Abstract
BACKGROUND
The field of prostate cancer has been stymied by the difficulty of cultivating patient-derived samples in the laboratory. In order to help circumvent this challenge, we sought to develop an in vitro assay of human prostate cancer initiation employing a prostate-associated mesenchymal feeder layer.
METHODS
Rat seminal vesicle mesenchyme (rSVM) harvested from male neonatal rats was plated in 12-well plates and then irradiated with 30 Gy after ~75% confluence. Single-cell suspensions of two human non-adherent prostate cancer xenograft lines (TRPC and LAPC9) were then plated on irradiated rSVM. At 3–4 weeks, three-dimensional solid structures, termed glandoids, were harvested and analyzed or transplanted singly into the renal capsule of immunodeficient mice. Animals were assessed for tumor formation 8–12 weeks after engraftment. Finally, clonality assays were performed to determine whether glandoids usually arise from a single cell and are therefore clonal in origin.
RESULTS
Glandoids form with reliable frequency (1/~300 plated cells), are constituted by relevant cell types (CK8+, CK5–, PSA+) and after implantation into immunocompromised mice, give rise to tumors that recapitulate original xenograft histology and cell composition; defining a glandoid as a tumor-initiating unit. In addition, assessment of red fluorescent protein (RFP)-labeled glandoids revealed either all red or non-red structures, with few areas of fusion, suggesting glandoids are clonal in origin.
CONCLUSIONS
The above assay describes an adjunct technique to readily cultivate cells from prostate cancer xenografts in vitro and as such provides a platform on which tumor-initiating cell studies and high-throughput drug discovery may be performed.
Keywords: prostate cancer, seminal vesicle mesenchyme, xenograft, tumor-initiating cell, cancer stem cell
INTRODUCTION
Progress in the field of human prostate tumor-initiating cell biology has been stymied by various experimental challenges. Due to downward stage migration as a result of PSA screening, the vast majority of prostate cancers in prostatectomy specimens today are often of low grade and stage and are difficult to identify grossly, making cancer tissue harvest for research purposes challenging. In addition, given current treatment paradigms, access to potentially tumor-initiating cell-enriched areas, such as metastatic or treatment refractory tissues, is uncommon. Furthermore, primary human prostate cancer is notoriously difficult to maintain in a laboratory environment, in vitro or in vivo, for any prolonged period of time. Of the obstacles facing researchers in the field, these three specific hurdles have impeded advancement the most. Notably, these particular issues pose minimal barriers in brain [1], breast [2], and colon cancer [3] research, areas where considerable discoveries have been already made in tumor-initiating cell biology.
Nevertheless, several investigators have recently reported significant breakthroughs. Collins et al. [4] have described the ability to isolate a specific subset of human prostate cancer cells, from surgical specimens, that are capable of forming colonies in vitro that possess self-renewal properties and that are composed of a mixed population of cells. Kasper and coworkers have been able to generate serially transplantable prostate tumors that recapitulate the parent human tumor from a clonal population of experimentally transformed human prostate cancer cells [5]. And Tang and coworkers have observed that only a small subset of cells from established prostate cancer cell lines and xenografts possess tumor initiating ability [6,7]. At present, no group has established an in vitro prostate tumor-initiating cell functional assay utilizing primary human tissue.
In an effort to improve the efficiency of in vitro modeling of human prostate cancer, numerous groups have described the use of fibroblasts as a feeder layer [8]. Such reports have revealed the ability to grow primary prostate spheres in culture albeit with varying tumorigenicity. It is clear that in addition to tissue culture conditions, such as media and feeder layer composition, the cell source (i.e., grade, stage, treatment history of the tumor) is critical to growing tumor-initiating spheres in vitro. As a first step toward developing a functional in vitro assay for prostate cancer initiation based upon primary tissues, we sought to establish an in vitro assay employing non-adherent xenograft human prostate cancer cell lines. Such an assay has the potential to hasten the investigation of human prostate tumor-initiating cell biology and, further, provide a platform on which high-throughput drug discovery may be performed. Borrowing from the seminal work of Cunha and Chung [9] and adapting concepts introduced by Isaacs and coworkers [10] and Witte and coworkers [11], we hypothesized that a feeder layer reflective of prostate stroma would be conducive to human prostate cancer tissue culture. To this end, we employed a feeder layer composed of rodent seminal vesicle mesenchyme (rSVM) to cultivate non-adherent human prostate cancer cells in vitro. Resultant three-dimensional colonies of cells were assayed for cell composition, tumorigenicity, and clonality.
