2 and MEKK3, which activate the only known MAP2K, MEK5, which then phosphorylates and activates ERK5. Once activated, ERK5 exerts its kinase activity on a number of other protein kinases and transcription factors in both the cytosol and the nucleus. Furthermore, unlike other MAPKs, ERK5 has been shown to function directly as a transcriptional activator (3, 193).
ERK5 signaling, in true MAPK fashion, is influenced by such things as scaffold proteins, docking sites, phosphatases, and other members of the MAPK family. However, because ERK5 is less well studied than the other MAPKs previously discussed, less is known about these forms of regulation. Adaptor and scaffold proteins such as Lck-associated adaptor (Lad) and Grb-2-associated binder 1 (Gab 1) as well as muscle specific A-kinase anchoring protein (mAKAP) have all been shown to play an integral role in ERK5 signaling (424). Furthermore, MEK5 (the MAP2K of ERK5) uses its Phox/Bem 1P (PB1) domain to bind and tether together the upstream MAP3K (MEKK2/3) and the downstream ERK5 to facilitate signaling (294, 295). While regulation of ERK5 activity has been shown to be regulated by specific protein phosphatases, such as MKP1 and -3 (192) and the phosphotyrosine specific phosphatases PTP-SL (59), much less is known about this type of regulation than is with the other MAPKs.
III. MITOGEN-ACTIVATED PROTEIN KINASES IN HEART DEVELOPMENT
Mammalian cardiogenesis is a complex and highly coordinated biological process. With the advancement of regenerative medicine and the utilization of stem cell therapy in treatment of cardiovascular diseases, understanding the basic biology behind cardiac development has become more important than ever. While there are many signaling events occurring during development, this review will focus only on the role that MAPKs play during this process (Fig. 3). For extensive coverage, readers are directed to a number of excellent recent reviews on this issue (56, 108, 308, 379, 380).
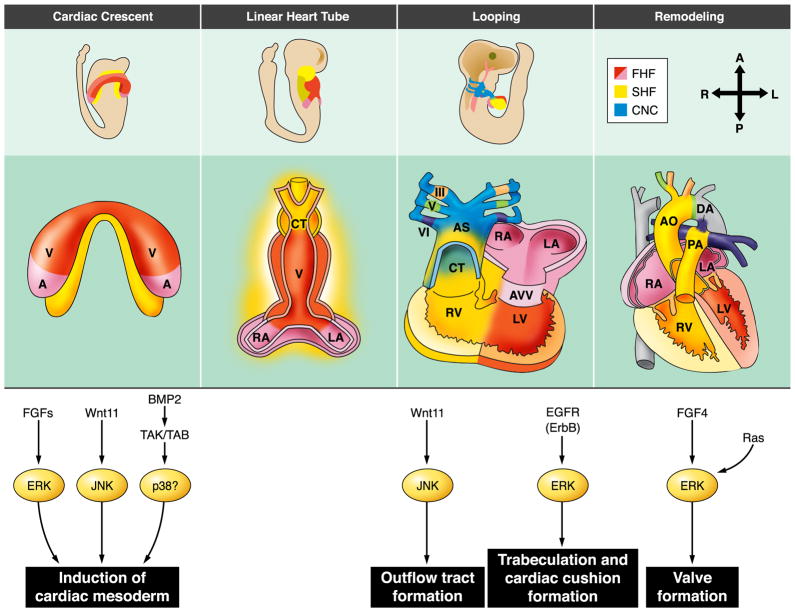
FIG. 3.
MAPK signaling during heart development. Proposed MAPK signaling events during various stages of heart development. FHF, first heart field; SHF, second heart field; CNC, cardiac neural crest; V, ventricle; A, atria; RA, right atrium; LA, left atrium; CT, conotruncus; RV, right ventricle; LV, left ventricle; AVV, atrioventricular valves; Ao, aorta; PA, pulmonary artery; DA, ductus arteriosus. [Modified from Srivastava (379).]
During development, the heart is the first organ to form. It does so by a series of well-defined processes that can broadly be grouped as 1) determination of cardiac cell fate at cardiac crescent and second heart field, 2) differentiation of cardiomyocytes, and 3) morphogenesis and growth (56, 137, 379, 396, 419) The entire process, including the simultaneous development of the non-muscle structures of the heart, results from the delicate balance between positive and negative regulatory signals coming from both within the structure and from the tissue surrounding the developing heart (108, 375). Numerous studies in myocardial development have elucidated a number of important signaling pathways and transcription factors that are involved in coordinating heart formation. Induction of cardiac fate involves the integration of a variety of signaling pathways, including Hedgehog, bone morphogenic protein (BMP), FGF, and Wnt (108). This signaling culminates in the induction of cardiogenic transcription factors including Nkx2.5, GATA4, serum response factor (SRF), Tbx5, and others. Much of the same signaling that activates cardiac induction continues throughout the subsequent morphogenesis and growth (reviewed in Ref. 51). In the following sections we look at how the various MAPK family members participate in this process.
A. ERK1/2
Most contributions of the ERK1/2 pathway to heart development are due to its role in growth factor signaling. FGFs are a large family of growth factors involved in a wide variety of cellular processes during development, including proliferation, differentiation, cell survival, apoptosis, and cell migration (50). FGF ligands differentially bind to and activate four different FGF receptors. These activated receptor tyrosine kinases transduce their signal through three main downstream pathways: the Ras/Raf/ERK pathway, the phospholipase C (PLC)-γ/Ca2+ pathway, or the phosphatidylinositol 3-kinase (PI3K)/Akt pathway (85).
FGF signaling contributes to cardiac development in a number of different ways. During early development, FGF signaling has been shown to be important in cardiogenic induction. Originally thought to be due only to signaling of BMPs, induction of progenitor cells to adopt a cardiac fate has more recently been shown to involve a cooperative interaction between BMPs and FGFs (32, 253). In both mouse and chicken models, various FGFs have been shown to cooperate with BMP-2 to induce mesodermal cells to adopt a cardiac cell fate. While the exact downstream mediators of FGF signaling in cardiac fate determination remain to be precisely elucidated, one study indicates that it may not be due to ERK signaling. In mouse P19CL6 cells, a type of embryonic carcinoma cells which retain mulitpotency (267), it was shown that the PI3K pathway is essential for early stage activation of Nkx2.5 and GATA4 and subsequent cardiac differentiation in this setting (292). Likewise, treatment of this cell line with PD98059, an ERK1/2 inhibitor, did not prevent cardiomyocyte differentiation in one study (91) and only partially prevented differentiation in another report (115). However, these in vitro observations may not fully reveal what is happening in vivo. This can be illustrated by the fact that ERK1/2 signaling has been shown to be vital to myocyte differentiation using other experimental models. In studies using embryonic stem (ES) cells isolated from both fgfr+/− or fgfr−/− mice, it was found that FGFR-1-deficient embryoid bodies (EBs) failed to differentiate into clusters of beating myocytes while those with one copy of the gene appeared to differentiate normally (94). These authors further elucidated the signaling involved in this differentiation process and found that the MEK1/2 inhibitor U0126 blocked cardiogenic differentiation of the fgfr+/− EBs. Interestingly, they found that use of the MEK1 inhibitor PD098059 did not affect differentiation, which may explain the results seen in P19CL6 cells previously discussed. The role of ERK1/2 signaling in this process was further supported by using the PKC inhibitor GF109, as PKC is known to regulate the Ras/Raf-1/MEK/ERK cascade at different levels (361). In the same study, GF109 also blocked cardiac differentiation of the fgfr+/− EBs, while treatment with phorbol ester, a PKC activator, partially rescues the differentiation of fgfr−/− EBs in a U0126-sensitive manner. These data suggest a role for FGF signaling via ERK1/2 in cardiogenic differentiation. Likewise, recent studies using mouse ES cells have also suggested that ERK plays a role in leukemia inhibitory factor (LIF)-BMP-2 mediated differentiation into cardiomyocytes (331) and other lineage commitment (215, 381). From these studies, PI3K/AKT/GSK in addition to LIF/JAK/STAT and BMP/Smad prove to be critical factors to maintain ES cell pluripotency and self-renewal and keep ES cells at a so-called “ground state.” Such effect is achieved at least in part by blocking FGF-mediated ERK activation and subsequent cell differentiation (130). While these in vitro studies supply us with some insight regarding induction of cardiomyocyte cell fate, it still remains to be determined what, if any, role ERK1/2 plays in FGF signaling during cardiac cell fate determination in vivo. Along with cardiac lineage induction, FGF signaling through the Ras/Raf/ERK pathway plays a role in morphogenesis and growth throughout cardiac development. FGFs and their receptors are expressed throughout development in the epicardium, endocardium, and myocardium (226, 386). In many cases, FGF signaling has been shown to occur in both autocrine and paracrine fashions. Sugi et al. (386) have shown that endocardium derived FGF-4 signals to the endocardium and endocardial mesenchyme leading to proliferation and expansion of the cushion mesenchyme during valve leaflet formation (386). While this particular study did not look at the specific signaling events taking place, recent studies have shown that, in assays of cells from endocardial cushions, FGF-4 treatment increases phosphorylated ERK1/2 (38, 246). Likewise, endocardium- and epicardium-derived FGF-9 has been shown to contribute to the regulation of myocyte differentiation and proliferation in the myocardium via FGFR1 and -2 (226). Therefore, FGF signaling may contribute to cardiac morphogenesis; however, the connection for ERK pathway in this process remains to be further established.
Other than FGF signaling, other growth factors have also been shown to promote cardiac differentiation via the ERK pathway. Using mouse ES cells, Chen et al. (68) have shown that VEGF promotes cardiomyocyte differentiation in an ERK-dependent manner (68). In this study, treatment of mES cells with either recombinant VEGF165 or VEGF cDNA resulted in a significant increase in expression of α-myosin heavy chain (MHC), cTn-I, and Nkx2.5. Corresponding to this, ERK1/2 phosphorylation was increased in VEGF-treated mES cells, and treatment with PD098059, an ERK inhibitor, significantly decreased VEGF-induced α-MHC expression. However, more in vivo evidence is needed to support a role of VEGF-mediated signaling in cardiomyocyte differentiation. Likewise, other receptor tyrosine kinases can utilize ERK1/2 signaling during heart development. Epidermal growth factor receptors (EGFRs), also known as ErbB receptors (ErbB1, -2, -3, and -4), are another group of important players in cardiac development. Genetic inactivation of ErbB receptors (ErbB2, -3, and -4) and one of its known ligands, neuregulin-1, leads to embryonic lethality between E10.5 and E13.5 due to cardiovascular defects in trabeculation and cardiac cushion formation (319). ErbB receptors are known to signal in part through the ras/raf/MEK1/ERK pathway. However, while numerous studies have verified the ERK pathway in ErbB signaling in neonatal and adult myocytes (135), only a few studies have looked at its exact role in embryonic heart development. One recent study by Lia and Pawson (223) has begun to shed some light on this question. By targeted inactivation of ShcA, an adaptor protein associated with RTKs (including ErbB receptors), they demonstrated that this protein is involved primarily in pTyr signaling during cardiovascular development. Furthermore, ShcA null embyros died by E11.5 with cardiovascular defects similar to those seen in the Neuregulin-1-, ErbB2-, and ErbB4-deficient embryos, including a thin left ventricular myocardium associated with decreased trabeculation and defective formation of the cardiac cushions. In these embryos, a significant decrease in phospho-ERK was observed in regions that correlate with cardiovascular development and the normal pattern of ShcA expression. These findings indicate that ERK signaling indeed might be part of the ErbB signaling during development.
One other area in cardiac development that has definitively pointed to the role of ERK1/2 signaling is during valve development. Normal valve formation involves a process by which endocardial cushions are initially formed in the atrioventricular canal (AVC) and the out-flow tract (OFT), followed by cell proliferation and differentiation and the eventual morphological remodeling (16). Development of cardiac cushions is a result of endothelial-mesenchymal transdifferentiation (EMT) from a subset of endothelial cells. During this process, ErbB signaling is critical for integration of signals from the extracellular matrix to regulate cardiac cushion proliferation and EMT (16). As discussed previously, inactivation of ErbB and the corresponding ERK signaling results in disruption of cardiac cushion formation. In the cardiac jelly, hyaluronic acid (HA) has been shown to induce ErbB signaling (269). Camenisch et al. (60) have shown that in embryos deficient for Has2, an enzyme responsible for HA synthesis, endocardial cells overlaying the cardiac cushion forming area display reduced EMT and migration (60), a phenotype rescued by a constitutively active Ras. Likewise, the same study found that transfection with a dominant negative Ras was able to block the ability of HA to promote EMT.
Other evidence for the role of ERK in valve development comes from situations where there is an overactivation of Ras. Neurofibromin (NF1) functions as a Ras-specific GTPase activating protein (GAP) to inactivate Ras activity. NF1 mutations cause the autosomal dominant disorder neurofibromatosis. Among other manifestations of the disease, ~2% of neurofibromatosis patients have been reported to suffer from cardiovascular malformations (245). NF1-deficient mice die in utero at E14.5 with severe cardiac defects including enlarged cardiac cushions and double-outlet right ventricles (54). Using cushion tissue explants from nf1−/− embryos at E10.5, Lakkis and Epstein (224) identified a Ras-dependent increase in EMT as the cause of the enlarged cardiac cushion. They further demonstrated that adenovirus transfection of the nf1−/− cushion explants with a dominant-negative form of Ras inhibited EMT while the transfection of wild-type explants with a constituently active form of Ras increased EMT.
In addition to NF1 mutations, missense mutations in Ptpn11, which encodes for the protein tyrosine phosphatase Shp2, have been discovered in 50% of individuals suffering from Noonan syndrome, an autosomal dominant disorder characterized by congenital heart defects, most commonly pulmonary valve stenosis (306, 400). Shp2 is generally a positive regulator of RTK signaling, and its recruitment is necessary for Ras activation, although the underlying molecular mechanisms remain unclear. By expressing a gain-of-function mutant, Ptpn11D61G, Araki et al. (14) were able to recapitulate many of the characteristics of Noonan syndrome in mice. Approximately 50% of the Ptpn11D61G transgenic embryos manifested multiple cardiac defects, the severity of which depended on the number of copies of the mutant transgene gene. Furthermore, increased levels of phospho-ERK in the cardiac cushion of Ptpn11D61G embryos were accompanied by an increase in cell proliferation and a decrease in apoptosis. These findings are in good agreement with similar findings by Krenz et al. (212), in which expression of a slightly different gain of function mutant, Shp2 (Q79R), resulted in proliferation of valve primordia mesenchymal cells in an ERK1/2-dependent manner. Furthermore, it has recently been shown that an inducible knock-in of Ptpn11D61G also overactivates ERK signaling in endothelial-derived cells, leading to extended EMT, a phenotype previously seen in the mouse model of Noonan syndrome (13).
In addition to valve defects, Nakamura et al. (296) have demonstrated that Shp2 gain-of-function mutations in cardiomyocytes during embryonic development lead to defects in ventricular compaction and ventral septal defects but have no impact when expressed after birth. Expression of the Shp2 mutant in embryonic cardiomyocytes resulted in specific ERK activation without any change in the activity of any of the other MAPKs or in the Akt, JAK/STAT, or RhoA pathways. Furthermore, cardiac defects observed in the Shp2 mutant embryos were rescued by crossing with ERK null allele (296). In addition to Shp2, mutations in other components of the Ras signaling pathway including K-Ras (63, 302, 363), Sos1 (343), and Raf1 (315, 334) are also found in cases of Noonan syndrome. Finally, mutations in H-Ras, K-Ras, B-Raf, and MEK1/2 have also been discovered to be involved in other genetic disorders with cardiac developmental defects, such as LEOPARD syndrome, cardio-facio-cutaneous (CFC) syndrome, and Costello syndrome (10, 302, 345). This is covered in more detail by several excellent reviews (11, 37, 364, 408).
While much evidence suggests that the ERK1/2 pathway plays an important role in cardiac development at various stages, several key questions remain to be clarified. The specific contribution of ERK1/2 pathway in cardiac development remains to be fully investigated in vivo. Genetic deletion of ERK1 does not affect cardiac development while ERK2 deletion is embryonic lethal, but that is due to developmental defects of extraembryonic ectoderm and ectoplacental cone, not the cardiovascular system (139). Temporally regulated, cell-specific targeted and multiloci genetic perturbation may be required to unravel the full function of ERK in different stages of cardiac development. Furthermore, the direct and indirect interaction between ERK pathway and other signaling pathways, such as AKT (359) and BMP (111), will also need to be examined as the compensatory feedback regulation among these players may contribute to the delicate outcome of heart development (111, 130, 139, 359). Therefore, there is much more to learn regarding the exact role the ERK1/2 signaling plays in cardiogenesis.
B. JNK
The role that JNK plays in heart development is best characterized for its function in noncanonical Wnt signaling. Wnts are a large family of secreted proteins that are involved in many developmental processes including proliferation, differentiatation, cell migration, cell fate determination, and establishment of cell polarity (298). Wnt ligands promote signal transduction through their receptors, the frizzled family of transmembrane proteins. In canonical Wnt signaling, the cytoplasmic protein Dishevelled removes the inhibitory effect of glycogen synthase kinase 3 (GSK3) on β-catenin, which subsequently translocates to the nucleus and activates transcription (reviewed in Refs. 35, 110, 130). Wnt can also signal through noncanonical pathways, one mediated through Ca2+/PKC signaling and the other mediated through Rho/JNK (418).
Both canonical and noncanonical Wnt signaling have been implicated in heart development. During early heart formation, a delicate mix of canonical Wnt activation and inhibition is thought to inhibit cardiogenesis in areas not fated to become heart tissue and promote induction of cardiac cell fate in areas where it is (79). More relevant to this review, noncanonical Wnt signaling by Wnt11 has been found to be required for the adoption of cardiac cell fate. Overexpression of Wnt11 in chick embryo posterior mesoderm, which normally does not adopt a cardiac fate, led to ectopically differentiated cardiomyocytes (109). Likewise, Pandur et al. (316) were able to block early cardiac gene expression in Xenopus using either a dominant negative Wnt11 mutant or a Wnt11 morpholino. Conversely, they were able to induce cardiac gene expression in pluripotent animal caps by treatment with wild-type Wnt11. In the same study, Wnt11 treatment led to an increase in phospho-JNK levels, and that inhibition of JNK signaling prevented Wnt11 induction of cardiac cell fate. Similarly, Wnt11 treatment of P19 cells, a mouse embryonic carcinoma cell line which can differentiate into cardiomyocytes with DMSO treatment, led to an induction of early cardiac genes GATA4 and Nkx2.5 and expression of cardiac-specific protein α-MHC (316). Promotion of cardiac differentiation via Wnt11 signaling has been shown in a number of settings. Recent studies using mouse ES cells have further demonstrated the role of Wnt11 during induction of cardiac cell fate (405, 412). However, the exact role of JNK was not investigated in these studies. In studies involving a population of circulating human endothelial progenitor cells, which have been shown to differentiate into cardiomyocytes under specific culture conditions, Wnt11 treatment increased the number of cells expressing α-sarcomeric actinin and troponin I (211). However, this was found to be dependent on noncanonical signaling via the PKC pathway with no involvement of JNK. Interestingly, it has been shown that noncanonical Wnt11 signaling is able to promote adult unfractionated bone marrow mononuclear cells (BMMNCs), which retain some cells in an uncommitted state, to adopt a cardiac phenotype (123). Treatment of BMMNCs with Wnt11 induced expression of cardiac specific genes (Nkx2.5, GATA-4, α- and β-myosin heavy chain, and cardiac troponin T) which was partially abolished by the JNK inhibitor SP600125. Furthermore, noncanonical Wnt signaling has also been implicated in the differentiation of cardiomyocytes from adipose-derived murine stromal vascular cells, but neither JNK nor PKC was directly implicated in the process (313).
Along with promoting adoption of cardiac cell fate, JNK signaling has been implicated in the morphogenesis of the developing heart. Zhou et al. (467) have reported that noncanonical Wnt11 signaling is required for proper outflow tract development in mouse (467). Using a combination of in situ hybridization and cell culture experiments, these authors identify JNK-mediated transcription of Tgfb2 as a downstream signaling event responsible for Wnt11-mediated morphogenesis. The combination of these findings would indicate that noncanonical Wnt signaling plays a clear role in determining cardiac cell fate and organ morphogenesis. What’s not clear is the extent that JNK signaling plays in Wnt function. JNK-deficient mice (either single gene knockouts or combinations thereof) display no significant defects in cardiogenesis (139). Therefore, the role of JNK signaling in cardiac development remains a correlative observation associated with noncanonical Wnt signaling, and its specific role in cardiac development remains speculative.
C. p38
While p38 has been shown to have an integral role in skeletal muscle development (198), its function in cardiac development has not been as extensively studied. Recent studies point to a possible role for p38 in BMP signaling during cardiac development. Other than Smads, BMPs can also signal via activation of the MAPK pathways (417). While p38 activation in response to BMP signaling has been shown, little work has been done to elucidate what, if any, role this particular downstream pathway plays in BMP-mediated regulation of cardiac development, including cardiac induction (32, 365), as well as OFT and ventricular formation (417). TAK1, a known MAPK upstream of p38 which can be induced by BMP (283), regulates a number of transcription factors, including MEF2C, GATA-2, and ATF-2, all critical to cardiac development (282, 355). While this correlation exists, work is just beginning to be done to better delineate p38’s role in cardiac development.
The idea that p38 is an important factor in cardiomyocyte differentiation is supported by a number of other recent studies. For example, p38 activity is required for cardiomyocyte differentiation of P19CL6 cells, and this is mediated via its activation of the transcription factor AP-1 (115). Interestingly, this same study, which demonstrated that ERK inhibition only partially blocked cardiomyocyte differentiation, also showed a cooperative role between p38 and ERK in the AP-1 activation necessary for differentiation. Further evidence of the importance of p38 in cardiomyocyte differentiation comes from the discovery that p38 promotes cardiogenesis over neurogenesis in ES cells (12). Aouadi et al. (12) reported that p38 activity appeared to be critical to promote cardiogenesis from embryonic body (EB) and to suppress spontaneous neuronal differentiation (12), thus implicating a role for p38 in early switch between cardiogenesis and neurogenesis. This is further supported by the evidence that p38-mediated activation of Hsp25 is required for cardiac differentiation from P19 cells while P19 differentiation into neurons had no such requirement (91). p38 has also been implicated in ROS-mediated cardiac differentiation from mES cells (99, 235, 432). While many studies have indicated that p38 is needed to promote cardiac differentiation of ES cells, two recent studies have demonstrated that use of the p38 specific small molecule inhibitor SB203580 at concentrations <10 μM promotes the differentiation of human ES cells into cardiomyocytes (142, 442). Interestingly, when they use the p38 inhibitor at concentrations ≥15 μM, cardiogenesis was strongly inhibited. Furthermore, p38 activity in ES cells undergoing cardiac differentiation has been shown to be a phasic event, with peak activity between days 3 and 10 of differentiation (100). When p38 was inhibited between days 3 and 7, cardiac differentiation was shown to be greatly reduced, while inhibition from day 5 on had only a slight effect. This is supported by a study in P19 cells which showed that p38 activity is only required during a particular window early in the differentiation process (91). This could reconcile some of the conflicting findings as the requirement of p38 activity during cardiac differentiation can be highly specific at different phases. Indeed, Engel et al. (112) have demonstrated in vivo p38 activity in the developing rat heart is biphasic; however, they only examined this during the later stages of gestation (E12-E21). Many of the transcription factors that are known to be involved in cardiac development, including GATA4 (403), MEF2C (149), and SRF (157), are known targets of p38. However, to date, no one has fully investigated p38’s role in regulating these key players during cardiac development. Immortalized E9.5 myocytes from p38α−/− embryos have diminished capacity to fully differentiate (158). However, expression of cardiac-specific genes GATA4 and MEF2C are not changed, indicating that their early expression is not p38 dependent. Still, these cells lack nuclear localization of MEF2C and exhibit a decrease in expression of some marker genes of cardiac differentiation, including ANF and myocardin. This suggests MEF2C at least in part contributes to p38-mediated regulation of cardiac differentiation.
Unfortunately, while cell culture studies have strongly implicated p38 as an important player in cardiogenesis, there is little to no in vivo data supporting this hypothesis. As mentioned previously, p38α−/− embryos are embryonic lethal due to defects in placental angiogenesis. However, cardiac-specific deletion of p38α results in normal development of the heart (139). As in vitro studies have shown, there seems to be a requirement for p38 activity at a very specific time during the developmental process. Since the cardiac specific knockout animals were created using the cre-lox system, by the time that the promoter driving the expression of the cardiac-specific cre is expressed p38 activity may no longer be an absolute requirement. Furthermore, total knockout of other p38 isoforms (β, δ, γ) produced no apparent cardiac phenotypes (Rose, Foster, and Wang, unpublished results; Ref. 139). To fully investigate the functional role of p38 in cardiac development, temporal inactivation of individual p38 isoforms or in combination will be needed.
D. ERK5
The role of ERK5 in cardiac development is more established. Deletion of either ERK5 or its upstream activator, MEK5, is embryonic lethal at approximately E10 due to an underdeveloped myocardium, disorganization of the trabeculae, and vascular defects (335, 423, 449). Subsequent studies utilizing targeted deletion of ERK5 in either endothelial cells or cardiomyocytes have shed more light on the role of ERK5. While endothelial cell specific deletion recapitulated the phenotype seen in the global knockout mice, mice with myocyte specific deletion developed normally (154). This suggests that the abnormal phenotype in ERK5 null animals is mostly secondary to vascular defects. Indeed, ERK5 null endothelial cells display abnormal morphology and survival, leading to dysfunctional vasculature and hemorrhage when the genetic inactivation is achieved in adult animals (154).
While the embryonic defects appear to be due to ERK5’s role in endothelial cells, this does not rule out the possibility that perhaps ERK5 activity in cardiomyocytes is required prior to expression of the cardiac specific gene (α-MHC) that is driving cre expression in the previous studies. ERK5 is a known activator of MEF2A, -C, and -D (194, 195, 451). As such, it is plausible that it may play a role in the early stages of cardiac differentiation, prior to the expression of α-MHC. Recent work in rat cardiac myoblasts has shown that retinoic acid (RA), a known promoter of proliferation during heart development, induces ERK5 activity and nuclear translocation, resulting in increased cellular proliferation (338). Therefore, ERK5 has an established role in vascular development, and its role in cardiomyocyte differentiation and proliferation is unclear. Further research needs to be done to better elucidate a more exact role for ERK5 in heart development.
IV. MITOGEN-ACTIVATED PROTEIN KINASES IN HEART FUNCTION AND DISEASE
Cardiovascular diseases affect one in three adults in the United States and account for approximately one-third of all deaths (1). Heart failure, in particular, has become a major cause of mortality and morbidity. Better understanding of the molecular mechanisms behind these diseases will allow for better treatment options in the future. The following sections focus on the role of MAPKs in various pathological aspects of cardiac diseases, with particular emphasis on hypertrophy, cardiac remodeling, and myocardial cell death.
Cardiac hypertrophy is a common response to external stressors, including mechanical overload, neurohor-monal stimulation, and oxidative stress. Hypertrophy can be a compensatory response to augment contractility and maintain cardiac output without adverse pathology. However, when stressors persist, this compensatory process can evolve into a decompensated state with profound changes in gene expression profile, contractile dysfunction, and extracellular remodeling (101, 369). Although physiological versus pathological hypertrophy can be clearly differentiated by a number of qualitative and quantitative parameters, the underlying mechanisms and their interrelationship remain controversial. Most importantly, the signaling mechanisms mediating the critical transition from compensated hypertrophy to decompensated heart failure remain poorly understood (104, 133, 369). Furthermore, while some cardiomyopathies are genetic and others idiopathic, many are the result of some sort of insult or injury to the myocardium. Myocardial ischemia and/or infarction due to partial or complete occlusion of a coronary artery and the subsequent reperfusion of the tissue (ischemia-reperfusion or IR) are among the most significant causes of injury to the heart. Most of the efforts in the past have focused on the underlying mechanisms of IR-induced myocardial injury or on cardiac protection offered by preconditioning or postconditioning (23). The signaling mechanisms involved in these events (injury versus cardiac protection) are distinctly different. However, recent work has shown that protective events are diminished in various pathological conditions commonly associated with cardiovascular disease (hyperglycemia, hypertension, cardiac hypertrophy, aging, obesity), pointing to the importance of understanding the signaling pathways involved (24). Finally, pathological manifestations in end-stage failing hearts share many common features regardless of the underlying etiologies, such as ventricular wall thinning, chamber dilation, cardiomyocyte dropout, and dramatically increased interstitial fibrosis (103), suggesting that intracellular signaling pathways elicited by different stressors may converge to some common targets. As highly conserved signaling pathways, MAPKs may be common mediators in these pathological remodeling processes. Although this review focuses primarily on MAPK function in cardiomyocytes, we fully appreciate the importance of other cardiac cell types in the development of heart failure. Normal cardiac function and pathological remodeling involve fibroblasts, the coronary vascular system, and inflammatory cells. Due to the limited scope of this review, the role of MAPKs in these other cell types is not discussed. However, given their importance in cardiac pathologies, the reader is directed to a number of excellent reviews regarding these topics (33, 131, 136, 160, 259, 325, 346). In this review, we highlight recent progress made in understanding these intricate roles for MAPKs in different aspects of hypertrophy, cardioprotection versus myocardial cell death, and general cardiac remodeling events, including chamber dilation, fibrosis, and changes in structural proteins and ion channels, as summarized in Table 1. Although much of the recent progresses are made through advanced wizardry of genetic manipulation in model organisms such as mice, it is important to appreciate some of the limitations of this powerful approach. Genetic manipulation through complete knockout and nonphysiological overexpression can produce a phenotype that may not truly reflect the functional role of the targeted molecule or pathway in a particular pathological condition. Compensatory, secondary, or off-target effects can arise from such nonphysiological manipulation to obscure correct interpretations. In addition, some of the genetic manipulation itself can lead to unwanted side effects, including cytotoxicity of GFP, Cre, and tamoxifen induction (58, 170, 206). Therefore, results from genetic studies should be interpreted with plenty of caution by taking into account some of these caveats.
TABLE 1.
