Abstract
Serotonin plays a significant role in the development of carcinoid heart disease, which primarily leads to fibrosis and contraction of right-sided heart valves. Recently, strong evidence has emerged that the use of specific drug classes such as ergot alkaloids (for migraine headaches), 5-hydroxytryptamine (5-HT or serotonin) uptake regulators/inhibitors (for weight reduction), and ergot-derived dopamine agonists (for Parkinson’s disease) can result in left-sided heart valve damage that resembles carcinoid heart disease. Recent studies suggest that both right- and left-sided drug-induced heart valve disease involves increased serotoninergic activity and in particular activation of the 5-HT receptors, including the 5-HT2B receptor subtype, which mediate many of the central and peripheral functions of serotonin. G-proteins that inhibit adenylate cyclase activity mediate the activity of the 5-HT2B receptor subunit which is widely expressed in a variety of tissues including liver, lung, heart, and coronary and pulmonary arteries; and it has also been reported in embryonic mouse heart, particularly on mouse heart valve leaflets. In this review we discuss the salient features of serotoninergic manifestations of both carcinoid heart disease and drug-induced cardiac valvulopathy with an emphasis on echocardiographic diagnosis.
Keywords: Carcinoid heart disease, Serotonin, Echocardiography
I. Carcinoid tumors and serotonin
Carcinoid tumors are relatively rare neuroendocrine malignancies with an incidence of 1/100,000 in the US. (1) First identified over 100 years ago as multiple tumors in the distal ileum of two patients at necropsy, carcinoid tumors are now known to behave in a more indolent fashion than typical adenocarcinomas. (2) Carcinoid tumors most commonly originate from enterochromaffin cells in the gastrointestinal tract. Enterochromaffin cells produce and contain about 90% of the serotonin stores and control intestinal motility, secretion, and absorption. The tumors typically grow slowly over many years, and commonly cause no symptoms until they become sufficiently large and/or metastasize. The typical non-cardiac manifestations of carcinoid disease include pulmonary and/or retroperitoneal fibrosis. Normally serotonin travels via the portal circulation to the liver where it is metabolized via monoamine oxidase and aldehyde dehydrogenase to 5-hydroxyindolacetic acid (5-HIAA), which is excreted into the urine. Carcinoid syndrome develops when the tumor cells metastasize to the liver and vasoactive substances including serotonin, histamine, and bradykinin are released into the systemic circulation via the venous system, causing the characteristic symptoms of flushing, wheezing, and diarrhea. The most accurate test for the diagnosis of carcinoid syndrome is measurement of 24-hour urinary 5-HIAA excretion, which has a sensitivity of 75% and a specificity of almost 100%.
II. Carcinoid heart disease
Systemic exposure to serotonin may lead to carcinoid heart disease. Carcinoid heart disease was initially recognized in 1954, and more recently the clinicopathologic findings were described in seminal work by Dr. William C. Roberts and colleagues. (3, 4) The characteristic plaque-like carcinoid deposits consist of fibrous tissue composed of smooth muscle cells and myofibrils within an extracellular matrix; collagen fibers and endothelial cells are abundant. (4) One of the classic studies described nine patients with carcinoid heart disease; all nine exhibited fibrous plaques on the tricuspid and pulmonic valves. Involvement of the left side of the heart occurred in three patients, and in none of these individuals was an intracardiac shunt found. In general the deposits of carcinoid heart disease occur nearly entirely on the ventricular aspects of the septal and posterior tricuspid leaflets and on the pulmonary arterial side of the pulmonic valve cusps. On the anterior tricuspid valve leaflet, the deposits can be present on both sides. (4) The consequences of carcinoid plaque deposition results in adherence and constriction of the leaflets to the underlying endocardium or to the underlying pulmonary arterial endothelium, resulting in a smaller than normal annulus of both right-sided cardiac valves and simultaneously diminished leaflet mobility. The hemodynamic consequences are usually the result from both tricuspid regurgitation and pulmonic stenosis. Although tricuspid regurgitation is the predominant lesion, some degree of tricuspid stenosis is also present; the predominant pulmonic valve lesion is pulmonic stenosis, although pulmonic regurgitation is frequently present. The combination of tricuspid regurgitation and pulmonic stenosis results in significant right ventricular pressure/volume overload and subsequently in right heart failure. (5, 6)
Several case-based studies have reported that carcinoid heart disease occurs in up to 66% of patients with carcinoid syndrome, and most often afflict middle-aged individuals. (7, 8) Since monoamine oxidase and serotonin transporter proteins in the pulmonary vascular bed degrade serotonin, left-sided heart valves are usually spared in carcinoid heart disease. Left-sided valvular pathology occurs in only 7 percent of patients with cardiac involvement, often in association with an atrial right-to-left shunt (such as a patent foramen ovale, which some authors believe should be assessed as a marker for carcinoid heart disease progression) or a primary bronchial carcinoid. (9). These abnormalities permit serotonin-rich blood to enter the left heart chambers without passing through the pulmonary capillaries where serotonin can be deactivated and degraded. Left-sided valve disease can also occur in patients with severe, poorly controlled carcinoid syndrome who have very high levels of circulating serotonin. (8)
Seminal work published from the Mayo Clinic described the echocardiographic features of 74 patients with carcinoid heart disease. (10) The tricuspid valve was mostly involved, with 97% of patients exhibiting shortened, thickened tricuspid leaflets. Tricuspid regurgitation was present in all 69 patients who underwent Doppler examination and was of moderate or severe degree in 90%. Severe tricuspid regurgitation in carcinoid heart disease is classically characterized by a dagger-shaped Doppler spectral profile with an early peak pressure followed by a rapid decline, indicative of markedly elevated right atrial pressures. The tricuspid valve inflow pressure half-time may be prolonged, indicative of associated tricuspid stenosis. The pulmonic valve appeared thickened, retracted, and immobile in 36 patients (49%). Among the 47 patients who underwent Doppler evaluation of the pulmonic valve, regurgitation was present in 81% and stenosis was present in 53%. Left-sided valvular involvement was present only in five patients (7%), four of whom had a patent foramen ovale or carcinoid tumor involving the lung. Typical echocardiographic images of carcinoid heart disease are shown on the figure and on the cine-loops (online supplement).
