Research with typically developing children (TD) has identified relations between language and motor domains in early development; and work with children experiencing developmental delays has reported that disruptions in one domain co-occur with disruptions in the other (see Iverson, 2010 and Hill, 2001 for reviews). One case in which this is observed is that of children with autism spectrum disorders (ASD): fine motor delays are often apparent and co-occur with the expressive language delays that are diagnostic of ASD (e.g. Stone & Yoder, 2001). The question we address here is whether this pattern is also observed in the later-born siblings of children with ASD (heightened-risk, HR). In addition to being at increased risk for an ASD diagnosis themselves (18.7% recurrence rate; Ozonoff et al., 2011), HR infants are at risk for language impairments (see Rogers, 2009). Here, we prospectively examine (1) whether fine motor delays are evident in HR infants in the first two years of life and (2) whether early fine motor skills can predict later expressive language.
Relations between Motor Development and Expressive Language Development
Among other things, producing language is a motor act (Thelen, 1991). As such, in the first few years of life, developments in the motor system relate to developments in productive language in TD children (Iverson & Thelen, 1999; see also Iverson, 2010). For instance, oral-motor control at 21 months relates to concurrent expressive language even after controlling for general cognitive skills (Alcock & Krawczyk, 2010). Similar relations have been found in atypical development. For example, children with language impairments demonstrate difficulties on a variety of motor tasks (see Hill, 2001), including tasks that tap fine motor and motor planning skills (e.g. Bishop & Edmundson, 1987).1
While the bulk of the research on motor – language links has focused on TD children and children exhibiting language impairments, relatively little attention has been devoted to children with ASD and their siblings. This is somewhat surprising in light of the fact that language difficulties are diagnostic of ASD and have been observed in HR infants without ASD.
Motor Skill and Expressive Language in ASD
ASD is a set of neurodevelopmental disorders characterized by a combination of symptoms that include impairments in social interaction and communication, as well as the presence of restricted interests and/or repetitive behaviors (American Psychiatric Association, 1994). Although not a diagnostic criterion, motor skill deficits are also widely observed among individuals with ASD (Bhat, Landa, & Galloway, 2011). Fournier and colleagues (2010) conducted a meta-analysis that included studies representing a wide range of participant ages, from toddlers to adults. Results revealed that individuals with ASD exhibited difficulties with motor coordination, upper- and lower-limb movements, and postural stability across all ages and levels of functioning. Of particular relevance to the present study, fine motor skill is one area in which delays have been reported and studies using both retrospective and prospective methods suggest that these delays emerge in infancy (Gernsbacher, Sauer, Geye, Schweigert, & Goldsmith, 2008; Landa & Garrett-Mayer, 2006; Lloyd, MacDonald, & Lord, 2011).
While communication impairments diagnostic of ASD may manifest as deficits in social communication, language, and/or pragmatic uses of language, expressive language delay seems to be prominent very early in development. For instance, Charman and colleagues (2005) reported that 85% of the children with ASD in their sample produced fewer than 5 words at age 2. This number decreased with age to 50% by age 3, and to 15% by age 7. Thus, many children with ASD exhibit growth in word production. However, when comparing their skills to same-aged TD children, difficulties are still observed through grade-school ages (Luyster, Qiu, Lopez, & Lord, 2007).
There is some evidence for relations between fine motor skills and later language skills in children with ASD. For instance, Stone and Yoder (2001) found that manual-motor skill at age 2 was the best predictor of expressive language skills at age 4. These relations extend into the school years. In a retrospective study with children ranging in age from pre-school to adolescence, Gernsbacher and colleagues (2008) found that children’s fine motor skills in the first two years predicted current-day speech fluency. Thus, consistent with findings from TD children and children with expressive language delay, there is some indication that the two domains are related in children with ASD.
Motor Skill and Expressive Language Development in HR infants
In this research we examine the case of later-born infant siblings of children with ASD (HR infants). While some HR infants exhibit pronounced delays, including language delays, others are indistinguishable from infants with no family history of ASD (Landa & Garrett-Mayer, 2006; Toth, Dawson, Meltzoff, Greenson, & Fein, 2007). Despite this variability, little research exists on within-child factors that may be related to expressive language development and that may be used to predict expressive language delay. Here we examine fine motor skill as a potential predictor.
Little research exists on the development of fine motor skill in HR infants. However, a handful of existing studies indicate that motor delays are apparent in HR infants (with and without ASD) in the first 14 months of life. For instance, Landa & Garrett-Mayer (2006) observed fine motor delays at 14 months in HR infants who later developed ASD as well as those who did not. Interestingly, these early delays persisted through 24 months (the oldest age reported) only for those HR infants who later developed ASD. Although they did not look at fine motor skills in particular, Iverson & Wozniak (2006) observed delays in other motor domains including gross motor milestone delays and postural instability in a sample of HR infants assessed between 5 and 14 months (they did not differentiate between infants with a subsequent ASD diagnosis and those without). Based on these studies, we predicted that HR infants would exhibit fine motor skill delays in the first 18 months, and that these early delays would be particularly pronounced for children who eventually received an ASD diagnosis.
At the group level, HR infants also exhibit language delays as early as 18 months (Gamliel, Yirmiya, & Sigman, 2007; Iverson & Wozniak, 2007), in pre-school (Toth et al., 2007), and through age 7 years (Gamliel, Yirmiya, Jaffe, Manor, & Sigman, 2009). Although there is large variability in skill level, more HR infants exhibit language delay (LD) than would be expected by chance, including those who do not have a later ASD diagnosis (Toth et al., 2007; Yirmiya, Gamliel, Shaked, & Sigman, 2007). What remains unclear from this literature is whether individual differences in infant fine motor development predict individual differences in expressive language development. The study by Landa and Garrett-Mayer (2006) is suggestive of a possible association between the two domains. The early fine motor delays reported in HR infants without a later ASD diagnosis were observed in a subgroup of children with LD at 24 months. However, they did not directly examine relations between motor and language domains. Further, it is unclear whether an association between early fine motor skill and language may extend to language at later ages. Combined with observed associations between motor and expressive language skills in TD children and children with language impairment (Hill, 2001; Iverson, 2010; Stone & Yoder, 2001), we predicted that the two domains would also be related in HR infants, with early fine motor skill predicting later expressive language at 36 months.
Current Study
To examine these predictions, we conducted a longitudinal study of fine motor and expressive language development in HR infants from 12 to 36 months. We utilized a measure of fine motor skills designed to tap motor planning and fine motor control at 12 and 18 months and a measure of expressive vocabulary at 36 months. Because both are parent report measures, we also used standardized observational measures of fine motor and expressive language skills as a complementary source of information. Parent report measures do not carry the additional task demands of interacting with an experimenter in a standardized testing situation; and while standardized observations carry these additional task demands, they permit direct observation of skills being assessed. Researchers and clinicians have emphasized the importance of assessing children’s skills using information from multiple contexts (see e.g. Tager-Flusberg et al., 2009, regarding expressive language in ASD). In Study 1, we asked whether HR infants (who did vs. did not receive a later ASD diagnosis) exhibit early fine motor delays relative to a normative comparison group of infants with no family history of ASD (low-risk, LR). In Study 2, we addressed whether early fine motor development predicts later expressive language skills at 3 years of age.