Mitogen-activated protein kinases in cardiac pathology
| Pathology | MAPK | Specific Protein Examined | Conclusion | Model | Reference Nos. |
|---|---|---|---|---|---|
| Hypertrophy | ERK1/2 | MEK1 | ↑ | CA and DN MEK1 expression in isolated myocytes; exposure to ET-1, PE, and ISP CA MEK1 TG hearts; natural development of concentric hypertrophy |
57, 414 |
| Ras | ↑ | CA MLC-2v-H-Ras-V12 and αMHC-H-Ras-V12 TG hearts; natural development of hypertrophy | 176, 466 | ||
| Raf | ↑ | DN Raf-1 TG hearts; subjected to TAC; resistant to development of hypertrophy | 150, 444 | ||
| ERK1/2 | ⇆ | ERK 1−/− KO and ERK2+/− (germline) mice and inducible DUSP6 TG hearts; subjected to TAC, swimming, ANG II and ISP; no comparable difference in hypertrophic response | 328 | ||
| RTK and GPCR | ↑ | ERK signaling in response to various RTK and GPCR stimulation both in vitro and in vivo. Please refer to text and references cited for more detail | 9, 70, 84, 197, 217, 255, 437, 438, 452, 457 | ||
| ERK | ↑ | ERK autophosphorylation in isolated cardiomyocytes exposed to ANG II and in hearts subjected to TAC ERK2 Thr188 mutant TG hearts; subjected to TAC |
252 | ||
| JNK | MKK4 | ↑ | DN MKK4 expression in isolated myocytes; exposure to ET-1 DN MKK4 adenoviral gene transfer to heart; subjected to TAC; induced hypertrophy inhibited |
73, 74 | |
| MKK4 | ↓a/⇆b |
aCKO of MKK4; subjected to TAC and chronic ISP; increased hypertrophic response bCKO of MKK4; subjected to swimming; no comparable difference in hypertrophic response |
250 | ||
| JNK | ↓ | DN JNK1/2 TG hearts; subjected to TAC; increased hypertrophic response KO of JNK1−/−, JNK2−/−, and JNK1±JNK2−/− subjected to TAC; increased hypertrophic response in JNK2−/− and JNK1±JNK2−/− |
239 | ||
| JNK | ⇆ | KO of JNK1−/−, JNK2−/− and JNK 3−/−, subjected to TAC; no comparable difference in hypertrophic response | 388 | ||
| MKK7 | ↑a/⇆b |
aCA MKK7 expression in isolated cardiomyocytes bCA MKK7 TG hearts; naturally develop heart failure but no significant difference in myocyte hypertrophy |
322, 426 | ||
| p38 | MKK3/6 | ↑a/⇆b |
aCA MKK3/6 expression in isolated myocytes bCA MKK3/6 TG hearts; naturally developed heart failure but no significant difference in hypertrophy |
242, 299, 425, 460 | |
| MKK3/6 | ↓ | DN MKK3/6 TG hearts; naturally developed concentric hypertrophy DN MKK3/6 TG hearts; subjected to acute abdominal aortic banding, and ANG II, ISP, and PE infusions; enhanced hypertrophy |
55 | ||
| p38 | ↑ | DN p38 expression in isolated cardiomyocytes Use of p38 inhibitors in isolated cardiomyocytes; exposure to ET-1, PE, LIF; blocked hypertrophic response |
240, 299, 434, 460 | ||
| p38 | ⇆a/↓b |
aDN p38 TG hearts; subjected to TAC and to swimming; no significant differences in hypertrophic response bDN p38 TG hearts; subjected to acute abdominal aortic banding, and ANG II, ISP, and PE infusions; enhanced hypertrophy |
55, 427, 464 | ||
| p38 | ⇆a/↓b |
aCKO of p38; subjected to TAC; so significant difference in hypertrophic response bCKO of p38; subjected to swimming; enhanced hypertrophy |
303, 398 | ||
| ERK5 | MEK5 | ↑ | CA MEK5 expression in isolated cardiomyocytes; myocyte elongation DN MEK5 expression in isolated cardiomyocytes; exposure to LIF; blocked myocyte elongation |
301 | |
| MEK5 | ↑a/⇆b |
aCA MEK5β TG hearts; naturally develop eccentric hypertrophy bCA MEK5α TG hearts; subjected to IR; no significant difference in hypertrophic response |
61, 301 | ||
| Myocardial cell death | ERK1/2 | MEK1 | ↓ | CA MEK1 expression in isolated cardiomyocytes; subjected to 2-deoxyglucose treatment; decreased apoptosis CA MEK1 TG hearts; subjected to IR; decreased infarct area |
57, 247 |
| Raf | ↓ | DN Raf-1 TG hearts; subjected to TAC; increased apoptosis | 148, 150 | ||
| ERK | ↓ | KO EKR1−/− and ERK2−/− subject to IR; increased infarct area (ERK2 specifically) | 247 | ||
| ERK | ↓ | ERK signaling as part of the reperfusion injury salvage kinase (RISK) pathway both in vivo and in vitro | 152, 153 | ||
| JNK | MKK7 | ↓ | CA MKK7 TG hearts; subjected to IR; decreased infarct size | 190 | |
| JNK | ↓ | DN JNK expression in isolated cardiomyocytes; exposure to nitric oxide and hypoxia/reoxygenation; increased apoptosis Use of JNK inhibitors in isolated cardiomyocytes; simulated IR; increased apoptosis |
5, 106, 114, 370, 410 | ||
| JNK | ↑ | JNK inhibition by antisense oligonucleotides in isolated cardiomyocytes; ischemia/reoxygenation; decreased apoptosis Use of JNK inhibitor in rat; subjected to IR; decreased infarct size |
119, 169, 273 | ||
| Pro-apoptotic proteins | ↑ | JNK interaction with multiple pro-apoptotic proteins has been seen in both in vitro and in vivo models (see text and citations) | 4, 8, 22, 208, 337, 376, 413, 463 | ||
| JNK | ↑ | DN JNK1/2 TG hearts; subjected to IR; decreased infarct size KO of JNK1 and JNK2; subjected to IR; decreased infarct size |
190 | ||
| p38 | p38 | ↓ | Use of p38 inhibitors in isolated perfused hearts; subjected to IR; increased infarct size, cardioprotective during ischemia (mostly in context of preconditioning) | 266, 279, 428, 460 | |
| p38 | ↑ | Use of p38 inhibitors in vivo, in isolated perfused hearts, and isolated myocytes; subjected to IR; decreased infarct size and apoptosis | 26, 36, 256, 257, 262, 291, 358 | ||
| p38 | ↓/↑ | Use of DN isoforms in TG hearts and isolated cardiomyocytes; subjected to IR; p38β imparts greater cardioprotection than p38α | 82, 189, 200, 201, 310, 425 | ||
| ERK5 | MEK5 | ↓ | CA MEK5 TG hearts, isolated perfused hearts subjected to IR; decreased infarct size CA MEK5 TG hearts, subjected to TAC; decreased apoptosis |
61, 373, 447 | |
| Remodeling | ERK1/2 | Ras | ↑ | αMHC-H-Ras-V12 TG hearts; exhibit early response gene induction, mitochondria dysfunction | 276 |
| Ras | ↑ | CA H-Ras-V12 expression in isolated cardiomyocytes; reduction in L-type Ca2+ currents αMHC-H-Ras-V12 TG hearts; SR Ca2+ defect Ras-ERK signaling involved in other aspects of modulating of Ca2+ handling in vitro and in vivo (see text and citations) |
145, 164, 349, 389, 466 | ||
| ERK | ↑ | ERK signaling involved in modulation of the Na+/H+ exchanger and K+ channels in myocytes (see text and citations) | 125, 126, 404, 420 | ||
| JNK | MKK7 | ↑ | CA MKK7 TG hearts; naturally develop selective extracellular matrix remodeling (no increase in collagen but significant increase in fibronectin) | 320 | |
| MKK7 | ↑ | CA MKK7 expression in isolated cardiomyocytes; loss of connexin43 and cell-cell communication CA MKK7 TG hearts; naturally develop loss of gap junctions |
321, 415 | ||
| JNK | ↓ | KO of JNK1−/−; subjected to TAC; increased fibrosis Use of JNK inhibitors in dilated cardiomyopathy model in vivo; increased fibrosis |
218, 388 | ||
| JNK | ↑ | JNK signaling promotes fibrosis as part of various signaling events in vitro and in vivo (see text and citations) | 71, 117, 179, 183, 374 | ||
| p38 | MKK3/6 | ↑ | CA MKK3/6 TG hearts; naturally develop restrictive cardiomyopathy with interstitial fibrosis |
242 236, 276, 402 |
|
| MKK3/6 | ↑ | CA MKK3/6 TG hearts; inflammatory and extracellular remodeling gene activation CA MKK3 adenoviral gene transfer to heart; cell cycle and inflammatory gene induction |
236, 276, 402 | ||
| p38 | ↓ | CKO of p38; subjected to TAC; significant increase in fibrosis | 303 | ||
| ERK5 | ERK5 | ↑ | Inducible KO of ERK5; loss of vascular integrity | 154, 178, 323 |
↑, Mitogen-activated protein kinase (MAPK) promotes hypertrophy, myocardial cell death, or remodeling; ↓, MAPK prevents hypertrophy, myocardial cell death, or remodeling; ⇆, MAPK had no effect on hypertrophy, cardioprotection/myocardial cell death, or remodeling. CA, constitutively active; DN, dominant negative; TG, transgenic; CKO, cardiac specific knockout; KO, germline knockout; ET-1, endothelin-1; PE, phenylephrine; LIF, leukemia inhibitory factor; ISP, isoproterenol; TAC, transverse aortic constriction; IR, ischemia/reperfusion; RTK, receptor tyrosine kinase; GPCR, G protein-coupled receptor.
A. ERK1/2
1. Cardiac hypertrophy
Many studies have implied a role for the Ras/Raf/MEK1/ERK signaling pathway in promoting cardiac hypertrophy. Hunter et al. (175) initially showed that transgenic expression of a constitutively active Ras (H-Ras-V12) in mouse heart led to left ventricular hypertrophy associated with cardiomyocyte hypertrophy but not increased cardiac fibrosis. Subsequently, Zheng et al. (466) observed characteristic features of familial hypertrophic cardiomyopathy (HCM) in another H-Ras-V12 transgenic model, including fetal-gene induction, myofilament disarray, and interstitial fibrosis which led to diastolic dysfunction (466). While both of these studies used the same constitutively activated H-Ras-V12 mutant, different promoters were used to drive its expression (MLC-2v versus α-MHC, respectively), possibly reflecting dose-dependent effects of Ras signaling driving hypertrophy versus cardiomyopathy. Gene expression profiling in temporally regulated αMHC-H-Ras-V12 transgenic mice suggests that overactivation of this pathway induces early response genes, loss of mitochondria function, and alteration in ion channel proteins, all of which lead to pathological changes in the extracellular matrix, reduced cardiac output, and electrophysiological abnormalities (276). Ras mRNA expression in HCM patients identified a positive correlation with the severity of hypertrophy (188). Likewise, patients suffering from so-called RAS/MAPK syndromes, a group of autosomal dominant disorders linked to mutations causing augmented Ras/Raf/MEK/ERK activity (e.g., Noonan and LEOPARD syndromes), exhibit hypertrophic cardiomyopathy (11). Finally, in response to mechanical unloading afforded by use of a left ventricular assist device (LVAD), reverse remodeling and reduction in myocyte hypertrophy in the post-LVAD heart is associated with decreased ERK activity (124). Conversely, an endogenous inhibitor of the ERK pathway, Sprouty-1, has been reported to be induced in human hearts during hypertrophy regression following LVAD support (174). Overexpression of MEK 1, the upstream activator of ERK1/2, has shown similar overactivation of Ras. Constitutively active MEK1 leads to cardiomyocyte hypertrophy in vitro, while dominant negative MEK1 attenuates this response (414). In vivo, cardiac-specific expression of constitutively activated MEK1 also promotes hypertrophy (57). However, unlike Ras overactivation, the MEK1 transgenic heart has no increase in fibrosis and displays preserved cardiac function, suggesting MEK-ERK may not be the critical downstream signaling pathway for Ras-induced pathological remodeling.
Complimentary to these gain-of-function approaches, Harris et al. (150) have demonstrated that inhibition of the ERK pathway via dominant negative Raf attenuated hypertrophy and fetal gene induction in response to pressure overload. Likewise, Yamaguchi et al. (444) have shown that cardiac specific deletion of c-raf-1 leads to heart failure without hypertrophy in the absence of external stress. Both groups found that, while there was an apparent lack of hypertrophy, there was a significant increase in apoptosis associated with Raf inactivation. This is consistent with the observation that overactivation of the ERK pathway causes both hypertrophy and a partial resistance to apoptosis (57). However, the antiapoptotic activity of Raf appears to be primarily due to Raf binding to and directly suppressing the proapoptotic kinases Ask1 and Mst2 independent of MEK/ERK activities (65, 66, 444). Similar results were also obtained when the protein tyrosine phosphatase Shp2 was deleted from the myocardium. As discussed previously, Shp2 is an essential component of RTK signaling through the Ras/Raf/MEK/ERK pathway, and a gain-of-function mutation in this protein causes craniofacial and cardiovascular defects in Noonan syndrome. Deletion of Shp2 in the myocardium leads to dilated cardiomyopathy without transition through hypertrophy at baseline or following pressure overload associated with diminished ERK activation (209). These findings, specifically the onset of dilated cardiomyopathy without transitioning through hypertrophy, are similar to what was observed in the c-raf-1 knockout animals. Furthermore, GSK3α has been shown to block cardiac hypertrophy both in vitro and in vivo via inhibition of ERK signaling (462). While all of these findings strongly suggest that ERK contributes to hypertrophy in the myocardium, one study by Purcell et al. (328) suggests that reduction in ERK activity is not sufficient to prevent hypertrophy in response to various forms of hypertrophic stimuli in vivo. Achieved by either overexpression of dual specific phosphatase 6 or deletion of ERK (ERK1−/− or ERK2+/−), these modifications led to an increase in apoptosis without a significant impact on hypertrophy. These results suggest that ERK activity is an important pathway for cardioprotection but that cardiac hypertrophy can proceed via ERK-independent mechanisms.
In addition to growth factor-mediated signaling through RTKs (77), signaling via G protein-coupled receptors (GPCRs) has also been shown to promote cardiac hypertrophy (352), and in a number of settings, this has been shown to be mediated via ERK signaling. β-Adrenergic agonists promote cardiomyocyte hypertrophy via direct interaction between ERK and β-arrestin (30, 352). Interestingly, signaling from β-adrenergic receptors, which can lead to detrimental effects in the failing heart, utilize β-arrestin to transactivate RTK signaling via ERK (305). This β-arrestin-dependent, G protein-independent signaling by those receptors is thought to be cardioprotective. This is exemplified by the recent discovery that carvedilol, a nonsubtype-selective β-adrenergic receptor antagonist that has been shown to be particularly effective in treatment of heart failure, promotes signaling via β-arrestin-dependent ERK1/2 activation in the absence of G protein activation (431). Likewise, other GPCRs, including α-adrenergic receptors (217, 437, 438), angiotensin receptors (9, 452), and endothelin receptors (70, 84, 197, 255, 457), have been shown to signal through ERK to promote cardiomyocyte hypertrophy. In addition to arrestin-mediated ERK activation, Wright et al. (435) provided another evidence that nuclear targeted α-adrenergic receptor might activate ERK located in caveolae, although the underlying molecular basis remains unclear. More recently, Lorenz et al. (252) have identified heterotrimeric G protein-mediated autophosphorylation of ERK as yet another hypertrophic signaling mechanism leading to ERK activation. In these studies, activation of Gq-coupled receptors was sufficient to mediate a protein-protein interaction between Gβγ and ERK, leading to autophosphorylation and translocation to the nucleus and activation of prohypertrophic substrates. This novel autophosphorylation-mediated ERK activation was sufficient to induce hypertrophy both in vitro and in vivo and was also shown to be present in failing human hearts. Finally, a recent report by Cervante et al. (64) suggests that crosstalk of GPCRs can be orchestrated by arrestin to achieve spatiotemporal activation of ERKs in nucleus versus cytoplasm, leading to different functional outcome. In addition to ligand-mediated mechanisms, Ras activation can be facilitated by direct oxidative modification of its thiol groups (217, 446), thus providing another possible molecular link between oxidative stress and the onset of cardiac hypertrophy. In short, Ras-Raf-MEK1-ERK1/2 pathway is generally regarded as a prohypertrophic and prosurvival pathway that can be a significant but not a necessary signaling component in cardiomyocyte hypertrophy.
2. Cardioprotection versus myocardial cell death
The cardioprotective effects of two classes of drugs commonly used to treat cardiac related diseases, Ca2+ channel blockers and β-adrenergic receptor blockers, have been reported to be mediated in part through ERK1/2 activity (210). In vivo studies in which c-Raf-1 activity in the heart was lost showed an increase in apoptosis both at baseline and in response to pressure overload (150, 444). Similarly, the Molkentin group has identified specific MEK-ERK2 signaling as a mediator of cardioprotection (247). In response to ischemia-reperfusion injury, MEK transgenic hearts were better protected from injury and apoptosis than wild-type controls, an effect that was lost when ERK2 was specifically deleted. Indeed, ERK1/2 signaling has been identified as one of the major components of the RISK (reperfusion injury salvage kinase) pathway. As a result of this idea, a plethora of studies have subsequently shown that activation of the ERK pathway by various stimuli leads to cardioprotection during reperfusion (reviewed in Ref. 152). While the role of ERK signaling in preventing reperfusion-induced injury is well established, its role in preconditioning is less well understood, and conflicting results have been reported (107, 153). ERK’s cardioprotective role has also been investigated in relation to the chemotherapeutic agent doxorubicin (DOX). DOX is known to induce myocardial damage, including cardiomyopathy and myocyte apoptosis (395). While the mechanism of DOX-induced cardiac damage is multifaceted, downregulation of ERK1/2 activity has been suggested to play a role. Indeed, DOX-induced cardiotoxicity was prevented by the administration of substrates that increased ERK1/2 activity (383, 436). Conversely, recent work done in cultured myocytes has suggested a functional link between ERK1/2 and p53 actually promotes apoptosis in response to DOX (248).
While much work has been done identifying upstream activators of cardioprotective ERK1/2 signaling, much less is known regarding the exact mechanism by which it imparts this protection. Multiple mechanisms may exist for prosurvival effects of ERK1/2. Work by Das et al. (90) has shown that the protective effect of ANG II-mediated preconditioning is due in part to ERK1/2 dissociating from caveolin. Similarly, ERK1/2 has been shown to play a role in cGMP-dependent protein kinase (PKG)-mediated cardioprotection in response to IR (87, 89). ERK1/2 activation in this case resulted in increase expression of inducible nitric oxide synthase (iNOS), endothelial NOS (eNOS), and Bcl-2. ERK1/2 has also proposed to exert its cardioprotective effects by phosphorylating and activating the transcription factor GATA4, which can then increase the expression of antiapoptotic proteins in neonatal ventricular myocytes (15, 205, 241, 284). However, recent work has shown that this does not hold true in adult cells. While GATA4 still promotes survival, it was found not to be downstream of ERK1/2 signaling in response to α1-adrenergic receptors, a previously described survival pathway in cardiomyocytes (171, 172). ERK1/2 may also promote survival of cardiomyocytes by interacting with other signaling pathways. IL-10 mediated ERK1/2 activation was shown to inhibit TNF-α-induced apoptotic signaling by blocking IKK phosphorylation and subsequent NFκB activation (96). Likewise, ERK1/2 has also been shown to compensate for loss of Akt activity in postinfarct myocardium and promote cardioprotection in response to erythropoietin (272). Finally, ERK1/2 has been found to suppress gap junction permeability in response to mitoKATP channel opening during IR, thus reducing myocardial damage (293). As noted above, the ERK-independent cardioprotective activity of Raf is mediated through direct suppression of proapoptotic kinases, Ask1 and Mst2 (65, 66, 444). In short, Ras-Raf-MEK-ERK1/2 may exert strong cardioprotective effects via multiple downstream targets, but much remains to be done to delineate their specific contribution under particular circumstances.
3. Cardiac remodeling
As discussed above, unregulated Ras-Raf-MEK-ERK signaling can lead to both hypertrophy and pathological remodeling in heart. Gene expression profiling in temporally regulated α-MHC-H-Ras-v12 transgenic mice suggests that overactivation of this pathway induces early response genes, loss of mitochondrial function, and alteration in ion channel proteins, all of which can contribute to extracellular matrix remodeling, reduced cardiac output, and electrophysiological abnormalities observed in HCM (276). In addition, Ras activation can have a direct impact on SR calcium cycling in ventricular myocytes both in vitro and in vivo. In vitro expression of Ha-Ras-V12 in cultured myocytes leads to downregulation of L-type Ca2+ channel expression and activity in an ERK-dependent manner (164). Furthermore, activation of Ras leads to decreased expression of SERCA in cultured myocytes (165). In vivo studies, while finding no change in L-type Ca2+ channels or sarcomeric structure, showed decreased Ca2+ transients secondary to suppressed SR Ca2+ uptake as a result of decreased SERCA expression and hypophosphorylation of phospholamban (466). More recently, Ruan et al. (349) reported that Giα1 induction and subsequent impairment of PKA signaling appears to be one of the key downstream mediators in Ras-induced SR calcium defects and arrhythmia. Yada et al. (443) also reported a role for another Ras-like small GTPase, Rad, in modulating calcium homeostasis and electrophysiological properties in ventricular myocytes. Therefore, unregulated Ras signaling may have a direct impact on cardiac function and electrophysiology, but downstream signaling appears to be different from the canonical MEK-ERK cascade. The molecular basis is just beginning to emerge. LIF, a hypertrophic stimulus in cardiomyocytes, leads to increased L-type Ca2+transients in an ERK-dependent manner (145, 389). These findings imply that the specific outcomes of ERK activation may be dependent on the activating stimulus, again demonstrating the complexity of these signaling networks. In addition to playing a role in Ca2+ channel regulation, ERK signaling also plays a role in regulation of other ion channels including potassium channels and the Na+/H+ exchanger in the myocardium (125, 126, 404, 420). In short, the Ras-Raf-MEK-ERK pathway can induce SR calcium defects and arrhythmias in the heart by modulating ion channels, exchangers, and pumps and serves as a potential contributor to the contractile defects and sudden cardiac arrest prevalent in hypertrophic cardiomyopathy.
In summary, both classic RTK-mediated and GPCR-mediated ERK activation have significant roles in cardiac hypertrophy and cardioprotection. However, the functional outcome of ERK activation can be modulated and altered by scaffolds in a specific spatiotemporal pattern. This complexity in ERK pathway leads to different phenotypes from “physiological” form of compensated hypertrophy and cardioprotection to pathological form of hypertrophic cardiomyopathy and remodeling. Therefore, the intricate ERK activation mechanisms must be carefully considered when we attempt to target ERK pathway as a potential therapy for heart failure.
B. JNK
1. Cardiac hypertrophy
The role that JNK plays in cardiac hypertrophy is less clear. JNK activity is substantially upregulated (as quickly as 10–15 min after application of pressure overload) and reaches a maximal level at ~30 min (122, 289). While JNK2 is activated in the cytosol, there is a significant increase in translocation of activated JNK1 to the nucleus (289). The transient activation of JNK is clearly seen in severe pressure overload (85% constriction of the aorta) but not volume-overloaded hearts. In pressure overload, activation peaks between 10 and 30 min (378). In contrast, mild pressure overload (35% constriction of the aorta) exhibits a transient increase in JNK activity at 30 min, but higher levels are seen at 1 and 2 days postconstriction. In contrast, another recent study in rat has shown that in response to pressure overload, JNK activity is actually decreased in the first 24 h postaortic banding (347). In this particular study, ERK activity was also decreased despite an upregulation of angiotensin receptors. These findings fit with other findings which show that there was no significant increase in JNK1/2 activity after 24 h in either pressure- or volume-overload experiments (277). Mechanical stretch of cultured myocytes also activates JNK in a rapid and phasic manner (288, 314). Furthermore, exercise, an acute form of stress, has been shown to also cause a rapid transient increase in JNK activity that is not present in exercised trained rats which exhibited a physiological cardiac hypertrophy (44). Taken together, these findings indicate that JNK activation is most likely a dynamic signaling event that can be influenced by the nature of the stimuli and that different JNK isoforms may play separate, nonredundant roles in the process.
Initial studies in cultured neonatal cardiomyocytes indicated that overactivation of JNK by MKK7, an upstream MAP2K, leads to a hypertrophic phenotype (426). Correspondingly, dominant negative MKK4, another upstream MAP2K, was able to attenuate the endothelin-1-induced hypertrophic response in cultured myocytes (74). Likewise, initial in vivo studies in rats showed that dominant negative MKK4 abrogated JNK activity and pressure overload-induced hypertrophy (73). These findings would suggest that JNK activity is responsible, in part, for the promotion of cell hypertrophy. In contrast, recent work by Lui et al. (250) has shown that cardiac-specific deletion of MKK4 sensitizes the myocardium to pathological hypertrophy following pressure overload or chronic β-adrenergic stimulation. Similarly, disruption of JNK activity (dominant negative JNK) in the heart contributes significantly to hypertrophy following pressure overload (239). This is shown to be due in part to the ability of JNK to inhibit the translocation of the prohypertrophic transcription factor NFAT into the nucleus (239, 250, 280). Deletion of JNK shows similar results. In one study, JNK1−/−, JNK2−/−, and JNK3−/− mice all show hypertrophy after pressure overload; however, the degree of hypertrophy is not significantly greater than wild-type mice (388). These same studies indicated that JNK1 in particular was required to maintain cardiac contractility and prevent heart failure under sustained mechanical overload. As mentioned previously, JNK1 is found to have increased translocation to the nucleus in response to pressure overload, which may explain why this particular isoform shows a more severe phenotype when deleted. This somewhat mirrors the JNK1 translocation to the nucleus that is also seen during IR (278). Interestingly, another group showed that loss of MEKK1-JNK signaling in the heart attenuates Gq-induced hypertrophy (275). However, subsequent studies using this model have shown that loss of MEKK1-JNK signaling does not prevent hypertrophy in response to pressure overload (351). Other studies have demonstrated that JNK signaling is an important part of endothelin-1-mediated hypertrophic signaling in cultured myocytes (177, 372). This further indicates that JNK may have differential roles dependent on the stimuli. Furthermore, in vivo JNK activation in various transgenic animal models failed to induce cardiac hypertrophy. Activation of JNK in the heart by overexpression of constitutively active MKK7 activated the fetal gene program and ventricular remodeling, but did not induce hypertrophy (320, 321). Utilizing a cre-loxP-mediated gene-switch approach, Petrich et al. (322) were able to temporally regulate JNK activation in the adult myocardium. As with other studies, JNK activation in this manner led to pathological remodeling in the absence of ventricular hypertrophy but with a marked induction of fetal gene expression.
Given the conflicting results that have been obtained from both in vitro and in vivo studies, it has been hard to delineate the exact mechanism behind the JNK signaling during hypertrophy. As mentioned previously, JNK’s regulation of NFAT may be one mechanism (239, 250). JunD, a downstream target of JNK, has been shown both in vivo and in vitro to block the cardiomyocyte hypertrophic response to both pressure overload and phenylephrine (PE) (161, 162, 341). It has been suggested that this may be one explanation for the lack of hypertrophy in response to JNK activation. However, at least in vitro, phosphorylation of JunD by JNK is not required for its ability to prevent PE-induced hypertrophy (161). Finally, recent work has shown that binding of growth arrest and DNA-damage-inducible beta (GADD45B) to MKK7 decreases its activity and prevents JNK-mediated cardiac hypertrophy (422). While studies in vitro have suggested that JNKs are prohypertrophic, the majority of studies in vivo do not support that conclusion; rather, JNKs appear to be more antihypertrophic, acting in part via excluding NF-ATs from the nucleus and upregulating JunD.
2. Cardioprotection versus myocardial cell death
Similar to the hypertrophy scenario, JNK’s role in IR is also unclear. Numerous studies both in vitro and in vivo have shown the JNK is activated as a result of reoxygenation upon reperfusion (134, 204, 222, 368, 455). While its activation during the ischemic phase is less well established, a few studies have suggested that it occurs (324, 371, 458). As a stress-induced signaling pathway, JNK has both protective and pathological roles in different cell types. This dichotomy is also observed in cardiomyocytes. JNK1, but not JNK2, has been shown to be proapoptotic during in vitro IR experiments (169). Likewise, treatment with various JNK-selective inhibitors reduces infarct size and apoptosis in response to IR (119, 273). Furthermore, JNK activity contributes to the detrimental effects of a number of proteins known to increase myocardial injury following IR, including the receptor for advanced glycation end-products (RAGE) (4), PKC-β (208), β-adrenergic receptors (337), uncleaved HB-EGF (413), Rho-kinase (463), and poly(ADP-ribose) polymerase (376). Most recently, JNK has been shown to be activated and promote apoptosis during IR by atrogin-1, an E3 ubiquitin ligase (439). Atrogin-1 targets MAPK phosphatase-1 (MKP-1) for degradation, resulting in a sustained activation of JNK and increased apoptosis through increasing cleaved caspase-9, caspase-3, and Bax and by decreasing Bcl-2. JNK has been reported to associate with the mitochondria, possibly interacting with proapoptotic proteins (8, 22), and has also been shown to mediate apoptosis-inducing factor (AIF) translocation from the mitochondria to the nucleus (376, 463). However, convincing as these data are, the complexity of the system is probably best exemplified by Kaiser et al. (190), who reported enhanced myocyte survival after IR with both JNK activation and inhibition. JNK has also been viewed as antiapoptotic in response to nitric oxide (NO) in vitro (5). Similarly, blocking JNK activity increased apoptosis and the activity of both caspase-9 (106) and caspase-3 (114) in another in vitro IR model. This has been proposed to be mediated by the interaction of JNK with Apaf-1, which delays the activation of caspase-9 by the apoptosome (410). It has also been suggested that part of JNK’s cardioprotective effect is due to activation of Akt, a key prosurvival protein in postischemic cardiomyocytes (370).
In short, like in hypertrophy, the role of the JNK pathway in IR injury remains controversial, perhaps reflecting the complexity of multistage, multitargeted signaling networks involved in this process.
3. Cardiac remodeling
Transgenic activation of the JNK pathway in the heart resulted in a lethal restrictive cardiomyopathy with selective extracellular matrix remodeling (320). (Collagen deposition was not increased, but fibronectin levels were markedly increased.) Prolonged activation of JNK activity in heart was also associated with abnormal gap junction structure, loss of the main component (connexin-43), and slowed conduction velocity in the heart (321). Recent evidence suggests that the loss of gap junctions in JNK-activated heart is associated with the loss of connexin-43 protein expression as well as improper intracellular targeting (415). On the other hand, deletion of JNK1 in the heart resulted in an increase in fibrosis following pressure overload (388). Similarly, chronic treatment with a JNK inhibitor led to increased apoptosis and cardiac fibrosis in the cardiomyopathic hamster model (218). Likewise, another study showed the loss of toll-like receptor 4 (TLR4) improved cardiac function and reduced cardiac remodeling following ischemic injury in the heart (340). In this study, the wild-type animals displayed a significant decrease in JNK activity following ischemia that did not occur in the TLR4 animals. Interestingly, the higher JNK level in the TLR knockout animals following ischemia was also accompanied by a significant decrease in calcineurin, indicating that the cross-talk between these two pathways as discussed previously may also play an important role in cardiac remodeling. These results indicate that JNK activity functions to keep some aspects of cardiac remodeling in check while promoting the dysregulation of others.
JNK has also been implicated in promoting cardiac remodeling downstream of various pathways. For example, ASK-1/JNK has been shown to play a role in β-adrenergic-induced cardiac remodeling and apoptosis in vivo (117). Hsp20, a protein with known cardioprotective effects (116), inhibited the activation of JNK in this setting. In a rat model of pressure overload, treatment with RA inhibited cardiac remodeling by inhibiting MAPK signaling, including JNK activity, by upregulating MKP-1 and MKP-2 (72). Likewise, in a model of ANG II-induced hypertrophy, mice deficient for ASK1-JNK signaling demonstrated an attenuation of cardiac fibrosis and remodeling (179). JNK activation downstream of ET-1 has also been implicated in perivascular remodeling in rat (183). Finally, matrix metalloproteinases (MMP) are well known to contribute to cardiac remodeling. Recent in vitro studies have shown that in response to β-adrenergic signaling, extracellular matrix metalloproteinase inducer (EMMPRIN) expression and MMP-2 activity was increased in a JNK-dependent manner in cardiomyocytes (374). These findings are supported by other in vitro work in which JNK activation in H9c2 cardiomyoblasts resulted in the up-regulation of MMP-2 (but not MMP-9) activity (71). Likewise, in vivo studies on the loss of β1-integrins showed increased JNK activity was associated with increased MMP-2 but not MMP-9 activity, which corresponded with less cardiac fibrosis in this setting (213). ROS signaling is emerging as an important player in cardiac remodeling. Since JNK activation is a downstream consequence of ROS induction, there might be a larger role for JNK in cardiac remodeling than originally thought (7, 167). Finally, ASK-1/JNK is a downstream pathway of ER stress signaling (290), which is also gaining more appreciation as an important aspect of stress signaling in the diseased heart (409, 440). For example, Kerkela and co-workers (127, 199) have found that ER stress and ensuing JNK activation is responsible for mitochondrial defects and severe cardiomyopathy as a result of anticancer therapy targeted to the tyrosine kinase c-abl.
These results may indicate that JNK’s role in cardiac pathological remodeling may depend on the activating stimuli. However, because hypertrophy and remodeling often go hand in hand, it is hard to determine whether the remodeling observed in some of these cases is a specific and direct result of JNK activation or secondary to the ensuing hypertrophy recruitment of other signaling pathways.
C. p38
1. Cardiac hypertrophy
Despite a great deal of interest in p38, much of its role in the heart is yet to be clarified. Initial in vitro studies of this pathway suggested that p38 promotes cardiac growth and hypertrophy. Inhibition of the pathway using small molecule inhibitors (SB203580 or SB202190) or dominant negative p38 adenovirus inhibits myocyte growth in response to hypertrophic stimuli (240, 299, 460). In addition, multiple groups have shown that over-activation of the p38 pathway induces hypertrophic changes in vitro (299, 425, 460). However, other studies suggest that p38 is not necessary for agonist-induced hypertrophy in cultured myocytes (74) and that p38 inhibition is actually associated with calcineurin induced hypertrophy via induced expression of MAPK phosphatase-1 (244).
