Abstract
Objective
To evaluate inpatient health care utilization for children with systemic lupus erythematosus (SLE) with and without kidney disease.
Methods
The Healthcare Cost and Utilization Project Kids’ Inpatient Database for the years 2000, 2003, and 2006 was used for this analysis. SLE hospitalizations from the 2006 cohort were identified and classified as those with versus without kidney involvement by International Classification of Diseases, Ninth Revision, Clinical Modification codes. Analyses were performed to examine determinants of hospitalization charges and changes in charges over time.
Results
In the US, 7,390 SLE-related pediatric hospitalizations generated $267 million in total charges in 2006. Of these, 4,193 discharges had kidney involvement. The average hospitalization charge was greater for SLE patients with kidney involvement compared to those without kidney involvement ($43,100 versus $28,500; P < 0.0001). In multivariate analysis, kidney involvement remained a significant predictor of hospitalization charges, independent of demographic and hospital characteristics (P < 0.0001). SLE-associated acute kidney failure, transplant, and end-stage kidney disease resulted in greater hospitalization charges than SLE without kidney involvement by $74,900 (P < 0.0001), $32,700 (P = 0.0002), and $27,400 (P < 0.0001), respectively.
Conclusion
In the US, >7,000 hospitalizations occurred in 2006 among children with SLE, with nearly 57% demonstrating kidney involvement. Kidney involvement is a major determinant of hospitalization charges for these children. This study represents one of the first large-scale assessments of in-hospital health care utilization by children with SLE.
INTRODUCTION
Systemic lupus erythematosus (SLE) is a chronic, relapsing, and remitting inflammatory autoimmune disease, with nearly 20% of cases presenting before age 18 years (1). Kidney involvement is present in 60–80% of pediatric SLE cases, with 10–50% progressing to end-stage kidney disease (ESKD) (2–10).
Studies of the adult SLE population demonstrate that patients with kidney involvement incur substantially higher treatment costs than those who do not have kidney involvement (11–16). In contrast, very little data exist examining the economic burden of treating SLE in children. A single study reported the costs of treating 119 children with SLE in 2 tertiary pediatric rheumatology centers in the US from 2001–2004. This study calculated the direct inpatient and outpatient cost of childhood-onset SLE per quality-adjusted life year to be $30,908 (17).
Given the paucity of pediatric data, we sought to further investigate the charges for treating children with SLE. Our primary objective was to evaluate health care utilization charges for hospitalized pediatric SLE patients and the relative contribution of kidney involvement to these charges. Our secondary objective was to identify demographic and socioeconomic factors associated with SLE hospitalization charges.
MATERIALS AND METHODS
Data source
For this analysis, we used the Healthcare Cost and Utilization Project Kids’ Inpatient Database (KID), which is sponsored by the US Agency for Healthcare Research and Quality. It is the only comprehensive, all-payer health care database for children in the US. KID data were compiled from hospitalization claims data every 3 years and reported at the level of discharge. To ensure an accurate representation of pediatric cases, a method of random sampling was used to select 10% of uncomplicated in-hospital births and 80% of complicated in-hospital births and other pediatric cases from each participating hospital. The hospital discharges are sorted by state, hospital, and diagnosis-related group. To obtain national estimates, the Healthcare Cost and Utilization Project (HCUP) developed weighting for hospital discharge numbers based on the American Hospital Association universe as the standard. Data in the 2006 KID were drawn from 3,739 hospitals in 38 reporting states for children ages ≤20 years. Data were drawn from 36 and 27 states for the years 2003 and 2000, respectively. For each year, data were collected from January 1 to December 31. Included in each discharge record were International Classification of Diseases, Ninth Revision, Clinical Modification (ICD-9-CM) diagnosis and procedure codes, patient and hospital demographics, length of stay, and hospital charges. This data set does not provide reimbursement data (18).
Because the KID contains deidentified data only, this study was reviewed and deemed not regulated by the University of Michigan Institutional Review Board.
Outcome variables of interest
The 2006 KID cohort discharges with a primary or secondary diagnosis of SLE (ICD-9-CM code 710.0) were identified. SLE with kidney involvement (SLE + KI) was defined by primary or secondary ICD-9-CM codes for nephritis, chronic kidney disease, dialysis, kidney transplant, acute kidney failure (AKF), or kidney biopsy listed on the same SLE claim (Table 1). SLE without kidney involvement (SLE – KI) was defined as all SLE discharges that did not fall into the SLE + KI cohort. We chose these codes based upon a manual review of ICD-9-CM codes and validated this selection based on prior studies that utilized ICD-9-CM codes to define lupus nephritis (12,13,15). Overall, the selected codes were consistent with the codes used in previously reported studies, except where the definition of kidney involvement was expanded by including codes for hypertension associated with kidney disease. To analyze the effect of kidney failure, we further categorized SLE + KI into the following groups based upon ICD-9-CM codes: AKF, ESKD, kidney transplant, and those without kidney failure. When AKF and ESKD codes were present on the same claim, priority was given to ESKD. Demographic and hospitalization data extracted included age, race, sex, insurance type (primary or secondary insurance was private, public, or other), median income for patient zip code, teaching status of each hospital, total charges, and length of stay. The primary analysis included here focused on the 2006 cohort. Data from 2000 and 2003 were used for trend analysis.
Table 1.
