Abstract
The outcome for patients with refractory or relapsed acute lymphoblastic leukemia (ALL) treated with conventional therapy is poor. Immunoconjugates present a novel approach and have recently been shown to have efficacy in this setting. Combotox is a mixture of two ricin-conjugated monoclonal antibodies (RFB4 and HD37) directed against CD19 and CD22, respectively, and has shown activity in pediatric and adult ALL. We created a murine xenograft model of advanced ALL using the NALM/6 cell line to explore whether the combination of Combotox with the cytotoxic agent cytarabine (Ara-C) results in better outcomes. In our model the combination of both low- and high-dose Combotox and Ara-C resulted in significantly longer median survival. Sequential administration of Ara-C and Combotox, however, was shown to be superior to concurrent administration. These findings have led to a phase I clinical trial exploring this combination in adults with relapsed or refractory B-lineage ALL (ClinicalTrials.gov identifier NCT01408160).
Keywords: Leukemia, acute lymphoblastic, immunoconjugate, cytarabine, ricin, animal model, mouse, NOD
Introduction
Relapsed or refractory (r/r) acute lymphoblastic leukemia (ALL) in adults has a poor prognosis when treated with conventional therapy. Only 7–12% of these patients become long-term survivors [1,2]. Novel approaches are urgently needed to improve the outcomes for this patient population. One promising new approach is exemplified by a class of anticancer agents termed immunoconjugates (ICs) or immunotoxins (ITs). Immunotoxins are composed of a potent toxin linked to a moiety designed to specifically target tumor cells [3,4]. These agents have two main advantages over cytotoxic agents: first, by directly targeting certain surface markers they maximize drug delivery to tumor cells without increasing toxicity to normal cells; second, they use a different mechanism to induce cell killing when compared with traditional cytotoxic drugs, thus potentially circumventing chemoresistance.
Combotox is such an IT. It is a 1:1 mixture of two murine monoclonal immunoglobulin G1 (IgG1) antibodies: HD37, directed against CD19, and RFB4, directed against CD22. They are coupled to a deglycosylated ricin-A chain (dgRTA) [5]. RTA inhibits protein synthesis by catalytically inactivating the ribosomal RNA. The antigens targeted with this drug, CD19 and CD22, are present on leukemic blasts in the vast majority of patients with B-lineage ALL [6,7]. Immunoconjugates targeting CD19, CD22 or both antigens at the same time have shown promising efficacy in r/r ALL in early clinical trials [8–13]. Although HD37-dgRTA and RFB4-dgRTA are active when given individually, their ability to kill tumor cells is additive when both are given together, and the combination is more efficacious than either agent alone in vivo [14].
The safety and efficacy of Combotox has been explored in two phase I clinical trials: one in pediatric patients and another in adult patients, both with r/r ALL [8,13]. Single-agent Combotox proved efficacious with an overall response rate (complete, partial and hematological response) of 53% for children and 31% for adults, but the responses were usually short-lived. There is in vivo evidence that the efficacy of immunotoxins can be further improved when they are combined with cytotoxic agents [15,16].
Our objective was to test the efficacy of combination therapy with Combotox and cytarabine (Ara-C), a cytotoxic agent commonly used in the treatment of ALL, and to explore whether concurrent or sequential administration results in improved efficacy in a murine xenograft model of advanced precursor B-cell ALL.
Materials and methods
Murine xenograft model
A human pre-B lymphoblast cell line NALM/6 [16] was cultured in RPMI 1640 medium with 15% fetal bovine serum and incubated at 37°C with 5% CO2 and 95% humidified air. The cells were maintained at a concentration of 4–9 × 105 cells/mL by adding fresh medium daily. Before inoculation, the cells were concentrated by centrifugation to 50 ×106 cells/mL and re-suspended in serum-free medium. Six-to-eight week old non-obese diabetic mice (NOD.Cg-Prkdcscid Il2rg tm1Wjl /SzJ) were purchased from The Jackson Laboratory (Bar Harbor, ME). These mice are maintained and used only under an animal use protocol approved by the Animal Institute Committee.