Results from in vivo studies showed that targeted activation of p38 in the heart did not produce any significant degree of cardiac hypertrophy (242). Instead, transgenic overexpression of either MKK3 or MKK6 in the heart increased interstitial fibrosis, ventricular wall thinning, and premature death due to cardiac failure. These findings are supported by Klein et al. (203) in which loss of PKC-ε resulted in increased activation of p38 in the myocardium following pressure overload. These animals displayed a similar phenotype to the MKK3/MKK6 animals, including no increase in hypertrophy but a significant increase in fibrosis and impaired diastolic function. These findings indicate that, in vivo, p38 activity alone is not sufficient to promote cardiomyocyte hypertrophy. Conversely, initial studies involving cardiac-specific p38 dominant negative transgenic mice showed that loss of p38 activity either had no effect on hypertrophy (464) or sensitized the heart to hypertrophy (55) in response to pressure overload. The enhanced hypertrophy in this setting is suggested to be the result of loss of p38’s antagonizing effect on NFAT-mediated transcriptional activity (55, 280, 453). Cardiac-specific deletion of p38 did not alter pressure overload hypertrophy. It resulted in a similar degree of myocyte hypertrophy between p38 CKO and wild-type animals following pressure overload (303). However, these mice did exhibit an increase in apoptosis, fibrosis, and chamber dilation as well as reduced LV function (303). Taken together, these findings would indicate that p38 activity is not involved in promoting hypertrophy in vivo, but may play an important role in pathological remodeling.
Recent findings have implicated p38 in physiological hypertrophy. In response to swimming, loss of p38, either by deletion of ASK1 (an upstream activator) or by conditional knockout of p38 from the myocardium, resulted in increased hypertrophy without increasing fibrosis (398). These authors proposed that the loss of p38 activity resulted in an increase in AKT activity, a known inducer of physiological hypertrophy. Conversely, p38 dominant negative transgenic mice did not experience an increase in hypertrophy in response to swimming (427). This may mirror the different results obtained from the dominant negative transgenic model and the conditional knockout model in response to pressure overload discussed above. Furthermore, an increase in p38 activation in response to loss of the regulatory protein 14–3-3 resulted in maladaptive hypertrophy with increased fibrosis and apoptosis in response to swimming (427).
While gain-of-function and loss-of-function studies through genetic manipulation have shown that p38 is not sufficient for hypertrophy, at least in vivo, studies involving upstream activators still imply a role for p38 in the hypertrophic process. Multiple studies have shown that the protective effects of estrogen on the myocardium are mediated in part through activation of the p38 pathway. In response to both pressure overload and activation of Gqα signaling, administration of estrogen in vivo blocks hypertrophy in a p38-mediated manner (19, 357). Likewise, p38 dominant negative transgenic female mice exhibited greater hypertrophy than either males or ovariectomized females in response to pressure overload (249). In vitro studies have produced similar results. In contrast, activation of thyroid receptor (TRα1) has been shown to cause myocyte hypertrophy via TAK-1/p38 activity in vitro (202). Likewise, leptin induces hypertrophy of cultured neonatal myocytes as a result of caveolae and RhoA-mediated phospho-p38 translocation to the nucleus (461). These results and others like them may indicate that the role of p38 in cardiac hypertrophy is a conflicting one. While acute activation of p38 appears to be prohypertrophic, chronic activation of p38 can lead to suppression of hypertrophic growth in heart. However, it is clear from both in vitro and in vivo studies that p38 activation has a detrimental effect on cardiac function and normal gene expression. Therefore, p38 induction is more closely related to pathological form of hypertrophy than to physiological compensation.
2. Cardioprotection versus myocardial cell death
The role of p38 during IR has been well reported in the literature. However, the conclusions are often contradictory, with some evidence pointing toward a protective role and other results indicating a detrimental role (76, 382). Similar conflicting results have also been noted regarding the role of p38 in ischemic preconditioning (36, 382). These contradictions are likely due, at least in part, to a number of variables including use of different protocols, species, method of p38 inhibition, and measured outcomes. That said, some studies that employed similar species and protocols still resulted in opposing conclusions (36).
Shortly after its identification, investigators demonstrated that p38 was robustly activated by ischemia in the isolated perfused rat heart (42). This activation is both rapid in its onset and transient in its duration after induction of ischemia. To date, however, the exact role that this temporal activation plays in ischemia still eludes investigators. A number of studies have indicated that activation of p38 during ischemia leads to increased injury (26, 36, 256, 257, 262, 291, 354, 358). Likewise, an equal number of studies have shown that p38 activity during ischemia serves a protective role (263, 266, 279, 428, 460). This last point is seen mostly in the context of ischemic preconditioning, the process by which small repeated periods of ischemia impart cardioprotection against more sustained ischemic periods. A number of investigators have shown that p38 activity increases during preconditioning (382). Interestingly, this too appears to be phasic in nature. Ping et al. (324) have shown that p38 activity is increased with brief periods of ischemia, but with repetitive cycles of ischemia and reperfusion, the level of p38 activity returns to baseline within 6 cycles. Furthermore, multiple cycles of ischemia and reperfusion lead to less p38 activation during sustained periods of ischemia following the preconditioning when compared with nonpreconditioned hearts (144, 260, 360). Furthermore, inhibiting p38 activity during prolonged periods of ischemia reduces infarct size only in nonpreconditioned hearts and that inhibition of p38 during preconditioning eliminates its cardioprotective effects (34, 144, 354). Although appearing to be contradictory, these results may be reconciled based on the hypothesis that a negative-feedback mechanism recruited by the modestly induced p38 activity during preconditioning contributes to minimize the detrimental impact of IR. Identifying the molecular basis of this preconditioning-induced protective mechanism remains a major challenge in the biology of ischemia and has the potential to translate this phenomenon into a viable therapy for ischemic disease. In addition, Engel et al. (113) have suggested that inhibiting p38 activity can help preserve cardiac function following ischemia by reducing scarring and promoting myocyte proliferation. This new aspect of p38-mediated cardioprotection opens up a new and exciting avenue by which p38 may be manipulated for therapeutic benefit in the heart.
Another confounding factor adding to the complexity of p38 study is the presence of multiple p38 isoforms in the heart. While p38α is the predominant isoform in the heart, p38β is also present and has been shown to have different physiological consequences. Initial studies in neonatal rat ventricular myocytes revealed that overex-pression of p38α imparts proapoptotic effects, while over-expression of p38β leads to myocyte hypertrophy (425). Studies investigating the role of different p38 isoforms during ischemia have discovered possible differential roles for the α- and β-isoforms. Following adenoviral-mediated overexpression and simulated ischemia, Saurin et al. (358) have shown that p38α activity is increased while p38β activity is decreased. Furthermore, these authors demonstrate that inhibition of only the α-isoform was protective. Subsequently, multiple studies have indicated that p38β may be the isoform that imparts cardio-protection (200, 201). Recently, in vivo studies utilizing overexpression of a cardiac-specific kinase dead p38β mutant revealed that loss of p38β, but not p38α, activity in the myocardium resulted in increased ischemic injury (82). This is similar to previous work in which mice either overexpressing a dominant negative form or lacking one allele of p38α were significantly protected from IR injury (189, 310). These results may provide some clue as to the discrepancy of previous results regarding the protective versus detrimental role of p38 during ischemia. Many early studies utilized p38 inhibitors that block both the α-and β-isoform. If the two isoforms do indeed have different activation profiles and functional roles during ischemia, then experimental results may be confounded by inadvertently blocking both isoforms. Furthermore, it has been shown that the most common p38 inhibitor, SB203580, can actually inhibit other kinases depending on the concentration used (46, 78, 146, 225). Finally, it has begun to be recognized that p38 activation can be mediated by more than the canonical kinase cascade as described earlier, but also via a noncanonical mechanism involving TAB-1 induced autophosphorylation (138). Indeed, TAB-1-mediated p38 activation is observed in ischemic heart and implicated in cardiac injury (234, 399). However, although one study showed TAB-1 induced p38 activation in cardiomycoyte caused apoptosis similar to canonically activated p38 activity, another showed TAB-1 interaction with p38 led to its intracellular localization and downstream signaling distinct from the canonical pathway (121, 254). Although mainly observed in brain and thymocytes, p38 can also function as an alternative mechanism of GSK-3β inactivation, thus further complicating the functional outcome of p38 activation/inactivation in the heart (407).
The upstream and downstream signaling that contributes to p38’s role in IR has yet to be fully determined. Multiple studies, however, have begun to examine such events. For instance, in a setting of insulin-induced cardioprotection, rat hearts treated with insulin followed by IR showed improved functional recovery and an increase in phospho-Hsp27 (233). This protective effect was abolished by treatment with the p38 inhibitor SB203580. p38-mediated Hsp27 activation has also been shown to play a protective role during IR in response to pretreatment of the hearts with H2O2 (40). In this case, the protective effect was determined to be due to Hsp27’s ability to prevent Ca2+-induced proteolysis of myofilament proteins. Hsp27 has been shown in other studies to have cardioprotective properties (166, 263, 264). p38’s ability to activate Hsp27 may explain some of the results showing that p38 is cardioprotective. However, transgenic expression of both wild-type and a nonphosphorylatable from of Hsp27 were both cardioprotective following IR, suggesting that phosphorylation by p38 is not an absolute requirement for its cardioprotective effects (166). Interestingly, Gorog et al. (141) have demonstrated that loss of MAP-KAPK2, the kinase that functions between p38 and Hsp27, resulted in the loss of ischemia-induced Hsp27 phosphorylation but did not exacerbate the ischemic injury.
While the previous examples discuss p38 activation and signaling in terms of cardioprotection, other studies have investigated how inhibition of p38 is cardioprotective. As mentioned previously, p38 activity in myocytes can be proapoptotic. Work by Dhingra et al. (97) has shown that p38 activity is important in TNF-α-mediated myocyte apoptosis, in part, due to ROS production. Moreover, p38 inhibition has also been shown to prevent mitochondrial ROS production and Ca2+ overload during IR (384). p38’s role in mitochondrial-mediated cell death events was also examined by Schwertz et al. (366). In their proteomic approach, they identified a number of proteins altered following IR in rabbit heart. Most interestingly, they noted that phosphorylation of VDAC-1, a mitochondrial porin with possible links to mitochondrial permeability transition pore (MPTP)-mediated cell death, was increased fourfold following IR. This phosphorylation was significantly decreased, and cardioprotection was increased with treatment with the p38 inhibitor PD169316. p38 inhibition has also been shown to impart its cardioprotective effects by altering glucose utilization (181, 182). Finally, endogenous p38 inhibition has also been shown to be cardioprotective during IR. Treatment of rats with the glucocoticoid dexamethasone was shown to be cardioprotective through induction of the dual-specific phosphatase MKP-1 which resulted in a decrease in p38 phosphorylation (118).
As can been seen, the role of p38 in cardiac protection and injury is a complicated one. The specific contribution of p38 kinase in ischemic injury and protection is determined by the level, duration, mode, and timing of induction involving different isoforms and upstream/downstream pathways. Dissecting the beneficial versus detrimental aspects of p38 signaling will be a major issue to be addressed in the future.
3. Cardiac remodeling
As with JNK, the other stress-activated MAPK, p38 appears to play an important role in cardiac remodeling after injury. Liao et al. (242) discovered that targeted p38 activation in the myocardium led to a restrictive cardiomyopathy with significant amounts of interstitial fibrosis. In this setting, p38 was shown to induce cytokine release from myocytes, including TNF-α and IL-6 (236). Interestingly, in these in vitro studies, blocking p38 activity did not appear to prevent cytokine production in the myocytes, only their release from the cell. Proinflammatory cytokines such as TNF-α and IL-6 are known to act in both autocrine and paracrine fashions. Acting in an autocrine fashion, they are known to have negative inotropic effects (326). Acting in a paracrine fashion, they play a large role in myocardial remodeling (300). These initial studies are supported by findings of Tenhunen et al. (402) in which DNA microarray analysis of animals with cardiac-specific overexpression of p38 revealed that genes related to inflammation and fibrosis were among the most significantly upregulated.
p38 is also activated by proinflammatory cytokines, including transforming growth factor (TGF)-β. This type of p38 activation also contributes to cardiac remodeling. Indeed, p38 is activated via a TGF-β1/TAK1-dependent mechanism in myocytes following myocardial infarction in rats (265). As discussed previously, p38 activity can induce cytokine production, thus creating a type of feed-forward mechanism for cytokine action and production. This autocrine and paracrine signaling can lead to recruitment and proliferation of cardiac fibroblasts and inflammatory cells resulting in remodeling. Recent work in aged hypertensive rats (which naturally develop significant amounts of cardiac fibrosis) has shown that treatment with a TGF-β antagonist dramatically reduces both hypertrophy and interstitial fibrosis (450). This was accompanied by a decrease in phospho-p38 levels. This fits with other studies that have shown inhibition of p38 reduces remodeling following myocardial infarction (251, 367). Interestingly, recent work by Frantz et al. (132) indicated that long-term (9 wk) inhibition of p38 starting 1 wk after induced cardiac ischemia reduced cytokine production but did not affect other aspects of cardiac remodeling, including collagen deposits (132). This finding, however, is in contrast to work by others which showed significant reduction in cardiac fibrosis and hypertrophy following IR with long-term p38 inhibition (207). While the protocols for these two studies were similar, the difference in outcome may be due to the different p38 inhibitors used (SB239063 and RWJ67657, respectively).
In addition to extracellular matrix remodeling, p38 can also regulate myocyte contractility. Activation of p38 has been shown to have negative inotropic effects in myocytes (69, 243, 460). Moreover, recent work by Szokodi et al. (387) has shown that increased contractility in response to ET-1 treatment was due to ERK signaling and was further augmented by inhibition of p38. In this setting, it appears that p38 may play a regulatory function by counterbalancing the effects of ERK. While the precise mechanism by which p38 influences myocyte contractility is not fully understood, it appears to be due, at least in part, to modifications of structural proteins. Initial work described p38’s role in contractility as Ca2+ transient independent (243), but suggested that it may be due to modifications of sarcomeric proteins (69). Most recently, Vahebi et al. (416) have suggested that a p38-mediated decreased phosphorylation of α-tropomyosin is one of the mechanisms involved. Furthermore, p38 has the potential to affect myocyte contractility by promoting transcription of the Na+/Ca2+ exchanger (NCX1), a key regulator of Ca2+ homeostasis in both the healthy and pathological myocardium (271). Interestingly, a recent study found that inhibiting NCX1 actually resulted in upregulation of the NCX1 gene in a p38-dependent manner and that this increase was accompanied by NCX-p38 complex formation (441). With the proposal of NCX1 inhibitors as a therapeutic treatment for heart failure, more work needs to be done to better understand the interplay between these two proteins. Finally, p38 has also been shown to down-regulate SERCA expression and prolong the Ca2+ transient in cultured myocytes (6). Although more work needs to be done, given the importance of Ca2+ handling in cardiac pathologies, it is important to better understand the role the p38 signaling may play on this front. In short, chronic induction of p38 activity in postinjury hearts plays an important role in cellular and myocardial remodeling by affecting both the contractility of myocytes and the extracellular matrix. Dissecting the underlying mechanisms involved in the myocyte cell-autonomous effects as well as the cross-talk interaction among myocytes, fibroblasts, and inflammatory cells should be a very promising area of future exploration.
D. ERK5
1. Cardiac hypertrophy
Relatively fewer studies involving ERK5 are published compared with the previously discussed MAPKs, so there is still much to learn about its role in hypertrophy. ERK5 is activated rapidly and transiently in myocytes by a variety of hypertrophic stimuli, including PE and ANG II (176, 301). In vivo studies examining hypertrophic signaling via GPCRs have demonstrated that ERK5 activity is increased during the hypertrophic phase, but not once the disease has progressed to congestive heart failure (CHF) (187). Similar findings were reported during chronic pressure overload in guinea pigs (393). Furthermore, this correlates with findings in humans in which ERK5 activity is decreased during heart failure (392).
In trying to determine its exact role, initial reports implicated ERK5 in promoting eccentric cardiac hypertrophy. Nicol et al. originally reported that activated MEK5, the ERK5 specific MAP2K, led to elongation of cultured myocytes as a result of an increase in serial sarcomere assembly (301), a phenotype similarly seen in myocytes stimulated with cytokines LIF or cardiotrophin-1 (CT-1) (433). ERK5’s role as a downstream pathway of LIF signaling in myocytes was further demonstrated by the ability of dominant negative MEK5 to block the serial, but not parallel, sarcomere assembly in cultured myocytes (301). The unique role for ERK5 in this particular type of cell hypertrophy was additionally supported by their in vivo studies. Transgenic mice expressing a cardiac-specific constitutively active MEK5β developed thinning and dilation of the ventricular chamber without a loss of cells or mass, indicating that these hearts undergo growth by eccentric hypertrophy. This mechanism has been further examined by Nakaoka et al. (297), who demonstrated that, in vitro, LIF-induced activation of ERK5 and subsequent elongation of myocytes is mediated by gp130 receptor signaling through Grb2-associated binder-1 (Gab-1) association with protein tyrosine phosphatase Shp2. Conversely, in recent work by Princen et al. (327), mice with a striated muscle-specific deletion of Shp2 actually exhibit an increase in ERK5 activity in response to LIF stimulation. These animals exhibit that same cardiac phenotype as the MEK5 transgenic mice. The difference in these results may be due to the greater complexity of signaling pathways and feedback mechanisms present in an in vivo setting, which are difficult to recapitulate in vitro. CT-1/gp130 signaling was also shown to mediate its hypertrophic response specifically via ERK5 in cultured myocytes (390). Furthermore, activation of ERK5 has also been associated with eccentric hypertrophy induced by intermittent hypoxia in rat (67). Interestingly, activation of a cardiac isoform MEK5α failed to induce hypertrophy in transgenic mice (61). MEK5β and MEK5α are splice variants that have distinct tissue distribution and cellular localization, which could explain the differences seen in the two different MEK5 transgenic lines. In short, ERK5 pathway located in different intracellular compartments regulated by different scaffolds can lead to specific functional outcome in heart during hypertrophy.
Currently, more work needs to be done to better understand the role of ERK5 in cardiac biology. The recent development of two small molecule inhibitors to MEK5 will help advance our current understanding of ERK5 signaling in the heart (401). Furthermore, the frequently used MEK inhibitors PD98059 and U0126 have also been shown to block MEK5 (192). Since many studies looking at ERK1/2 signaling in cardiac myocytes utilized these inhibitors, it is not impossible to hypothesize that ERK5 may be playing a larger role in hypertrophy than originally thought.
2. Cardioprotection versus myocardial cell death
While little work has been done investigating the role of ERK5 in cardiac hypertrophy, even less has been done concerning IR. ERK5 signaling has been proposed to be cardioprotective after ischemic injury. It has been shown that ischemia transiently activates ERK5, peaking around 30 min (391, 394). Cameron et al. have shown that MEK5α transgenic animals demonstrate greater functional recovery after ischemia that is accompanied by a corresponding decrease in caspase-3 activation and reduced infarct size (61). Preconditioning not only increases ERK5’s maximal activity, but its peak activation occurs much more rapidly (394). Taken together, these data suggest that ERK5 activation functions in a cardioprotective role during IR. While the exact mechanism that underlies ERK5’s antiapoptotic effects is not known, recent studies are beginning to shed some light on the question. Previous studies have shown that the feedback loop between phosphodiesterase 3A (PDE3A) and inducible cAMP early repressor (ICER) is involved in promoting apoptosis in cardiomyocytes (98). Yan et al. (447) have elucidated that ERK5 activity induced by insulin-like growth factor I (IGF-I) leads to the inhibition of this PDE3A/ICER feedback loop and attenuates isoproterenol-induced apoptosis in vitro. These same studies also demonstrated that in vivo cardiac specific constitutively active MEK5 inhibited pressure overload or doxorubicin-mediated apoptosis via inhibition of this feedback loop. Furthermore, SUMOylation is found to significantly inhibit ERK5 transciptional activity (434). Recent work by Shishido et al. (373) have shown that in a diabetic mouse model, ERK5-SUMOylation exacerbated LV dysfunction following myocardial infarction. Likewise, they demonstrated that both H2O2 and high glucose treatment of cardiomyocytes in vitro increases ERK5-SUMOylation resulting in decreased transcriptional activity and increased apoptosis. Thus, taken together, although data are fairly limited, ERK5 does seem to protect against ischemic injury.
3. Cardiac remodeling
While little work has been done regarding the role of ERK5 in cardiac remodeling, it does play a physiological role in a number of aspects that occur during cardiac stress. As discussed previously, ERK5 appears to be important to maintaining the vascular integrity that is critically important for cardiac function. Targeted deletion of ERK5 in adult mice was shown to result in hemorrhages in multiple organs, including the heart, due to increased endothelial cell apoptosis (154). A healthy and functional vascular system is necessary for the proper growth and maintenance of the myocardium, and vascular growth often accompanies cardiac pathologies. ERK5’s prosur-vival role in endothelial cells thus may be an important component of cardiovascular physiology (154, 323). Similarly, ERK5 has also been shown to be important in smooth muscle cells, another important component of the heart’s vascular network (178). Along with its role in the vascular system, ERK5 has also been shown to be important in myocytes. As discussed previously, postnatal growth of the heart occurs by hypertrophy and limited hyperplasia. While its role in these events has not been exclusively studied, ERK5 is known to activate many of the immediate-early response genes known to be involved in cardiac growth, including fos, myc, jun, and MEF2 (258, 424). Likewise, the ERK5 signaling pathway is activated by a number of cardiac growth factors. Therefore, it is plausible that ERK5 may play a role in postnatal cardiac growth, including hypertrophic growth in response to stress. Indeed, findings by Nicol et al. (301) suggest that ERK5 may have a specific role in directing myocyte growth by serial sarcomere addition and inhibiting parallel assembly. Furthermore, ERK5 may play an integrating role in pathways activated under various situations. cAMP is a vital second messenger molecule in cardiac signaling, playing a role in PKA signaling, activating ion channels, and guanine nucleotide exchange factors such as Epac (49). cAMP activity is under spatiotemporal control that results from a complex that contains both adenylyl cyclases which activate it and phosphodiesterases which metabolize it. ERK5 has been found to be part of one of these complexes, suppressing PDE4DE activity when it is associated with the complex (102). Likewise, ERK5 has recently been shown to also play a role in a feedback loop involving the phosphodiesterase PDE3A (447). Because cAMP signaling is such an integral part of normal cardiac physiology, it may be that ERK5 functions to help control the specification and duration of this signaling. In short, ERK5 may also impact on cardiac remodeling through its effect on vascular integrity and myocyte growth by integrating important cardiac signaling pathways, including GPCRs and RTKs (307).
V. MITOGEN-ACTIVATED PROTEIN KINASE IN THERAPIES
When reading the literature that employs small molecule inhibitors of protein kinases, it is essential to understand the limitations of this approach. Paramount among them is the inherent nonselectivity of the agents employed. The vast majority of small molecule inhibitors of protein kinases target the pocket within the kinase to which ATP binds (i.e., they are ATP-competitive antagonists) (129). However, this region is highly conserved across the ~500 kinases in the human genome, making it unlikely that any small molecule inhibitor, especially the early-generation ones that have been used to date in experimental studies, will be truly selective. This issue has been highlighted over the past several years by the development of kinase inhibitors for the treatment of various cancers. Many of these were believed to be highly selective for specific targets, but as the technologies evolved to test selectivity of agents against very large numbers of kinases (currently >250), so-called “off-target” inhibition of kinases was virtually always seen. For example, some United States Food and Drug Administration-approved kinase inhibitors (e.g., sorafenib and sunitinib) inhibit upwards of 25–50 kinases. This is not a new concept, since for years the Cohen laboratory has performed detailed studies exposing the nonselectivity of reportedly selective inhibitors (20, 21, 92). This has led Cohen to create a list of “controls” for studies employing kinase inhibitors that first and foremost suggests that concordant findings be demonstrated with the use of two (or more) structurally unrelated inhibitors of a specific target in order for that target to be preliminarily validated as causing a particular phenotype (e.g., enhanced ischemic injury). As is seen, this minimum requirement is very rarely achieved in the cardiovascular literature. The second problem relates to the fact that different isoforms of a kinase often play specific roles in a disease process (as discussed above), yet isoform selectivity is rarely achieved with inhibitors, and when it is, it is only relative. Thus identification of specific functions for specific isoforms will usually require genetic or RNAi approaches.
All of this said, some of the better small molecule inhibitors available have targeted the ERK and p38-MAPK pathways, and much can be gleaned concerning the roles of the kinases in various pathologies by a review of studies employing them. In the section that follows, we discuss the relevance of using this methodology to determine the role of MAPKs in the heart. Furthermore, we discuss possible clinical implications of targeting MAPKs in various disease states.
A. ERK Inhibitors
Several commercially available inhibitors exist for the ERK signaling pathway. Two of the most commonly utilized are PD98059 and U0126. These agents are unusual for small molecule inhibitors since they appear to have an exceptionally good selectivity profile. This is due in large part to the fact that they are so-called type III inhibitors that target regions distinct from the ATP pocket. Thus these are very good agents when used in appropriate concentrations. However, use of these inhibitors in studies is somewhat complicated by the fact that both of them inhibit all MEKs upstream of the ERKs. Thus MEK1, MEK2, and MEK5 are potently inhibited by these drugs. Some selectivity is achieved by using PD184352, a less potent inhibitor of MEK5, but the selectivity is only relative. As a result, the specificity of one pathway may be masked by the inadvertent inhibition of another. This is of significance given the sometimes conflicting results obtained in various studies.
As discussed previously, ERKs have been shown to be cardioprotective. However, the majority of the studies suggesting a protective effect of ERKs in cardiomyocytes have involved somewhat artificial settings in which reversal of protective effects of exogenously administered growth factors was seen with ERK inhibition. For example, antiapoptotic effects of IGF-I, CT-1, serotonin, hepatocyte growth factor, basic FGF, and catecholamines were reduced by ERK inhibition (152). However, broad conclusions should not necessarily be drawn from such findings. For example, Li et al. (232) administered an NO donor during reperfusion of isolated perfused hearts. While U0126 blocked the protective effects of the NO donor, U0126 had no effect on caspase-3 cleavage or recovery of function in the absence of the NO donor, thus demonstrating that the cardioprotective effects of ERK inhibition are highly complex and most likely a small part of a much bigger picture involving multiple signaling pathways.
One of the first clear demonstrations of a protective role for ERKs in ischemic injury in cardiomyocytes was shown in neonatal rat ventricular myocytes (NRVMs) in culture and in the isolated perfused heart exposed to global ischemia (458). These studies showed increased apoptosis following ERK inhibition in both models which was partially reversed by either of two p38 inhibitors, SB203580 (which also inhibits JNK2) and SB242719. More recently, however, the concept that ERK inhibition is detrimental has been challenged both in cardiomyocytes in culture exposed to simulated ischemia and reperfusion (114) as well as in a model of global low-flow IR injury (168). In these studies, PD98059 had no effect on cardiomyocyte survival in vitro, and U0126 had no effect on contractile dysfunction or infarct size. Given the potentially contradicting role of Ras-Raf-Mek-ERK pathway in cardioprotection and pathological remodeling, it would be necessary to perhaps inhibit only the selective components of the pathway in a defined disease state so the benefit can outweigh the undesirable effects.
In general, studies employing ERK inhibitors tend to agree with findings discussed above in genetically modified mice and confirm a cardioprotective role of ERK signaling. This is probably not surprising given the good selectivity and minimal off-target effects of these inhibitors.
B. JNK Inhibitors
As discussed earlier, JNK activity is implicated in both cardiac protection and injury during IR. Unfortunately, studies with pharmacological inhibitors have not allowed a consensus nor, for the most part, have they reduced the confusion. This is due in large part to the over-reliance on SP600125, a relatively nonselective inhibitor with poor pharmacokinetics, limiting its utility in vivo. Models exist in which JNK inhibition is detrimental and also where it is beneficial. While this may seem contradictory, both sides of the argument are most likely correct, that is, in specific instances, JNK inhibition can be either detrimental or beneficial depending on the stressor, the severity and duration of the stress, the completeness of inhibition, and how JNK is able to interact with their many downstream targets which, as discussed above, belong to both pro- and antiapoptotic families. To delineate JNKs’ effects, multiple approaches and targets need to be examined. For example, Shao et al. (370) employed multiple techniques to inhibit JNK in vitro (small molecule inhibitors, gene transfer of dominant inhibitory mutants of MKK4, and a selective cell-permeant peptide inhibitor) to demonstrate a protective role. This cardioprotection was mediated at least in part via phosphorylation of a novel site in the COOH-terminal tail of Akt, T450, which played a role in activating Akt early post-IR. Furthermore, they found that, in vivo, a selective p38 MAPK inhibitor protected from IR injury, whereas a combination JNK/p38-MAPK inhibitor was detrimental, suggesting JNK is protective in this setting. Thus it is abundantly clear that JNK, even in the same cell type, can signal both death and survival.
Models in which JNK inhibition is detrimental include oxidant stress, with or without glucose restriction (5, 75, 274), hypoxia/reperfusion (H/R) (75, 88, 105), and chemical anoxia (CN/2-DOG) (114). The converse has also been seen with H/R (156, 169, 214). In contrast, Ferrandi et al. (119) have employed a novel small molecule inhibitor of JNK to demonstrate protection from ischemic injury in vivo, but this compound (AS601245) also potently inhibits GSK3β, a kinase with known protective effects on mitochondria in the setting of ischemia (287). Recently, a novel cell-permeate, protease-resistant peptide based on the JNK-binding sequence of JNK-interacting protein 1 (JIP1) has been developed by Bonny et al. (45). This peptide (now called XG-102) blocks JNK/substrate interactions by a direct competitive mechanism. The peptide also inhibits MEK4 and MEK7, since they also contain JNK binding domains, but does not inhibit activity of 40 other kinases tested. The peptide has been shown to protect in stroke models as well as in isolated perfused hearts and in vivo (47, 273, 430). At present, these data, when taken together with the studies in mice deleted for either JNK1 or JNK2, are probably the most definitive in terms of encouraging proceeding with drug development targeting JNK for ischemic syndromes in the heart. However, this may not be true for all organs since the SP compound aggravated IR injury in the liver (231).
JNKs have a large number of substrates, most of which promote apoptosis, but they also have a limited number of factors that promote survival. Whether JNK induces death or promotes survival in any particular cell type following a particular stressor is a net balance of activation/inhibition of all these pro/antiapoptotic factors. To better understand this balance, more work needs to be done. This will require work with both small molecule inhibitors and genetic manipulations and will need to look at downstream mediators and cross-talk with other pathways, a systems biology approach.
C. p38 Inhibitors
In the early 1990s, Smith-Kline Beacham identified a series of compounds they called cytokine-suppressive anti-inflammatory drugs (CSAIDs), based on their ability to suppress cytokine production in an in vitro model system. In a remarkable series of studies, they cloned the protein to which the drug bound and identified that protein as p38 MAPK. Thus the SB compounds suppressed cytokine production by inhibiting p38. Therefore, it is important to remember that any phenotype resulting from p38 inhibition in the heart in vivo could be due to direct effects on the cardiomyocyte and/or to suppression of cytokine production.
Although there are contradictory viewpoints, the vast majority of work with p38 MAPK inhibitors suggests p38 MAPK activation is detrimental in IR injury. The first two reports, which appeared almost simultaneously in the literature, demonstrated that SB203580 reduced hypoxia-induced apoptotic and necrotic cell death in NRVMs (257) and reduced IR injury in isolated, perfused hearts (256). These findings were important not only for demonstrating protection, but also in demonstrating that direct cardiomyocyte-protective effects, as opposed to inhibition of cytokine production in the periphery, were at least one mechanism of protection. Furthermore, a third critical paper employed adenovirus-mediated gene transfer of an SB203580-resistant p38α mutant to demonstrate that inhibition of p38, as opposed to some unknown target of the drug, was indeed responsible for the reduced injury in cardiomyocytes following simulated ischemia (262). However, as discussed earlier, a few reports have emerged suggesting protective effects of p38 MAPK activation. These studies have primarily been done in model systems in vitro, and when work has been done in vivo or in isolated perfused hearts, generally inhibition of p38 MAPKs has been protective. In contrast, however, Kaiser et al. (191) demonstrated protection by SB239063 in the mouse but no protection in the pig. Although this might suggest different roles of p38 in larger mammals, the models had additional differences (e.g., the pigs received amiodarone, which has both antiarrythmic and β-blocking properties) that confounded the analysis. Furthermore, Barancik et al. (26) showed protection in the pig with SB203580, although this compound is less selective than SB239063. Along with the SB compounds, studies have also been done with other p38 inhibitors, including SD-282 (238), SC-409 (251), RWJ-67657 (367), and FR167653 (218). Although some conflicting results exist, the vast majority of studies show a protective effect of p38 inhibition (76), which is corroborated with studies in the p38α+/− mouse that exhibited a 40% reduction in p38 activity and was protected from ischemic injury (310).