Figure.
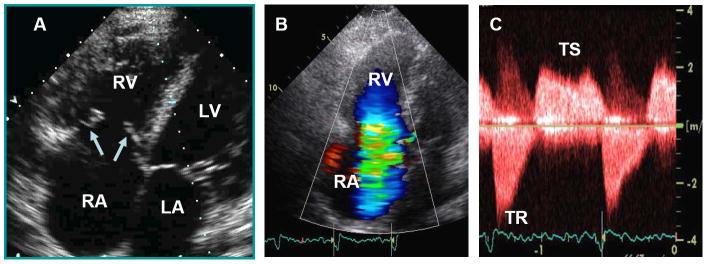
Characteristic echocardiographic images from a patient with carcinoid heart disease. Panel A. Apical 4-chamber view showing marked right atrial (RA) and right ventricular (RV) enlargement. The tricuspid leaflets (arrows) are thickened, retracted and fixed leading to both tricuspid regurgitation (TR) and tricuspid stenosis (TS). Panel B. Apical 4-chamber view showing severe TR by color flow Doppler. Panel C. Continuous-wave Doppler showing both TS and TR. On the TR jet notice the classic dagger-shaped Doppler spectral profile with an early peak pressure followed by a rapid decline, indicative of markedly increased RA pressure.
III. Drug-induced cardiac valvulopathy
A. Ergot alkaloids
Ergot alkaloids are a class of chemicals with vasoconstrictive properties derived from Claviceps purpurea, a type of mold that infects wheat and rye. Methysergide is a synthetically derived ergot alkaloid introduced clinically in the 1960s for the treatment of migraines. Serotonin and methysergide have similar chemical compositions. The first review of methysergide-induced valvular heart disease was published in 1967, describing 27 cases of retroperitoneal fibrosis, with 4 developing significant cardiac murmurs during treatment. (11) One patient developed significant aortic regurgitation after 6 months of methysergide treatment and at cardiac surgery was found to have a thick, fibrotic ring involving the root of the aorta with thickened aortic valve leaflets. Another patient without history of heart disease developed heart failure and clinical findings consistent with mitral stenosis, aortic stenosis, and aortic regurgitation within 3 years of methysergide use. A separate group reported a case of a young woman who developed severe mitral regurgitation after treatment with methysergide for 4 years. At the time of mitral valve replacement the chordae tendineae were fused and 3 large masses were noted on the mitral valve; the valve leaflets were thickened and shiny. (12)
An early echocardiographic case-report of methysergide induced valvulopathy involved a case of a 51 year old woman that presented with dyspnea on exertion, paroxysmal nocturnal dyspnea, and orthopnea after taking methysergide for 19 months. (13) Transthoracic echocardiography revealed markedly thickened mitral valve leaflets with severe wide open mitral regurgitation; the aortic valve leaflets and proximal aortic root were also thickened with moderate to severe aortic insufficiency. There was reverse doming of the aortic valve leaflets at end systole. The tricuspid valve also appeared thickened, with moderate tricuspid regurgitation and severe pulmonary hypertension (pulmonary artery systolic pressure ~70mmHg). Left and right ventricular size and function were normal. The patient underwent aortic valve and mitral valve repair, and pathologic examination of the resected posterior mitral valve leaflet revealed gross thickening, restriction, and a thick fibrotic layer covering the leaflet but without leaflet destruction. Multiple additional case reports suggest a link between prolonged methysergide exposure and development of cardiac valvulopathy. The gross examination and histological findings from these patients reveal striking similarities to carcinoid heart disease, except that these findings predominantly involve left-sided heart valves (Table 1, refs 11, 12, 14, 15).
Table 1.