The mice were inoculated via tail vein injection with 8–10×106 NALM/6 cells to establish a murine xenograft model of advanced B-lineage ALL. About 50 μL of blood from each mouse was taken from a facial vein on the day of administration of the first treatment. The presence of leukemic blasts in the blood was confirmed microscopically (Figure 1).
Figure 1.
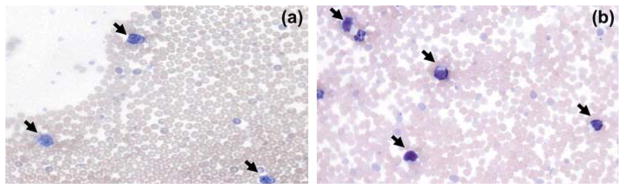
Lymphoblasts (black arrows) in peripheral blood of a NOD mouse injected with NALM/6 cells on days 7 (a) and 14 (b) after inoculation.
Combotox
Combotox is a 1:1 mixture of anti-CD19 (HD37)-dgRTA and anti-CD22 (RFB4)-dgRTA. Both murine monoclonal IgG1 antibodies RFB4 (anti-CD22) and HD37 (anti-CD19) are coupled to the deglycosylated ricin-A chain (dgRTA) via a heterobifunctional, thiol-containing cross-linker, N-succinimidyl-oxycarbonyl-a-methyl-a-(2-pyridyldithio)toluene (SMPT) [5].
RFB4-dgRTA and HD37-dgRTA were prepared in the Good Manufacturing Practice (GMP) laboratory at the University of Texas Southwestern Medical Center (UTSWMC) and shipped overnight on dry ice. They were stored in a −85°C freezer. On the day of planned administration, the vials were thawed and mixed in a 1:1 ratio to give Combotox. The infusions were held at 4 °C until administered.
Cytarabine
Cytarabine (or Ara-C) was purchased from Hospira (Hospira ©, Inc., Lake Forest, IL) as vials of 100 mg dry powder for injection. The powder was then reconstituted for injection on the day of administration with normal saline.
Treatment on a concurrent schedule
In the first experiment mice were divided into four groups and treated daily with intravenous injection of: (1) Ara-C, (2) Combotox, (3) Ara-C concurrent with Combotox or (4) normal saline (vehicle control), respectively. The doses were 100 mg/kg daily × 6 for Ara-C and 0.8 mg/kg anti-CD19-dgRTA plus 0.5 mg/kg anti-CD22-dgRTA per injection daily × 6 for Combotox [Figure 2(A)]. Treatment started on day 12, counted from the day of NALM/6 inoculation.
Figure 2.
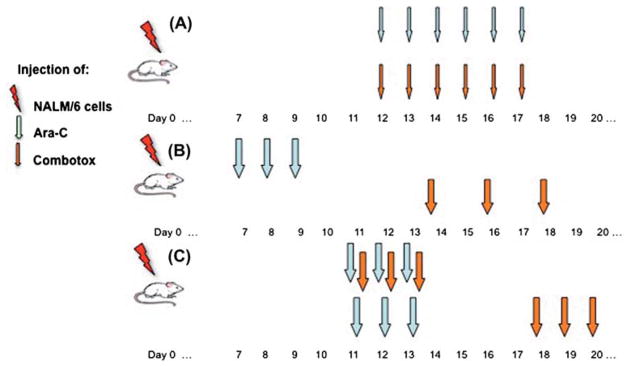
Schema demonstrating administration schedule for each experiment: (A) experiment 1 (concurrent administration of cytarabine [Ara-C] and Combotox), (B) experiment 2 (sequential administration) and (C) experiment 3 (comparison of concurrent and sequential administration). Thin arrows indicate lower doses, whereas bolder arrows indicate higher doses of Ara-C and Combotox (see text).