Study 1
In Study 1 we investigated whether HR infants exhibited early delays in fine motor skill. We begin by examining between group (HR and LR) differences in fine motor skill. We then explore individual differences within the HR group by comparing fine motor skills in infants who received a diagnosis of ASD at 3 years (HR-ASD) to infants with no such diagnosis (HR-ND).
Participants
Study participants included 34 HR infants (18 males) who have an older sibling with an autism diagnosis. The older sibling’s autism diagnosis was independently confirmed through a University Autism Research Program where a trained clinician administered the Autism Diagnostic Observation Schedule (ADOS; Lord et al., 2000). For an infant to qualify for the study, the older sibling had to score above the threshold for Autism on the ADOS and receive clinical judgment of Autism based on DSM-IV criteria.
HR infants participated in this study as part of an ongoing longitudinal investigation of development between 5 and 36 months in infants who have an older sibling with an autism diagnosis. Families were recruited through a University Autism Research Program, parent support organizations, and local agencies and schools serving families of children with ASD.
At 36 months, HR children came to the lab for an outcome classification session during which a trained clinician blind to all previous study data administered the ADOS and evaluated whether the child met DSM-IV criteria for an ASD. Using these criteria, 7 children (4 male, 3 female) received an ASD diagnosis at 36 months (HR-ASD subgroup); the remaining 27 children (13 male, 14 female) did not (HR-ND subgroup). Table 1 presents participant characteristics for each HR subgroup.
Table 1.
Participant Characteristics for HR-ND and HR-ASD subgroups
| HR-ND Group | HR-ASD Group | Subgroup Difference | |
|---|---|---|---|
| N | 27 | 7 | |
| Sex | 13 male | 4 male | |
| 36-month MSEL ELC SS1 | 107.61 (SD=19.64) 77–141; Mdn=109 |
73.00 (SD=21.63) 55–97; Mdn=67 |
p=.030 |
| 36-month MSEL VR | 58.52 (SD=14.49) 24–80; Mdn=60 |
37.75 (SD=17.63) 20–60; Mdn=35.50 |
p=.031 |
| 36-month MSEL FM | 47.58 (SD=15.94) 20–78; Mdn=46.50 |
25.67 (SD=8.45) 20–38; Mdn=20.50 |
p=.003 |
| 36-month MSEL EL | 56.43 (SD=11.05) 36–78; Mdn=56.00 |
30.60 (SD=13.07) 20–52; Mdn=30 |
p=.003 |
| 36-month MSEL RL | 52.15 (SD=11.60) 35–75; Mdn=51.00 |
24.80 (SD=10.73) 20–44; Mdn=20 |
p=.001 |
Note. Table includes group mean, SD, range, and median for the Mullen Scales of Early Learning (MSEL) Early Learning Composite (ELC) standard score (SS) and T-scores for each MSEL cognitive subscale (Visual Reception, VR; Fine Motor, FM; Expressive Language, EL; and Receptive Language, RL).
Scores are unavailable for: 8 children on the ELC (4 HR-ND, 4 HR-ASD); 3 HR-ASD children on VR, 4 children on FM (3 HR-ND, 1 HR-ASD); 6 children on EL (4 HR-ND, 2 HR-ASD); 2 HR-ASD children on RL. The reported significance level is for a Mann-Whitney test of differences between HR-ASD and HR-ND subgroups.
Study 1 also included a comparison group of 25 infants (10 males) with no family history of ASD (i.e., no first- or second degree relatives diagnosed with ASD; low-risk, LR group). This LR sample was selected from a larger group participating in a separate, completed longitudinal study of infants between the ages of 2 and 19 months conducted by the second author. These infants served as a comparison group for the Infant Oral- and Manual-Motor Interview (IOM) for which norms are unavailable. For all other measures, normative data are available and are used to characterize HR group skills.
To participate in the study, all participants in both the HR and LR samples had to be from full-term, uncomplicated pregnancies and have a 5-minute Apgar score within the normal range. In addition, all HR and LR participants came from monolingual, English-speaking households. Both samples had similar demographic characteristics: most infants in both groups (97%) were Caucasian and came from middle- to upper-middle-class families. Most parents in both groups had college education and beyond (Mothers: HR = 38% college, 38% post-college; LR = 36% college, 52% post-college) (Fathers: HR = 26.5% college, 44% post-college; LR = 40% college, 40% post-college) and father occupational prestige (HR = 61.42, LR = 57.61; t(50) = 0.92, p = .36; Nakao & Treas, 1994) was similar across samples. We did not calculate occupational prestige for mothers because at least a third of mothers in both samples did not work outside the home (HR = 50%; LR = 36%).
Procedure
Infants and primary caregivers were videotaped at home for approximately 45 minutes each month. For HR infants, data collection began when the infant was 5 months old and continued at monthly intervals to the age of 14 months, with follow-up visits at 18, 24, and 36 months. In Study 1, we focus on data collected at the sessions when children were 12, 18, and 24 months of age. Comparison data were obtained from the LR sample from comparable monthly visits. However, because the study in which LR infants participated ended at 19 months, no 24-month data are available for the LR group. Each session included naturalistic observation, semi-structured play, and standardized assessments. We also included diagnostic status of HR infants (ASD or ND) from the 36-month outcome classification session as a factor in our analyses. All study procedures received approval from the institutional review board. In addition, informed consent was obtained for each infant prior to the first observation.
Measures
Infant Oral- and Manual Motor Interview
We assessed infant fine motor skill at 12 and 18 months using items taken from a parent interview that has been previously used to assess fine motor skills in children from 6 to 36 months (Gernsbacher et al., 2008). We created a composite score by selecting 12- and 18-month items from the interview for which we had sufficient variability and data available from our samples of HR and LR children. For instance, we excluded a 12-month item that asked parents whether their child banged objects at 12 months because all parents responded “yes”. We also excluded a 12-month item that asked whether the child could scribble because there were insufficient data available (many parents reported that they had not given their child the opportunity to scribble). Our resulting Infant Oral- and Manual-Motor Composite (IOM Composite) contained 7 items; these are listed in Table 2. All 7 items were yes/no questions and 1 point was given for each “yes” response. Thus, the range of scores on the IOM Composite was 0 – 7. The IOM Composite showed acceptable internal reliability (Cronbach’s alpha = .701) and all items were correlated r > .27 with total scale, with r > .3 for 5 items (Cronbach, 1951). The composite contains one oral-motor item, and this item showed good correlation with the total scale (r = .337)
Table 2.
IOM Composite Items
| 12-month Items | 18-month Items |
|---|---|
| Child can drink from a sippy-cup* | Child can draw horizontal lines* |
| Child can stack a tower of children’s blocks* | Child can draw vertical lines* |
| Child can clap with a controlled movement* | Child can point distally on request with index finger |
| Child can point proximally on request |
Note. Only items with an * were included in the IOM Composite-Short measure. Unlike standardized ASD assessments, the pointing composite items that were excluded from the IOM Composite-Short measure do not specify the function for which the point is produced.