ICD-9-CM codes used to define disease subgroups*
| Diagnosis groups | ICD-9-CM diagnosis and procedure codes |
|---|---|
| SLE | 710.0 |
| SLE + KI | |
| Diagnosis codes | |
| Nephritis | 580.0, 580.4, 580.8, 580.81, 580.89, 580.9, 581.0, 581.1, 581.2, 581.3, 581.8, 581.81, 581.89, 581.9, 582.0, 582.1, 582.2, 582.4, 582.8, 582.81, 582.89, 582.9, 583.0, 583.1, 583.2, 583.4, 583.6, 583.7, 583.8, 583.81, 583.89, 583.9 |
| Chronic kidney disease | 585, 585.1, 585.2, 585.3, 585.4, 283.11, 403.00, 403.10, 403.90, 404.00, 404.10, 404.90, 404.01, 404.11, 404.91, 587, 588.8, 588.9, 642.1, 642.2, 794.4 |
| Dialysis | v56.0, v56.8, v45.11, v56.1, v56.2, v56.3, v45.12, 792.5, v45.1, v56.31, v56.32 |
| Kidney transplant | v42.0, 996.81 |
| AKF | 584, 584.5, 584.6, 584.7, 584.8, 584.9 |
| ESKD | 585.5, 585.6, 585.9, 586, 403.01, 403.11, 403.91, 404.02, 404.12, 404.92, 404.03, 404.13, 404.93 |
| Procedure codes | |
| Dialysis | 39.95, 54.98, 39.93, 39.94, 39.42, 39.27 |
| Kidney transplant | 55.69, 55.53, 55.52, 55.51, 55.54 |
| Kidney biopsy | 55.23, 55.24, 55.39, 55.21 |
| SLE – KI | Any 710.0 patient not included in SLE + KI |
| Kidney failure groups | |
| SLE with transplant | All kidney transplant diagnosis or procedure codes |
| SLE with ESKD | All ESKD codes except those with a transplant code |
| All dialysis codes and/or dialysis procedure codes except those with transplant | |
| SLE with AKF | All AKF codes except those with transplant or ESKD codes |
ICD-9-CM = International Classification of Diseases, Ninth Revision, Clinical Modification; SLE = systemic lupus erythematosus; SLE + KI = SLE with kidney involvement; AKF = acute kidney failure; ESKD = end-stage kidney disease; SLE – KI = SLE without kidney involvement.
Statistical analysis
Frequencies and percentages were calculated for categorical demographic data, including age, race, sex, teaching status of hospital, patient insurance status, and median income by patient zip code for discharges, including a diagnosis of SLE. Sums, SDs, means, and SEMs were computed for the number of discharges and charges related to SLE. Chi-square tests and t-tests were used to assess differences between the groups. Linear regression models were used to assess predictors of charges and length of stay. Independent variables included in the models were age, race, sex, teaching status of hospital, insurance status, and median income by patient zip code. Appropriate survey weights and domain, cluster, and stratum statements were specified in all analyses to provide accurate national estimates and assure unbiased variance estimates. When conducting analysis of trends in charges over time, we converted charges from 2000 and 2003 into 2006 dollars to adjust for increases in inflation using the Consumer Price Index for All Urban Consumers. Consequently, all results comparing trends in charges are shown in 2006 dollars (19). All analyses were performed with SAS, version 9.2.
RESULTS
Demographics
In 2006, a total of 7.5 million pediatric discharges generated cumulative charges of $96.4 billion in the US (Table 2). SLE accounted for 7,390 discharges, of which 4,193 (57%) had SLE + KI.
Table 2.
Comparison of pediatric hospitalizations in the US in 2006, categorized by SLE and kidney involvement status*
| Pediatric discharges |
Pediatric SLE discharges |
|||||
|---|---|---|---|---|---|---|
| Non-SLE | SLE | P | SLE + KI | SLE – KI | P | |
| Age, years | ||||||
| <15 | 80.6 ± 0.2 | 26.2 ± 1.5 | < 0.0001 | 27.2 ± 1.8 | 24.8 ± 1.8 | 0.4773 |
| 15–18 | 10.0 ± 0.1 | 37.1 ± 1.2 | 36.6 ± 1.6 | 37.8 ± 1.5 | ||
| 19–20 | 9.4 ± 0.1 | 36.7 ± 1.7 | 36.2 ± 2.1 | 37.4 ± 1.9 | ||
| Race | ||||||
| White | 50.9 ± 0.8 | 23.0 ± 1.6 | < 0.0001 | 18.0 ± 1.8 | 29.9 ± 2.0 | < 0.0001 |
| African American | 15.1 ± 0.5 | 41.1 ± 2.1 | 43.9 ± 2.7 | 37.3 ± 2.2 | ||
| Hispanic | 24.5 ± 0.9 | 25.6 ± 2.2 | 26.3 ± 2.6 | 24.6 ± 2.4 | ||
| Other | 9.5 ± 0.4 | 10.3 ± 1.1 | 11.8 ± 1.6 | 8.2 ± 1.0 | ||
| Sex | ||||||
| Male | 47.2 ± 0.1 | 14.5 ± 0.8 | < 0.0001 | 16.3 ± 1.2 | 12.2 ± 0.9 | 0.0042 |
| Female | 52.8 ± 0.1 | 85.5 ± 0.8 | 83.7 ± 1.2 | 87.3 ± 0.9 | ||
| Teaching status | ||||||
| Nonteaching | 46.5 ± 0.9 | 18.6 ± 1.6 | < 0.0001 | 14.5 ± 1.6 | 23.9 ± 1.9 | < 0.0001 |
| Teaching | 53.5 ± 0.9 | 81.4 ± 1.6 | 85.5 ± 1.6 | 76.1 ± 1.9 | ||
| Insurance status | ||||||
| Private | 46.4 ± 0.6 | 38.0 ± 1.5 | < 0.0001 | 37.0 ± 1.9 | 39.4 ± 1.8 | 0.4998 |
| Public | 45.5 ± 0.6 | 52.6 ± 1.5 | 53.5 ± 1.9 | 51.5 ± 1.9 | ||
| Other | 8.1 ± 0.3 | 9.4 ± 0.8 | 9.6 ± 0.9 | 9.2 ± 1.0 | ||
| Median income by zip code | ||||||
| $1–37,999 | 29.7 ± 0.6 | 35.3 ± 1.8 | 0.0001 | 33.8 ± 2.2 | 37.1 ± 2.0 | 0.1984 |
| $38,000–46,999 | 24.9 ± 0.4 | 25.8 ± 1.2 | 27.0 ± 1.6 | 24.2 ± 1.5 | ||
| $47,000–61,999 | 23.8 ± 0.4 | 20.9 ± 1.1 | 21.8 ± 1.6 | 19.7 ± 1.3 | ||
| ≥$62,000 | 21.5 ± 0.6 | 18.1 ± 1.4 | 17.4 ± 1.7 | 19.0 ± 1.6 | ||
Values are the mean ± SEM percentage. Total numbers of discharges are 7,558,812 for non-SLE, 7,390 for SLE, 4,193 for SLE + KI, and 3,197 for SLE – KI. Chi-square comparisons between non-SLE vs. SLE and SLE + KI vs. SLE – KI were made within characteristic groups. SLE = systemic lupus erythematosus; SLE + KI = SLE with kidney involvement; SLE – KI = SLE without kidney involvement.