MATERIALS AND METHODS
Prostate Cancer Xenograft Tumor Lines
In accordance with the University of Rochester School of Medicine's Research Subject's Review Board and following informed consent, fresh tissue obtained at surgery from a man with Gleason sum 10 prostate cancer was placed into immunocompromised mice for xenograft establishment. The patient had previously received radiation therapy, hormone therapy, and docetaxel chemotherapy; however, subsequently developed locally progressive disease without overt metastasis. For palliation the patient underwent radical cystoprostatectomy. Upon surgical removal of the tumor, a xenograft was established by placing 2–3 mm sections subcutaneously in castrated male severe combined immunodeficient mice (SCID; National Cancer Institute at Frederick). The resultant xenograft, treatment refractory prostate cancer (TRPC), has been maintained for more than 2 years and nine generations. A more detailed characterization of this line is being prepared in a separate manuscript. The LAPC9 xenograft was supplied by the Laboratory of Robert E. Reiter at UCLA.
Xenograft Tumor Dissociation
LAPC9 and TRPC xenograft tumors were excised and placed in Petri dishes containing 10% fetal bovine serum (FBS, Gibco, 10437) in RPMI 1640 (with GlutaMAX-l and phenol red, Gibco, 61870). Tumors were cut into 2–3 mm sections, resuspended in HBSS (Invitrogen, 14175) containing 200 U/ml collagenase type I (Worthington Biochemical, Lakewood, NJ, LS004197), and stirred at 37°C for 20 min. Dissociated cells were passed through a 40 μm nylon strainer and resuspended in 7.4% FBS in RPMI. Filtered cells were examined with an Olympus CKX31 inverted microscope to confirm total dissociation.
Primary Mesenchymal Cell Culture
Timed-pregnant female Sprague–Dawley rats were purchased from Harlan Laboratories. All animals used in this work were maintained according to the guidelines of the University Committee on Animal Resources at the University of Rochester. Seminal vesicles were excised from neonatal rats using a Leica MZ9.5 dissecting stereomicroscope and collected in RPMI containing 10% FBS. Seminal vesicles were resuspended in HBSS containing 10 BTEE units/ml trypsin (Sigma, T4799) for 1 hr at 4°C. The trypsin was then neutralized by resuspension of the seminal vesicles in RPMI containing 10% FBS and <0.5 mg/ml deoxyribonuclease I (Sigma, DN25-1G). Epithelium was teased away from each seminal vesicle with 25-gauge needles under the dissecting stereomicroscope. The remaining mesenchyme was resuspended in RPMI containing 200 U/ml collagenase type I and gently rocked at room temperature for 20 min. Dissociated cells were maintained in RPMI containing 10% FBS and 0.1 mg/ml Primocin (Invitrogen, ant-pm-1) and incubated at 37°C in humidified air containing 5% CO2. Due to technical issues with cultivating and maintaining rodent urogenital mesenchyme (UGM) in vitro, UGM was not utilized in these studies as a feeder layer.
Glandoid Culture
Monolayers of rSVM cells were grown to ~75% confluence in 12-well culture plates (Corning, 3513), either directly or on cover glasses (VWR, 48380-046), and irradiated with 30 Gy of gamma radiation from a cesium-137 source. As controls, xenograft cells were also plated either directly on plastic or upon a STO cell (mouse embryonic fibroblast) feeder layer. The rSVM growth medium was replaced with RPMI containing 7.4% FBS and 0.1 mg/ml Primocin. Cells freshly dissociated from human prostate cancer xenograft tumors were plated at densities of 800–3,200 cells/cm2 over the feeder layer. After 14 days of culture, the medium was replenished if acidification was noted (i.e., color change). Due to technical considerations, limiting dilution assays were not performed.
Lentiviral Infection of Dissociated Glandoid Cells
A pLentiRed plasmid containing the gene DsRed-N2, which encodes a red fluorescing protein, was transfected into 293FT cells using Lipofectamine 2000 according to manufacturer's instructions (Invitrogen, 11668). Cell culture medium containing the shed viral particles was harvested every 8 hr, filtered through a 0.45-μm filter, and stored at –80°C. Thawed medium was then used to replace the growth medium in 12-well culture plates of irradiated rSVM feeder layer. To obtain cells, glandoids were collected one at a time with pipette tips of sufficient bore diameter to allow passage. Adherent glandoids were first dislodged from the substrate with a blunt glass probe. Each glandoid was removed to 200 μl of 37°C trypsin–EDTA (0.25%, Gibco, 25200) and pipetted vigorously for 3–6 min until completely dissociated. Resultant single cells from a number of TRPC glandoids were mixed, counted on a hemocytometer, and plated at a density of 800 cells/cm2 over the feeder layer, and the medium containing lentivirus served as the growth medium until red fluorescing glandoids appeared. Culture wells were subsequently photographed with an Olympus IX70 inverted microscope.
Engraftment of Glandoids Into Immunocompromised Mice
Glandoids were engrafted beneath the murine renal capsule using the method described at the following website: http://mammary.nih.gov/tools/mousework/Cunha001. Each glandoid remained intact during handling and was positioned beneath the renal capsule with a blunt glass probe, two to four glandoids per kidney in distinct locations. Glandoid position was easily observed using a Leica MZ9.5 dissecting stereomicroscope. TRPC glandoids were engrafted into castrated male SCID mice. LAPC9 glandoids were engrafted into intact non-obese diabetic/SCID (NOD/SCID; National Cancer Institute at Frederick) males supplemented with 9 mg testosterone pellets placed subcutaneously. Grafts were harvested at 6 or 8 weeks.