Case reports of Methysergide induced valvulopathy
| Methysergide Duration (study author, ref #) | Valvulopathy | Histopathology |
|---|---|---|
| 6 months (Graham, ref #11) | Aortic regurgitation | Fibrotic ring at root of aortic valve, thickened aortic valve leaflets |
| 3 years (Graham, ref #11) | Mitral stenosis, aortic stenosis and regurgitation | Aortic and mitral valve thickening with an orderly distribution of fibrous tissue |
| 4 years (Munroe, ref #12) | Mitral regurgitation | Fused chordae tendineae, masses on mitral valve, thick and shiny leaflets |
| 3 years (Mason, ref #14) | Tricuspid regurgitation | Fibrotic thickening of the tricuspid valve |
| 4 years (Misch, ref #15) | Mitral regurgitation | Glistening white plaque on chordae tendineae with thickening on the surface of leaflets. Microscopy with cellular fibrous tissue covering mitral valve leaflets |
Ergotamine, an ergot alkaloid, is structurally similar to methysergide and has also been used for the treatment of migraines. A 1992 report described 5 cases of valvular heart disease associated with ergotamine use. (16) Echocardiography revealed thickening and diastolic doming of the anterior mitral leaflet with thickening and immobility of the posterior leaflet. Subvalvular involvement was characterized by thickening and shortening of the chordae tendineae. Involvement of the aortic valves was characterized by thickening and retraction of the leaflets. Microscopically the lesions were characterized by irregular proliferation of myofibroblasts within an avascular myxoid or collagenous matrix that encased the leaflets and chordal structures but with little disruption of the valve. Thus, ergotamine use has also been implicated in cardiac valvulopathy, exhibiting pathologic and echocardiographic findings that are similar to those caused by use of methysergide (Table 2, refs 17-19).
Table 2.
Cases of Ergotamine induced valvulopathy
| Ergotamine Duration,(study author, ref #) | Valvulopathy | Echocardiographic or pathological findings |
|---|---|---|
| 20 years (Austin, ref #17) | Mitral regurgitation and stenosis | Thickened mitral valve with restricted mobility |
| 18 months (Flaherty, ref #18) | Mitral regurgitation, aortic insufficiency | Thickened mitral valve |
| 5 years (Wilke, ref #19) | Mitral regurgitation, tricuspid stenosis | Aortic valve thickening, tricuspid valve commissural fusion, mitral valve leaflet thickening |
B. Phentermine, Fenfluramine, and Dexfenfluramine
Appetite suppression medications such as phentermine and fenfluramine have been prescribed for the treatment of obesity. Phentermine, approved for use in the US in 1959, is a monoamine oxidase inhibitor that inhibits serotonin degradation within vascular cells. Fenfluramine, approved in 1973, is a serotonin releaser and re-uptake inhibitor. Both medications induce a centrally-mediated anorectic effect that lead to weight loss; both drugs were intended for shortterm use (i.e., less than 3 months). In 1996, dexfenfluramine, the dextro-isomer of fenfluramine, was approved in the US for the long-term treatment of obesity.
A couple of seminal publications in the 1990s linked these drugs to both pulmonary hypertension and left-sided heart valve disease. (20-21) Left-side heart valve disease was described in 24 women without a history of cardiac disease who had been treated with the combination of fenfluramine and phentermine (average doses: fenfluramine 60 mg/day and phentermine 30 mg/day; mean duration of treatment 11 months) and who presented with cardiovascular symptoms and/or a new heart murmur. (20) Aortic and/or mitral regurgitation was present in all 24, and 5 (28%) required valve replacement. On pathologic examination the valves exhibited a glistening white appearance. Histopathologic findings included plaque-like encasement of the leaflets and chordal structures with intact valve architecture, characteristic of those seen in carcinoid heart disease.
On July 8, 1997, the Food and Drug Administration (FDA) issued a public health advisory after a report that 33 women who had taken combination fenfluramine-phentermine (treatment duration: 1-28 months) had unusual heart-valve morphology and regurgitation, which was characterized by multivalvular disease involving the mitral, aortic, and tricuspid valves. (22) Dexfenfluramine and fenfluramine were subsequently withdrawn from the market, although phentermine was not since it had not been directly implicated as a culprit, but most likely acted synergistically with fenfluramine to enhance serotonergic activity.
Subsequent studies have reported the overall risk and prevalence of heart valve regurgitation in the general population and that associated with the use of these anorectic medications. (Table 3, refs 23-28) The FDA criterion for significant drug-induced heart valve regurgitation is aortic regurgitation severity mild or greater and/or mitral regurgitation severity moderate or greater. (29) According to the FDA criteria, the overall prevalence of significant heart valve regurgitation in the general population is 4.8% for aortic insufficiency and 1.6% for mitral regurgitation. (23) An echocardiographic study of 1072 overweight patients performed within one month after discontinuation of drug treatments showed that those who had been randomly assigned to receive dexfenfluramine (n=366), sustained-release dexfenfluramine (n=352), or placebo (n=354) for an average duration of 72 days found no statistically significant differences among the groups when using the above-described FDA criteria for heart valve regurgitation. (24)
Table 3.