SLE-related discharges were older (P < 0.0001) and more likely to be of a minority race or ethnicity (P < 0.0001), reside in a lower-income zip code (P = 0.0001), and be female (P < 0.0001) compared to non-SLE pediatric discharges. Additionally, SLE discharges were more likely to receive their care in a teaching hospital environment (P < 0.0001) and be covered by public insurance (P < 0.0001).
When assessing SLE discharges with and without kidney involvement, SLE + KI discharges included a lower percentage of white race (18.0% versus 29.9%; P < 0.0001), a higher percentage of males (16.3% versus 12.2%; P = 0.004), and a higher percentage who received care at a teaching hospital (85.5% versus 76.1%; P < 0.0001) when compared to SLE – KI discharges.
Analysis of charges
Table 3 provides the mean SLE-associated charge per discharge. Mean charges were $43,100 for SLE + KI versus $28,500 for SLE – KI (P < 0.0001). The mean length of stay was longer with SLE + KI compared to SLE – KI (7.1 days versus 5.5 days; P < 0.0001).
Table 3.
2006 pediatric charges per hospitalization for children with SLE*
| All SLE | SLE + KI | SLE − KI | |
|---|---|---|---|
| All pediatric patients with SLE | 36.8 ± 1.5 | 43.1 ± 2.1 | 28.5 ± 1.6 |
| Age, years | |||
| <15 | 35.3 ± 3.1 | 36.7 ± 4.2† | 33.2 ± 4.0 |
| 15–18 | 34.4 ± 2.0‡ | 40.8 ± 2.9† | 26.3 ± 2.4 |
| 19–20 (reference) | 40.3 ± 2.0 | 50.3 ± 3.2 | 27.6 ± 1.7 |
| Race | |||
| African American | 35.3 ± 2.3‡ | 39.2 ± 3.0 | 29.0 ± 2.8 |
| Hispanic | 43.9 ± 3.1§ | 54.4 ± 4.7† | 28.9 ± 2.6 |
| Other | 45.4 ± 4.3† | 47.4 ± 5.6 | 41.5 ± 6.3† |
| White (reference) | 29.4 ± 2.3 | 35.3 ± 4.6 | 24.4 ± 1.9 |
| Sex | |||
| Female | 35.9 ± 1.6‡ | 42.2 ± 2.3 | 27.9 ± 1.6 |
| Male (reference) | 42.5 ± 3.3 | 48.0 ± 4.6 | 32.9 ± 4.0 |
| Teaching status | |||
| Nonteaching | 30.9 ± 1.9‡ | 40.1 ± 3.6 | 23.5 ± 1.7‡ |
| Teaching (reference) | 38.3 ± 1.8 | 43.7 ± 2.4 | 30.2 ± 2.0 |
| Insurance status | |||
| Private | 35.0 ± 2.1 | 38.4 ± 3.0 | 30.9 ± 2.7 |
| Other | 39.8 ± 3.9 | 47.0 ± 6.1 | 29.9 ± 2.9 |
| Public (reference) | 37.3 ± 1.9 | 45.3 ± 2.8 | 26.4 ± 1.9 |
| Median income by zip code | |||
| $1–37,999 | 35.4 ± 2.6 | 43.2 ± 3.9 | 25.9 ± 2.2 |
| $38,000–46,999 | 37.4 ± 2.0 | 43.8 ± 3.0 | 27.9 ± 2.6 |
| $47,000–61,999 | 36.8 ± 2.6 | 40.8 ± 3.7 | 31.0 ± 3.6 |
| ≥$62,000 (reference) | 38.8 ± 3.3 | 43.9 ± 4.9 | 32.6 ± 3.7 |
Values are the mean ± SEM in thousands of dollars. Within each disease category, statistical comparisons were performed in characteristic groups and were compared to the corresponding reference group. SLE = systemic lupus erythematosus; SLE + KI = SLE with kidney involvement; SLE – KI = SLE without kidney involvement.
P< 0.01.
P< 0.05.
P< 0.0001.
Among all SLE discharges, the following characteristics were associated with higher charges: ages 19–20 years; African American, Hispanic, and other races; male sex; and discharges from a teaching hospital. Among SLE + KI discharges, ages 19–20 years and Hispanic race were associated with higher charges. Insurance status and median income of zip code were not associated with charges in either group.