Treatment on a sequential schedule
In the second experiment, mice were also divided into four groups. The treatment was similar to the first experiment, except that the combination treatment with Ara-C and Combotox was on a sequential schedule. The treatment schedule was as follows: (1) Ara-C on days 7–9 after inoculation; (2) Combotox on days 14, 16 and 18; (3) Ara-C on days 7–9 followed by Combotox on days 14, 16 and 18; or (4) normal saline (control) on days 7–9, 14, 16 and 18. The doses were 200 mg/kg daily ×3 for Ara-C and 2.4 mg/kg anti-CD19-dgRTA plus 1.5 mg/kg anti-CD22-dgRTA per injection every other day ×3 for Combotox [Figure 2(B)].
Comparison of concurrent and sequential schedules
In the third experiment, we compared the combination treatment between the concurrent and sequential schedules. The mice were randomly divided into three groups of eight mice each on day 11. Two groups were treated with tail vein injections of 200 mg/kg daily Ara-C for 3 days (days 11–13) and Combotox (2.4 mg/kg anti-CD19 +1.5 mg/kg anti-CD22 daily ×3). Combotox was given either concurrently with Ara-C on days 11–13 (concurrent schedule) or sequentially on days 18–20 (sequential schedule). The third group of mice was injected intravenously with an equal volume of normal saline and served as vehicle control [Figure 2(C)].
Statistics
The median survival time (MST) for each treatment group was calculated. Overall survival was plotted as a Kaplan–Meier curve and a two-sided log-rank test was performed. A p-value <0.05 indicated statistical significance. Animals that died immediately after injection of treatment agents (accidental deaths) were excluded from the analysis. The statistical analysis was performed using GraphPad Prism(R) version 5.0 (GraphPad Software, La Jolla, CA).
Results
Inoculation of NALM/6 cells into NOD mice results in an in vivo model of advanced disease
Since relapsed or refractory ALL usually presents in advanced stages with increased peripheral blasts, we wanted to develop an in vivo animal model that represented this stage of the human disease. Therefore, a large number of NALM/6 cells (8–10 ×106) were injected in the tail veins of NOD mice. Peripheral smears of animals were tested at various days after inoculation. We were able to detect numerous peripheral blood leukemic cells from day 7 onward, mimicking advanced disease in humans. Thus, we determined that this would be an adequate time to test the therapeutic potential of the antileukemic agents Combotox and cytarabine.
Concurrent administration of low doses of chemo- and immunotherapy leads to increased survival
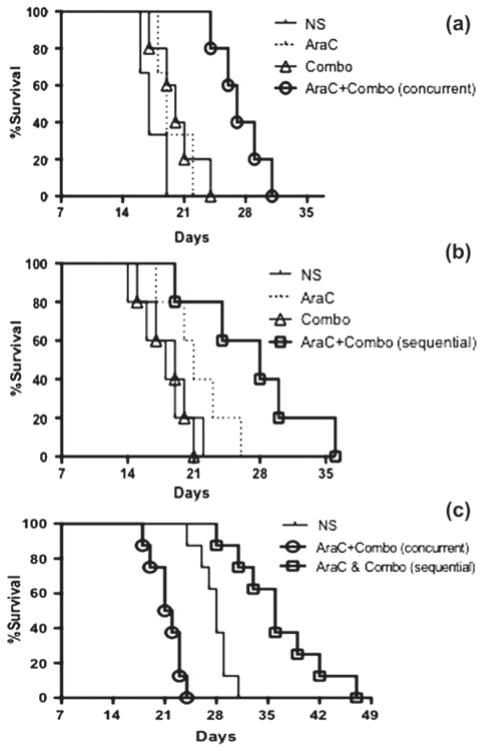
For the first cohort we used 100 mg/m 2 of Ara-C given daily for 6 days and 0.8 mg/kg anti-CD19-dgRTA plus 0.5 mg/kg anti-CD22-dgRTA (Combotox) per injection daily for 6 days. These regimens were tested alone and concurrently with each other. We observed that combination therapy led to increased survival, with a MST of 27 days in the cohort that received concurrent chemoimmunotherapy compared to 17, 19 and 20 days, respectively, in the placebo, Ara-C only and Combotox only treated groups [p-value <0.05; Table I and Figure 3(a)].