Prevalence of FDA grade regurgitation with anorectic drug use.
| Study Author (ref #) | Medication | Number of patients | Treatment duration (mean) | FDA-grade AR Prevalence | FDA-grade MR Prevalence | ||
|---|---|---|---|---|---|---|---|
|
| |||||||
| Cases | Controls | Cases | Controls | ||||
| Weissman, ref #24 | Dexfenfluramine | 342 | 72 Days | 5.0% | 3.6% | 1.7% | 1.2% |
| Dexfenfluramine | 329 | 71 Days | 5.8% | 3.6% | 1.8% | 1.2% | |
| Combined Dex. groups | 671 | 72 Days | 5.4% | 3.6% | 1.8% | 1.2% | |
| Burger, ref #27 | Fenfluramine-phentermine | 226 | 13 months | 6.6% | 4.9%** | 1.3% | 1.6%** |
| Shivley, ref # 26 | Dexfenfluramine | 223 | 7 months | 6.3% * | 1.6% | 1.3% | 0.5% |
| Gardin, ref #28 | Dexfenfluramine | 479 | 6 months | 8.9% * | 4.1% | 4.9% | 3.2% |
| Fenfluramine-phentermine | 455 | 12 months | 13.7% * | 4.1% | 5.1% | 3.2% | |
AR, aortic regurgitation; MR, mitral regurgitation.
Statistically significant prevalence (vs normal controls).
Framingham Heart Study Prevalence (controls not used in this study)
In summary, the prevalence of significant phentermine, fenfluramine, and dexfenfluramine drug-induced left-sided heart valve regurgitation ranges from 1.3% to 5.1% for mitral valves and from 5.0% to 13.7% for aortic valves (Table 3). Most studies suggest an increased risk of valvular regurgitation with the use of combination fenfluramine-dexfenfluramine, or with either medication used in combination with phentermine.
C. Pergolide and Cabergoline
Pergolide and cabergoline are ergot-derived dopamine agonists used in the treatment of Parkinson’s disease and restless leg syndrome. Pergolide has agonistic activity at dopaminergic receptors (i.e., D1 and D2 receptors) and stimulates postsynaptic dopamine receptors in the nigrostriatal pathway, temporizing some of the motor disorders commonly associated with this disease. Cabergoline has higher affinity for the D2 receptor and is FDA-approved for the treatment of hyperprolactinemia. An association between pergolide use and heart valve disease was first described in 2002 in 3 patients. (30) By echocardiography, severe tricuspid valve regurgitation was seen in all three individuals and significant left-sided valve regurgitation was noted in 2 of the patients. Histology of the explanted valves revealed fibroproliferative lesions with preserved underlying valve architecture, suggestive of carcinoid heart disease. A subsequent report described 4 cases of heart valve disease in patients taking either pergolide or cabergoline for Parkinson’s disease. Echocardiography showed aortic, mitral, and tricuspid regurgitation with other echocardiographic features similar to those seen in carcinoid heart disease; histology showed myofibroblast proliferation. (31)
Several subsequent publications raised significant concern in regards to pergolide and cabergoline causing valvulopathy localized to the mitral and aortic valves (Table 4, refs 32-34). Shortly after the publication of these reports, pergolide was voluntarily withdrawn from the US market. Cabergoline is still available in the US, but its use is limited by the FDA only for hyperprolactinemia, and the recommended dose is less than that used to treat Parkinson’s disease.
Table 4.
Summary of studies investigating pergolide and cabergoline induced valvulopathy.
| Medication (study author, ref #) | Average Duration of Use | Significant Echocardiographic Findings | Significant Results |
|---|---|---|---|
| Pergolide (Van Camp, ref #32) | 18 months | Restrictive disease of the mitral valve | Restrictive valvular heart disease (any type) present in 33% of the Pergolide group and in none of the controls (p=0.003). A significant correlation noted between cumulative doses of Pergolide and tenting areas of the mitral valves (ρ=0.41, p=0.02). |
| Pergolide and Cabergoline (Schade, ref #33) | Greater than 6 months | Mitral and aortic regurgitation | Risk of cardiac valve regurgitation was significantly increased with current use of Pergolide (incidence-rate ratio [IRR] 7.1, 95% CI 2.3-22.3) and Cabergoline (IRR 4.9, 95% CI 1.5-15.6) but not with other dopamine agonists. |
| Pergolide and Cabergoline (Zanettini, ref #34) | 63 months for pergolide, 24 months for cabergoline | Mitral and aortic regurgitation | Relative risk of clinically significant valvular disease for Pergolide was 6% for MR (p = 0.008), 4% for AR (p= 0.01). For Cabergoline, the relative risk was 5% for MR (p = 0.09), 7% for AR (p<0.001). Mean composite score for patients taking cabergoline or pergolide who had no leaflet thickening was significantly higher than the score in the control group (4.4±1.5 vs. 3.27±2.02, p<0.001). |
AR, aortic regurgitation; MR, mitral regurgitation.