In addition to our overall IOM Composite, we looked at subsets of items from the IOM Composite to describe the nature of infants’ fine motor skills. We examined three sets of scores: a) 12-month (3 items); b) 18-month (4 items); and c) a 5-item abbreviated version of the composite, the IOM Composite-Short, which excluded the two 18-month pointing items (score range = 0–5; see Table 2). Although the production of pointing requires fine motor skill, it is also included in standard assessments for ASD and we wanted to avoid overlap between our items and those used to assess ASD. However, we included both pointing items in our IOM Composite because both showed good correlation with the total scale (distal point with index finger on request: r = .593; proximal point on request: r = .382) and, as will be reported, similar patterns of results were found for both the IOM Composite-Short and the IOM Composite.
Mullen Scales of Early Learning (MSEL)
The Mullen Scales of Early Learning was administered to all 34 HR infants at 24 and 36 months (MSEL; Mullen, 1995) and to a subset of HR infants at 18 months (n = 23). The MSEL was not administered to LR infants. However, there were no parent or experimenter concerns about language or cognitive development for any LR infants. The MSEL is an experimenter-administered standardized assessment comprising 5 subscales: Gross Motor and 4 cognitive subscales (Visual Reception, Receptive Language, Expressive Language, and Fine Motor). Both a raw score and a standardized T-score are available for each subscale (T-score M = 50, SD = 10). In addition, all 4 cognitive scales can be combined into an Early Learning Composite for which standard scores are available (M = 100, SD = 15). Table 1 presents 36-month ELC standard scores as a measure of general developmental level at study completion for HR infants. T-scores for each of the 4 cognitive subscales are also included in Table 12.
The MSEL is observation-based and thus we used the 18-month Fine Motor (FM) subscale to verify our parent report IOM for the subset of HR infants for whom 18-month scores were available (23 of the 34 infants). Consistent with prior findings of relations between parent-report FM skill and the MSEL FM (Lloyd et al., 2011), IOM Composite scores significantly related to 18-month MSEL FM scores (rho = .53, p = .01). In addition to using MSEL FM scores to verify infant fine motor skill on the IOM, we used the FM subscale at 24 months (the earliest age at which data are available for the full sample) as a complement to the IOM Composite. We used the MSEL FM subscale percentile scores to examine performance for the HR group relative to the normative sample.
Results
Do HR infants exhibit delays in fine motor skill?
We first examined whether HR infants exhibit early fine motor delays relative to a comparison group of LR infants. All analyses employed non-parametric Mann-Whitney tests.3 IOM Composite scores were significantly lower for the HR group (M = 3.62, SD = 1.86) than the LR group (M = 5.20, SD = 1.41) (U = 215.0, p = .001). Analysis of the additional three IOM measures confirmed that the difference was significant at both 12 months (HR: M = 1.68 (SD = 0.73); LR: M = 2.20 (SD = 0.71); U = 295.5, p = .004) and 18 months (HR: M = 1.94 (SD = 1.43); LR: M = 3.00 (SD = 1.08); U = 241.5, p = .003). It also remained significant after excluding the two pointing items from the composite (IOM Composite-Short) (HR M = 2.62 (SD = 1.35); LR M = 3.36 (SD = 1.25); U = 293.5, p = .039).
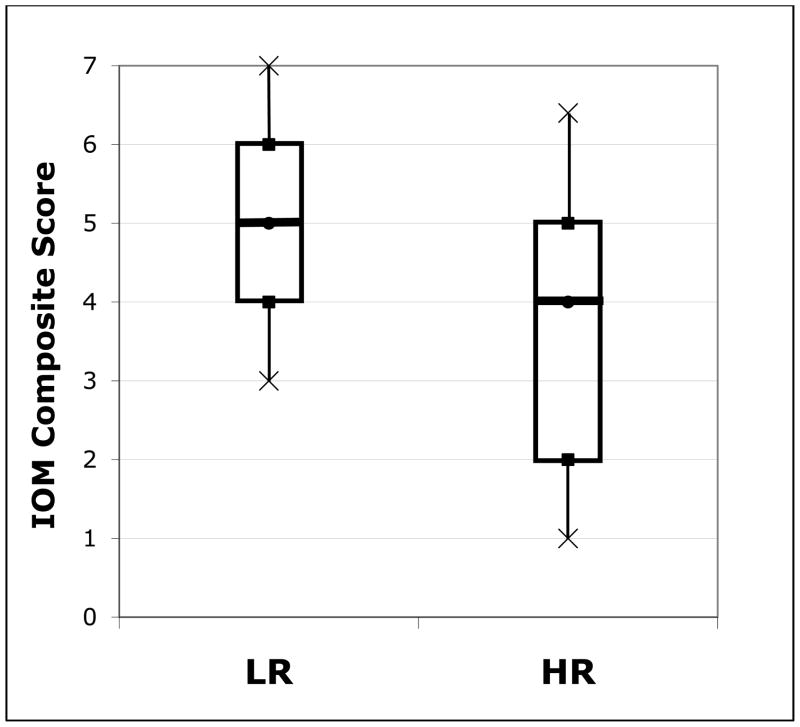
We next examined group distributions to determine whether this group-level pattern held for individual children. While HR infants as a group scored lower than LR infants on the IOM Composite, there was large variability within the HR group. Box plots representing the distributions of IOM Composite Scores for each group are presented in Figure 1. As is apparent, the LR group tended to have higher and less variable IOM Composite scores than the HR group. We conducted a series of binomial tests to examine whether more HR children than would be expected by chance had low scores that may be indicative of delay. We based chance level on the LR distribution of scores and used the 10th percentile4 of the LR group as our cut-off. A larger percentage of HR children exhibited delay on the IOM Composite than would be expected by chance (47%, p < .001). The same pattern was apparent on the other three IOM measures (12-months: 35%, 18-months: 47%; IOM Composite-Short: 24%; all p < .02).
Figure 1.
IOM Composite Scores for HR and LR Groups. Distribution of IOM Composite scores (range 0 – 7) for the LR group and HR group. The boxes in the graph represent the interquartile range for each group; the line in the middle of each box represents the median and the crosses represent the 10th and 90th percentiles.
We complemented the IOM Composite findings for the HR group using the standardized, observation-based MSEL FM subscale and found a similar pattern of fine motor delay in toddler fine motor skill. IOM Composite scores (at 12 and 18 months) significantly related to 24-month MSEL FM subscale score (rho = .383, p = .031). Similar to the IOM Composite, FM T-scores at 24 months (M = 42.91, SD = 12.17) tended to be low and variable, with binomial tests revealing that more HR infants had a T-score at or below the 10th percentile than would be expected by chance (28%, p = .003).
HR infant subgroups
To explore whether the HR and LR group differences reported above were driven by the children who eventually received an ASD diagnosis, we repeated our previous analyses, but this time examined data separately for each HR infant subgroup: those subsequently receiving an ASD diagnosis at 36 months (n = 7) and those who did not (n = 27).
Descriptive data for both HR infant subgroups are provided in Table 3. The HR-ND subgroup scored significantly lower on the IOM Composite than did the LR group (U = 205.0, p = .013). Analysis of the additional three IOM measures confirmed that this difference was significant at 12 months (U = 226.0, p =.02) and 18 months (U = 232.5, p = .043), but not for the Fine-Motor Composite-Short (U = 260.5, p = .148). By contrast, differences between the HR-ASD and LR groups were significant on the IOM Composite (U = 10.0, p < .001) and on all three additional IOM measures (all p-values < .02) such that the HR-ASD subgroup had consistently lower scores than the LR group.