SLE + KI accounted for 57% of SLE discharges and 67% of SLE total charges (Table 4). When SLE + KI discharges were subdivided according to kidney failure status, a diagnosis code for AKF generated the highest mean charge per discharge of $100,000 versus $63,700 for transplant (P = 0.004) and $58,200 for ESKD (P = 0.0001). ESKD was present in 1,180 discharges, constituting 15% of SLE and 28% of SLE + KI discharges. Charges for SLE + KI, but no kidney failure (AKF, ESKD, or kidney transplant), were not significantly different from charges for SLE − KI (P = 0.09).
Table 4.
Impact of kidney disease on pediatric SLE hospitalizations*
| SLE + KI |
|||||
|---|---|---|---|---|---|
| SLE – KI | AKF | ESKD | Kidney transplant |
No kidney failure |
|
| No. of discharges | 3,197 | 433 | 1,180 | 111 | 2,469 |
| Total charges, million $ | 89.3 | 42.5 | 67.2 | 7.0 | 60.9 |
| Length of stay, mean ± SEM days | 5.5 ± 0.3 | 13.8 ± 1.2 | 9.5 ± 0.8 | 6.8 ± 0.6 | 4.7 ± 0.3 |
| No. of dialysis events | 0 | 83 | 701 | 34 | 0 |
| Charge/discharge, mean ± SEM thousand $ | 28.5 ± 1.6 | 100.0 ± 9.8† | 58.2 ± 4.3† | 63.7 ± 8.1† | 25.0 ± 1.6 |
SLE = systemic lupus erythematosus; SLE – KI = SLE without kidney involvement; SLE + KI = SLE with kidney involvement; AKF = acute kidney failure; ESKD = end-stage kidney disease.
P< 0.0001 for comparison of mean charge/discharge for each SLE + KI subgroup with SLE – KI.
Multivariate analysis of charges
In the multivariate analysis of the SLE population, age, race, sex, and presence of kidney involvement were all independent predictors of charges (Table 5). Charges were greatest for young adults ages 19–20 years, those of Hispanic and other races, males, and discharges that included a diagnosis code for kidney involvement. When the analysis cohort was restricted to SLE + KI, race, admission to a teaching hospital, and kidney failure were independent predictors of charges.
Table 5.
Adjusted multivariate analysis of hospitalization charges within SLE and SLE + KI disease subgroups*
| All SLE |
SLE + KI |
|||
|---|---|---|---|---|
| Mean ± SEM | P | Mean ± SEM | P | |
| Age, years | ||||
| <15 | −7.9 ± 3.5 | 0.023 | −1.5 ± 5.8 | NS |
| 15–18 | −8.5 ± 2.7 | 0.002 | −2.3 ± 4.7 | NS |
| 19–20 | Reference | Reference | ||
| Race | ||||
| African American | 4.3 ± 3.5 | NS | −1.2 ± 5.2 | NS |
| Hispanic | 13.9 ± 4.1 | 0.001 | 18.7 ± 6.5 | 0.004 |
| Other | 15.8 ± 5.1 | 0.002 | 11.7 ± 6.5 | NS |
| White | Reference | Reference | ||
| Sex | ||||
| Female | −7.6 ± 3.7 | 0.042 | −6.2 ± 5.4 | NS |
| Male | Reference | Reference | ||
| Teaching status | ||||
| Nonteaching | −5.0 ± 2.9 | NS | −12.6 ± 4.8 | 0.009 |
| Teaching | Reference | Reference | ||
| Insurance status | ||||
| Private | −0.4 ± 3.0 | NS | 1.1 ± 4.3 | NS |
| Other | 1.1 ± 5.1 | NS | 2.6 ± 7.5 | NS |
| Public | Reference | Reference | ||
| Median income by zip code | ||||
| $1–37,999 | −5.7 ± 4.0 | NS | −2.7 ± 5.9 | NS |
| $38,000–46,999 | −5.5 ± 3.8 | NS | −7.3 ± 5.8 | NS |
| $47,000–61,999 | −4.5 ± 4.0 | NS | −7.2 ± 6.1 | NS |
| ≥$62,000 | Reference | Reference | ||
| Kidney involvement | ||||
| SLE + KI | 12.7 ± 2.6 | < 0.0001 | ||
| SLE – KI | Reference | |||
Values are in thousand US dollars and compared to the reference for each characteristic. SLE = systemic lupus erythematosus; SLE + KI = SLE with kidney involvement; NS = not significant; SLE − KI = SLE without kidney involvement.
In the SLE population, further regression analysis of the role of kidney failure was performed. Compared to SLE − KI discharges, SLE with AKF generated charges that were $74,900 more (P < 0.0001), ESKD generated charges that were $27,400 more (P < 0.0001), and kidney transplant generated charges that were $32,700 more (P = 0.0002). SLE + KI discharges without kidney failure (AKF, ESKD, or kidney transplant) generated charges that were $5,500 less (P = 0.02) than SLE − KI (data not shown).