IV. Potential mechanisms of serotonin-induced heart valve disease
A. Serotonin and cardiac 5-HT2B receptors
As previously discussed, serotonin and serotoninergic drugs play an important role in the development of right- and left-sided drug-induced heart valve disease. Recent studies have shown that the human heart possesses several receptors for serotonin (i.e., 5HT1A, 5HT1B, 5HT2B, 5HT3, and 5HT4). (35) It is not entirely clear why drug-induced valvular heart disease tends to predominantly affect left-sided heart valves. While it appears that drug-induced valvular disease is more prominent in left-sided valves with phentermine-fenfluramine, methysergide, or ergotamine use, right-sided involvement appears more common in patients who have taken cabergoline or pergolide. (32-34) Two separate studies have shown that the metabolites of fenfluramine, ergotamine, and methysergide have high affinity for the 5HT2B receptor and that pergolide and cabergoline also have agonistic activity at the same receptor. In addition the 5HT-2B receptors are located on all four heart valves. (35-38) Activation of the 5HT2B receptor is a G-protein mediated process. Once the receptor is activated it leads to dissociation of the G-protein, whose subunits can then activate phospholipase C-β, phosphokinase-C, steroid receptor co-activator, and extracellular regulated kinases, a process that engages mitogenic pathways. G-protein activation may also enhance the activity of transforming growth factor-β (TGFB), augmenting 5HT2B-stimulated mitogenesis. (39) Up-regulation of TGFB has been shown to lead to increased extracellular matrix, including collagen and glycosaminoglycans in human aortic valve interstitial cells, potentially playing a significant role in increasing fibrosis and subsequent valvulopathy. (40) The final common pathway for mitogenesis via 5HT2B stimulation probably involves the phosphorylation of the retinoblastoma protein. Excessive cell division and proliferation leads to an overgrowth valvulopathy and subsequent dysfunction as normally quiescent cells become activated. (39)
B. 5HT2B receptor-induced heart valve disease in animal models
Inactivation of the serotonin transporter protein (5HTT) has been proposed as one mechanism for heart valve disease. 5HTT is a key protein responsible for serotonin uptake and subsequent inactivation in the lungs and thus in normal concentrations this protein limits systemic availability of serotonin. 5HTT-deficient mice develop structural cardiac abnormalities including marked interstitial, perivascular, and valvular fibrosis, thus establishing a link between 5HTT deficiency, serotonin and heart valve fibrosis. (41) Rats exposed to long-term serotonin administration (injected once a day for 3 months) exhibit a 10-fold increase in plasma serotonin levels and a statistically significant weight loss compared to controls, consistent with the known anorectic effects of serotonin. Furthermore, six of 10 rats exhibited pulmonic and/or aortic insufficiency by echocardiography, and a significant increase in heart weight compared to controls (p<0.02). (42) Histopathologic examination revealed shortened and thickened aortic cusps and carcinoidlike plaques.
In another study, rats were given daily injections of pergolide, serotonin, or placebo for 5 months. (43) At 20 weeks, echocardiography demonstrated significant aortic and/or mitral regurgitation in serotonin-treated rats in 86% and 57%, respectively, and 67% aortic and mitral regurgitation for pergolide-treated rats, compared to none in placebo control group. Histologic examination revealed diffusely thickened and myxoid aortic, mitral, and tricuspid valves in both pergolide- and serotonin-treated animals, but not in the controls.
V. How about selective serotonin re-uptake inhibitors (SSRIs)?
Since serotonin release and serotonin reuptake inhibitors are involved in heart valve disease, concerns have been raised regarding the use of selective serotonin re-uptake inhibitors (SSRI) such as fluoxetine, sertraline, paroxetine, and similar drugs used for the treatment of depression as potentially causing heart valve disease. One study examined the association between valvular regurgitation and treatment with SSRI’s in 292 patients and compared these to 5,145 consecutively hospitalized patients as controls. (44) The unadjusted prevalence of left-sided and/or overall heart valve regurgitation was slightly less common among SSRI-treated patients than controls, thus arguing for a lack of an association between SSRI’s and heart valve disease. These data are in agreement with the current thinking that serotonergic activity alone is probably not sufficient to induce heart valve disease, and that activation of the 5HT2B receptor is also required. A host of medications with serotonergic activity do not activate the 5HT2B receptor, and none of these have been directly implicated with heart valve disease (Table 5).
Table 5.