Table 3.
Fine Motor Skill in HR-ND and HR-ASD subgroups
| Measure | HR-ND (n=27) | HR-ASD (n=7) | Subgroup Difference |
|---|---|---|---|
| IOM Composite (maximum possible=7) | 4.04 (SD=1.74) 1–7; Mdn=4 |
2.00 (SD=1.41) 0–4; Mdn=2 |
p=.01 |
| IOM 12-month (maximum possible=3) | 1.78 (SD=0.70) 0–3; Mdn=2 |
1.29 (SD=0.76) 0–2; Mdn=1 |
p=.122 |
| IOM 18-month (maximum possible=4) | 2.26 (SD=1.40) 0–4; Mdn=2 |
0.71 (SD=0.76) 0–2; Mdn=1 |
p=.008 |
| IOM Composite-Short (maximum possible=5) | 2.85 (SD=1.26) 1–5; Mdn=3 |
1.71 (SD=1.38) 0–4; Mdn=1 |
p=.052 |
| MSEL FM 24-month | 24.00 (SD=1.89) 21–29; Mdn=24 |
18.29 (SD=3.15) 14–22; Mdn=18 |
p<.001 |
Note. Table includes group mean, SD, range, and median for IOM measures and MSEL FM subscale raw score. The reported statistical significance levels are for Mann-Whitney tests of differences between HR-ASD and HR-ND subgroups. MSEL FM scores are unavailable for 2 children in the HR-ND subgroup at 24-months.
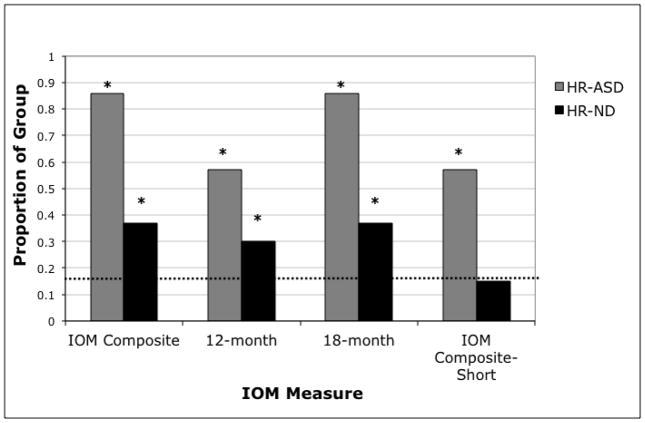
Distributional analyses were performed to examine whether these differences held at the level of individual children. Figure 2 presents the percentages of HR-ASD and HR-ND infants whose scores fell at or below the 10th percentile for the LR infants. Binomial tests confirmed that for both groups, more HR children in both subgroups had IOM Composite scores at or below the 10th percentile than expected by chance (HR-ND: p < .001; HR-ASD: p < .001). A similar finding held for all three additional IOM measures for the ASD group (all p-values < .01) and on two of the measures for the HR-ND group (12-month: p = .004, 18-month: p < .001, IOM Composite-Short: p = .282).
Figure 2.
Proportion of HR-ND and HR-ASD subgroups at or below the LR 10th percentile. LR 10th percentile score for each measure: IOM Composite = 3, 12-month = 1.6, 18-month = 1.6, IOM Composite-Short = 1.6. Chance prediction is 10 percent of each group (dashed line). *p < .05.
After examining whether the HR-ND and HR-ASD subgroups differed from the LR group, we next asked whether they differed from one another. As seen in Table 3, the HR-ASD subgroup had significantly lower scores on the IOM Composite than did the HR-ND subgroup (U = 35.0, p = .01). Group differences were also significant when looking separately at the 18-month items (18-month U = 34.5, p = .008) and were nearly significant for the IOM Composite-Short (U = 50, p = .052), but they were not significant for the 12-month items (12-month U = 62, p = .122). Fisher’s Exact Tests indicated that a significantly greater proportion of HR-ASD infants exhibited fine motor delays on the IOM Composite (scores at or below the 10th percentile) relative to HR-ND infants (p = .035).
Lastly, we complemented the HR subgroup findings using the MSEL FM subscale raw score at 24 months (see Table 3). At the group level, the HR-ASD subgroup had significantly lower MSEL FM raw scores than did the HR-ND subgroup (U = 5.5, p < .01). We also examined score distributions within each subgroup. Binomial tests revealed that delay (defined as T-score at or below the 10th percentile) was observed at greater than chance levels for the HR-ASD subgroup (86%, p < .001), but not for the HR-ND subgroup (12%, p = .463). Further, the HR-ASD subgroup had greater likelihood for delay than the HR-ND subgroup (Fishers exact test: p = .001).
Study 2
Our findings from Study 1 indicate that HR infants exhibited fine motor delays between 12 and 24 months and that degree of delay was a distinguishing characteristic of infants who were subsequently diagnosed with ASD. We also observed a large range of variability within the HR group. Distributional analyses revealed that while HR infants both with and without ASD were at risk for early fine motor delay, some HR infants had fine motor skills within the typical range. In Study 2, we ask whether individual differences in early fine motor skill in HR infants predict subsequent individual differences in expressive language. In addition, it is possible that differences in motor skill observed in Study 1 arose from differences in nonverbal cognition and, thus, we control for nonverbal cognition in our Study 2 analyses. Based on prior work with TD children and children with language impairment (Hill, 2001; Iverson, 2010; Stone & Yoder, 2001), we predicted significant relations between motor skill and expressive language.
Participants
Study 2 included the 34 HR participants who participated in Study 1. We focus on data collected at the 12-, 18-, 24-, and 36-month sessions. We used fine motor measures at 12, 18, and 24 months to predict individual differences in expressive language measures at 36 months.
Measures
Fine Motor Composite
Our measure of fine motor skill in Study 2 is a Fine Motor Composite (FM Composite) that combines complementary parent report and standardized observational measures of fine motor skill between 12 and 24 months to provide a comprehensive measure of early fine motor skill. The FM Composite includes the IOM Composite score (measured at 12 and 18 months) and the MSEL FM raw score at 24 months. We transformed IOM Composite scores and 24-month MSEL FM raw scores into z-scores and then calculated the average of the two scores for each child (Cronbach’s alpha = .639). As described earlier, examination of raw scores confirmed that IOM Composite and 24-month MSEL FM scores were significantly related.
MacArthur-Bates Communicative Development Inventories (CDI)
We assessed expressive vocabulary at 36 months using the CDI, a parent-report measure of child communication and language development that is widely used and has been validated with observational data (Fenson et al., 2007). We chose the CDI because we were interested in examining individual differences as well as risk for delay and the CDI has been used extensively for both purposes (e.g. Fenson et al., 1994; Heilmann et al., 2005). We used the CDI-III vocabulary checklist at the 36-month visit as our measure of expressive vocabulary.
MSEL Expressive Language (EL) Subscale
As a complementary source of information, we also utilized EL subscale scores from the standardized observational MSEL measure (described earlier) at 36 months. Whereas the CDI vocabulary checklist measures lexical skills, the MSEL EL subscale is a general measure of expressive language that encompasses different aspects of expressive language including phonological, lexical, syntactic, and semantic skills (Mullen, 1995).