Immunofluorescent and Histological Analysis
Harvested xenografts were fixed in 10% neutral-buffered formalin and embedded in paraffin. Glandoids were prepared for paraffin processing by fixation in 10% neutral-buffered formalin for 1 hr and resuspension in 100 μl of type I rat tail collagen. The collagen was pipetted as a round button into a Petri dish, allowed to polymerize for 30 min at 37°C, and immersed in formalin overnight before paraffin embedding. Sections were cut on a microtome and mounted on microscope slides (VWR, 48311). For staining of intact structures, glandoids were left adherent to their culture wells or cover glasses and fixed in –20°C methanol for 10 min.
Conventional hematoxylin and eosin staining was performed for histological analysis. For indirect immunofluorescent analysis of sections, antigen unmasking was performed with pre-heated (95–99°C) citrate buffer (Vector, H-3300) in a Black and Decker steamer (HS800) for 30 min. Sections were permeabilized in 0.2% Triton X-100 (Sigma, T9284) in 2% normal goat serum for 30 min, and all materials were blocked with 5% normal goat serum in PBS for 2 hr to bind nonspecific sites. A monoclonal mouse antibody raised against cytokeratin 8 (Santa Cruz, sc-8020, 1:200) was used in combination with rabbit polyclonal antibodies raised against cytokeratin 5 (Santa Cruz, sc-66856, 1:200) or prostate-specific antigen (PSA) (Genetex, GTX72905, 1:100) in 5% normal goat serum and incubated overnight at room temperature. Secondary antibodies Alexa Fluor 546 goat anti-mouse IgG (Invitrogen, A-11003, 1:200) and Alexa Fluor 488 donkey anti-rabbit IgG (Invitrogen, A-21206, 1:200) diluted in PBS were then conjugated to the primary antibodies in a 40-min incubation at room temperature. DAPI staining was performed separately or with Vectashield HardSet Mounting Medium (Vector; H-1500). Sections were photographed with a Leica DM5000 B microscope. Glandoids stained in the culture wells were photographed with an Olympus IX70 inverted microscope.
A rabbit polyclonal antibody raised against chromogranin A (Dako, A0430, 1:1,000) was used in combination with mouse monoclonal anti-human nuclear antigen (Chemicon, MAB1281, 1:50) and incubated overnight at room temperature on the original cover glasses. Secondary antibodies Alexa Fluor 546 goat anti-mouse IgG and Alexa Fluor 633 F(ab)2 fragment of goat anti-rabbit IgG (Invitrogen, A-21072, 1:200) were then conjugated to the primary antibodies in a 40-min incubation at room temperature. This stain was photographed with a Leica TCS SP confocal microscope.
Conventional immunohistochemical staining was performed on glandoids adherent to their culture wells. Endogenous peroxidase activity was blocked with 0.3% hydrogen peroxide in methanol for 30 min; nonspecific sites were blocked with 5% normal swine serum in PBS for 2 hr. Anti-PSA antibody was diluted in 5% normal swine serum, and glandoids were incubated overnight at 4°C. Biotinylated polyclonal swine anti-rabbit secondary antibody (Dako, E0353, 1:500) was diluted in 5% normal swine serum, and the culture wells were incubated for 40 min at room temperature. Subsequently, the material was incubated with avidin–biotin complex (Vector, PK-6100) for 30 min at room temperature. Immunoreactivity was visualized with diaminobenzidine (DAB) (Dako, K3465); the material was lightly counterstained with hematoxylin.
RESULTS
In Vitro Assay: Technique
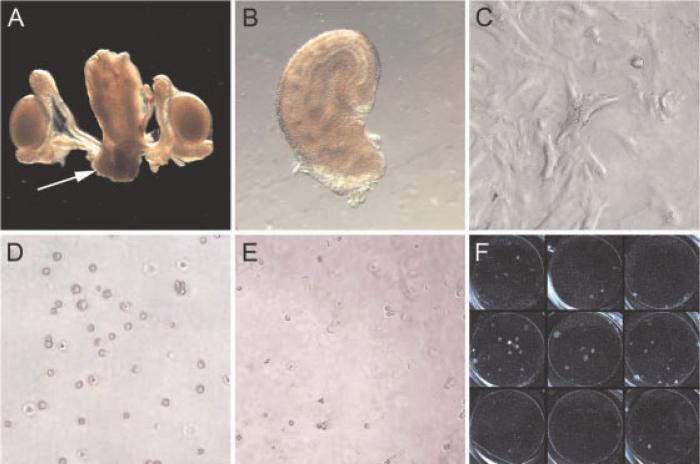
We obtained rSVM from male neonatal rats (day of life 1–4) via microdissection (see Fig. 1), as previously described. After harvest, rSVM was plated in 12-well plates and allowed to adhere and grow. At ~75% confluence, rSVM was irradiated with 30 Gy to prevent overgrowth. This radiation dose is enough to halt cell proliferation without causing cell death. Single-cell suspensions of human prostate cancer cells were then applied on top of this feeder layer to create a floating co-culture system. At 21–28 days, three-dimensional colonies we have termed glandoids are grossly visible (Fig. 1, panel F). No glandoids were visualized when xenograft cells were plated either alone or upon an STO cell feeder layer (data not shown).