Economic trends in hospitalization expenditures
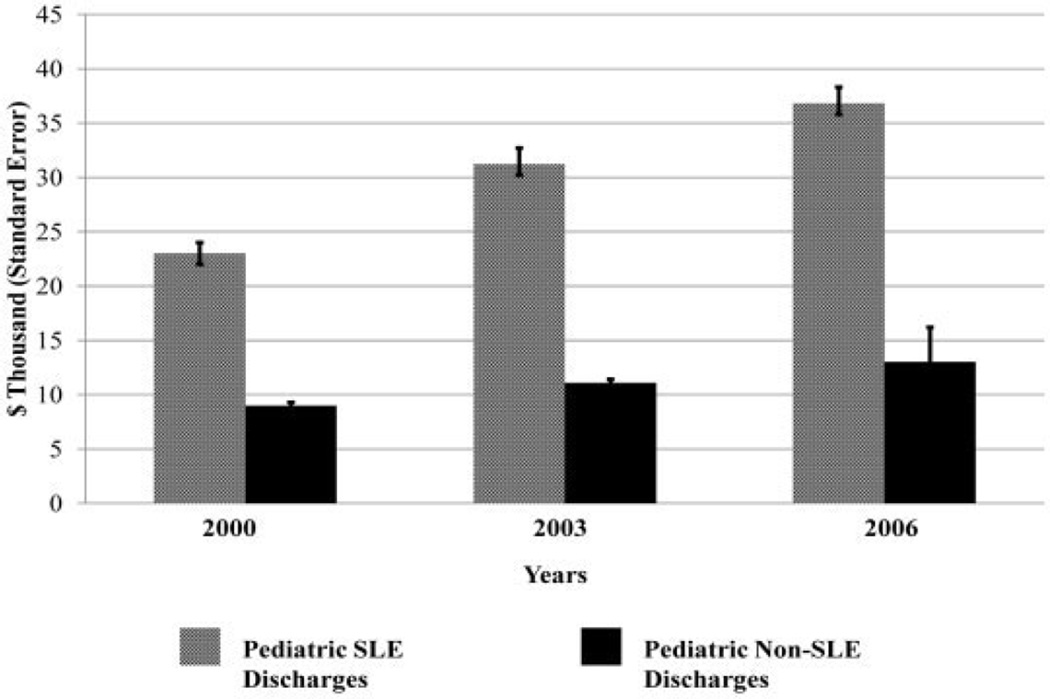
Figure 1 shows the mean charge per discharge for general pediatric and SLE-related pediatric discharges in 2000, 2003, and 2006, adjusted for inflation. The figure demonstrates a rise in charges for both groups from 2000–2006, but the fraction of SLE-related charges relative to general pediatric charges rose over this timeframe (P < 0.0001). There was also a small, but significant, increase in the length of stay for SLE discharges over that time period (from 5.6 days to 6.4 days; P < 0.01).
Figure 1.
Mean charge per discharge trend data for pediatric systemic lupus erythematosus (SLE) and non-SLE discharges from 2000–2006.
DISCUSSION
This study presents one of the first large-scale analyses of pediatric inpatient health care charges for children with SLE. Using the KID, we found that mean hospitalization charges were significantly greater for children with SLE compared to other pediatric discharges. Among pediatric SLE discharges, the presence of kidney involvement, especially kidney failure, significantly predicted higher charges.
A coded diagnosis of AKF was associated with the highest charges and longest hospitalizations among the SLE cohort. The KID does not provide data regarding the cause of AKF. Consequently, we cannot specify whether AKF was secondary to lupus nephritis. Regardless, these results were not surprising, since the increased charges associated with AKF were consistent with other studies of hospitalized patients who develop AKF, regardless of etiology (20,21).
While SLE with a kidney transplant represented a relatively small proportion of SLE discharges (1.5%), the mean charge per discharge associated with a kidney transplant diagnosis was substantial and greater than SLE-associated ESKD. Approximately 30% of transplant-related discharges included the code for the kidney transplant procedure, implying that the transplant was performed during that hospitalization. It is probable that the transplant operation and postoperative care contributed significantly to the charges for the group as a whole.
Compared to AKF and transplant, ESKD accounted for the least expensive hospitalizations, but the greatest number of discharges and total charges. ESKD contributed to 15% of SLE discharges and generated 28% of all SLE-associated charges. The high charges may be a combination of the high proportion of discharges and the prolonged length of hospital stay. At 9.5 ± 0.8 days, this group had the second-longest mean ± SD length of stay. Analysis of the primary reason for admission in a prospective study may provide more insight into these hospitalizations and potentially provide a target for reduction in health care resource utilization.
Hospitalization for SLE + KI without kidney failure resulted in slightly lower charges than SLE – KI. The mean ± SD length of stay was shortest for this group at 4.7 ± 0.3 days, compared to 5.5 ± 0.3 days for SLE – KI and 6.8 ± 0.6, 9.5 ± 0.8, and 13.8 ± 1.2 days for kidney transplant, ESKD, and AKF, respectively. Some of the SLE + KI without kidney failure hospitalizations may have included brief, elective hospitalizations for diagnostic and therapeutic procedures such as kidney biopsy or medication infusion. Regardless, these data support the hypothesis that the presence of kidney failure, not solely kidney involvement, is the key determinant of increased hospitalization charges for SLE patients.
We examined the relationship between demographic and socioeconomic factors and inpatient charges, postulating that factors that lead to worse disease severity will result in greater charges. Multiple studies of patients with SLE have shown worse generalized SLE and kidney-related disease severity and outcomes in those of male sex, nonwhite race, and low socioeconomic status (8,22–24). Data also exist demonstrating that both general SLE and kidney-related disease damage are associated with higher direct costs in the care of patients with SLE (14,16,25).
Based on these studies, one would conclude that sex, race, socioeconomic status, and severe kidney disease would all significantly affect health care utilization. In our study, this was only partially true. When examining all SLE discharges, sex and race were associated with higher charges in both bivariate and multivariate analyses, while socioeconomic factors such as insurance status and median income of patient zip code were not. In SLE + KI discharges, race was associated with higher charges on both bivariate and multivariate analyses, whereas socioeconomic factors were not.