Table I.
Median survival times (MSTs) for each cohort of each experiment.
| Group | MST | p-Value* |
|---|---|---|
| Concurrent (experiment 1) | ||
| 1 (Ara-C; n =5) | 19 | <0.05 |
| 2 (Combotox; n =5) | 20 | |
| 3 (Ara-C and Combotox; n =5) | 27 | |
| 4 (normal saline; n =5) | 17 | |
| Sequential (experiment 2) | ||
| 1 (Ara-C; n =5) | 20 | <0.05† |
| 2 (Combotox; n =5) | 18 | |
| 3 (Ara-C and Combotox; n =5) | 28 | |
| 4 (normal saline; n =5) | 19 | |
| Concurrent vs. sequential (experiment 3) | ||
| 1 (Ara-C and Combotox concurrent; n =8) | 22 | <0.001 |
| 2 (Ara-C and Combotox sequential; n =8) | 36 | |
| 3 (normal saline; n =8) | 28 | |
p-Value calculated for overall survival difference with two-sided log-rank test.
p-Value for survival analysis calculated excluding mice that died accidentally on day of facial vein blood draw (two per group). If these mice were included, p-value would be 0.13.
Figure 3.
(a) Concurrent schedule (experiment 1) of Ara-C and Combotox. (b) Sequential schedule (experiment 2) of Ara-C and Combotox. (c) Sequential versus concurrent schedule of Ara-C and Combotox (experiment 3). Ara-C, cytarabine; NS, normal saline; Combo, Combotox.
Sequential administration of high doses leads to increased survival
For the second and third experiments we increased the total dose of Combotox to reflect the equivalence of human doses that have been used in the clinical setting [13]. The dose of Combotox was 2.4 mg/kg anti-CD19-dgRTA plus 1.5 mg/kg anti-CD22-dgRTA per injection × 3, which was derived from calculation of the maximum tolerated dose (MTD) in humans. Additionally, the daily dose of Ara-C was increased to 200 mg/m2 and treatment was given over a shorter period of time, thereby mimicking regimens used in the treatment of acute leukemias in humans.
In the second experiment we tested these higher doses alone or sequentially in mice with advanced disease. We observed significantly improved MST in the combination group (MST 28 days) compared to 19, 20 and 18 days, respectively, in the placebo, Ara-C only and Combotox only treated groups [p-value < 0.05; Table I and Figure 3(b)].
Having demonstrated the efficacy of the low-dose concurrent combination and high-dose sequential combination, we next sought to perform a head-to-head comparison of these two regimens at the higher dose level. Thus, mice in the third experiment were treated by either Ara-C given concurrently with Combotox on days 11–13 or Ara-C given by itself on days 11–13 followed by Combotox on days 18–20. In this experiment only the sequential administration of Ara-C and Combotox resulted in a survival advantage compared with saline-injected controls (p <0.001). No improved survival could be seen when Ara-C and high-dose Combotox were given concurrently [Table I and Figure 3(C)].
Discussion
Our results demonstrate that combined administration of the cytotoxic agent cytarabine and the immunotoxin Combotox has synergistic activity in an in vivo model of advanced B-lineage ALL. On direct comparison, sequential administration of both drugs at doses calculated to simulate human maximum tolerated doses resulted in increased survival when compared to concurrent administration.
These findings provide further evidence that chemoimmunotherapy results in superior outcomes when compared to cytotoxic therapy alone. There is ample evidence that combining cytotoxic agents with immunotherapy results in superior efficacy. For many hematologic malignancies, combining cytotoxic agents with immunotherapy has become the standard of care. The most notable example is the successful addition of the CD20-directed antibody rituximab to various chemotherapeutic backbones in indolent and aggressive CD20-positive B-cell malignancies, including mature B-cell ALL [17–22].