Fig. 1.
In vivo assay technique. Urogenital tract from a neonatal rat male pup (day of life1) is shown in panel A with arrow pointing to seminal vesicle mesenchyme (SVM). Microdissected rat SVM (panel B) is then plated in a 12-well plate and allowed to reach ~75% confluence (panel C) at which point 30 Gy is administered to prevent further outgrowth. A single-cell suspension of human prostate cancer cells (panel D) is then placed on top of the irradiated rSVM feeder layer (panel E). Approximately 21–28 days later, three-dimensional colonies (“glandoids”) are grossly visible (panel F).
Non-Adherent Xenograft Prostate Cancer Cell Lines: TRPC and LAPC9
The non-adherent xenograft cell lines TRPC and LAPC9 were utilized for our studies. TRPC was isolated by our lab from a patient who had locally recurrent/progressive disease despite radiation therapy, hormone ablation, and docetaxel chemotherapy (see the Materials and Methods Section). Efforts to cultivate this line in vitro alone or with a fibroblast feeder layer (STO cells) have been unsuccessful (data not shown). LAPC9 is a well-established human prostate cancer xenograft cell line derived from a hormone refractory metastatic lesion [12]. Due to the difficulty of maintaining it in cell culture, it has largely been studied in vivo as a xenograft. Notably, some have reported in vitro colony-forming ability when LAPC9 is plated upon a feeder layer of fibroblasts [6]. Based upon the relatively poor performance of TRPC and LAPC9 in cell culture, we elected to use these non-adherent xenograft lines to test the efficacy of our in vitro assay of prostate tumor initiation. Cell composition and histology of the xenograft lines are shown in Figure 2.
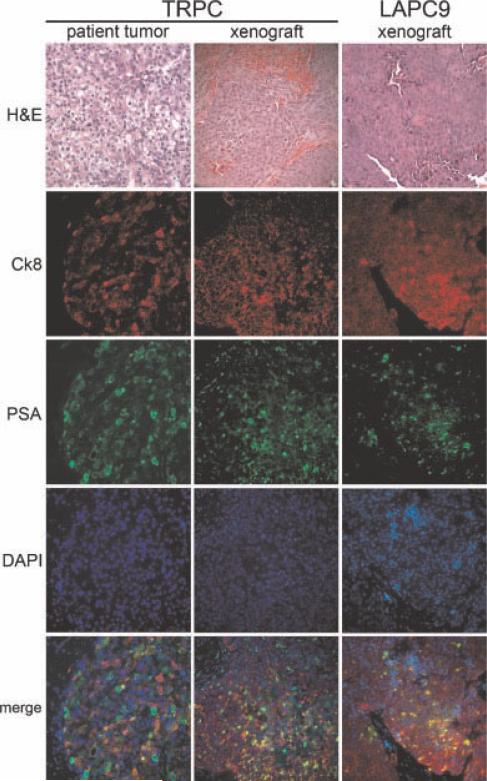
Fig. 2.
Non-adherent xenograft celllines TRPC and LAPC9. Treatment refractory prostate cancer (TRPC) and LAPC-9 histology and cell composition demonstrate xenografts that express cytokeratin 8 and PSA in the majority of cells. Cytokeratin 5 expression was not observed in either the TRPC xenograft or in TRPC glandoids; one LAPC9 glandoid was observed to have a small population of cytokeratin-5-positive cells (data not shown). Consistency of cell composition between TRPC directly from the patient and from a xenograft >8 generations later is alsonoted. DAPI = nuclear stain.
TRPC and LAPC9 Form Glandoids In Vitro
TRPC and LAPC9 glandoids were grossly visible by ~21 days in culture (see Figs. 3 and 4). TRPC-derived glandoids were observed to be more rounded while LAPC9 glandoids tended to be more flattened. Overall, we detected a relatively consistent glandoid forming rate for each cell line: TRPC 300–600 plated cells/glandoid and LAPC9 200–300 plated cells/glandoid. Glandoids were found to be composed of all cell types found in the parent xenograft (see Figs. 3 and 4). No cytokeratin-5-positive cells (basal cells) were detected in the TRPC xenograft or in TRPC glandoids that were examined. While cytokeratin-5-positive cells were not observed in the LAPC9 xenograft, one LAPC9 glandoid was found to have a small cytokeratin 5 positive population (data not shown).