D. MAPKs as Therapeutic Targets
Protein kinases are often the target of drug development. The vast majority of kinase inhibitor development is in the cancer field (128), and that is the case with ERK inhibitors (2, 281, 344). Indeed, we could find no clinical trials of ERK inhibitors outside of this use. However, there remains a moderate level of interest in other (17, 39, 128, 173, 199, 216, 465) MAPKs as drug targets for disease states other than cancer. For example, in addition to the studies noted above with the peptide JNK inhibitor, JNK inhibition has been tested as a target for both hearing loss (385, 421) and neurodegenerative diseases (48, 339). The greatest amount of interest has been with p38 inhibitors. These agents have been in trials for many inflammatory diseases, reasoning that a broader inhibition of cytokine production, as opposed to the monocloncal antibodies, which are cytokine-targeted monotherapeutics, would be more efficacious. Disease states include rheumatoid arthritis (80, 86), Crohn’s disease (362), endotoxemia (52, 53), and atherosclerosis. Although many p38 antagonists have been limited by dose-dependent hepatotoxicity, recent studies in patients with rheumatoid arthritis and acute coronary syndromes (ACS) demonstrated tolerability (80, 86, 356). However, at the tolerated dose, efficacy in rheumatoid arthritis was limited. In contrast, a significant and persistent reduction in C-reactive protein levels, possibly indicating reduced inflammation at the level of the atherosclerotic plaque, was reported in the ACS trial (356). Whether this translates into clinical efficacy remains to be determined.
In summary, the use of MAPK blockade as a therapeutic treatment is exciting and daunting at the same time. Given the discordant findings utilizing kinase inhibitors, the lack of true specificity, and the unanswered questions that remain about precise mechanisms of action, use of MAPK inhibitors as therapeutics will have to proceed with caution. As we have seen, MAPKs play an integral and complex role in myocardial signaling. As a result, use of MAPK inhibitors or other therapies that hit the MAPK pathways may continue to have cardiovascular (and other) side effects. Much more work needs to be done at the basic science levels to better understand these pathways and to develop more specific pharmaceuticals.
VI. CONCLUSIONS AND PERSPECTIVES
It is difficult to conclude a drama without revealing the identities of all the villains and heroes. In this sense, the quest continues in the study of MAPKs in heart. However, as literature has abundantly demonstrated, the complexity of the signaling transduction network makes it impossible and imprudent to label any particular molecule as definitively “bad” or “good.” Using genetic approaches to achieve either a complete inactivation (knockout) or constitutive activation (overexpression) of signaling pathways, although very powerful, has major limitations in uncovering their intricate roles in the dynamic process of stress response. A detrimental pathway in one setting can be critically beneficial in another. Thus it remains to be a daunting challenge for us to harness the beneficial activities of MAPK pathways while containing the detrimental effects of the “rogue” members in combating heart diseases. Looking forward, we can expect that the significant progress will have to come from different fronts. First, we will need to develop better approaches to investigate signaling network in the context of specific pathophysiological environment. By focusing on the network interaction rather than signal molecules themselves, we have a better chance to identify critical regulatory nodules that can be manipulated to influence the outcome. This so-called systems biology approach has indeed made great in-roads in understanding complex traits in metabolic diseases and has potential to do the same in the cardiovascular field. Second, we will need better tools to understand the function of individual players in signal transduction network. In addition to biochemical measurement of protein activity from a mixture, the temporal and spatial resolution of our analysis must be improved with the help of modern imaging and detection techniques. Lastly, more efforts will be needed to translate molecular and genetic studies in model organisms like rodents to humans, which will require better interaction among basic and clinical scientists with a common mission. On an optimistic note, rapid technological advancement and integration of multidisciplinary approaches are the norm rather than exception in cardiac research. There will be more exciting episodes with unexpected twists and turns to be played out in the coming years.
Acknowledgments
GRANTS
This review was supported by grants from the National Institutes of Health and the American Heart Association. B. Rose is a recipient of a training fellowship from UCLA Vascular Biology from the National Institutes of Health.
Footnotes
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the authors.
References
- 1.Heart disease and stroke statistics: 2008 update. American Heart Association; 2008. [Google Scholar]
- 2.Adjei AA, Cohen RB, Franklin W, Morris C, Wilson D, Molina JR, Hanson LJ, Gore L, Chow L, Leong S, Maloney L, Gordon G, Simmons H, Marlow A, Litwiler K, Brown S, Poch G, Kane K, Haney J, Eckhardt SG. Phase I pharmacokinetic and pharma-codynamic study of the oral, small-molecule mitogen-activated protein kinase kinase 1/2 inhibitor AZD6244 (ARRY-142886) in patients with advanced cancers. J Clin Oncol. 2008;26:2139–2146. doi: 10.1200/JCO.2007.14.4956. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Akaike M, Che W, Marmarosh N, Ohta S, Osawa M, Ding B, Berk B, Yan C, Abe J. The hinge-helix 1 region of peroxisome proliferator-activated receptor gamma 1 (PPARgamma1) mediates interaction with extracellular signal-regulated kinase 5 and PPAR-gamma1 transcriptional activation: involvement in flow-induced PPARgamma activation in endothelial cells. Mol Cell Biol. 2004;24:8691–8704. doi: 10.1128/MCB.24.19.8691-8704.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Aleshin A, Ananthakrishnan R, Li Q, Rosario R, Lu Y, Qu W, Song F, Bakr S, Szabolcs M, D’Agati V, Liu R, Homma S, Schmidt AM, Yan SF, Ramasamy R. RAGE modulates myocardial injury consequent to LAD infarction via impact on JNK and STAT signaling in a murine model. Am J Physiol Heart Circ Physiol. 2008;294:H1823–H1832. doi: 10.1152/ajpheart.01210.2007. [DOI] [PubMed] [Google Scholar]
- 5.Andreka P, Zang J, Dougherty C, Slepak TI, Webster KA, Bishopric NH. Cytoprotection by Jun kinase during nitric oxide-induced cardiac myocyte apoptosis. Circ Res. 2001;88:305–312. doi: 10.1161/01.res.88.3.305. [DOI] [PubMed] [Google Scholar]
- 6.Andrews C, Ho PD, Dillmann WH, Glembotski CC, Mc-Donough PM. The MKK6-p38 MAPK pathway prolongs the cardiac contractile calcium transient, downregulates SERCA2, and activates NF-AT. Cardiovasc Res. 2003;59:46–56. doi: 10.1016/s0008-6363(03)00329-8. [DOI] [PubMed] [Google Scholar]
- 7.Anilkumar N, Sirker A, Shah A. Redox sensitive signaling pathways in cardiac remodeling, hypertrophy and failure. Front Biosci. 2009;14:3168–1387. doi: 10.2741/3443. [DOI] [PubMed] [Google Scholar]
- 8.Aoki H, Kang PM, Hampe J, Yoshimura K, Noma T, Matsuzaki M, Izumo S. Direct activation of mitochondrial apoptosis machinery by c-Jun N-terminal kinase in adult cardiac myocytes. J Biol Chem. 2002;277:10244–10250. doi: 10.1074/jbc.M112355200. [DOI] [PubMed] [Google Scholar]
- 9.Aoki H, Richmond M, Izumo S, Sadoshima J. Specific role of the extracellular signal-regulated kinase pathway in angiotensin II-induced cardiac hypertrophy in vitro. Biochem J. 2000;347:275–284. [PMC free article] [PubMed] [Google Scholar]
- 10.Aoki Y, Niihori T, Kawame H, Kurosawa K, Ohashi H, Tanaka Y, Filocamo M, Kato K, Suzuki Y, Kure S. Germline mutations in HRAS proto-oncogene cause Costello syndrome. Nat Genet. 2005;37:1038–1040. doi: 10.1038/ng1641. [DOI] [PubMed] [Google Scholar]
- 11.Aoki Y, Niihori T, Narumi Y, Kure S, Matsubara Y. The RAS/MAPK syndromes: novel roles of the RAS pathway in human genetic disorders. Hum Mutat. 2008;29:992–1006. doi: 10.1002/humu.20748. [DOI] [PubMed] [Google Scholar]
- 12.Aouadi M, Bost F, Caron L, Laurent K, Le Marchand Brustel Y, Binetruy B. p38 mitogen-activated protein kinase activity commits embryonic stem cells to either neurogenesis or cardiomyogenesis. Stem Cells. 2006;24:1399–1406. doi: 10.1634/stemcells.2005-0398. [DOI] [PubMed] [Google Scholar]
- 13.Araki T, Chan G, Newbigging S, Morikawa L, Bronson RT, Neel B. Noonan syndrome cardiac defects are caused by PTPN11 acting in endocardium to enhance endocardial-mesenchymal transformation. Proc Natl Acad Sci USA. 2009;106:4736–4741. doi: 10.1073/pnas.0810053106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Araki T, Mohi MG, Ismat FA, Bronson RT, Williams IR, Kutok JL, Yang W, Pao LI, Gilliland DG, Epstein JA, Neel BG. Mouse model of Noonan syndrome reveals cell type- and gene dosage-dependent effects of Ptpn11 mutation. Nat Med. 2004;10:849–857. doi: 10.1038/nm1084. [DOI] [PubMed] [Google Scholar]
- 15.Aries A, Paradis P, Lefebvre C, Schwartz RJ. Essential role of GATA-4 in cell survival and drug-induced cardiotoxicity. Proc Natl Acad Sci USA. 2004;101:6975–6980. doi: 10.1073/pnas.0401833101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Armstrong EJ, Bischoff J. Heart valve development: endothelial cell signaling and differentiation. Circ Res. 2004;95:459–470. doi: 10.1161/01.RES.0000141146.95728.da. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Atallah E, Durand J, Kantarjian H, Cortes J. Congestive heart faliure is a rare event in patients receiving imatinib therapy. Blood. 2007;110:1233–1237. doi: 10.1182/blood-2007-01-070144. [DOI] [PubMed] [Google Scholar]
- 18.Avruch J. MAP kinase pathways: the first twenty years. Biochim Biophys Acta. 2007;1773:1150–1160. doi: 10.1016/j.bbamcr.2006.11.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Babiker FA, Lips D, Meyer R, Delvaux E, Zandberg P, Janssen B, van Eys G, Grohe C, Doevendans PA. Estrogen receptor β protects the murine heart against left ventricular hypertrophy. Arterioscler Thromb Vasc Biol. 2006;26:1524–1530. doi: 10.1161/01.ATV.0000223344.11128.23. [DOI] [PubMed] [Google Scholar]
- 20.Bain J, McLauchlan H, Elliott M, Cohen P. The specificities of protein kinase inhibitors: an update. Biochem J. 2003;371:199–204. doi: 10.1042/BJ20021535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Bain J, Plater L, Elliott M, Shpiro N, Hastie CJ, McLauchlan H, Klevernic I, Arthur JSC, Alessi DR, Cohen P. The selectivity of protein kinase inhibitors: a further update. Biochem J. 2007;408:297–315. doi: 10.1042/BJ20070797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Baines CP, Zhang J, Wang GW, Zheng YT, Xiu JX, Cardwell EM, Bolli R, Ping P. Mitochondrial PKCε and MAPK form signaling modules in the murine heart: enhanced mitochondrial PKCε-MAPK interactions and differential MAPK activation in PKCε-induced cardioprotection. Circ Res. 2002;90:390–397. doi: 10.1161/01.res.0000012702.90501.8d. [DOI] [PubMed] [Google Scholar]
- 23.Balakumar P, Rohilla A, Singh M. Pre-conditioning and postconditioning to limit ischemia-reperfusion-induced myocardial injury: what could be the next footstep? Pharmacol Res. 2008;57:403–412. doi: 10.1016/j.phrs.2008.05.006. [DOI] [PubMed] [Google Scholar]
- 24.Balakumar P, Singh H, Singh M, Anand-Srivastava MB. The impairment of preconditioning-mediated cardioprotection in pathological conditions. Pharmacol Res. 2009;60:18–23. doi: 10.1016/j.phrs.2009.03.002. [DOI] [PubMed] [Google Scholar]
- 25.Bao JE, Dixon GZ, Zhao Q. Components of a new human protein kinase signal transduction pathway. J Biol Chem. 1995;270:12665–12669. doi: 10.1074/jbc.270.21.12665. [DOI] [PubMed] [Google Scholar]
- 26.Barancik M, Htun P, Strohm C, Kilian S, Schaper W. Inhibition of the cardiac p38-MAPK pathway by SB230580 delays ischemic cell death. J Cardiovasc Pharmacol. 2000;35:474–483. doi: 10.1097/00005344-200003000-00019. [DOI] [PubMed] [Google Scholar]
- 27.Bardwell AJ, Abdollahi M, Bardwell L. Docking sites on mitogen-activated protein kinase (MAPK) kinases, MAPK phosphatases and the Elk-1 transcription factor compete for MAPK binding and are crucial for enzymic activity. Biochem J. 2003;370:1077–1085. doi: 10.1042/BJ20021806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Bardwell AJ, Flatauer LJ, Matsukuma K, Thorner J, Bardwell L. A conserved docking site in MEKs mediates high-affinity binding to MAP kinases and cooperates with a scaffold protein to enhance signal transmission. J Biol Chem. 2001;276:10374–10386. doi: 10.1074/jbc.M010271200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Bardwell AJ, Frankson E, Bardwell L. Selectivity of docking sites in MAPK kinases. J Biol Chem. 2009;284:13165–13173. doi: 10.1074/jbc.M900080200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Barki-Harrington L, Perrino C, Rockman HA. Network integration of the adrenergic system in cardiac hypertrophy. Cardiovasc Res. 2004;63:391–402. doi: 10.1016/j.cardiores.2004.03.011. [DOI] [PubMed] [Google Scholar]
- 31.Barr RK, Bogoyevitch MA. The c-Jun N-terminal protein kinase family of mitogen-activated protein kinases (JNK MAPKs) Int J Biochem Cell Biol. 2001;33:1047–1063. doi: 10.1016/s1357-2725(01)00093-0. [DOI] [PubMed] [Google Scholar]
- 32.Barron M, Gao M, Lough J. Requirement for BMP and FGF signaling during cariogenic induction in non-precardiac mesoderm is specific, transient and cooperative. Dev Dyn. 2000;218:383–393. doi: 10.1002/(SICI)1097-0177(200006)218:2<383::AID-DVDY11>3.0.CO;2-P. [DOI] [PubMed] [Google Scholar]
- 33.Baudino TA, Carver W, Giles W, Borg TK. Cardiac fibroblasts: friend or foe? Am J Physiol Heart Circ Physiol. 2006;291:H1015–H1026. doi: 10.1152/ajpheart.00023.2006. [DOI] [PubMed] [Google Scholar]
- 34.Béguin PC, Belaidi E, Godin-Ribuot D, Lévy P, Ribuot C. Intermittent hypoxia-induced delayed cardioprotection is mediated by PKC and triggered by p38 MAP kinase and Erk1/2. J Mol Cell Cardiol. 2007;42:343–351. doi: 10.1016/j.yjmcc.2006.11.008. [DOI] [PubMed] [Google Scholar]
- 35.Bejsovec A. Wnt pathway activation: new relations and locations. Cell. 2005;120:11–14. doi: 10.1016/j.cell.2004.12.021. [DOI] [PubMed] [Google Scholar]
- 36.Bell JR, Eaton P, Shattock MJ. Role of p38-mitogen-activated protein kinase in ischaemic preconditioning in rat heart. Clin Exp Pharmacol Physiol. 2008;35:126–134. doi: 10.1111/j.1440-1681.2007.04794.x. [DOI] [PubMed] [Google Scholar]
- 37.Bentires-Alj M, Kontaridis MI, Neel BG. Stops along the RAS pathway in human genetic disease. Nat Med. 2006;12:283–285. doi: 10.1038/nm0306-283. [DOI] [PubMed] [Google Scholar]
- 38.Bin Zhao LE, Hinton RB, Jr, Benson WD. BMP and FGF regulatory pathways in semilunar valve precursor cells. Dev Dyn. 2007;236:971–980. doi: 10.1002/dvdy.21097. [DOI] [PubMed] [Google Scholar]
- 39.Bird B, Swain S. Cardiac toxicity in breast cancer survivors: review of potential cardiac problems. Clin Cancer Res. 2008;14:14–24. doi: 10.1158/1078-0432.CCR-07-1033. [DOI] [PubMed] [Google Scholar]
- 40.Blunt BC, Creek AT, Henderson DC, Hofmann PA. H2O2 activation of HSP25/27 protects desmin from calpain proteolysis in rat ventricular myocytes. Am J Physiol Heart Circ Physiol. 2007;293:H1518–H1525. doi: 10.1152/ajpheart.00269.2006. [DOI] [PubMed] [Google Scholar]
- 41.Bogoyevitch MA. The isoform-specific functions of the c-jun N-terminal kinases (JINKs): differences revealed by gene targeting. Bioessays. 2006;28:923–934. doi: 10.1002/bies.20458. [DOI] [PubMed] [Google Scholar]
- 42.Bogoyevitch MA, Gullespie-Brown J, Ketterman A, Fuller S, Ben-Levy R, Ashworth A, Marshall CJ, Sugden P. Stimulation of the stress-activated mitogen-activated protein kinase subfamilies in perfused heart: p38/RK mitogen-activated protein kinases and c-Jun N-terminal kinases are activated by ischemia/reperfusion. Circ Res. 1996;79:162–173. doi: 10.1161/01.res.79.2.162. [DOI] [PubMed] [Google Scholar]
- 43.Bogoyevitch MA, Kobe B. Uses for JNK: the many and varied substrates of the c-Jun N-terminal kinases. Microbiol Mol Biol Rev. 2006;70:1061–1095. doi: 10.1128/MMBR.00025-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Boluyt MO, Loyd AM, Roth MH, Randall MJ, Song EYM. Activation of JNK in rat heart by exercise: effect of training. Am J Physiol Heart Circ Physiol. 2003;285:H2639–H2647. doi: 10.1152/ajpheart.00596.2003. [DOI] [PubMed] [Google Scholar]
- 45.Bonny C, Oberson A, Negri S, Sauser C, Schorderet D. Cell-permeable peptide inhibitors of JNK. Diabetes. 2001;50:77–82. doi: 10.2337/diabetes.50.1.77. [DOI] [PubMed] [Google Scholar]
- 46.Borsch-Haubold AG, Pasquet S, Watson SP. Direct inhibition of cyclooxygenase-1 and -2 by the kinase inhibitors SB 203580 and PD 98059. SB 203580 also inhibits thromboxane synthase. J Biol Chem. 1998;273:28766–28772. doi: 10.1074/jbc.273.44.28766. [DOI] [PubMed] [Google Scholar]
- 47.Borsello T, Clarke P, Hirt L, Vercelli A, Repici M, Schorderet D, Bogousslavsky J, Bonny C. A peptide inhibitor of c-Jun N-terminal kinase protects against cytotoxicity and cerebral ischemia. Nat Med. 2003;9:1180–1186. doi: 10.1038/nm911. [DOI] [PubMed] [Google Scholar]
- 48.Borsello T, Forloni G. JNK signalling: a possible target to prevent neurodegeneration. Curr Pharm Des. 2007;13:1875–1886. doi: 10.2174/138161207780858384. [DOI] [PubMed] [Google Scholar]
- 49.Bos JL. Epac: a new cAMP target and new avenues in cAMP research. Nat Rev Mol Cell Biol. 2003;4:733–738. doi: 10.1038/nrm1197. [DOI] [PubMed] [Google Scholar]
- 50.Bottcher R, Niehrs C. Fibroblast growth factor signaling during early vertebrate development. Endocr Rev. 2005;26:63–77. doi: 10.1210/er.2003-0040. [DOI] [PubMed] [Google Scholar]
- 51.Brand T. Heart development: molecular insights into cardiac specification and early morphogenesis. Dev Biol. 2003;258:1–19. doi: 10.1016/s0012-1606(03)00112-x. [DOI] [PubMed] [Google Scholar]
- 52.Branger J, van den Blink B, Weijer S, Gupta A, van Deventer SJH, Hack CE, Peppelenbosch MP, van der Poll T. Inhibition of coagulation, fibrinolysis, and endothelial cell activation by a p38 mitogen-activated protein kinase inhibitor during human endotoxemia. Blood. 2003;101:4446–4448. doi: 10.1182/blood-2002-11-3338. [DOI] [PubMed] [Google Scholar]
- 53.Branger J, van den Blink B, Weijer S, Madwed J, Bos CL, Gupta A, Yong CL, Polmar SH, Olszyna DP, Hack CE, van Deventer SJH, Peppelenbosch MP, van der Poll T. Anti-inflammatory effects of a p38 mitogen-activated protein kinase inhibitor during human endotoxemia. J Immunol. 2002;168:4070–4077. doi: 10.4049/jimmunol.168.8.4070. [DOI] [PubMed] [Google Scholar]
- 54.Brannan C, Perkins A, Vogel K, Ratner N, Nordlund M, Reid S, Buchberg A, Jenkins N, Parada L, Copeland N. Targeted disruption of the nerurofibromatosis type-1 gene leads to developmental abnormalities in heart and various neural crest-derived tissues. Genes Development. 1994;8:1019–1029. doi: 10.1101/gad.8.9.1019. [DOI] [PubMed] [Google Scholar]
- 55.Braz JC, Bueno OF, Liang Q, Wilkins BJ, Dai YS, Parsons S, Braunwart J, Glascock BJ, Klevitsky R, Kimball TF, Hewett TE, Molkentin JD. Targeted inhibition of p38 MAPK promotes hypertrophic cardiomyopathy through upregulation of calcineurin-NFAT signaling. J Clin Invest. 2003;111:1475–1486. doi: 10.1172/JCI17295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Buckingham M, Meilhac S, Zaffran S. Building the mammalian heart from two sources of myocardial cells. Nat Rev Genet. 2005;6:826–837. doi: 10.1038/nrg1710. [DOI] [PubMed] [Google Scholar]
- 57.Bueno O, De Windt LJ, Tymitz KM, Witt SA, Kimball T, Klevitsky R, Hewett T, Jones S, Lefer D, Peng C, Kitsis R, Molkentin J. The MEK1-ERK1/2 signaling pathway promotes compensated cardiac hypertrophy in transgenic mice. EMBO J. 2000;19:6341–6350. doi: 10.1093/emboj/19.23.6341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Buerger A, Rozhitskaya O, Sherwood MC, Dorfman AL, Bisping E, Abel ED, Pu WT, Izumo S, Jay PY. Dilated cardiomyopathy resulting from high-level myocardial expression of Cre-recombinase. J Card Fail. 2006;12:392–398. doi: 10.1016/j.cardfail.2006.03.002. [DOI] [PubMed] [Google Scholar]
- 59.Buschbeck M, Eickhoff J, Sommer MN, Ullrich A. Phosphotyrosine-specific phosphatase PTP-SL regulates the ERK5 signaling pathway. J Biol Chem. 2002;277:29503–29509. doi: 10.1074/jbc.M202149200. [DOI] [PubMed] [Google Scholar]
- 60.Camenisch T, Spicer A, Brehm-Gibson T, Biesterfeldt J, Augustine M, Calabro A, Kiubalak S, Klewer S, McDonald J. Disruption of hyaluronan synthase-2 abrogates normal cardiac morphogenesis and hyaluronan-mediated transformation of epithelium to mesenchyme. J Clin Invest. 2000;106:349–360. doi: 10.1172/JCI10272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Cameron SJ, Itoh S, Baines CP, Zhang C, Ohta S, Che W, Glassman M, Lee JD, Yan C, Yang J, Abe Ji. Activation of big MAP kinase 1 (BMK1/ERK5) inhibits cardiac injury after myocardial ischemia and reperfusion. FEBS Lett. 2004;566:255–260. doi: 10.1016/j.febslet.2004.03.120. [DOI] [PubMed] [Google Scholar]
- 62.Canagarajah B, Khokhlatchev A, Cobb M, Goldsmith E. Activation mechanism of the MAP kinase ERK2 by dual phosphoyrlation. Cell. 1997;90:859–869. doi: 10.1016/s0092-8674(00)80351-7. [DOI] [PubMed] [Google Scholar]
- 63.Carta C, Pantaleoni F, Bocchinfuso G, Stella L, Vasta I, Sarkozy A, Digilio C, Palleschi A, Pizzuti A, Grammatico P, Zampino G, Dallapiccola B, Gelb BD. Germline missense mutations affecting KRAS isoform B are associated with a severe Noonan syndrome phenotype. Am J Hum Genet. 2006;79:129–135. doi: 10.1086/504394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Cervantes D, Crosby C, Xiang Y. Arrestin orchestrates crosstalk between G protein-coupled receptors to modulate the spatiotemporal activation of ERK MAPK. Circ Res. 106:79–88. doi: 10.1161/CIRCRESAHA.109.198580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Chen C, Sytkowski AJ. Apoptosis-linked gene-2 connects the Raf-1 and ASK1 signalings. Biochem Biophys Res Commun. 2005;333:51–57. doi: 10.1016/j.bbrc.2005.05.074. [DOI] [PubMed] [Google Scholar]
- 66.Chen J, Fujii K, Zhang L, Roberts T, Fu H. Raf-1 promotes cell survival by antagonizing apoptosis signal-regulating kinase 1 through a MEK-ERK independent mechanism. Proc Natl Acad Sci USA. 2001;98:7783–7788. doi: 10.1073/pnas.141224398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Chen L, Kuo W, Yang J, Wang S, Yeh Y, Tsai F, Ho Y, Chang M, Huang C, Lee S. Eccentric cardiac hypertrophy was induced by long-term intermittent hypoxia in rats. Exp Physiol. 2007;92:409–416. doi: 10.1113/expphysiol.2006.036590. [DOI] [PubMed] [Google Scholar]
- 68.Chen Y, Amende I, Hampton TG, Yang Y, Ke Q, Min JY, Xiao YF, Morgan JP. Vascular endothelial growth factor promotes cardiomyocyte differentiation of embryonic stem cells. Am J Physiol Heart Circ Physiol. 2006;291:H1653–H1658. doi: 10.1152/ajpheart.00363.2005. [DOI] [PubMed] [Google Scholar]
- 69.Chen Y, Rajashree R, Liu Q, Hofmann P. Acute p38 MAPK activation decreases force development in ventricular myocytes. Am J Physiol Heart Circ Physiol. 2003;285:H2578–H2586. doi: 10.1152/ajpheart.00365.2003. [DOI] [PubMed] [Google Scholar]
- 70.Cheng TH, Shih NL, Chen SY, Lin JW, Chen YL, Chen CH, Lin H, Cheng CF, Chiu WT, Wang DL, Chen JJ. Nitric oxide inhibits endothelin-1-induced cardiomyocyte hypertrophy through cGMP-mediated suppression of extracellular-signal regulated kinase phosphorylation. Mol Pharmacol. 2005;68:1183–1192. doi: 10.1124/mol.105.014449. [DOI] [PubMed] [Google Scholar]
- 71.Cheng YC, Kuo WW, Wu HC, Lai TY, Wu CH, Hwang JM, Wang WH, Tsai FJ, Yang JJ, Huang CY, Chu CH. ZAK induces MMP-2 activity via JNK/p38 signals and reduces MMP-9 activity by increasing TIMP-1/2 expression in H9c2 cardiomyoblast cells. Mol Cell Biochem. 2009;325:69–77. doi: 10.1007/s11010-008-0021-1. [DOI] [PubMed] [Google Scholar]
- 72.Choudhary R, Palm-Leis A, Scott RC, III, Guleria RS, Rachut E, Baker KM, Pan J. All-trans retinoic acid prevents development of cardiac remodeling in aortic banded rats by inhibiting the renin-angiotensin system. Am J Physiol Heart Circ Physiol. 2008;294:H633–H644. doi: 10.1152/ajpheart.01301.2007. [DOI] [PubMed] [Google Scholar]
- 73.Choukroun G, Hajjar R, Fry S, del Monte F, Haq S, Guerrero J, Picard M, Rosenzweig A, Force T. Regulation of cardiac hypertrophy in vivo by the stress-activated protein kinases/c-Jun NH2-terminal kinases. J Clin Invest. 1999;104:391–398. doi: 10.1172/JCI6350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Choukroun G, Hajjar R, Kyriakis JM, Bonventre JV, Rosenzweig A, Force T. Role of the stress-activated protein kinases in endothelin-induced cardiomyocyte hypertrophy. J Clin Invest. 1998;102:1311–1320. doi: 10.1172/JCI3512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Cicconi S, Ventura N, Pastore D, Bonini P, Nardo PD, Lauro R, Marlier LNJL. Characterization of apoptosis signal transduction pathways in HL-5 cardiomyocytes exposed to ischemia/reperfusion oxidative stress model. J Cell Physiol. 2003;195:27–37. doi: 10.1002/jcp.10219. [DOI] [PubMed] [Google Scholar]
- 76.Clark JE, Sarafraz N, Marber MS. Potential of p38-MAPK inhibitors in the treatment of ischaemic heart disease. Pharmacol Ther. 2007;116:192–206. doi: 10.1016/j.pharmthera.2007.06.013. [DOI] [PubMed] [Google Scholar]
- 77.Clerk A, Aggeli IKS, Stathopoulou K, Sugden PH. Peptide growth factors signal differentially through protein kinase C to extracellular signal-regulated kinases in neonatal cardiomyocytes. Cell Signal. 2006;18:225–235. doi: 10.1016/j.cellsig.2005.04.005. [DOI] [PubMed] [Google Scholar]
- 78.Clerk A, Sugden P. The p38-MAPK inhibitor, SB 203580, inhibits cardiac stress-activated protein kinases/c-jun N-terminal kinases (SAPKs/JNKs) FEBS Lett. 1998;426:93–96. doi: 10.1016/s0014-5793(98)00324-x. [DOI] [PubMed] [Google Scholar]
- 79.Cohen ED, Tian Y, Morrisey EE. Wnt signaling: an essential regulator of cardiovascular differentiation, morphogenesis and progenitor self-renewal. Development. 2008;135:789–798. doi: 10.1242/dev.016865. [DOI] [PubMed] [Google Scholar]
- 80.Cohen SB, Cheng TT, Chindalore V, Damjanov N, Burgos-Vargas R, DeLora P, Zimany K, Travers H, Caulfield JP. Evaluation of the efficacy and safety of pamapimod, a p38 MAP kinase inhibitor, in a double-blind, methotrexate-controlled study of patients with active rheumatoid arthritis. Arthritis Rheumatism. 2009;60:335–344. doi: 10.1002/art.24266. [DOI] [PubMed] [Google Scholar]
- 81.Coulombe P, Meloche S. Atypical mitogen-activated protein kinases: structure, regulation and functions. Biochim Biophys Acta. 2007;1773:1376–1387. doi: 10.1016/j.bbamcr.2006.11.001. [DOI] [PubMed] [Google Scholar]