Another antigen, CD19, is present on virtually all malignant lymphoblasts in patients with B-lineage ALL, whereas the CD22 epitope is expressed on 80% of the blast population [23]. This makes these antigens excellent targets for immunotherapy in B-cell ALL. Both in vitro and in vivo experiments provide evidence for the activity of CD19 and CD22 antibody–drug conjugates against B-lineage leukemic lymphoblasts [14,16,24–28]. The combination of cytotoxic drugs and agents targeting CD19 and/or CD22 was shown in in vitro experiments and xenograft models of early ALL to result in synergy [16,29].
Whereas these earlier experiments attempted to recapitulate stages of ALL with less disease burden by treating xenografted mice with antileukemic agents only 24 h after inoculation with the malignant cell line NALM/6 [16,25,27], our goal was to mimic an advanced leukemic phenotype. Ara-C and/ or Combotox was administered only when advanced disease with peripheral blasts was established. At this time leukemic blasts were readily detectable in the peripheral blood. Therefore the leukemic burden in our model was much higher, and our experiment more reliably recapitulates the clinical picture encountered with human relapsed disease.
The observation that single-agent administration of only Ara-C or Combotox showed no significant survival difference in advanced disease as compared to placebo is noteworthy, but not entirely surprising. This is in line with the generally accepted fact that, in patients with advanced ALL, single-agent therapy is usually insufficient: outcomes are disappointing and the multi-agent approach needed to control this aggressive malignancy represents the current accepted standard of care [30–35].
The combination of cytarabine and Combotox is novel, and has been explored for the first time in this study. We assessed the antileukemic effect of the administered agents on the basis of median survival time. The xenografted mice could have died of either uncontrolled leukemia or toxicity of the administered drugs. As expected, none of the animals exhibited any visible signs of vascular leak syndrome, which is the most severe toxicity of Combotox, thus indicating that they died of disease progression. It is also reassuring that mice consistently survived longer when treated with chemoimmunotherapy in all three experiments. This suggests a powerful antileukemic effect from the combination of cytarabine and Combotox, which resulted in longer survival. On the other hand, the observation that concurrent administration of high-dose Ara-C and Combotox resulted in survival similar to that of placebo-treated mice suggests that the significant toxicity of the concomitant therapy could have negated any antileukemic effect at these dose levels.
In summary, chemoimmunotherapy with Ara-C and Combotox improved survival when given sequentially in a murine model of advanced B-lineage ALL. This is a novel combination that has not been previously studied. The findings of our study have led to the design of a current phase I clinical trial that seeks to explore the safety and efficacy of Ara-C combined with Combotox in adults with relapsed or refractory ALL (ClinicalTrials.gov identifier NCT01408160).
Acknowledgments
The work was supported in part by the Paul Calabresi Career Development Award for Clinical Oncology (K12CA132783-03 grant).
The authors thank Ellen S. Vitetta, PhD and Victor Ghetie, PhD at the Cancer Immunobiology Center of the University of Texas Southwestern Medical Center for their scientific support and expert advice.
Footnotes
Potential conflict of interest: Disclosure forms provided by the authors are available with the full text of this article at www.informahealthcare.com/lal.