Expressive Language Composite
Finally, we combined the CDI and MSEL EL measures to create an Expressive Language Composite (EL Composite) at 36 months. CDI expressive vocabulary scores significantly related to MSEL EL scores (rho = .689; p < .001). We calculated the EL Composite in the same manner as the Fine Motor Composite described earlier (Cronbach’s alpha = .837).
MSEL Visual Reception (VR) Subscale
We used raw scores at 36 months on the MSEL Visual Reception (VR) subscale as a covariate in regression analyses of fine motor and expressive language relations. The VR subscale provides a measure of nonverbal cognition and allows us to examine whether variance in expressive language skill is accounted for by fine motor skills above and beyond any variance accounted for by general nonverbal skill. Although the ELC is a composite measure available for the MSEL, it includes both MSEL FM and MSEL EL subscales scores. We therefore utilized the MSEL VR subscale as our nonverbal cognition covariate because it is independent of the measures in our regression analyses.
Results
Expressive language in HR infants
Table 4 contains descriptive information for CDI raw expressive vocabulary scores at 36 months. To determine whether more children exhibited expressive vocabulary delays than would be expected by chance, we performed binomial tests, with delay defined as scores at or below the 10th percentile of CDI norms (e.g., Heilmann et al., 2005). Significantly more HR infants exhibited delays at 36 months than would be expected by chance (65%, p < .001). We then looked separately at the HR-ND and HR-ASD subgroups to examine whether these effects were driven by the HR-ASD subgroup. This was not the case: binomial tests were significant for both the HR-ND subgroup (62%, p < .001) and the HR-ASD subgroup, (80%, p < .001).
Table 4.
HR Sample Expressive Vocabulary Raw Scores at 36 Months
| Measure | HR Group | HR subgroups | ||
|---|---|---|---|---|
| HR-ND | HR-ASD | Subgroup Difference | ||
| CDI | 51.97 (SD=25.83) 0–98, Mdn=47 |
57.58 (SD=21.36) 26–98, Mdn=53.5 |
22.80 (SD=29.74) 0–73, Mdn=18 |
p=.016 |
| MSEL EL | 31.07 (SD=9.45) 3–46, Mdn=32 |
34.52 (SD=5.51) 25–46, Mdn=34 |
17.83 (SD=10.07) 3–32, Mdn=18.5 |
p=.001 |
Note. Table includes mean, SD, range, and median for CDI Vocabulary Checklist raw score and MSEL EL subscale raw score. The reported statistical significance levels are for Mann-Whitney tests of differences between HR-ASD and HR-ND subgroups. CDI data are unavailable for 3 children (1 HR-ND, 2 HR-ASD). MSEL EL data are unavailable for 5 children (4 HR-ND, 1 HR-ASD).
Finally, we complemented our findings using MSEL EL subscale raw score at 36 months (Table 4). For the entire HR group, the likelihood of delay (at or below the 10th percentile of MSEL norms) was not greater than chance level (binomial tests: 18%, p = .14). However, subgroup analyses revealed greater than chance levels of delay for the HR-ASD subgroup (80%, p < .001), but not for the HR-ND subgroup (4%, p = .315).
Relating early fine motor skill to later expressive language in HR infants
Prior to conducting analyses using the FM Composite as a predictor of EL Composite scores at 36 months, we first used Spearman correlations to explore relations between the component measures included in the two composites. Both expressive language measures positively and significantly related to both the IOM Composite (at 12 and 18 months) and the 24-month MSEL FM subscale (Table 5).
Table 5.
Correlations Between Fine Motor and Expressive Language Measures
| 24-month MSEL FM | IOM Composite (12–18 months) | |
|---|---|---|
| 36-month CDI | .374 (p=.046) | .420 (p=.019) |
| 36-month MSEL EL | .568 (p=.002) | .418 (p=.024) |
Note: The table presents Spearman correlation statistics. All are significant at alpha = .05.
We now turn to relations between our fine motor and expressive language composite measures. As is evident in Figure 3, there was a significant positive correlation between the FM Composite and 36-month EL Composite (rho = .613, p = .001). To determine whether these relations held after accounting for nonverbal developmental level, we performed multiple regression analyses that included 36-month MSEL VR subscale raw score as a predictor in the models5. The mean MSEL VR raw score for the full HR infant sample was 37.36 (SD = 6.76) with a score range of 20 to 47 (HR-ND: mean = 38.67, SD = 5.72, range = 26–47; HR-ASD: mean = 29.50, SD = 8.02, range = 20–39).
Figure 3.
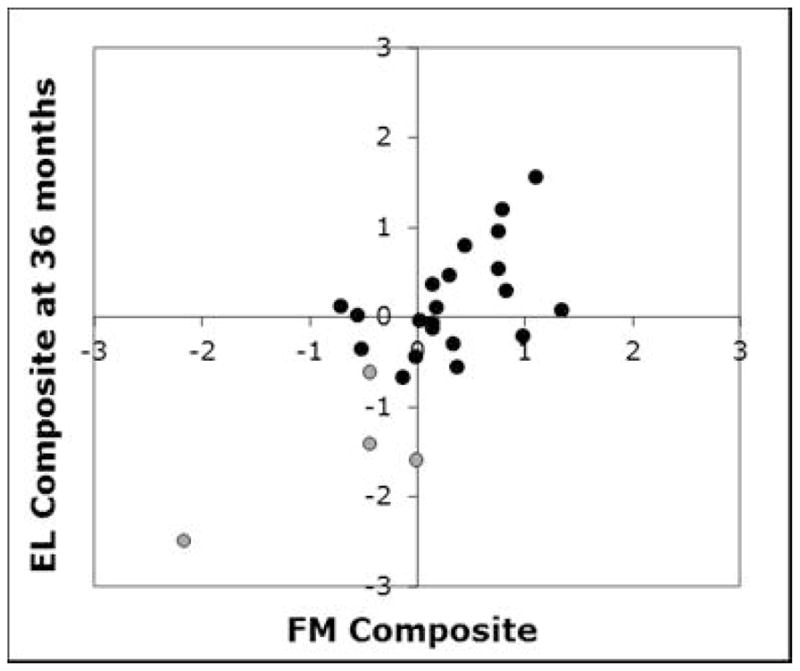
Relation between FM Composite and EL Composite in the HR sample. Grey indicates HR-ASD group. Black indicates HR-ND group. 9 children (3 in HR-ASD group; 6 in HR-ND group) are not included because FM Composite and/or 36-month EL Composite data are unavailable.
When accounting for 36-month MSEL VR, FM composite continued to significantly predict later EL Composite at 36 months (p = .009) (Table 6)6. Because HR-ASD group scores tended to be lower than HR-ND scores on fine motor and expressive language measures, we also examined whether these relations held when including HR subgroup (HR-ASD or HR-ND) in the regression models. As presented in Table 6, FM composite continued to be a significant predictor of 36-month EL Composite (p = .04).
Table 6.