- 82.Cross H, Li M, Petrich B, Murphy E, Wang Y, Steenbergen C. Effect of p38 MAP kinases on contractility and ischmeic injury in intact heart. Acta Physiol Hung. 2009;96:307–323. doi: 10.1556/APhysiol.96.2009.3.5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Cuenda A, Rousseau S. p38 MAP-kinases pathway regulation, function and role in human diseases. Biochim Biophys Acta. 2007;1773:1358–1375. doi: 10.1016/j.bbamcr.2007.03.010. [DOI] [PubMed] [Google Scholar]
- 84.Cullingford T, Markou T, Fuller S, Giraldo A, Pikkarainen S, Zoumpoulidou G, Alsafi A, Ekere C, Kemp T, Dennis J, Game L, Sugden PH, Clerk A. Temporal regulation of expression of immediate early and second phase transcripts by endothelin-1 in cardiomyocytes. Genome Biol. 2008;9:R32. doi: 10.1186/gb-2008-9-2-r32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Daily L, Ambrosetti L, Mansukhani A, Basilico C. Mechanisms underlying differential responses to FGF signaling. Cytokine Growth Factor Rev. 2005;16:233–247. doi: 10.1016/j.cytogfr.2005.01.007. [DOI] [PubMed] [Google Scholar]
- 86.Damjanov N, Kauffman RS, Spencer-Green GT. Efficacy, pharmacodynamics, and safety of VX-702, a novel p38 MAPK inhibitor, in rheumatoid arthritis: results of two randomized, double-blind, placebo-controlled clinical studies. Arthritis Rheumatism. 2009;60:1232–1241. doi: 10.1002/art.24485. [DOI] [PubMed] [Google Scholar]
- 87.Das A, Salloum FN, Xi L, Rao YJ, Kukreja RC. ERK phosphorylation mediates sildenafil-induced myocardial protection against ischemia-reperfusion injury in mice. Am J Physiol Heart Circ Physiol. 2009;296:H1236–H1243. doi: 10.1152/ajpheart.00100.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Das A, Smolenski A, Lohmann SM, Kukreja RC. Cyclic GMP-dependent protein kinase Ialpha attenuates necrosis and apoptosis following ischemia/reoxygenation in adult cardiomyocyte. J Biol Chem. 2006;281:38644–38652. doi: 10.1074/jbc.M606142200. [DOI] [PubMed] [Google Scholar]
- 89.Das A, Xi L, Kukreja RC. Protein Kinase G-dependent cardioprotective mechanism of phosphodiesterase-5 inhibition involves phosphorylation of ERK and GSK3β. J Biol Chem. 2008;283:29572–29585. doi: 10.1074/jbc.M801547200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Das M, Das S, Das DK. Caveolin and MAP kinase interaction in angiotensin II preconditioning of the myocardium. J Cell Mol Med. 2007;11:788–797. doi: 10.1111/j.1582-4934.2007.00067.x. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 91.Davidson SM, Morange M. Hsp25 and the p38 MAPK pathway are involved in differentiation of cardiomyocytes. Dev Biol. 2000;218:146–160. doi: 10.1006/dbio.1999.9596. [DOI] [PubMed] [Google Scholar]
- 92.Davies SP, Reddy H, Caivano M, Cohen P. Specificity and mechanism of action of some commonly used protein kinase inhibitors. Biochem J. 2000;351:95–105. doi: 10.1042/0264-6021:3510095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Davis RJ. Signal transduction by the JNK group of MAP kinases. Cell. 2000;103:239–252. doi: 10.1016/s0092-8674(00)00116-1. [DOI] [PubMed] [Google Scholar]
- 94.Dell’Era P, Ronca R, Coco L, Nicoli S, Metra M, Presta M. Fibroblast growth factor receptor-1 is essential for in vitro cardiomyocyte development. Circ Res. 2003;93:414–420. doi: 10.1161/01.RES.0000089460.12061.E1. [DOI] [PubMed] [Google Scholar]
- 95.Dhanasekaran D, Kashef K, Lee C, Xu H, Reddy E. Scaffold proetin of MAPK-kinase modules. Oncogene. 2007;26:3185–3202. doi: 10.1038/sj.onc.1210411. [DOI] [PubMed] [Google Scholar]
- 96.Dhingra S, Sharma AK, Arora RC, Slezak J, Singal PK. IL-10 attenuates TNF-α-induced NFκB pathway activation and cardiomyocyte apoptosis. Cardiovasc Res. 2009;82:59–66. doi: 10.1093/cvr/cvp040. [DOI] [PubMed] [Google Scholar]
- 97.Dhingra S, Sharma AK, Singla DK, Singal PK. p38 and ERK1/2 MAPKs mediate the interplay of TNF-α and IL-10 in regulating oxidative stress and cardiac myocyte apoptosis. Am J Physiol Heart Circ Physiol. 2007;293:H3524–H3531. doi: 10.1152/ajpheart.00919.2007. [DOI] [PubMed] [Google Scholar]
- 98.Ding B, Abe J, Wei H, Huang Q, Walsh RA, Molina CA, Zhao A, Sadoshima J, Blaxall BC, Berk B, Yan C. Functional role of phosphodiesterase 3 in cardiomyocyte apoptosis: implication in heart failure. Circulation. 2005;111:2469–2476. doi: 10.1161/01.CIR.0000165128.39715.87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Ding L, Liang X, Hu Y, Zhu D, Lou Y. Involvement of p38MAPK and reactive oxygen species in icariin-induced cardiomyocyte differentiation of murine embryonic stem cells in vitro. Stem Cells Dev. 2008;17:751–760. doi: 10.1089/scd.2007.0206. [DOI] [PubMed] [Google Scholar]
- 100.Ding L, Liang X, Lou Y. Time-dependence of cardiomyocyte differentiation disturbed by peroxisome proliferator-activated receptor a inhibitor GW6471 in murine embryonic stem cells in vitro. Acta Pharmacol Sinica. 2007;28:634–642. doi: 10.1111/j.1745-7254.2007.00558.x. [DOI] [PubMed] [Google Scholar]
- 101.Diwan A, Dorn GW., II Decompensation of cardiac hypertrophy: cellular mechanisms and novel therapeutic targets. Physiology. 2007;22:56–64. doi: 10.1152/physiol.00033.2006. [DOI] [PubMed] [Google Scholar]
- 102.Dodge-Kafka KL, Soughayer J, Pare GC, Carlisle Michel JJ, Langeberg LK, Kapiloff MS, Scott JD. The protein kinase A anchoring protein mAKAP coordinates two integrated cAMP effector pathways. Nature. 2005;437:574–578. doi: 10.1038/nature03966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Dorn GW. Novel pharmacotherapies to abrogate postinfarction ventricular remodeling. Nat Rev Cardiol. 2009;6:283–291. doi: 10.1038/nrcardio.2009.12. [DOI] [PubMed] [Google Scholar]
- 104.Dorn GW, Force T. Protein kinase cascases in the regulation of cardiac hypertrophy. J Clin Invest. 2005;115:527–537. doi: 10.1172/JCI24178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Dougherty CJ, Kubasiak LA, Frazier DP, Li H, Xiong WC, Bishopric NH, Webster KA. Mitochondrial signals initiate the activation of c-Jun N-terminal kinase (JNK) by hypoxia-reoxygenation. FASEB J. 2004;18:1060–1070. doi: 10.1096/fj.04-1505com. [DOI] [PubMed] [Google Scholar]
- 106.Dougherty CJ, Kubasiak LA, Prentice H, Andreka P, Bishopric NH, Webster KA. Activation of c-Jun N-terminal kinase promotes survival of cardiac myocytes after oxidative stress. Biochem J. 2002;362:561–571. doi: 10.1042/0264-6021:3620561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Downey J, Davis A, Cohen M. Signaling pathways in ischemic preconditioning. Heart Fail Rev. 2007;12:181–188. doi: 10.1007/s10741-007-9025-2. [DOI] [PubMed] [Google Scholar]
- 108.Dunwoodie SL. Combinatorial signaling in the heart orchestrates cardiac induction, lineage specification and chamber formation. Semin Cell Dev Biol. 2007;18:54–66. doi: 10.1016/j.semcdb.2006.12.003. [DOI] [PubMed] [Google Scholar]
- 109.Eisenberg C, Eisenberg L. WNT11 promotes cardiac tissue formation of early mesoderm. Dev Dyn. 1999;216:45–58. doi: 10.1002/(SICI)1097-0177(199909)216:1<45::AID-DVDY7>3.0.CO;2-L. [DOI] [PubMed] [Google Scholar]
- 110.Eisenberg LM, Eisenberg CA. Wnt signal transduction and the formation of the myocardium. Dev Biol. 2006;293:305–315. doi: 10.1016/j.ydbio.2006.02.014. [DOI] [PubMed] [Google Scholar]
- 111.Eivers E, Fuentealba LC, De Robertis EM. Integrating positional information at the level of Smad1/5/8. Curr Opin Genet Dev. 2008;18:304–310. doi: 10.1016/j.gde.2008.06.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Engel F, Schebesta M, Duong M, Lu G, Ren S, Madwed J, Jiang H, Wang Y, Keating M. p38 MAP kinase inhibition enables proliferation of adult mammalian cardiomyocytes. Genes Dev. 2005;19:1175–1187. doi: 10.1101/gad.1306705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Engel FB, Hsieh PC, Lee RT, Keating MT. FGF1/p38 MAP kinase inhibitor therapy induces cardiomyocyte mitosis, reduces scarring, and rescues function after myocardial infarction. Proc Natl Acad Sci USA. 2006;103:15546–15551. doi: 10.1073/pnas.0607382103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Engelbrecht AM, Niesler C, Page C, Lochner A. p38 and JNK have distinct regulatory functions on the development of apoptosis during simulated ischaemia and reperfusion in neonatal cardiomyocytes. Basic Res Cardiol. 2004;99:338–350. doi: 10.1007/s00395-004-0478-3. [DOI] [PubMed] [Google Scholar]
- 115.Eriksson M, Leppa S. Mitogen-activated protein kinases and activator protein 1 are required for proliferation and cardiomyocyte differentiation of p19 embryonal carcinoma cells. J Biol Chem. 2002;277:15992–16001. doi: 10.1074/jbc.M107340200. [DOI] [PubMed] [Google Scholar]
- 116.Fan GC, Chu G, Kranias EG. Hsp20 and its cardioprotection. Trends Cardiovasc Med. 2005;15:138–141. doi: 10.1016/j.tcm.2005.05.004. [DOI] [PubMed] [Google Scholar]
- 117.Fan GC, Yuan Q, Song G, Wang Y, Chen G, Qian J, Zhou X, Lee YJ, Ashraf M, Kranias EG. Small heat-shock protein Hsp20 attenuates β-agonist-mediated cardiac remodeling through apoptosis signal-regulating kinase 1. Circ Res. 2006;99:1233–1242. doi: 10.1161/01.RES.0000251074.19348.af. [DOI] [PubMed] [Google Scholar]
- 118.Fan WJ, Genade S, Genis A, Huisamen B, Lochner A. Dexa-methasone-induced cardioprotection: a role for the phosphatase MKP-1? Life Sci. 2009;84:838–846. doi: 10.1016/j.lfs.2009.03.014. [DOI] [PubMed] [Google Scholar]
- 119.Ferrandi C, Ballerio R, Gaillard P, Carboni S, Vitte P, Gotteland J, Cirillo R. Inhibition of c-Jun N-terminal kinase decreases cardiomyocyte apoptosis and infarct size after myocardial ischemia and reperfusion in anaesthetized rats. Br J Pharmacol. 2004;142:953–960. doi: 10.1038/sj.bjp.0705873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Ferrell JE., Jr Tripping the switch fantastic: how a protein kinase cascade can convert graded inputs into switch-like outputs. Trends Biochem Sci. 1996;21:460–466. doi: 10.1016/s0968-0004(96)20026-x. [DOI] [PubMed] [Google Scholar]
- 121.Fiedler B, Feil R, Hofmann F, Willenbockel C, Drexler H, Smolenski A, Lohmann SM, Wollert KC. cGMP-dependent protein kinase type I inhibits TAB1-p38 mitogen-activated protein kinase apoptosis signaling in cardiac myocytes. J Biol Chem. 2006;281:32831–32840. doi: 10.1074/jbc.M603416200. [DOI] [PubMed] [Google Scholar]
- 122.Fischer TA, Ludwig S, Flory E, Gambaryan S, Singh K, Finn P, Pfeffer MA, Kelly RA, Pfeffer JM. Activation of cardiac c-Jun NH2-terminal kinases and p38-mitogen-activated protein kinases with abrupt changes in hemodynamic load. Hypertension. 2001;37:1222–1228. doi: 10.1161/01.hyp.37.5.1222. [DOI] [PubMed] [Google Scholar]
- 123.Flaherty MP, Abdel-Latif A, Li Q, Hunt G, Ranjan S, Ou Q, Tang XL, Johnson RK, Bolli R, Dawn B. Noncanonical Wnt11 signaling is sufficient to induce cardiomyogenic differentiation in unfractionated bone marrow mononuclear cells. Circulation. 2008;117:2241–2252. doi: 10.1161/CIRCULATIONAHA.107.741066. [DOI] [PubMed] [Google Scholar]
- 124.Flesch M, Margulies KB, Mochmann HC, Engel D, Sivasubramanian N, Mann DL. Differential regulation of mitogen-activated protein kinases in the failing human heart in response to mechanical unloading. Circulation. 2001;104:2273–2276. doi: 10.1161/hc4401.099449. [DOI] [PubMed] [Google Scholar]
- 125.Fliegel L. Molecular biology of the myocardial Na+/H+ exchanger. J Mol Cell Cardiol. 2008;44:228–237. doi: 10.1016/j.yjmcc.2007.11.016. [DOI] [PubMed] [Google Scholar]
- 126.Fliegel L. Regulation of the Na+/H+ exchanger in the healthy and diseased myocardium. Expert Opin Ther Targets. 2009;13:55–68. doi: 10.1517/14728220802600707. [DOI] [PubMed] [Google Scholar]
- 127.Force T, Kerkela R. Cardiotoxicity of the new cancer therapeutics-mechanisms of and approaches to the problem. Drug Discov Today. 2008;17–18:778–784. doi: 10.1016/j.drudis.2008.05.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Force T, Krause DS, Van Etten RA. Molecular mechanisms of cardiotoxicity of tyrosine kinase inhibition. Nat Rev Cancer. 2007;7:332–344. doi: 10.1038/nrc2106. [DOI] [PubMed] [Google Scholar]
- 129.Force T, Kuida K, Namchuk M, Parang K, Kyriakis JM. Inhibitors of protein kinase signaling pathways: emerging therapies for cardiovascular disease. Circulation. 2004;109:1196–1205. doi: 10.1161/01.CIR.0000118538.21306.A9. [DOI] [PubMed] [Google Scholar]
- 130.Force T, Woodgett JR. Unique and overlapping functions of GSK-3 isoforms in cell differentiation and proliferation and cardiovascular development. J Biol Chem. 2009;284:9643–9647. doi: 10.1074/jbc.R800077200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Frangogiannis NG. The immune system and cardiac repair. Pharmacol Res. 2008;58:88–111. doi: 10.1016/j.phrs.2008.06.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Frantz S, Berh T, Hu K, Fraccarollo D, Strotmann J, Goldberg E, Ertl G, Angermann C, Bauersachs J. Role of p38 mitogen-activated protein kinase in cardiac remodeling. Br J Pharmacol. 2007;150:130–135. doi: 10.1038/sj.bjp.0706963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Frey N, Olson EN. Cardiac hypertrophy: the good, the bad, and the ugly. Annu Rev Physiol. 2003;65:45–79. doi: 10.1146/annurev.physiol.65.092101.142243. [DOI] [PubMed] [Google Scholar]
- 134.Fryer RM, Patel HH, Hsu AK, Gross GJ. Stress-activated protein kinase phosphorylation during cardioprotection in the ischemic myocardium. Am J Physiol Heart Circ Physiol. 2001;281:H1184–H1192. doi: 10.1152/ajpheart.2001.281.3.H1184. [DOI] [PubMed] [Google Scholar]
- 135.Fuller SJ, Sivarajah K, Sugden PH. ErbB receptors, their ligands, and the consequences of their activation and inhibition in the myocardium. J Mol Cell Cardiol. 2008;44:831–854. doi: 10.1016/j.yjmcc.2008.02.278. [DOI] [PubMed] [Google Scholar]
- 136.Galkina E, Ley K. Immune and inflammatory mechanisms of atherosclerosis. Annu Rev Immunol. 2009;27:165–197. doi: 10.1146/annurev.immunol.021908.132620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Garcia-Martinez V, Schoenwolf G. Primitive-streak origin of the cardiovascular system in avian embryos. Dev Biol. 1993;159:706–719. doi: 10.1006/dbio.1993.1276. [DOI] [PubMed] [Google Scholar]
- 138.Ge B, Gram H, Di Padova F, Huang B, New L, Ulevitch RJ, Luo Y, JH MAPKK-independent activation of p38alpha mediated by TAB1-dependent autophosphorylation of p38alpha. Science. 2002;295:1291–1294. doi: 10.1126/science.1067289. [DOI] [PubMed] [Google Scholar]
- 139.Gerits N, Kostenko S, Moens U. In vivo functions of mitogen-activated protein kinases: conclusions from knock-in and knockout mice. Transgenic Res. 2007;16:281–314. doi: 10.1007/s11248-006-9052-0. [DOI] [PubMed] [Google Scholar]
- 140.Goldsmith ZG, Dhanasekaran DN. G Protein regulation of MAPK networks. Oncogene. 26:3122–3142. doi: 10.1038/sj.onc.1210407. [DOI] [PubMed] [Google Scholar]
- 141.Gorog DA, Jabr R, Tanno M, Sarafraz N, Clark JE, Fisher S, Cao XB, Bellahcene M, Dighe K, Kabir AMN, Quinlan RA, Kato K, Gaestel M, Marber MS. MAPKAPK-2 modulates p38-MAPK localization and small heat shock protein phosphorylation but does not mediate the injury associated with p38-MAPK activation during myocardial ischemia. Cell Stress Chaperones. 2009;14:477–489. doi: 10.1007/s12192-009-0101-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Graichen R, Xu X, Braam S, Balakrishnan T, Norfiza S, Sieh S, Soo S, Tham S, Mummery C, Colman A, Zweigerdt R, Davidson B. Enhanced cardiomyogenesis of human embryonic stem cells by a small molecular inhibitor of p38 MAPK. Differentiation. 2008;76:357–370. doi: 10.1111/j.1432-0436.2007.00236.x. [DOI] [PubMed] [Google Scholar]
- 143.Grewal S, Molina DM, Bardwell L. Mitogen-activated protein kinase (MAPK)-docking sites in MAPK kinases function as tethers that are crucial for MAPK regulation in vivo. Cell Signal. 2006;18:123–134. doi: 10.1016/j.cellsig.2005.04.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Gysembergh A, Simkhovich B, Kloner R, Przyklenk K. p38 MAPK activity is not increased early during sustained coronary artery occulusion in preconditioned versus control rabbit heart. J Mol Cell Cardiol. 2001;33:681–690. doi: 10.1006/jmcc.2000.1331. [DOI] [PubMed] [Google Scholar]
- 145.Hagiwara Y, Miyoshi S, Fukuda K, Nishiyama N, Ikegami Y, Tanimoto K, Murata M, Takahashi E, Shimoda K, Hirano T, Mitamura H, Ogawa S. SHP2-mediated signaling cascade through gp130 is essential for LIF-dependent ICaL, [Ca2+]i transient, APD increase in cardiomyocytes. J Mol Cell Cardiol. 2007;43:710–716. doi: 10.1016/j.yjmcc.2007.09.004. [DOI] [PubMed] [Google Scholar]
- 146.Hall-Jackson C, Goedert M, Hedge P, Cohen P. Effects of SB 203580 on the activity of c-Raf in vitro and in vivo. Oncogene. 1999;18:2047–2054. doi: 10.1038/sj.onc.1202603. [DOI] [PubMed] [Google Scholar]
- 147.Han J, Lee J, Bibbs L, Ulevitch R. A MAP kinase targeted by endotoxin and hyperosmolarity in mammalian cells. Science. 1994;265:808–811. doi: 10.1126/science.7914033. [DOI] [PubMed] [Google Scholar]
- 148.Han J, Lee J, Tobias P, Ulevitch R. Endotoxin induces rapid protein tyrosine phosphorylation in 70Z/3 cells expressing CD14. J Biol Chem. 1993;268:25009–25014. [PubMed] [Google Scholar]
- 149.Han J, Molkentin JD. Regulation of MEF2 by p38 MAPK and its implication in cardiomyocyte biology. Trends Cardiovasc Med. 2000;10:19–22. doi: 10.1016/s1050-1738(00)00039-6. [DOI] [PubMed] [Google Scholar]
- 150.Harris IS, Zhang S, Treskov I, Kovacs A, Weinheimer C, Muslin AJ. Raf-1 kinase is required for cardiac hypertrophy and cardiomyocyte survival in response to pressure overload. Circulation. 2004;110:718–723. doi: 10.1161/01.CIR.0000138190.50127.6A. [DOI] [PubMed] [Google Scholar]
- 151.Hatano N, Mori Y, Oh-hora M, Kosugi A, Fujikawa T, Nakai N, Niwa H, Miyazaki J, Hamaoka T, Ogata M. Essential role for ERK2 mitogen-activated protein kinase in placental development. Genes Cells. 2003;8:847–856. doi: 10.1046/j.1365-2443.2003.00680.x. [DOI] [PubMed] [Google Scholar]
- 152.Hausenloy D, Yellon D. Reperfusion injury salvage kinase signalling: taking a RISK for cardioprotection. Heart Fail Rev. 2007;12:217–234. doi: 10.1007/s10741-007-9026-1. [DOI] [PubMed] [Google Scholar]
- 153.Hausenloy DJ, Tsang A, Yellon DM. The reperfusion injury salvage kinase pathway: a common target for both ischemic preconditioning and postconditioning. Trends Cardiovasc Med. 2005;15:69–75. doi: 10.1016/j.tcm.2005.03.001. [DOI] [PubMed] [Google Scholar]
- 154.Hayashi M, Kim S, Imanaka-Yoshida K, Yoshida T, Abel E, Eliceiri B, Yang Y, RJU, Lee J. Targeted deletion of BMK1/ERK5 in adult mice perturbs vascular integrity and leads to endothelial failure. J Clin Invest. 2004;113:1138–1148. doi: 10.1172/JCI19890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Hayashi M, Lee J. Role of the BMK/ERK5 signaling pathway: lessons from knockout mice. J Mol Med. 2004;82:800–808. doi: 10.1007/s00109-004-0602-8. [DOI] [PubMed] [Google Scholar]
- 156.He H, Li HL, Lin A, Gottlieb RA. Activation of the JNK pathway is important for cardiomyocyte death in response to simulated ischemia. Cell Death Differ. 1999;6:987–991. doi: 10.1038/sj.cdd.4400572. [DOI] [PubMed] [Google Scholar]
- 157.Heidenreich O, Neininger A, Schratt G, Zinck R, Cahill MA, Engel K, Kotlyarov A, Kraft R, Kostka S, Gaestel M, Nordheim A. MAPKAP kinase 2 phosphorylates serum response factor in vitro and in vivo. J Biol Chem. 1999;274:14434–14443. doi: 10.1074/jbc.274.20.14434. [DOI] [PubMed] [Google Scholar]
- 158.Hernández-Torres F, Martínez-Fernández S, Zuluaga S, Nebreda Á, Porras A, Aránega AE, Navarro F. A role for p38α mitogen-activated protein kinase in embryonic cardiac differentiation. FEBS Lett. 2008;582:1025–1031. doi: 10.1016/j.febslet.2008.02.050. [DOI] [PubMed] [Google Scholar]
- 159.Hibi M, Lin A, Smeal T, Minden A, Karin M. Identification of an oncoprotein- and UV-responsive protein kinase that binds and potentiates the c-Jun activation domain. Genes Dev. 1993;7:2135–2148. doi: 10.1101/gad.7.11.2135. [DOI] [PubMed] [Google Scholar]
- 160.Higashi Y, Noma K, Yoshizum M, Kihara Y. Endothelial function and oxidative stress in cardiovascular diseases. Circ J. 2009;73 doi: 10.1253/circj.cj-08-1102. [DOI] [PubMed] [Google Scholar]
- 161.Hilfiker-Kleiner D, Hilfiker A, Castellazzi M, Wollert KC, Trautwein C, Schunkert H, Drexler H. JunD attenuates phenylephrine-mediated cardiomyocyte hypertrophy by negatively regulating AP-1 transcriptional activity. Cardiovasc Res. 2006;71:108–117. doi: 10.1016/j.cardiores.2006.02.032. [DOI] [PubMed] [Google Scholar]
- 162.Hilfiker-Kleiner D, Hilfiker A, Kaminski K, Schaefer A, Park JK, Michel K, Quint A, Yaniv M, Weitzman JB, Drexler H. Lack of JunD promotes pressure overload-induced apoptosis, hypertrophic growth, and angiogenesis in the heart. Circulation. 2005;112:1470–1477. doi: 10.1161/CIRCULATIONAHA.104.518472. [DOI] [PubMed] [Google Scholar]
- 163.Ho DT, Bardwell AJ, Grewal S, Iverson C, Bardwell L. Interacting JNK-docking sites in MKK7 promote binding and activation of JNK mitogen-activated protein kinases. J Biol Chem. 2006;281:13169–13179. doi: 10.1074/jbc.M601010200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164.Ho PD, Fan JS, Hayes NL, Saada N, Palade PT, Glembotski CC, McDonough PM. Ras reduces L-type calcium channel current in cardiac myocytes : corrective effects of L-channels and SERCA2 on [Ca2+ regulation and cell morphology. Circ Res. 2001;88:63–69. doi: 10.1161/01.res.88.1.63. [DOI] [PubMed] [Google Scholar]
- 165.Ho PD, Zechner D, He H, Dillmann W, Glembotski C, Mc-Donough PM. The Raf-MEK-ERK cascalde represents a common pathway for alteration of intracellular calcium by Ras and protein kinase C in cardiac myocytes. J Biol Chem. 1998;273:21730–21735. doi: 10.1074/jbc.273.34.21730. [DOI] [PubMed] [Google Scholar]
- 166.Hollander J, Martin J, Belke D, Scott B, Swanson E, Krishnamoorthy V, Dillmann W. Overexpression of wild-type heat shock protein 27 and a nonphosphorylatable heat shock protein 27 mutant protects against ischemia/reperfusion injury in a transgenic mouse model. Circulation. 2004;110:3544–3552. doi: 10.1161/01.CIR.0000148825.99184.50. [DOI] [PubMed] [Google Scholar]
- 167.Hori M, Nishida K. Oxidative stress and left ventricular remodelling after myocardial infarction. Cardiovasc Res. 2009;81:457–464. doi: 10.1093/cvr/cvn335. [DOI] [PubMed] [Google Scholar]
- 168.House SL, Branch K, Newman G, Doetschman T, Schultz JEJ. Cardioprotection induced by cardiac-specific overexpression of fibroblast growth factor-2 is mediated by the MAPK cascade. Am J Physiol Heart Circ Physiol. 2005;289:H2167–H2175. doi: 10.1152/ajpheart.00392.2005. [DOI] [PubMed] [Google Scholar]
- 169.Hreniuk D, Garay M, Gaarde W, Monia BP, McKay RA, Cioffi CL. Inhibition of c-Jun N-terminal kinase 1, but not c-Jun N-terminal kinase 2, suppresses apoptosis induced by ischemia/reoxygenation in rat cardiac myocytes. Mol Pharmacol. 2001;59:867–874. doi: 10.1124/mol.59.4.867. [DOI] [PubMed] [Google Scholar]
- 170.Huang WY, Aramburu J, Douglas PS, Izumo S. Transgenic expression of green fluorescence protein can cause dilated cardiomyopathy. Nat Med. 2000;6:482–483. doi: 10.1038/74914. [DOI] [PubMed] [Google Scholar]
- 171.Huang Y, Wright CD, Kobayashi S, Healy CL, Elgethun M, Cypher A, Liang Q, O’Connell TD. GATA4 is a survival factor in adult cardiac myocytes but is not required for α1A-adrenergic receptor survival signaling. Am J Physiol Heart Circ Physiol. 2008;295:H699–H707. doi: 10.1152/ajpheart.01204.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 172.Huang Y, Wright CD, Merkwan CL, Baye NL, Liang Q, Simpson PC, O’Connell TD. An α1A-adrenergic-extracellular signal-regulated kinase survival signaling pathway in cardiac myocytes. Circulation. 2007;115:763–772. doi: 10.1161/CIRCULATIONAHA.106.664862. [DOI] [PubMed] [Google Scholar]
- 173.Hudis C. Trastuzumab: mechanism of action and use in clinical practice. N Engl J Med. 2007;357:39–51. doi: 10.1056/NEJMra043186. [DOI] [PubMed] [Google Scholar]
- 174.Huebert RC, Li Q, Adhikari N, Charles NJ, Han X, Ezzat MK, Grindle S, Park S, Ormaza S, Fermin D, Miller LW, Hall JL. Identification and regulation of Sprouty1, a negative inhibitor of the ERK cascade, in the human heart. Physiol Genomics. 2004;18:284–289. doi: 10.1152/physiolgenomics.00098.2004. [DOI] [PubMed] [Google Scholar]
- 175.Hunter J, Tanaka N, Rockman H, Ross J, Jr, Chien KR. Ventricular expression of a MLC-2v-ras fusion gene induces cardiac hypertorphy and selective diastolic dysfunction in transgenic mice. J Biol Chem. 1995;270:23173–23178. doi: 10.1074/jbc.270.39.23173. [DOI] [PubMed] [Google Scholar]
- 176.Ikeda Y, Aihara Ki Sato T, Akaike M, Yoshizumi M, Suzaki Y, Izawa Y, Fujimura M, Hashizume S, Kato M, Yagi S, Tamaki T, Kawano H, Matsumoto T, Azuma H, Kato S, Matsumoto T. Androgen receptor gene knockout male mice exhibit impaired cardiac growth and exacerbation of angiotensin II-induced cardiac fibrosis. J Biol Chem. 2005;280:29661–29666. doi: 10.1074/jbc.M411694200. [DOI] [PubMed] [Google Scholar]
- 177.Irukayama-Tomobe Y, Miyauchi T, Sakai S, Kasuya Y, Ogata T, Takanashi M, Iemitsu M, Sudo T, Goto K, Yamaguchi I. Endothelin-1-induced cardiac hypertrophy is inhibited by activation of peroxisome proliferator-activated receptor-α partly via blockade of c-Jun NH2-terminal kinase pathway. Circulation. 2004;109:904–910. doi: 10.1161/01.CIR.0000112596.06954.00. [DOI] [PubMed] [Google Scholar]
- 178.Izawa Y, Yoshizumi M, Ishizawa K, Fujita Y, Kondo S, Kagami S, Kawazoe K, Tsuchiya K, Tomita S, Tamaki T. Big mitogen-activated protein kinase 1 (BMK1)/extracellular signal regulated kinase 5 (ERK5) is involved in platelet-derived growth factor (PDGF)-induced vascular smooth muscle cell migration. Hypertens Res. 2007;30:1107–1117. doi: 10.1291/hypres.30.1107. [DOI] [PubMed] [Google Scholar]
- 179.Izumiya Y, Kim S, Izumi Y, Yoshida K, Yoshiyama M, Matsuzawa A, Ichijo H, Iwao H. Apoptosis signal-regulating kinase 1 plays a pivotal role in angiotensin II-induced cardiac hypertrophy and remodeling. Circ Res. 2003;93:874–883. doi: 10.1161/01.RES.0000100665.67510.F5. [DOI] [PubMed] [Google Scholar]
- 180.Jacobs D, Glossip D, Xing H, Muslin A, Kornfeld K. Multiple docking sites on substrate proteins form a modular system that mediates recognition by ERK MAP kinase. Genes Dev. 1999;13:163–175. [PMC free article] [PubMed] [Google Scholar]
- 181.Jaswal JS, Gandhi M, Finegan BA, Dyck JRB, Clanachan AS. Inhibition of p38 MAPK and AMPK restores adenosine-induced cardioprotection in hearts stressed by antecedent ischemia by altering glucose utilization. Am J Physiol Heart Circ Physiol. 2007;293:H1107–H1114. doi: 10.1152/ajpheart.00455.2007. [DOI] [PubMed] [Google Scholar]
- 182.Jaswal JS, Gandhi M, Finegan BA, Dyck JRB, Clanachan AS. p38 mitogen-activated protein kinase mediates adenosine-induced alterations in myocardial glucose utilization via 5′-AMP-activated protein kinase. Am J Physiol Heart Circ Physiol. 2007;292:H1978–H1985. doi: 10.1152/ajpheart.01121.2006. [DOI] [PubMed] [Google Scholar]
- 183.Jesmin S, Hattori Y, Maeda S, Zaedi S, Sakuma I, Miyauchi T. Subdepressor dose of benidipine ameliorates diabetic cardiac remodeling accompanied by normalization of upregulated endothelin system in rats. Am J Physiol Heart Circ Physiol. 2006;290:H2146–H2154. doi: 10.1152/ajpheart.01142.2005. [DOI] [PubMed] [Google Scholar]