References
- 1.Fielding AK, Richards SM, Chopra R, et al. Outcome of 609 adults after relapse of acute lymphoblastic leukemia (ALL); an MRC UKALL12/ ECOG 2993 study. Blood. 2007;109:944–950. doi: 10.1182/blood-2006-05-018192. [DOI] [PubMed] [Google Scholar]
- 2.Faderl S, O’Brien S, Pui C-H, et al. Adult acute lymphoblastic leukemia. Cancer. 2010;116:1165–1176. doi: 10.1002/cncr.24862. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.FitzGerald DJ, Wayne AS, Kreitman RJ, et al. Treatment of hematologic malignancies with immunotoxins and antibody-drug conjugates. Cancer Res. 2011;71:6300t–6309. doi: 10.1158/0008-5472.CAN-11-1374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Schindler J, Sausville E, Messmann R, et al. The toxicity of deglycosylated ricin A chain-containing immunotoxins in patients with non-Hodgkin’s lymphoma is exacerbated by prior radiotherapy: a retrospective analysis of patients in five clinical trials. Clin Cancer Res. 2001;7:255–258. [PubMed] [Google Scholar]
- 5.Messmann RA, Vitetta ES, Headlee D, et al. A phase I study of combination therapy with immunotoxins IgG-HD37-deglycosylated ricin A chain (dgA) and IgG-RFB4-dgA (Combotox) in patients with refractory CD19(+), CD22(+) B cell lymphoma. Clin Cancer Res. 2000;6:1302–1313. [PubMed] [Google Scholar]
- 6.Jegalian AG, Wayne AS, Kreitman RJ, et al. CD22 expression in pediatric B-lineage acute lymphoblastic leukemia. Blood. 2009;114(Suppl 1):Abstract 4119. [Google Scholar]
- 7.May RD, Vitetta ES, Moldenhauer G, et al. Selective killing of normal and neoplastic human B cells with anti-CD19- and anti-CD22-ricin A chain immunotoxins. Cancer Drug Deliv. 1986;3:261–272. doi: 10.1089/cdd.1986.3.261. [DOI] [PubMed] [Google Scholar]
- 8.Herrera L, Bostrom B, Gore L, et al. A phase 1 study of Combotox in pediatric patients with refractory B-lineage acute lymphoblastic leukemia. J Pediatr Hematol Oncol. 2009;31:936–941. doi: 10.1097/MPH.0b013e3181bdf211. [DOI] [PubMed] [Google Scholar]
- 9.Topp MS, Zugmaier G, Goekbuget N, et al. Report of a phase II trial of single-agent BiTE(R) antibody blinatumomab in patients with minimal residual disease (MRD) positive B-precursor acute lymphoblastic leukemia (ALL) Blood. 2009;114(Suppl 1):Abstract 840. [Google Scholar]
- 10.Topp MS, Kufer P, Gökbuget N, et al. Targeted therapy with the T-cell-engaging antibody blinatumomab of chemotherapy-refractory minimal residual disease in B-lineage acute lymphoblastic leukemia patients results in high response rate and prolonged leukemia-free survival. J Clin Oncol. 2011;29:2493–2498. doi: 10.1200/JCO.2010.32.7270. [DOI] [PubMed] [Google Scholar]
- 11.Dominietto A, Raiola AM, Van Lint MT, et al. Factors influencing haematological recovery after allogeneic haemopoietic stem cell transplants: graft-versus-host disease, donor type, cytomegalovirus infections and cell dose. Br J Haematol. 2001;112:219–227. doi: 10.1046/j.1365-2141.2001.02468.x. [DOI] [PubMed] [Google Scholar]
- 12.Jabbour E, O’Brien S, Thomas D, et al. Inotuzumab ozogamicin (IO; CMC544), a CD22 monoclonal antibody attached to calicheamycin, produces complete response (CR) plus complete marrow response (mCR) of greater than 50% in refractory relapse (R-R) acute lymphocytic leukemia (ALL) J Clin Oncol. 2011;29(Suppl):Abstract 6507. [Google Scholar]