Regression Models Predicting Expressive Language Composite at 36 months
| Model 1 | Model 2 | |
|---|---|---|
| Intercept | −0.29 (0.83) | −0.94 (0.86) |
| MSEL VR | 0.01 (0.02) | 0.004 (0.02) |
| FM Composite | 0.66** (0.23) | 0.51* (0.23) |
| HR Subgroup | 0.79~ (0.43) | |
| R-squared Statistic (%) | 33.8* | 43.9* |
Note: Table provides the unstandardized beta and standard error for each predictor. HR subgroups coded as HR-ND = 1, HR-ASD = 0.
significant at .01 level,
significant at .05
General Discussion
The pattern of results presented above indicates that HR infants exhibit early fine motor delays. These delays were not limited to HR infants who eventually received an ASD diagnosis, although they appeared to be more pronounced in these infants. Further, fine motor skills predicted later expressive language at 3 years of age.
Fine motor skill in HR infants
Fine motor delays were apparent among HR infants in the first 18 months of life, as indicated by the IOM Composite at 12 and 18 months. Further, IOM item analyses revealed that these delays emerged as early as 12 months of age. However, there were large individual differences in fine motor skill, with some HR infants demonstrating typical fine motor skills and others exhibiting delay. This is consistent with other work that has reported large variability in the early development of HR infants (see e.g. Gamliel et al., 2007). Nonetheless, more HR infants exhibited fine motor delay than would be expected by chance, and this finding held for both infants who did and did not later receive an ASD diagnosis. A similar pattern of results was obtained with the observation-based MSEL FM subscale at 24 months, and infant IOM Composite scores at 12 and 18 months were positively and significantly related to MSEL FM scores at 24 months.
Looking specifically at HR-ND children, delays were apparent on the IOM Composite in the first 18 months. However, we found no evidence of subsequent delays at 24 months on the MSEL FM subscale. Because IOM Composite and MSEL FM findings are both across context and across age, a direct comparison cannot be made between the results from these measures. However, in combination with previous literature, this finding raises the possibility that fine motor delays in HR infants without ASD may be transient. This pattern is consistent with other work conducted with HR infants. For instance, Landa & Garrett-Mayer (2006) observed MSEL FM delays at 14 months but not at 24 months in HR-ND infants.
With regard to HR-ASD children, we found that while both HR subgroups exhibited delays on the IOM Composite in the first 18 months of life, these delays were more pronounced in children who were later diagnosed with ASD. This pattern of lower scores was also apparent on the 24-month MSEL FM subscale. This suggests that the severity and persistence of delay may be characteristic of emerging HR-ASD in comparison to HR-ND. In typical development, motor planning skill develops in a continuous manner from 12 months through grade school (Dewey, 1995; Kools & Tweedie, 1975). Our findings suggest that the fine motor and motor planning difficulties observed in pre-school and grade-school aged children with ASD may begin to emerge as early as 12 months, and this may have implications for understanding the role of fine motor skills in ASD (e.g. Dziuk et al., 2007).
Our findings of fine motor delays in HR infants with and without ASD also have practical implications. They suggest that it may be useful to assess fine motor skills in the first 18 months, particularly in children who are at risk for developmental delays. Identification of fine motor difficulties in the first 18 months may support design of developmentally appropriate interventions. For instance, motor difficulties themselves can be addressed as part of a child’s intervention program. As discussed below, they may also have direct and indirect implications for supporting children’s communication (both verbal and nonverbal; Iverson, 2010).
Predicting expressive language development
We observed that differences in fine motor development between 12 and 24 months related to individual differences in later expressive language at 36 months. Further, we found that this relation held even after including nonverbal cognition scores and ASD subgroup status as covariates in our models. Theoretically, our findings add to the literature highlighting relations between motor and expressive language development in children at risk for or exhibiting language delays (Hill, 2001). By revealing relations between these two domains in our sample of HR infants, they also contribute to understanding of expressive language and relations between developing domains in this population—a population characterized by risk for language and other delays (see Rogers, 2009 for review).
Why might motor and expressive language skills relate? One possibility is that motor planning and coordination difficulties may underlie delays in both domains (Iverson & Thelen, 1999). However, it is also possible that the two skills are related because developing motor skills impact language development. Iverson (2010) reviewed several studies suggesting that motor development in the first 18 months may have cascading consequences for developing language skills. Developing motor skills shape children’s experiences with the world and, by influencing children’s learning opportunities, may have an impact on language development. As an example, one such learning opportunity provided by advances in fine motor abilities is in object manipulation and exploration behaviors. Studies with TD infants have found relations between increasing complexity and refinement in infants’ object exploration activities and attainments in language development (Lifter & Bloom, 1989). Interestingly, lags in object exploration behaviors have been observed in HR infants in the first year (Koterba, Leezenbaum, & Iverson, 2012; Mulligan & White, 2012). Although not necessary for language development, delays in motor experiences may nonetheless constrain learning opportunities.
One limitation of the current study is that standardized cognitive measures were not available at 12 and 18 months. Thus, although we accounted for variability in nonverbal cognition at 24 and 36 months in the present study, we were unable to address whether nonverbal cognition at earlier ages may contribute to motor delays and relations with expressive language. It is important to note that some HR infants may exhibit other delays in addition to fine motor and language delays, as indicated by low and variable MSEL ELC standard scores, an observation that is consistent with previous work with HR infants (e.g. Landa & Garrett-Mayer, 2006; Toth et al., 2007). It is likely that delays in a variety of domains jointly contribute to the behavioral manifestations of ASD and delay in HR infants.
Nonetheless, the possibility that fine motor skills may be one of these domains makes it one of several avenues for future research. Future studies can examine fine motor skills at even earlier ages than were examined in this study. Evidence of lags in object exploration at 6 months suggests that there may be corresponding lags in fine motor development within the first year (Koterba et al., 2012).
Practically, our findings suggest that fine motor skill may be a useful predictor of expressive language at 3 years of age. That these fine motor skills were observed as early as the first 18 months adds to the possible benefits for early identification since expressive language itself is still highly variable in the second year (see e.g. Bates et al., 1994). Further, early identification affords early intervention, which may facilitate expressive language development (Stone & Yoder, 2001). Future research with larger samples can further examine the utility of fine motor skill as a predictor of language delay in HR infants as a group and within each subgroup (HR-ASD and HR-ND). Future research can also examine whether fine motor skill continues to predict expressive language at older ages. For many HR-ND children, early delays resolve, but they persist in some domains for some individuals (Gamliel et al., 2009).
Future Directions
Future directions include extension of this work to additional motor domains as well as the use of more precise measures of motor and language skills. First, there is work suggesting motor delays in HR-ASD and HR-ND infants may not be limited to fine motor skills, but may also extend to gross motor skills (Bhat, Galloway, & Landa, 2012; Iverson & Wozniak, 2007; Lloyd et al., 2000). Because the MSEL does not provide T-scores for the Gross Motor subscale at 36 months, they are not included in Table 1. However, 24-month Gross Motor T-scores for our sample were particularly low for the HR-ASD group and significantly lower than for the HR-ND group (HR-ASD: M = 25, Mdn = 20, SD = 6.46, range = 20 – 35; HR-ND: M = 44, Mdn = 44, SD = 12.54, range = 20 – 80; p = .001). Recent research suggests that relations may also exist between gross motor and language skills (Bhat et al., 2012). The extension of our investigation of fine motor skills to gross motor abilities is a clear avenue for future research.