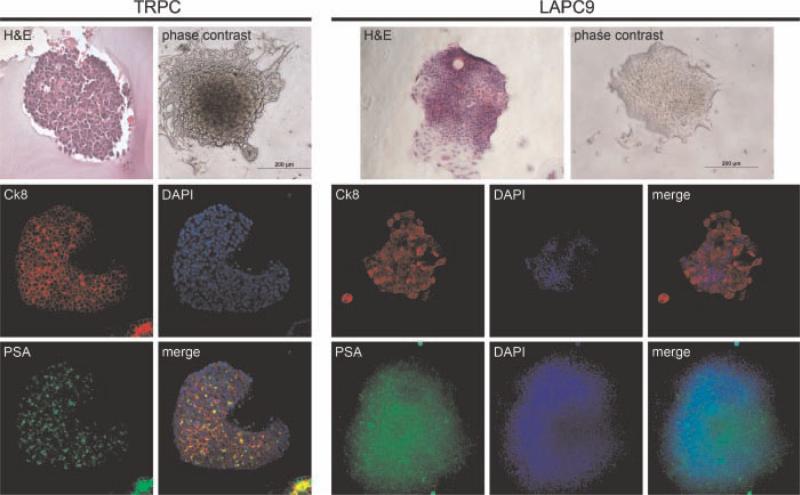
Fig. 3.
TRPC- and LAPC9-derived glandoids. Characterization of glandoids obtained from TRPC demonstrates fidelity with regard to H&E (glandoid embedded in collagen after retrieval from plate to facilitate handling), cytokeratin 8, and PSA staining when compared to thexenograft line. Cytokeratin 5 expression was not observed (data not shown). Similarly, characterization of glandoids obtained from LAPC9 demonstrate uniformity with regard to H&E, cytokeratin 8, and PSA staining when compared to the xenograft line.
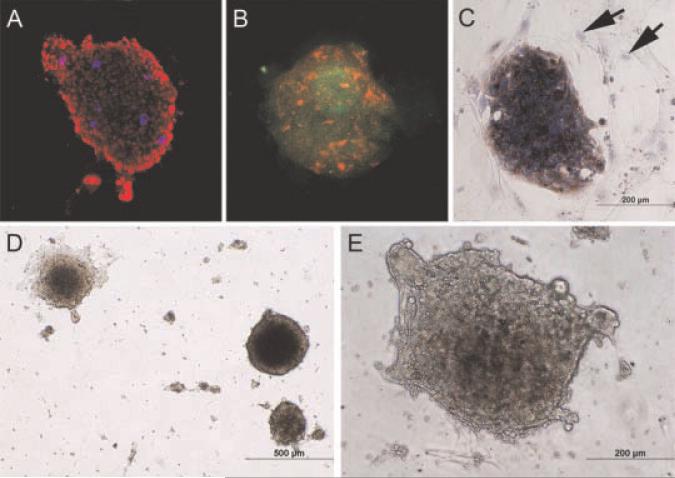
Fig. 4.
Additional glandoid characterization.Panel A depicts human nuclear antigen staining (red) and chromogranin A expression (blue) within a TRPC-derive dglandoid. Panels B and C demonstrate glandoids obtained directly from the patient whose tumor became TRPC (panel B: green = PSA,red = cytokeratin 8; Panel C: brown = PSA, blue = hematoxylin counter stain, arrows denoter SVM stromal cells). Panels D (low power) and E (high power) show phase-contrast images of TRPC glandoids in culture.
TRPC and LAPC9 Glandoids Are Tumorigenic
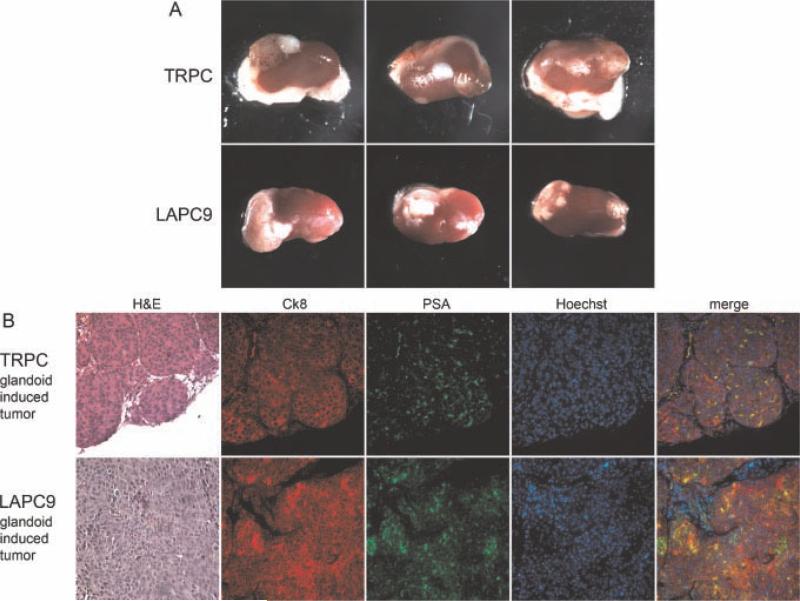
To assess tumorigenicity, we micro-surgically transplanted single glandoids without rSVM into the renal capsule of immunodeficient mice. At 6 or 8 weeks, engrafted kidneys were harvested and analyzed. Gross tumors developed in 6/9 TRPC and 9/9 LAPC9 glandoids implanted (see Fig. 5, panel A). No obvious evidence of metastasis was noted on harvest. Immunocytochemical analysis of glandoid-induced tumors (GITs) revealed cell composition similar to that of the parent glandoid and xenograft cell line (see Fig. 5, panel B). Moreover, GITs and the parental xenograft appeared histologically similar.