Our analysis only examined inpatient charges, and it is possible that examination of outpatient expenditures would find an association between socioeconomic status and charges. It may be that once a patient is admitted to the hospital, their disease has become severe enough that socioeconomic differences affecting disease severity become less important in predicting charges. It may also be that insurance status and income by zip code are not a complete reflection of the socioeconomic factors that affect disease management and severity. Petri et al examined the relationship between race, socioeconomic status, and morbidity in SLE and found that poor scores rating patient compliance with the prescribed treatment regimen were associated with kidney disease severity, while other standard measures of socioeconomic status such as income were not directly associated with disease status (26). Data on patient compliance were not available in the KID for inclusion in our analysis. Another explanation may be that the KID employs categorical data to characterize socioeconomic status and that utilization of continuous variables would reveal a linear association or threshold effect on hospitalization charges.
It was not surprising that discharges from a teaching hospital were associated with higher charges. Hospitalization at a teaching hospital has traditionally been associated with higher costs and charges due to more complex cases, specialized services, and time directed to graduate medical education (27,28).
Prior studies have demonstrated an association between disease severity and age at presentation. Descloux et al examined the effect of age at disease onset on disease severity, dividing by prepubertal (<9 years), peripubertal (9–14 years), and postpubertal (>14 years), and found an inverse relationship between age and extent of overall disease damage regardless of disease duration, although this relationship did not apply to renal damage specifically (29). In our study, to analyze whether age effect translated into higher charges, age was divided into 3 groups: <15 years to represent the pre- and peripubescent population, 15–18 years to represent the postpubescent population, and 19–20 years to represent young adults. Initially, the age cutoff for the youngest group was <10 years, but this resulted in a very small sample size, precluding the ability to make meaningful associations with this group. We also deviated from prior publications, since this HCUP study included prevalent and incident SLE patients with age at hospitalization documented, not age at SLE onset.
On multivariate analysis, older age was associated with higher charges for all SLE discharges, but this was not true for the SLE + KI subgroup. This contradicts the presumed hypothesis that the younger patients with more severe disease would generate higher charges. Further analysis of this question would require more detailed analysis than performed here, but one could postulate that this contradiction may be explained by non–SLE-related factors such as location of service (19–20-year-olds may be admitted to adult wards), higher rates of obstetrical care in 19–20-year-old patients, or a greater burden of SLE-associated complications in survivors of early-onset SLE. The lack of an association on multivariate analysis for age in the SLE + KI subgroup is consistent with past publications (by Baqi et al and Descloux et al) that also failed to demonstrate an association between age and development of ESKD (5,29).
One study has reviewed pediatric SLE health care utilization in the past. Brunner et al evaluated SLE costs from 2 pediatric centers, examining variables such as inpatient versus outpatient care, medications, laboratory testing, and dialysis (17). Our analysis was different in that we examined inpatient care only, analyzing demographic and kidney disease–specific variables. Both the study by Brunner et al and our study examined the role of dialysis/ESKD on charges. Brunner et al examined the cost of dialysis in their population (n = 119), finding that dialysis patients constituted 2.5% of their study population, but contributed to 11% of the total direct costs. In our study of 7,558,812 pediatric and 7,390 pediatric SLE hospitalizations in 2006, we found that ESKD contributed to 15% of SLE discharges and 28% of all SLE-associated charges. While the 2 analyses are not directly comparable, they both demonstrate that ESKD is responsible for a disproportionate percentage of total SLE health care resources.
Several studies have examined health care utilization by adults with SLE on a national level and demonstrated results consistent with our study. Pelletier et al reported data from a 2006–2008 US claims database that adult SLE patients with kidney involvement were hospitalized more frequently than those without kidney involvement (30.3% versus 13.6%; P < 0.001) and had greater annual followup costs in both inpatient and outpatient settings ($30,652 versus $12,029; P < 0.001) (15). Clarke et al surveyed adult SLE patients at 6 centers in the US, Canada, and the UK on their reported use of medical services and calculated an estimated cost based on market costs. They reported that the 4-year (1995–1999) cumulative direct medical cost of SLE patients with kidney involvement was $27,869–99,544 versus $20,337 in those without kidney involvement (16). Li et al examined a Medicaid database (combined pediatric and adult data) and reported that the mean annual cost over a 5-year period ranged from $27,463–50,578 in patients with kidney involvement versus $13,014–16,638 in patients without kidney involvement. Patients that developed ESKD had mean costs ranging from $47,660–106,982 per annum (13).
Our data indicated that the fraction of pediatric SLE hospitalization charges compared to other pediatric hospitalization charges was rising between 2000 and 2006 (P < 0.0001). Consequently, we cannot attribute the rising charges to inflation alone. Review of the data demonstrated a small, but significant, increase in the length of stay between 2000 and 2006 (from 5.6 days to 6.4 days). Possible explanations may be that novel therapies targeted for SLE are disproportionately expensive and that survival of complex patients is increasing the length of stay.
The greatest strength of this study was the size and scope of the KID. The use of a national claims database offered data that eliminate single-center biases. The KID provided sufficient data that permitted us to move beyond demographic summaries and examine disease-specific factors such as kidney failure and its contribution to charges. Apart from the analysis by Li et al examining the effects of ESKD on charges reported in a Medicaid database (13), few other studies have been able to subdivide the kidney involvement population to examine this specific factor.
However, there were also limitations to using this database. First, assignment of billing codes is operator dependent, and therefore may be subjective. For that reason, we kept our definition of kidney involvement broad to ensure we included anyone who may have experienced any kidney complication. We reviewed ICD-9-CM codes in other articles to inform the codes used to define SLE and kidney involvement in this study (12,13,15). Additionally, a separate study by Chibnik et al validated the efficacy of ICD-9-CM codes in a Medicaid population to identify patients with lupus nephritis (30). In the study by Chibnik et al, the positive predictive value for a patient having lupus nephritis was 88%. They required greater than two 710.0 codes for SLE, greater than 2 nephrology visits, and greater than 2 renal ICD-9-CM codes. Our methods were different in that we were unable to require 2 separate encounters, since the KID data are shown by discharge rather than by patient. Also, the sensitivity and specificity for the pediatric population in the present study, which includes all payers, may be slightly different from the predominantly adult Medicaid population in the study by Chibnik et al.