Common serotonergic medications and their actions.
| Medication | Indication | Role with Serotonin |
|---|---|---|
| Trazadone Tricyclic’s | Sedation/Depression Antidepressants | Serotonin Antagonist Inhibits reuptake of serotonin |
| Lithium | Bipolar disorder | Decreases reuptake of serotonin |
| Monoamine Oxidase Inhibitors | Depression | Inhibits metabolism of serotonin |
| Mirtazipine | Depression | Blocks 5HT2 and 5HT3 receptors |
| Triptans | Migraines | 5-HT1B and 5-HT1D receptor agonist |
| Ondansetron | Antiemetic | Selective 5-HT3 Receptor Antagonist |
VI. Summary
Human and animal data provide support for a role of serotonin and other serotonergic agents to induce heart valve disease in a fashion similar to that seen in carcinoid heart disease. Although some of these agents have been taken off the US market (i.e., methysergide, dexfenfluramine, fenfluramine, and pergolide), others still remain, and clinicians should be aware of their potential toxicity. Ergotamine is rarely used in the US due to the popularity of the triptans for the treatment of migraines; it has a black box warning for risk of peripheral ischemia but interestingly not for heart valve disease. Phentermine is still available since it has not been directly implicated in causing heart valve disease, likely because it does not activate the 5HT2B receptor. Cabergoline is available in the United States only for the treatment of hyperprolactinemia and the recommended dosage used is less than that used for treatment of Parkinson’s disease. Although no consensus recommendations exist regarding the use of these medications, it appears reasonable to recommend that medications that activate the 5HT2B receptor should be avoided when possible, and that patients taking serotoninergic medications are at risk of developing heart valve disease and thus routine evaluation with echocardiography may be reasonable.
Supplementary Material
Acknowledgments
Supported by grants from the Barnes-Jewish Hospital Foundation to the Cardiovascular Imaging and Clinical Research Core Laboratory.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Modlin IM, Sandor A. An analysis of 8305 cases of carcinoid tumors. Cancer. 1997;79:813–829. doi: 10.1002/(sici)1097-0142(19970215)79:4<813::aid-cncr19>3.0.co;2-2. [DOI] [PubMed] [Google Scholar]
- 2.Kulke MH, Meyer RJ. Carcinoid Tumors. N Engl J Med. 1999;340:858–68. doi: 10.1056/NEJM199903183401107. [DOI] [PubMed] [Google Scholar]
- 3.Thorson A, Biorck G, Bjorkman G, Waldenstrom J. Malignant carcinoid of the small intestine with metastases to the liver, valvular disease of the right side of the heart (pulmonary stenosis and tricuspid regurgitation without septal defects), peripheral vasomotor symptoms, bronchoconstriction, and an unusual type of cyanosis: a clinical and pathologic syndrome. Am Heart J. 1954;47:795–817. doi: 10.1016/0002-8703(54)90152-0. [DOI] [PubMed] [Google Scholar]
- 4.Roberts WC, Sjoerdsma A. The Cardiac Disease Associated with the Carcinoid Syndrome (Carcinoid Heart Disease) Am J Med. 1964;36:5–34. doi: 10.1016/0002-9343(64)90145-7. [DOI] [PubMed] [Google Scholar]
- 5.Roberts WC. A unique heart disease associated with a unique cancer: carcinoid heart disease. Am J Cardiol. 1997;80:251–256. doi: 10.1016/s0002-9149(97)00340-8. [DOI] [PubMed] [Google Scholar]
- 6.Pearlman AS. Carcinoid heart disease: echocardiographic recognition and differential diagnosis. Am Heart Hosp J. 2005;3(2):132–5. doi: 10.1111/j.1541-9215.2005.04044.x. [DOI] [PubMed] [Google Scholar]
- 7.Bhattacharyya S, Davar J, Dreyfus G, Caplin ME. Carcinoid Heart Disease. Circulation. 2007;116:2860–5. doi: 10.1161/CIRCULATIONAHA.107.701367. [DOI] [PubMed] [Google Scholar]
- 8.Lundin L, Norheim I, Landelius J, Oberg K, Theodorsson-Norheim E. Relationship of circulating vasoactive substances to ultrasound detectable cardiac abnormalities. Circulation. 1988;77:264–9. doi: 10.1161/01.cir.77.2.264. [DOI] [PubMed] [Google Scholar]
- 9.Mansencal N, Mitry E, Forissier JF, Martin F, Redheuil A, Lepère C, et al. Assessment of patent foramen ovale in carcinoid heart disease. Am Heart J. 2006 May;151(5):1129.e1–6. doi: 10.1016/j.ahj.2006.02.019. [DOI] [PubMed] [Google Scholar]