Fig. 5.
Glandoids induce in vivo tumors. Singly implanted (without rSVM) TRPC and LAPC9 glandoids generate gross tumors within the renal capsule of immunodeficient mice by 8 weeks (panel A). Glandoid-induced tumors (GITs) derived from both TRPC and LAPC9 demonstrate identical histology and expression of cytokeratin 8 and PSA as that seen in the parent xenograft (panel B). DAPI = nuclear stain.
Glandoids Show Evidence of Clonality
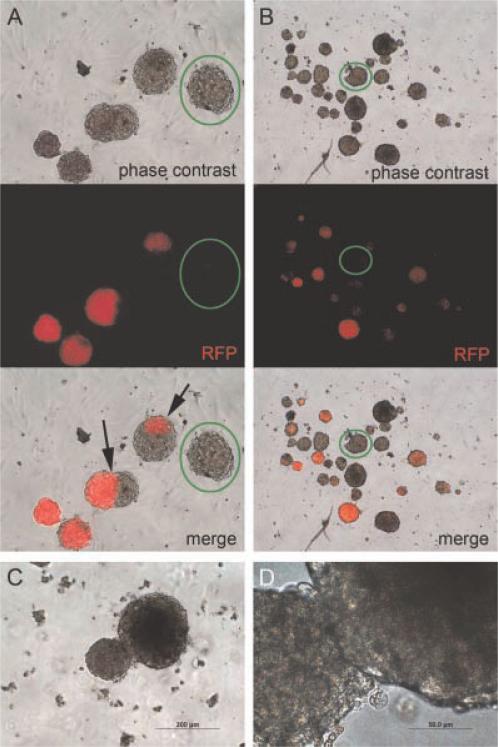
One possible way by which glandoids form is simply through aggregation of input cells. This type of event would yield heterogeneous populations, but would not represent a true tumor-initiating cell assay, which is a central goal of our work. To begin to evaluate whether glandoids arise from a single-initiating cell versus a cellular aggregate, we employed a cell autonomous marking strategy. Specifically, primary TRPC glandoids were harvested, pooled together, and gently treated with trypsin to create a single-cell suspension. The cells were then transduced with a lentivirus vector encoding the red fluorescent protein (RFP), and plated in the glandoid assay. As shown in Figure 6 resultant glandoids were observed to be one of three kinds: (i) all red, (ii) completely colorless, or (iii) composed of a small pocket of a minority color of cells (either red or colorless depending on the majority cell color). Isolated clusters of uniformly colored cells within glandoids are thought to have occurred via glandoid fusion. The fact that the no red speckled or polka dot appearing glandoids were detected suggests that glandoids are clonal and not the result of cell aggregation.
Fig. 6.
Glandoids and clonality. RFP (red fluorescent protein) lenti-virus-treated TRPC cells generated glandoids that were either all red or all not red (columns A and B). Arrows point to clustered areas of red cells suggesting fusion of separate glandoids. Green circles denote RFP negative glandoids.The lack of red speckled or red polka dot appearing glandoids suggests that cell aggregation is not likely to account for glandoid formation and provides evidence in support of glandoid clonality. Panels C (10X) and D (40×) depict the adhesion of two neighboring glandoids.
DISCUSSION
Methodologies that enable laboratory modeling of primary prostate cancer are desperately needed. Such systems would allow the interrogation of tumor-initiating cell biology as well as drug discovery, among other things, from clinically relevant tissue samples. In this report, we describe an in vitro functional assay of prostate cancer initiation that utilizes rSVM as a feeder layer. Employing this technique with non-adherent human prostate cancer cells plated on the surface, we observed the formation of clusters of dense cellular spheres that we have termed glandoids, at a reproducible frequency. Additionally, we have shown that glandoids share cell composition with the parent xenograft and are tumorigenic, an important observation that defines glandoids as tumor initiating units. Furthermore, we have presented evidence suggesting that glandoids are clonal; thus, demonstrating a way the presented technique may be used to gain insights into prostate tumor-initiating cell biology.
For decades investigators have sought to develop in vitro culture systems that are conducive to studying non-adherent patient-derived human prostate cancer cells. To be sure, the concept of an in vitro feeder layer is not new and has been used widely in other organ systems to facilitate primary cell culture [13–16]. In the prostate, the mouse embryonic fibroblast cell line 3T3 has been employed and has met with some success vis-à-vis supporting in vitro growth of primary or xenograft prostate cancer cell lines [6,8]. Immortalized 3T3 cells (STO cells) and mesenchymal stem cells have been used to cultivate primary epithelial cells from other organs but have not been fully explored in the prostate [17,18]. We elected to utilize rSVM as a mesenchymal feeder layer given the tremendous success of this tissue to support modeling of human prostate tissue in vivo with tissue recombination. To our knowledge, this is the first report of using rSVM to facilitate in vitro culture of non-adherent human prostate cancer cells.