In addition, the use of more precise measures of fine motor skill may allow better understanding of early delays and improve the predictive value of fine motor skill on expressive language. As was the case for our expressive language measures, our fine motor skill measures also differed along several dimensions. In addition to varying across context and age of assessment, the underlying skills tapped also varied: the parent report IOM composite contained items designed to assess motor planning skill, but the standardized observational MSEL FM subscale was less specific. The lack of MSEL data for a third of our sample at 18 months limited our ability to investigate potential differences in our findings between contexts, but it may be a focus of future research. In addition, given that motor planning may be an area of particular difficulty in ASD, the use of tasks specifically tapping motor planning skills may be fruitful as a complement to our IOM composite (Bhat et al., 2011). Current work in our laboratory is taking this approach, utilizing a set of experimental tasks with novel technology to collect kinematic data on upper limb movements to permit detailed characterization of the organization of actions that vary in precision demands (Taffoni et al., 2012).
Lastly, to gain a more complete understanding of expressive language development, it will be useful to obtain multiple measures of expressive language development in multiple contexts (Tager-Flusberg et al., 2009). We investigated relations between fine motor and expressive language skills using composite measures that incorporated both parent report and standardized observational measures. However, as suggested above for fine motor skill, it may be useful to look more specifically at expressive language across contexts and domains. The measures of expressive language employed here vary on several dimensions. In addition to context differences, the CDI expressive vocabulary measure assesses lexical skills, while the MSEL EL subscale assesses a broad range of expressive language skills. Future work using more precise measures of specific aspects of expressive language may contribute to a better understanding of specific strengths, weaknesses, and relations with fine motor skills (Eigsti, de Marchena, Shuh, & Kelley, 2011).
Acknowledgments
This work was supported by grants from Autism Speaks and R01 HD054979 and R01 HD41677 to Jana M. Iverson. We thank D. Williams, N. Minshew, and the NICHD-funded University of Pittsburgh-Carnegie Mellon Collaborative Program of Excellence in Autism Research (HD35469 and HD055748 to N. Minshew) for supporting assessments and assistance with participant recruitment; J. Hetherington for administrative assistance; and members of the Infant Communication Lab for assistance with data collection. We offer special thanks to the families and infants who enthusiastically participated in the research.
Footnotes
The observed relations may be both direct and indirect. For instance, relations may be indirect such that developments in motor systems may influence language development through the sorts of learning experiences they afford (e.g. Iverson, 2010 and Lifter & Bloom, 1989; see also work on gross motor development and advances in communication, e.g. Campos et al., 2000 and Karasik, Tamis-LeMonda, & Adolph, 2011). Although this work provides additional motivation for our current study, this study was designed to provide an initial examination of whether relations between fine motor abilities and later language indeed exist in HR infants with and without ASD. Investigation of potential mechanisms that may underlie these relations is an important topic that awaits future study.
The MSEL does not provide T-scores for the Gross Motor subscale at 36 months. Thus, these scores are not included in Table 1.
We used Mann-Whitney tests for all analyses of group differences because this non-parametric test is less sensitive to between-group differences in score variance and is appropriate for use with small samples and unequal sample sizes (Siegel & Castellan, 1988). However, as a check of our findings, we also examined group differences using independent sample t-tests, and findings were unchanged.
As has been done in other developmental research studies, we used the 10th percentile as a cut-off for the motor measures (see e.g. Darrah, Redfern, Maguire, Beaulne, & Watt, 1998) as well as the Study 2 language measures (see e.g. Heilmann, Ellis Weismer, Evans, & Hollar, 2005).
Inspection of the data did not reveal any evidence of violations of the assumptions of multiple regression for any of the analyses.
If we use 24-month MSEL VR subscale raw score as a covariate instead of 36-month score, we obtain similar results. FM Composite significantly predicted EL Composite in a model including 24-month MSEL VR subscale (p = .008) and in a model including both 24-month MSEL VR subscale and HR subgroup (p = .021).
Contributor Information
Eve Sauer LeBarton, Department of Psychology, University of Pittsburgh.
Jana M. Iverson, Department of Psychology, University of Pittsburgh
References
- American Psychiatric Association. Diagnostic and statistical manual of mental disorders. 4. Washington, D.C: American Psychiatric Association; 1994. [Google Scholar]
- Alcock KJ, Krawczyk K. Individual differences in language development: Relation with motor skill at 21 months. Developmental Science. 2010;13:677–691. doi: 10.1111/j.1467-7687.2009.00924.x. [DOI] [PubMed] [Google Scholar]
- Bates E, Marchman V, Thal D, Fenson L, Dale P, Reznick JS, Hartung J. Developmental and stylistic variation in the composition of early vocabulary. Journal of Child Language. 1994;21:85–123. doi: 10.1017/s0305000900008680. [DOI] [PubMed] [Google Scholar]
- Bhat AN, Galloway JC, Landa RJ. Relation between early motor delay and later communication delay in infants at risk for autism. Infant Behavior and Development. 2012;35:838–846. doi: 10.1016/j.infbeh.2012.07.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bhat A, Landa R, Galloway J. Current perspectives on motor functioning in infants, children, and adults with autism spectrum disorders. Physical Therapy. 2011;91:1116–1129. doi: 10.2522/ptj.20100294. [DOI] [PubMed] [Google Scholar]
- Bishop DVM, Edmundson A. Specific language impairment as a maturational lag: Evidence from longitudinal data on language and motor development. Developmental Medicine and Child Neurology. 1987;29:442–459. doi: 10.1111/j.1469-8749.1987.tb02504.x. [DOI] [PubMed] [Google Scholar]
- Campos J, Anderson D, Barbu-Roth M, Hubbard E, Hertenstein M, Witherington D. Travel broadens the mind. Infancy. 2000;1:149–219. doi: 10.1207/S15327078IN0102_1. [DOI] [PubMed] [Google Scholar]
- Charman T, Taylor E, Drew A, Cockerill H, Brown J, Baird G. Outcomes at 7 years of children diagnosed with autism at age 2: Predictive validity of assessments conducted at 2 and 3 years of age and pattern of symptom change over time. Journal of Child Psychology and Psychiatry. 2005;46:500–513. doi: 10.1111/j.1469-7610.2004.00377.x. [DOI] [PubMed] [Google Scholar]
- Cronbach LJ. Coefficient alpha and the internal structure of tests. Psychometrika. 1951;16:297–334. [Google Scholar]
- Darrah J, Redfern L, Maguire TO, Beaulne AP, Watt J. Intra-individual stability of rate of gross motor development in full-term infants. Early Human Development. 1998;52:169–179. doi: 10.1016/s0378-3782(98)00028-0. [DOI] [PubMed] [Google Scholar]