- 10.Pellikka PA, Tajik AJ, Khandheria BK, Seward JB, Callahan JA, Pitot HC, et al. Carcinoid heart disease. Clinical and echocardiographic spectrum in 74 patients. Circulation. 1993;87:1188–96. doi: 10.1161/01.cir.87.4.1188. [DOI] [PubMed] [Google Scholar]
- 11.Graham JR. Cardiac and pulmonary fibrosis during Methysergide therapy for headache. Trans Am Clin Climatol Assoc. 1967;78:79–92. [PMC free article] [PubMed] [Google Scholar]
- 12.Munroe DS, Allen P, Cox AR. Mitral regurgitation occurring during methysergide (Sansert) therapy. Can Med Assoc J. 1969;101:62–5. [PMC free article] [PubMed] [Google Scholar]
- 13.Joseph T, Tam SK, Kamat BR, Mangion JR. Successful repair of aortic and mitral incompetence induced by methylsergide maleate: confirmation by intraoperative transesophageal echocardiography. Echocardiography. 2003;3:283–7. doi: 10.1046/j.1540-8175.2003.03027.x. [DOI] [PubMed] [Google Scholar]
- 14.Mason JW, Billingham ME, Friedman JP. Methysergide-Induced heart disease: A case of multivalvular and myocardial fibrosis. Circulation. 1977;56:889–890. doi: 10.1161/01.cir.56.5.889. [DOI] [PubMed] [Google Scholar]
- 15.Misch K, et al. Development of heart valve lesions during Methysergide therapy. British Medical Journal. 1974;2:365–366. doi: 10.1136/bmj.2.5915.365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Redfield MM, Nicholson WJ, Edwards WD, Tajik AJ. Valve disease associated with ergot alkaloid use: echocardiographic and pathologic correlations. Ann Intern Med. 1992;117:50–2. doi: 10.7326/0003-4819-117-1-50. [DOI] [PubMed] [Google Scholar]
- 17.Austin SM, el-Hayek A, Comianos M, Tamulonis DJ. Mitral valve disease associated with long-term ergotamine use. Southern Med J. 1993;86:1179–81. doi: 10.1097/00007611-199310000-00024. [DOI] [PubMed] [Google Scholar]
- 18.Flaherty KR, Bates JR. Mitral regurgitation caused by chronic ergotamine use. Am Heart J. 1996;131:603–6. doi: 10.1016/s0002-8703(96)90544-x. [DOI] [PubMed] [Google Scholar]
- 19.Wilke A, Hesse H, Hufnagel G, Maisch B. Mitral, aortic and tricuspid valvular heart disease associated with ergotamine therapy for migraine. Eur Heart J. 1997;18:701. doi: 10.1093/oxfordjournals.eurheartj.a015324. [DOI] [PubMed] [Google Scholar]
- 20.Atanassoff PG, Weiss BM, Schmid ER, Tornic M. Pulmonary hypertension and dexfenfluramine. Lancet. 1992;339:436. [PubMed] [Google Scholar]
- 21.Connolly HM, Crary JL, McGoon MD, Hensrud DD, Edwards BS, Edwards WD, et al. Valvular heart disease associated with fenfluramine-phentermine. N Engl J Med. 1997;337:581–8. doi: 10.1056/NEJM199708283370901. [DOI] [PubMed] [Google Scholar]
- 22.Cardiac valvulopathy associated with exposure to fenfluramine or dexfenfluramine: U.S. Department of Health and Human Services interim public health recommendations. MMWR Morb Mortal Wkly Rep. 1997 Nov;46:1061–6. [PubMed] [Google Scholar]
- 23.Singh JP, Evans JC, Levy D, Larson MG, Freed LA, Fuller DL, et al. Prevalence and clinical determinants of mitral, tricuspid, and aortic regurgitation (the Framingham Heart Study) Am J Cardiol. 1999;83:897–902. doi: 10.1016/s0002-9149(98)01064-9. [DOI] [PubMed] [Google Scholar]
- 24.Weissman NJ, Tighe JF, Jr, Gottdiener JS, Gwynne JT. An assessment of heartvalve abnormalities in obese patients taking Dexfenfluramine, sustained-release Dexfenfluramine, or placebo. N Engl J Med. 1998;339:725–32. doi: 10.1056/NEJM199809103391103. [DOI] [PubMed] [Google Scholar]
- 25.Khan MA, Herzog CA, St Peter JV, Hartley GG, Madlon-Kay R, Dick CD, et al. The prevalence of cardiac valvular insufficiency assessed by transthoracic echocardiography in obese patients treated with appetite-suppressant drugs. N Engl J Med. 1998;339:713–8. doi: 10.1056/NEJM199809103391101. [DOI] [PubMed] [Google Scholar]
- 26.Shively BK, Roldan CA, Gill EA, Najarian T, Loar SB. Prevalence and determinants of valvulopathy in patients treated with dexfenfluramine. Circulation. 1999;100:2161–7. doi: 10.1161/01.cir.100.21.2161. [DOI] [PubMed] [Google Scholar]
- 27.Burger AJ, Sherman HB, Charlamb MJ, Kim J, Asinas LA, Flickner SR, et al. Low prevalence of valvular heart disease in 226 phentermine-fenfluramine protocol subjects prospectively followed for up to 30 months. J Am Coll Cardiol. 1999;34:1153–8. doi: 10.1016/s0735-1097(99)00321-6. [DOI] [PubMed] [Google Scholar]