- 184.Jiang Y, Chen C, Li Z, Guo W, Gergner J, Lin S, Han J. Characterization of the structure and function of a new mitogen-activated protein kinase (p38b) J Biol Chem. 1996;271:17920–17926. doi: 10.1074/jbc.271.30.17920. [DOI] [PubMed] [Google Scholar]
- 185.Johnson GL, Lapadat R. Mitogen-activated protein kinase pathways mediated by ERK, JNK, and p38 protein kinases. Science. 2002;298:1911–1912. doi: 10.1126/science.1072682. [DOI] [PubMed] [Google Scholar]
- 186.Juntilla M, Li S, Westermarck J. Phosphatase-mediated crosstalk between MAPK signaling pathways in the regulation of cell survival. FASEB J. 2008;22:954–964. doi: 10.1096/fj.06-7859rev. [DOI] [PubMed] [Google Scholar]
- 187.Kacimi R, Gerdes AM. Alterations in G protein and MAP kinase signaling pathways during cardiac remodeling in hypertension and heart failure. Hypertension. 2003;41:968–977. doi: 10.1161/01.HYP.0000062465.60601.CC. [DOI] [PubMed] [Google Scholar]
- 188.Kai H, Muraishi A, Sugiu Y, Nishi H, Seki Y, Kuwahara F, Kimura A, Kato H, Imaizumi T. Expression of proto-oncogenes and gene mutation of sarcomeric proteins in patients with hypertrophic cardiomyopathy. Circ Res. 1998;83:594–601. doi: 10.1161/01.res.83.6.594. [DOI] [PubMed] [Google Scholar]
- 189.Kaiser R, Bueno O, Lips D, Doevendans P, Jones F, Kimball T, Molkentin J. Targeted inhibition of p38 mitogen-activated protein kinase antagonizes cardiac injury and cell death following ischemia-reperfusion in vivo. J Biol Chem. 2004;279:15524–15530. doi: 10.1074/jbc.M313717200. [DOI] [PubMed] [Google Scholar]
- 190.Kaiser RA, Liang Q, Bueno O, Huang Y, Lackey T, Klevitsky R, Hewett TE, Molkentin JD. Genetic inhibition or activation of JNK1/2 protects the myocardium from ischemia-reperfusion-induced cell death in vivo. J Biol Chem. 2005;280:32602–32608. doi: 10.1074/jbc.M500684200. [DOI] [PubMed] [Google Scholar]
- 191.Kaiser RA, Lyons JM, Duffy JY, Wagner CJ, McLean KM, O’Neill TP, Pearl JM, Molkentin JD. Inhibition of p38 reduces myocardial infarction injury in the mouse but not pig after ischemia-reperfusion. Am J Physiol Heart Circ Physiol. 2005;289:H2747–H2751. doi: 10.1152/ajpheart.01280.2004. [DOI] [PubMed] [Google Scholar]
- 192.Kamakura S, Moriguchi T, Nishida E. Activation of the protein kinase ERK5/BMK1 by receptor tyrosine kinases. Identification and characterization of a signaling pathway to the nucleus. J Biol Chem. 1999;274:26563–26571. doi: 10.1074/jbc.274.37.26563. [DOI] [PubMed] [Google Scholar]
- 193.Kasler H, Victoria J, Duramad O, Winoto A. ERK5 is a novel type of mitogen-activated protein kinase containing a transciptional activation domain. Mol Cell Biol. 2000;20:8382–8389. doi: 10.1128/mcb.20.22.8382-8389.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 194.Kato Y, Kravchenko V, Tapping R, Han J, Ulevitch R, Lee J. BMK1/ERK5 regulates serum-induced early gene expression through transcription factor MEF2C. EMBO J. 1997;16:7054–7066. doi: 10.1093/emboj/16.23.7054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 195.Kato Y, Zhao M, Morikawa A, Sugiyama T, Chakravortty D, Koide N, Yoshida T, Tapping RI, Yang Y, Yokochi T, Lee JD. Big mitogen-activated kinase regulates multiple members of the MEF2 protein family. J Biol Chem. 2000;275:18534–18540. doi: 10.1074/jbc.M001573200. [DOI] [PubMed] [Google Scholar]
- 196.Katz M, Amit I, Yarden Y. Regulation of MAPKs by growth factors and receptor tyrosine kinases. Biochim Biophys Acta. 2007;1773:1161–1176. doi: 10.1016/j.bbamcr.2007.01.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 197.Kennedy RA, Kemp TJ, Sugden PH, Clerk A. Using U0126 to dissect the role of the extracellular signal-regulated kinase 1/2 (ERK1/2) cascade in the regulation of gene expression by endothelin-1 in cardiac myocytes. J Mol Cell Cardiol. 2006;41:236–247. doi: 10.1016/j.yjmcc.2006.04.011. [DOI] [PubMed] [Google Scholar]
- 198.Keren A, Tamir Y, Bengal E. The p38 MAPK signaling pathway: a major regulator of skeletal muscle development. Mol Cell Endocrinol. 2006;252:224–230. doi: 10.1016/j.mce.2006.03.017. [DOI] [PubMed] [Google Scholar]
- 199.Kerkela R, Grazette L, Yacobi R, Iliescu C, Beahm C, Walters B, Shevtsov S, Pesant S, Clubb F, Rosenzweig A, Salomon R, Van Etten RA, Alroy J, Durand J, Force T. Cardiotoxicity of the cancer therapeutic agent imatinib mesylate. Nat Med. 2006;12 doi: 10.1038/nm1446. [DOI] [PubMed] [Google Scholar]
- 200.Kim H, Wang X, Zhang J, Suh G, Benjamin I, Ryter S, Choi A. Heat shock protein-70 mediates the cytoprotective effect of carbon monoxide: involvement of p38 beta MAPK and heat shock factor 1. J Immunol. 2005;175:2622–2629. doi: 10.4049/jimmunol.175.4.2622. [DOI] [PubMed] [Google Scholar]
- 201.Kim J, Pedram A, Razandi M, Leven E. Estrogen prevents cardiomyocyte apoptosis through inhibition of reactiove oxygen species and differentiatial regulation of p38 kinase isoforms. J Biol Chem. 2006;281:6760–6767. doi: 10.1074/jbc.M511024200. [DOI] [PubMed] [Google Scholar]
- 202.Kinugawa K, Jeong MY, Bristow MR, Long CS. Thyroid hormone induces cardiac myocyte hypertrophy in a thyroid hormone receptor α1-specific manner that requires TAK1 and p38 mitogen-activated protein kinase. Mol Endocrinol. 2005;19:1618–1628. doi: 10.1210/me.2004-0503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 203.Klein G, Schaefer A, Hilfiker-Kleiner D, Oppermann D, Shukla P, Quint A, Podewski E, Hilfiker A, Schroder F, Leitges M, Drexler H. Increased collagen deposition and diastolic dysfunction but preserved myocardial hypertrophy after pressure overload in mice lacking PKCε. Circ Res. 2005;96:748–755. doi: 10.1161/01.RES.0000161999.86198.1e. [DOI] [PubMed] [Google Scholar]
- 204.Knight RJ, Buxton DB. Stimulation of c-Jun kinase and mitogen-activated protein kinase by ischemia and reperfusion in the perfused rat heart. Biochem Biophys Res Commun. 1996;218:83–88. doi: 10.1006/bbrc.1996.0016. [DOI] [PubMed] [Google Scholar]
- 205.Kobayashi S, Lackey T, Huang Y, Bisping E, Pu WT, Boxer LM. Transcription factor GATA4 regulates cardiac BCL2 gene expression in vitro and in vivo. FASEB J. 2006;20:800–802. doi: 10.1096/fj.05-5426fje. [DOI] [PubMed] [Google Scholar]
- 206.Koitabashi N, Bedja D, Zaiman AL, Pinto YM, Zhang M, Gabrielson KL, Takimoto E, Kass DA. Avoidance of transient cardiomyopathy in cardiomyocyte-targeted tamoxifen-induced Mer-CreMer gene deletion models. Circ Res. 2009;105:12–15. doi: 10.1161/CIRCRESAHA.109.198416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 207.Kompa AR, See F, Lewis DA, Adrahtas A, Cantwell DM, Wang BH, Krum H. Long-term but not short-term p38 mitogen-activated protein kinase inhibition improves cardiac function and reduces cardiac remodeling post-myocardial infarction. J Pharmacol Exp Ther. 2008;325:741–750. doi: 10.1124/jpet.107.133546. [DOI] [PubMed] [Google Scholar]
- 208.Kong L, Andrassy M, Chang JS, Huang C, Asai T, Szabolcs MJ, Homma S, Liu R, Zou YS, Leitges M, Yan SD, Ramasamy R, Schmidt AM, Yan SF. PKCβ modulates ischemia-reperfusion injury in the heart. Am J Physiol Heart Circ Physiol. 2008;294:H1862–H1870. doi: 10.1152/ajpheart.01346.2007. [DOI] [PubMed] [Google Scholar]
- 209.Kontaridis MI, Yang W, Bence KK, Cullen D, Wang B, Bodyak N, Ke Q, Hinek A, Kang PM, Liao R, Neel BG. Deletion of Ptpn11 (Shp2) in cardiomyocytes causes dilated cardiomyopathy via effects on the extracellular signal-regulated kinase/mitogen-activated protein kinase and RhoA sSignaling pathways. Circulation. 2008;117:1423–1435. doi: 10.1161/CIRCULATIONAHA.107.728865. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 210.Kovacs K, Hanto K, Bognar Z, Tapodi A, Bognar E, Kiss G, Szabo A, Rappai G, Kiss T, Sumegi B, Gallyas F. Prevalent role of Akt and ERK activation in cardioprotective effect of Ca2+ channel- and beta-adrenergic receptor blockers. Mol Cell Biochem. 2009;321:155–164. doi: 10.1007/s11010-008-9929-8. [DOI] [PubMed] [Google Scholar]
- 211.Koyanagi M, Haendeler J, Badorff C, Brandes RP, Hoffmann J, Pandur P, Zeiher AM, Kuhl M, Dimmeler S. Non-canonical Wnt signaling enhances differentiation of human circulating progenitor cells to cardiomyogenic cells. J Biol Chem. 2005:M500323200. doi: 10.1074/jbc.M500323200. [DOI] [PubMed] [Google Scholar]
- 212.Krenz M, Yutzey KE., JR Noonan syndrome mutation Q79R in Shp2 increases proliferation of valve primordia mesenchymal cells via extracellular signal-regulated kinase 1/2 signaling. Circ Res. 2005;97:813–820. doi: 10.1161/01.RES.0000186194.06514.b0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 213.Krishnamurthy P, Subramanian V, Singh M, Singh K. β1 Integrins modulate β-adrenergic receptor-stimulated cardiac myocyte apoptosis and myocardial remodeling. Hypertension. 2007;49:865–872. doi: 10.1161/01.HYP.0000258703.36986.13. [DOI] [PubMed] [Google Scholar]
- 214.Kumar Y, Tatu U. Stress protein flux during recovery from simulated ischemia: induced heat shock protein 70 confers cytoprotection by suppressing JNK activation and inhibiting apoptotic cell death. Proteomics. 2003;3:513–526. doi: 10.1002/pmic.200390065. [DOI] [PubMed] [Google Scholar]
- 215.Kunath T, Saba-El-Leil MK, Almousailleakh M, Wray J, Meloche S, Smith A. FGF stimulation of the Erk1/2 signalling cascade triggers transition of pluripotent embryonic stem cells from self-renewal to lineage commitment. Development. 2007;134:2895–2902. doi: 10.1242/dev.02880. [DOI] [PubMed] [Google Scholar]
- 216.Kuramochi Y, Guo X, Sawyer DB. Neuregulin activates erbB2-dependent src/FAK signaling and cytoskeletal remodeling in isolated adult rat cardiac myocytes. J Mol Cell Cardiol. 2006;41:228–235. doi: 10.1016/j.yjmcc.2006.04.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 217.Kuster G, Pimentel D, Adachi T, Ido Y, Brenner D, Cohen R, Liao R, Siwik D, Colucci W. Alpha-adrenergic receptor-stimulated hypertrophy in adult rat ventricular myocytes is mediated via thioredoxin-1-sensitive oxidative modification of thiols on Ras. Circulation. 2005;111:1192–1198. doi: 10.1161/01.CIR.0000157148.59308.F5. [DOI] [PubMed] [Google Scholar]
- 218.Kyoi S, Otani H, Matsuhisa S, Akita Y, Tatsumi K, Enoki C, Fujiwara H, Imamura H, Kamihata H, Iwasaka T. Opposing effect of p38 MAP kinase and JNK inhibitors on the development of heart failure in the cardiomyopathic hamster. Cardiovasc Res. 2006;69:888–898. doi: 10.1016/j.cardiores.2005.11.015. [DOI] [PubMed] [Google Scholar]
- 219.Kyriakis J, Avruch J. pp54 microtubule-associated protein 2 kinase. A novel serine/threonine protein kinase regulated by phosphorylation and stimulated by poly-L-lysine. J Biol Chem. 1990;265:17355–17363. [PubMed] [Google Scholar]
- 220.Kyriakis J, Brautigan D, Ingebritsen T, Avruch J. pp54 microtubule-associated protein-2 kinase requires both tyrosine and serine/threonine phosphorylation for activity. J Biol Chem. 1991;266:10043–10046. [PubMed] [Google Scholar]
- 221.Kyriakis JM, Avruch J. Mammalian mitogen-activated protein kinase signal transduction pathways activated by stress and inflammation. Physiol Rev. 2001;81:807–869. doi: 10.1152/physrev.2001.81.2.807. [DOI] [PubMed] [Google Scholar]
- 222.Laderoute KR, Webster KA. Hypoxia/reoxygenation stimulates jun kinase activity through redox signaling in cardiac myocytes. Circ Res. 1997;80:336–344. doi: 10.1161/01.res.80.3.336. [DOI] [PubMed] [Google Scholar]
- 223.Lai K, Pawson T. The ShcA phosphotryosine docking protein sensitizes cardiovascular signaling in the mouse embryo. Genes Dev. 2000;14 [PMC free article] [PubMed] [Google Scholar]
- 224.Lakkis M, Epstein J. Neurofibromin modulation of ras activity is required for normal endocardial-mesenchymal transformation in the developing heart. Development. 1998;125:4359–4367. doi: 10.1242/dev.125.22.4359. [DOI] [PubMed] [Google Scholar]
- 225.Lali FV, Hunt AE, Turner SJ, Foxwell BMJ. The pyridinyl imidazole inhibitor Sb203580 blocks phosphoinositide-dependent protein kinase activity, protein kinase B phosphorylation, retino-blastoma hyperphosphorylation in interleukin-2-stimulated T cells independently of p38 mitogen-activated protein kinase. J Biol Chem. 2000;275:7395–7402. doi: 10.1074/jbc.275.10.7395. [DOI] [PubMed] [Google Scholar]
- 226.Lavine KJ, Yu K, White AC, Zhang X, Smith C, Partanen J, Ornitz DM. Endocardial and epicardial derived FGF signals regulate myocardial proliferation and differentiation in vivo. Dev Cell. 2005;8:85–95. doi: 10.1016/j.devcel.2004.12.002. [DOI] [PubMed] [Google Scholar]
- 227.Lawler S, Fleming Y, Goedert M, Cohen P. Synergistic activation of SAPK1/JNK1 by two MAP kinase kinases in vitro. Curr Biol. 1998;8:1387–1390. doi: 10.1016/s0960-9822(98)00019-0. [DOI] [PubMed] [Google Scholar]
- 228.Lechner C, Zahalaka M, Giot J, Moler N, Ullrich A. ERK6, a mitogen-activated protein kinase involvled in C2C12 myoblast differentiation. Proc Natl Acad Sci USA. 1996;93:4355–4359. doi: 10.1073/pnas.93.9.4355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 229.Lee J, Laydon J, McDonnell P, Gallagher T, Kumar S, Green D, McNulty D, Blumenthal M, Keys J, Landvatter S, Strickler J, McLaughlin M, Siemens I, Fisher S, Livi G, White J, Adams J, Young P. A protein kinase involved in the regulation of inflammatory cytokine biosynthesis. Nature. 1994;372:739–746. doi: 10.1038/372739a0. [DOI] [PubMed] [Google Scholar]
- 230.Lee JD, Ulevitch RJ, Han JH. Primary structure of BMK1: a new mammalian MAP kinase. Biochem Biophys Res Commun. 1995;213:715–724. doi: 10.1006/bbrc.1995.2189. [DOI] [PubMed] [Google Scholar]
- 231.Lee KH, Kim SE, Lee YS. SP600125, a selective JNK inhibitor, aggravates hepatic ischemia-reperfusion injury. Exp Mol Med. 2006;38:408–416. doi: 10.1038/emm.2006.48. [DOI] [PubMed] [Google Scholar]
- 232.Li D, Tao L, Lui H, Christopher T, Lopez B, Ma X. Role of ERK1/2 in the anti-apoptotic and cardioprotective effects of nitric oxide after myocardial ischemia and reperfusion. Apoptosis. 2006;11:923–930. doi: 10.1007/s10495-006-6305-6. [DOI] [PubMed] [Google Scholar]
- 233.Li G, Ali IS, Currie RW. Insulin-induced myocardial protection in isolated ischemic rat hearts requires p38 MAPK phosphorylation of Hsp27. Am J Physiol Heart Circ Physiol. 2008;294:H74–H87. doi: 10.1152/ajpheart.00675.2007. [DOI] [PubMed] [Google Scholar]
- 234.Li J, Miler E, Ninomiya-Tsuji J, Russell R, Young L. AMP-activated protein kinase activates p38 mitogen-activated protein kinase by increasing recruitment of p38 MAPK to TAB1 in the ischemic heart. Circ Res. 2005;97:872–879. doi: 10.1161/01.RES.0000187458.77026.10. [DOI] [PubMed] [Google Scholar]
- 235.Li J, Stouffs M, Serrander L, Banfi B, Bettiol E, Charnay Y, Steger K, Krause KH, Jaconi ME. The NADPH oxidase NOX4 drives cardiac differentiation: role in regulating cardiac transcription factors and MAP kinase activation. Mol Biol Cell. 2006;17:3978–3988. doi: 10.1091/mbc.E05-06-0532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 236.Li M, Georgakopoulos D, Lu G, Hester L, Kass D, Hasday J, Wang Y. p38 MAP kinase mediates inflammatory cytokine induction in cardiomyocytes and extracellular matrix remodeling in heart. Circulation. 2005;111:2494–2502. doi: 10.1161/01.CIR.0000165117.71483.0C. [DOI] [PubMed] [Google Scholar]
- 237.Li Z, Jiang Y, Ulevitch R, Han J. The primary sturcture of p38gamma: a new member of the p38 group of MAP kinase. Biochem Biophys Res Commun. 1996;228:334–340. doi: 10.1006/bbrc.1996.1662. [DOI] [PubMed] [Google Scholar]
- 238.Li Z, Ma JY, Kerr I, Chakravarty S, Dugar S, Schreiner G, Protter AA. Selective inhibition of p38α MAPK improves cardiac function and reduces myocardial apoptosis in rat model of myocardial injury. Am J Physiol Heart Circ Physiol. 2006;291:H1972–H1977. doi: 10.1152/ajpheart.00043.2006. [DOI] [PubMed] [Google Scholar]
- 239.Liang Q, Bueno O, Wilkins BJ, Kuan C, Xia Y, Molkentin J. c-Jun N-terminal kinases (JNK) antagonize cardiac growth through cross-talk with calcineurin-NFAT signaling. EMBO J. 2003;22:5079–5089. doi: 10.1093/emboj/cdg474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 240.Liang Q, Molkentin J. Redefining the roles of p38 and JNK signaling in cardiac hypertrophy: dichotomy between cultured myocytes and animal models. J Mol Cell Cardiol. 2003;35:1385–1394. doi: 10.1016/j.yjmcc.2003.10.001. [DOI] [PubMed] [Google Scholar]
- 241.Liang Q, Wiese RJ, Bueno OF, Dai YS, Markham BE, Molkentin JD. The transcription factor GATA4 is activated by extracellular signal-regulated kinase 1- and 2-mediated phosphorylation of serine 105 in cardiomyocytes. Mol Cell Biol. 2001;21:7460–7469. doi: 10.1128/MCB.21.21.7460-7469.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 242.Liao P, Georgakopoulos D, Kovacs A, Zheng M, Lerner D, Pu H, Saffitz J, Chien KR, Xiao R, Kass D, Wang Y. The in vivo role of p38 MAP kinases in cardiac remodeling and restrictive cardiomyopathy. Proc Natl Acad Sci USA. 2001;98:12283–12288. doi: 10.1073/pnas.211086598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 243.Liao P, Wang SQ, Wang S, Zheng M, Zheng M, Zhang SJ, Cheng H, Wang Y, Xiao RP. p38 Mitogen-activated protein kinase mediates a negative inotropic effect in cardiac myocytes. Circ Res. 2002;90:190–196. doi: 10.1161/hh0202.104220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 244.Lim H, New L, Han J, Molkentin J. Calcineurin enhances MAPK phosphatase-1 expression and p38 MAPK inactivation in cardiac myocytes. J Biol Chem. 2001;276:15913–15919. doi: 10.1074/jbc.M100452200. [DOI] [PubMed] [Google Scholar]
- 245.Lin A, Birch P, Korf B, Tenconi R, Niimura M, Poyhonen M, Armfield Uhas K, Sigorini M, Virdis R, Romano C, Bonioli E, Wolkenstein P, Pivnick E, Lawrence M, Friedman J. Cardiovascular malformations and other cardiovascular abnormalities in neurofibromatosis 1. Am J Med Genet. 2000;95:108–117. doi: 10.1002/1096-8628(20001113)95:2<108::aid-ajmg4>3.0.co;2-0. [DOI] [PubMed] [Google Scholar]
- 246.Lincoln J, Alfieri C, Yutzey K. BMP and FGF regulatory pathways control cell lineage diversification of heart valve precursor cells. Dev Biol. 2006;292:292–302. doi: 10.1016/j.ydbio.2005.12.042. [DOI] [PubMed] [Google Scholar]
- 247.Lips D, Bueno O, Wilkins BJ, Purcell N, Kaiser R, Lorenz J, Voisin L, Saba-El-Leill M, Meloche S, Pouyssegur J, Pages G, De Windt LJ, Doevendans P, Molkentin J. MEK1-ERK2 signaling pathway protects myocardium from ischemic injury in vivo. Circulation. 2004;109:1938–1941. doi: 10.1161/01.CIR.0000127126.73759.23. [DOI] [PubMed] [Google Scholar]
- 248.Liu J, Mao W, Ding B, Liang CS. ERKs/p53 signal transduction pathway is involved in doxorubicin-induced apoptosis in H9c2 cells and cardiomyocytes. Am J Physiol Heart Circ Physiol. 2008;295:H1956–H1965. doi: 10.1152/ajpheart.00407.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 249.Liu J, Sadoshima J, Zhai P, Hong C, Yang G, Chen W, Yan L, Wang Y, Vatner SF, Vatner DE. Pressure overload induces greater hypertrophy and mortality in female mice with p38α MAPK inhibition. J Mol Cell Cardiol. 2006;41:680–688. doi: 10.1016/j.yjmcc.2006.07.007. [DOI] [PubMed] [Google Scholar]
- 250.Liu W, Zi M, Jin J, Prehar S, Oceandy D, Kimura TE, Lei M, Neyses L, Weston AH, Cartwright EJ, Wang X. Cardiac-specific deletion of Mkk4 reveals its role in pathological hypertrophic remodeling but not in physiological cardiac growth. Circ Res. 2009;104:905–914. doi: 10.1161/CIRCRESAHA.108.188292. [DOI] [PubMed] [Google Scholar]
- 251.Liu YH, Wang D, Rhaleb NE, Yang XP, Xu J, Sankey SS, Rudolph AE, Carretero OA. Inhibition of p38 mitogen-activated protein kinase protects the heart against cardiac remodeling in mice with heart failure resulting from myocardial infarction. J Cardiac Failure. 2005;11:74–81. doi: 10.1016/j.cardfail.2004.04.004. [DOI] [PubMed] [Google Scholar]
- 252.Lorenz K, Schmitt JP, Schmitteckert EM, Lohse MJ. A new type of ERK1/2 autophosphorylation causes cardiac hypertrophy. Nat Med. 2009;15:75–83. doi: 10.1038/nm.1893. [DOI] [PubMed] [Google Scholar]
- 253.Lough J, Barron M, Brogley M, Sugi Y, Bolender D, Zhu X. BMP-2 and FGF-4, but neither factor alone, induces cardiogenesis in non-precardiac embryonic mesoderm. Dev Biol. 1996;178:198–202. doi: 10.1006/dbio.1996.0211. [DOI] [PubMed] [Google Scholar]
- 254.Lu G, Kang Y, Han J, Herschman H, Stefani E, Wang Y. TAB-1 modulates intracellular localization of p38 MAP kinase and downstream signaling. J Biol Chem. 2006;281:6087–6095. doi: 10.1074/jbc.M507610200. [DOI] [PubMed] [Google Scholar]
- 255.Lu Y, Shioda N, Han F, Kamata A, Shirasaki Y, Qin Z, Fukunaga K. DY-9760e inhibits endothelin-1-induced cardiomyocyte hypertrophy through inhibition of CaMKII and ERK activities. Cardiovasc Ther. 2009;27:17–27. doi: 10.1111/j.1755-5922.2008.00068.x. [DOI] [PubMed] [Google Scholar]
- 256.Ma X, Kumar S, Gao F, Louden C, Lopez B, Christopher T, Wang C, Lee J, Feuerstein GZ, Yue TL. Inhibition of p38 mitogen-activated protein kinase decreases cardiomyocyte apoptosis and improves cardiac function after myocaridal ischemia and reperfusion. Circulation. 1999;99:1685–1691. doi: 10.1161/01.cir.99.13.1685. [DOI] [PubMed] [Google Scholar]
- 257.Mackay K, Mochly-Rosen D. An inhibitor of p38 mitogen-activated protein kinase protects neonatal cardiac myocytes from ischemia. J Biol Chem. 1999;274:6272–6279. doi: 10.1074/jbc.274.10.6272. [DOI] [PubMed] [Google Scholar]
- 258.MacLellan W, Schneider M. Genetic dissection of cardiac growth control pathways. Annu Rev Physiol. 2000;62:289–319. doi: 10.1146/annurev.physiol.62.1.289. [DOI] [PubMed] [Google Scholar]
- 259.Mann DL. Inflammatory mediators and the failing heart: past, present and the foreseeable future. Circ Res. 2002;91:988–998. doi: 10.1161/01.res.0000043825.01705.1b. [DOI] [PubMed] [Google Scholar]
- 260.Marais E, Genade S, Huisamin B, Strijdom J, Moolman J, Lochner A. Activation of p38 MAPK induced by a multi-cycle ischaemic preconditioning protocol is associated with attenuated p38 MAPK activity during sustained ischaemia and reperfusion. J Mol Cell Cardiol. 2001;33:769–778. doi: 10.1006/jmcc.2001.1347. [DOI] [PubMed] [Google Scholar]
- 261.Martin H, Flández M, Nombela C, Molina M. Protein phosphatases in MAPK signalling: we keep learning from yeast. Mol Microbiol. 2005;58:6–16. doi: 10.1111/j.1365-2958.2005.04822.x. [DOI] [PubMed] [Google Scholar]
- 262.Martin J, Avkiran M, Quinlan RA, Cohen P, Marber MS. Anti-ischemic effects of SB203580 are mediated through the inhibition of p38α mitogen-activated protein kinase: evidence from ectopic expression of an inhibition-resistant kinase. Circ Res. 2001;89:750–752. doi: 10.1161/hh2101.099504. [DOI] [PubMed] [Google Scholar]
- 263.Martin J, Hickey E, Weber L, Dillmann WH, Mestril R. Influence of phosphorylation and oligomerization on the protective role of the small heat shock protein 27 in rat adult cardiomyocytes. Gene Exp. 1999;7:349–355. [PMC free article] [PubMed] [Google Scholar]
- 264.Martin J, Mestril R, Hilal-Dandan R, Brunton L, Dillmann W. Small heat shock proteins and protection against ischemic injury in cardiac myocytes. Circulation. 1997;96:4343–4348. doi: 10.1161/01.cir.96.12.4343. [DOI] [PubMed] [Google Scholar]
- 265.Matsumoto-Ida M, Takimoto Y, Aoyama T, Akao M, Takeda T, Kita T. Activation of TGF-β1-TAK1-p38 MAPK pathway in spared cardiomyocytes is involved in left ventricular remodeling after myocardial infarction in rats. Am J Physiol Heart Circ Physiol. 2006;290:H709–H715. doi: 10.1152/ajpheart.00186.2005. [DOI] [PubMed] [Google Scholar]
- 266.Maulik N, Yoshida T, Zu YL, Sato M, Banerjee A, Das DK. Ischemic preconditioning triggers tyrosine kinase signaling: a potential role for MAPKAP kinase 2. Am J Physiol Heart Circ Physiol. 1998;275:H1857–H1864. doi: 10.1152/ajpheart.1998.275.5.H1857. [DOI] [PubMed] [Google Scholar]
- 267.McBurney M, Jones-Villeneuve E, Edwards M, Anderson P. Control of muscle and neuronal differentiation in a cultured embryonal carcinoma cell line. Nature. 1982;299:165–167. doi: 10.1038/299165a0. [DOI] [PubMed] [Google Scholar]
- 268.McCaw BJ, Chow SY, Wong ESM, Tan KL, Guo H, Guy GR. Identification and characterization of mErk5-T, a novel Erk5/Bmk1 splice variant. Gene. 2005;345:183–190. doi: 10.1016/j.gene.2004.11.011. [DOI] [PubMed] [Google Scholar]
- 269.McDonald J, Camenisch T. Hyaluronan: genetic insights into the complex biology of a simple polysaccharide. Glycoconj J. 2002;19:331–339. doi: 10.1023/A:1025369004783. [DOI] [PubMed] [Google Scholar]
- 270.McKay MM, Morrison DK. Integrating signals from RTKs to ERK/MAPK. Oncogene. 26:3113–3121. doi: 10.1038/sj.onc.1210394. [DOI] [PubMed] [Google Scholar]
- 271.Menick D, Renaud L, Buchholz A, Muller J, Zhou H, Kappler C, Kubalak S, Conway S, Xu L. Regulation of Ncx1 gene expression in the normal and hypertrophic heart. Ann NY Acad Sci. 2007;1099:195–203. doi: 10.1196/annals.1387.058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 272.Miki T, Miura T, Tanno M, Nishihara M, Naitoh K, Sato T, Takahashi A, Shimamoto K. Impairment of cardioprotective PI3K-Akt signaling by post-infarct ventricular remodeling is compensated by an ERK-mediated pathway. Basic Res Cardiol. 2007;102:163–170. doi: 10.1007/s00395-006-0622-3. [DOI] [PubMed] [Google Scholar]
- 273.Milano G, Morel S, Bonny C, Samaja M, von Segesser LK, Nicod P, Vassalli G. A peptide inhibitor of c-Jun NH2-terminal kinase reduces myocardial ischemia-reperfusion injury and infarct size in vivo. Am J Physiol Heart Circ Physiol. 2007;292:H1828–H1835. doi: 10.1152/ajpheart.01117.2006. [DOI] [PubMed] [Google Scholar]
- 274.Minamino T, Yujiri T, Papst PJ, Chan ED, Johnson GL, Terada N. MEKK1 suppresses oxidative stress-induced apoptosis of embryonic stem cell-derived cardiac myocytes. Proc Natl Acad Sci USA. 1999;96:15127–15132. doi: 10.1073/pnas.96.26.15127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 275.Minamino T, Yujiri T, Terada N, Taffet G, Michael L, Johnson G, Schneider M. MEKK1 is essential for cardiac hypertrophy and dysfunction induced by Gq. Proc Natl Acad Sci USA. 2002;99:3866–3871. doi: 10.1073/pnas.062453699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 276.Mitchell S, Ota A, Foster W, Zhang B, Fang Z, Patel S, Nelson SF, Horvath S, Wang Y. Distinct gene expression profiles in adult mouse heart following targeted MAP kinase activation. Physiol Genomics. 2006;25:50–59. doi: 10.1152/physiolgenomics.00224.2005. [DOI] [PubMed] [Google Scholar]
- 277.Miyamoto T, Takeishi Y, Takahashi H, Shishido T, Arimoto T, Tomoike H, Kubota I. Activation of distinct signal transduction pathways in hypertrophied hearts by pressure and volume overload. Basic Res Cardiol. 2004;99:328–337. doi: 10.1007/s00395-004-0482-7. [DOI] [PubMed] [Google Scholar]
- 278.Mizukami Y, Yoshioka K, Morimoto S, Yoshida KI. A novel mechanism of JNK1 activation. Nuclear translocation and activation of JNK1 during ischemia and reperfusion. J Biol Chem. 1997;272:16657–16662. doi: 10.1074/jbc.272.26.16657. [DOI] [PubMed] [Google Scholar]
- 279.Mocanu MM, Baxter GF, Yue Y, Critz SD, Yellon DM. The p38 MAPK inhibitor, SB203580, abrogates ischaemic preconditioning in rat heart but timing of administration is critical. Basic Res Cardiol. 2000;95:472–478. doi: 10.1007/s003950070023. [DOI] [PubMed] [Google Scholar]
- 280.Molkentin JD. Calcineurin-NFAT signaling regulates the cardiac hypertrophic response in coordination with the MAPKs. Cardiovasc Res. 2004;63:467–475. doi: 10.1016/j.cardiores.2004.01.021. [DOI] [PubMed] [Google Scholar]