- Dewey D. What is developmental dyspraxia? Brain and Cognition. 1995;29:254–274. doi: 10.1006/brcg.1995.1281. [DOI] [PubMed] [Google Scholar]
- Dziuk MA, Gidley Larson JC, Apostu A, Mahone EM, Denckla MB, Mostofsky SH. Dyspraxia in autism: association with motor, social, and communicative deficits. Developmental Medicine and Child Neurology. 2007;49:734–739. doi: 10.1111/j.1469-8749.2007.00734.x. [DOI] [PubMed] [Google Scholar]
- Eigsti I, Marchena A, Schuh J, Kelley E. Language acquisition in autism spectrum disorders: A developmental review. Research in Autism Spectrum Disorders. 2011;5:681–691. [Google Scholar]
- Fenson L, Dale PS, Reznick JS, Bates E, Thal D, Pethick S. Variability in early communicative development. Monographs of the Society for Research in Child Development. 1994;59(5) Serial No. 242. [PubMed] [Google Scholar]
- Fenson L, Marchman VA, Thal DJ, Dale PS, Reznick JS, Bates E. The MacArthur Communicative Development Inventories: User’s guide and technical manual. 2. Baltimore: Brookes; 2007. [Google Scholar]
- Gamliel I, Yirmiya N, Jaffe DH, Manor O, Sigman M. Developmental trajectories of siblings of children with autism: Cognition and language from 4 months to 7 years. Journal of Autism and Developmental Disorders. 2009;39:1131–1144. doi: 10.1007/s10803-009-0727-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gamliel I, Yirmiya N, Sigman M. The development of young siblings of children with autism from 4 to 54 months. Journal of Autism and Developmental Disorders. 2007;37:171–183. doi: 10.1007/s10803-006-0341-5. [DOI] [PubMed] [Google Scholar]
- Gernsbacher MA, Sauer E, Geye H, Kees E, Goldsmith HH. Infant and toddler oral and manual-motor skills predict later speech fluency in autism. Journal of Child Psychology and Psychiatry. 2008;49:43–50. doi: 10.1111/j.1469-7610.2007.01820.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heilmann J, Ellis Weismer S, Evans J, Hollar C. Utility of the MacArthur-Bates Communicative Development Inventory in identifying language abilities of late-talking and typically developing toddlers. American Journal of Speech-Language Pathology. 2005;14:40–51. doi: 10.1044/1058-0360(2005/006). [DOI] [PubMed] [Google Scholar]
- Hill EL. Non-specific nature of specific language impairment: a review of the literature with regard to concomitant motor impairments. International Journal of Language and Communication Disorders. 2001;36:149–171. doi: 10.1080/13682820010019874. [DOI] [PubMed] [Google Scholar]
- Iverson JM. Developing language in a developing body: the relationship between motor development and language development. Journal of Child Language. 2010;37:229–261. doi: 10.1017/S0305000909990432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iverson JM, Thelen E. Hand, mouth, and brain. They dynamic emergence of speech and gesture. Journal of Consciousness Studies. 1999;6(11–12):19–40. [Google Scholar]
- Iverson JM, Wozniak RH. Variation in vocal-motor development in infant siblings of children with autism. Journal of Autism and Developmental Disorders. 2007;37:158–170. doi: 10.1007/s10803-006-0339-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karasik LB, Tamis-LeMonda CS, Adolph KE. Transition from crawling to walking and infants’ actions with objects and people. Child Development. 2011;82:1199–1209. doi: 10.1111/j.1467-8624.2011.01595.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kools JA, Tweedie D. Development of praxis in children. Perceptual and Motor Skills. 1975;40:11–19. doi: 10.2466/pms.1975.40.1.11. [DOI] [PubMed] [Google Scholar]
- Koterba E, Leezenbaum NB, Iverson JM. Object exploration at 6 and 9 months in infants with and without risk for autism. Autism. 2012 doi: 10.1177/1362361312464826. Advance online publication. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Landa R, Garrett-Mayer E. Development in infants with autism spectrum disorders: a prospective study. Journal of Child Psychology and Psychiatry. 2006;47:629–638. doi: 10.1111/j.1469-7610.2006.01531.x. [DOI] [PubMed] [Google Scholar]
- Lifter K, Bloom L. Object knowledge and the emergence of language. Infant Behavior and Development. 1989;12:395–423. [Google Scholar]
- Lloyd M, MacDonald M, Lord C. Motor skills of toddlers with autism spectrum disorders. Autism. 2013;17:133–146. doi: 10.1177/1362361311402230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lord C, Risi S, Lambrecht L, Cook EH, Jr, Leventhal BL, DiLavore PC, Rutter M. The Autism Diagnostic Observation Schedule-Generic: A standard measure of social and communication deficits associated with the spectrum of autism. Journal of Autism and Developmental Disorders. 2000;30:205–223. [PubMed] [Google Scholar]
- Luyster R, Qiu S, Lopez K, Lord C. Predicting outcomes of children referred for autism using the MacArthur-Bates communicative development inventory. Journal of Speech, Language, and Hearing Research. 2007;50:667–681. doi: 10.1044/1092-4388(2007/047). [DOI] [PubMed] [Google Scholar]
- Mulligan S, White BP. Sensory and motor behaviors of infant siblings of children with and without autism. The American Journal of Occupational Therapy. 2012;66:556–566. doi: 10.5014/ajot.2012.004077. [DOI] [PubMed] [Google Scholar]
- Mullen EM. Mullen Scales of Early Learning. Circle Pines, MN: American Guidance Service, Inc; 1995. (AGS Edition) [Google Scholar]
- Nakao K, Treas J. Updating occupational prestige and socioeconomic scores: How the new measures measure up. Sociological Methodology. 1994;24:1–72. [Google Scholar]
- Ozonoff S, Young GS, Carter A, Messinger D, Yirmiya N, Zwaigenbaum L, Stone WL. Recurrence risk for autism spectrum disorders: A baby siblings research consortium study. Pediatrics. 2011;128:e488–e495. doi: 10.1542/peds.2010-2825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Siegel S, Castellan NJ., Jr . Nonparametric Statistics for the Behavioral Sciences. 2. New York: McGraw-Hill; 1988. [Google Scholar]
- Stone W, Yoder P. Predicting spoken language level in children with autism spectrum disorders. Autism. 2001;5:341–361. doi: 10.1177/1362361301005004002. [DOI] [PubMed] [Google Scholar]
- Toth K, Dawson G, Meltzoff A, Greenson J, Fein D. Early social, imitation, play, and language abilities of young non-autistic siblings of children with autism. Journal of Autism and Developmental Disorders. 2007;37:145–157. doi: 10.1007/s10803-006-0336-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taffoni F, Focaroli V, Formica D, Guglielmelli E, Keller F, Iverson JM. Sensor-based technology in the study of motor skills in infants at risk for ASD. Proceedings of the Fourth IEEE RAS/EMBS International Conference on Biomedical Robotics and Biomechatronics. 2012:1879–1883. doi: 10.1109/BioRob.2012.6290922. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tager-Flusberg H, Rogers S, Cooper J, Landa R, Lord C, Paul R, Yoder P. Defining spoken language benchmarks and selecting measures of expressive language development for young children with autism spectrum disorders. Journal of Speech, Language, and Hearing Research. 2009;52:643–652. doi: 10.1044/1092-4388(2009/08-0136). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yirmiya N, Gamliel I, Shaked M, Sigman M. Cognitive and verbal abilities of 24- to 36-month-old siblings. Journal of Autism and Developmental Disorders. 2007;37:218–229. doi: 10.1007/s10803-006-0163-5. [DOI] [PubMed] [Google Scholar]