It is notable that ICD-9-CM codes do not distinguish between neonatal lupus and SLE. This is complicated by the fact that SLE can present as early as infancy. Zulian et al summarized the clinical courses of 13 patients with infantile SLE presenting between ages 6 and 11 months (31). An examination of the age distribution of patients in our cohort demonstrated 57 discharges for patients ages <3 years who were at risk for misclassification. When these discharges were removed from the analysis, no significant change in charges was found. Given that SLE can present in infancy, the total number of discharges at age <3 years was so small and their impact on charges so insignificant that all pediatric ages were included in the analysis.
Other limitations of this data set include the fact that it is based on the number of discharges, not the number of patients. As a result, we cannot make conclusions on medical care charges per capita. Finally, this database is limited to an analysis of inpatient charges and is not inclusive of outpatient or emergency costs. Consequently, our analysis reflects only a portion of the full expense of managing patients with SLE.
This study represents a step toward a better understanding of in-hospital health care utilization by children with SLE and SLE-associated kidney disease. Our data demonstrated that hospitalization of SLE patients with kidney involvement, and more specifically kidney failure, resulted in significant health care expenditures and higher charges. A more detailed examination of resource utilization for SLE patients with kidney failure in addition to an assessment of outpatient expenditures for all aspects of SLE would complement this study and provide a more complete description of SLE-related health care utilization and charges.
Significance & Innovations.
In systemic lupus erythematosus (SLE), kidney involvement can cause significant morbidity. Adult studies have demonstrated that care for SLE patients with kidney involvement results in significantly greater expenditures than care for those without kidney disease.
This study is one of the first large-scale analyses of pediatric health care utilization for children in the US with SLE and kidney involvement.
In this article, we demonstrate that kidney involvement is present in 57% of children hospitalized with SLE. Charges for hospitalizations with SLE-associated kidney failure are 2–3 times greater than for SLE hospitalizations without kidney failure.
Improved understanding of charges can help lead to more efficient utilization of health care resources for children with SLE.
Footnotes
AUTHOR CONTRIBUTIONS
All authors were involved in drafting the article or revising it critically for important intellectual content, and all authors approved the final version to be published. Dr. Tanzer had full access to all of the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis.
Study conception and design. Tanzer, Tran, Messer, Herreshoff, Wickman, Gipson.
Acquisition of data. Messer, Kroeker, Harkness.
Analysis and interpretation of data. Tanzer, Tran, Messer, Wickman, Song, Gipson.
REFERENCES
- 1.Petty RE, Laxer RM. Systemic lupus erythematosus. In: Cassidy JT, Petty RE, Laxer RM, Lindsley CB, editors. Textbook of pediatric rheumatology. 5th ed. Philadelphia: Elsevier Saunders; 2005. pp. 342–385. [Google Scholar]
- 2.Hagelberg S, Lee Y, Bargman J, Mah G, Schneider R, Laskin C, et al. Longterm followup of childhood lupus nephritis. J Rheumatol. 2002;29:2635–2642. [PubMed] [Google Scholar]
- 3.Yang LY, Chen WP, Lin CY. Lupus nephritis in children: a review of 167 patients. Pediatrics. 1994;94:335–340. [PubMed] [Google Scholar]
- 4.White PH. Pediatric systemic lupus erythematosus and neonatal lupus. Rheum Dis Clin North Am. 1994;20:119–127. [PubMed] [Google Scholar]
- 5.Baqi N, Moazami S, Singh A, Ahmad H, Balachandra S, Tejani A. Lupus nephritis in children: a longitudinal study of prognostic factors and therapy. J Am Soc Nephrol. 1996;7:924–929. doi: 10.1681/ASN.V76924. [DOI] [PubMed] [Google Scholar]
- 6.Papadimitraki ED, Isenberg DA. Childhood- and adult-onset lupus: an update of similarities and differences. Expert Rev Clin Immunol. 2009;5:391–403. doi: 10.1586/eci.09.29. [DOI] [PubMed] [Google Scholar]
- 7.Cameron JS. Lupus nephritis in childhood and adolescence. Pediatr Nephrol. 1994;8:230–249. doi: 10.1007/BF00865490. [DOI] [PubMed] [Google Scholar]
- 8.Gibson KL, Gipson DS, Massengill SA, Dooley MA, Primack WA, Ferris MA, et al. Predictors of relapse and end stage kidney disease in proliferative lupus nephritis: focus on children, adolescents, and young adults. Clin J Am Soc Nephrol. 2009;4:1962–1967. doi: 10.2215/CJN.00490109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Tucker LB, Uribe AG, Fernandez M, Vila LM, McGwin G, Apte M, et al. Adolescent onset of lupus results in more aggressive disease and worse outcomes: results of a nested matched case-control study within LUMINA, a multiethnic US cohort (LUMINA LVII) Lupus. 2008;17:314–322. doi: 10.1177/0961203307087875. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Hiraki LT, Benseler SM, Tyrrell PN, Hebert D, Harvey E, Silverman ED. Clinical and laboratory characteristics and long-term outcome of pediatric systemic lupus erythematosus: a longitudinal study. J Pediatr. 2008;152:550–556. doi: 10.1016/j.jpeds.2007.09.019. [DOI] [PubMed] [Google Scholar]