- 281.Montagut C, Settleman J. Targeting the RAF-MEK-ERK pathway in cancer therapy. Cancer Lett. 2009;283:125–134. doi: 10.1016/j.canlet.2009.01.022. [DOI] [PubMed] [Google Scholar]
- 282.Monzen K, Hiroi Y, Kudoh S, Akazawa H, Oka T, Takimoto E, Hayashi D, Hosoda T, Kawabata M, Miyazono K, Ishii S, Yazaki Y, Nagai R, Komuro I. Smads, TAK1, their common target ATF-2 play a critical role in cardiomyocyte differentiation. J Cell Biol. 2001;153:687–698. doi: 10.1083/jcb.153.4.687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 283.Monzen K, Shiojima I, Hiroi Y, Kudoh S, Oka T, Takimoto E, Hayashi D, Hosoda T, Habara-Ohkubo A, Nakaoka T, Fujita T, Yazaki Y, Komuro I. Bone morphogenetic proteins induce cardiomyocyte differentiation through the mitogen-activated protein kinase kinase kinase TAK1 and cardiac transcription factors Csx/Nkx-2.5 and GATA-4. Mol Cell Biol. 1999;19:7096–7105. doi: 10.1128/mcb.19.10.7096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 284.Morimoto T, Hasegawa K, Kaburagi S, Kakita T, Wada H, Yanazume T. Phosphorylation of GATA-4 is involved in alpha 1-adrenergic agonist-responsive transcription of the endothelin-1 gene in cardiac myocytes. J Biol Chem. 2000;275:13721–13726. doi: 10.1074/jbc.275.18.13721. [DOI] [PubMed] [Google Scholar]
- 285.Morrison DK, Davis R. Regulation of MAP kinase signaling modules by scaffold proteins in mammals. Annu Rev Cell Dev Biol. 2003;19:91–118. doi: 10.1146/annurev.cellbio.19.111401.091942. [DOI] [PubMed] [Google Scholar]
- 286.Mudgett J, Ding J, Guh-Siesel L, Chartrain N, Yang L, Gopal S, Chen M. Essential role for p38α mitogen-activated protein kinase in placental angiogenesis. Proc Natl Acad Sci USA. 2000;97:10454–10459. doi: 10.1073/pnas.180316397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 287.Murphy E, Steenbergen C. Does inhibition of glycogen synthase kinase protect in mice? Circ Res. 2008;103:226–228. doi: 10.1161/CIRCRESAHA.108.181602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 288.Nadruz W, Jr, Corat MAF, Marin TM, Guimaraes Pereira GA, Franchini KG. Focal adhesion kinase mediates MEF2 and c-Jun activation by stretch: role in the activation of the cardiac hypertrophic genetic program. Cardiovasc Res. 2005;68:87–97. doi: 10.1016/j.cardiores.2005.05.011. [DOI] [PubMed] [Google Scholar]
- 289.Nadruz W, Jr, Kobarg CB, Kobarg J, Franchini KG. c-Jun is regulated by combination of enhanced expression and phosphorylation in acute-overloaded rat heart. Am J Physiol Heart Circ Physiol. 2004;286:H760–H767. doi: 10.1152/ajpheart.00430.2003. [DOI] [PubMed] [Google Scholar]
- 290.Nagai H, Noguchi T, Takeda K, Ichijo H. Pathophysiological roles of ASK1-MAP kinase signaling pathways. J Biochem Mol Biol. 2007;40:1–6. doi: 10.5483/bmbrep.2007.40.1.001. [DOI] [PubMed] [Google Scholar]
- 291.Nagarkatti D, Sha’afi R. Role of p38 MAP kinase in myocardial stress. J Mol Cell Cardiol. 1998;30:1651–1664. doi: 10.1006/jmcc.1998.0733. [DOI] [PubMed] [Google Scholar]
- 292.Naito AT, Tominaga A, Oyamada M, Oyamada Y, Shiraishi I, Monzen K, Komuro I, Takamatsu T. Early stage-specific inhibitions of cardiomyocyte differentiation and expression of Csx/Nkx-2.5 and GATA-4 by phosphatidylinositol 3-kinase inhibitor LY294002. Exp Cell Res. 2003;291:56–69. doi: 10.1016/s0014-4827(03)00378-1. [DOI] [PubMed] [Google Scholar]
- 293.Naitoh K, Ichikawa Y, Miura T, Nakamura Y, Miki T, Ikeda Y, Kobayashi H, Nishihara M, Ohori K, Shimamoto K. MitoKATP channel activation suppresses gap junction permeability in the ischemic myocardium by an ERK-dependent mechanism. Cardiovasc Res. 2006;70:374–383. doi: 10.1016/j.cardiores.2006.01.023. [DOI] [PubMed] [Google Scholar]
- 294.Nakamuara K, Uhlik M, Johnson N, Hahn K, Johnson G. PB1 domain-dependent signaling complex is required for extracellular signal-regulated kinase 5 activation. Mol Cell Biol. 2006;26:2065–2079. doi: 10.1128/MCB.26.6.2065-2079.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 295.Nakamura K, Johnson G. PB1 domains of MEKK2 and MEKK3 interact with the MEK5 PB1 domain for activation of the ERK5 pathway. J Biol Chem. 2003;278:36989–36992. doi: 10.1074/jbc.C300313200. [DOI] [PubMed] [Google Scholar]
- 296.Nakamura T, Colbert M, Krenz M, Molkentin J, Hahn H, Dorn G, Robbins J. Mediating ERK1/2 signaling rescues congenital heart defects in a mouse model of Noonan syndrome. J Clin Invest. 2007;117:2123–2132. doi: 10.1172/JCI30756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 297.Nakaoka Y, Nishida K, Fujio Y, Izumi M, Terai K, Oshima Y, Sugiyama S, Matsuda S, Koyasu S, Yamauchi-Takihara K, Hirano T, Kawase I, Hirota H. Activation of gp130 transduces hypertrophic signal through interaction of scaffolding/docking protein Gab1 with tyrosine phosphatase SHP2 in cardiomyocytes. Circ Res. 2003;93:221–229. doi: 10.1161/01.RES.0000085562.48906.4A. [DOI] [PubMed] [Google Scholar]
- 298.Nelson W, Nusse R. Convergence of Wnt, beta-catenin, and cadherin pathways. Science. 2004;303:1483–1487. doi: 10.1126/science.1094291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 299.Nemoto S, Sheng Z, Lin A. Opposing effects of jun kinase and p38 mitogen-activated protein kinases on cardiomyocyte hypertrophy. Mol Cell Biol. 1998;18:3518–3526. doi: 10.1128/mcb.18.6.3518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 300.Nian M, Lee P, Khaper N, Liu P. Inflammatory cytokines and postmyocardial infarction remodeling. Circ Res. 2004;94:1543–1553. doi: 10.1161/01.RES.0000130526.20854.fa. [DOI] [PubMed] [Google Scholar]
- 301.Nicol R, Frey N, Pearson G, Cobb M, Richardson J, Olson E. Activated MEK5 induces serial assembly of sarcomeres and eccentric cardiac hypertrophy. EMBO J. 2001;20:2757–2767. doi: 10.1093/emboj/20.11.2757. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 302.Niihori T, Aoki Y, Narumi Y, Neri G, Cave H, Verloes A, Okamoto N, Hennekam RC, Gillessen-Kaesbach G, Wieczorek D, Kavamura MI, Kurosawa K, Ohashi H, Wilson L, Heron D, Bonneau D, Corona G, Kaname T, Naritomi K, Baumann C, Matsumoto N, Kato K, Kure S. Germline KRAS and BRAF mutations in cardio-facio-cutaneous syndrome. Nat Genet. 2006;38:294–296. doi: 10.1038/ng1749. [DOI] [PubMed] [Google Scholar]
- 303.Nishida K, Yamaguchi O, Hirotani S, Hikoso S, Higuchi Y, Watanabe T, Takeda T, Osuka S, Morita T, Kondoh G, Uno Y, Kashiwase K, Taniike M, Nakai A, Matsumura Y, Miyazaki Ji Sudo T, Hongo K, Kusakari Y, Kurihara S, Chien KR, Takeda J, Hori M, Otsu K. p38α Mitogen-activated protein kinase plays a critical role in cardiomyocyte survival but not in cardiac hypertrophic growth in response to pressure overload. Mol Cell Biol. 2004;24:10611–10620. doi: 10.1128/MCB.24.24.10611-10620.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 304.Nishimoto S, Nishida E. MAPK signalling ERK5 versus ERK1/2. EMBO. 2006;7:782–786. doi: 10.1038/sj.embor.7400755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 305.Noma T, Lemaire A, Naga Prasad S, Barki-Harrington L, Tilley D, Chen J, Le Corvoisier P, Violin J, Wei H, Lefkowitz R, Rockman H. Beta-arrestin mediated beta(1)-adreneregic receptor trasnsactivation of the EGFR confers cardioprotection. J Clin Invest. 2007;117:2445–2458. doi: 10.1172/JCI31901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 306.Noonan J. Noonan syndrome and related disorders: alterations in growth and puberty. Rev Endocr Metab Disord. 2006;7:251–255. doi: 10.1007/s11154-006-9021-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 307.Obara Y, Okano Y, Ono S, Yamauchi A, Hoshino T, Kurose H, Nakahata N. βγ-Subunits of Gi/o suppress EGF-induced ERK5 phosphorylation, whereas ERK1/2 phosphorylation is enhanced. Cell Signal. 2008;20:1275–1283. doi: 10.1016/j.cellsig.2008.02.016. [DOI] [PubMed] [Google Scholar]
- 308.Olson EN. Gene regulatory networks in the evolution and development of the heart. Science. 2006;313:1922–1927. doi: 10.1126/science.1132292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 309.Ono K, Han J. The p38 signal transduction pathway activation and function. Cell Signal. 2000;12:1–13. doi: 10.1016/s0898-6568(99)00071-6. [DOI] [PubMed] [Google Scholar]
- 310.Otsu K, Yamashita N, Nishida K, Hirotani S, Yamaguchi O, Watanabe T, Hikoso S, Higuchi Y, Matsumura Y, Maruyama M, Sudo T, Osada H, Hori M. Disruption of a single copy of the p38alpha MAP kinase gene leads to cardioprotection against ischemia-reperfusion. Biochem Biophys Res Commun. 2003;302:56–60. doi: 10.1016/s0006-291x(03)00096-2. [DOI] [PubMed] [Google Scholar]
- 311.Owens DM, Keyse SM. Differential regulation of MAP kinase signalling by dual-specificity protein phosphatases. Oncogene. 26:3203–3213. doi: 10.1038/sj.onc.1210412. [DOI] [PubMed] [Google Scholar]
- 312.Pages G, Guerin S, Grall D, Bonino F, Smith A, Anjuere F, Auberger P, Pouyssegur J. Defective thymocyte maturation in p44MAP kinase (Erk1) knockout mice. Science. 1999;286:1374–1377. doi: 10.1126/science.286.5443.1374. [DOI] [PubMed] [Google Scholar]
- 313.Palpant N, Yasuda S, MacDougald O, Metzger J. Non-canonical Wnt signaling enhances differentiation of Sca1+/c-kit+ adipose-derived murine stromal vascular cells into spontaneously beating cardiac myocytes. J Mol Cell Cardiol. 2007;43:362–370. doi: 10.1016/j.yjmcc.2007.06.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 314.Pan J, Singh U, Takahashi T, Oka Y, Palm-Leis A, Herbelin B, Baker K. PKC mediates cyclic stretch-induced cardiac hypertrophy through Rho family GTPases and mitogen-activated protein kinases in cardiomyocytes. J Cell Physiol. 2005;2002:536–553. doi: 10.1002/jcp.20151. [DOI] [PubMed] [Google Scholar]
- 315.Pandit B, Sarkozy A, Pennacchio LA, Carta C, Oishi K, Martinelli S, Pogna EA, Schackwitz W, Ustaszewska A, Landstrom A, Bos JM, Ommen SR, Esposito G, Lepri F, Faul C, Mundel P, Lopez Siguero JP, Tenconi R, Selicorni A, Rossi C, Mazzanti L, Torrente I, Marino B, Digilio MC, Zampino G, Ackerman MJ, Dallapiccola B, Tartaglia M, Gelb BD. Gain-of-function RAF1 mutations cause Noonan and LEOPARD syndromes with hypertrophic cardiomyopathy. Nat Genet. 2007;39:1007–1012. doi: 10.1038/ng2073. [DOI] [PubMed] [Google Scholar]
- 316.Pandur P, Lasche M, Eisenberg LM, Kuhl M. Wnt-11 activation of a non-canonical Wnt signalling pathway is required for cardiogenesis. Nature. 2002;418:636–641. doi: 10.1038/nature00921. [DOI] [PubMed] [Google Scholar]
- 317.Pandur P, Maurus D, Kuhl M. Increasingly complex: new players enter the Wnt signaling network. Bioessays. 2002;24 doi: 10.1002/bies.10164. [DOI] [PubMed] [Google Scholar]
- 318.Pearson G, Robinson F, Beers Gibson T, Xu Be Karandikar M, Berman K, Cobb MH. Mitogen-activated protein (MAP) kinase pathways: regulation and physiological functions. Endocr Rev. 2001;22:153–183. doi: 10.1210/edrv.22.2.0428. [DOI] [PubMed] [Google Scholar]
- 319.Pentassuglia L, Sawyer DB. The role of Neuregulin-1β/ErbB signaling in the heart. Exp Cell Res. 2009;315:627–637. doi: 10.1016/j.yexcr.2008.08.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 320.Petrich BG, Eloff BC, Lerner DL, Kovacs A, Saffitz JE, Rosenbaum DS, Wang Y. Targeted activation of c-Jun N-terminal kinase in vivo induces restrictive cardiomyopathy and conduction defects. J Biol Chem. 2004;279:15330–15338. doi: 10.1074/jbc.M314142200. [DOI] [PubMed] [Google Scholar]
- 321.Petrich BG, Gong X, Lerner DL, Wang X, Brown JH, Saffitz JE, Wang Y. c-Jun N-terminal kinase activation mediates down-regulation of connexin43 in cardiomyocytes. Circ Res. 2002;91:640–647. doi: 10.1161/01.res.0000035854.11082.01. [DOI] [PubMed] [Google Scholar]
- 322.Petrich BG, Molkentin JD, Wang Y. Temporal activation of c-Jun N-terminal kinase in adult transgenic heart via cre-loxP-mediated DNA recombination. FASEB J. 2003:02–0438fje. doi: 10.1096/fj.02-0438fje. [DOI] [PubMed] [Google Scholar]
- 323.Pi X, Yan C, Berk BC. Big mitogen-activated protein kinase (BMK1)/ERK5 protects endothelial cells from apoptosis. Circ Res. 2004;94:362–369. doi: 10.1161/01.RES.0000112406.27800.6F. [DOI] [PubMed] [Google Scholar]
- 324.Ping P, Zhang J, Huang S, Cao X, Tang XL, Li RCX, Zheng YT, Qiu Y, Clerk A, Sugden P, Han J, Bolli R. PKC-dependent activation of p46/p54 JNKs during ischemic preconditioning in conscious rabbits. Am J Physiol Heart Circ Physiol. 1999;277:H1771–H1785. doi: 10.1152/ajpheart.1999.277.5.H1771. [DOI] [PubMed] [Google Scholar]
- 325.Porter KE, Turner NA. Cardiac fibroblasts: at the heart of myocardial remodeling. Pharmacol Ther. 2009;123:255–278. doi: 10.1016/j.pharmthera.2009.05.002. [DOI] [PubMed] [Google Scholar]
- 326.Prabhu S. Cytokine-induced modulation of cardiac function. Circ Res. 2004;95:1140–1153. doi: 10.1161/01.RES.0000150734.79804.92. [DOI] [PubMed] [Google Scholar]
- 327.Princen F, Bard E, Sheikh F, Zhang SS, Wang J, Zago WM, Wu D, Trelles RD, Bailly-Maitre B, Kahn CR, Chen Y, Reed JC, Tong GG, Mercola M, Chen J, Feng GS. Deletion of Shp2 tyrosine phosphatase in muscle leads to dilated cardiomyopathy, insulin resistance, and premature death. Mol Cell Biol. 2009;29:378–388. doi: 10.1128/MCB.01661-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 328.Purcell N, Wilkins BJ, York A, Saba-El-Leil M, Meloche S, Robbins J, Molkentin J. Genetic inhibition of cardiac ERK1/2 promotes stress-induced apoptosis and heart failure but has no effect on hypertrophy in vivo. Proc Natl Acad Sci USA. 2007;104:14074–14079. doi: 10.1073/pnas.0610906104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 329.Qi M, Elion EA. MAP kinase pathways. J Cell Sci. 2005;118:3569–3572. doi: 10.1242/jcs.02470. [DOI] [PubMed] [Google Scholar]
- 330.Raingeaud J, Gupta S, Rogers J, Dickens M, Han J, Ulevitch R, Davis R. Pro-inflammatory cytokines and environmental stress cause p38 mitogen-activated protein kinase transactivation by dual phosphorylation on tyrosine and threonine. J Biol Chem. 1995;270:7420–7426. doi: 10.1074/jbc.270.13.7420. [DOI] [PubMed] [Google Scholar]
- 331.Rajasingh J, Bord E, Hamada H, Lambers E, Qin G, Losordo DW, Kishore R. STAT3-dependent mouse embryonic stem cell differentiation into cardiomyocytes: analysis of molecular signaling and therapeutic efficacy of cardiomyocyte precommitted mES transplantation in a mouse model of myocardial infarction. Circ Res. 2007;101:910–918. doi: 10.1161/CIRCRESAHA.107.156786. [DOI] [PubMed] [Google Scholar]
- 332.Raman M, Chen W, Cobb MH. Differential regulation and properties of MAPKs. Oncogene. 2007;26:3100–3112. doi: 10.1038/sj.onc.1210392. [DOI] [PubMed] [Google Scholar]
- 333.Ramos JW. The regulation of extracellular signal-regulated kinase (ERK) in mammalian cells. Int J Biochem Cell Biol. 2008;40:2707–2719. doi: 10.1016/j.biocel.2008.04.009. [DOI] [PubMed] [Google Scholar]
- 334.Razzaque MA, Nishizawa T, Komoike Y, Yagi H, Furutani M, Amo R, Kamisago M, Momma K, Katayama H, Nakagawa M, Fujiwara Y, Matsushima M, Mizuno K, Tokuyama M, Hirota H, Muneuchi J, Higashinakagawa T, Matsuoka R. Germline gain-of-function mutations in RAF1 cause Noonan syndrome. Nat Genet. 2007;39:1013–1017. doi: 10.1038/ng2078. [DOI] [PubMed] [Google Scholar]
- 335.Regan C, Li W, Boucher D, Spatz S, Su M, Kuida K. Erk5 null mice display multiple extraembryonic vascular and embryonic cardiovascular defects. Proc Natl Acad Sci USA. 2002;99:9248–9253. doi: 10.1073/pnas.142293999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 336.Remenyi A, Good M, Bhayyacharyya R, Lim W. The role of docking interaction in mediating signaling input, output, and discrimination in the yeast MAPK network. Mol Cell. 2005;20:951–962. doi: 10.1016/j.molcel.2005.10.030. [DOI] [PubMed] [Google Scholar]
- 337.Remondino A, Kwon SH, Communal C, Pimentel DR, Sawyer DB, Singh K, Colucci WS. β-Adrenergic receptor-stimulated apoptosis in cardiac myocytes is mediated by reactive oxygen species/c-Jun NH2-terminal kinase-dependent activation of the mitochondrial pathway. Circ Res. 2003;92:136–138. doi: 10.1161/01.res.0000054624.03539.b4. [DOI] [PubMed] [Google Scholar]
- 338.Ren X, Ma X, Li Y. All-trans retinoic acid regulates c-jun expression via ERK5 in cardiac myoblasts. J Nutr Biochem. 2007;18:832–838. doi: 10.1016/j.jnutbio.2006.12.023. [DOI] [PubMed] [Google Scholar]
- 339.Repici M, Borsello T. JNK pathway as therapeutic target to prevent degeneration in the central nervous system. Adv Exp Med Biol. 2006;588:145–155. doi: 10.1007/978-0-387-34817-9_13. [DOI] [PubMed] [Google Scholar]
- 340.Riad A, Jager S, Sobirey M, Escher F, Yaulema-Riss A, Westermann D, Karatas A, Heimesaat MM, Bereswill S, Dragun D, Pauschinger M, Schultheiss HP, Tschope C. Toll-like receptor-4 modulates survival by induction of left ventricular remodeling after myocardial infarction in mice. J Immunol. 2008;180:6954–6961. doi: 10.4049/jimmunol.180.10.6954. [DOI] [PubMed] [Google Scholar]
- 341.Ricci R, Eriksson U, Oudit G, Eferl R, Akhmedov A, Sumara I, Sumara G, Kassiri Z, David J, Bakiri L, Sasse B, Idarraga M, Rath M, Kurz D, Theussl H, Perriard J, Backx P, Penninger J, Wagner E. Distinct functions of junD in cardiac hypertrophy and heart failure. Genes Dev. 2005;19:208–213. doi: 10.1101/gad.327005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 342.Rincón M, Davis R. Regulation of the immune response by stress-activated protein kinases. Immunol Rev. 2009;228:212–224. doi: 10.1111/j.1600-065X.2008.00744.x. [DOI] [PubMed] [Google Scholar]
- 343.Roberts AE, Araki T, Swanson KD, Montgomery KT, Schiripo TA, Joshi VA, Li L, Yassin Y, Tamburino AM, Neel BG. Germline gain-of-function mutations in SOS1 cause Noonan syndrome. Nat Genet. 2007;39:70–74. doi: 10.1038/ng1926. [DOI] [PubMed] [Google Scholar]
- 344.Roberts PJ, Der CJ. Targeting the Raf-MEK-ERK mitogen-activated protein kinase cascade for the treatment of cancer. Oncogene. 26:3291–3310. doi: 10.1038/sj.onc.1210422. [DOI] [PubMed] [Google Scholar]
- 345.Rodriguez-Viciana P, Tetsu O, Tidyman WE, Estep AL, Conger BA, Cruz M, McCormick F. Germline mutations in genes within the MAPK pathway cause cardio-facio-curtaneious syndrome. Science. 2006;311 doi: 10.1126/science.1124642. [DOI] [PubMed] [Google Scholar]
- 346.Ross R. Atherosclerosis: an inflammatory disease. N Engl J Med. 1999;340:115–126. doi: 10.1056/NEJM199901143400207. [DOI] [PubMed] [Google Scholar]
- 347.Roussel É, Gaudreau M, Plante É, Drolet MC, Breault C, Couet J, Arsenault M. Early responses of the left ventricle to pressure overload in Wistar rats. Life Sci. 2008;82:265–272. doi: 10.1016/j.lfs.2007.11.008. [DOI] [PubMed] [Google Scholar]
- 348.Roux P, Blenis J. ERK and p38 MAPK-activated protein kinases: a family of protein kinases with diverse biological functions. Microbiol Mol Biol Rev. 2004;68:320–344. doi: 10.1128/MMBR.68.2.320-344.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 349.Ruan H, Mitchell S, Vainoriene M, Lou Q, Xie LH, Ren S, Goldhaber JI, Wang Y. Giα1-mediated cardiac electrophysiological remodeling and arrhythmia in hypertrophic cardiomyopathy. Circulation. 2007;116:596–605. doi: 10.1161/CIRCULATIONAHA.106.682773. [DOI] [PubMed] [Google Scholar]
- 350.Saba-El-Leill M, Vella F, Vernay B, Voisin L, Chen L, Labrecque N, Ang S, Meloche S. An essential function of the mitogen-activated protein kinase Erk2 in mouse trophoblast development. EMBO J. 2003;4:964–986. doi: 10.1038/sj.embor.embor939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 351.Sadoshima J, Montagne O, Wang Q, Yang G, Warden J, Lui J, Takagi G, Karoor V, Hong C, Johnson G, Vatner D, Vatner S. The MEKK1-JNK pathway plays a protective role in pressure overload but does not mediate cardiac hypertrophy. J Clin Invest. 2002;110:271–279. doi: 10.1172/JCI14938. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 352.Salazar NC, Chen J, Rockman HA. Cardiac GPCRs: GPCR signaling in healthy and failing hearts. Biochim Biophys Acta. 2007;1768:1006–1018. doi: 10.1016/j.bbamem.2007.02.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 353.Salvador JM, Mittelstadt PR, Guszczynski T, Copeland TD, Yamaguchi H, Appella E, Fornace AJJ. Alternative p38 activation pathway mediated by T cell receptor-proximal tyrosine kinases. Nat Immunol. 2005;6:390–395. doi: 10.1038/ni1177. [DOI] [PubMed] [Google Scholar]
- 354.Sanada S, Kitakaze M, Papst P, Hatanaka K, Asanuma H, Aki T, Shinozaki Y, Ogita H, Node K, Takashima S, Asakura M, Yamada T, Fukushima T, Ogai A, Kuzuya T, Mori H, Terada N, Yoshida K, Hori M. Role of phasic dynamism of p38 mitogen-activated protein kinase activation in ischemic preconditioning of the canine heart. Circ Res. 2001;88:175–180. doi: 10.1161/01.res.88.2.175. [DOI] [PubMed] [Google Scholar]
- 355.Sano Y, Harada J, Tashiro S, Gotoh-Mandeville R, Maekawa T, Ishii S. ATF-2 is a common nuclear target of Smad and TAK1 pathways in transforming growth factor-beta signaling. J Biol Chem. 1999;274:8949–8957. doi: 10.1074/jbc.274.13.8949. [DOI] [PubMed] [Google Scholar]
- 356.Sarov-Blat L, Morgan J. Vascular disease. J Am Coll Cardiol. 2009;53:A419–458. [Google Scholar]
- 357.Satoh M, Matter CM, Ogita H, Takeshita K, Wang CY, Dorn GW, II, Liao JK. Inhibition of apoptosis-regulated signaling kinase-1 and prevention of congestive heart failure by estrogen. Circulation. 2007;115:3197–3204. doi: 10.1161/CIRCULATIONAHA.106.657981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 358.Saurin A, Martin J, Heads R, Mockridge J, Foley C, Wright M, Wang Y, Marber MS. The role of differential activation of p38-mitogen-activated protein kinase in preconditioned ventricular myocytes. FASEB J. 2000;14:2237–2246. doi: 10.1096/fj.99-0671com. [DOI] [PubMed] [Google Scholar]
- 359.Scheid M, Woodgett J. PKB/Akt: functional insights from genetic models. Nat Rev Mol Cell Biol. 2001;2:760–768. doi: 10.1038/35096067. [DOI] [PubMed] [Google Scholar]
- 360.Schneider S, Chen W, Hou J, Steenbergen C, Murphy E. Inhibition of p38 MAPK alpha/beta reduces ischemic injury and does not block protective effects of preconditioning. Am J Physiol Heart Circ Physiol. 2001;280:H499–H508. doi: 10.1152/ajpheart.2001.280.2.H499. [DOI] [PubMed] [Google Scholar]
- 361.Schonwasser DC, Marais RM, Marshall CJ, Parker PJ. Activation of the mitogen-activated protein kinase/extracellular signal-regulated kinase pathway by conventional, novel, and atypical protein kinase C isotypes. Mol Cell Biol. 1998;18:790–798. doi: 10.1128/mcb.18.2.790. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 362.Schreiber S, Feagan B, D’Haens G, Colombel JF, Geboes K, Yurcov M, Isakov V, Golovenko O, Bernstein CN, Ludwig D, Winter T, Meier U, Yong C, Steffgen J. Oral p38 mitogen-activated protein kinase inhibition with BIRB 796 for active Crohn’s disease: a randomized, double-blind, placebo-controlled trial. Clin Gastroenterol Hepatol. 2006;4:325–334. doi: 10.1016/j.cgh.2005.11.013. [DOI] [PubMed] [Google Scholar]
- 363.Schubbert S, Zenker M, Rowe SL, Boll S, Klein C, Bollag G, van der Burgt I, Musante L, Kalscheuer V, Wehner LE, Nguyen H, West B, Zhang KY, Sistermans E, Rauch A, Niemeyer CM, Shannon K. Germline KRAS mutations cause Noonan syndrome. Nat Genet. 2006;38:331–336. doi: 10.1038/ng1748. [DOI] [PubMed] [Google Scholar]
- 364.Schubbert S, Bollag G, Shannon K. Deregulated Ras signaling in developmental disorders: new tricks for an old dog. Curr Opin Genet Dev. 2007;17:15–22. doi: 10.1016/j.gde.2006.12.004. [DOI] [PubMed] [Google Scholar]
- 365.Schultheiss T, Burch J, Lassar A. A role for bone morphogenetic proteins in the induction of cardiac myogenesis. Genes Dev. 1997;11:451–462. doi: 10.1101/gad.11.4.451. [DOI] [PubMed] [Google Scholar]
- 366.Schwertz H, Carter J, Abdudureheman M, Russ M, Buerke U, Schlitt A, Muller-Werdan U, Prondzinsky R, Werdan K. Myocardial ischemia/reperfusion causes VDAC phosphorylation which is reduced by cardioprotection with a p38 MAP kinase inhibitor. Proteomics. 2007;7:4579–4588. doi: 10.1002/pmic.200700734. [DOI] [PubMed] [Google Scholar]
- 367.See F, Thomas W, Way K, Tzanidis A, Kompa A, Lewis D, Itescu S, Krum H. p38 Mitogen-activated protein kinase inhibition improves cardiac function and attenuates left ventricular remodeling following myocardial infarction in the rat. J Am Coll Cardiol. 2004;44:1679–1689. doi: 10.1016/j.jacc.2004.07.038. [DOI] [PubMed] [Google Scholar]
- 368.Seko Y, Takahashi N, Tobe K, Kadowaki T, Yazaki Y. Hypoxia and hypoxia/reoxygenation activate p65PAK, p38 mitogen-activated protein kinase (MAPK), stress-activated protein kinase (SAPK) in cultured rat cardiac myocytes. Biochem Biophys Res Commun. 1997;239:840–844. doi: 10.1006/bbrc.1997.7570. [DOI] [PubMed] [Google Scholar]
- 369.Selvetella G, Hirsch E, Notte A, Tarone G, Lembo G. Adaptive and maladaptive hypertrophic pathways: points of convergence and divergence. Cardiovasc Res. 2004;63:373–380. doi: 10.1016/j.cardiores.2004.04.031. [DOI] [PubMed] [Google Scholar]
- 370.Shao Z, Bhattacharya K, Hsich E, Park L, Walters B, Germann U, Wang YM, Kyriakis J, Mohanlal R, Kuida K, Namchuk M, Salituro F, Yao Ym Hou Wm Chen X, Aronovitz M, Tsichlis PN, Bhattacharya S, Force T, Kilter H. c-Jun N-terminal kinases mediate reactivation of Akt and cardiomyocyte survival after hypoxic injury in vitro and in vivo. Circ Res. 2006;98:111–118. doi: 10.1161/01.RES.0000197781.20524.b9. [DOI] [PubMed] [Google Scholar]
- 371.Shimizu N, Yoshiyama M, Omura T, Hanatani A, Kim S, Takeuchi K, Iwao H, Yoshikawa J. Activation of mitogen-activated protein kinases and activator protein-1 in myocardial infarction in rats. Cardiovasc Res. 1998;38:116–124. doi: 10.1016/s0008-6363(97)00327-1. [DOI] [PubMed] [Google Scholar]
- 372.Shimojo N, Jesmin S, Zaedi S, Maeda S, Soma M, Aonuma K, Yamaguchi I, Miyauchi T. Eicosapentaenoic acid prevents endothelin-1-induced cardiomyocyte hypertrophy in vitro through the suppression of TGF-beta1 and phosphorylated JNK. Am J Physiol Heart Circ Physiol. 2006;291:H835–H845. doi: 10.1152/ajpheart.01365.2005. [DOI] [PubMed] [Google Scholar]
- 373.Shishido T, Woo CH, Ding B, McClain C, Molina CA, Yan C, Yang J, Abe Ji. Effects of MEK5/ERK5 association on small ubiquitin-related modification of ERK5: implications for diabetic ventricular dysfunction after myocardial infarction. Circ Res. 2008;102:1416–1425. doi: 10.1161/CIRCRESAHA.107.168138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 374.Siwik DA, Kuster GM, Brahmbhatt JV, Zaidi Z, Malik J, Ooi H, Ghorayeb G. EMMPRIN mediates β-adrenergic receptor-stimulated matrix metalloproteinase activity in cardiac myocytes. J Mol Cell Cardiol. 2008;44:210–217. doi: 10.1016/j.yjmcc.2007.07.054. [DOI] [PubMed] [Google Scholar]