- 13.Schindler J, Gajavelli S, Ravandi F, et al. A phase I study of a combination of anti-CD19 and anti-CD22 immunotoxins (Combotox) in adult patients with refractory B-lineage acute lymphoblastic leukaemia. Br J Haematol. 2011;154:471–476. doi: 10.1111/j.1365-2141.2011.08762.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Herrera L, Farah RA, Pellegrini VA, et al. Immunotoxins against CD19 and CD22 are effective in killing precursor-B acute lymphoblastic leukemia cells in vitro. Leukemia. 2000;14:853–858. doi: 10.1038/sj.leu.2401779. [DOI] [PubMed] [Google Scholar]
- 15.Ghetie MA, Podar EM, Gordon BE, et al. Combination immunotoxin treatment and chemotherapy in SCID mice with advanced, disseminated Daudi lymphoma. Int J Cancer. 1996;68:93–96. doi: 10.1002/(SICI)1097-0215(19960927)68:1<93::AID-IJC16>3.0.CO;2-7. [DOI] [PubMed] [Google Scholar]
- 16.Herrera L, Stanciu-Herrera C, Morgan C, et al. Anti-CD19 immunotoxin enhances the activity of chemotherapy in severe combined immunodeficient mice with human pre-B acute lymphoblastic leukemia. Leuk Lymphoma. 2006;47:2380–2387. doi: 10.1080/10428190600821989. [DOI] [PubMed] [Google Scholar]
- 17.Griffin TC, Weitzman S, Weinstein H, et al. A study of rituximab and ifosfamide, carboplatin, and etoposide chemotherapy in children with recurrent/refractory B-cell (CD20 +) non-Hodgkin lymphoma and mature B-cell acute lymphoblastic leukemia: a report from the Children’s Oncology Group. Pediatr Blood Cancer. 2009;52:177–181. doi: 10.1002/pbc.21753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Coiffier B, Lepage E, Briere J, et al. CHOP chemotherapy plus rituximab compared with CHOP alone in elderly patients with diffuse large-B-cell lymphoma. N Engl J Med. 2002;346:235–242. doi: 10.1056/NEJMoa011795. [DOI] [PubMed] [Google Scholar]
- 19.Pfreundschuh M, Trümper L, Österborg A, et al. CHOP-like chemotherapy plus rituximab versus CHOP-like chemotherapy alone in young patients with good-prognosis diffuse large-B-cell lymphoma: a randomised controlled trial by the MabThera International Trial (MInT) Group. Lancet Oncol. 2006;7:379–391. doi: 10.1016/S1470-2045(06)70664-7. [DOI] [PubMed] [Google Scholar]
- 20.Oriol A, Ribera JM, Bergua J, et al. High-dose chemotherapy and immunotherapy in adult Burkitt lymphoma: comparison of results in human immunodeficiency virus-infected and noninfected patients. Cancer. 2008;113:117–125. doi: 10.1002/cncr.23522. [DOI] [PubMed] [Google Scholar]
- 21.Corazzelli G, Frigeri F, Russo F, et al. RD-CODOX-M/IVAC with rituximab and intrathecal liposomal cytarabine in adult Burkitt lymphoma and “unclassifiable” highly aggressive B-cell lymphoma. Br J Haematol. 2012;156:234–244. doi: 10.1111/j.1365-2141.2011.08947.x. [DOI] [PubMed] [Google Scholar]
- 22.Thomas DA, Faderl S, O’Brien S, et al. Chemoimmunotherapy with hyper-CVAD plus rituximab for the treatment of adult Burkitt and Burkitt-type lymphoma or acute lymphoblastic leukemia. Cancer. 2006;106:1569–1580. doi: 10.1002/cncr.21776. [DOI] [PubMed] [Google Scholar]
- 23.Raponi S, Stefania De Propris M, Intoppa S, et al. Flow cytometric study of potential target antigens (CD19, CD20, CD22, CD33) for antibody-based immunotherapy in acute lymphoblastic leukemia: analysis of 552 cases. Leuk Lymphoma. 2011;52:1098–1107. doi: 10.3109/10428194.2011.559668. [DOI] [PubMed] [Google Scholar]