- 28.Gardin JM, Schumacher D, Constantine G, Davis KD, Leung C, Reid CL. Valvular abnormalities and cardiovascular status following exposure to dexfenfluramine or phentermine/fenfluramine. JAMA. 2000;283:1703–9. doi: 10.1001/jama.283.13.1703. [DOI] [PubMed] [Google Scholar]
- 29.FDA analysis of cardiac valvular dysfunction with use of appetite suppressants. Washington, D.C: Food and Drug Administration; 1997. [Google Scholar]
- 30.Pritchett AM, Morrison JF, Edwards WD, Schaff HV, Connolly HM, Espinosa RE. Valvular heart disease induced by drugs. Mayo Clin Proc. 2002;77:1280–6. doi: 10.4065/77.12.1280. [DOI] [PubMed] [Google Scholar]
- 31.Horvath J, Fross RD, Kleiner-Fisman G, Lerch R, Stalder H, Liaudat S, et al. Severe multivalvular heart disease: A new complication of the Ergot derivative Dopamine agonists. Movement Disorders. 2004;19:656–662. doi: 10.1002/mds.20201. [DOI] [PubMed] [Google Scholar]
- 32.Van Camp G, Flamez A, Cosyns B, Weytjens C, Muyldermans L, Van Zandijcke M, et al. Treatment of Parkinson’s disease with Pergolide and relation to restrictive valvular heart disease. Lancet. 2004;363:1179–83. doi: 10.1016/S0140-6736(04)15945-X. [DOI] [PubMed] [Google Scholar]
- 33.Schade R, Andersohn F, Suissa S, Haverkamp W, Garbe E. Dopamine agonists and the risk of cardiac-valve regurgitation. N Engl J Med. 2007;356:29–38. doi: 10.1056/NEJMoa062222. [DOI] [PubMed] [Google Scholar]
- 34.Zanettini R, Antonini A, Gatto G, Gentile R, Tesei S, Pezzoli G. Valvular heart disease and the use of dopamine agonists for Parkinson’s disease. N Engl J Med. 2007;356:39–46. doi: 10.1056/NEJMoa054830. [DOI] [PubMed] [Google Scholar]
- 35.Kauman AJ, Levy FO. 5-Hydroxytryptamine receptors in the human cardiovascular system. Pharmacology and Therapeutics. 2006;111:674–706. doi: 10.1016/j.pharmthera.2005.12.004. [DOI] [PubMed] [Google Scholar]
- 36.Fitzgerald LW, Burn TC, Brown BS, Patterson JP, Corjay MH, Valentine PA, et al. Possible role of valvular serotonin 5-HT(2B) receptors in the cardiopathy associated with fenfluramine. Mol Pharmacol. 2000;57:75–81. [PubMed] [Google Scholar]
- 37.Rothman RB, Baumann MH, Savage JE, Rauser L, McBride A, Hufeisen SJ, et al. Evidence for possible involvement of 5-HT(2B) receptors in the cardiac valvulopathy associated with fenfluramine and other serotonergic medications. Circulation. 2000;102:2836–41. doi: 10.1161/01.cir.102.23.2836. [DOI] [PubMed] [Google Scholar]
- 38.Jähnichen S, Horowski R, Pertz HH. Agonism at 5-HT2B receptors is not a class effect of the ergolines. European Journal of Pharmacology. 2005;513:225–8. doi: 10.1016/j.ejphar.2005.03.010. [DOI] [PubMed] [Google Scholar]
- 39.Roth BL. Drugs and valvular heart disease. N Engl J Med. 2007;356:6–9. doi: 10.1056/NEJMp068265. [DOI] [PubMed] [Google Scholar]
- 40.Jian B, Xu J, Connolly J, Savani RC, Narula N, Liang B, et al. Serotonin-induced upregulation of transforming growth factor- β1 via G-protein signal transduction in aortic valve interstitial cells. Am J Pathol. 2002;161:2111–2121. doi: 10.1016/s0002-9440(10)64489-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Mekontso-Dessap A, Brouri F, Pascal O, Lechat P, Hanoun N, Lanfumey L, et al. Deficiency of the 5-hydroxytryptamine transporter gene leads to cardiac fibrosis and valvulopathy in mice. Circulation. 2006;113:81–89. doi: 10.1161/CIRCULATIONAHA.105.554667. [DOI] [PubMed] [Google Scholar]
- 42.Gustafsson BI, Tømmerås K, Nordrum I, Loennechen JP, Brunsvik A, Solligård E, et al. Long-term serotonin administration induces heart valve disease in rats. Circulation. 2005;111:1517–1522. doi: 10.1161/01.CIR.0000159356.42064.48. [DOI] [PubMed] [Google Scholar]
- 43.Droogmans S, Franken PR, Garbar C, Weytjens C, Cosyns B, Lahoutte T, et al. In vivo model of drug-induced valvular heart disease in rats: pergolide-induced valvular heart disease demonstrated with echocardiography and correlation with pathology. Eur Heart J. 2007;28:2156–2162. doi: 10.1093/eurheartj/ehm263. [DOI] [PubMed] [Google Scholar]
- 44.Mast ST, Gersing KR, Anstrom KJ, Krishnan KR, Califf RM, Jollis JG. Association between selective serotonin-reuptake inhibitor therapy and heart valve regurgitation. Am J Cardiol. 2001;87:89–93. doi: 10.1016/s0002-9149(01)01435-7. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