- 375.Smith TK, Bader DM. Signals from both sides: control of cardiac development by the endocardium and epicardium. Semin Cell Dev Biol. 2007;18:84–89. doi: 10.1016/j.semcdb.2006.12.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 376.Song Z, Ji X, Li X, Wang S, Wang S. Inhibition of the activity of poly (ADP-ribose) polymerase reduces heart ischaemia/reperfusion injury via suppressing JNK-mediated AIF translocation. J Cell Mol Med. 2008;12:1220–1228. doi: 10.1111/j.1582-4934.2008.00183.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 377.Songyang Z, Lu K, Kwon Y, Tsai L, Filhol O, Cochet C, Brickey D, Soderling T, Bartleson C, Graves D, DeMaggio A, Hoekstra M, Blenis J, Hunter T, Cantley L. A structural basis for substrate specificities of protein Ser/Thr kinases: primary sequence preference of casein kinases I and II, NIMA, phosphorylase kinase, calmodulin-dependent kinase II, CDK5, and Erk1. Mol Cell Biol. 1996;16:6486–6493. doi: 10.1128/mcb.16.11.6486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 378.Sopontammarak S, Aliharoob A, Ocampo C, Arcilla R, Gupta M, Gupta M. Mitogen-activated protein kinases (p38 and c-Jun NH2-terminal kinase) are differentially regulated during cardiac volume and pressure overload hypertrophy. Cell Biochem Biophys. 2005;43:61–76. doi: 10.1385/CBB:43:1:061. [DOI] [PubMed] [Google Scholar]
- 379.Srivastava D. Making or breaking the heart: from lineage determination to morphogenesis. Cell. 2006;126:1037–1048. doi: 10.1016/j.cell.2006.09.003. [DOI] [PubMed] [Google Scholar]
- 380.Srivastava D, Olson EN. A genetic blueprint for cardiac development. Nature. 2000;407:221–226. doi: 10.1038/35025190. [DOI] [PubMed] [Google Scholar]
- 381.Stavridis MP, Lunn JS, Collins BJ, Storey KG. A discrete period of FGF-induced Erk1/2 signalling is required for vertebrate neural specification. Development. 2007;134:2889–2894. doi: 10.1242/dev.02858. [DOI] [PubMed] [Google Scholar]
- 382.Steenbergen C. The role of p38 mitogen-activated protein kinase in myocardial ischemia/reperfusion injury: relationship to ischemic preconditioning. Basic Res Cardiol. 2002;97:276–285. doi: 10.1007/s00395-002-0364-9. [DOI] [PubMed] [Google Scholar]
- 383.Su HF, Samsamshariat A, Fu J, Shan YX, Chen YH, Piomelli D, Wang PH. Oleylethanolamide activates Ras-Erk pathway and improves myocardial function in doxorubicin-induced heart failure. Endocrinology. 2006;147:827–834. doi: 10.1210/en.2005-1098. [DOI] [PubMed] [Google Scholar]
- 384.Sucher R, Gehwolf P, Kaier T, Hermann M, Maglione M, Oberhuber R, Ratschiller T, Kuznetsov AV, Bösch F, Kozlov AV, Ashraf MI, Schneeberger S, Brandacher G, Öllinger R, Margreiter R, Troppmair J. Intracellular signaling pathways control mitochondrial events associated with the development of ischemia/reperfusion-associated damage. Transplant Int. 2009;22:922–930. doi: 10.1111/j.1432-2277.2009.00883.x. [DOI] [PubMed] [Google Scholar]
- 385.Suckfuell M, Canis M, Strieth S, Scherer H, Haisch A. Intra-tympanic treatment of acute acoustic trauma with a cell-permeable JNK ligand: a prospective randomized phase I/II study. Acta Otolaryngol. 2007;127:938–942. doi: 10.1080/00016480601110212. [DOI] [PubMed] [Google Scholar]
- 386.Sugi Y, Ito N, Szebenyi G, Myers K, Fallon JF, Mikawa T, Markwald RR. Fibroblast growth factor (FGF)-4 can induce proliferation of cardiac cushion mesenchymal cells during early valve leaflet formation. Dev Biol. 2003;258:252–263. doi: 10.1016/s0012-1606(03)00099-x. [DOI] [PubMed] [Google Scholar]
- 387.Szokodi I, Kerkela R, Kubin AM, Sarman B, Pikkarainen S, Konyi A, Horvath IG, Papp L, Toth M, Skoumal R, Ruskoaho H. Functionally opposing roles of extracellular signal-regulated kinase 1/2 and p38 mitogen-activated protein kinase in the regulation of cardiac contractility. Circulation. 2008;118:1651–1658. doi: 10.1161/CIRCULATIONAHA.107.758623. [DOI] [PubMed] [Google Scholar]
- 388.Tachibana H, Perrino C, Takaoka H, Davis RJ, Naga Prasad SV, Rockman HA. JNK1 is required to preserve cardiac function in the early response to pressure overload. Biochem Biophys Res Commun. 2006;343:1060–1066. doi: 10.1016/j.bbrc.2006.03.065. [DOI] [PubMed] [Google Scholar]
- 389.Takahashi E, Fukuda K, Miyoshi S, Murata M, Kato T, Ita M, Tanabe T, Ogawa S. Leukemia inhibitory factor activates cardiac L-type Ca2+ channels via phosphorylation of serine 1829 in the rabbit Cav1.2 subunit. Circ Res. 2004;94:1242–1248. doi: 10.1161/01.RES.0000126405.38858.BC. [DOI] [PubMed] [Google Scholar]
- 390.Takahashi N, Saito Y, Kuwahara K, Harada M, Tanimoto K, Nakagawa Y, Kawakami R, Nakanishi M, Yasuno S, Usami S, Yoshimura A, Nakao K. Hypertrophic responses to cardiotrophin-1 are not mediated by STAT3, but via a MEK5-ERK5 pathway in cultured cardiomyocytes. J Mol Cell Cardiol. 2005;38:185–192. doi: 10.1016/j.yjmcc.2004.10.016. [DOI] [PubMed] [Google Scholar]
- 391.Takeishi Y, Abe Ji Lee JD, Kawakatsu H, Walsh RA, Berk BC. Differential regulation of p90 ribosomal S6 kinase and big mitogen-activated protein kinase 1 by ischemia/reperfusion and oxidative stress in perfused guinea pig hearts. Circ Res. 1999;85:1164–1172. doi: 10.1161/01.res.85.12.1164. [DOI] [PubMed] [Google Scholar]
- 392.Takeishi Y, Huang Q, Abe Ji Che W, Lee JD, Kawakatsu H, Hoit BD, Berk BC, Walsh RA. Activation of mitogen-activated protein kinases and p90 ribosomal S6 kinase in failing human hearts with dilated cardiomyopathy. Cardiovasc Res. 2002;53:131–137. doi: 10.1016/s0008-6363(01)00438-2. [DOI] [PubMed] [Google Scholar]
- 393.Takeishi Y, Huang Q, Abe Ji Glassman M, Che W, Lee JD, Kawakatsu H, Lawrence EG, Hoit BD, Berk BC, Walsh RA. Src and multiple MAP kinase activation in cardiac hypertrophy and congestive heart failure under chronic pressure-overload: comparison with acute mechanical stretch. J Mol Cell Cardiol. 2001;33:1637–1648. doi: 10.1006/jmcc.2001.1427. [DOI] [PubMed] [Google Scholar]
- 394.Takeishi Y, Huang Q, Wang T, Glassman M, Yoshizumi M, Baines CP, Lee JD, Kawakatsu H, Che W, Lerner-Marmarosh N, Zhang C, Yan C, Ohta S, Walsh RA, Berk BC, Abe JI. Src family kinase and adenosine differentially regulate multiple MAP kinases in ischemic myocardium: modulation of MAP kinases activation by ischemic preconditioning. J Mol Cell Cardiol. 2001;33:1989–2005. doi: 10.1006/jmcc.2001.1463. [DOI] [PubMed] [Google Scholar]
- 395.Takemura G, Fujiwara H. Doxorubicin-induced cardiomyopathy: from the cardiotoxic mechanisms to management. Prog Cardiovasc Dis. 2007;49:330–352. doi: 10.1016/j.pcad.2006.10.002. [DOI] [PubMed] [Google Scholar]
- 396.Tam P, Parameswaran M, Kinder S, Weinberger R. The allocation of epiblast cells to the embryonic heart and other mesodermal lineages: the role of ingression and tissue movement during gastrulation. Development. 1997;124:1631–1642. doi: 10.1242/dev.124.9.1631. [DOI] [PubMed] [Google Scholar]
- 397.Tamura K, Sudo T, Senftleben U, Dadak A, Johnson R, Karin M. Requirement for p38alpha in erythropoietin expression: a role for stress kinases in erythropoiesis. Cell. 2000;102:221–231. doi: 10.1016/s0092-8674(00)00027-1. [DOI] [PubMed] [Google Scholar]
- 398.Taniike M, Yamaguchi O, Tsujimoto I, Hikoso S, Takeda T, Nakai A, Omiya S, Mizote I, Nakano Y, Higuchi Y, Matsumura Y, Nishida K, Ichijo H, Hori M, Otsu K. Apoptosis signal-regulating kinase 1/p38 signaling pathway negatively regulates physiological hypertrophy. Circulation. 2008;117:545–552. doi: 10.1161/CIRCULATIONAHA.107.710434. [DOI] [PubMed] [Google Scholar]
- 399.Tanno M, Bassi R, Gorog DA, Saurin AT, Jiang J, Heads RJ, Martin JL, Davis RJ, Flavell RA. Diverse mechanisms of myocardial p38 mitogen-activated protein kinase activation: evidence for MKK-independent activation by a TAB1-associated mechanism contributing to injury during myocardial ischemia. Circ Res. 2003;93:254–261. doi: 10.1161/01.RES.0000083490.43943.85. [DOI] [PubMed] [Google Scholar]
- 400.Tartaglia M, Mehler EL, Goldberg R, Zampino G, Brunner HG, Kremer H, van der Burgt I, Crosby AH, Ion A, Jeffery S, Kalidas K, Patton MA, Kucherlapati RS, Gelb BD. Mutations in PTPN11, encoding the protein tyrosine phosphatase SHP-2, cause Noonan syndrome. Nat Genet. 2001;29:465–468. doi: 10.1038/ng772. [DOI] [PubMed] [Google Scholar]
- 401.Tatake RJ, O’Neill MM, Kennedy CA, Wayne AL, Jakes S, Wu D, Kugler SZ, Jr, Kashem MA, Kaplita P, Snow RJ. Identification of pharmacological inhibitors of the MEK5/ERK5 pathway. Biochem Biophys Res Commun. 2008;377:120–125. doi: 10.1016/j.bbrc.2008.09.087. [DOI] [PubMed] [Google Scholar]
- 402.Tenhunen O, Rysa J, Ilves M, Soini Y, Ruskoaho H, Leskinen H. Identification of cell cycle regulatory and inflammatory genes as predominant targets of p38 mitogen-activated protein kinase in the heart. Circ Res. 2006;99:485–493. doi: 10.1161/01.RES.0000238387.85144.92. [DOI] [PubMed] [Google Scholar]
- 403.Tenhunen O, Sarman B, Kerkela R, Szokodi I, Papp L, Toth M, Ruskoaho H. Mitogen-activated protein kinases p38 and ERK 1/2 mediate the wall stress-induced activation of GATA-4 binding in adult heart. J Biol Chem. 2004;279:24852–24860. doi: 10.1074/jbc.M314317200. [DOI] [PubMed] [Google Scholar]
- 404.Teos LY, Zhao A, Alvin Z, Laurence GG, Li C, Haddad GE. Basal and IGF-I-dependent regulation of potassium channels by MAP kinases and PI3-kinase during eccentric cardiac hypertrophy. Am J Physiol Heart Circ Physiol. 2008;295:H1834–H1845. doi: 10.1152/ajpheart.321.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 405.Terami H, Hidaka K, Katsumata T, Iio A, Morisaki T. Wnt11 facilitates embryonic stem cell differentiation to Nkx2.5-positive cardiomyocytes. Biochem Biophys Res Commun. 2004;325:968–975. doi: 10.1016/j.bbrc.2004.10.103. [DOI] [PubMed] [Google Scholar]
- 406.Thornton T, Rincón M. Non-classical p38 map kinase functions: cell cycle checkpoints and survival. Int J Biol Sci. 2008;5:44–52. doi: 10.7150/ijbs.5.44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 407.Thornton TM, Pedraza-Alva G, Deng B, Wood CD, Aronshtam A, Clements JL, Sabio G, Davis RJ, Matthews DE, Doble B, Rincon M. Phosphorylation by p38 MAPK as an alternative pathway for GSK3beta inactivation. Science. 2008;320:667–670. doi: 10.1126/science.1156037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 408.Tidyman WE, Rauen KA. Noonan, Costello and cardio/facio/cutaneous syndromes: dysregulation of the Ras/MAPK pathway. Expert Rev Mol Med. 2008;10 doi: 10.1017/S1462399408000902. [DOI] [PubMed] [Google Scholar]
- 409.Toth A, Nickson P, Mandl A, Bannister ML, Toth K, Erhardt P. Endoplasmic reticulum stress as a novel therapeutic target in heart diseases. Cardiovasc Hematol Disord Drug Targets. 2007;7:205–218. doi: 10.2174/187152907781745260. [DOI] [PubMed] [Google Scholar]
- 410.Tran TH, Andreka P, Rodrigues CO, Webster KA, Bishopric NH. Jun kinase delays caspase-9 activation by interaction with the apoptosome. J Biol Chem. 2007;282:20340–20350. doi: 10.1074/jbc.M702210200. [DOI] [PubMed] [Google Scholar]
- 411.Turjanski AG, Vaque JP, Gutkind JS. MAP kinases and the control of nuclear events. Oncogene. 26:3240–3253. doi: 10.1038/sj.onc.1210415. [DOI] [PubMed] [Google Scholar]
- 412.Ueno S, Weidinger G, Osugi T, Kohn A, Golob J, Pabon L, Reinecke H, Moon R, Murry C. Biphasic role for Wnt/b-catenin signaling in cardiac specification in zebrafish and embryonic stem cells. Proc Natl Acad Sci USA. 2007;104:9685–9690. doi: 10.1073/pnas.0702859104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 413.Uetani T, Nakayama H, Okayama H, Okura T, Higaki J, Inoue H, Higashiyama S. Insufficiency of Pro-heparin-binding epidermal growth factor-like growth factor shedding enhances hypoxic cell death in H9c2 cardiomyoblasts via the activation of caspase-3 and c-Jun N-terminal kinase. J Biol Chem. 2009;284:12399–12409. doi: 10.1074/jbc.M900463200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 414.Ueyama T, Kawashima S, Sakoda T, Rikitake Y, Ishida T, Kawai M, Yamashita T, Ishido S, Hotta H, Yokoyama M. Requirement of activation of the extracellular signal-regulated kinase cascade in myocardial cell hypertrophy. J Mol Cell Cardiol. 2000;32:947–960. doi: 10.1006/jmcc.2000.1135. [DOI] [PubMed] [Google Scholar]
- 415.Ursitti JA, Petrich BG, Lee PC, Resneck WG, Ye X, Yang J, Randall WR, Bloch RJ. Role of an alternatively spliced form of alphaII-spectrin in localization of connexin 43 in cardiomyocytes and regulation by stress-activated protein kinase. J Mol Cell Cardiol. 2007;42:572–581. doi: 10.1016/j.yjmcc.2006.11.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 416.Vahebi S, Ota A, Li M, Warren CM, de Tombe PP, Wang Y, Solaro RJ. p38-MAPK induced dephosphorylation of α-tropomyosin is associated with depression of myocardial sarcomeric tension and ATPase activity. Circ Res. 2007;100:408–415. doi: 10.1161/01.RES.0000258116.60404.ad. [DOI] [PubMed] [Google Scholar]
- 417.Van Wijk B, Moorman AFM, van den Hoff MJB. Role of bone morphogenetic proteins in cardiac differentiation. Cardiovasc Res. 2007;74:244–255. doi: 10.1016/j.cardiores.2006.11.022. [DOI] [PubMed] [Google Scholar]
- 418.Veeman M, Axelrod J, Moon R. A second canon. Functions and mechanisms of beta-catenin-independent Wnt signaling. Dev Cell. 2003;5:367–377. doi: 10.1016/s1534-5807(03)00266-1. [DOI] [PubMed] [Google Scholar]
- 419.Wagner M, Siddiqui MAQ. Signal transduction in early heart development (I): cardiogenic induction and heart tube formation. Exp Biol Med. 2007;232:852–865. [PubMed] [Google Scholar]
- 420.Walsh KB, Sweet JK, Parks GE, Long KJ. Modulation of outward potassium currents in aligned cultures of neonatal rat ventricular myocytes during phorbol ester-induced hypertrophy. J Mol Cell Cardiol. 2001;33:1233–1247. doi: 10.1006/jmcc.2001.1386. [DOI] [PubMed] [Google Scholar]
- 421.Wang J, Van De Water T, Bonny C, de Ribaupierre F, Puel J, Zine A. A peptide inhibitor of c-Jun N-terminal kinase protects against both aminoglycoside and acoustic trauma-induced hair cell death and hearing loss. J Neurosci. 2003;23:8596–8607. doi: 10.1523/JNEUROSCI.23-24-08596.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 422.Wang J, Wang H, Chen J, Wang X, Sun K, Wang Y, Wang J, Yang X, Song X, Xin Y, Liu Z, Hui R. GADD45B inhibits MKK7-induced cardiac hypertrophy and the polymorphisms of GADD45B is associated with inter-ventricular septum hypertrophy. Biochem Biophys Res Commun. 2008;372:623–628. doi: 10.1016/j.bbrc.2008.05.122. [DOI] [PubMed] [Google Scholar]
- 423.Wang X, Merritt A, Seyfried J, Guo C, Papadakis E, Finegan K, Kayahara M, Dixon J, Boot-Handford R, Cartwright E, Mayer U, Tournier C. Targeted deletion of mek5 causes early embryonic death and defects in the extracellular signal-regulated kinase 5/myocyte enhancer factor 2 cell survival pathway. Mol Cell Biol. 2005;25:336–345. doi: 10.1128/MCB.25.1.336-345.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 424.Wang X, Tournier C. Regulation of cellular functions by the ERK5 signalling pathway. Cell Signal. 2006;18:753–760. doi: 10.1016/j.cellsig.2005.11.003. [DOI] [PubMed] [Google Scholar]
- 425.Wang Y, Huang S, Sah V, Ross J, Jr, Brown JH, Han J, Chien KR. Cardiac muscle cell hypertrophy and apoptosis induced by distinct members of the p38 mitogen-activated protein kinase family. J Biol Chem. 1998;273:2161–2168. doi: 10.1074/jbc.273.4.2161. [DOI] [PubMed] [Google Scholar]
- 426.Wang Y, Su B, Sah V, Brown JH, Han J, Chien KR. Cardiac hypertrophy induced by mitogen-activated protein kinase kinase 7, a specific activator for c-Jun NH2-terminal kinase in ventricular muscle cells. J Biol Chem. 1998;273:5423–5426. doi: 10.1074/jbc.273.10.5423. [DOI] [PubMed] [Google Scholar]
- 427.Watanabe KI, Ma M, Hirabayashi KI, Gurusamy N, Veeraveedu PT, Prakash P, Zhang S, Muslin AJ, Kodama M, Aizawa Y. Swimming stress in DN 14–3-3 mice triggers maladaptive cardiac remodeling: role of p38 MAPK. Am J Physiol Heart Circ Physiol. 2007;292:H1269–H1277. doi: 10.1152/ajpheart.00550.2006. [DOI] [PubMed] [Google Scholar]
- 428.Weinbrenner C, Liu GS, Cohen MV, Downey JM. Phosphorylation of tyrosine 182 of p38 mitogen-activated protein kinase correlates with the protection of preconditioning in the rabbit heart. J Mol Cell Cardiol. 1997;29:2383–2391. doi: 10.1006/jmcc.1997.0473. [DOI] [PubMed] [Google Scholar]
- 429.Widmann C, Gibson S, Jarpe MB, Johnson GL. Mitogen-activated protein kinase: conservation of a three-kinase module from yeast to human. Physiol Rev. 1999;79:143–180. doi: 10.1152/physrev.1999.79.1.143. [DOI] [PubMed] [Google Scholar]
- 430.Wiegler K, Bonny C, Coquoz D, Hirt L. The JNK inhibitor XG-102 protects from ischemic damage with delayed intravenous administration also in the presence of recombinant tissue plasminogen activator. Cerebrovasc Dis. 2008;26:360–366. doi: 10.1159/000151639. [DOI] [PubMed] [Google Scholar]
- 431.Wisler JMS, DeWire S, Whalen E, Violin J, Drake M, Ahn S, Shenoy S, Lefkowitz R. A unique mechanism of β-blocker action: carvedilol stimulates β-arrestin signaling. Proc Natl Acad Sci USA. 2007;104 doi: 10.1073/pnas.0707936104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 432.Wo Y, Zhu D, Hu Y, Wang Z, Liu J, Lou Y. Reactive oxygen species involved in prenylflavonoids, icariin and icaritin, initiating cardiac differentiation of mouse embryonic stem cells. J Cell Biochem. 2008;103:1536–1550. doi: 10.1002/jcb.21541. [DOI] [PubMed] [Google Scholar]
- 433.Wollert KC, Taga T, Saito M, Narazaki M, Kishimoto T, Glembotski C, Vernallis A, Heath J, Pennica D, Wood W, Chien KR. Cardiotrophin-1 activates a distinct form of cardiac muscle cell hypertophy: assembly of sarcomeric units in series via gp130/leukemia inhibitory factor receptor dependent pathways. J Biol Chem. 1996;271:5783–5791. doi: 10.1074/jbc.271.16.9535. [DOI] [PubMed] [Google Scholar]
- 434.Woo CH, Shishido T, McClain C, Lim JH, Li JD, Yang J, Yan C, Abe JI. Extracellular signal-regulated kinase 5 sumoylation antagonizes shear stress-induced antiinflammatory response and endothelial nitric oxide synthase expression in endothelial cells. Circ Res. 2008;102:538–545. doi: 10.1161/CIRCRESAHA.107.156877. [DOI] [PubMed] [Google Scholar]
- 435.Wright CD, Chen Q, Baye NL, Huang Y, Healy CL, Kasinathan S, O’Connell TD. Nuclear alpha1-adrenergic receptors signal activated ERK localization to caveolae in adult cardiac myocytes. Circ Res. 2008;103:992–1000. doi: 10.1161/CIRCRESAHA.108.176024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 436.Xiang P, Deng H, Li K, Huang G, Chen Y, Tu L, Ng P, Pong N, Zhao H, Zhang L, Sung R. Dexrazoxane protects against doxorubicin-induced cardiomyopathy: upregulation of Akt and Erk phosphorylation in a rat model. Cancer Chemother Pharmacol. 2009;63:343–349. doi: 10.1007/s00280-008-0744-4. [DOI] [PubMed] [Google Scholar]
- 437.Xiao L, Pimental DR, Amin JK, Singh K, Sawyer DB, Colucci WS. MEK1/2-ERK1/2 mediates α1-adrenergic receptor-stimulated hypertrophy in adult rat ventricular myocytes. J Mol Cell Cardiol. 2001;33:779–787. doi: 10.1006/jmcc.2001.1348. [DOI] [PubMed] [Google Scholar]
- 438.Xiao L, Pimentel DR, Wang J, Singh K, Colucci WS, Sawyer DB. Role of reactive oxygen species and NAD(P)H oxidase in alpha 1-adrenoceptor signaling in adult rat cardiac myocytes. Am J Physiol Cell Physiol. 2002;282:C926–C934. doi: 10.1152/ajpcell.00254.2001. [DOI] [PubMed] [Google Scholar]
- 439.Xie P, Guo S, Fan Y, Zhang H, Gu D, Li H. Atrogin-1/MAFbx enhances simulated ischemia/reperfusion-induced apoptosis in cardiomyocytes through degradation of MAPK phosphatase-1 and sustained JNK activation. J Biol Chem. 2009;284:5488–5496. doi: 10.1074/jbc.M806487200. [DOI] [PubMed] [Google Scholar]
- 440.Xu C, Bailly-Maitre B, Reed JC. Endoplasmic reticulum stress: cell life and death decisions. J Clin Invest. 2005;115:2656–2664. doi: 10.1172/JCI26373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 441.Xu L, Kappler C, Mani S, Shepherd N, Renaud L, Snider P, Conway S, Menick D. Chronic administration of KB-R7943 induces up-regulation of cardiac NCX1. J Biol Chem. 2009;284:27265–27272. doi: 10.1074/jbc.M109.022855. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 442.Xu X, Graichen R, Soo S, Balakrishnan T, Norfiza BTE, Rahmat S, Sieh S, Tham S, Freund C, Moore J, Mummery C, Colman A, Zweigerdt R, Davidson B. Chemically defined medium supporting cardiomyocyte differentiation of human embryonic stem cells. Differentiation. 2008;76:958–970. doi: 10.1111/j.1432-0436.2008.00284.x. [DOI] [PubMed] [Google Scholar]
- 443.Yada H, Murata M, Shimoda K, Yuasa S, Kawaguchi H, Ieda M, Adachi T, Murata M, Ogawa S, Fukuda K. Dominant negative suppression of Rad leads to QT prolongation and causes ventricular arrhythmias via modulation of L-type Ca2+ channels in the heart. Circ Res. 2007;101:69–77. doi: 10.1161/CIRCRESAHA.106.146399. [DOI] [PubMed] [Google Scholar]
- 444.Yamaguchi O, Watanabe T, Nishida K, Kashiwase K, Higuchi Y, Takeda T, Hikoso S, Hirotani S, Asahi M, Taniike M, Nakai A, Tsujimoto I, Matsumura Y, Miyazaki J, Chien KR, Matsuzawa A, Sadamitsu C, Ichijo H, Baccarini M, Hori M. Cardiac-specific disruption of the c-raf-1 gene induces cardiac disfunction and apoptosis. J Clin Invest. 2004;114:937–943. doi: 10.1172/JCI20317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 446.Yamamoto M, Yang G, Hong C, Liu J, Holle E, Yu X, Wagner T, Vatner S, Sadoshima J. Inhibition of endogenous thioredoxin in the heart increases oxidative stress and cardiac hypertrophy. J Clin Invest. 2003;112:1395–1406. doi: 10.1172/JCI17700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 447.Yan C, Ding B, Shishido T, Woo CH, Itoh S, Jeon KI, Liu W, Xu H, McClain C, Molina CA, Blaxall BC, Abe JI. Activation of extracellular signal-regulated kinase 5 reduces cardiac apoptosis and dysfunction via inhibition of a phosphodiesterase 3A/inducible cAMP early repressor feedback loop. Circ Res. 2007;100:510–519. doi: 10.1161/01.RES.0000259045.49371.9c. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 448.Yan C, Luo H, Lee JD, Abe Ji, Berk BC. Molecular cloning of mouse ERK5/BMK1 splice variants and characterization of ERK5 functional domains. J Biol Chem. 2001;276:10870–10878. doi: 10.1074/jbc.M009286200. [DOI] [PubMed] [Google Scholar]
- 449.Yan L, Carr J, Ashby P, Murry-Tait V, Thompson C, Arthur JS. Knockout of ERK5 causes multiple defects in placental and embryonic development. BMC Dev Biol. 2003;3:11. doi: 10.1186/1471-213X-3-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 450.Yan W, Wang P, Zhao CX, Tang J, Xiao X, Wang DW. Decorin gene delivery inhibits cardiac fibrosis in spontaneously hypertensive rats by modulation of transforming growth factor-β/Smad and p38 mitogen-activated protein kinase signaling pathways. Human Gene Therapy. doi: 10.1089/hum.2008.204. In press. [DOI] [PubMed] [Google Scholar]
- 451.Yang C, Ornatsky O, McDermott J, Cruz T, Prody C. Interaction of myocyte enhancer factor 2 (MEF2) with a mitogen-activated protein kinase, ERK5/BMK1. Nucl Acids Res. 1998;26:4771–4777. doi: 10.1093/nar/26.20.4771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 452.Yang L, Zou X, Liang Q, Chen H, Feng J, Yan L, Wang Z, Zhou D, Li S, Yao S, Zheng Z. Sodium tanshinone IIA sulfonate depresses angiotensin II-induced cardiomyocyte hypertrophy through MEK/ERK pathway. Exp Mol Med. 2007;39:65–73. doi: 10.1038/emm.2007.8. [DOI] [PubMed] [Google Scholar]
- 453.Yang T, Xiong Q, Enslen H, RJD, Chow C. Phosphorylation of NFATc4 by p38 mitogen-activated protein kinases. Mol Cell Biol. 2002;22:3892–3904. doi: 10.1128/MCB.22.11.3892-3904.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 454.Yao Y, Li W, Wu J, Germann U, Su M, Kuida K, Boucher D. Extracellular signal-regulated kinase 2 is necessary for mesoderm differentiation. Proc Natl Acad Sci USA. 2003;100:12759–12764. doi: 10.1073/pnas.2134254100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 455.Yin T, Sandhu G, Wolfgang CD, Burrier A, Webb RL, Rigel DF, Hai T, Whelan J. Tissue-specific pattern of stress kinase activation in ischemic/reperfused heart and kidney. J Biol Chem. 1997;272:19943–19950. doi: 10.1074/jbc.272.32.19943. [DOI] [PubMed] [Google Scholar]
- 456.Yoon S, Seger R. The extracellular signal-regulated kinase: multiple substrates regulate diverse cellular functions. Growth Factors. 2006;21:21–44. doi: 10.1080/02699050500284218. [DOI] [PubMed] [Google Scholar]
- 457.Yue TL, Gu JL, Wang C, Reith AD, Lee JC, Mirabile RC, Kreutz R, Wang Y, Maleeff B, Parsons AA, Ohlstein EH. Extracellular signal-regulated kinase plays an essential role in hypertrophic agonists, endothelin-1 and phenylephrine-induced cardiomyocyte hypertrophy. J Biol Chem. 2000;275:37895–37901. doi: 10.1074/jbc.M007037200. [DOI] [PubMed] [Google Scholar]
- 458.Yue TL, Wang C, Gu JL, Ma XL, Kumar S, Lee JC, Feuerstein GZ, Thomas H, Maleeff B, Ohlstein EH. Inhibition of extracellular signal-regulated kinase enhances ischemia/reoxygenation-induced apoptosis in cultured cardiac myocytes and exaggerates reperfusion injury in isolated perfused heart. Circ Res. 2000;86:692–699. doi: 10.1161/01.res.86.6.692. [DOI] [PubMed] [Google Scholar]
- 459.Zarubin T, Han J. Activation and signaling of the p38 MAP kinase pathway. Cell Res. 2005;15:11–18. doi: 10.1038/sj.cr.7290257. [DOI] [PubMed] [Google Scholar]
- 460.Zechner D, Thuerauf DJ, Hanford DS, McDonough PM, Glembotski CC. A role for the p38 mitogen-activated protein kinase pathway in myocardial cell growth, sarcomeric organization, and cardiac-specific gene expression. J Cell Biol. 1997;139:115–127. doi: 10.1083/jcb.139.1.115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 461.Zeidan A, Javadov S, Chakrabarti S, Karmazyn M. Leptin-induced cardiomyocyte hypertrophy involves selective caveolae and RhoA/ROCK-dependent p38 MAPK translocation to nuclei. Cardiovasc Res. 2008;77:64–72. doi: 10.1093/cvr/cvm020. [DOI] [PubMed] [Google Scholar]
- 462.Zhai P, Gao S, Holle E, Yu X, Yatani A, Wagner T, Sadoshima J. Glycogen synthase kinase-3α reduces cardiac growth and pressure overload-induced cardiac hypertrophy by inhibition of extracellular signal-regulated kinases. J Biol Chem. 2007;282:33181–33191. doi: 10.1074/jbc.M705133200. [DOI] [PubMed] [Google Scholar]
- 463.Zhang J, Li XX, Bian HJ, Liu XB, Ji XP, Zhang Y. Inhibition of the activity of Rho-kinase reduces cardiomyocyte apoptosis in heart ischemia/reperfusion via suppressing JNK-mediated AIF translocation. Clin Chim Acta. 2009;401:76–80. doi: 10.1016/j.cca.2008.11.016. [DOI] [PubMed] [Google Scholar]
- 464.Zhang S, Weinheimer C, Courtois M, Kovacs A, Zhang C, Cheng A, Wang Y, Muslin A. The role of the Grb2-p38 MAPK signaling pathway in cardiac hypertrophy and fibrosis. J Clin Invest. 2003;111:833–841. doi: 10.1172/JCI16290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 465.Zhao Y, Sawyer DB, Baliga R, Opel D, Han X, Marchionni M, Kelly RA. Neuregulins promote survival and growth of cardiac myocytes. J Biol Chem. 1998;273:10261–10269. doi: 10.1074/jbc.273.17.10261. [DOI] [PubMed] [Google Scholar]
- 466.Zheng M, Dilly K, Dos Santos Cruz J, Li M, Gu Y, Ursitti JA, Chen J, Ross J, Jr, Chien KR, Lederer JW, Wang Y. Sarcoplasmic reticulum calcium defect in Ras-induced hypertrophic cardiomyopathy heart. Am J Physiol Heart Circ Physiol. 2004;286:H424–H433. doi: 10.1152/ajpheart.00110.2003. [DOI] [PubMed] [Google Scholar]
- 467.Zhou W, Lin L, Majumdar A, Li X, Zhang X, Liu W, Etheridge L, Shi Y, Martin J, Van de Ven W, Kaartinen V, Wynshaw-Boris A, McMahon AP, Rosenfeld MG, Evans SM. Modulation of morphogenesis by noncanonical Wnt signaling requires ATF/CREB family-mediated transcriptional activation of TGFβ2. Nat Genet. 2007;39:1225–1234. doi: 10.1038/ng2112. [DOI] [PMC free article] [PubMed] [Google Scholar]