- 11.Zhu TY, Tam LS, Lee VW, Lee KK, Li EK. The impact of flare on disease costs of patients with systemic lupus erythematosus. Arthritis Rheum. 2009;61:1159–1167. doi: 10.1002/art.24725. [DOI] [PubMed] [Google Scholar]
- 12.Carls G, Li T, Panopalis P, Wang S, Mell AG, Gibson TB, et al. Direct and indirect costs to employers of patients with systemic lupus erythematosus with and without nephritis. J Occup Environ Med. 2009;51:66–79. doi: 10.1097/JOM.0b013e31818a405a. [DOI] [PubMed] [Google Scholar]
- 13.Li T, Carls GS, Panopalis P, Wang S, Gibson TB, Goetzel RZ. Long-term medical costs and resource utilization in systemic lupus erythematosus and lupus nephritis: a five-year analysis of a large Medicaid population. Arthritis Rheum. 2009;61:755–763. doi: 10.1002/art.24545. [DOI] [PubMed] [Google Scholar]
- 14.Sutcliffe N, Clarke AE, Taylor R, Frost C, Isenberg DA. Total costs and predictors of costs in patients with systemic lupus erythematosus. Rheumatology (Oxford) 2001;40:37–47. doi: 10.1093/rheumatology/40.1.37. [DOI] [PubMed] [Google Scholar]
- 15.Pelletier EM, Ogale S, Yu E, Brunetta P, Garg J. Economic outcomes in patients diagnosed with systemic lupus erythematosus with versus without nephritis: results from an analysis of data from a US claims database. Clin Ther. 2009;31:2653–2664. doi: 10.1016/j.clinthera.2009.11.032. [DOI] [PubMed] [Google Scholar]
- 16.Clarke AE, Panopalis P, Petri M, Manzi S, Isenberg DA, Gordon C, et al. SLE patients with renal damage incur higher health care costs. Rheumatology (Oxford) 2008;47:329–333. doi: 10.1093/rheumatology/kem373. [DOI] [PubMed] [Google Scholar]
- 17.Brunner HI, Sherrard TM, Klein-Gitelman MS. Cost of treatment of childhood-onset systemic lupus erythematosus. Arthritis Rheum. 2006;55:184–188. doi: 10.1002/art.21845. [DOI] [PubMed] [Google Scholar]
- 18.Healthcare Cost and Utilization Project (HCUP) Overview of the Kids’ Inpatient Database (KID) URL: www.hcup-us.ahrq.gov/kidoverview.jsp.
- 19.United States Department of Labor, Bureau of Labor Statistics. Consumer Price Index. URL: http://www.bls.gov/cpi/
- 20.Chertow GM, Burdick E, Honour M, Bonventre JV, Bates DW. Acute kidney injury, mortality, length of stay, and costs in hospitalized patients. J Am Soc Nephrol. 2005;16:3365–3370. doi: 10.1681/ASN.2004090740. [DOI] [PubMed] [Google Scholar]
- 21.Schneider J, Khemani R, Grushkin C, Bart R. Serum creatinine as stratified in the RIFLE score for acute kidney injury is associated with mortality and length of stay for children in the pediatric intensive care unit. Crit Care Med. 2010;38:933–939. doi: 10.1097/CCM.0b013e3181cd12e1. [DOI] [PubMed] [Google Scholar]
- 22.Kamphuis S, Silverman ED. Prevalence and burden of pediatric-onset systemic lupus erythematosus. Nat Rev Rheumatol. 2010;6:538–546. doi: 10.1038/nrrheum.2010.121. [DOI] [PubMed] [Google Scholar]
- 23.Cooper GS, Treadwell EL, St.Clair EW, Gilkeson GS, Dooley MA. Sociodemographic associations with early disease damage in patients with systemic lupus erythematosus. Arthritis Rheum. 2007;57:993–999. doi: 10.1002/art.22894. [DOI] [PubMed] [Google Scholar]
- 24.Ward MM. Medical insurance, socioeconomic status, and age of onset of endstage renal disease in patients with lupus nephritis. J Rheumatol. 2007;34:2024–2027. [PubMed] [Google Scholar]
- 25.Lacaille D, Clarke AE, Bloch DA, Danoff D, Esdaile JM. The impact of disease activity, treatment and disease severity on short-term costs of systemic lupus erythematosus. J Rheumatol. 1994;21:448–453. [PubMed] [Google Scholar]
- 26.Petri M, Perez-Gutthann S, Longenecker JC, Hochberg M. Morbidity of systemic lupus erythematosus: role of race and socioeconomic status. Am J Med. 1991;91:345–353. doi: 10.1016/0002-9343(91)90151-m. [DOI] [PubMed] [Google Scholar]
- 27.Mechanic R, Coleman K, Dobson A. Teaching hospital costs: implications for academic missions in a competitive market. JAMA. 1998;280:1015–1019. doi: 10.1001/jama.280.11.1015. [DOI] [PubMed] [Google Scholar]
- 28.Foley JK, Mulhausen RO. The cost of complexity: the teaching hospital. Hosp Health Serv Adm. 1986;31:96–109. [PubMed] [Google Scholar]
- 29.Descloux E, Durieu I, Cochat P, Vital-Durand D, Ninet J, Fabien N, et al. Influence of age at disease onset in the outcome of paediatric systemic lupus erythematosus. Rheumatology (Oxford) 2009;48:779–784. doi: 10.1093/rheumatology/kep067. [DOI] [PubMed] [Google Scholar]
- 30.Chibnik LB, Massarotti EM, Costenbader KH. Identification and validation of lupus nephritis cases using administrative data. Lupus. 2010;19:741–743. doi: 10.1177/0961203309356289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Zulian F, Pluchinotta F, Martini G, Da Dalt L, Zacchello G. Severe clinical course of systemic lupus erythematosus in the first year of life. Lupus. 2008;17:780–786. doi: 10.1177/0961203308090992. [DOI] [PubMed] [Google Scholar]