Human prostasphere forming assays utilizing non-adherent cell culture conditions derived from primary surgical specimens have been reported. These studies have been able to demonstrate that prostaspheres cultivated in this manner possess phenotypic characteristics (e.g., PSA expression, cytokeratin 8 expression, TMPRSS/ERG gene fusion) of cancer and functional attributes (e.g., self-renewal) of tumor-initiating cells in vitro [19]. Importantly, to date, no one has reported the ability to form prostaspheres directly from patient samples that are themselves tumorigenic in vivo. The reasons for this are likely multifold and may be related to (i) the tissue source (i.e., grade/stage, treatment history), (ii) tissue/cell handling (i.e., pathological manipulation, cell separation techniques), (iii) in vitro culture conditions (i.e., feeder layer, media, growth factors), and (iv) the immunodeficient animal used (i.e., nude vs. NOD vs. NOD/SCID vs. NOD/SCID gamma vs. Rag2 knockout mice). As an initial attempt to circumvent these obstacles we tested the ability of rSVM to act as a feeder layer to non-adherent human prostate cancer xenograft lines in vitro and found that the resultant sphere-like structures (“glandoids”) were tumorigenic.
Based on these findings, we propose that rSVM may serve as a viable feeder layer for primary human prostate cancer cells in vitro, perhaps owing to either a secreted or cell surface factor. Further studies that seek to understand the precise mechanisms by which rSVM can support human prostate cancer cell growth in vitro are needed. In addition, experiments that aim to grow glandoids directly from patient samples and that assay glandoids for tumorigenicity need to be performed to validate this technique for use with primary patient samples. Nevertheless, the assay as presently described has potential to interrogate tumor-initiating cell properties and explore therapeutic targets in xenograft prostate cancer cell lines.
CONCLUSION
Here, we report on the capacity of rSVM to serve as a feeder layer to non-adherent human prostate cancer cells in vitro. Xenograft maintained cells that are grown in this way form three-dimensional structures that (i) comprised all relevant cell types, (ii) give evidence for clonality, and (iii) are tumorigenic. The described methodology may prove useful in studying tumor-initiating cell biology and drug susceptibility of xenograft maintained cancer cell lines and if validated with primary cells, may dramatically improve our ability to model human prostate cancer in the laboratory.
ACKNOWLEDGMENTS
G.S.P. is funded by Department of Defense award W81XWH-08-1-0508, AUA Foundation Rising Stars in Urology award, NE section-AUA Young Investigators award, and the Prostate Cancer Foundation. J.H. is supported by grants from American Cancer Society (RSG-07-092-01-TBE), Department of Defense Prostate Cancer Research Program (PC061456), a developmental research award from UCLA SPORE in prostate cancer, and a challenge award from the Prostate Cancer Foundation.
Grant sponsor: Prostate Cancer Foundation; Grant sponsor: American Cancer Society; Grant number: RSG-07-092-01-TBE; Grant sponsor: Department of Defense Prostate Cancer Research Program; Grant number: PC073121, PC061456.
REFERENCES
- 1.Singh SK, Hawkins C, Clarke ID, Squire JA, Bayani J, Hide T, Henkelman RM, Cusimano MD, Dirks PB. Identification of human brain tumour initiating cells. Nature. 2004;432(7015):396–401. doi: 10.1038/nature03128. [DOI] [PubMed] [Google Scholar]
- 2.Ginestier C, Hur MH, Charafe-Jauffret E, Monville F, Dutcher J, Brown M, Jacquemier J, Viens P, Kleer CG, Liu S, Schott A, Hayes D, Birnbaum D, Wicha MS, Dontu G. ALDH1 is a marker of normal and malignant human mammary stem cells and a predictor of poor clinical outcome. Cell Stem Cell. 2007;1(5):555–567. doi: 10.1016/j.stem.2007.08.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.O'Brien CA, Pollett A, Gallinger S, Dick JE. A human colon cancer cell capable of initiating tumour growth in immunodeficient mice. Nature. 2007;445(7123):106–110. doi: 10.1038/nature05372. [DOI] [PubMed] [Google Scholar]
- 4.Collins AT, Berry PA, Hyde C, Stower MJ, Maitland NJ. Prospective identification of tumorigenic prostate cancer stem cells. Cancer Res. 2005;65(23):10946–10951. doi: 10.1158/0008-5472.CAN-05-2018. [DOI] [PubMed] [Google Scholar]