- 24.Ghetie M-A, May RD, Till M, et al. Evaluation of ricin A chain-containing immunotoxins directed against CD19 and CD22 antigens on normal and malignant human B-cells as potential reagents for in vivo therapy. Cancer Res. 1988;48:2610–2617. [PubMed] [Google Scholar]
- 25.Herrera L, Yarbrough S, Ghetie V, et al. Treatment of SCID/human B cell precursor ALL with anti-CD19 and anti-CD22 immunotoxins. Leukemia. 2003;17:334–338. doi: 10.1038/sj.leu.2402790. [DOI] [PubMed] [Google Scholar]
- 26.de Vries JF, Zwaan CM, De Bie M, et al. The novel calicheamicin-conjugated CD22 antibody inotuzumab ozogamicin (CMC-544) effectively kills primary pediatric acute lymphoblastic leukemia cells. Leukemia. 2012;26:255–264. doi: 10.1038/leu.2011.206. [DOI] [PubMed] [Google Scholar]
- 27.Liu X-Y, Pop LM, Tsai L, et al. Chimeric, divalent and tetravalent anti-CD19 monoclonal antibodies with potent in vitro and in vivo antitumor activity against human B-cell lymphoma and pre-B acute lymphoblastic leukemia cell lines. Int J Cancer. 2011;129:497–506. doi: 10.1002/ijc.25695. [DOI] [PubMed] [Google Scholar]
- 28.Dreier T, Baeuerle PA, Fichtner I, et al. T cell costimulus-independent and very efficacious inhibition of tumor growth in mice bearing subcutaneous or leukemic human B cell lymphoma xenografts by a CD19-/CD3- bispecific single-chain antibody construct. J Immunol. 2003;170:4397–4402. doi: 10.4049/jimmunol.170.8.4397. [DOI] [PubMed] [Google Scholar]
- 29.Stanciu-Herrera C, Morgan C, Herrera L. Anti-CD19 and anti-CD22 monoclonal antibodies increase the effectiveness of chemotherapy in pre-B acute lymphoblastic leukemia cell lines. Leuk Res. 2008;32:625–632. doi: 10.1016/j.leukres.2007.07.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Advani A, Gundacker H, Sala-Torra O, et al. Southwest Oncology GroupstudyS0530:aphase2trialofclofarabine/cytarabineforrelapsed/ refractory acute lymphocytic leukemia. Blood. 2009;114(Suppl 1):Abstract 3094. [Google Scholar]
- 31.Larson RA, Dodge RK, Linker CA, et al. A randomized controlled trial of filgrastim during remission induction and consolidation chemotherapy for adults with acute lymphoblastic leukemia: CALGB study 9111. Blood. 1998;92:1556–1564. [PubMed] [Google Scholar]
- 32.Ludwig W-D, Rieder H, Bartram CR, et al. Immunophenotypic and genotypic features, clinical characteristics, and treatment outcome of adult pro-B acute lymphoblastic leukemia: results of the German multicenter trials GMALL 03/87 and 04/89. Blood. 1998;92:1898–1909. [PubMed] [Google Scholar]
- 33.Rowe JM, Buck G, Burnett AK, et al. Induction therapy for adults with acute lymphoblastic leukemia: results of more than 1500 patients from the international ALL trial: MRC UKALL XII/ECOG E2993. Blood. 2005;106:3760–3767. doi: 10.1182/blood-2005-04-1623. [DOI] [PubMed] [Google Scholar]
- 34.Ribera J, Oriol A, Bethencourt C, et al. Comparison of intensive chemotherapy, allogeneic or autologous stem cell transplantation as post-remission treatment for adult patients with high-risk acute lymphoblastic leukemia. Results of the PETHEMA ALL-93 trial. Haematologica. 2005;90:1346–1356. [PubMed] [Google Scholar]
- 35.Bassan R, Hoelzer D. Modern therapy of acute lymphoblastic leukemia. J Clin Oncol. 2011;29:532–543. doi: 10.1200/JCO.2010.30.1382. [DOI] [PubMed] [Google Scholar]