- 5.Gu G, Yuan J, Wills M, Kasper S. Prostate cancer cells with stem cell characteristics reconstitute the original human tumor in vivo. Cancer Res. 2007;67(10):4807–4815. doi: 10.1158/0008-5472.CAN-06-4608. [DOI] [PubMed] [Google Scholar]
- 6.Patrawala L, Calhoun T, Schneider-Broussard R, Li H, Bhatia B, Tang S, Reilly JG, Chandra D, Zhou J, Claypool K, Coghlan L, Tang DG. Highly purified CD44+ prostate cancer cells from xenograft human tumors are enriched in tumorigenic and metastatic progenitor cells. Oncogene. 2006;25(12):1696–1708. doi: 10.1038/sj.onc.1209327. [DOI] [PubMed] [Google Scholar]
- 7.Patrawala L, Calhoun-Davis T, Schneider-Broussard R, Tang DG. Hierarchical organization of prostate cancer cells in xenograft tumors: The CD44+alpha2beta1+ cell population is enriched in tumor-initiating cells. Cancer Res. 2007;67(14):6796–6805. doi: 10.1158/0008-5472.CAN-07-0490. [DOI] [PubMed] [Google Scholar]
- 8.Miki J, Rhim JS. Prostate cell cultures as in vitro models for the study of normal stem cells and cancer stem cells. Prostate Cancer Prostatic Dis. 2008;11(1):32–39. doi: 10.1038/sj.pcan.4501018. [DOI] [PubMed] [Google Scholar]
- 9.Cunha GR, Chung LW. Stromal-epithelial interactions—I. Induction of prostatic phenotype in urothelium of testicular feminized (Tfm/y) mice. J Steroid Biochem. 1981;14(12):1317–1324. doi: 10.1016/0022-4731(81)90338-1. [DOI] [PubMed] [Google Scholar]
- 10.Litvinov IV, Vander Griend DJ, Xu Y, Antony L, Dalrymple SL, Isaacs JT. Low-calcium serum-free defined medium selects for growth of normal prostatic epithelial stem cells. Cancer Res. 2006;66(1):8598–8607. doi: 10.1158/0008-5472.CAN-06-1228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Lawson DA, Xin L, Lukacs RU, Cheng D, Witte ON. Isolation and functional characterization of murine prostate stem cells. Proc Natl Acad Sci USA. 2007;104(1):181–186. doi: 10.1073/pnas.0609684104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Craft N, Chhor C, Tran C, Belldegrun A, DeKernion J, Witte ON, Said J, Reiter RE, Sawyers CL. Evidence for clonal outgrowth of androgen-independent prostate cancer cells from androgen-dependent tumors through a two-step process. Cancer Res. 1999;59(19):5030–5036. [PubMed] [Google Scholar]
- 13.Matouskova E, Dudorkinova D, Krasna L, Vesely P. Temporal in vitro expansion of the luminal lineage of human mammary epithelial cells achieved with the 3T3 feeder layer technique. Breast Cancer Res Treat. 2000;60(3):241–249. doi: 10.1023/a:1006409605067. [DOI] [PubMed] [Google Scholar]
- 14.Tenchini ML, Ranzati C, Malcovati M. Culture techniques for human keratinocytes. Burns. 1992;18(Suppl 1):S11–S16. doi: 10.1016/0305-4179(92)90104-3. [DOI] [PubMed] [Google Scholar]
- 15.Paraskeva C, Buckle BG, Sheer D, Wigley CB. The isolation and characterization of colorectal epithelial cell lines at different stages in malignant transformation from familial polyposis coli patients. Int J Cancer. 1984;34(1):49–56. doi: 10.1002/ijc.2910340109. [DOI] [PubMed] [Google Scholar]
- 16.Whitehead RH, Brown A, Bhathal PS. A method for the isolation and culture of human colonic crypts in collagen gels. In Vitro Cell Dev Biol. 1987;23(6):436–442. doi: 10.1007/BF02623860. [DOI] [PubMed] [Google Scholar]
- 17.Park SP, Lee YJ, Lee KS, Ah Shin H, Cho HY, Chung KS, Kim EY, Lim JH. Establishment of human embryonic stem cell lines from frozen-thawed blastocysts using STO cell feeder layers. Hum Reprod (Oxford, Engl) 2004;19(3):676–684. doi: 10.1093/humrep/deh102. [DOI] [PubMed] [Google Scholar]
- 18.Shamblott MJ, Axelman J, Wang S, Bugg EM, Littlefield JW, Donovan PJ, Blumenthal PD, Huggins GR, Gearhart JD. Derivation of pluripotent stem cells from cultured human primordial germ cells. Proc Natl Acad Sci USA. 1998;95(23):13726–13731. doi: 10.1073/pnas.95.23.13726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Guzman-Ramirez N, Voller M, Wetterwald A, Germann M, Cross NA, Rentsch CA, Schalken J, Thalmann GN, Cecchini MG. In vitro propagation and characterization of neoplastic stem/progenitor-like cells from human prostate cancer tissue. Prostate. 2009;69(15):1683–1693. doi: 10.1002/pros.21018. [DOI] [PubMed] [Google Scholar]